1. Introduction
Alopecia is the phenomenon of hair loss from one’s head or other parts of the body by various causes including genetics, stress, and diseases [
1]. This condition causes psychological effects such as low self-esteem in patients [
1]. Among several types of alopecia, androgenetic alopecia (AGA) is the most common and causes the most progressive hair loss [
2]. Ordinarily, hair growth cycles comprise of four phases including anagen, catagen, telogen, and exogen [
3]. The cycling of these phases are dependent on the condition of the follicles to produce hair [
1]. The most important phase for hair growth is the anagen phase, of which approximately 90% of hair growth in a healthy scalp occurs in this phase [
4]. Under a short anagen phase, the hairs progressively become weaker and resemble villus hairs [
1].
Androgen effects on the human skin include the growth and differentiation of sebaceous glands, wound healing, hair growth, and regulation of epidermal barrier functions [
5]. Although thyroid hormones and glucocorticoids are involved in hair growth [
6], the key hormones are androgens to regulate the inhibition of terminal hair growth [
2]. Paradoxically, androgen dependent areas are large but the hormone suppresses hair growth and promote a short anagen stage in scalp follicles [
2]. Hair follicle dermal papilla cells (HFDPCs) play various roles including maintenance of epithelial cell growth, mediation of androgenic signals by paracrine growth factors, and productive modulation of factors from the epithelial cells of hair follicles [
7,
8,
9]. Androgen action in hair follicles depends on local bioavailability. Even if androgens are at a normal level of in the circulatory system, in androgenic alopecia both testosterone and dihydrotestosterone (DT) are increased in local regions.
Hemp,
Cannabis sativa, has been cultured for industrial purposes including uses in food, paper, rope, textiles, clothing, bio-plastics, biofuel, and animal feed [
10,
11]. Of the bioactive compounds in hemp, cannabinoids are the most notable molecules. Although at least 113 phytocannabinoids in hemp have been isolated, six key cannabinoids are tetrahydrocannabinol (THC), cannabidiol (CBD), cannabigerol (CBG), cannabinol (CBN), cannabichromene (CBC), and tetrahydrocannabivarin (THCV) [
12]. In alopecia, CBD, THCV, and cannabidivarin (CBDV) are involved in hair regrowth therapies [
13] and hemp seed oil improves hair growth [
14]; however, there is no research on alopecia with germinated hemp seeds extract (GHSE) and exosomes derived from the callus of the germinated seeds.
Callus is a mass of parenchymal cells and is induced from explants using culture medium supplemented with various hormones including auxin, cytokinin, and gibberellin [
15]. The ratio between 6-benzylaminopurine (6-BA, cytokinin) and 1-naphthaleneacetic acid (NAA, auxin family) is very important for the induction and growth of hemp calli [
16]. Recent research suggests that callus derived exosomes impact various bioactive functions including the activation of osteogenic differentiation and immunomodulation [
17]. However, there is no research on the bioactive function of callus derived exosomes from germinated hemp seeds.
Exosomes, approximately 40–100 nm in size are secreted by almost all types of cells in organisms [
18,
19]. Exposure to some conditions can induce cells to secrete exosomes containing diversely altered components such as proteins, carbohydrates, mRNA, microRNA (miRNA), and DNA molecules [
20]. Through delivery of these altered components, the surrounding cells receive various stimulations by the induced exosomes. Owing to these characteristics, exosomes have the potential to be applied as biomaterials in various fields, including foods, pharmaceuticals, and cosmetics [
21].
This study aims to demonstrate functions of GHSE and exosomes derived from calli of germinated hemp seeds for the prevention of alopecia.
2. Results
We documented the biological functions of two biomaterials including the activation of cellular differentiation, modulation of alopecia stimulation between HFDPCs and immune cells, and activation of immune cells against pathogens despite exposure to DT.
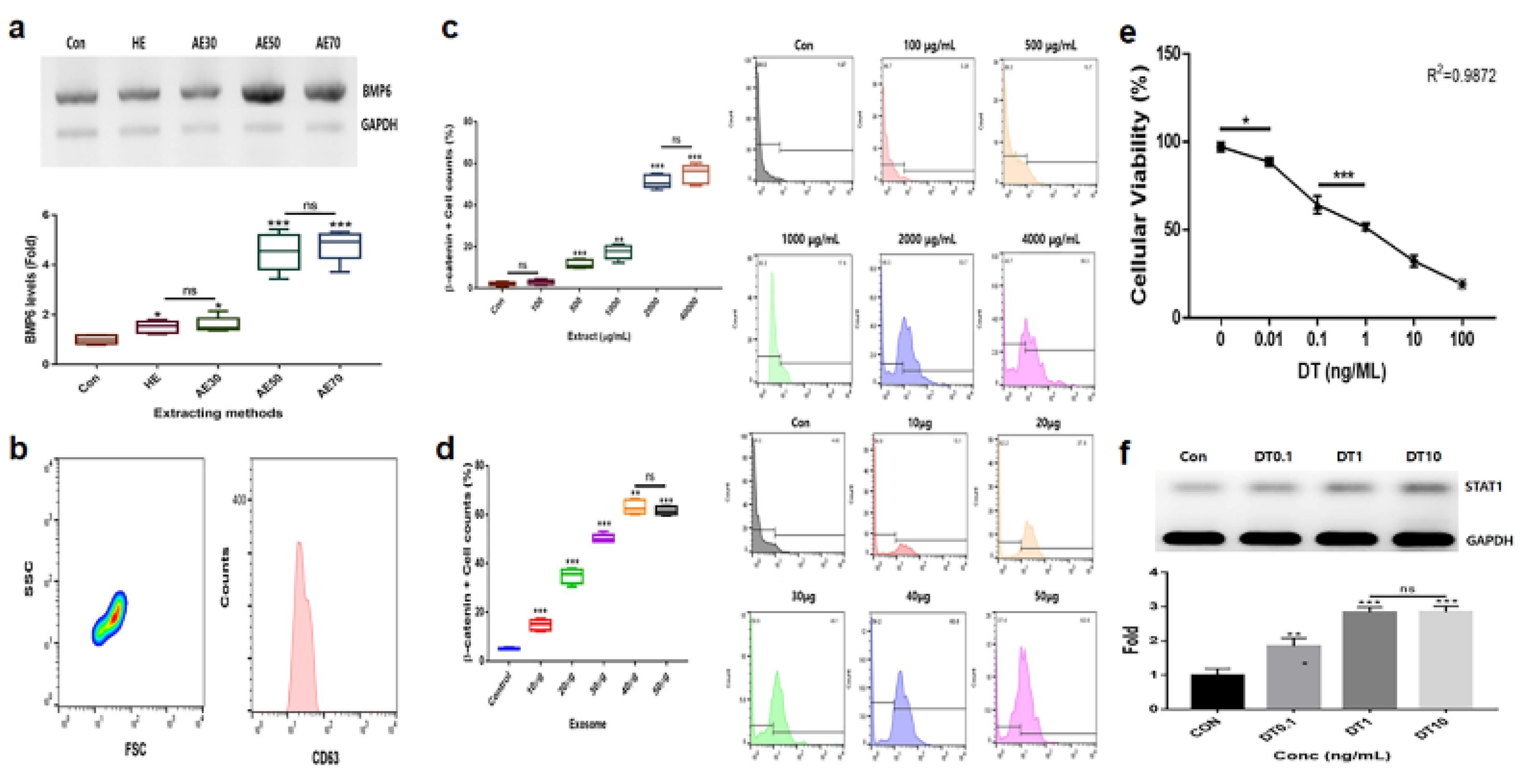
Comparison of hydrothermal extracts (HE) and alcoholic extracts (AE; AE%) for effective upregulation of the marker levels b. Purification of induced exosomes derived from calli of germinated hemp seeds c-f. Establishment of exposure concentrations with the germinated extract (c), induced exosome (d), and dihydrotestosterone (DT; e and f) using evaluations of effective doses. ns, not significant; (* p < 0.05; ** p < 0.01; *** p < 0.001).
2-1. Modulation of Alopecia Markers in Hair Follicle Dermal Papilla Stem Cells by Two Materials
In the results to establish effective materials (
Figure 1), alcoholic extracts (AE)50 (extraction of the germinated hemp seeds with 50% ethanol) was the most effective material among various extracts (
Figure 1a). Based on evaluating the results for cytotoxicity, established exposure doses of two materials, AE50 and the induced exosome derived from calli of germinated hemp seeds, were 2000 μg/mL and 40 ng/mL, respectively, (
Figure 1b, c, d) for hair follicle dermal papilla stem cells (HFDPSC). In DT, the dose was established as 1 ng/mL for HFDPSCs (
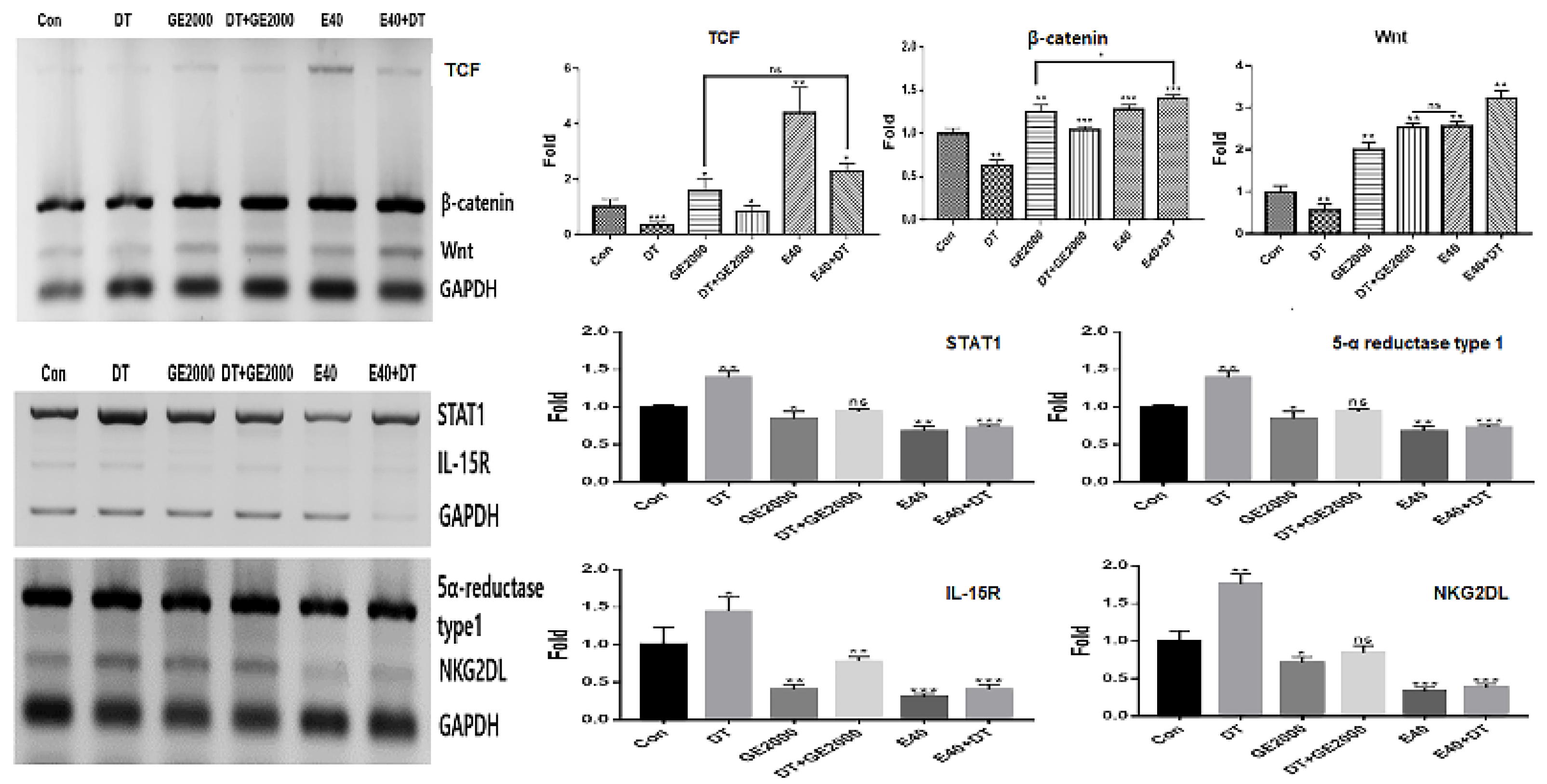
Figure 1e, f). For alopecia modulating markers, though exposed to DT1, the two materials (GE 2000 and E40) upregulated alopecia by preventing activation of genes for proteins including TCF (T cell factor), β-catenin, and Wnt in HFDPSCs. Notably, E40 dramatically upregulated genes in HFDPSCs (
Figure 2). Contrary to these results, alopecia activating genes were down regulated by two materials in HFDPSC (
Figure 2). Compared with DT, TCF, Wnt, IL-15R, and NKG2DL genes were dramatically modulated by E40 in HFDPSC (
Figure 2).
These results show the levels of alopecia markers in hair follicle dermal papilla stem cells (HFDPSCs) under the germinated hemp seeds extract (GHSE; 2000 ug/mL, GE2000), dihydrotestosterone (DT) 1 ng/mL, induces exosomes 40 ng/mL, DT1 after exposure to GE2000 (DT+GE2000), DT1 after exposure to E40 (E40+DT). ns, not significant; (* p < 0.05; ** p < 0.01; *** p < 0.001).
2-2. Activation and Protection of Differentiation in HFDPSCs by Two Materials
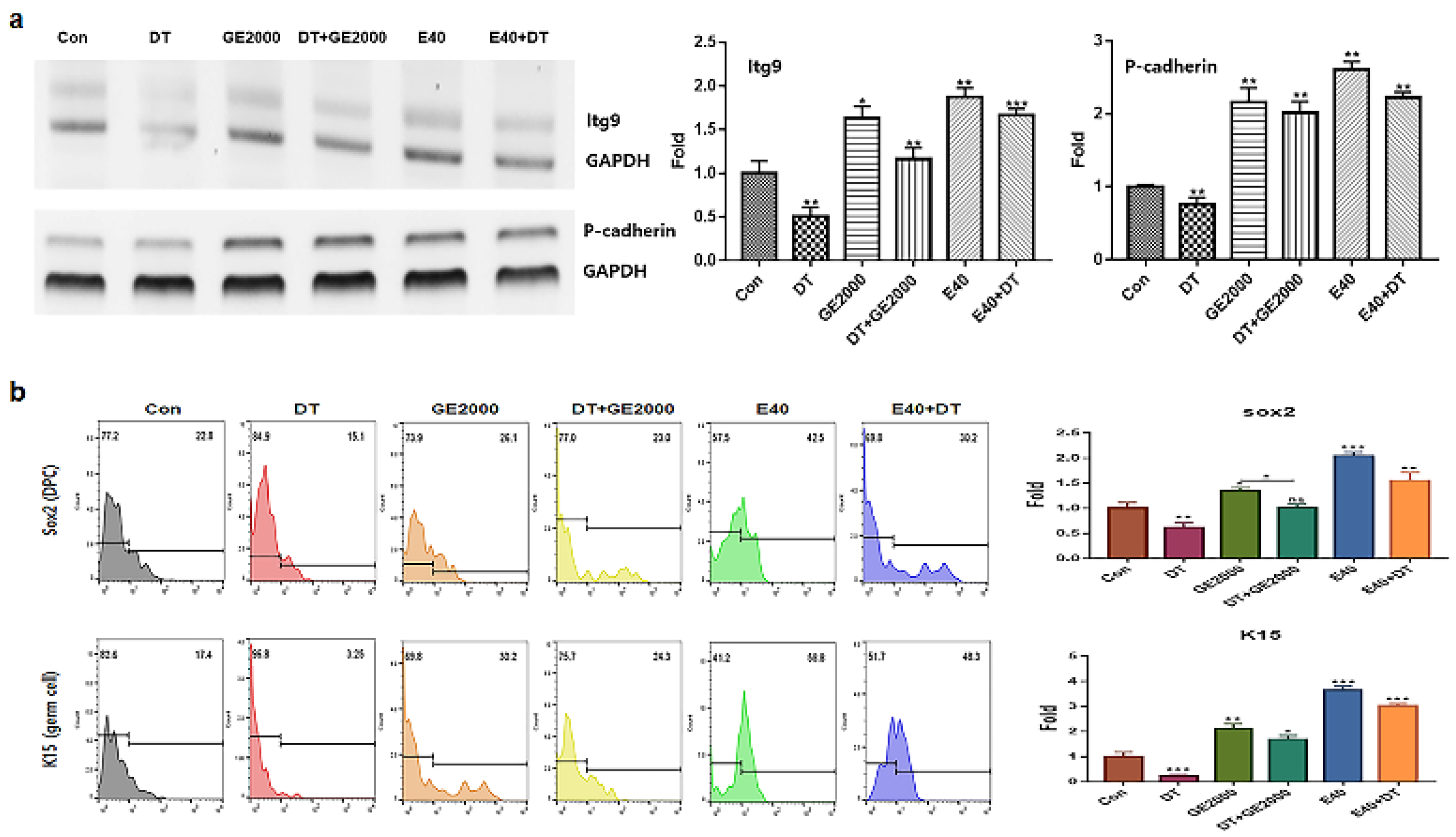
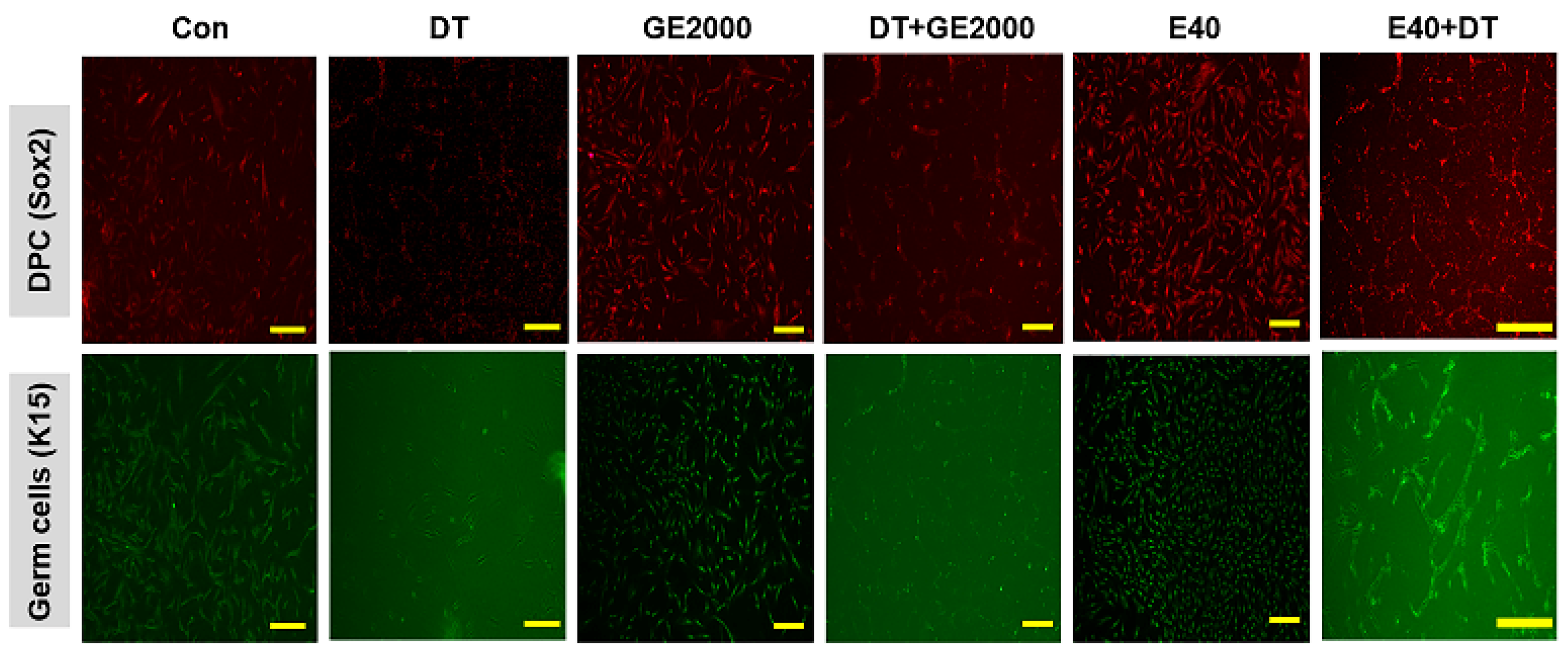
Contrary to DT1, two materials activated and protected the differentiation of HFDPSCs to HFDPCs and germ cells (
Figure 3a, b). Compared with GE2000, E40 more strongly activated and protected their differentiation. The effects of E40 were approximately 1.6 times higher than that of GE2000 (
Figure 3b). Notably, E40 more intensely activated HFDPSC differentiation to germ cells than to HFDPCs (
Figure 3a, b). The flow cytometry results from the evaluation of E 40 and GE 2000 induction (
Figure 3b) corresponded with their immunocytochemistry results (
Figure 4).
Levels of markers for Itg9 (hair follicle dermal papilla cells ) and P-cadherin (hair germ cells) genes under various conditions (Con, control; DT, dihydrotestosterone; GE2000, germinated hemp seeds extract 2000 ug/mL; DT+GE2000, DT 1 ng/mL after exposure to GE2000; E40, induces exosomes 40 ng/mL derived from the calli of germinated hemp seeds; E40+DT, DT1 after exposure to E40) b. Expression of markers for Sox2 and K15 proteins under various conditions. ns; not significant; (* p < 0.05; ** p < 0.01; *** p < 0.001).
The results for differentiated cells from hair follicle dermal papilla stem cells under different conditions (Con, control; DT, dihydrotestosterone; GE2000, germinated hemp seeds extract 2000 ug/mL; DT+GE2000, DT 1 ng/mL after exposure to GE2000; E40, induces exosomes 40 ng/mL derived from the calli of germinated hemp seeds; E40+DT, DT1 after exposure to E40) (the scale bars = 30 um).
2-3. Prevention of Alopecia by Two Materials in Immune Cells
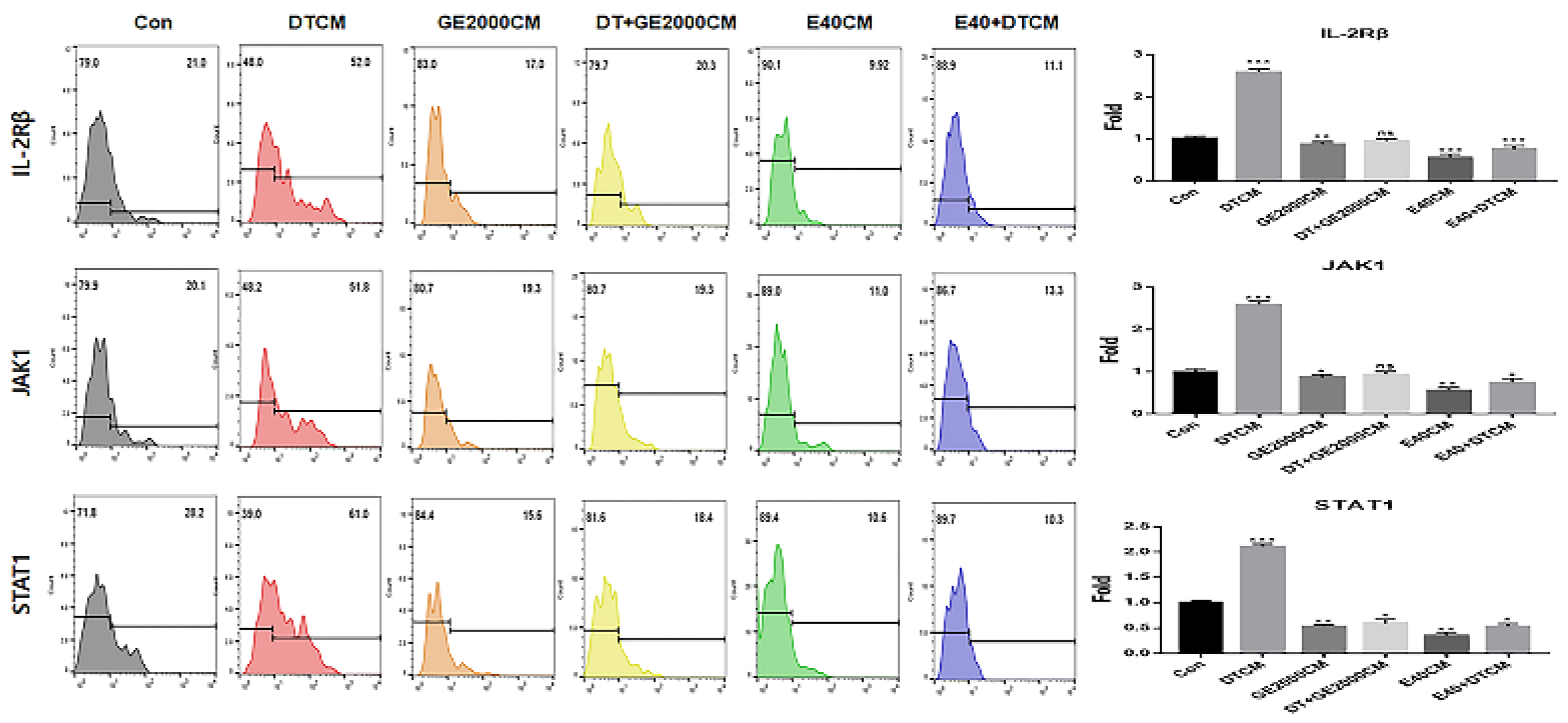
In CD8
+ T cells, two materials down regulated alopecia activating markers including IL2-Rβ, JAK1, and STAT1 under DT1 influence (
Figure 5). Compared with the other two genes, STAT1 protein expression was dramatically downregulated by activated HFDPSCs with the two materials despite exposure to DT1 in CD8
+ T cells (
Figure 5). On average, the intensities for STAT1 expression were 3.8 times higher than those expressing DTCM (
Figure 5).
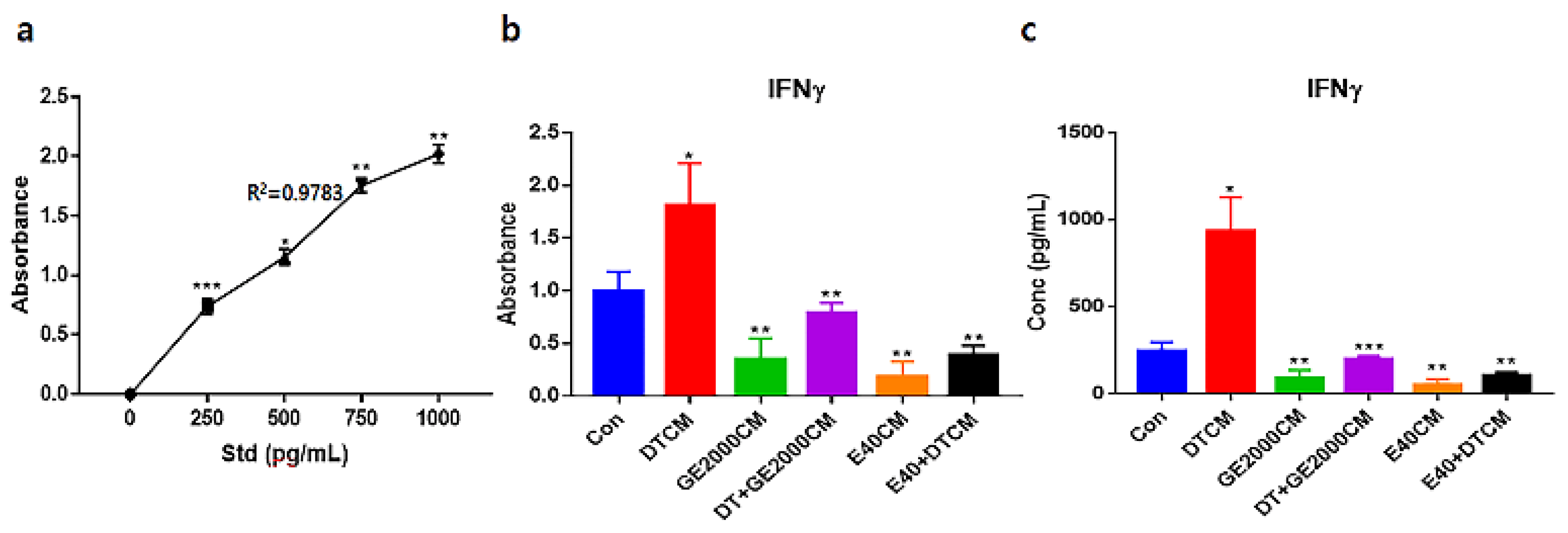
Under the various conditioned media, CD8
+ T cells modulated their secretion of IFNγ (
Figure 6). Notably, when exposed to E40CM, the T cells suppressed secretion of IFNγ than those under DT1. The secreted concentrations of IFNγ under E40CM was approximately 8.2 times lower than those under DTCM influence (
Figure 6c).
The results for expression of alopecia activators in CD8+ T cells exposed to various supernatants from HFDPSCs under different conditions (Con, control; DT, dihydrotestosterone; GE2000, germinated hemp seeds extract 2000 ug/mL; DT+GE2000, DT 1 ng/mL after exposure to GE2000; E40, induces exosomes 40 ng/mL derived from calli of germinated hemp seeds; E40+DT, DT1 after exposing to E40). Theses histograms are derived from the CD8+ population flow cytometry results. CM, conditioned medium; ns, not significant; (* p < 0.05; ** p < 0.01; *** p < 0.001).
Standard curves (a) and evaluating absorbances for interferon gamma (IFNγ) from hair follicle dermal papilla stem cells (HFDPSCs) exposed to various conditioned media (b). Evaluating the concentrations of the IFNγs based on a and b panels. R, correlation coefficient (* p < 0.05; ** p < 0.01; *** p < 0.001).
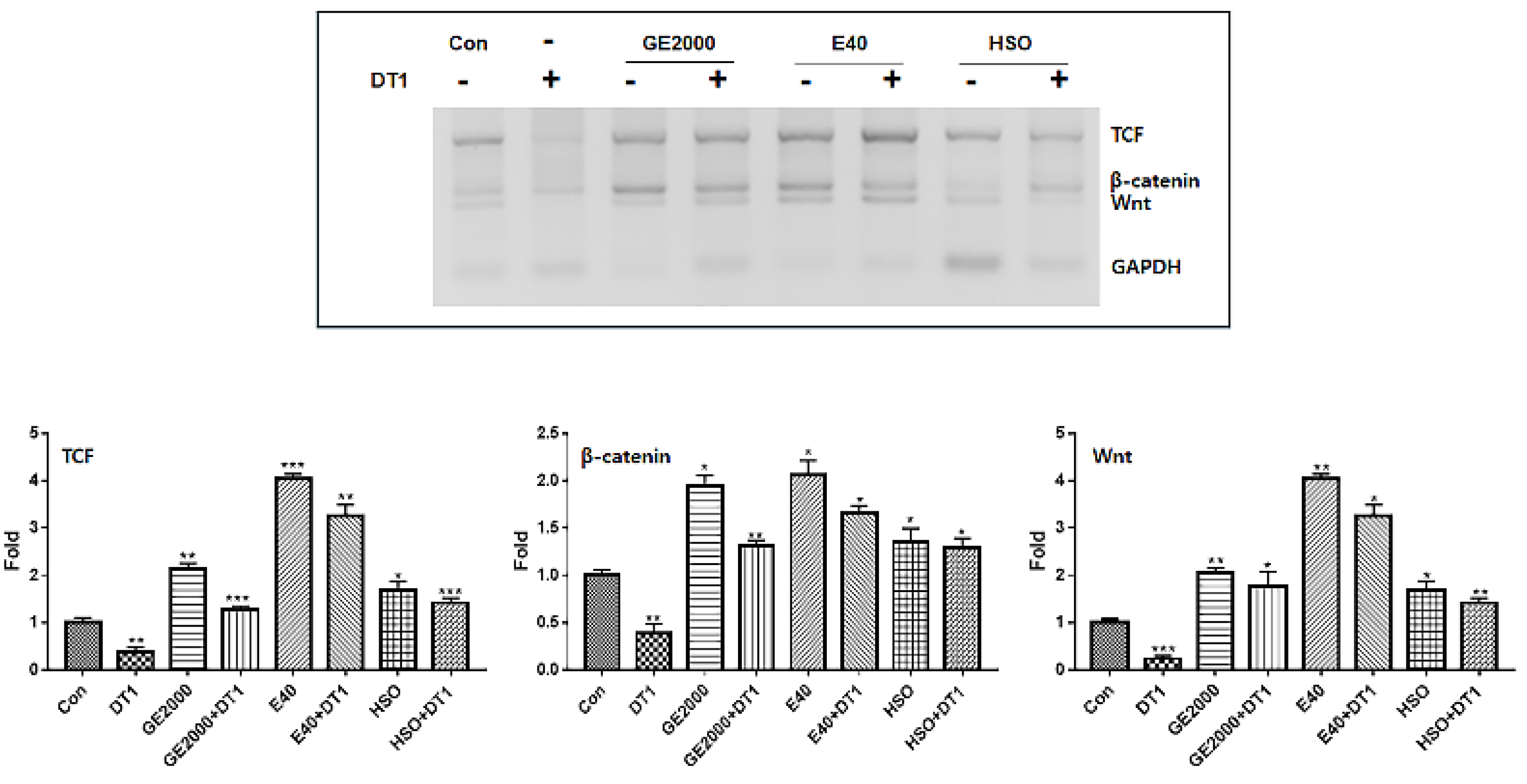
Levels of alopecia preventing genes in hair follicle dermal papilla stem cells (HFDPSCs) exposed to three materials (GE2000, germinated hemp seeds extract 2000 μg/mL; E40, induces exosomes 40 ng/mL from HFDPSCs exposed to 50% ethanolic extract; HSO, hemp seed oil 1000 μg/mL) under dihydrotestosterone 1 ng/mL (DT1) (* p < 0.05; ** p < 0.01; *** p < 0.001).
2-4. Comparison of Effects between Hemp Seed Oil and Two Materials
Although hemp seed oil (HSO) is effective for preventing alopecia based on recent reports [
22,
23], this study suggests that there are more effective bio materials against alopecia inducing compound, DT (
Figure 7). Compared with HSO, two materials upregulated the alopecia preventing genes more intensely including the genes for TCF, β-catenin, and Wnt in HFDPSCs despite exposure to DT1 (
Figure 7). Although the preventive effects of GE 2000 were slightly higher than that of HSO, the effects under E40 were approximately 2.2 times higher than that of HSO (
Figure 7).
3. Discussion
Although utilization of hemp has been restricted by cannabis regulation in many countries, in recent, many countries have eased or abolished the regulations to aid development of the hemp industry [
24]. Besides, more than 30 countries have cultured and exported industrial hemp; the industrial hemp has been applied in the development of various products [
25].
Though not life-threatening, androgenic alopecia has a negative effect on the quality of life, mental health, and work productivity [
26]. For these reasons, development of materials with effective and eco-friendly results, without side-effects is greatly required in public health. In some reports [
27,
28,
29], seed oil and CBD in hemp are associated with hair regrowth. However, there are disadvantages of these materials including difficulties in application, usage limitations, and side effects, which limit the impact on preventing androgenic alopecia [
29,
30]. Further, there are no reports for hemp derived materials associated with the activation of follicle cell differentiation from HFDPSC and protection against DT.
This research reveals preventive functions of two biomaterials (GE2000 and E40) for androgenic alopecia through three biochemical categories including genetic modulation in HFDPSCs, cellular differentiation, and modulation of the immune system.
First, in genetic modulation of HFDPSCs, two materials modulated the alopecia associated genes including genes for TCF, β-catenin, Wnt, STAT1, 5α-reductase type 1, IL-15R, and NKG2DL. Notably, E40 dramatically upregulated TCF, β-catenin, and Wnt levels in HFDPSCs despite exposure to DT (
Figure 2). Additionally, the material intensely down regulated alopecia activating genes including genes for IL-15R and NKG2DL (
Figure 2). In recent reports, Wnt signaling pathway is a crucial key in hair growth [
31,
32]. Various types of Wnt molecules (Wnt1a, Wnt3a, Wnt4, Wnt5a, Wnt7b, and Wnt 10a/b) play crucial roles including hair follicle formation, damage repair, anagen gene expression, regeneration, and wound healing in the hair growth cycle [
31,
32]. Further, cascadic signals associated with Wnt, activation of β-catenin, and TCF activates hair growth [
31,
32]. Additionally, expressive suppression of STAT1, 5α-reductase type 1, IL-15R, NKG2DL molecules in follicular epithelial cells is very important to prevent alopecia [
31]. The down regulation of 5α-reductase type 1 in hair follicular epithelial cells suggests suppression of androgenic alopecia [
32]. Notably, expression of STAT1, IL-15R, and NKG2DL in the epithelial cells activates alopecia by communication with CD8
+ T cells [
31]. These reports suggest that two biomaterials prevent androgenic alopecia through modulation of alopecia associated genes in HFDPSCs and dramatic effectivity of E40 indicates the possibility for pharmaceutical materials to prevent androgenic alopecia.
Second, from the results on cellular differentiation, two materials accelerate germ cells and HFDPC differentiation from HFDPSCs despite exposure to DT. Based on the results of
Figure 3 and 4, two materials activated germ cells and HFDPC differentiation from HFDPSC. Notably, differentiation to germ cells was intensely activated. Ordinally, Itg9 and Sox2 molecules are marker proteins for HFDPC and P-cadherin and K15 also are markers for hair germ cells [
33]. A trigger of hair regeneration is a hair bulb located at the lowest part of the hair follicle where the hair germ is positioned. The hair germ cells communicate with HFDPCs and induce the stem cell activation in the hair bulge and the anagen phase initiation [
34]. Therefore, two biomaterials are worth applying as a key material for alopecia preventing items in cosmetic and pharmaceutic markets.
Third, alopecia activating molecules in CD8
+ T cells were modulated by HFDPSCs activated with two molecules. Signal pathways associated with IL2-Rβ, JAK1, and STAT1 in CD8
+ T cells activated alopecia [
31]. Beside IFNγ, various other cytokines are pivotal players in alopecia. IFNγ and γ
c cytokines (IL-2, IL-7, and IL-15) are involved in activation of Janus kinases (JAKs) and signal transducers and activators of transcription (STATs) [
35]. Inhibition of this signal pathway is a crucial mechanism of alopecia prevention and therapy. From the results of
Figure 5 and 6, two materials suppressed expression of these molecules in CD8
+ T cells despite exposure to DT. Notably, E40 suppressed them strongly in CD8
+ T cells. These results suggest that E40 can be a possible bio-pharmaceutical material.
Consequently, although HSO prevented androgenic alopecia (
Figure 7
), E40 and GE2000 effectively prevented DT stress and activated mechanisms for hair formation through biological activities in three categories.
4. Materials and Methods
4-1. Callus Induction and Purification of Exosomes and Extraction of Germinated Hemp Seeds
After immersion of sterilized hemp seeds (Cheongsam cultured in Andong city, Korea) 5 g in 1% H2O2 for a day, the hydrated seeds were germinated for 3 days at 24 °C on Murashige & Skoog (MS; KisanBio, Seoul, Korea) germinating media containing gibberellic acid (GA3, KisanBio), indole-3-acetic acid (IAA; KisanBio), and sucrose (KisanBio). The meristematic tissues were isolated from the germinated hemp seeds and then the tissues were cultured on MS (KisanBio) callus inducing media containing 6-benzylaminopurine (6BAP; KisanBio), IAA, and sucrose. After culturing the calli for 5 weeks on callus transfer medium, the exosomes were isolated and purified from the calli using exoEasy Maxi Kit (DE; QIAGEN, Hilden, Germany), CD68 Exo-Flow Capture Kit (SBI, Mountainview, CA, USA), exosome standards kit (STL; Sigma-Aldrich, St. Louis, MO, USA). The GHSEs were prepared through 50% ethyl alcohol (AE), 12 h incubation at 50% ethanol and hydrothermal treatment, 12 h incubation at DW, 70 °C (hydrothermal extract; HE) with the germinated seed.
4-2. Establishment of Treating Dosage in Human Follicle Dermal Papilla Stem Cells
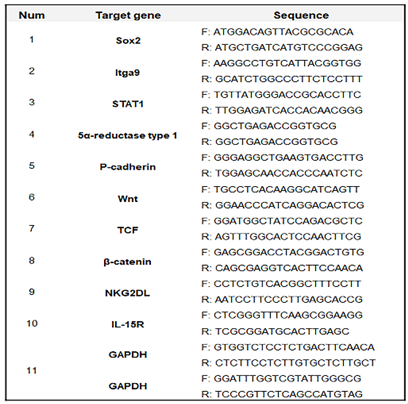
Proliferated HFDPSC (Sku: M36007-08S; Celprogen, Torrance, CA, USA) were cultured on specific growth media (SKU: 36007-08; Celprogen) for 1 day at 37 °C and 5% under various conditions (HE, AE30 to 70, and exosomes extracts) for establishment of treatment dosages. To evaluate and compare the effects between hydrothermal and alcoholic extraction, total RNAs of the exposed cells were isolated using RiboEx reagent (GeneAll, Seoul, Korea), and cDNA was synthesized from the isolated RNA using Maxime RT PreMix (iNtRON, Seongnam, Korea). The cDNA was amplified with primers (
Table 1) at the cycling parameters: 1 min at 95 °C, followed by 35 cycles for 35 s at 59 °C, and 1 min at 72 °C. The amplified DNA was estimated using iBright FL1000 and iBright Analysis Software 4.0.0 (Invitrogen, Waltham, MA, USA).
4-4. Evaluating the Differentiating Patterns of HFDPSC under the Two Materials
After exposure to various conditions (Con, DT, GE2000, DT+ GE2000, E40, and E40+DT) for a day, the total RNA in HFDPSCs (Sku: M36007-08S; Celprogen) were isolated from cells using RiboEx reagent (GeneAll), and cDNA was synthesized from the isolated RNA using Maxime RT PreMix (iNtRON). The cDNA was amplified with the primers (
Table 1) at the cycling parameters: 1 min at 95 °C, followed by 35 cycles for 35 s at 59 °C, and 1 min at 72 °C. The amplified DNA was estimated using iBright FL1000 and iBright Analysis Software 4.0.0 (Invitrogen). For evaluation of the flow cytometry results, the cultured cells were fixed with 2% paraformaldehyde for 4 h and treated with 0.02% Tween 20 for 5 min. The treated cells were incubated with fluorescence-conjugated antibodies (three immunoglobulins), FITC-anti-K15 (Novus Biologicals, Centennial, CO, USA), and APC-anti- Sox2 (Abcam) at 37 °C for 2 days. The stained cells were evaluated using a flow cytometer (BD FACScalibur), FlowJo 10.6.1 (BD Biosciences), and Prism 7 (GraphPad).
4-5. Images for Differentiated HFDPCs and Germ Cells
For evaluating the immunocytochemical results, the cultured cells exposed to various conditions (Con, DT, GE2000, DT+ GE2000, E40, E40+DT, and HSO 1000 μg/mL) were fixed with 2% paraformaldehyde for 4 h and treated with 0.02% Tween 20 for 5 min. The treated cells were incubated with fluorescence-conjugated antibodies (three immunoglobulins), FITC-anti-K15 (Novus Biologicals), and APC-anti- Sox2 at 37 °C for 2 days. The stained cells were evaluated using a flow cytometer (BD FACScalibur), FlowJo 10.6.1 (BD Biosciences), and Prism 7 (GraphPad).
4-6. Modulation of CD8+ T Cells by the Two Materials
To evaluate the down regulation of inactivating factors, the proliferated T cells (MOLT-4; ATCC, Manassas, VA, USA) were exposed to conditioned media (Con, GE2000CM, DTCM, DT+GE2000CM, E40CM, and E40+DTCM) and then were fixed with 2% paraformaldehyde for 4 h and treated with 0.02% Tween 20 for 5 min. The treated cells were incubated with fluorescence-conjugated antibodies (three immunoglobulins), FITC-anti-CD8 (BD Biosciences), PE-anti-IL-2Rβ (BioLegend, San Diego, CA, USA), PerCP-anti-JAK1 (ThermoFisher, Waltham, MA, USA), and APC-anti- STAT1(Abcam) at 37 °C for 2 days. With gated populations (CD8+ populations), the stained cells were evaluated using a flow cytometer (BD FACScalibur), FlowJo 10.6.1 (BD Biosciences), fluorescence microscope (Eclipse Ts-2; Nikon, Shinagawa, Japan), imaging and cell count software (NIS-elements V5.11; Nikon), and Prism 7 (GraphPad).
4-7. Concentration of IFNγ in CD8+ T Cells by the Two Materials
For evaluating IFNγ regulation, the proliferated T cells (MOLT-4; ATCC) were exposed to conditioned media (Con, GE2000CM, DTCM, DT+GE2000CM, E40CM, and E40+DTCM) and then the secreted IFNγ from stimulated CD8+ T cells were evaluated using IFNγ ELISA kits (ThermoFisher), AMR-100(ALLSHENG, Hangzhou, China) and Prism 7 (GraphPad).
Table 1.
Sequences for PCR primers.
Table 1.
Sequences for PCR primers.