3.2. Experimental Section
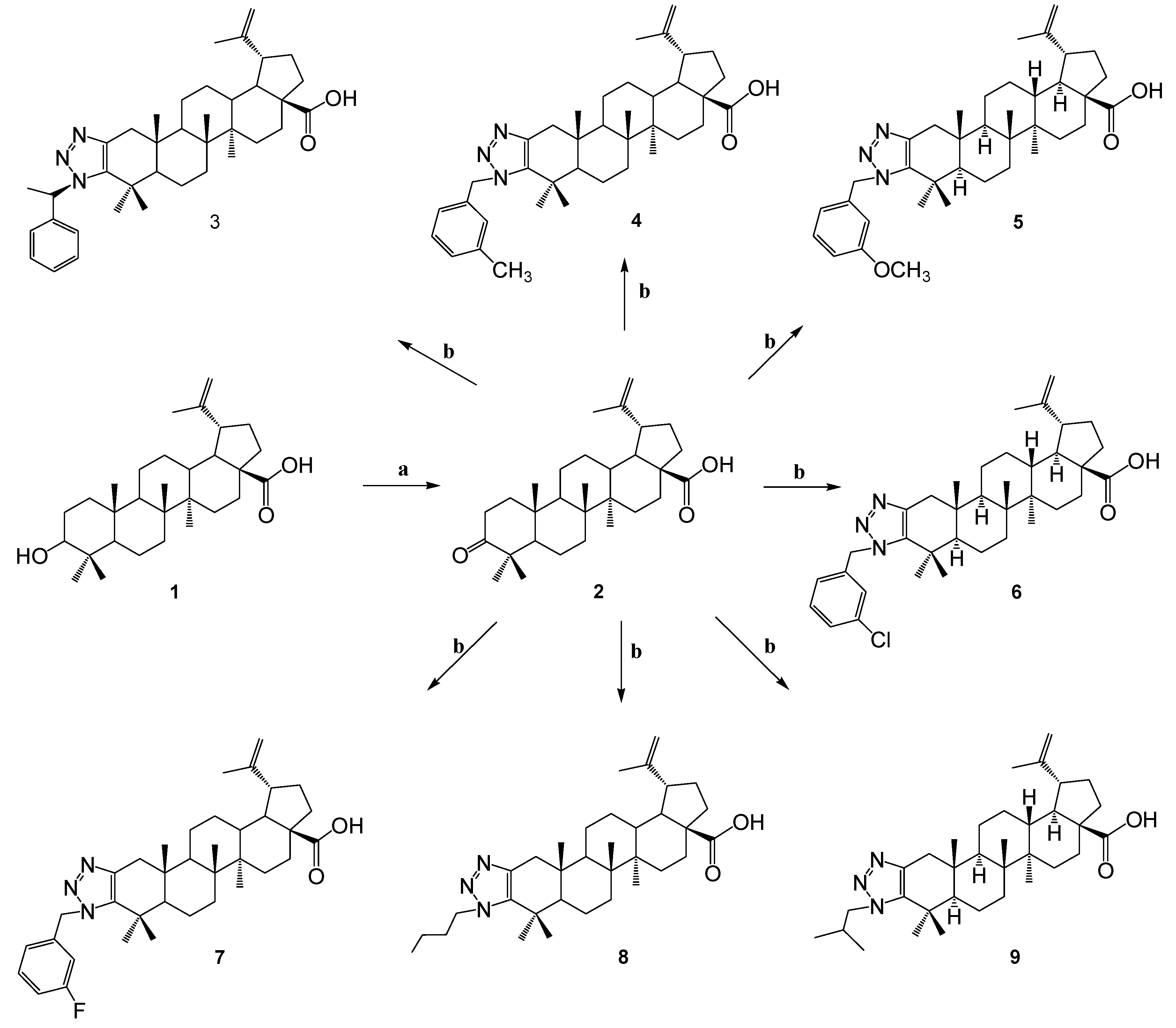
3-Oxo-lup-20(29)-en-28-oic Acid (Betulonic Acid, 2). Betulin (5.0 g, 11.3 mmol) was placed in a 0.5 l flask and filled with acetone (150 ml) and dissolved in an ultrasonic water bath. The freshly prepared Jones reagent (6.65 g Na2Cr2O7 and 6 ml H2SO4 in 50 ml water) was added drop by drop to the cooled (on an ice bath) solution. The color of the solution began to change. The reaction mixture was allowed to heat up to room temperature and continued to be stirred for 5 hours. The course of the reaction was checked by the TLC. Then MeOH (100 ml) was first added to the reaction mixture, and then water (50 ml). The resulting precipitate was filtered and washed with water (50 ml). The crude product was dried, then dissolved in Et2O (60 ml) and washed with water (30 ml), 7.5% hydrochloric acid (20 ml), water (20 ml), saturated aqueous solution NaHCO3 (20 ml) and water (20 ml). The ether layer was driven off on a rotary evaporator, and the remaining residue was purified by column chromatography (silica gel), a mixture of heptane and ethyl acetate (80:20) was used as an eluent. Compound 2 is collorless powder. The product yield is 1.55 g (30.2%).
1H NMR (400 MHz, CDCl3): δ 4.74 (d, J = 2.3 Hz, 1H), 4.66 – 4.58 (m, 1H), 3.01 (td, J = 10.7, 4.6 Hz, 1H), 2.56 – 2.35 (m, 2H), 2.33 – 2.17 (m, 2H), 2.07 – 1.83 (m, 2H), 1.70 (s, 4H), 1.64 (t, J = 11.4 Hz, 1H), 1.59 – 1.14 (m, 14H), 1.13 – 1.04 (m, 4H), 1.03 – 0.95 (m, 9H), 0.93 (s, 3H).
13C NMR (101 MHz, CDCl3): δ 218.23, 181.72, 150.32, 109.79, 56.37, 54.94, 49.85, 49.19, 47.34, 46.89, 42.49, 40.64, 39.61, 38.51, 37.04, 36.92, 34.13, 33.60, 32.10, 30.55, 29.68, 26.64, 25.49, 21.37, 21.00, 19.63, 19.37, 15.96, 15.82, 14.63.
1′-((S)-1-Phenylethyl)-1H′-lup-2-eno-[2,3-d]-[
1,
2,
3]
-triazole-28 oic Acid (3). Betulonic acid (1.0 g, 1 equiv, 2.2 mmol), 4-nitrophenyl azide (360 mg, 1.3 equiv, 2.86 mmol), (S)- (‒)-α-methylbenzylamine (350 mg, 1.3 equiv, 2.86 mmol) and 4 Å molecular sieves (200 mg) were added to a dried reaction tube with a screw cap equipped with a magnetic stirrer. The mixture was dissolved in dry toluene (5 ml) and the reaction mixture was stirred at 100 °C for 24 hours. A mixture of H
2SO
4 and ethanol (10:90) was used to visualize the TLC plates. The crude reaction mixture was purified directly using column chromatography (silica gel), first using dichloromethane to remove all 4-nitroaniline formed during the reaction, and then a mixture of petroleum ether and ethyl acetate was used as an eluent. Compound
3 is powdery with a yellowish tinge, m.p. 325-327
oC. Yield 790 mg (61 %).
1H NMR (400 MHz, CDCl3): δ 7.33 – 7.25 (m, 3H), 7.25 – 7.19 (m, 2H), 5.72 (q, J = 7.0 Hz, 1H), 4.77 (d, J = 2.3 Hz, 1H), 4.64 (m, 1H), 3.03 (td, J = 10.6, 4.5 Hz, 1H), 2.96 (d, J = 15.3 Hz, 1H), 2.32 – 2.20 (m, 2H), 2.15 (d, J = 15.3 Hz, 1H), 2.05 – 1.95 (m, 5H), 1.81-1.60 (m, 5H), 1.61 – 1.32 (m, 11H), 1.26 (s, 5H), 1.10 (s, 3H), 0.99 (s, 6H), 0.81 (s, 3H).
13C NMR (101 MHz, CDCl3): δ 181.07, 150.22, 141.79, 141.05, 137.53, 128.64, 127.56, 126.24, 109.88, 59.28, 56.38, 54.78, 49.34, 49.21, 46.90, 42.45, 40.60, 38.91, 38.53, 38.32, 37.04, 33.80, 33.41, 32.08, 30.59, 29.80, 28.89, 25.50, 23.73, 21.36, 21.31, 19.42, 18.91, 16.25, 15.70, 14.65.
1′-(3-Methylbenzyl)-1H′-lup-2-eno-[2,3-d]-[
1,
2,
3]
-triazole-28- oic Acid (4). Betulonic acid
2 (100 mg, 1 equiv, 0.22 mmol), 4-nitrophenyl azide (72 mg, 2 equiv, 0.44 mmol), 3-methylbenzylamine (74.6 mg, 2.8 equiv, 0.616 mmol) and 4 Å molecular sieves (50 mg) were added to a dried reaction tube with a screw cap equipped with a magnetic stirrer. The mixture was dissolved in dry toluene (1 ml) and the reaction mixture was stirred at 100 °C for 24 hours. A mixture of H
2SO
4 and ethanol (10:90) was used to visualize the TLC plates. The crude reaction mixture was purified directly using column chromatography (silica gel), first using dichloromethane to remove all 4-nitroaniline formed during the reaction, and then a mixture of petroleum ether and ethyl acetate (10:1) was used as an eluent. Compound
4 is pale yellow powder, m.p. 261-264
oC. Yield 71.7 mg (68.3 %).
1H NMR (400 MHz, CDCl3): δ 7.17 (t, J = 7.6 Hz, 1H), 7.06 (d, J = 7.6 Hz, 1H), 6.85 (s, 1H), 6.81 (d, J = 7.7 Hz, 1H), 5.62 (m, 2H), 4.76 (d, J = 2.3 Hz, 1H), 4.64 (m, 1H), 4.12 (q, J = 7.2 Hz, 0H), 3.04 (td, J = 10.6, 4.5 Hz, 1H), 2.96 (d, J = 15.3 Hz, 1H), 2.25 (m, 6H), 1.99 (m, 2H), 1.77 (d, J = 12.8 Hz, 1H), 1.71 (s, 3H), 1.45 (m, 13H), 1.17 (s, 3H), 1.04 (s, 3H), 0.99 (d, J = 9.2 Hz, 6H), 0.90 (m, 1H), 0.78 (s, 3H).
13C NMR (101 MHz, CDCl3): δ 181.38, 150.25, 141.84, 138.49, 138.00, 136.38, 128.59, 128.52, 127.03, 123.46, 109.86, 56.40, 54.59, 52.81, 49.29, 49.19, 46.90, 42.44, 40.56, 38.97, 38.51, 38.36, 37.04, 33.71, 33.35, 32.08, 30.58, 29.79, 28.77, 25.50, 21.41, 21.37, 21.31, 19.42, 18.91, 16.08, 15.70, 14.65.
HRMS (ESI+): m/z calculated for C38H54N3O2 [M+H]+: 584.42160, found: 584.4216.
1′-(3-Methoxybenzyl)-1H′-lup-2-eno-[2,3-d]-[
1,
2,
3]
-triazole-28- oic Acid (5). Betulonic acid (100 mg, 1 equiv, 0.22 mmol), 4-nitrophenyl azide (72 mg, 2 equiv, 0.44 mmol), 3-methoxybenzylamine (84.5 mg, 2.8 equiv, 0.616 mmol) and 4 Å molecular sieves (50 mg) were added to a dried reaction tube with a screw cap equipped with a magnetic stirrer. The mixture was dissolved in dry toluene (1 ml) and the reaction mixture was stirred at 100 °C for 18 hours. A mixture of H
2SO
4 and ethanol (10:90) was used to visualize the TLC plates. The crude reaction mixture was purified directly using column chromatography (silica gel), first using dichloromethane to remove all 4-nitroaniline formed during the reaction, and then a mixture of petroleum ether and ethyl acetate was used as an eluent. Compound
5 is powdery with a yellowish tinge, m.p. 205-208
oC. Yield 78 mg (60 %).
1H NMR (400 MHz, CDCl3): δ 7.21 (td, J = 7.9, 3.3 Hz, 1H), 6.83 – 6.75 (m, 1H), 6.65 – 6.54 (m, 3H), 5.62 (d, J = 3.8 Hz, 3H), 4.76 (d, J = 2.3 Hz, 1H), 4.67 – 4.63 (m, 1H), 3.74 (d, J = 1.6 Hz, 4H), 3.09 – 2.92 (m, 2H), 2.26 (ddd, J = 15.5, 9.7, 3.5 Hz, 2H), 2.21 – 2.14 (m, 1H), 2.06 – 1.82 (m, 3H), 1.81 – 1.75 (m, 2H), 1.71 (s, 3H), 1.68 (s, 0H), 1.61 – 1.36 (m, 10H), 1.35 – 1.18 (m, 4H), 1.17 (d, J = 2.6 Hz, 3H), 1.04 (s, 3H), 1.02 – 0.96 (m, 8H), 0.81 (s, 1H), 0.77 (s, 3H).
13C NMR (101 MHz, CDCl3): δ 180.81, 159.97, 150.24, 141.89, 138.09, 138.04, 129.78, 118.65, 113.31, 111.96, 109.87, 56.36, 55.23, 54.79, 54.59, 52.74, 52.66, 49.30, 49.19, 46.88, 44.09, 42.45, 40.56, 40.36, 38.98, 38.48, 38.37, 37.03, 33.72, 33.35, 32.07, 30.57, 29.79, 28.73, 25.50, 21.38, 21.31, 19.42, 18.91, 16.08, 15.71, 15.19, 14.65.
HRMS (ESI+): m/z calculated for C38H54N3O3 [M+H]+: 600.4160, found: 600.4194.
1′-(3-Chlorobenzyl)-1H′-lup-2-eno-[2,3-d]-[
1,
2,
3]
-triazole-28- oic Acid (6). Betulonic acid
2 (100 mg, 1 equiv, 0.22 mmol), 4-nitrophenyl azide (72 mg, 2 equiv, 0.44 mmol), 3-chlorobenzylamine (87 mg, 2.8 equiv, 0.616 mmol) and 4 Å molecular sieves (50 mg) were added to a dried reaction tube with a screw cap equipped with a magnetic stirrer. The mixture was dissolved in dry toluene (1 ml) and the reaction mixture was stirred at 100 °C for 24 hours. A mixture of H
2SO
4 and ethanol (10:90) was used to visualize the TLC plates. The crude reaction mixture was purified directly using column chromatography (silica gel), first using dichloromethane to remove all 4-nitroaniline formed during the reaction, and then a mixture of petroleum ether and ethyl acetate (1:1) was used as an eluent. Compound
6 is pale yellow powder, m.p. 288-290
oC. Yield 65 mg (62 %).
1H NMR (400 MHz, CDCl3): δ 7.28 – 7.19 (m, 2H), 7.03 (d, J = 2.0 Hz, 1H), 6.89 (dt, J = 5.7, 2.1 Hz, 1H), 5.61 (d, J = 5.5 Hz, 2H), 4.76 (d, J = 2.3 Hz, 1H), 4.64 (t, J = 1.9 Hz, 1H), 3.04 (td, J = 10.6, 4.6 Hz, 1H), 2.96 (d, J = 15.4 Hz, 1H), 2.28 (ddd, J = 14.7, 9.0, 3.2 Hz, 2H), 2.23 – 2.15 (m, 1H), 2.00 (ddd, J = 17.8, 11.6, 5.5 Hz, 2H), 1.83 – 1.73 (m, 1H), 1.71 (s, 3H), 1.67 – 1.19 (m, 13H), 1.17 (s, 4H), 1.06 – 0.95 (m, 9H), 0.78 (s, 3H).
13C NMR (101 MHz, CDCl3): δ 181.63, 150.23, 142.05, 130.06, 128.07, 126.53, 124.55, 109.87, 56.41, 54.51, 52.16, 49.27, 49.20, 46.91, 42.44, 40.55, 38.98, 38.53, 38.29, 37.03, 33.69, 33.31, 32.07, 30.58, 29.80, 28.83, 25.47, 21.41, 21.37, 19.42, 18.88, 16.09, 15.73, 14.64.
HRMS (ESI+): m/z calculated for C37H51ClN3O2 [M+H]+: 604.36698, found: 604.3661.
1’-(3-Fluorobenzyl)-1H′-lup-2-eno-[2,3-d]-[
1,
2,
3]
-triazole-28- oic Acid (7). Betulonic acid (100 mg, 1 equiv, 0.22 mmol), 4-nitrophenyl azide (72 mg, 2 equiv, 0.44 mmol), 3-fluorobenzylamine (77 mg, 2.8 equiv, 0.616 mmol) and 4 Å molecular sieves (50 mg) were added to a dried reaction tube with a screw cap equipped with a magnetic stirrer. The mixture was dissolved in dry toluene (1 ml) and the reaction mixture was stirred at 100 °C for 22 hours. A mixture of H
2SO
4 and ethanol (10:90) was used to visualize the TLC plates. The crude reaction mixture was purified directly using column chromatography (silica gel), first using dichloromethane to remove all 4-nitroaniline formed during the reaction, and then a mixture of petroleum ether and ethyl acetate was used as an eluent. Compound
7 is powdery with a yellowish tinge, m.p. 302-305
oC. Yield 82 mg (64 %).
1H NMR (400 MHz, CDCl3): δ 7.27 (d, J = 5.5 Hz, 2H), 7.00 – 6.92 (m, 1H), 6.81 (ddd, J = 7.8, 1.8, 0.9 Hz, 1H), 6.71 (dt, J = 9.6, 2.2 Hz, 1H), 5.68 – 5.58 (m, 2H), 4.76 (d, J = 2.3 Hz, 1H), 4.67 – 4.62 (m, 1H), 3.03 (td, J = 10.6, 4.6 Hz, 1H), 2.96 (d, J = 15.4 Hz, 1H), 2.27 (td, J = 12.7, 11.3, 3.3 Hz, 2H), 2.23 – 2.15 (m, 1H), 2.07 – 1.93 (m, 2H), 1.77 (dd, J = 11.4, 4.1 Hz, 1H), 1.71 (s, 3H), 1.66 (t, J = 11.4 Hz, 1H), 1.62 – 1.33 (m, 9H), 1.33 – 1.18 (m, 3H), 1.17 (s, 3H), 1.04 (s, 3H), 0.99 (d, J = 8.9 Hz, 6H), 0.78 (s, 3H).
13C NMR (101 MHz, CDCl3): δ 181.18, 150.22, 142.05, 138.10, 130.41, 130.32, 121.99, 113.63, 113.40, 109.88, 56.38, 54.53, 52.24, 49.28, 49.20, 46.91, 42.45, 40.56, 38.98, 38.53, 38.31, 37.02, 33.69, 33.32, 32.07, 30.58, 29.80, 28.78, 25.48, 21.37, 19.42, 18.89, 16.10, 15.73, 14.65.
HRMS (ESI+): m/z calculated for C37H51FN3O2 [M+H]+: 588.3960, found: 588.3966.
1′-Butyl-1H′-lup-2-eno-[2,3-d]-[
1,
2,
3]
-triazole-28- oic Acid (8). Betulonic acid (100 mg, 1 equiv, 0.22 mmol), 4-nitrophenyl azide (36 mg, 1 equiv, 0.22 mmol), n-butylamine (21 mg, 1.3 equiv, 0.286 mmol) and 4 Å molecular sieves (50 mg) were added to a dried reaction tube with a screw cap equipped with a magnetic stirrer. The mixture was dissolved in dry toluene (1 ml) and the reaction mixture was stirred at 100 °C for 24 hours. A mixture of H
2SO
4 and ethanol (10:90) was used to visualize the TLC plates. The crude reaction mixture was purified directly using column chromatography (silica gel), first using dichloromethane to remove all 4-nitroaniline formed during the reaction, and then a mixture of petroleum ether and ethyl acetate was used as an eluent. Compound
8 is powdery with a yellowish tinge, m.p. 288-290
oC. Yield 74 mg (62.8 %).
1H NMR (400 MHz, CDCl3): δ 4.76 (d, J = 2.3 Hz, 1H), 4.67 – 4.61 (m, 1H), 4.29 (td, J = 7.1, 1.6 Hz, 2H), 3.04 (td, J = 10.5, 4.5 Hz, 1H), 2.91 (d, J = 15.3 Hz, 1H), 2.34 – 2.20 (m, 2H), 2.13 (d, J = 15.4 Hz, 1H), 2.06 – 1.93 (m, 4H), 1.80 – 1.73 (m, 1H), 1.71 (s, 3H), 1.65 (d, J = 11.5 Hz, 1H), 1.62 – 1.33 (m, 11H), 1.30 (s, 3H), 1.26 (s, 4H), 1.18 (s, 3H), 1.04 – 0.93 (m, 10H), 0.93 – 0.80 (m, 1H), 0.77 (s, 3H).
13C NMR (101 MHz, CDCl3): δ 181.21, 150.24, 141.03, 109.86, 56.39, 54.68, 49.37, 49.30, 49.21, 46.90, 42.46, 40.59, 38.98, 38.54, 38.27, 37.05, 33.66, 33.39, 32.85, 32.09, 30.60, 29.81, 29.71, 28.68, 25.50, 21.33, 20.18, 19.43, 18.98, 16.06, 15.72, 14.69, 13.70.
HRMS (ESI+): m/z calculated for C34H54N3O2 [M+H]+: 536.4211, found: 536.4214.
1′-Isobutyl-1H′-lup-2-eno-[2,3-d]-[
1,
2,
3]
-triazole-28- oic Acid (9). Betulonic acid
2 (100 mg, 1 equiv, 0.22 mmol), 4-nitrophenyl azide (36 mg, 1 equiv, 0.22 mmol), isobutylamine (21 mg, 1.3 equiv, 0.286 mmol) and 4 Å molecular sieves (50 mg) were added to a dried reaction tube with a screw cap equipped with a magnetic stirrer. The mixture was dissolved in dry toluene (1 ml) and the reaction mixture was stirred at 100 °C for 24 hours. A mixture of H
2SO
4 and ethanol (10:90) was used to visualize the TLC plates. The crude reaction mixture was purified directly using column chromatography (silica gel), first using dichloromethane to remove all 4-nitroaniline formed during the reaction, and then a mixture of petroleum ether and ethyl acetate (10:1) was used as an eluent. Compound
9 is pale yellow powder, m.p. 299-301
oC. Yield 67.8 mg (64.6 %).
1H NMR (400 MHz, CDCl3): δ 4.76 (d, J = 2.3 Hz, 1H), 4.64 (t, J = 1.9 Hz, 1H), 4.16 – 4.03 (m, 2H), 3.04 (td, J = 10.6, 4.6 Hz, 1H), 2.92 (d, J = 15.3 Hz, 1H), 2.54 (dq, J = 13.8, 6.9 Hz, 1H), 2.35 – 2.19 (m, 2H), 2.14 (d, J = 15.3 Hz, 1H), 2.08 – 1.94 (m, 2H), 1.81 – 1.73 (m, 1H), 1.71 (s, 3H), 1.69 – 1.32 (m, 10H), 1.30 (s, 3H), 1.24 (d, J = 16.4 Hz, 3H), 1.17 (s, 3H), 1.15 – 1.03 (m, 1H), 1.03 – 0.93 (m, 13H), 0.93 – 0.83 (m, 1H), 0.77 (s, 3H).
13C NMR (101 MHz, CDCl3): δ 181.59, 150.26, 140.94, 137.79, 109.83, 56.56, 56.42, 54.74, 49.30, 49.22, 46.91, 42.45, 40.58, 38.90, 38.55, 38.28, 37.05, 33.73, 33.38, 32.10, 30.61, 29.82, 29.38, 28.90, 25.50, 21.57, 21.36, 20.25, 20.21, 19.43, 19.00, 16.04, 15.74, 14.68.
HRMS (ESI+): m/z calculated for C34H54N3O2 [M+H]+: 536.42160, found: 536.4209.
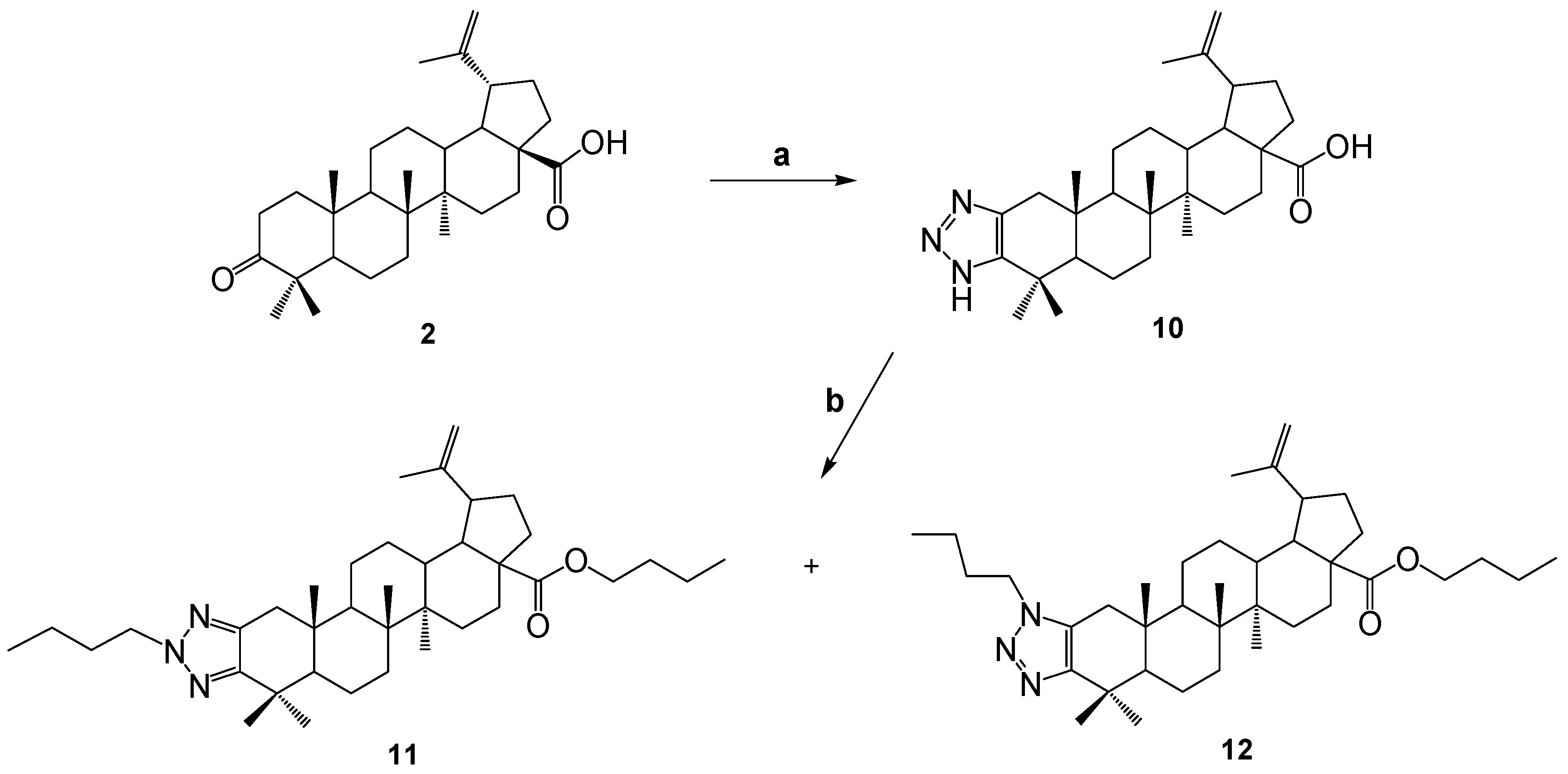
1H′-Lup-2-eno-[2,3-d]-[
1,
2,
3]
-triazole-28-oic Acid (10). Betulonic acid
2 (100 mg, 1 equiv, 0.22 mmol), ammonium acetate (84.8 mg, 5 equiv, 1.1 mmol) and 4-nitrophenyl azide (45.8 mg, 1.3 equiv, 0.28 mmol) were dissolved in dry DMF (1 mL). The reaction mixture was stirred at 80 °C for 24 hours. A mixture of H
2SO
4 and ethanol (10:90) was used to visualize the TLC plates. The crude reaction mixture was purified directly using column chromatography (silica gel), first using dichloromethane to remove all 4-nitroaniline formed during the reaction, and then a mixture of petroleum ether and ethyl acetate (10:1) was used as an eluent. Compound
10 is pale yellow powder, m.p. 158
oC. Yield 48.3 mg (46 %). Spectroscopic data for compound
10 was consistent with previously reported data for this compound.
1H NMR (400 MHz, CDCl3): δ 4.77 (d, J = 2.5 Hz, 1H), 4.64 (d, J = 1.9 Hz, 1H), 3.05 (td, J = 10.7, 4.6 Hz, 1H), 2.90 (d, J = 15.5 Hz, 1H), 2.36 – 2.22 (m, 2H), 2.19 – 2.09 (m, 1H), 2.08 – 1.93 (m, 2H), 1.83 – 1.74 (m, 1H), 1.72 (s, 3H), 1.68 – 1.36 (m, 15H), 1.32 (s, 4H), 1.30 – 1.18 (m, 4H), 1.18 – 1.04 (m, 1H), 1.01 (d, J = 10.2 Hz, 6H), 0.97 – 0.80 (m, 6H), 0.77 (s, 3H).
13C NMR (101 MHz, CDCl3): δ 181.38, 150.35, 149.92, 140.40, 109.80, 56.40, 53.42, 49.22, 49.09, 46.95, 42.51, 41.35, 40.75, 39.04, 38.51, 37.32, 37.07, 33.72, 33.38, 33.30, 32.16, 31.01, 30.62, 29.80, 29.06, 27.67, 25.51, 23.76, 22.63, 21.41, 20.45, 19.42, 19.16, 16.27, 15.69, 14.68.
Compound (11) and (12). Compound 10 (181 mg) was dissolved in dry methanol (1.5 ml) then added potassium tret-butoxide (PTB) (100.08 mg, 0.91 mmol). The reaction mixture was stirred ar room temperature for 3 hours. Arter a 1-bromobutane was slowly added the reaction mixture and stirred at 60oC for 12 hours. The solvent was distilled in a rotary evaporator. The crude reaction mixture was purified directly using column chromatography (silica gel), using a mixture of petroleum ether and ethyl acetate was used as an eluent. Compound 11 is colorless substance. Yield 102.9 mg (46.4 %).
1H NMR (400 MHz, CDCl3): δ 4.76 (d, J = 2.3 Hz, 1H), 4.63 (t, J = 1.8 Hz, 1H), 4.32 (t, J = 7.3 Hz, 2H), 4.09 (qt, J = 10.8, 6.6 Hz, 2H), 3.04 (td, J = 10.8, 4.5 Hz, 1H), 2.83 (d, J = 15.4 Hz, 1H), 2.29 (td, J = 12.4, 11.7, 3.6 Hz, 2H), 2.07 (d, J = 15.4 Hz, 1H), 1.96 – 1.85 (m, 4H), 1.80 – 1.72 (m, 1H), 1.71 (s, 3H), 1.68 – 1.30 (m, 15H), 1.29 (d, J = 4.0 Hz, 4H), 1.21 (s, 2H), 1.17 (d, J = 5.7 Hz, 4H), 1.15 – 1.02 (m, 1H), 1.00 (s, 3H), 0.98 (d, J = 4.3 Hz, 4H), 0.96 – 0.81 (m, 11H), 0.79 (s, 3H).
13C NMR (101 MHz, CDCl3): δ 176.22, 150.89, 150.54, 141.49, 109.63, 63.72, 56.58, 54.41, 53.50, 49.35, 49.17, 47.01, 42.46, 40.76, 38.90, 38.35, 37.62, 37.05, 33.50, 33.39, 32.14, 32.05, 31.08, 30.81, 30.68, 29.72, 25.61, 23.82, 21.44, 19.84, 19.44, 19.32, 19.21, 16.26, 15.63, 14.67, 13.72, 13.59.
HRMS (ESI+): m/z calculated for C38H62N3O2 [M+H]+: 592.48420, found: 592.4850.
Compound 12 is colorless substance. Yield 45 mg (20.3 %).
1H NMR (400 MHz, CDCl3) δ 4.75 (d, J = 2.4 Hz, 1H), 4.61 (m, 1H), 4.17 (m, 1H), 4.10 (m, 3H), 3.05 (td, J = 10.8, 4.4 Hz, 1H), 2.61 (d, J = 15.3 Hz, 1H), 2.37 – 2.24 (m, 2H), 2.05 – 1.98 (m, 1H), 1.97 – 1.85 (m, 2H), 1.84 – 1.73 (m, 3H), 1.70 (s, 3H), 1.68 – 1.37 (m, 13H), 1.33 (d, J = 10.5 Hz, 4H), 1.27 – 1.20 (m, 6H), 1.18 – 1.03 (m, 2H), 1.01 (s, 3H), 0.98 (d, J = 5.2 Hz, 3H), 0.95 (d, J = 6.8 Hz, 4H), 0.93 – 0.82 (m, 5H), 0.79 (s, 3H).
13C NMR (101 MHz, CDCl3) δ 176.17, 150.68, 150.15, 129.16, 109.61, 63.76, 56.52, 53.37, 49.34, 49.27, 47.36, 47.00, 42.49, 40.87, 39.24, 38.31, 37.03, 36.06, 33.65, 33.36, 32.16, 32.09, 30.81, 30.70, 30.59, 29.70, 25.58, 23.08, 21.59, 19.86, 19.38, 19.31, 19.00, 16.63, 15.63, 14.65, 13.72, 13.60.
HRMS (ESI+): m/z calculated for C38H62N3O2 [M+H]+: 592.48420, found: 592.4844.
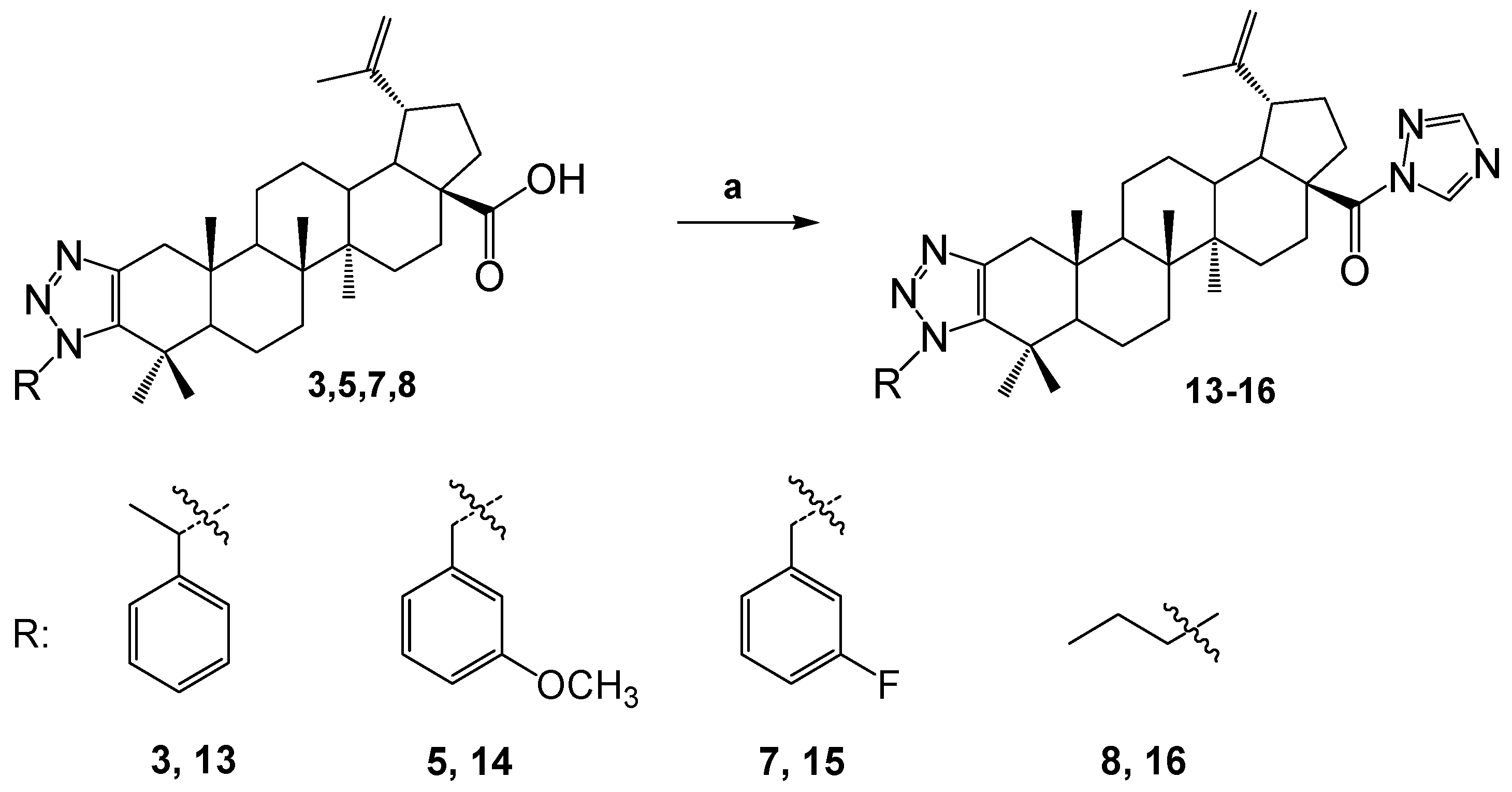
1′-((S)-1-Phenylethyl)-1H′-lup-2-eno-[2,3-d]-[
1,
2,
3]
-triazole-28-(1H-triazol-1-yl) (13). Compound
3 (100 mg, 1 equiv, 0.171 mmol) was dissolved in 2 ml of THF and 1,1′-carbonyldi-(1,2,4-triazole) (112.1 mg, 4 equiv, 0.684 mmol) was added. The reaction mixture was stirred at 70
oC. The reaction was carried out for 5 hours. The solvent was distilled into a rotary evaporator. The remainder was chromatographed with silica gel in a column. When the column was eluted with a mixture of petroleum ether and ethyl acetate (5:3), compound
13 was isolated. Compound
13 is a colorless powder, m.p. 232-235
oC. Yield 116 mg (107.4 %).
1H NMR (400 MHz, CDCl3): δ 8.93 (s, 1H), 7.99 (s, 1H), 7.34 – 7.26 (m, 3H), 7.24 – 7.18 (m, 3H), 5.72 (q, J = 7.0 Hz, 1H), 4.79 (d, J = 2.1 Hz, 1H), 4.68 (t, J = 1.8 Hz, 1H), 3.04 – 2.90 (m, 3H), 2.74 – 2.55 (m, 2H), 2.20 – 2.09 (m, 2H), 2.02 (d, J = 7.0 Hz, 3H), 1.90 – 1.75 (m, 3H), 1.73 (s, 3H), 1.72 – 1.57 (m, 2H), 1.57 – 1.30 (m, 6H), 1.30 – 1.13 (m, 9H), 1.09 (s, 3H), 0.99 (d, J = 10.7 Hz, 6H), 0.95 – 0.86 (m, 1H), 0.85 (s, 3H).
13C NMR (101 MHz, CDCl3): δ 152.19, 145.19, 141.02, 128.64, 127.55, 126.23, 110.20, 59.28, 58.45, 54.84, 50.92, 49.54, 45.65, 42.33, 40.62, 38.94, 38.41, 37.19, 36.26, 33.81, 33.38, 31.47, 30.54, 29.97, 28.89, 25.53, 23.74, 21.52, 21.32, 19.40, 18.90, 16.29, 15.67, 14.65.
HRMS (ESI+): m/z calculated for C40H55N6O [M+H]+: 635.4432, found: 635.4393.
1′-(3-Methoxybenzyl)-1H′-lup-2-eno-[2,3-d]-[
1,
2,
3]
-triazole-28-(1H-triazol-1-yl) (14). Compound
5 (93 mg, 1 equiv, 0.155 mmol) was dissolved in 2 ml of THF and 1,1′-carbonyldi-(1,2,4-triazole) (152.6 mg, 6 equiv, 0.93 mmol) was added. The reaction mixture was stirred at 70
oC. The reaction was carried out for 24 hours. The solvent was distilled into a rotary evaporator. The remainder was chromatographed with silica gel in a column. When the column was eluted with a mixture of petroleum ether and ethyl acetate (4:1), compound
14 was isolated. Compound
14 is a colorless powder, m.p. 279-282
oC. Yield 52 mg (51.6 %).
1H NMR (400 MHz, CDCl3): δ 8.92 (s, 1H), 7.99 (s, 1H), 7.21 (t, J = 7.9 Hz, 1H), 6.82 – 6.77 (m, 1H), 6.63 – 6.58 (m, 1H), 6.56 (t, J = 2.1 Hz, 1H), 5.62 (s, 2H), 4.80 (d, J = 2.2 Hz, 1H), 4.71 – 4.64 (m, 1H), 3.74 (s, 3H), 3.05 – 2.90 (m, 3H), 2.68 (td, J = 12.4, 3.4 Hz, 1H), 2.60 (dd, J = 12.9, 7.3 Hz, 1H), 2.17 (s, 5H), 1.92 – 1.75 (m, 3H), 1.74 (s, 3H), 1.72 – 1.64 (m, 1H), 1.63 (d, J = 1.9 Hz, 2H), 1.60 – 1.34 (m, 5H), 1.35 – 1.19 (m, 2H), 1.18 (s, 3H), 1.06 (s, 3H), 1.03 (s, 3H), 0.97 (s, 4H), 0.80 (s, 3H).
13C NMR (101 MHz, CDCl3): δ 173.39, 159.97, 152.21, 149.83, 145.18, 141.87, 138.10, 138.00, 129.78, 118.65, 113.27, 112.00, 110.20, 58.44, 55.23, 54.66, 52.74, 50.91, 49.49, 45.65, 42.33, 40.60, 39.01, 38.48, 37.18, 36.27, 33.74, 33.32, 31.47, 30.54, 29.96, 28.73, 25.53, 21.54, 21.35, 19.41, 18.91, 16.13, 15.65, 14.66.
HRMS (ESI+): m/z calculated for C40H55N6O2 [M+H]+: 651.4381, found: 651.4378.
1’-(3-Fluorobenzyl)-1H′-lup-2-eno-[2,3-d]-[
1,
2,
3]
-triazole-28-(1H-triazol-1-yl) (15). Compound
7 (50 mg, 1 equiv, 0.085 mmol) was dissolved in 2 ml of THF and 1,1′-carbonyldi-(1,2,4-triazole) (55.7 mg, 4 equiv, 0.34 mmol) was added. The reaction mixture was stirred at 70
oC. The reaction was carried out for 2.5 hours. The solvent was distilled into a rotary evaporator. The formed compound was washed three times with cold acetone. Compound
15 is a colorless powder, m.p. 307-310
oC. Yield 60.7 mg (111.9 %).
1H NMR (400 MHz, CDCl3): δ 8.92 (s, 1H), 7.99 (s, 1H), 7.31 – 7.23 (m, 2H), 7.00 – 6.91 (m, 1H), 6.81 (dt, J = 7.8, 1.3 Hz, 1H), 6.72 (dt, J = 9.6, 2.1 Hz, 1H), 5.64 (d, J = 3.0 Hz, 2H), 4.80 (d, J = 2.2 Hz, 1H), 4.68 (t, J = 1.8 Hz, 1H), 3.75 (tdd, J = 5.8, 2.5, 1.6 Hz, 1H), 3.04 – 2.90 (m, 3H), 2.68 (td, J = 12.4, 3.4 Hz, 1H), 2.64 – 2.55 (m, 1H), 2.20 (d, J = 15.4 Hz, 1H), 1.91 – 1.75 (m, 4H), 1.74 (s, 3H), 1.70 – 1.34 (m, 8H), 1.25 (ddd, J = 25.0, 8.5, 3.2 Hz, 3H), 1.17 (s, 4H), 1.07 (s, 3H), 1.03 (s, 3H), 0.97 (s, 3H), 0.81 (s, 3H).
13C NMR (101 MHz, CDCl3): δ 173.38, 164.29, 161.83, 152.21, 149.82, 145.18, 142.04, 138.07, 130.40, 130.32, 121.94, 114.94, 113.65, 110.21, 67.98, 58.44, 54.61, 52.25, 50.91, 49.49, 45.65, 42.34, 40.60, 39.01, 38.42, 37.18, 36.27, 33.72, 33.30, 31.46, 30.53, 29.96, 28.79, 25.52, 21.54, 21.41, 19.40, 18.90, 16.16, 15.65, 14.66.
HRMS (ESI+): m/z calculated for C39H52FN6O [M+H]+: 639.4181, found: 639.4180.
1′-Butyl-1H′-lup-2-eno-[2,3-d]-[
1,
2,
3]
-triazole-28-(1H-triazol-1-yl) (16). Compound
8 (50 mg, 1 equiv, 0.0934 mmol) was dissolved in 2 ml of THF and 1,1′-carbonyldi-(1,2,4-triazole) (61.2 mg, 4 equiv, 0.3736 mmol) was added. The reaction mixture was stirred at 70
oC. The reaction was carried out for 2 hours. The solvent was distilled into a rotary evaporator. The formed compound was washed three times with cold acetone. Compound
16 is a colorless powder, m.p. 281-283
oC. Yield 59 mg (107.7 %).
1H NMR (400 MHz, CDCl3): δ 8.93 (s, 1H), 8.00 (s, 1H), 7.27 (s, 1H), 4.79 (d, J = 2.2 Hz, 1H), 4.71 – 4.63 (m, 1H), 4.29 (td, J = 7.1, 1.4 Hz, 2H), 3.05 – 2.89 (m, 3H), 2.68 (td, J = 12.4, 3.4 Hz, 1H), 2.63 – 2.56 (m, 1H), 2.19 – 2.10 (m, 2H), 1.97 (pd, J = 7.1, 1.4 Hz, 2H), 1.89 – 1.75 (m, 3H), 1.71 (d, J = 17.7 Hz, 5H), 1.66 – 1.34 (m, 7H), 1.30 (s, 3H), 1.27 – 1.18 (m, 4H), 1.12 (td, J = 13.0, 4.3 Hz, 1H), 1.03 (s, 3H), 1.01 – 0.94 (m, 6H), 0.80 (s, 3H).
13C NMR (101 MHz, CDCl3): δ 173.39, 152.21, 149.82, 145.19, 141.02, 137.26, 110.20, 58.45, 54.75, 50.92, 49.50, 49.36, 45.65, 42.34, 40.62, 39.01, 38.39, 37.19, 36.27, 33.68, 33.36, 32.86, 31.48, 30.54, 29.98, 28.68, 25.53, 21.52, 21.36, 20.18, 19.40, 18.97, 16.11, 15.67, 14.70, 13.70.
HRMS (ESI+): m/z calculated for C36H55N6O [M+H]+: 587.4432, found: 587.4438.
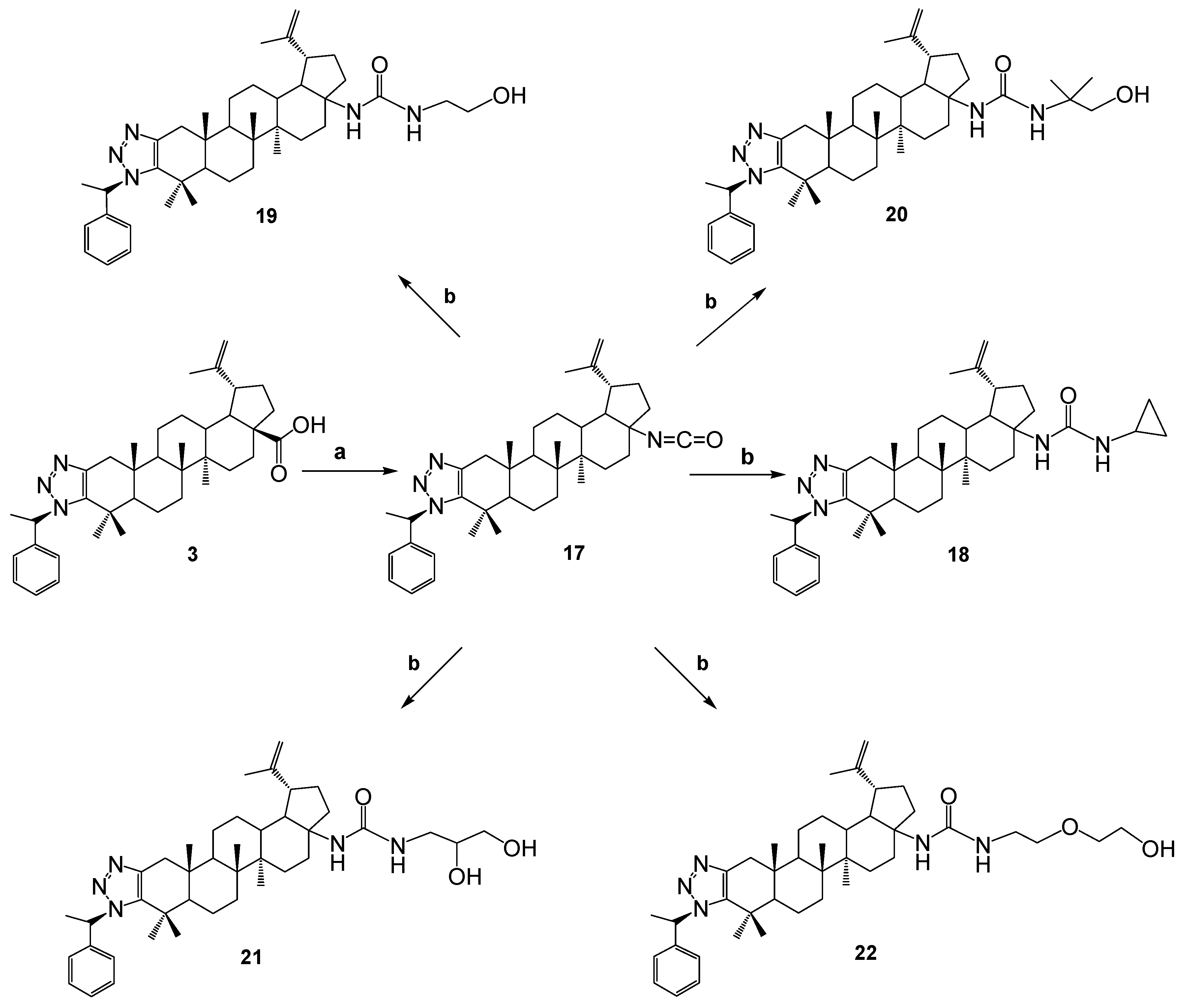
1′-((S)-1-Phenylethyl)-1H′-lup-2-eno-[2,3-d]-[
1,
2,
3]
-triazole-isocyanate (17). Compound
3 (200 mg, 1 equiv., 0.34 mmol) was dissolved in toluene (6 ml) in an ultrasonic bath, then triethylamine (0.0464 ml, 1 equiv., 0.34 mmol) and diphenylphosphoryl azide (0.0772 ml, 1 equiv., 0.34 mmol) were added. The reaction was carried out at room temperature (21
oC) for 6 hours. At the end of the reaction, the solvent was distilled on a rotary evaporator. The remainder was chromatographed with silica gel in a column. Compound
17 colorless powder, m.p. 197-200
oC. Yield 120 mg (60.9 %).
1H NMR (400 MHz, CDCl3): δ 7.29 (m, 3H), 7.22 (m, 2H), 5.73 (q, J = 7.0 Hz, 1H), 4.77 (d, J = 2.1 Hz, 1H), 4.67 (m, 1H), 2.97 (d, J = 15.3 Hz, 1H), 2.56 (td, J = 10.9, 5.8 Hz, 1H), 2.13 (m, 2H), 2.02 (d, J = 7.0 Hz, 3H), 1.84 (m, 5H), 1.70 (d, J = 1.2 Hz, 3H), 1.52 (m, 8H), 1.29 (s, 3H), 1.20 (m, 2H), 1.11 (d, J = 1.8 Hz, 6H), 0.94 (s, 3H), 0.84 (m, 3H).
13C NMR (101 MHz, CDCl3): δ 148.71, 141.83, 140.99, 137.51, 128.64, 127.55, 126.24, 121.63, 110.63, 71.59, 59.27, 54.80, 49.32, 49.18, 48.10, 42.06, 40.66, 39.27, 39.17, 38.91, 38.42, 33.81, 33.57, 33.46, 29.29, 28.91, 27.81, 24.91, 23.74, 21.36, 21.34, 19.52, 18.89, 16.29, 15.69, 14.44.
HRMS (ESI+): m/z calculated for C38H53N4O [M+H]+: 581.4214, found: 581.4218.
1′-((S)-1-Phenylethyl)-1H′-lup-2-eno-[2,3-d]-[
1,
2,
3]
-triazole-28-oic Acid derivative -1. (18). Compound
3 (100 mg, 1 equiv., 0.17 mmol) was dissolved in toluene (3 ml) in an ultrasonic bath, then triethylamine (0.024 ml, 1 equiv., 0.17 mmol) and diphenylphosphoryl azide (0.039 ml, 1 equiv., 0.17 mmol) were added. The reaction was carried out at room temperature (20
oC) for 7 hours. After cyclopropylamine (0.177 ml, 10 equiv., 1.7 mmol) in 0.5 ml of toluene was added to the reaction mixture. Then it was heated at 110°C for 4 hours. At the end of the reaction, the solvent was distilled on a rotary evaporator. The remainder was chromatographed with silica gel in a column. During elution of the column with ethyl acetate, a colorless substance
18 with a melting point of 200-203
oC was isolated. The yield was 65.9 mg (61 %).
1H NMR (400 MHz, CDCl3): δ 7.34 – 7.25 (m, 3H), 7.23 – 7.18 (m, 2H), 5.73 (q, J = 7.0 Hz, 1H), 4.95 (s, 1H), 4.75 (d, J = 2.1 Hz, 1H), 4.69 – 4.60 (m, 2H), 2.96 (d, J = 15.2 Hz, 1H), 2.67 (dt, J = 13.2, 3.3 Hz, 1H), 2.54 (dd, J = 12.5, 8.1 Hz, 1H), 2.44 (tq, J = 7.9, 4.6, 4.1 Hz, 2H), 2.16 (d, J = 15.2 Hz, 1H), 2.07 – 1.91 (m, 4H), 1.82 – 1.64 (m, 7H), 1.62 – 1.16 (m, 11H), 1.10 (d, J = 9.6 Hz, 6H), 0.99 (s, 3H), 0.95 – 0.75 (m, 6H), 0.69 – 0.56 (m, 2H).
13C NMR (101 MHz, CDCl3): δ 158.04, 149.40, 141.76, 140.94, 137.53, 128.66, 127.59, 126.23, 110.27, 63.72, 59.28, 54.74, 49.31, 49.23, 48.33, 42.07, 40.64, 38.89, 38.42, 38.37, 35.19, 33.83, 33.15, 29.86, 29.33, 28.89, 27.34, 25.11, 23.72, 22.70, 21.48, 21.35, 19.28, 18.90, 16.29, 15.58, 14.48, 7.82, 7.35.
HRMS (ESI+): m/z calculated for C41H60N5O [M+H]+: 638.4792, found:638.4750.
1′-((S)-1-Phenylethyl)-1H′-lup-2-eno-[2,3-d]-[
1,
2,
3]
-triazole-28-oic Acid derivative -2. (19). Compound
3 (100 mg, 1 equiv., 0.17 mmol) was dissolved in toluene (3 ml) in an ultrasonic bath, then triethylamine (0.024 ml, 1 equiv., 0.17 mmol) and diphenylphosphoryl azide (0.039 ml, 1 equiv., 0.17 mmol) were added. The reaction was carried out at room temperature (21
oC) for 2 hours. After ethanolamine (0.1026 ml, 10 equiv., 1.7 mmol) in 0.5 ml of toluene was added to the reaction mixture. Then it was heated at 110°C for 7 hours. At the end of the reaction, the solvent was distilled on a rotary evaporator. The remainder was chromatographed with silica gel in a column. During elution of the column with ethyl acetate, a colorless substance
19 with a melting point of 202-204
oC was isolated. The yield was 126.2 mg (115.78 %).
1H NMR (400 MHz, CDCl3): δ 7.35 – 7.23 (m, 2H), 7.27 – 7.14 (m, 2H), 5.79 (s, 1H), 5.75 (q, J = 6.9 Hz, 1H), 5.18 (s, 1H), 4.69 (d, J = 2.3 Hz, 1H), 4.62 (t, J = 1.9 Hz, 1H), 4.12 (q, J = 7.2 Hz, 1H), 3.77 – 3.60 (m, 3H), 3.60 – 3.45 (m, 1H), 3.24 (s, 2H), 2.91 (d, J = 15.3 Hz, 1H), 2.68 – 2.60 (m, 1H), 2.55 (td, J = 11.0, 5.0 Hz, 1H), 2.46 (dd, J = 12.2, 8.1 Hz, 1H), 2.21 – 2.15 (m, 1H), 2.14 (d, J = 14.3 Hz, 1H), 2.08 (s, 1H), 2.03 (d, J = 12.8 Hz, 2H), 2.02 – 1.92 (m, 2H), 1.86 – 1.75 (m, 1H), 1.74 (s, 1H), 1.70 (s, 3H), 1.65 (d, J = 11.6 Hz, 1H), 1.62 – 1.54 (m, 1H), 1.57 – 1.47 (m, 4H), 1.47 – 1.35 (m, 1H), 1.38 – 1.28 (m, 1H), 1.28 (s, 4H), 1.26 (s, 9H), 1.30 – 1.19 (m, 1H), 1.13 (s, 3H), 1.19 – 1.03 (m, 2H), 1.02 (s, 3H), 0.96 (s, 3H), 0.96 – 0.85 (m, 2H), 0.89 – 0.81 (m, 2H), 0.79 (s, 3H).
13C NMR (101 MHz, CDCl3): δ 174.49, 159.57, 149.75, 141.44, 140.91, 137.89, 128.73, 127.74, 126.14, 110.03, 64.03, 63.73, 59.28, 54.73, 49.53, 49.31, 47.47, 43.51, 42.07, 40.65, 38.86, 38.31, 37.51, 35.61, 33.85, 33.23, 31.93, 29.79, 29.71, 29.66, 29.37, 28.88, 27.43, 25.02, 23.58, 22.70, 21.49, 21.35, 19.23, 18.90, 16.35, 15.70, 14.41, 14.13.
HRMS (ESI+): m/z calculated for C40H60N5O2 [M+H]+: 642.4742, found: 642.470.
1′-((S)-1-Phenylethyl)-1H′-lup-2-eno-[2,3-d]-[
1,
2,
3]
-triazole-28-oic Acid derivative -3. (20). Compound
3 (100 mg, 1 equiv., 0.17 mmol) was dissolved in toluene (3 ml) in an ultrasonic bath, then triethylamine (0.024 ml, 1 equiv., 0.17 mmol) and diphenylphosphoryl azide (0.039 ml, 1 equiv., 0.17 mmol) were added. The reaction was carried out at room temperature (21
oC) for 3 hours. After 2-amino-2-methyl-1-propanol (0.162 ml, 10 equiv., 1.7 mmol) in 0.5 ml of toluene was added to the reaction mixture. Then it was heated at 110°C for 3.5 hours. At the end of the reaction, the solvent was distilled on a rotary evaporator. The remainder was chromatographed with silica gel in a column. During elution of the column with ethyl acetate, a colorless substance
20 with a melting point of 176-178
oC was isolated. The yield was 95.9 mg (84.3 %).
1H NMR (400 MHz, CDCl3): δ 7.30 (dd, J = 8.2, 6.3 Hz, 3H), 7.23 – 7.16 (m, 2H), 6.45 (s, 1H), 5.75 (q, J = 7.0 Hz, 1H), 5.25 (s, 1H), 5.05 (s, 1H), 4.65 (d, J = 2.3 Hz, 1H), 4.60 (t, J = 1.9 Hz, 1H), 3.55 (s, 2H), 2.92 (d, J = 15.2 Hz, 1H), 2.67 (dt, J = 13.4, 3.4 Hz, 1H), 2.44 (ddd, J = 20.1, 14.0, 9.6 Hz, 2H), 2.17 (d, J = 15.2 Hz, 1H), 2.08 (s, 0H), 2.02 (d, J = 7.0 Hz, 3H), 1.99 – 1.90 (m, 1H), 1.75 (td, J = 14.7, 14.1, 8.5 Hz, 2H), 1.68 (s, 3H), 1.62 (d, J = 11.7 Hz, 1H), 1.62 – 1.40 (m, 5H), 1.39 – 1.24 (m, 7H), 1.23 (d, J = 1.9 Hz, 6H), 1.09 (d, J = 22.1 Hz, 8H), 0.96 (s, 3H), 0.93 – 0.78 (m, 7H).
13C NMR (101 MHz, CDCl3): δ 158.88, 149.68, 141.54, 140.91, 128.70, 127.69, 126.18, 109.99, 72.29, 63.89, 59.30, 54.78, 54.76, 49.51, 49.35, 47.37, 42.04, 40.62, 38.89, 38.36, 37.52, 35.61, 33.85, 33.24, 29.84, 29.55, 28.89, 27.40, 25.26, 25.22, 25.04, 23.60, 21.50, 21.35, 19.29, 18.89, 16.33, 15.74, 14.45.
HRMS (ESI+): m/z calculated for C42H64N5O2 [M+H]+: 670.5055, found: 670.5093.
1′-((S)-1-Phenylethyl)-1H′-lup-2-eno-[2,3-d]-[
1,
2,
3]
-triazole-28- oic Acid derivative -4. (21). Compound
3 (100 mg, 1 equiv., 0.17 mmol) was dissolved in toluene (3 ml) in an ultrasonic bath, then triethylamine (0.024 ml, 1 equiv., 0.17 mmol) and diphenylphosphoryl azide (0.039 ml, 1 equiv., 0.17 mmol) were added. The reaction was carried out at room temperature (22
oC) for 3 hours. After 3-amino-1,2-propanediol (0.1317 ml, 10 equiv., 1.7 mmol) was added to the reaction mixture. Then it was heated at 110°C for 2 hours. At the end of the reaction, the solvent was distilled on a rotary evaporator. The remainder was chromatographed with silica gel in a column. Elution of a column with a mixture of ethyl acetate and methanol (10:1) isolated a colorless substance
21 with a melting point of 182-185
oC. The yield was 89.1 mg (78 %).
1H NMR (400 MHz, CDCl3): δ 7.35 – 7.23 (m, 3H), 7.18 (td, J = 7.3, 1.9 Hz, 2H), 5.95 (s, 1H), 5.76 (q, J = 7.3 Hz, 1H), 5.35 – 5.18 (m, 1H), 4.68 (s, 1H), 4.64 – 4.57 (m, 1H), 4.12 (q, J = 7.2 Hz, 1H), 3.82 – 3.70 (m, 1H), 3.70 – 3.58 (m, 1H), 3.59 – 3.39 (m, 2H), 3.26 (dt, J = 16.1, 5.3 Hz, 2H), 2.92 (dd, J = 20.5, 15.2 Hz, 1H), 2.69 – 2.59 (m, 1H), 2.54 (ddq, J = 16.3, 11.8, 6.9, 5.2 Hz, 1H), 2.42 (dd, J = 12.0, 8.1 Hz, 1H), 2.24 – 2.10 (m, 1H), 2.06 (d, J = 10.6 Hz, 1H), 2.01 (d, J = 6.9 Hz, 3H), 1.97 – 1.71 (m, 1H), 1.69 (s, 3H), 1.67 – 1.30 (m, 5H), 1.30 – 1.19 (m, 7H), 1.12 (d, J = 8.0 Hz, 4H), 1.03 – 0.89 (m, 6H), 0.89 – 0.80 (m, 1H), 0.78 (s, 2H).
13C NMR (101 MHz, CDCl3): δ 159.68, 149.68, 141.37, 140.94, 138.02, 128.76, 128.68, 127.79, 126.18, 126.08, 110.09, 71.98, 63.85, 63.83, 63.30, 59.27, 54.71, 49.45, 49.31, 47.39, 42.50, 42.06, 40.63, 38.86, 38.29, 37.50, 35.72, 33.86, 33.22, 30.84, 29.71, 28.85, 27.41, 25.62, 25.01, 23.63, 23.52, 21.52, 21.34, 19.22, 18.89, 16.40, 15.68, 14.43, 14.20.
HRMS (ESI+): m/z calculated for C41H62N5O3 [M+H]+: 672.4847, found: 672.4858.
1′-((S)-1-Phenylethyl)-1H′-lup-2-eno-[2,3-d]-[
1,
2,
3]
-triazole-28-oic Acid derivative -5. (22). Compound
3 (100 mg, 1 equiv., 0.17 mmol) was dissolved in toluene (3 ml) in an ultrasonic bath, then triethylamine (0.024 ml, 1 equiv., 0.17 mmol) and diphenylphosphoryl azide (0.039 ml, 1 equiv., 0.17 mmol) were added. The reaction was carried out at room temperature (22
oC) for 3 hours. After 2-(2-aminoethoxy-ethanol (0.1705 ml, 10 equiv., 1.7 mmol) in 0.5 ml of toluene was added to the reaction mixture. Then it was heated at 110°C for 2 hours. At the end of the reaction, the solvent was distilled on a rotary evaporator. The remainder was chromatographed with silica gel in a column. Elution of a column with a mixture of ethyl acetate and methanol (20:1) isolated a colorless substance
22 with a melting point of 165-167
oC. The yield was 76.2 mg (65 %).
1H NMR (400 MHz, CDCl3): δ 7.37 – 7.23 (m, 3H), 7.22 – 7.16 (m, 2H), 5.74 (q, J = 7.0 Hz, 1H), 5.49 (s, 1H), 4.76 – 4.67 (m, 2H), 4.62 (t, J = 1.9 Hz, 1H), 3.83 – 3.68 (m, 3H), 3.68 – 3.60 (m, 3H), 3.60 – 3.51 (m, 4H), 3.36 (d, J = 5.1 Hz, 2H), 2.93 (d, J = 15.2 Hz, 1H), 2.71 – 2.61 (m, 1H), 2.49 (ddt, J = 20.7, 12.4, 6.5 Hz, 2H), 2.16 (d, J = 15.2 Hz, 1H), 2.06 (d, J = 8.7 Hz, 0H), 2.01 (d, J = 7.0 Hz, 3H), 1.76 (d, J = 11.2 Hz, 2H), 1.70 (s, 3H), 1.68 – 1.28 (m, 7H), 1.27 (s, 7H), 1.19 – 1.02 (m, 7H), 0.96 (s, 3H), 0.90 – 0.82 (m, 5H), 0.80 (s, 3H).
13C NMR (101 MHz, CDCl3): δ 158.08, 149.69, 141.63, 140.96, 137.71, 128.69, 127.65, 126.19, 110.06, 72.36, 70.81, 63.60, 61.66, 61.14, 59.30, 54.74, 49.50, 49.27, 47.52, 42.07, 41.35, 40.63, 40.18, 38.87, 38.32, 37.63, 35.71, 33.83, 33.25, 29.81, 29.77, 28.88, 27.39, 25.03, 23.67, 22.63, 21.46, 21.35, 19.26, 18.91, 16.32, 15.71, 14.42.
HRMS (ESI+): m/z calculated for C42H64N5O3 [M+H]+: 686.5004, found: 686.4954.