1. Introduction
Intracranial hemorrhage (ICH) involves bleeding within different areas of the brain, including the ventricles, parenchyma, or surrounding meningeal spaces and can occur before birth [
1]. Although estimates indicate that ICH affects 1 in 10,000 pregnancies, the precise prevalence remains uncertain [
2]. Despite advancements in prenatal imaging techniques like ultrasound (US) and magnetic resonance imaging (MRI) that assist in detecting ICH, challenges persist in accurately diagnosing this condition prenatally. Fetal ICH is often unexpectedly identified during later stages of pregnancy through US scans conducted following a routine second-trimester examination [
3].
The primary risk factors associated with fetal ICH include maternal trauma and the use of anticoagulant medications. Additional risk factors encompass fetal coagulation disorders, thrombocytopenia, severe fetal hypoxia, and fetal infections [
4]. However, in many instances, the specific cause of fetal ICH remains unidentified. Identifying fetal ICH during pregnancy is essential as it influences pregnancy management, informs decisions for future pregnancies, and predicts the likelihood of recurrence [
5]. These factors collectively contribute to optimizing treatment strategies, improving fetal survival, and reducing brain damage. This report presents two cases of prenatal diagnosis of fetal ICH and their postnatal outcomes.
2. Cases Presentation
2.1. Case 1
A 30-year-old pregnant woman (gravida 2, para 1) was referred for a detailed examination following an abnormal routine fetal ultrasonography. She had no significant medical history, surgical interventions, medication use, or reported trauma. Prenatal care was irregular with infrequent check-ups. However, she did report one prior uncomplicated vaginal delivery two years ago with no documented fetal abnormalities.
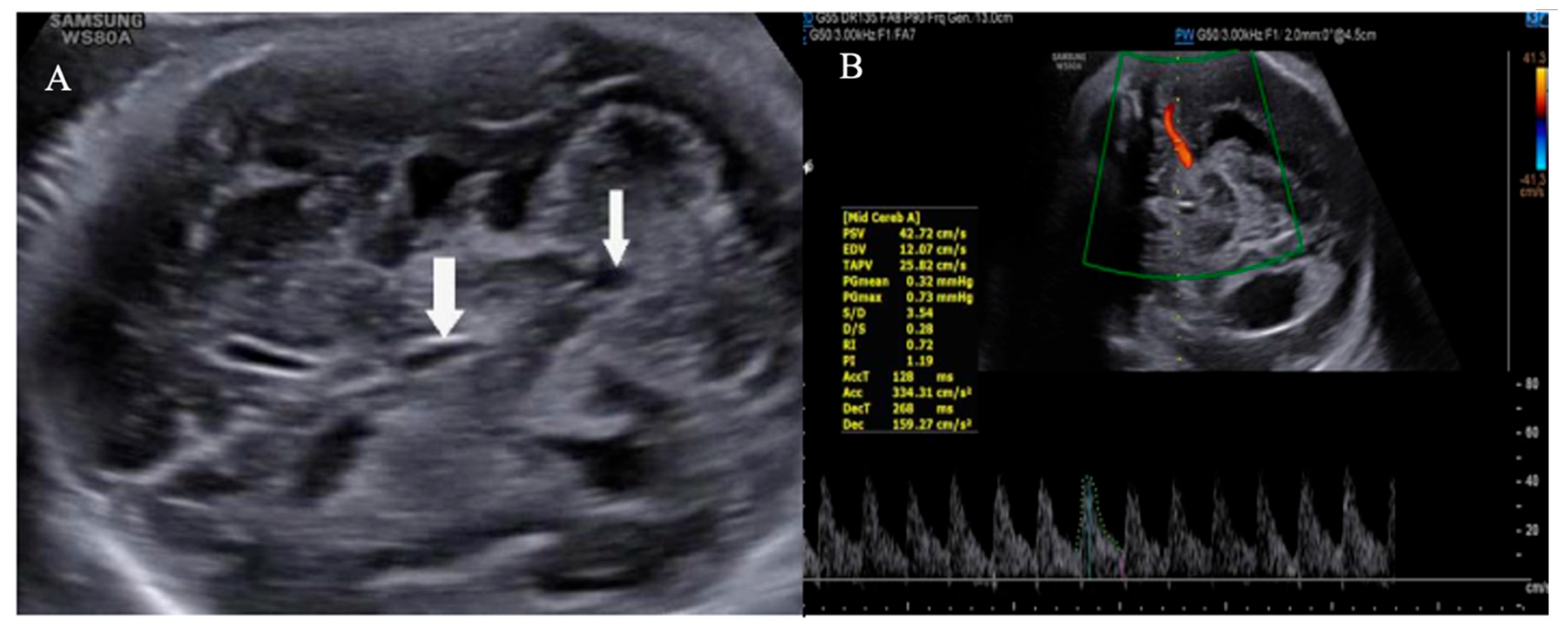
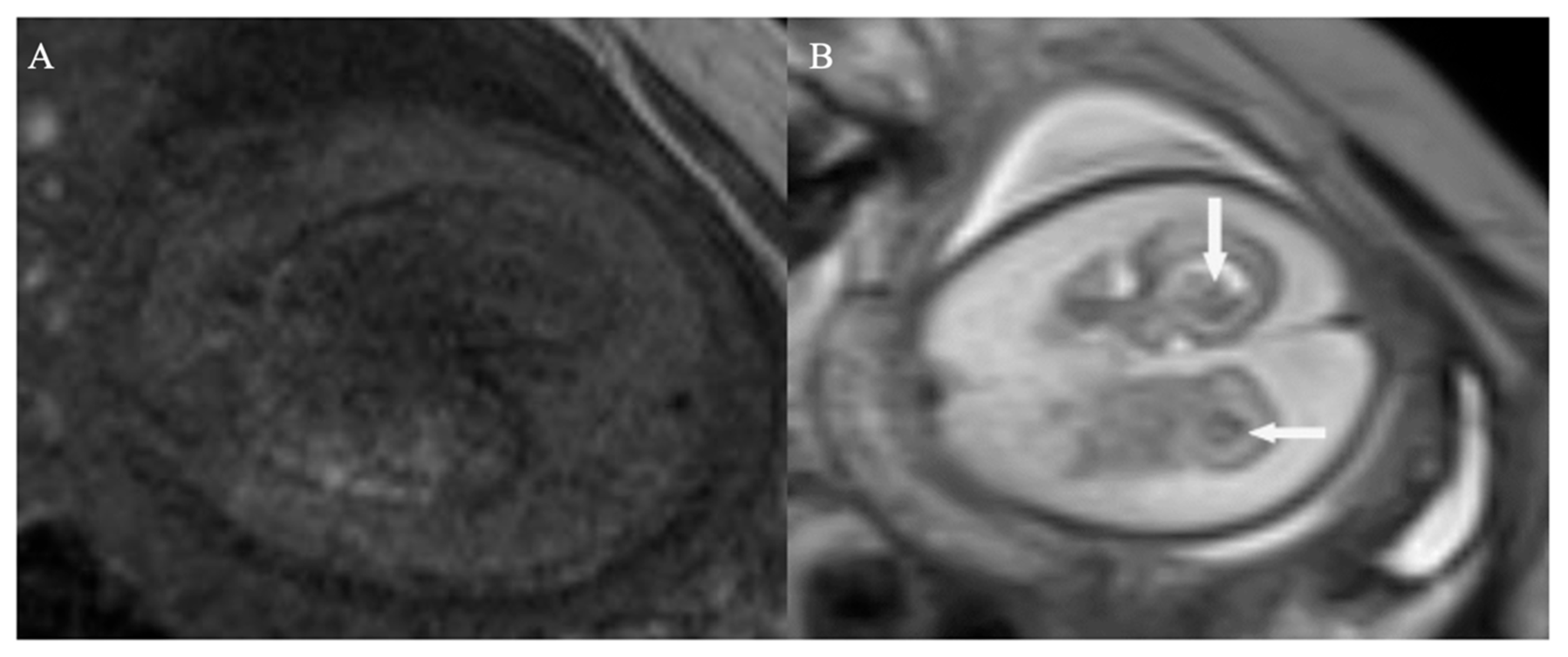
Transabdominal US at 32 weeks gestation revealed concerning features suggestive of ICH with ventricular dilatation. Findings included bilateral ventriculomegaly with dilation of both frontal and occipital horns, irregular ventricular walls, and multiple hyperechoic regions with indistinct margins within the ventricular lumens. Additionally, the choroid plexus exhibited a roughened appearance (
Figure 1 and
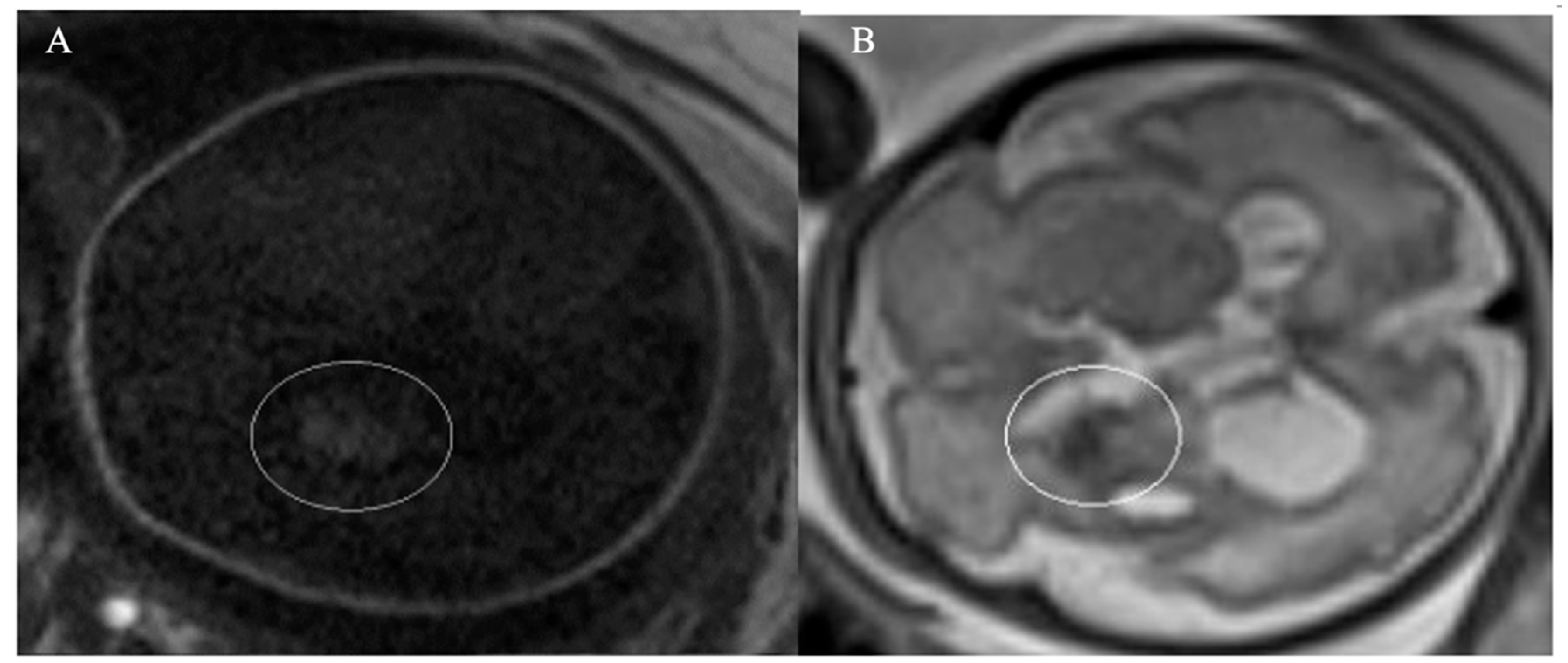
Figure 2). To confirm the diagnosis, an MRI was performed confirming ICH with bilateral ventricular enlargement (
Figure 3).
A comprehensive workup for potential underlying causes was conducted, including evaluation for infectious diseases (using the TORCH panel), platelet count, coagulation abnormalities (prothrombin time and partial thromboplastin time), fibrinolysis (plasminogen levels), platelet function (von Willebrand factor, factor V Leiden, protein S, protein C), and antiplatelet antibodies. All tests returned within normal ranges. After discussions with specialists in neonatology and pediatric neurology regarding potential neurological consequences, the patient and her family decided to proceed with the pregnancy. Follow-up US showed no progression of ventricle dilation.
At 36 weeks of gestation, the patient underwent an elective cesarean section due to signs of fetal distress, resulting in the delivery of a male infant weighing 3160 grams. The newborn had low Apgar scores of 1, 5, and 7 at 1, 5, and 10 minutes, respectively, prompting immediate intubation and positive-pressure ventilation, followed by transfer to the neonatal intensive care unit (NICU). Three days after birth, the infant underwent successful placement of a ventriculoperitoneal shunt (VPS) and showed subsequent clinical improvement. Extubation was conducted five days later, and the infant was discharged home after three weeks. The follow-up examination at three months postnatally by US showed a reduction in ventricle diameter. However, ongoing assessments revealed clinical signs of epilepsy and hemiparesis. The infant is currently receiving regular outpatient monitoring and care from pediatric specialists..
2.2. Case 2
A 27-year-old woman (gravida 1, para 0) with a history of mitral valve stenosis requiring mechanical valve replacement 10 years ago attended our clinic for a routine follow-up at 20 weeks of pregnancy. In the first trimester, she received subcutaneous low-molecular-weight heparin as anticoagulation due to the contraindication of warfarin during pregnancy. By the 12th week, she transitioned back to acenocoumarol aiming for a target Prothrombin Time by International Normalized Ratio (INR) of 2.3-3.0.
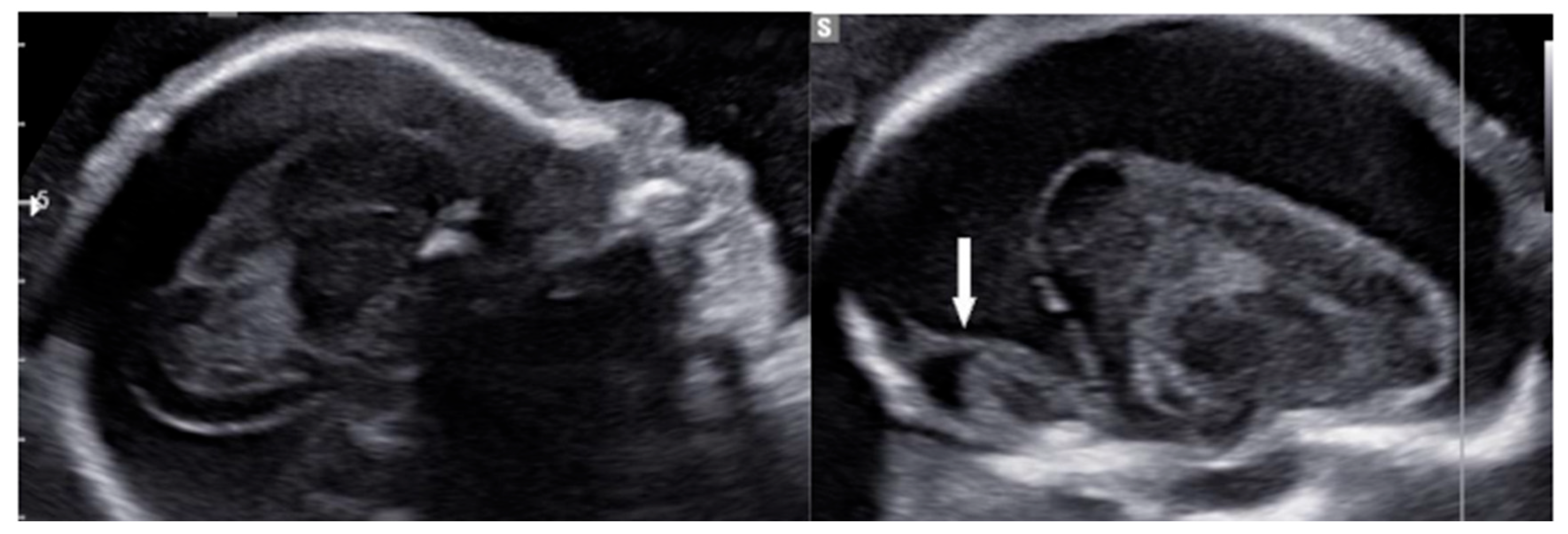
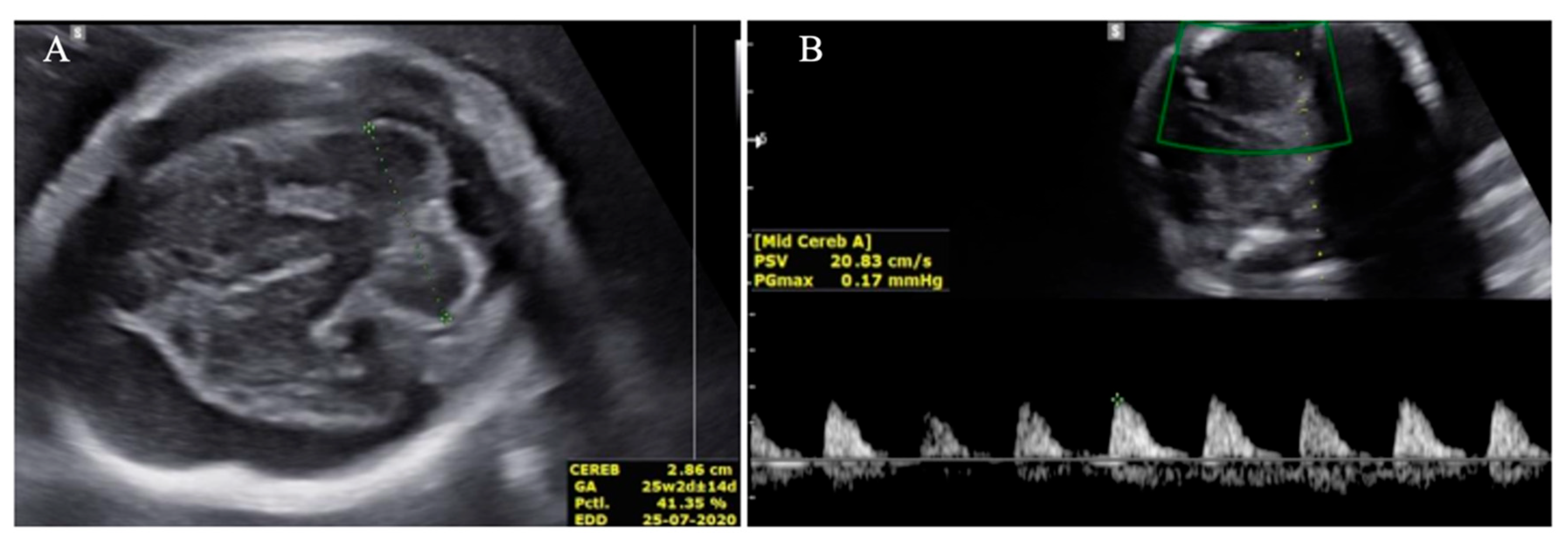
Transabdominal US identified a significant hyperechoic area adjacent to the brain parenchyma, exerting pressure towards the center and raising suspicion of subdural hemorrhage (
Figure 4 and
Figure 5). To confirm the diagnosis, MRI was indicated and validated the initial concern by revealing both subdural hemorrhage and blood clots within the brain's ventricles (
Figure 6).
The maternal TORCH panel test showed negative results, and the patient reported no history of injury during pregnancy. At the time of diagnosis, the INR was 2.7. Following consultations with neonatology and pediatric neurology specialists regarding potential neurological complications, the patient opted to terminate the pregnancy. Labor was induced, resulting in the delivery of a stillborn male infant weighing 1530 grams. Postmortem autopsy of the fetus was not performed based on the mother's and her family's wishes. The mother was discharged from the hospital on postpartum day 5.
In both cases, the US was performed using a high-resolution transabdominal probe (WS80A Samsung) by a radiologist with over 5 years of experience in fetal imaging. To confirm the diagnosis, MRI was indicated and performed using a 1.5 Tesla MRI scanner (Philips Ingenia) with sequences including T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), and susceptibility-weighted imaging (SWI). The MRI images were interpreted by a neuroradiologist with 10 years of experience in fetal neuroimaging.
3. Discussion
Intracranial hemorrhage (ICH) is a significant cause of morbidity and mortality in newborns, with approximately 40% of cases resulting in fetal or neonatal death [
6]. The clinical outcome of ICH primarily depends on the severity of bleeding and the presence of associated factors such as white matter injury, parenchymal infarction, ventriculomegaly, and posthemorrhagic syndrome. ICH categorized as grade III or grade IV tends to have a worse prognosis compared to grade I or grade II hemorrhage [
7].
In our cases, prenatal ultrasound (US) and magnetic resonance imaging (MRI) were essential tools for diagnosing and assessing the severity of fetal ICH. The first case revealed bilateral ventricular dilatation, irregular edges of the choroid plexus, hyperechoic nodes, and areas in the occipital horn of the lateral ventricle, and a thickened hyperechoic wall of the lateral ventricle, consistent with grade 3 hemorrhage. The second case showed poorly echogenic areas outside the brain parenchyma with compression of the brain parenchyma, no dilation of the lateral ventricles, or disruption of the midline, indicating a subdural hemorrhage causing brain parenchymal compression. The MRI results confirmed the US findings and provided additional information on the extent and stage of the hemorrhage.
In the diagnosis of ICH, MRI is recommended in cases when US findings are inconclusive, as it provides detailed information on the location, size, stage of bleeding, extent of spread, and compression of surrounding structures, facilitating a more accurate determination of the underlying cause of bleeding. Additionally, MRI is superior to US for evaluating the posterior fossa area [
8]. The optimal timing for MRI to assess brain structure is typically around 32 weeks of gestation and beyond. MRI sequences utilized in diagnosing ICH include T1-weighted (T1W), T2-weighted (T2W), T2*-weighted (T2*), and diffusion-weighted imaging (DWI) [
9].
A case by Gao et al. reported an unexplained fetal ICH diagnosed at 31 weeks of gestation, similar to our cases where prenatal diagnosis was crucial for management decisions [
10]. Gao's study emphasized the use of both US and MRI for detecting extensive and multifocal hemorrhagic lesions, corroborating our approach of utilizing both imaging modalities for comprehensive assessment. Additionally, Shan et al described a warfarin-associated fetal ICH in a woman with a mechanical heart valve, illustrating the challenges of managing anticoagulation therapy during pregnancy [
11]. This case underscores the necessity of individualized anticoagulation strategies and intensive fetal monitoring, paralleling our management of a patient on anticoagulants for a mechanical heart valve. These comparative cases reinforce the critical role of early detection and tailored management in improving outcomes for fetal ICH.
Management of fetal ICH poses significant challenges. While termination of pregnancy is often considered due to the high risk of severe neurological deficits, counseling should highlight the limitations of predicting neonatal outcomes solely based on prenatal US findings. For cases where continuing the pregnancy is chosen, regular ultrasounds are essential to monitor hemorrhage progression. If an underlying cause for the ICH is identified, targeted prenatal treatments such as maternal vitamin K supplementation for deficiency [
12], switching maternal anticoagulation from warfarin to heparin or intravenous immunoglobulin for fetal alloimmune thrombocytopenia may be options [
13]. The potential benefits of fetal interventions like platelet or red blood cell transfusions for ICH with fetal anemia or thrombocytopenia are uncertain and must be carefully considered against risks such as preterm delivery, stillbirth, and exacerbation of fetal hemorrhage, particularly in cases with suspected coagulopathy [
14].
The optimal timing and method of delivery for fetal ICH remain uncertain, aiming to balance the risks of premature birth, fetal loss, and worsening bleeding. Cesarean section is frequently chosen due to concerns about prematurity, fetal distress, and potential exacerbation of intracranial bleeding during vaginal delivery [
15]. However, it should be noted that this approach lacks supporting solid evidence. Therefore, after comprehensive parental counseling, both vaginal delivery and Caesarean section should be considered as viable options. Following delivery, neonatal blood testing is crucial to evaluate complications associated with fetal ICH, including anemia, thrombocytopenia, or disseminated intravascular coagulation [
5].
Study limitations
There are some limitations that should be acknowledged in our study. First, the study is based on a small sample size of only two cases, which may not fully represent the broader population experiencing prenatal ICH. Second, the study reflected outcomes and management strategies specific to a single healthcare center, potentially limiting the applicability of findings to other settings with different resources and expertise. Additionally, the report lacks long-term follow-up data on neurodevelopmental outcomes for the infants following prenatal ICH diagnosis and management.
4. Conclusions
The clinical presentation of fetal ICH encompasses a range of severity with diverse prognostic implications. Diagnosis and grading can be reliably accomplished using US, although the underlying cause often remains unidentified. Fetal cranial MRI holds promise for elucidating the etiology of fetal ICH. Management strategies for ICH remain controversial; however, termination of pregnancy may be considered appropriate in severe cases due to the unfavorable prognosis associated with significant hemorrhage. Further research and clinical guidelines are warranted to refine management approaches and optimize outcomes in cases of fetal ICH.
Funding
The author received no specific funding for this work.
Abbreviations
| CS |
cesarean section |
NICU |
neonatal intensive care unit |
| DWI |
Diffusion-weighted images. |
T1W |
T1-weighted signal |
| ICH |
intracranial hemorrhage |
T2W |
T2-weighted signal |
| INR |
international normalized ratio |
US |
ultrasonography |
| MRI |
magnetic resonance imaging |
VPS |
ventriculoperitoneal shunt |
Acknowledgments
The authors gratefully acknowledge the Obstetrics and Gynecology Department and other members of Radiology Department at Diamond Healthcare Center for their assistance in managing the patient.
Declaration
Ethics approval and consent to participate: The study was approved by the Ethical Committee at Diamond Healthcare center, No: 155/HĐĐĐ/Diamond, signed 5 May 2023.
Consent for publication: Written informed consents were obtained from the patients for the publication of this research and copies of the written consents are available for review by the Editor-in-Chief of this journal on request.
Availability of data and material: The data of patients is available from the corresponding author upon reasonable request.
Competing Interest: The author report no declarations of interest.
Author Contribion: Dr. Vu Thi Hai Yen is solely responsible for all stages of this research.
References
- Adiego B, Martínez-Ten P, Bermejo C, Estévez M, Recio Rodriguez M, Illescas T. Fetal intracranial hemorrhage. Prenatal diagnosis and postnatal outcomes. J Matern Fetal Neonatal Med. Jan 2019;32(1):21-30. [CrossRef]
- Sanapo L, Whitehead MT, Bulas DI, et al. Fetal intracranial hemorrhage: role of fetal MRI. Prenat Diagn. Aug 2017;37(8):827-836. [CrossRef]
- Monteagudo A. Intracranial Hemorrhage. Am J Obstet Gynecol. Dec 2020;223(6):B34-b37. [CrossRef]
- Kutuk MS, Yikilmaz A, Ozgun MT, et al. Prenatal diagnosis and postnatal outcome of fetal intracranial hemorrhage. Childs Nerv Syst. Mar 2014;30(3):411-8. [CrossRef]
- Sileo FG, Zöllner J, D'Antonio F, Islam S, Papageorghiou AT, Khalil A. Perinatal and long-term outcome of fetal intracranial hemorrhage: systematic review and meta-analysis. Ultrasound in Obstetrics & Gynecology. 2022;59(5):585-595. [CrossRef]
- Owens R. Intraventricular hemorrhage in the premature neonate. Neonatal Netw. May-Jun 2005;24(3):55-71. [CrossRef]
- Monteagudo A. Intracranial Hemorrhage. American Journal of Obstetrics & Gynecology. 2020;223(6):B34-B37. [CrossRef]
- Epstein KN, Kline-Fath BM, Zhang B, et al. Prenatal Evaluation of Intracranial Hemorrhage on Fetal MRI: A Retrospective Review. AJNR Am J Neuroradiol. Dec 2021;42(12):2222-2228. [CrossRef]
- Baburaj R, Rangasami R, Chandrasekharan A, Suresh I, Suresh S, Seshadri S. Utility of Various Ultrafast Magnetic Resonance Sequences in the Detection of Fetal Intracranial Hemorrhage. Ann Indian Acad Neurol. Oct-Dec 2018;21(4):275-279. [CrossRef]
- Gao B, Zhang L, Wei Q. An unexplained fetal intracranial hemorrhage with extensive and multifocal hemorrhagic lesions: A case report. Medicine (Baltimore). Jun 24 2022;101(25):e29335. [CrossRef]
- Shan D, Ji Y, Hu Y, Li T. Treasure to the mother and threat to the fetus: case report of warfarin-associated fetal intracranial hemorrhage and review of literature. J Int Med Res. Aug 2023;51(8):3000605231192773. [CrossRef]
- Sakai M, Yoneda S, Sasaki Y, Saito S. Maternal total parenteral nutrition and fetal subdural hematoma. Obstet Gynecol. May 2003;101(5 Pt 2):1142-4. [CrossRef]
- Oswal K, Agarwal A. Warfarin-induced fetal intracranial subdural hematoma. J Clin Ultrasound. Sep 2008;36(7):451-3. [CrossRef]
- Espinoza JP, Caradeux J, Norwitz ER, Illanes SE. Fetal and neonatal alloimmune thrombocytopenia. Rev Obstet Gynecol. 2013;6(1):e15-21.
- Andersson NG, Chalmers EA, Kenet G, Ljung R, Mäkipernaa A, Chambost H. Mode of delivery in hemophilia: vaginal delivery and Cesarean section carry similar risks for intracranial hemorrhages and other major bleeds. Haematologica. Oct 2019;104(10):2100-2106. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).