Submitted:
06 June 2024
Posted:
07 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sampling and Determination of Melatonin
2.3. Statistical Processing
3. Results
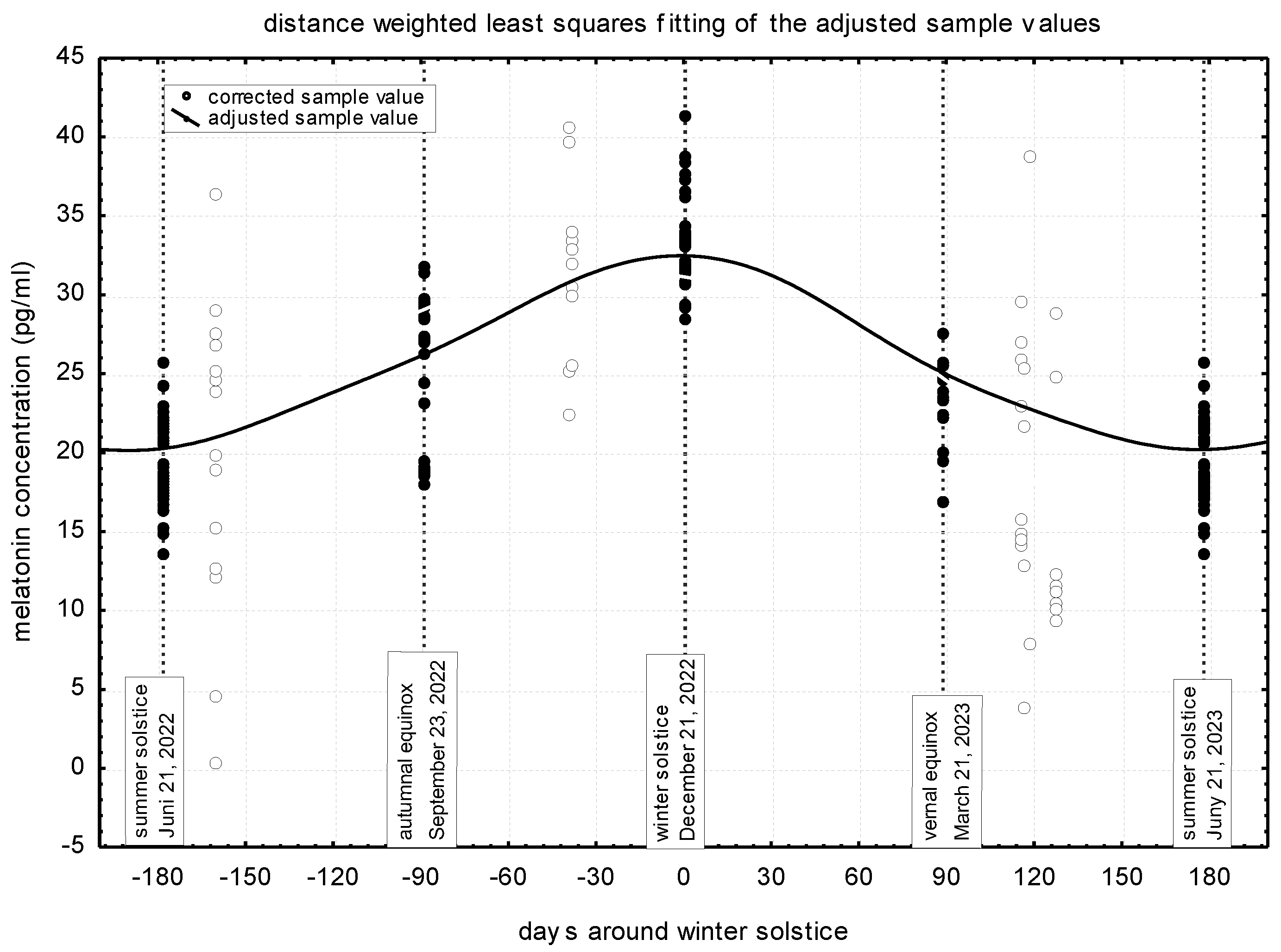
3.1. Circannual Rhythm
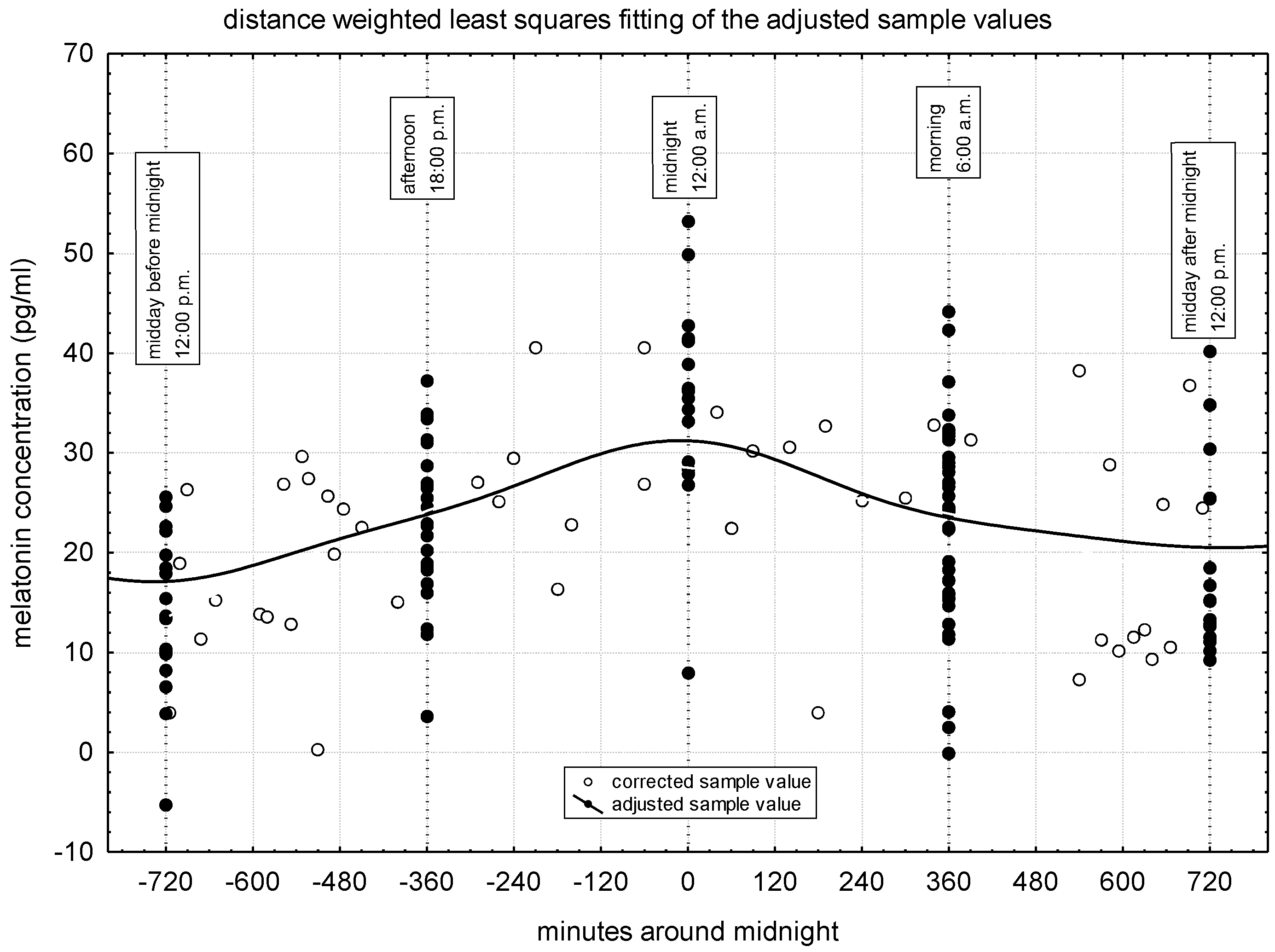
3.2. Circadian Rhythm
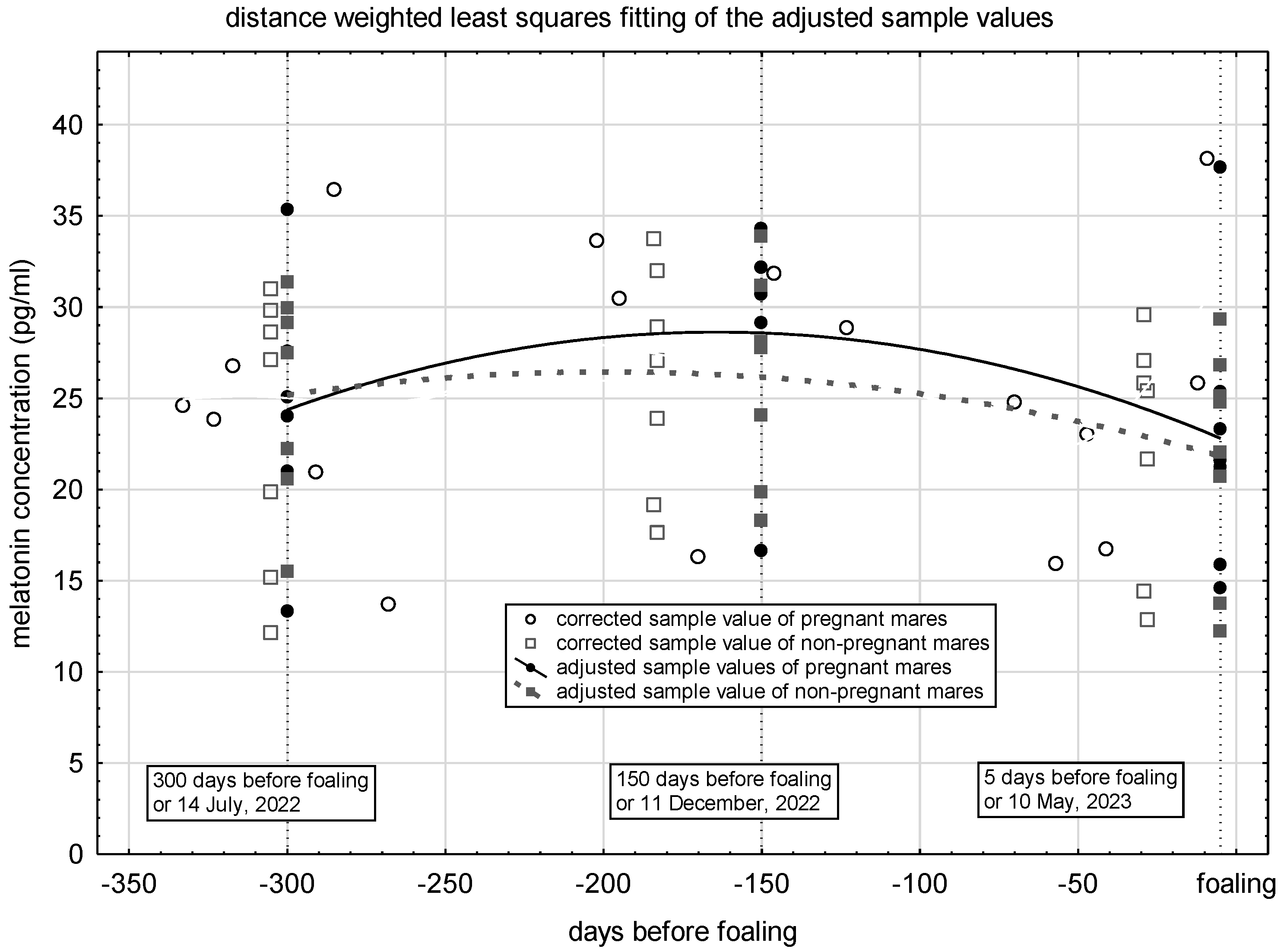
3.2. Gestational Age
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schroeder, M.M.; Harrison, K.R.; Jaeckel, E.R.; Berger, H.N.; Zhao, X.; Flannery, M.P.; St Pierre, E.C.; Pateqi, N.; Jachimska, A.; Chervenak, A.P.; Wong, K.Y. The roles of rods, cones, and melanopsin in photoresponses of M4 intrinsically photosensitive retinal ganglion cells (ipRGCs) and optokinetic visual behavior. Front Cell Neurosci. 2018, 12:203. [CrossRef]
- Talpur, H.S.; Chandio, I.B.; Brohi, R.D.; Worku, T.; Rehman, Z.; Bhattarai, D.; Ullah, F.; JiaJia, L.; Yang, L. Research progress on the role of melatonin and its receptors in animal reproduction: A comprehensive review. Reprod Domest Anim. 2018, 53:831–849. [CrossRef]
- Gao, Y.; Zhao, S.; Zhang, Y.; Zhang, Q. Melatonin Receptors: A Key Mediator in Animal Reproduction. Vet Sci. 2022, 9:309. [CrossRef]
- Sharp, D.C.; Grubaugh, W.R. Use of push-pull perfusion techniques in studies of gonadotropin-releasing hormone secretion in mares. J Reprod Fertil Suppl. 1987, 35:289–296.
- Hart, P.J.; Squires, E.L.; Imel, K.J.; Nett, T.M. Seasonal variation in hypothalamic content of gonadotropin-releasing hormone (GnRH), pituitary receptors for GnRH, and pituitary content of luteinizing hormone and follicle-stimulating hormone in the mare. Biol Reprod. 1984, 30:1055–1062. [CrossRef]
- Grubaugh, W.; Sharp, D.C.; Berglund, L.A.; McDowell, K.J.; Kilmer, D.M.; Peck, L.S.; Seamans, K.W. Effects of pinealectomy in Pony mares. J Reprod Fertil Suppl. 1982, 32:293–295.
- Sharp, D.C.; Vernon, M.W.; Zavy, M.T. Alteration of seasonal reproductive patterns in mares following superior cervical ganglionectomy. J Reprod Fertil Suppl. 1979, 27:87–93.
- Kilmer, D.M.; Sharp, D.C.; Berglund, L.A.; Grubaugh, W.; McDowell, K.J.; Peck, L.S. Melatonin rhythms in Pony mares and foals. J Reprod Fertil Suppl. 1982, 32:303–307.
- Nagy, P.; Guillaume, D.; Daels, P. Seasonality in mares. Anim Reprod Sci. 2000, 60-61:245–262. [CrossRef]
- Murphy, B.A. Circadian and circannual regulation in the horse: internal timing in an elite athlete. J Equine Vet Sci. 2019, 76:14–24. [CrossRef]
- Fielding, D. Reproductive characteristics of the jenny donkey - Equus asinus: A review. Trop Anim Health Prod. 1988, 20:161–166. [CrossRef]
- Galisteo, J.; Perez-Marin, C.C. Factors affecting gestation length and estrus cycle characteristics in Spanish donkey breeds reared in southern Spain. Theriogenology. 2010, 74:443–450. [CrossRef]
- Carluccio, A.; Gloria, A.; Robbe, D.; Veronesi, M.C.; De Amicis, I.; Cairoli, F.; Contri, A. Reproductive characteristics of foal heat in female donkeys. Animal. 2017, 11:461–465. [CrossRef]
- Cozzi, B.; Morei, G.; Ravault, J.P.; Chesneau, D.; Reiter, R.J. Circadian and seasonal rhythms of melatonin production in mules (Equus asinus x Equus caballus). J Pineal Res. 1991, 10:130–135. [CrossRef]
- O’Brien, C.; Darcy-Dunne, M.R.; Murphy, B.A. The effects of extended photoperiod and warmth on hair growth in ponies and horses at different times of year. PLoS ONE. 2020, 15:e0227115. [CrossRef]
- Altinsaat, Ç.; Üner, A.G.; Sulu, N.; Ergün, A.: Seasonal variations in serum concentrations of melatonin, testosterone, and progesterone in Arabian horse. Ankara Üniv Vet Fak Derg. 2009, 56:19–24. [CrossRef]
- Guerin, M.V.; Deed, J.R.; Kennaway, D.J.; Matthews, C.D. Plasma melatonin in the horse: Measurements in natural photoperiod and in acutely extended darkness throughout the year. J Pineal Res. 1995, 19:7–15. [CrossRef]
- Haritou, S.J.A.; Zylstra, R.; Ralli, C.; Turner, S.; Tortonese, D.J. Seasonal changes in circadian peripheral plasma concentrations of melatonin, serotonin, dopamine and cortisol in aged horses with Cushing's disease under natural photoperiod. J Neuroendocrinol. 2008, 20:988–996. [CrossRef]
- Rapacz, A.; Lewczuk, B.; Prusik, M.; Raś, A. Diurnal rhythm of plasma melatonin level in mares from spring equinox to summer solstice. Bull Vet Inst Pulawy. 2010, 54:693–699.
- Guillaume, D.; Zarazaga, L.; Malpaux, B.; Chemineau, P. Variability of plasma melatonin level in pony mares (Equus caballus), comparison with the hybrid: mules and with jennies (Equus asinus). Reprod Nutr Dev. 2006, 46:633–639. [CrossRef]
- González-Arto, M.; Vicente-Carrillo, A.; Martínez-Pastor, F.; Fernández-Alegre, E.; Roca, J.; Miró, J.; Rigau, T.; Rodríguez-Gil, J.E., Pérez-Pé, R.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; Casao, A. Melatonin receptors MT1 and MT2 are expressed in spermatozoa from several seasonal and nonseasonal breeder species. Theriogenology. 2016, 86(8):1958–1968. [CrossRef]
- Messias, T.B.O.N.; Sant’Ana, A.M.S.; Araújo, E.O.M.; Rangel, A.H.N.; Vasconcelos, A.S.E.; Salles, H.O.; Morgano, M.A.; Silva, V.S.N.; Pacheco, M.T.B.; Queiroga, R.C.R.E. Milk from Nordestina donkey breed in Brazil: Nutritional potential and physicochemical characteristics in lactation. Int Dairy J. 2022, 127:105291. [CrossRef]
- Ernst, J. The donkey. In: Living Heritage, Old Historical Hungarian Livestock; Bodó, I. ed.; Agroinform Publishing and Printing Ltd., Budapest, Hungary, 2004; pp. 30–33.
- Lénárt, Z.; Ernst, M.; Gáspárdy, A. Preliminary results on body conformation of Hungarian Fallow Donkey. Danub Anim Genet Resour. 2017, 2:53–58.
- Harmat, L.; Kuncicky, A.; Lénárt, Z.; Ernst, M.; Nagy, J.; Gáspárdy, A. Conformation traits of Hungarian Fallow Donkey mares according to their basic colour. Danub Anim Genet Resour. 2022, 7:17–21.
- Pedigree viewer (2015) version 6.5f. http://bkinghor@une.edi.au.
- TIBCO Software Inc. (2020) Data Science Workbench. Statistica version 14. http://tibco.com.
- Murphy, B.A.; Elliott, J.A.; Sessions, D.R.; Vick, M.M.; Kennedy, E.L.; Fitzgerald, B.P. Rapid phase adjustment of melatonin and core body temperature rhythms following a 6-h advance of the light/dark cycle in the horse. J Circadian Rhythms. 2007, 5:5. [CrossRef]
- Piccione, G.; Giannetto, C.; Bertolucci, C.; Refinetti, R. Daily rhythmicity of circulating melatonin is not endogenously generated in the horse. Biol Rhythm Res. 2013, 44:143–149. [CrossRef]
- Murphy, B.A.; Martin, A.-M.; Furney, P.; Elliott, J.A. Absence of a serum melatonin rhythm under acutely extended darkness in the horse. J Circadian Rhythms. 2011, 9:3. [CrossRef]
- Gáspárdy, A.; Gallagher, G.; Bartha, B.; Cseh, S.; Fekete, S.G.; Somoskői, B. Plasma melatonin concentration during the early post-partum period in Thoroughbred mares and their foals. Acta Vet Hung. 2023, 71:119–127. [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.A.; Manchester, L.C.; Tan, D.-X. Peripheral reproductive organ health and melatonin: ready for prime time. Int J Mol Sci. 2013, 14:7231–7272. [CrossRef]
- Tarocco, A.; Caroccia, N.; Morciano, G.; Wieckowski, M.R.; Ancora, G.; Garani, G.; Pinton, P. Melatonin as a master regulator of cell death and inflammation: molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 2019, 10:317. [CrossRef]
- Kivelä, A. Serum melatonin during human pregnancy. Acta Endocrinol (Copenh). 1991, 124:233-237. [PubMed]
- Ejaz, H.; Figaro, J.K.; Woolner, A.M.F.; Thottakam, B.M.V.; Galley, H.F. Maternal Serum Melatonin Increases During Pregnancy and Falls Immediately After Delivery Implicating the Placenta as a Major Source of Melatonin. Front Endocrinol (Lausanne). 2021, 11:623038. [CrossRef]
- Cagnacci, A.; Soldani, R.; Melis, G.B.; Volpe, A. Diurnal rhythms of labor and delivery in women: Modulation by parity and seasons. Am J Obstet Gynecol. 1998, 178:140–145. [CrossRef]
- Mårtensson, L.G.; Andersson, R.G.; Berg, G. Melatonin together with noradrenaline augments contractions of human myometrium. Eur J Pharmacol. 1996, 316:273–275. [CrossRef]
- Karpovitch, A.E.; Inna, E.; Moiseevich, K.I. In Melatonin: pregnancy and childbirth. MOJ Curr Res & Rev. 2018, 1:206‒210. [CrossRef]
- Wilhelmsen, M.; Amirian, I.; Reiter, R.J.; Rosenberg, J.; Gönegur, I. Analgesic effects of melatonin: a review of current evidence from experimental and clinical studies. J Pineal Res. 2011, 51:270–277. [CrossRef]
- Olcese, J.; Lozier, S.; Paradise, C. Melatonin and the circadian timing of human parturition. Reprod Sci. 2013, 20:168‒174. [CrossRef]
|
Event p < 0.001 |
Melatonin concentration mean, pg/ml |
SEM* |
| Summer solstice, Juni 22, 2022, 12 a.m. | 27.67a | 0.459 |
| Autumnal equinox, September 24, 2022, 12 a.m. | 33.35b | 1.071 |
| Winter solstice, December 22, 2022, 12 a.m. | 42.18c | 0.523 |
| Vernal equinox, March 21, 2023, 12 a.m. | 31.23b | 0.683 |
|
Dayparts p < 0.001 |
Melatonin concentration mean, pg/ml |
SEM* |
| Midday before midnight, 12:00 p.m., December 21, 2022 | 23.85a | 1.981 |
| Afternoon, 18:00 p.m., December 21, 2022 | 33.17b | 1.607 |
| Midnight, 12:00 a.m., December 22, 2022 | 45.16c | 2.460 |
| Morning, 06:00 a.m., December 22, 2022 | 32.63b | 1.652 |
| Midday after midnight, 12:00 p.m., December 22, 2022 | 28.81ab | 2.371 |
|
Days to foaling p = 0.136 |
Melatonin concentration mean, pg/ml |
SEM* |
| 300 days before foaling, p = 0.830 | 38.80 | 1.74 |
| Pregnant | 38.37 | 2.971 |
| Non-pregnant | 39.17 | 2.218 |
| 150 days before foaling, p = 0.523 | 41.17 | 1.757 |
| Pregnant | 42.58 | 3.107 |
| Non-pregnant | 40.16 | 2.169 |
| 5 days before foaling, p = 0.771 | 36.09 | 1.754 |
| Pregnant | 36.67 | 2.922 |
| Non-pregnant | 35.58 | 2.248 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





