Submitted:
07 June 2024
Posted:
10 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Subjects
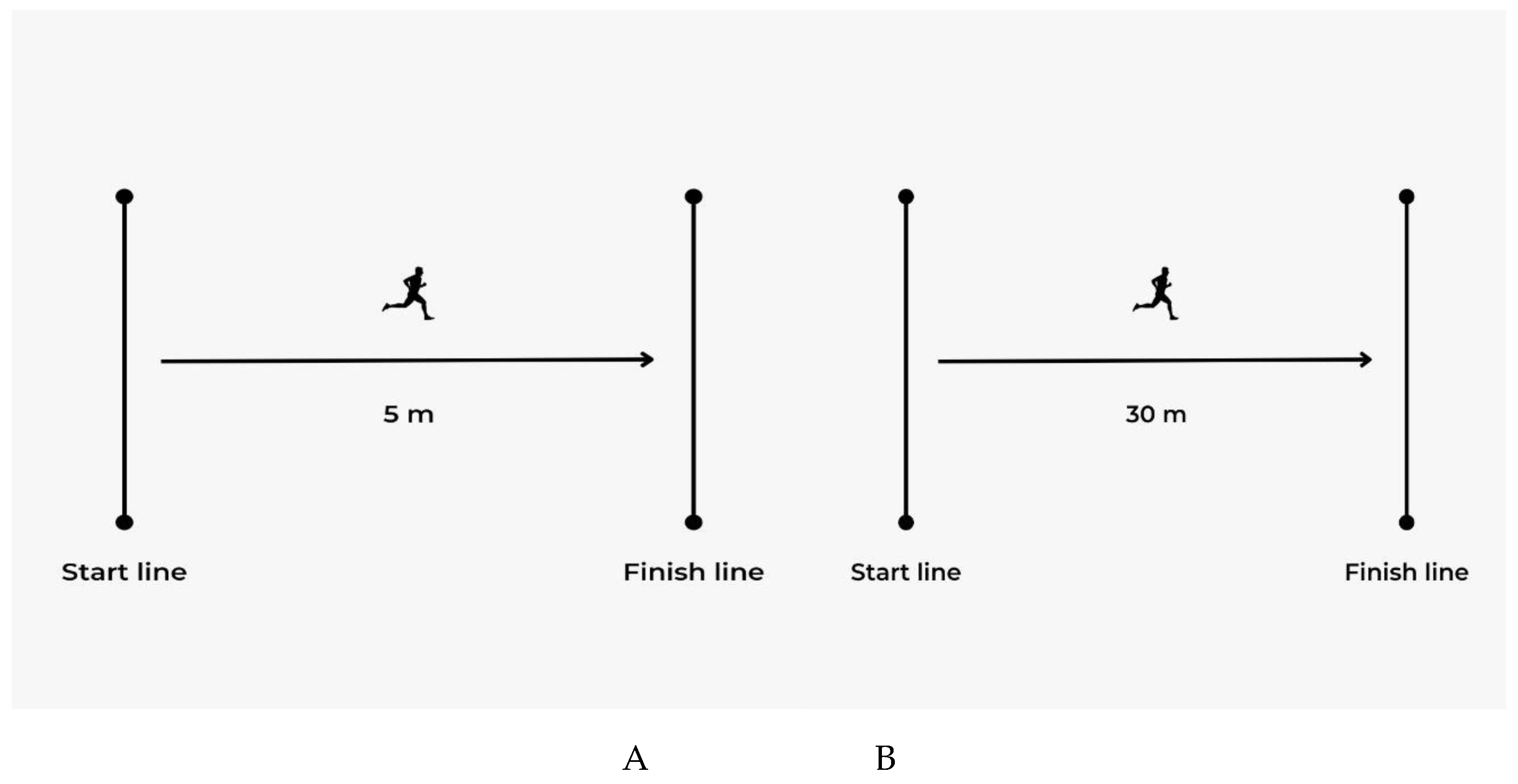
2.3. Sprint Test
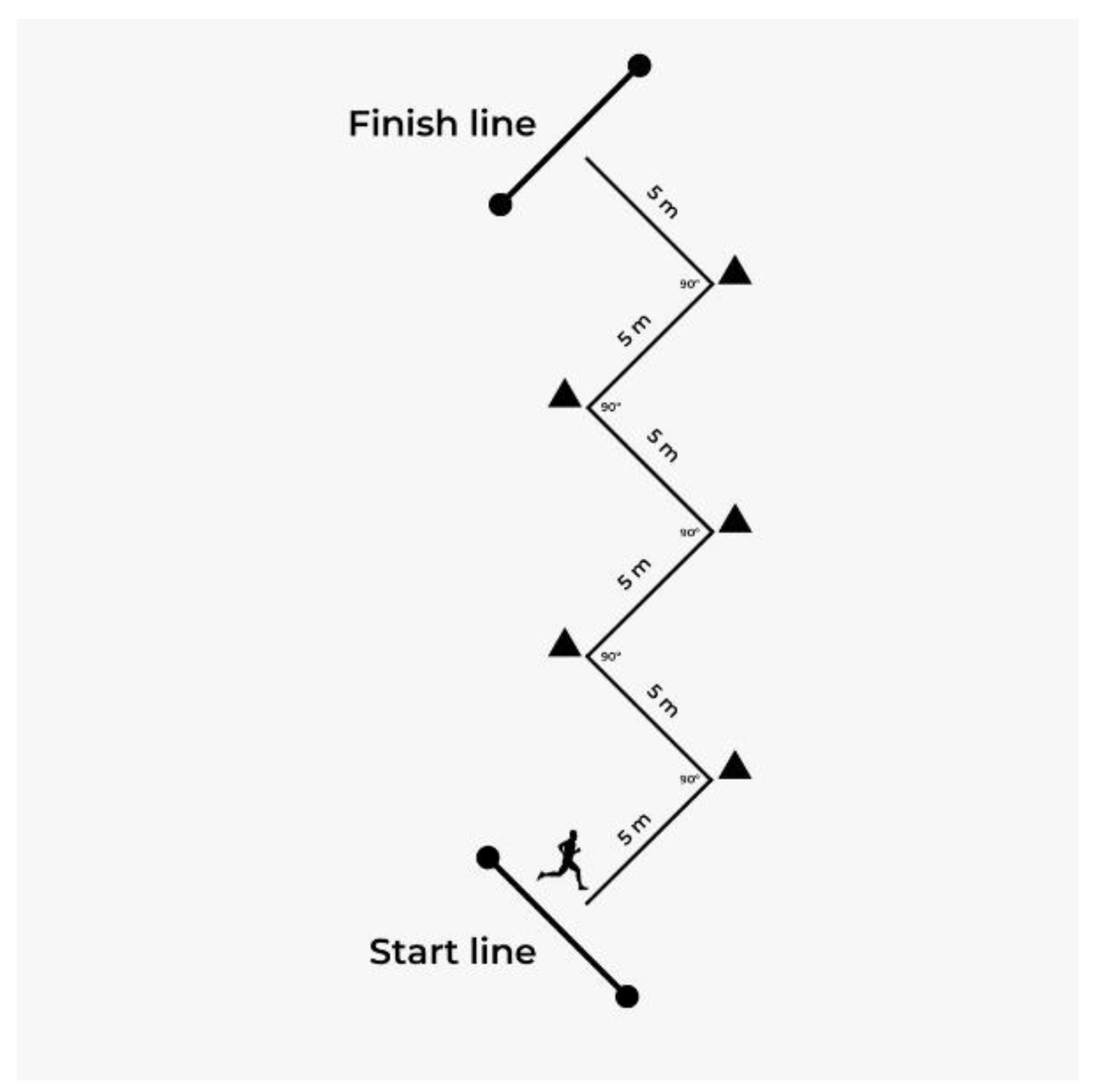
2.4. Change of Direction Test
2.5. Change of Direction Deficits
2.6. Vitamin D Serum Analysis
2.7. Statistical Analysis
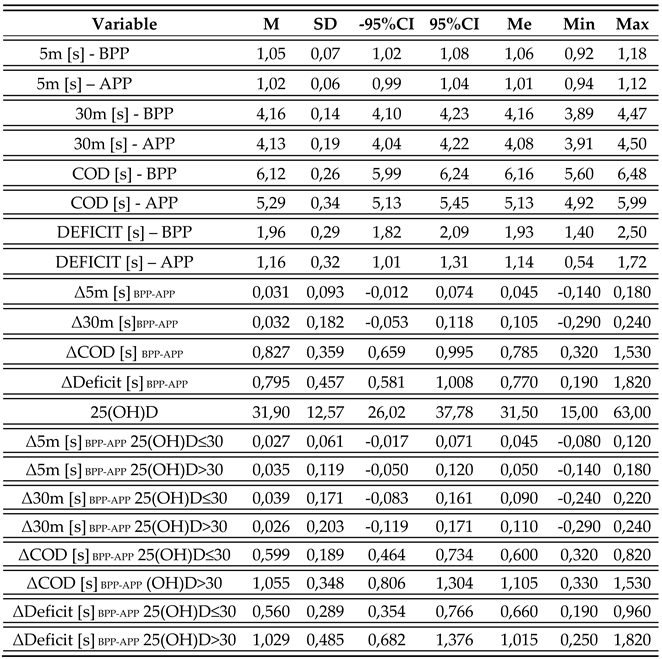
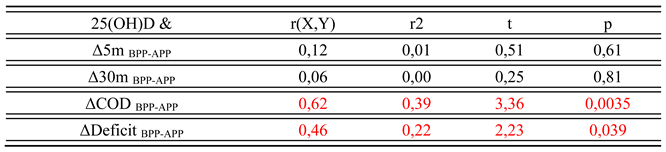
3. Results
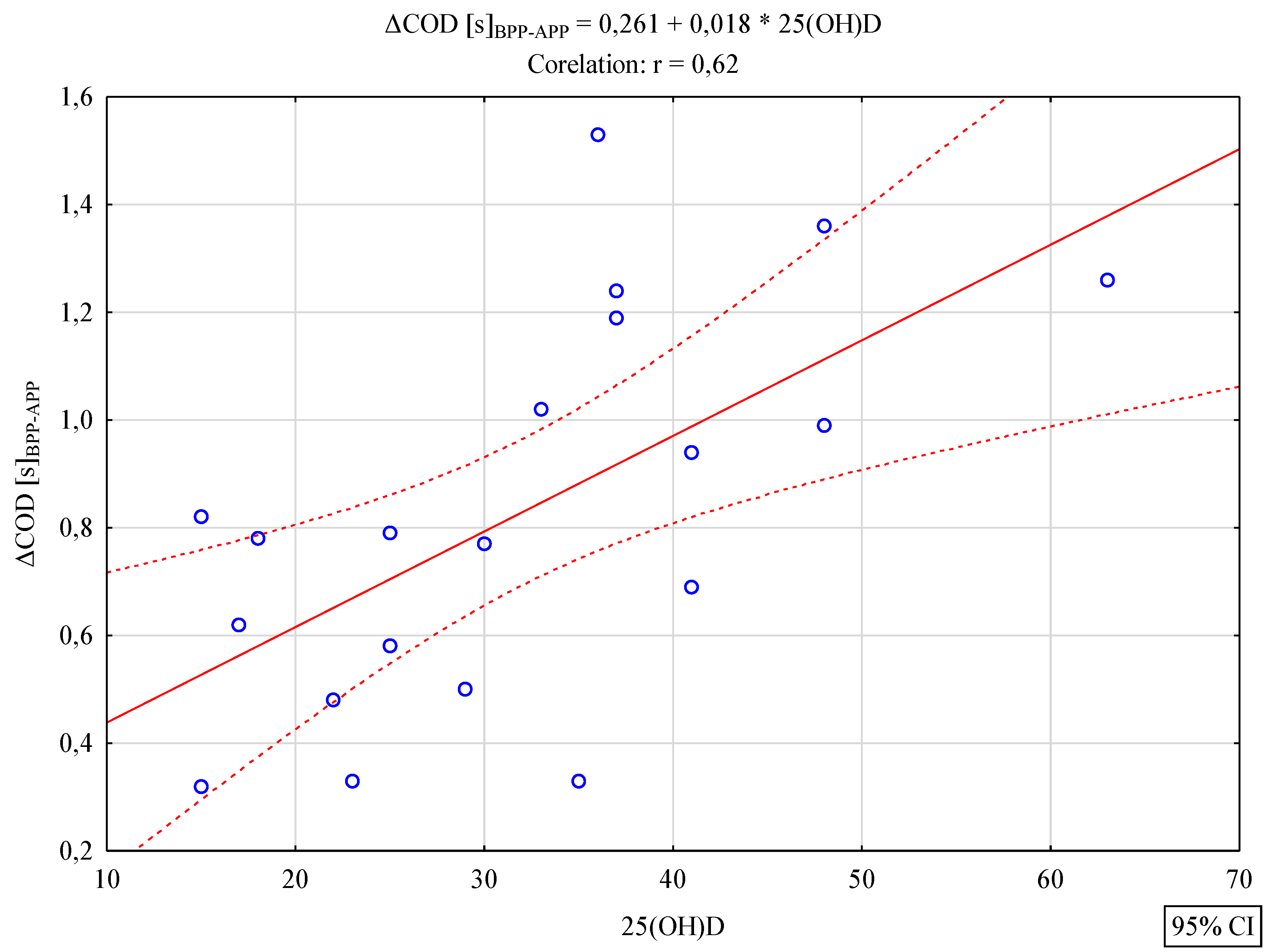
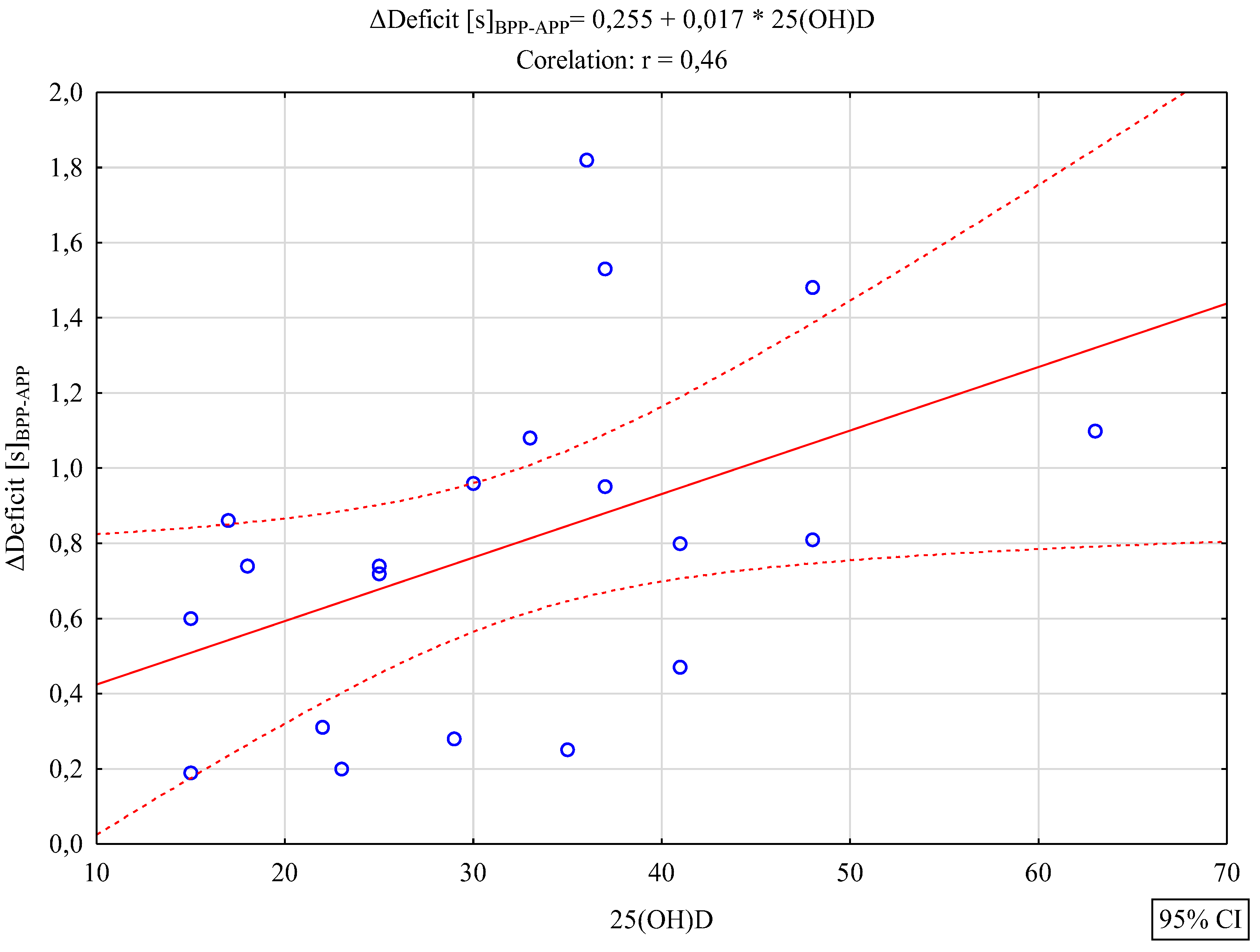
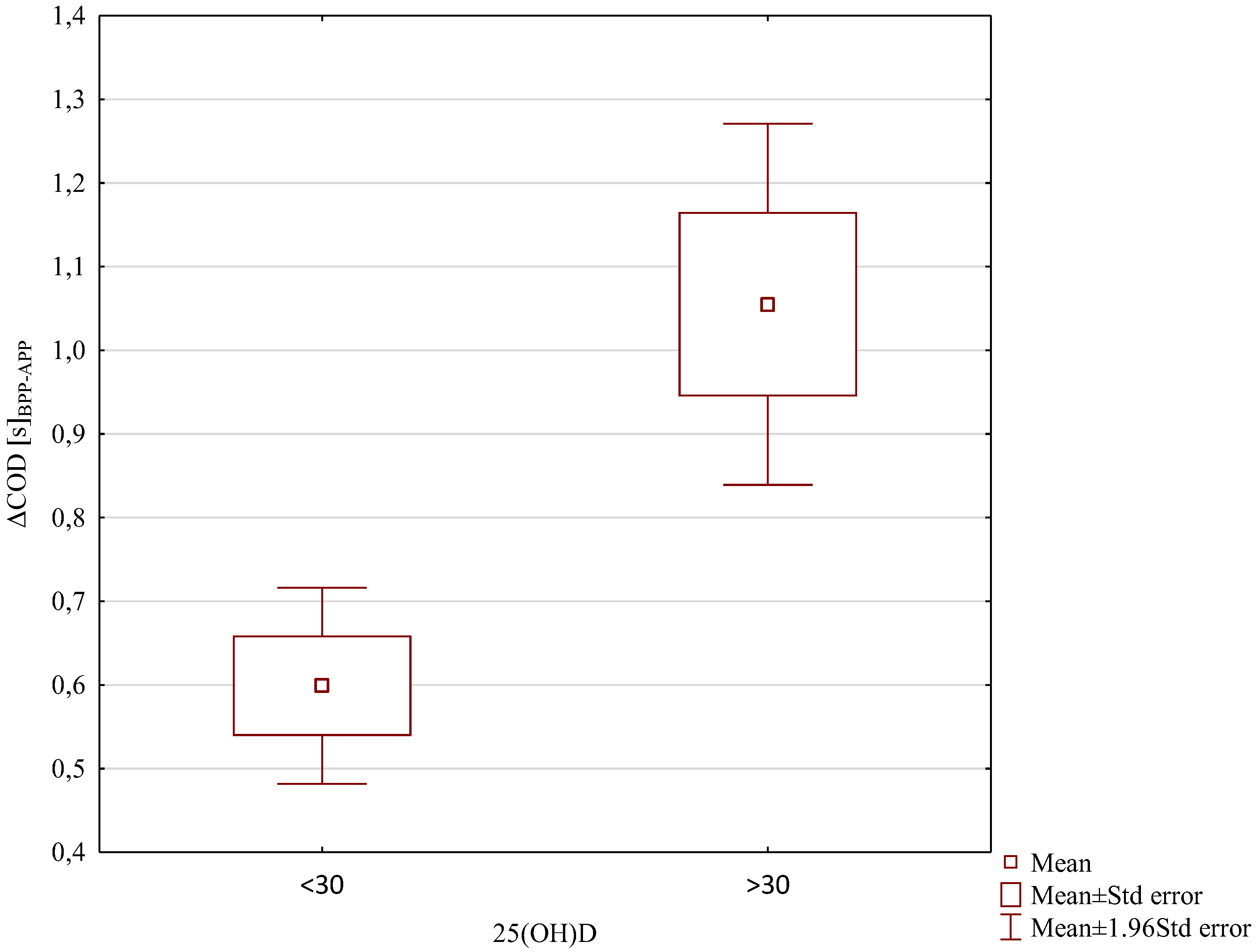
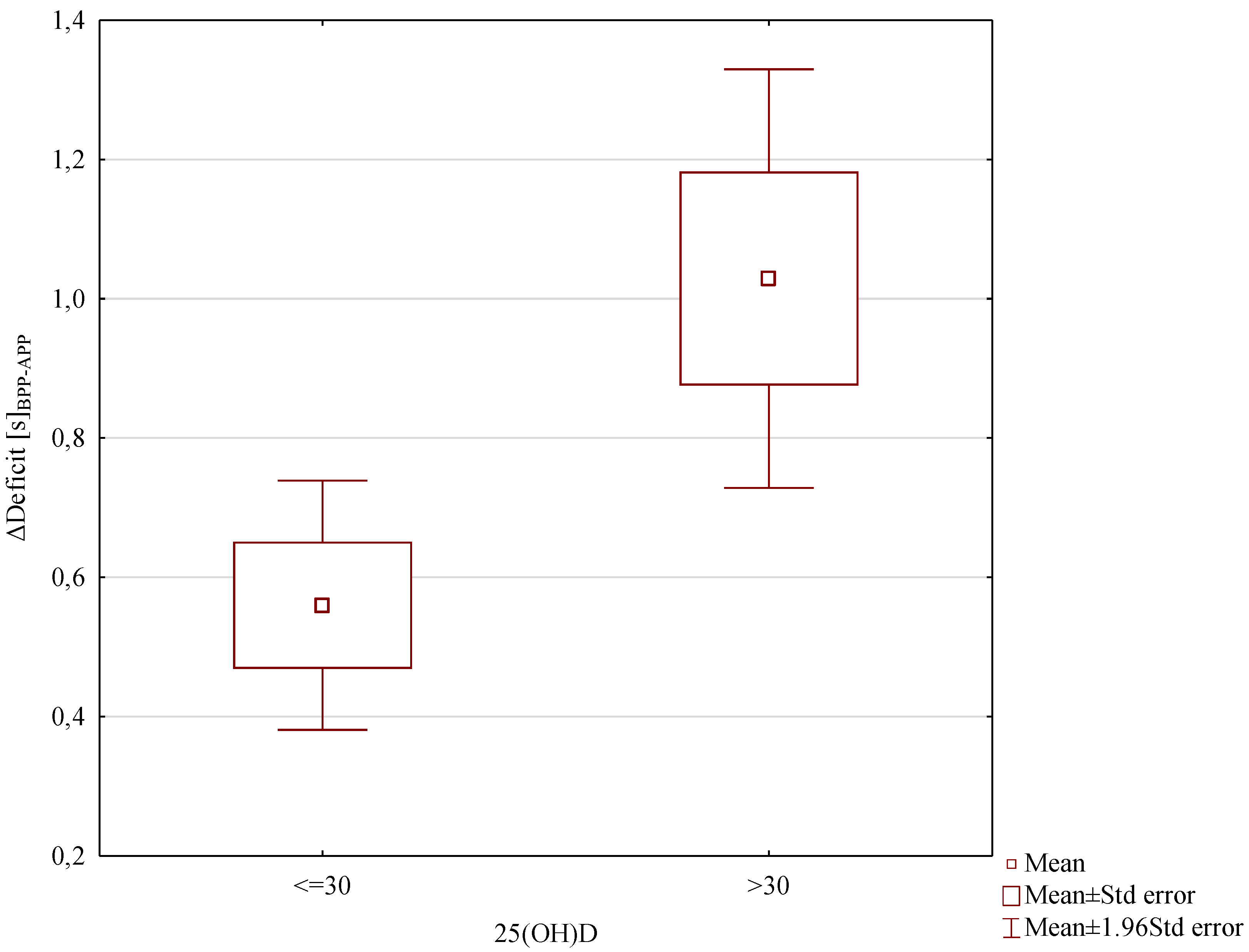
4. Discussion
Vitamin D and Performance
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006, 92, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Rupprecht, M.; Wagenpfeil, S.; Schöpe, J.; Vieth, R.; Vogt, T.; Reichrath, J. Meta-Analysis of European Clinical Trials Characterizing the Healthy-Adult Serum 25-hydroxyvitamin D Response to Vitamin D Supplementation. Nutrients. 2023, 15, 3986. [Google Scholar] [CrossRef] [PubMed]
- Peterlik, M.; Cross, H.S. Vitamin D and calcium insufficiency-related chronic diseases: molecular and cellular pathophysiology. Eur J Clin Nutr. 2009, 63, 1377–86. [Google Scholar] [CrossRef]
- Brännström, A.; Yu, J.G.; Jonsson, P. , Åkerfeldt, T.; Stridsberg, M.; Svensson, M. Vitamin D in relation to bone health and muscle function in young female soccer players. Eur J Sport Sci. 2017, 17, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Lips, P. Vitamin D physiology. Prog Biophys Mol Biol. 2006, 92, 4–8. [Google Scholar] [CrossRef]
- Tanaka, M.; Kishimoto, K.N.; Okuno, H.; Saito, H.; Itoi, E. Vitamin D receptor gene silencing effects on differentiation of myogenic cell lines. Muscle Nerve. 2014, 49, 700–708. [Google Scholar] [CrossRef]
- Verstuyf, A.; Carmeliet, G.; Bouillon, R. ; Mathieu, C “Vitamin D: a pleiotropic hormone”. Kidney Int. 2010, 78, 140–145. [Google Scholar] [CrossRef]
- Dahlquist, D.T.; Dieter, B.P.; Koehle, M.S. Plausible ergogenic effects of vitamin D on athletic performance and recovery. J Int Soc Sports Nutr. 2015, 12, 33, eCollection 2015. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020, 12, 2097. [Google Scholar] [CrossRef]
- Zittermann, A. “Vitamin D in preventive medicine: are we ignoring the evidence?”. Br J Nutr. 2003, 89, 552–572. [Google Scholar] [CrossRef] [PubMed]
- Polak, M.A.; Houghton, L.A.; Reeder, A.I.; Harper, M.J.; Conner, T.S. Serum 25-hydroxyvitamin D concentrations and depressive symptoms among young adult men and women. Nutrients. 2014, 6, 4720–30. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, K.R.; Cruzat, V.; Carlessi, R.; Newsholme, P. Mechanisms of vitamin D action in skeletal muscle. Nutr Res Rev. 2019, 32, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Kwon, J.; Kim, H.; Shimada, H.; Yoshida, Y.; Iwasa, H.; Yoshida, H. Low serum 25-hydroxyvitamin D levels associated with falls among Japanese community-dwelling elderly. J Bone Miner Res. 2008, 23, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.; Henriksen, V.T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Schneider, E.D.; Dixon, B.M.; Brian, M.; Weaver, L.K. Higher serum 25-hydroxyvitamin D concentrations associate with a faster recovery of skeletal muscle strength after muscular injury. Nutrients. 2013, 5, 1253–1275. [Google Scholar] [CrossRef] [PubMed]
- Most, A.; Dorr, O.; Nef, H.; Hamm, C.; Bauer, T.; Bauer, P. Influence of 25-hydroxy-vitamin D insufficiency on maximal aerobic power in elite indoor athletes: a cross-sectional study. Sports Med - Open, 7.
- Hamilton, B.; Whiteley, R.; Farooq, A.; Chalabi, H. Vitamin d concentration in 342 professional football players and association with lower limb isokinetic function. J. Sci. Med. Sport. 2014, 17, 139–143. [Google Scholar] [CrossRef]
- Girgis, C.M.; Clifton-Bligh, R.J.; Hamrick, M.W.; Holick, M.F.; Gunton, J.E. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev. 2013, 34, 33–83. [Google Scholar] [CrossRef] [PubMed]
- Close, G.L.; Russell, J.; Cobley, J.N.; Owens, D.J.; Wilson, G.; Gregson, W.; Fraser, W.D.; Morton, J.P. Assessment of vitamin D concentration in non-supplemented professional athletes and healthy adults during the winter months in the UK: Implications for skeletal muscle function. J. Sports Sci. 2013, 31, 344–353. [Google Scholar] [CrossRef]
- Gilic, B.; Kosor, J.; Jimenez-Pavon, D.; Markic, J.; Karin, Z.; Domic, D.S.; Sekulic, D. Associations of Vitamin D Levels with Physical Fitness and Motor Performance; A Cross-Sectional Study in Youth Soccer Players from Southern Croatia. Biology. 2021, 10, 751. [Google Scholar] [CrossRef]
- Nimptsch, K.; Platz, E.A.; Willett, W.C.; Giovannucci, E. Association Between Plasma 25-oh Vitamin D and Testosterone Levels in Men. Clin. Endocrinol. 2012, 77, 106–112. [Google Scholar] [CrossRef]
- Michalczyk, M.M.; Golas, A.; Maszczyk, A.; Zajac, A. Influence of sunlight and oral D(3) supplementation on serum 25(OH)D concentration and exercise performance in elite soccer players. Nutrients. 2020, 12, 1311. [Google Scholar] [CrossRef] [PubMed]
- Ameri, P.; Giusti, A.; Boschetti, M.; Murialdo, G.; Minuto, F.; Ferone, D. Interactions between Vitamin D and IGF-I: From Physiology to Clinical Practice. Clin. Endocrinol. 2013, 79, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, L.Y.; Wortsman, J.; Dannenberg, M.J.; Hollis, B.W.; Lu, Z.; Holick, M.F. Clothing prevents ultraviolet-B radiation-dependent photosynthesis of vitamin D3. J. Clin. Endocrinol. Metab. 1992, 75, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Holden, J.M.; Lemar, L.E.; Exler, J. Vitamin D in foods: development of the US Department of Agriculture database. Am J Clin Nutr. 2008, 87, 1092S–6S. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; Rudenka, E.; Misiorowski, W.; Zakharova, I.; Rudenka, A.; Łukaszkiewicz, J.; Marcinowska-Suchowierska, E.; Łaszcz, N.; Abramowicz, P.; Bhattoa, H.P.; Wimalawansa, S.J. Vitamin D supplementation guidelines. J Steroid Biochem Mol Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Hollis, W.B. Circulating 25-Hydroxyvitamin D levels indicative of Vitamin D insufficiency: Implications of establishing a new effective dietary intake. Recommendations for Vitamin, D. J Nutr. 2005; 135, 317–22. [Google Scholar]
- Cashman, K.D.; Dowling, K.G.; Skrabakova, Z.; Gonzalez-Gross, M.; Valtuena, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Molgaard, C.; et al. Vitamin D deficiency in Europe-Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Ksiazek, A.; Zagrodna, A.; Dziubek, W.; Pietraszewski, B.; Ochmann, B.; Słowińska-Lisowska, M. 25(OH)D3 levels relative to muscle strength and maximum oxygen uptake in athletes. J Hum Kinet. 2016, 50, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Ksiazek, A.; Zagrodna, A.; Slowinska-Lisowska, M.; Lombardi, G. Relationship between metabolites of vitamin D, free 25-(OH)D, and physical performance in indoor and outdoor athletes. Front Physiol. 2022, 13, 909086, eCollection 2022. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.P.; Iqbal, Z.; Drust, B.; Burgess, D.; Close, G.L.; Brukner, P.D. Seasonal variation in vitamin D status in professional soccer players of the English premier league. Appl. Physiol. Nutr. Metab. 2012, 37, 798–802. [Google Scholar] [CrossRef]
- Bezuglov, E.; Tikhonova, A.; Zueva, A.; Khaitin, V.; Lyubushkina, A.; Achkasov, E.; Waśkiewicz, Z.; Gerasimuk, D.; Żebrowska, A.; Nikolaidis, P.T.; Rosemann, T.; Knechtle, B. The Dependence of Running Speed and Muscle Strength on the Serum Concentration of Vitamin D in Young Male Professional Football Players Residing in the Russian Federation. Nutrients. 2019, 11, 1960. [Google Scholar] [CrossRef]
- Bezuglov, E.; Tikhonova, A.; Zueva, A.; Khaitin, V.; Waśkiewicz, Z.; Gerasimuk, D.; Żebrowska, A.; Rosemann, T.; Nikolaidis, P.; Knechtle, B. Prevalence and Treatment of Vitamin D Deficiency in Young Male Russian Soccer Players in Winter. Nutrients. 2019, 11, 2405. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B. Vitamin D and Human Skeletal Muscle. Scand. J. Med. Sci. Sports. 2010, 20, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N.E.; Karl, J.P.; Cable, S.J.; Williams, K.W.; Rood, J.C.; Young, A.J.; Lieberman, H.R.; McClung, J.P. Vitamin D status in female military personnel during combat training. J Int Soc Sports Nutr. 2010, 14, 7–38. [Google Scholar] [CrossRef] [PubMed]
- Von Hurst, P.R.; Beck, K.L. Vitamin D and skeletal muscle function in athletes. Curr. Opin. Clin. Nutr. Metab. Care. 2014, 17, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Ardestani, A.; Parker, B.; Mathur, S.; Clarkson, P.; Pescatello, L.S.; Hoffman, H.J.; Polk, D.M.; Thompson, P.D. Relation of vitamin d level to maximal oxygen uptake in adults. Am J Cardiol. 2011, 107, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Butscheidt, S.; Rolvien, T.; Ueblacker, P.; AmLing, M.; Barvencik, F. Impact of Vitamin D in Sports: Does vitamin D insufficiency compromise athletic performance? Sportverletz. Sportschaden. 2017, 31, 37–44. [Google Scholar] [PubMed]
- Agergaard, J.; Trøstrup, J.; Uth, J.; Iversen, J.V.; Boesen, A.; Andersen, J.L.; Schjerling, P.; Langberg, H. Does Vitamin-D Intake During Resistance Training Improve the Skeletal Muscle Hypertrophic and Strength Response in Young and Elderly men?—A Randomized Controlled Trial. Nutr. Metab. 2015, 2, 32. [Google Scholar] [CrossRef] [PubMed]
- Giminiani, R.D.; Visca, C. Explosive strength and endurance adaptations in young elite soccer players during two soccer seasons. PLoS One. 2017; 12, e0171734, Published online 2017 Feb 13. [Google Scholar] [CrossRef]
- Zajac, A.; Golas, A.; Chycki, J.; Halz, M.; Michalczyk, M. The Effects of Long-Term Magnesium Creatine Chelate Supplementation on Repeated Sprint Ability (RAST) in Elite Soccer Players. Nutrients. 2020, 12, 2961. [Google Scholar] [CrossRef]
- Jastrzębska, J.; Skalska, M.; Radzimiński, Ł.; López-Sánchez, G.F.; Weiss, K.; Hill, L.; Knechtle, B. Changes of 25(OH)D Concentration, Bone Resorption Markers and Physical Performance as an Effect of Sun Exposure, Supplementation of Vitamin D and Lockdown among Young Soccer Players during a One-Year Training Season. Nutrients. 2022, 14, 521. [Google Scholar] [CrossRef]
- Askar, P.V.; Pais, V.; Mohan, N.; Saad, S.; Shaikhji, N.M. Effectivness of eccentric training, dynamic range of motion exercise and static stretching on flexibility of hamstring muscle among football players. Int J Physio, 2015; 2, 1012–1018. [Google Scholar]
- Papla, M.; Latocha, A.; Grzyb, W.; Golas, A. Relationship between lower limb power output, sprint and change of direction performance in soccer players. Balt J Health Phys Act. 2022, 14, 3. [Google Scholar] [CrossRef]
- Koundourakis, N.E.; Androulakis, N.E.; Malliaraki, N.; Margioris, A.N. Vitamin D and exercise performance in professional soccer players. PLoS ONE. 2014, 9, e101659. [Google Scholar] [CrossRef] [PubMed]
- Bezrati, I.; Ben Fradj, M.K.; Hammami, R.; Ouerghi, N.; Padulo, J.; Feki, M. A Single Mega Dose of Vitamin D(3) Improves Selected Physical Variables in Vitamin D-Deficient Young Amateur Soccer Players: A Randomized Controlled Trial. Appl. Physiol. Nutr. Metab. 2020, 45, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Jastrzebska, M.; Kaczmarczyk, M.; Michalczyk, M.; Radziminski, L.; Stepien, P.; Jastrzebska, J.; Wakuluk, D.; Suarez, A.D.; Lopez Sanchez, G.F.; Cieszczyk, P.; Godlewski, P.; Król, P.; Jastrzębski, Z. Can Supplementation of Vitamin D Improve Aerobic Capacity in Well Trained Youth Soccer Players? J. Hum. Kinet. 2018, 61, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębska, M.; Kaczmarczyk, M.; Jastrzębski, Z. The effect of vitamin d supplementation on training adaptation in well trained soccer players. J. Strength Cond. Res. 2016, 30, 2648–2655. [Google Scholar] [CrossRef] [PubMed]
- Kopeć, A.; Solarz, K.; Majda, F.; Słowińska-Lisowska, M.; Mędraś, M. An evaluation of the levels of vitamin d and bone turnover markers after the summer and winter periods in polish professional soccer players. J Hum Kinet. 2013, 38, 135–40, eCollection 2013. [Google Scholar] [CrossRef] [PubMed]
- Chun, R.F.; Liu, P.T.; Modlin, R.L.; Adams, J.S.; Hewison, M. Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Front Physiol. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M. Immune function in sport and exercise. J Appl Physiol. 2007, 103, 693–9. [Google Scholar] [CrossRef] [PubMed]
- Solarz, K.; Kopeć, A.; Pietraszewska, J.; Majda, F.; Słowińska-Lisowska, M.; Mędraś, M. An evaluation of the levels of 25-hydroxyvitamin D3 and bone turnover markers in professional football players and in physically inactive men. Physiol. Res. 2014, 63, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Barcal, J.N.; Thomas, J.T.; Hollis, B.W.; Austin, K.J.; Alexander, B.M.; Larson-Meyer, D.E. Vitamin D and weight cycling: Impact on injury, illness, and inflammation in collegiate wrestlers. Nutrients. 2016, 8, E775. [Google Scholar] [CrossRef]
- Skalska, M.; Nikolaidis, P.T.; Knechtle, B.; Rosemann, T.J.; Radzimiński, Ł.; Jastrzębska, J.; Kaczmarczyk, M.; Myśliwiec, A.; Dragos, P.; López-Sánchez, G.F.; Jastrzębski, Z. Vitamin D Supplementation and Physical Activity of Young Soccer Players during High-Intensity Training. Nutrients. 2019, 11, E349. [Google Scholar] [CrossRef]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of vitamin D on skeletal muscle function: oxidative stress, energy metabolism and anabolic state. Eur J Appl Physiol. 2019, 119, 825–839, Published online 2019 Mar 4. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, M.W.; Brinkmeier, H.; Muntener, M. Calcium Ion in Skeletal Muscle: Its Crucial Role for MuscleFunction, Plasticity, and Disease. Physiol. Rev. 2000, 80, 1215–1265. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Adamkiewicz, D.; Adamkiewicz, M.; Śniegocki, M.; Podhorecka, M.; Szychta, P.; Malinowski, B. Impact of Vitamin D on Physical Efficiency and Exercise Performance- A Review. Nutrients. 2019, 11, 2826, Published online 2019 Nov 19. [Google Scholar] [CrossRef] [PubMed]
- Santillán, G.; Katz, S.; Vazquez, G.; Boland, R.L. TRPC3-Like Protein and Vitamin D Receptor Mediate 1alpha,25(OH)2D3-Induced SOC Influx in Muscle Cells. Int. J. Biochem. Cell Biol. 2004, 36, 1910–1918. [Google Scholar] [CrossRef]
- Deschenes, M.R.; Kraemer, W.J. Performance and Physiologic Adaptations to Resistance Training. Am. J. Phys. Med. Rehabil. 2002, 81, S3–S16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





