Submitted:
06 June 2024
Posted:
10 June 2024
You are already at the latest version
Abstract
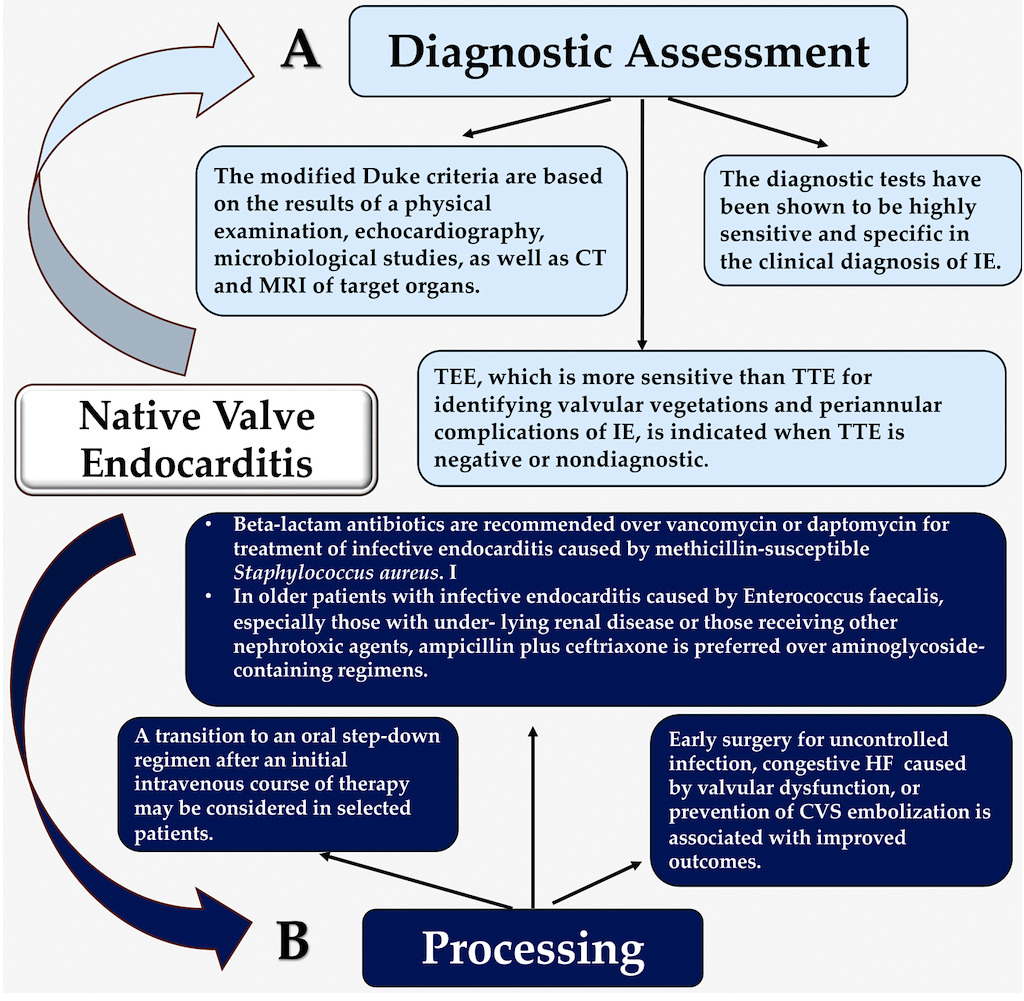
Keywords:
1. Introduction: An Overview of the Epidemiological, Pathophysiological, and Clinical Features of Disease
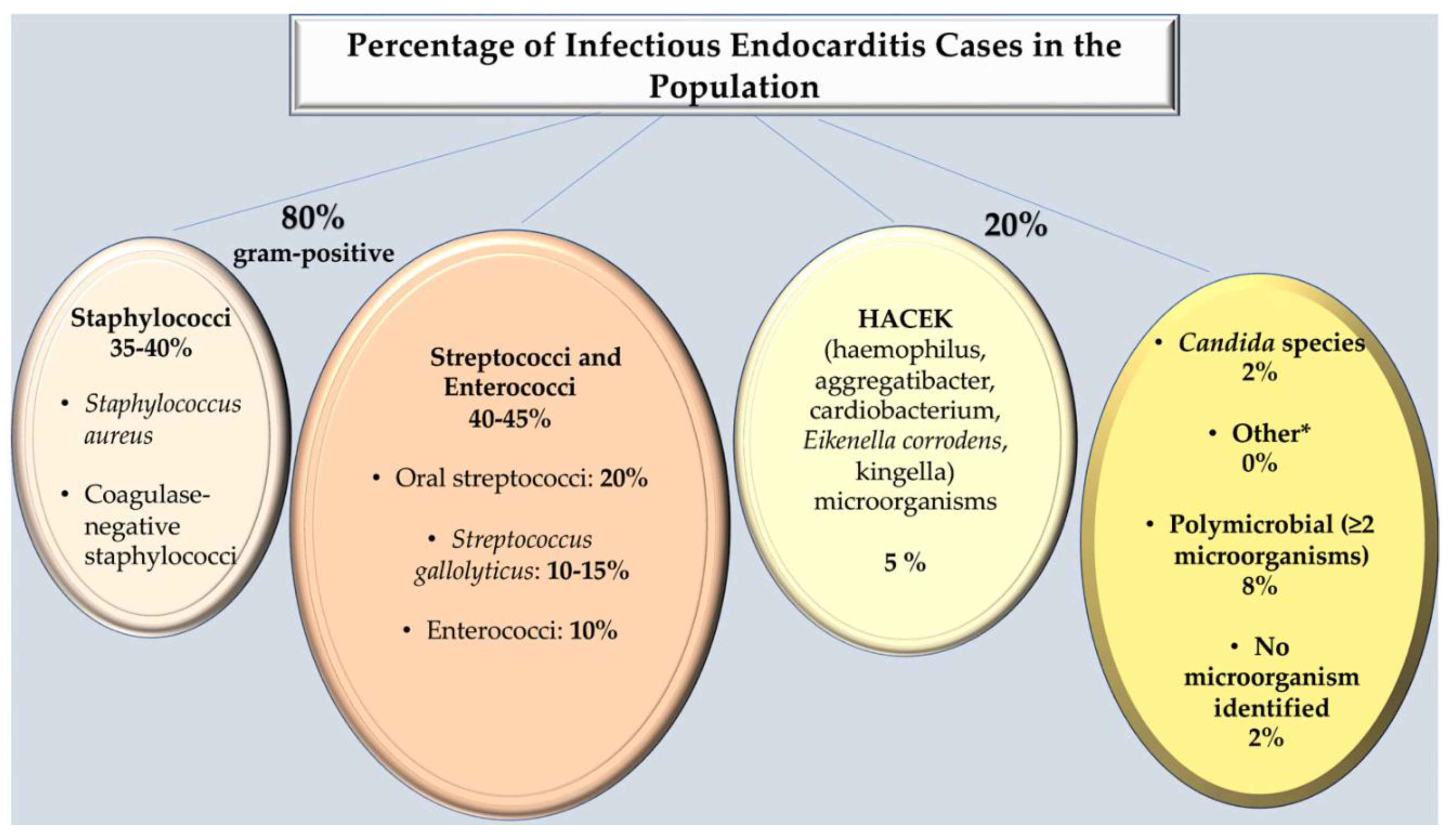
2. Microbiological Characteristics
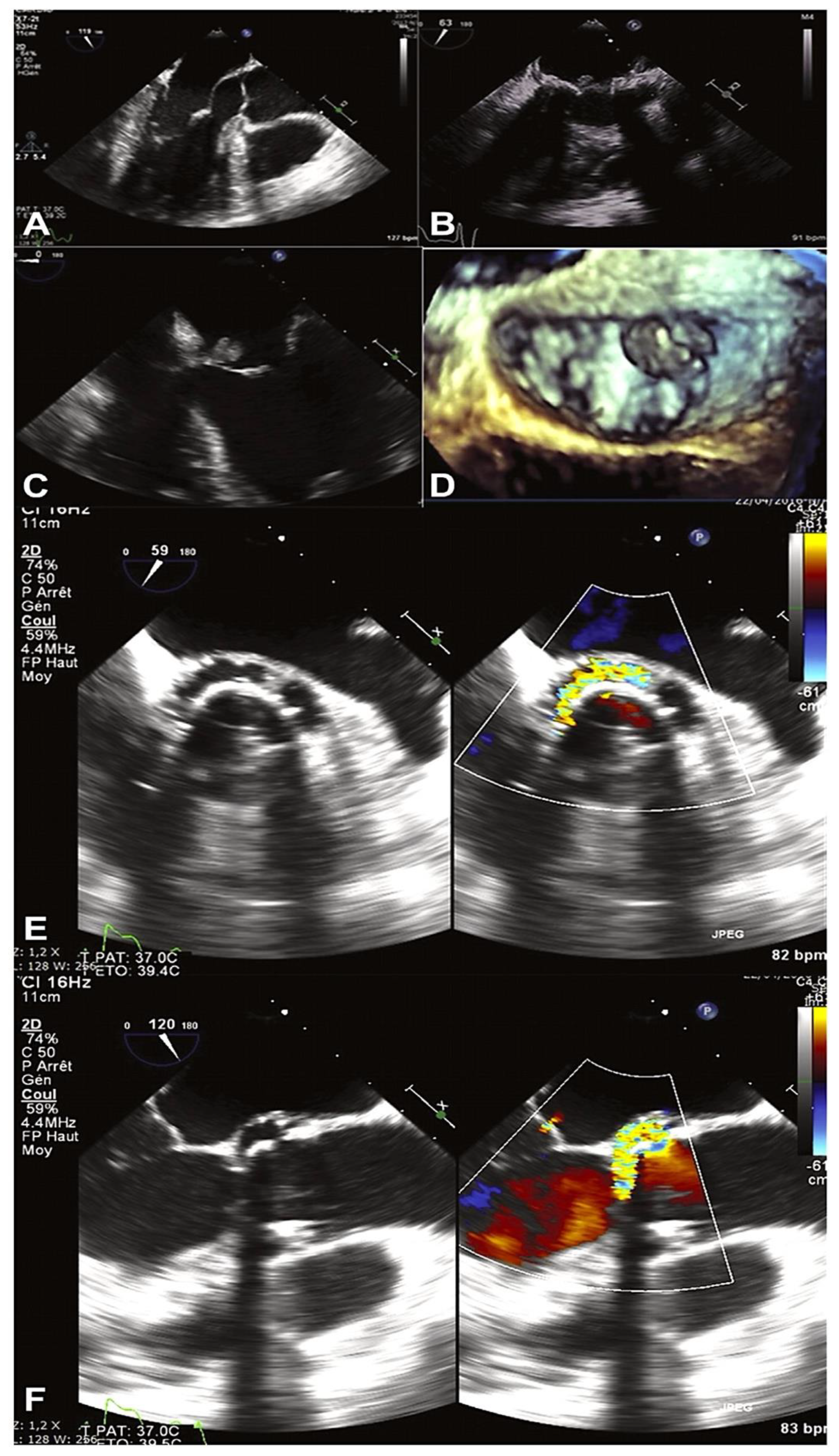
3. Strategy Assessment and Proof of Concept
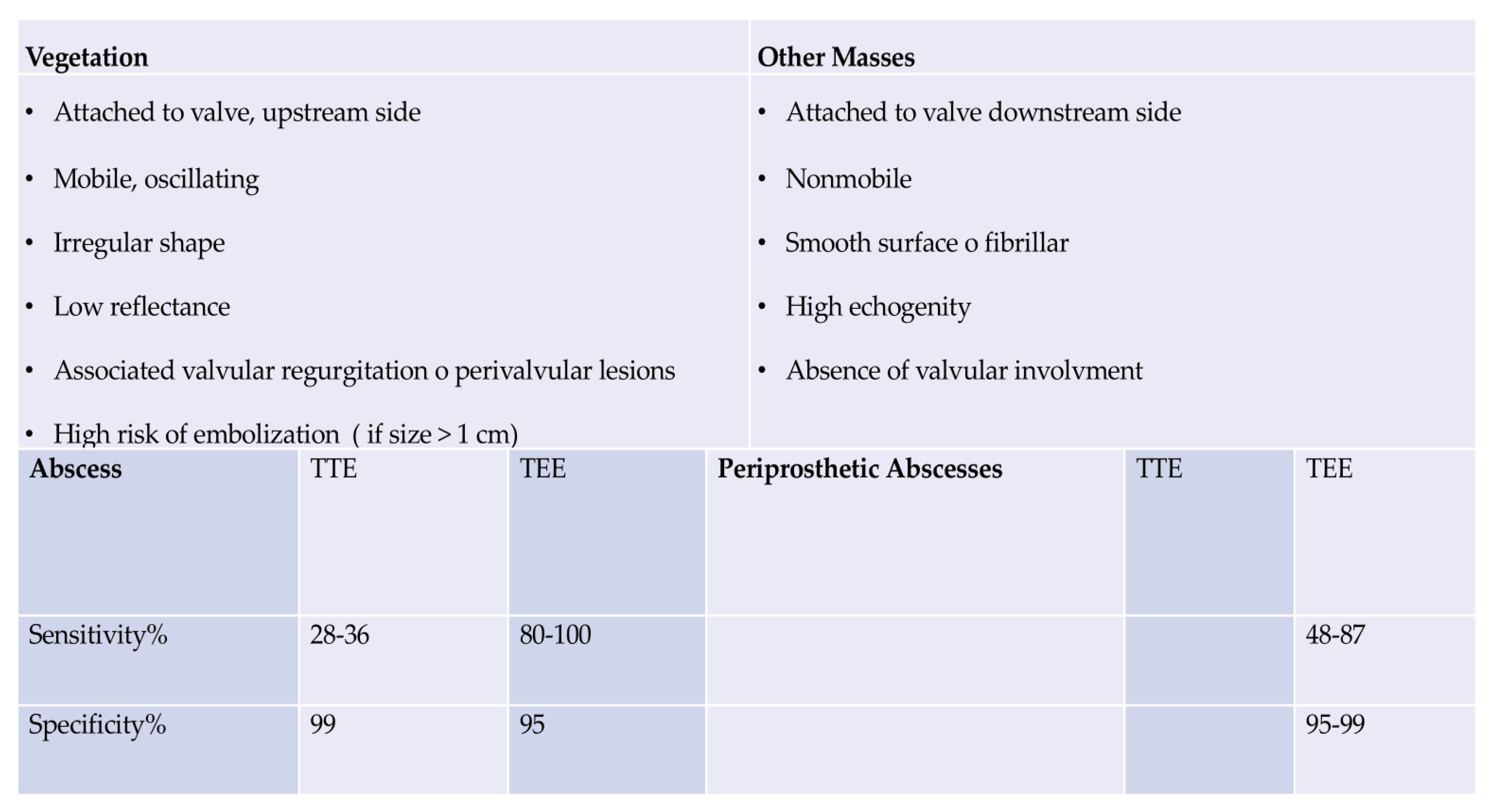
- Clinical Evidence: Imaging Criteria
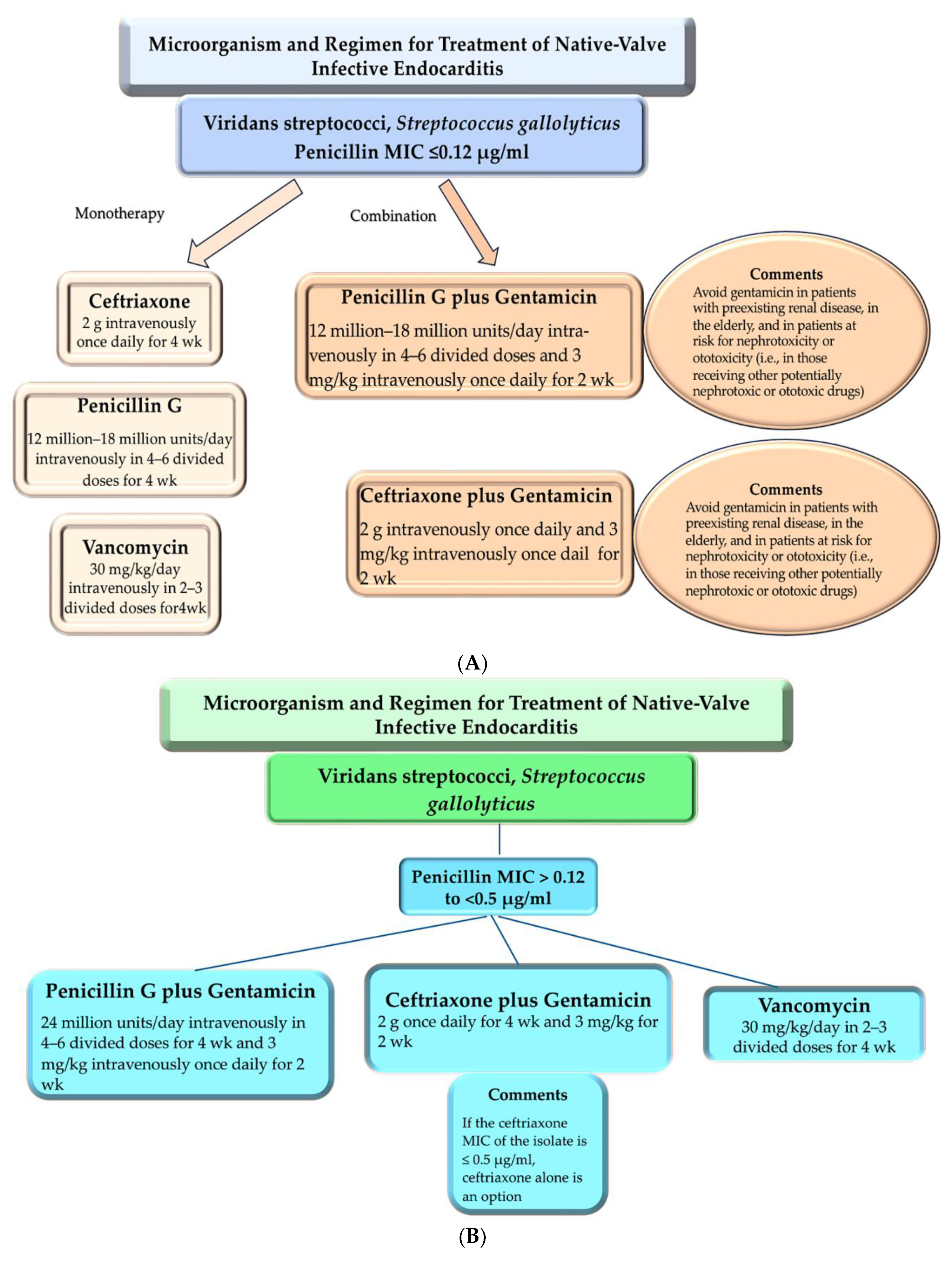
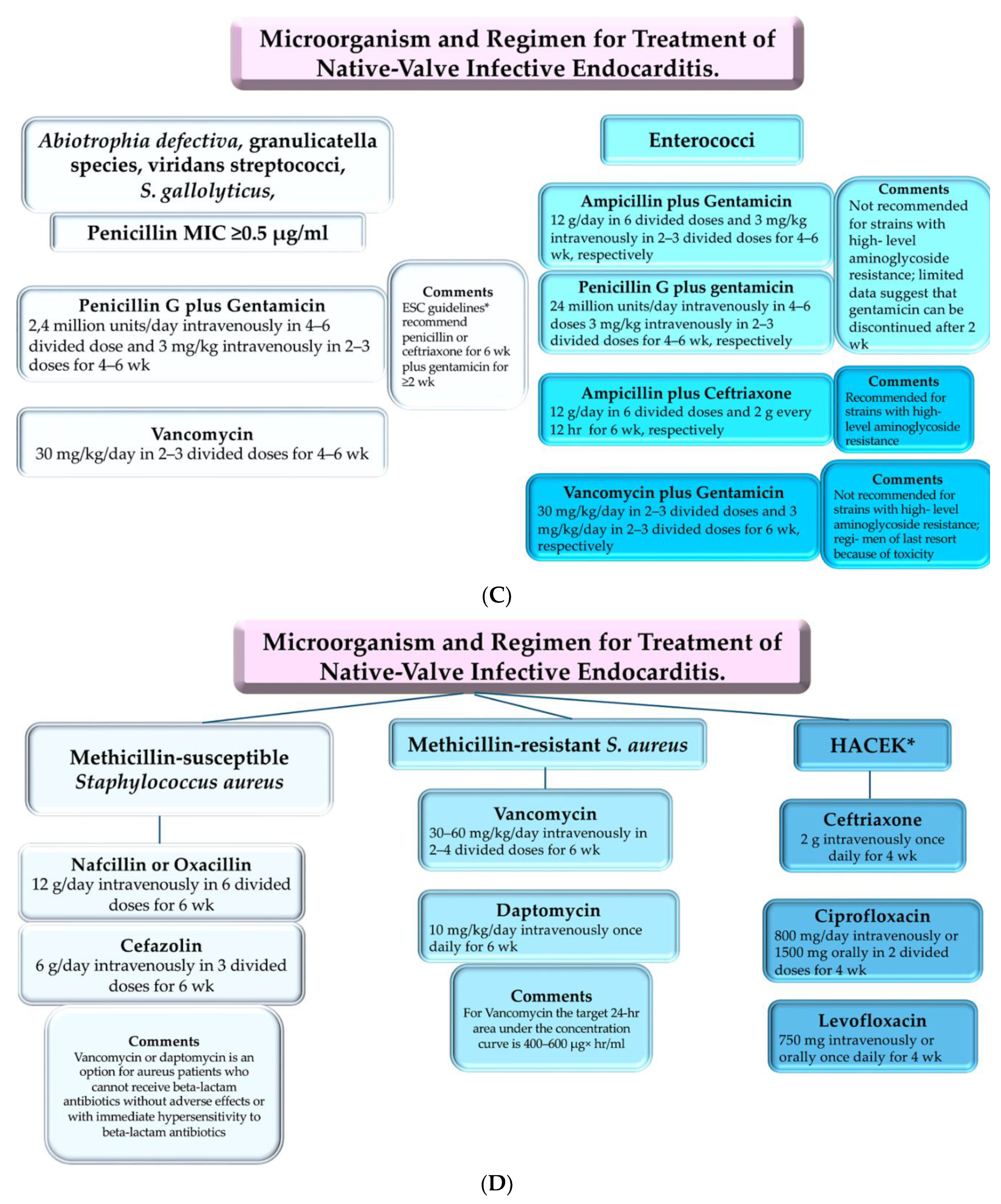
4. Clinical Use: Antimicrobial Therapies
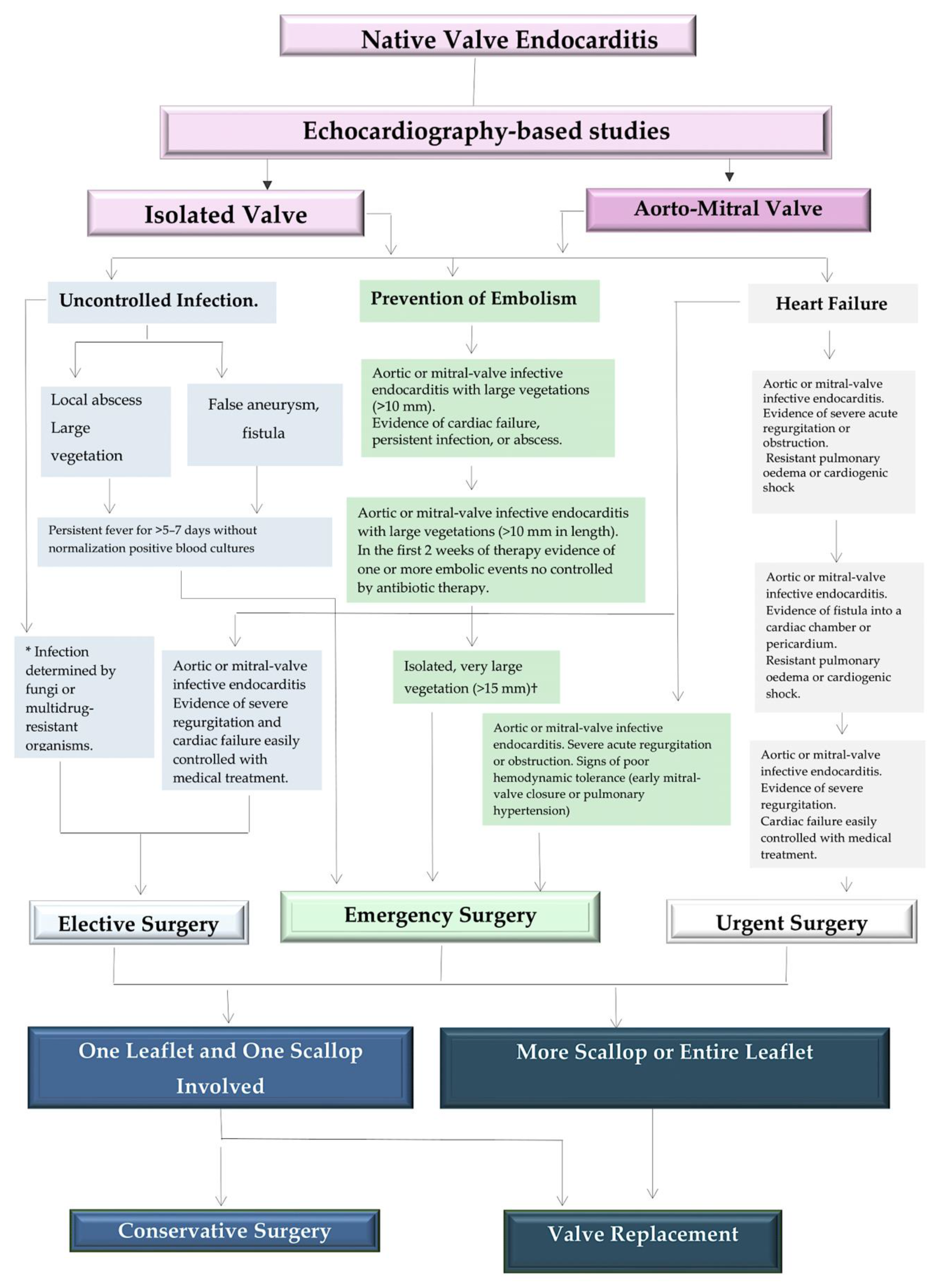
5. Clinical Use: Surgical Handling
6. A Cursory Examination of Areas of Incertitude
7. How Should We Interpret the Guidelines?
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nappi, F.; Spadaccio, C.; Mihos, C. Infective endocarditis in the 21st century. Ann Transl Med. 2020, 8, 1620. [Google Scholar] [CrossRef]
- Cahill, T.J.; Prendergast, B.D. Infective endocarditis. Lancet 2016, 387, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Bin Abdulhak, A.A.; Baddour, L.M.; Erwin, P.J.; Hoen, B.; Chu, V.H.; Mensah, G.A.; Tleyjeh, I.M. Global and regional burden of infective endocarditis, 1990 2010: a systematic review of the literature. Glob Heart 2014, 9, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Spadaccio, C.; Dreyfus, J.; Attias, D.; Acar, C.; Bando, K. Mitral endocarditis: A new management framework. J. Thorac. Cardiovasc. Surg. 2018, 156, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Avtaar Singh, S.S. Host-Bacterium Interaction Mechanisms in Staphylococcus aureus Endocarditis: A Systematic Review. Int J Mol Sci. 2023, 24, 11068. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miró, J.M.; Fowler, V.G., Jr.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; Chambers, S.T.; Chu, V.H.; Tattevin, P.; Olaison, L.; Freiberger, T.; Hurley, J.; Hannan, M.M.; Chu, V.; Hoen, B. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009, 169, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Martuscelli, G.; Bellomo, F.; Avtaar Singh, S.S.; Moon, M.R. Infective Endocarditis in High-Income Countries. Metabolites. 2022, 12, 682. [Google Scholar] [CrossRef] [PubMed]
- Ambrosioni, J.; Hernandez-Meneses, M.; Téllez, A.; Tattevin, P.; Olaison, L.; Freiberger, T.; Hurley, J.; Hannan, M.M.; Chu, V.; Hoen, B.; et al. The changing epidemiology of infective endocarditis in the twenty-first century. Curr Infect Dis Rep 2017, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F. Current Knowledge of Enterococcal Endocarditis: A Disease Lurking in Plain Sight of Health Providers. Pathogens. 2024, 13, 235. [Google Scholar] [CrossRef]
- Nappi, F.; Iervolino, A.; Singh, S.S.A. The New Challenge for Heart Endocarditis: From Conventional Prosthesis to New Devices and Platforms for the Treatment of Structural Heart Disease. Biomed Res Int. 2021, 2021, 7302165. [Google Scholar] [CrossRef]
- Pettersson, G.B.; Hussain, S.T.; Shrestha, N.K.; Gordon, S.; Fraser, T.G.; Ibrahim, K.S.; Blackstone, E.H. Infective endocarditis:an atlas of disease progression for describing, staging, coding, and understanding the pathology. J Thorac Cardiovasc Surg 2014, 147, 1142–1149.e2. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Witten, J.C.; Lowry, A.M.; Shrestha, N.K.; Blackstone, E.H.; Unai, S.; Pettersson, G.B.; Wierup, P.; Endocarditis Study Group. Isolated mitral valve endocarditis: Patient, disease, and surgical factors that influence outcomes. J Thorac Cardiovasc Surg. 2024, 167, 127–140.e15. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Martínez, A.; Domínguez, F.; Muñoz, P.; Marín, M.; Pedraz, Á.; Fariñas, M.C.; Tascón, V.; de Alarcón, A.; Rodríguez-García, R.; Miró, J.M.; Goikoetxea, J.; Ojeda-Burgos, G.; Escrihuela-Vidal, F.; Calderón-Parra, J.; GAMES investigators. Clinical presentation, microbiology, and prognostic factors of prosthetic valve endocarditis. Lessons learned from a large prospective registry. PLoS One. 2023, 18, e0290998. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Derumeaux, G.; Avierinos, J.F.; Casalta, J.P.; Jamal, F.; Volot, F.; Garcia, M.; Lefevre, J.; Biou, F.; Maximovitch-Rodaminoff, A.; Fournier, P.E.; et al. Value and limitations of the Duke criteria for the diagnosis of infective endocarditis. J Am Coll Cardiol 1999, 33, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G., Jr.; Ryan, T.; Bashore, T.; Corey, G.R. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000, 30, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, B.D. Diagnostic criteria and problems in infective endocarditis. Heart 2004, 90, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Madershahian, N.; Strauch, J.T.; Breuer, M.; Bruhin, R.; Straube, E.; Wahlers, T. Polymerase chain reaction amplification as a diagnostic tool in culture-negative multiple-valve endocarditis. Ann Thorac Surg. 2005, 79, e21–e22. [Google Scholar] [CrossRef] [PubMed]
- Mihos, C.G.; Nappi, F. A narrative review of echocardiography in infective endocarditis of the right heart. Ann. Transl. Med. 2020, 8, 1622. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; et al. Infective endocarditis in adults: diag- nosis, antimicrobial therapy, and manage- ment of complications: a scientific state- ment for healthcare professionals from the American HeartAssociation. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; et al. 2015 ESC Guidelines for the man- agement of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC) — endorsed by: Euro- pean Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015, 36, 3075–3128. [Google Scholar]
- Fournier, P.-E.; Gouriet, F.; Casalta, J.-P.; Lepidi, H.; Chaudet, H.; Thuny, F.; Collart, F.; Habib, G.; Raoult, D. Blood culture-negative endocarditis: improving the diagnostic yield using new diagnostic tools. Medicine (Baltimore) 2017, 96, e8392. [Google Scholar] [CrossRef] [PubMed]
- Subedi, S.; Jennings, Z.; Chen, S.C. Laboratory Approach to the Diagnosis of Culture-Negative Infective Endocarditis. Heart Lung Circ. 2017, 26, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Bannon, L.; Choshen, G.; Giladi, M.; Ablin, J. Bartonella endocarditis masquerading as systemic vasculitis with rapidly progressive glomerulonephritis (aka 'Löhlein nephritis'). BMJ Case Rep. 2019, 12, e231413. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.A.; Aziz, K.T.; Korbet, S. Bartonella henselae Infective Endocarditis: A Rare Cause of Pauci-Immune Necrotizing Glomerulonephritis-A Case Report. Can J Kidney Health Dis. 2023, 10, 20543581221150554. [Google Scholar] [CrossRef]
- Kitamura, M.; Dasgupta, A.; Henricks, J.; Parikh, S.V.; Nadasdy, T.; Clark, E.; Bazan, J.A.; Satoskar, A.A. Clinicopathological differences between Bartonella and other bacterial endocarditis-related glomerulonephritis - our experience and a pooled analysis. Front Nephrol. 2024, 3, 1322741. [Google Scholar] [CrossRef] [PubMed]
- Liesman, R.M.; Pritt, B.S.; Maleszewski, J.J.; Patel, R. Laboratory diagnosis of infective endocarditis. J Clin Microbiol 2017, 55, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Delgado, V.; Ajmone Marsan, N.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the management of endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [PubMed]
- Fowler, V.G.; Durack, D.T.; Selton-Suty, C.; Athan, E.; Bayer, A.S.; Chamis, A.L.; Dahl, A.; Di Bernardo, L.; Durante-Mangoni, E.; Duval, X.; et al. The 2023 Duke-International Society for Cardiovascular Infectious Diseases Criteria for Infective Endocarditis: Updating the Modified Duke Criteria. Clin. Infect. Dis. 2023, 77, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Afonso, L.; Kottam, A.; Reddy, V.; Penumetcha, A. Echocardiography in infective endocarditis: state of the art. Curr Cardiol Rep 2017, 19, 127. [Google Scholar] [CrossRef]
- Avtaar Singh, S.S.; Costantino, M.F.; D'Addeo, G.; Cardinale, D.; Fiorilli, R.; Nappi, F. A narrative review of diagnosis of infective endocarditis-imaging methods and comparison. Ann Transl Med. 2020, 8, 1621. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C.; Moon, M.R. A management framework for left sided endocarditis: a narrative review. Ann Transl Med. 2020, 8, 1627. [Google Scholar] [CrossRef]
- Habib, G.; Badano, L.; Tribouilloy, C.; Vilacosta, I.; Zamorano, J.L.; Galderisi, M.; Voigt, J.U.; Sicari, R.; Cosyns, B.; Fox, K.; Aakhus, S.; European Association of Echocardiography. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr 2010, 11, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Bai, A.D.; Steinberg, M.; Showler, A.; Burry, L.; Bhatia, R.S.; Tomlinson, G.A.; Bell, C.M.; Morris, A.M. Diagnostic accuracy of transthoracic echocardiography for infective endocarditis findings using transesophageal echocardiography as the reference standard: a meta-analysis. J Am Soc Echocardiogr 2017, 30, 639–646.e8. [Google Scholar] [CrossRef]
- Gomes, A.; Glaudemans, A.W.J.M.; Touw, D.J.; et al. Diagnostic value of imaging in infective endocarditis: a systematic review. Lancet Infect Dis 2017, 17, e1–e14. [Google Scholar] [CrossRef]
- Wong, D.; Rubinshtein, R.; Keynan, Y. Alternative cardiac imaging modalities to echocardiography for the diagnosis of infective endocarditis. Am J Cardiol 2016, 118, 1410–1418. [Google Scholar] [CrossRef]
- Scialla, S.; Martuscelli, G.; Nappi, F.; Singh, S.S.A.; Iervolino, A.; Larobina, D.; Ambrosio, L.; Raucci, M.G. Trends in Managing Cardiac and Orthopaedic Device-Associated Infections by Using Therapeutic Biomaterials. Polymers (Basel). 2021, 13, 1556. [Google Scholar] [CrossRef] [PubMed]
- Behmanesh, B.; Gessler, F.; Schnoes, K.; Dubinski, D.; Won, S.Y.; Konczalla, J.; Seifert, V.; Weise, L.; Setzer, M. Infective endocarditis in patients with pyogenic spondylodiscitis: Implications for diagnosis and therapy. Neurosurg. Focus 2019, 46, E2. [Google Scholar] [CrossRef]
- De Castro, S.; Cartoni, D.; d’Amati, G.; Beni, S.; Yao, J.; Fiorell, M.; Gallo, P.; Fedele, F.; Pandian, N.G. Diagnostic accuracy of transthoracic and multiplane transesophageal echocardiography for valvular perforation in acute infective endocarditis: Correlation with anatomic findings. Clin. Infect. Dis. 2000, 30, 825–826. [Google Scholar] [CrossRef] [PubMed]
- Khalique, O.K.; Veillet-Chowdhury, M.; Choi, A.D.; Feuchtner, G.; Lopez-Mattei, J. Cardiac computed tomography in the contemporary evaluation of infective endocarditis. J. Cardiovasc. Comput. Tomogr. 2021, 15, 304–312. [Google Scholar] [CrossRef]
- Nappi, F.; Singh, S.S.A.; Nappi, P.; Spadaccio, C.; Nenna, A.; Gentile, F.; Chello, M. Heart Valve Endocarditis. Surg Technol Int. 2020, 37, 203–215. [Google Scholar]
- Nappi, F.; Spadaccio, C.; Mihos, C.; Shaikhrezai, K.; Acar, C.; Moon, M.R. The quest for the optimal surgical management of tricuspid valve endocarditis in the current era: a narrative review. Ann Transl Med. 2020, 8, 1628. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Avtaar Singh, S.S.; Jitendra, V.; Fiore, A. Bridging Molecular and Clinical Sciences to Achieve the Best Treatment of Enterococcus faecalis Endocarditis. Microorganisms. 2023, 11, 2604. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Guittet, L.; Hamon, M.; Hamon, M. Comparative value of cardiac CT and transesophageal echocardiography in infective endocarditis: A systematic review and meta-analysis. Radiol. Cardiothorac. Imaging 2020, 2, e190189. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.K.M.; Sánchez-Nadales, A.; Igbinomwanhia, E.; Cremer, P.; Griffin, B.; Xu, B. Diagnosis of infective endocarditis by subtype using (18)F-fluorodeoxyglucose positron emission tomography/computed tomography: A contemporary meta- analysis. Circ. Cardiovasc. Imaging 2020, 13, e010600. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, U.; Spadaccio, C.; Gentile, F.; Moon, M.R.; Nappi, F. A narrative review of early surgery versus conventional treatment for infective endocarditis: Do we have an answer? Ann. Transl. Med. 2020, 8, 1626. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Singh, S.S.A.; Spadaccio, C.; Acar, C. Revisiting the guidelines and choice the ideal substitute for aortic valve endocarditis. Ann. Transl. Med. 2020, 8, 952. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, S.E.; Vigliani, G.A.; Fowler VGJr Abrutyn, E.; Corey, G.R.; Levine, D.P.; Rupp, M.E.; Chambers, H.F.; Karchmer, A.W.; Boucher, H.W. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endo- carditis is nephrotoxic. Clin Infect Dis 2009, 48, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, G.E.; Scarborough, M.; Szubert, A.; Nsutebu, E.; Tilley, R.; Greig, J.; Wyllie, S.A.; Wilson, P.; Auckland, C.; Cairns, J.; Ward, D.; Lal, P.; Guleri, A.; Jenkins, N.; Sutton, J.; Wiselka, M.; et al. Adjunctive rifampicin for Staphylococcus aureus bacteraemia(ARREST): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2018, 391, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, S.; Ohara, T.; Ashihara, K.; Izumi, C.; Iwanaga, S.; Eishi, K.; Okita, Y.; Daimon, M.; Kimura, T.; Toyoda, K.; Nakase, H.; Nakano, K.; Higashi, M.; Mitsutake, K.; et al. JCS 2017 guideline on prevention and treatment of infective endocarditis. Circ J 2019, 83, 1767–1809. [Google Scholar] [CrossRef]
- Rindone, J.P.; Mellen, C.K. Meta-analysis of trials comparing cefazolin to antistaphylococcal penicillins in the treatment of methicillin-sensitive Staphylococcus aureus bacteraemia. Br J Clin Pharmacol. 2018, 84, 1258–1266. [Google Scholar] [CrossRef]
- Weis, S.; Kesselmeier, M.; Davis, J.S.; Morris, A.M.; Lee, S.; Scherag, A.; Hagel, S.; Pletz, M.W. Cefazolin versus anti-staphylococcal penicillins for the treatment ofpatients with Staphylococcus aureus bacteraemia. Clin Microbiol Infect 2019, 25, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Nannini, E.C.; Stryjewski, M.E.; Singh, K.V.; Bourgogne, A.; Rude, T.H.; Corey, G.R.; Fowler, V.G., Jr.; Murray, B.E. Inoculum effect with cefazolin among clinical isolates of methicillin-susceptible Staphylococcus aureus: frequency and possible cause of cefazolin treatment failure. Antimicrob Agents Chemother 2009, 53, 3437–3441. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.R.; Seas, C.; Carvajal, L.P.; Diaz, L.; Echeverri, A.M.; Ferro, C.; Rios, R.; Porras, P.; Luna, C.; Gotuzzo, E.; Munita, J.M.; Nannini, E.; Carcamo, C.; Reyes, J.; Arias, C.A. The cefazolin inoculum effect is associ- ated with increased mortality inmethicillin-susceptible Staphylococcus aureus bacteremia. Open Forum Infect Dis 2018, 5, ofy123. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.G., Jr.; Boucher, H.W.; Corey, G.R.; Abrutyn, E.; Karchmer, A.W.; Rupp, M.E.; Levine, D.P.; Chambers, H.F.; Tally, F.P.; Vigliani, G.A.; Cabell, C.H.; Link, A.S.; DeMeyer, I.; et al. Daptomycin versus standard therapy for bacteremia andendocarditis caused by Staphylococcus aureus. N Engl J Med 2006, 355, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Holland, T.L.; Arnold, C.; Fowler, V.G., Jr. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA 2014, 312, 1330–1341. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Lye, D.C.; Yahav, D.; Sud, A.; Robinson, J.O.; Nelson, J.; Archuleta, S.; Roberts, M.A.; Cass, A.; Paterson, D.L.; Foo, H.; Paul, M.; Guy, S.D.; Tramontana, A.R.; Walls, G.B.; McBride, S.; Bak, N.; Ghosh, N.; Rogers, B.A.; Ralph, A.P.; Davies, J.; Ferguson, P.E.; et al. Effect of vancomycin or daptomycin with vs without an antistaphylococcal β-lactam on mortality, bacteremia, relapse, or treatment failure in patients with MRSA bacteremia: a randomized clinical trial. JAMA 2020, 323, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Geriak, M.; Haddad, F.; Rizvi, K.; Rose, W.; Kullar, R.; LaPlante, K.; Yu, M.; Vasina, L.; Ouellette, K.; Zervos, M.; Nizet, V.; Sakoulas, G. Clinical data on daptomycin plus ceftaro- line versus standard of care monotherapy in the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2019, 63, e02483-18. [Google Scholar] [CrossRef] [PubMed]
- Gritsenko, D.; Fedorenko, M.; Ruhe, J.J.; Altshuler, J. Combination therapy with vancomycin and ceftaroline forrefractory methicillin-resistant Staphylococcus aureus bacteremia: a case series. Clin Ther 2017, 39, 212–218. [Google Scholar] [CrossRef]
- Holubar, M.; Meng, L.; Deresinski, S. Bacteremia due to methicillin-resistant Staphylococcus aureus: new therapeutic approaches. Infect Dis Clin North Am 2016, 30, 491–507. [Google Scholar] [CrossRef]
- Fernández-Hidalgo, N.; Almirante, B.; Gavaldà, J.; Gurgui, M.; Peña, C.; de Alarcón, A.; Ruiz, J.; Vilacosta, I.; Montejo, M.; Vallejo, N.; López-Medrano, F.; Plata, A.; López, J.; et al. Ampicillin plus ceftriaxone is as effective as ampicillin plus gentamicin for treating enterococcusfaecalis infective endocarditis. Clin Infect Dis 2013, 56, 1261–1268. [Google Scholar] [CrossRef]
- Dahl, A.; Rasmussen, R.V.; Bundgaard, H.; Hassager, C.; Bruun, L.E.; Lauridsen, T.K.; Moser, C.; Sogaard, P.; Arpi, M.; Bruun, N.E. Enterococcus faecalis infective endocarditis: a pilot study of the relationship between duration of gentamicin treatment and outcome. Circulation 2013, 127, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Olaison, L.; Schadewitz, K. Enterococcal endocarditis in Sweden, 1995-1999: can shorter therapy with aminoglycosides be used? Clin Infect Dis 2002, 34, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F. Current Knowledge of Enterococcal Endocarditis: A Disease Lurking in Plain Sight of Health Providers. Pathogens. 2024, 13, 235. [Google Scholar] [CrossRef] [PubMed]
- Bartash, R.; Nori, P. Beta-lactam combination therapy for the treatment of Staphylococcus aureus and Enterococcus species bacteremia: A summary and appraisal of the evidence. Int. J. Infect. Dis. 2017, 63, 7–12. [Google Scholar] [PubMed]
- Li, G.; Walker, M.J.; De Oliveira, D.M.P. Vancomycin Resistance in Enterococcus and Staphylococcus aureus. Microorganisms 2022, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Rangama, S.; Lidbury, I.D.E.A.; Holden, J.M.; Borsetto, C.; Murphy, A.R.J.; Hawkey, P.M.; Wellington, E.M.H. Mechanisms Involved in the Active Secretion of CTX-M-15 β-Lactamase by Pathogenic Escherichia coli ST131. Antimicrob. Agents Chemother. 2021, 65, e0066321. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, A.M.; Hussein, S.A.; Husain, V.I. Detection of Antibiotic Resistance Genes (CTX-M, Van A and Van B) of Enterococcus faecalis Isolated from Children with Bacteremia by RT-PCR. Arch. Razi Inst. 2023, 78, 73–77. [Google Scholar] [CrossRef]
- Almahdawy, O.T.; Pricop, R.; Sadik, O.; Najee, H.; Pircalabioru, G.G.; Marutescu, L.; Czobor Barbu, I.; Banu, O.; Cristea, V.; Grigore, R.; et al. Description of vancomycin resistance genes in Enterococcus sp. clinical strains isolated from Bucharest, Romania. Rom. Biotechnol. Lett. 2019, 24, 395–399. [Google Scholar] [CrossRef]
- Guzman Prieto, A.M.; van Schaik, W.; Rogers, M.R.; Coque, T.M.; Baquero, F.; Corander, J.; Willems, R.J. Global emergence and dissemination of enterococci as nosocomial pathogens: Attack of the clones? Front. Microbiol. 2016, 7, 788. [Google Scholar] [CrossRef]
- Hammerum, A.M.; Justesen, U.S.; Pinholt, M.; Roer, L.; Kaya, H.; Worning, P.; Nygaard, S.; Kemp, M.; Clausen, M.E.; Nielsen, K.L.; et al. Surveillance of vancomycin-resistant enterococci reveals shift in dominating clones and national spread of a vancomycin-variable vanA Enterococcus faecium ST1421-CT1134 clone, Denmark, 2015 to March 2019. Eurosurveillance 2019, 24, 1900503. [Google Scholar] [CrossRef]
- Rivas, J.M.; Speziale, P.; Patti, J.M.; Hook, M. MSCRAMM-targeted vaccines and immunotherapy for staphylococcal infection. Curr. Opin. Drug Discov. Dev. 2004, 7, 223–227. [Google Scholar]
- Marston, H.D.; Dixon, D.M.; Knisely, J.M.; Palmore, T.N.; Fauci, A.S. Antimicrobial resistance. JAMA 2016, 316, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Avtaar Singh, S.S.; Timofeeva, I. Learning From Controversy: Contemporary Surgical Management of Aortic Valve Endocarditis. Clin Med Insights Cardiol. 2020, 14, 1179546820960729. [Google Scholar] [CrossRef] [PubMed]
- Pollari, F.; Spadaccio, C.; Cuomo, M.; Chello, M.; Nenna, A.; Fischlein, T.; Nappi, F. Sharing of decision-making for infective endocarditis surgery: a narrative review of clinical and ethical implications. Ann Transl Med. 2020, 8, 1624. [Google Scholar] [CrossRef] [PubMed]
- Satriano, U.M.; Nenna, A.; Spadaccio, C.; Pollari, F.; Fischlein, T.; Chello, M.; Nappi, F. Guidelines on prosthetic heart valve management in infective endocarditis: a narrative review comparing American Heart Association/American College of Cardiology and European Society of Cardiology guidelines. Ann Transl Med. 2020, 8, 1625. [Google Scholar] [CrossRef]
- Habib, G.; Erba, P.A.; Iung, B.; et al. Clini- cal presentation, aetiology and outcome of infective endocarditis: results of the ESC-EORP EURO-ENDO (European infec- tive endocarditis) registry: a prospective cohort study. Eur Heart J 2019, 40, 3222–3232. [Google Scholar] [CrossRef]
- AATS Surgical Treatment of Infective Endocarditis Consensus Guidelines Writing Committee. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg 2017, 153, 1241–1258. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-H.; Kim, Y.-J.; Kim, S.-H.; et al. Early surgery versus conventional treatment for infective endocarditis. N EnglJ Med 2012, 366, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Anantha Narayanan, M.; Mahfood Haddad, T.; Kalil, A.C.; et al. Early versus late surgical intervention or medical manage- ment for infective endocarditis: a system- atic review and meta-analysis. Heart 2016, 102, 950–957. [Google Scholar] [CrossRef]
- Liang, F.; Song, B.; Liu, R.; Yang, L.; Tang, H.; Li, Y. Optimal timing for early surgery in infective endocarditis: a meta-analysis. Interact Cardiovasc Thorac Surg 2016, 22, 336–345. [Google Scholar] [CrossRef]
- Richards, M.J.; Edwards, J.R.; Culver, D.H.; Gaynes, R.P. Nosocomial infections in combined medical surgical intensive care units in the United States. Infect. Control Hosp. Epidemiol. 2000, 21, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.; Roques, F.; Michel, P.; et al. European system for cardiac operative risk evaluation (EuroSCORE). European journal of cardio-thoracic surgery. 1999, 16, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Gaca, J.G.; Sheng, S.; Daneshmand, M.A.; et al. Outcomes for endocarditis surgery in North America : a simplified risk scoring system. J Thorac Cardiovasc Surg 2011, 141, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cisneros, A.; Hernández-Meneses, M.; Llopis, J.; Sandoval, E.; Pereda, D.; Alcocer, J.; Barriuso, C.; Castellá, M.; Ambrosioni, J.; Pericàs, J.M.; Vidal, B.; Falces, C.; Ibáñez, C.; Perdomo, J.; Rovira, I.; García-de-la-María, C.; Moreno, A.; Almela, M.; Perisinotti, A.; Dahl, A.; Castro, P.; Miró, J.M.; Quintana, E.; Equip d’Endocarditis de l’Hospital Clínic de Barcelona. Risk scores' performance and their impact on operative decision-making in left-sided endocarditis: a cohort study. Eur J Clin Microbiol Infect Dis. 2023, 42, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Ejiofor, J.I.; Yammine, M.; Ando, M.; Camuso, J.M.; Youngster, I.; Nelson, S.B.; Kim, A.Y.; Melnitchouk, S.I.; Rawn, J.D.; MacGillivray, T.E.; Cohn, L.H.; Byrne, J.G.; Sundt, T.M. , 3rd. Surgical outcomes of infective endocarditis among intravenous drug users. J Thorac Cardiovasc Surg. 2016, 152, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Ejiofor, J.I.; Yammine, M.; Camuso, J.M.; Walsh, C.W.; Ando, M.; et al. Are homografts superior to conventional prosthetic valves in the setting of infective en- docarditis involving the aortic valve? J Thorac Cardiovasc Surg. 2016, 151, 1239–1246. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C. Simplest solutions are not always the cleverest: Can we stitch in an infected annulus? Should we rethink the current guidelines? J. Thorac. Cardiovasc. Surg. 2017, 154, 1899–1900. [Google Scholar] [CrossRef]
- Nappi, F.; Spadaccio, C. keep fumbling around in the dark when it comes to infective endocarditis, or produce new, reliable data to redesign the guidelines? J. Thorac. Cardiovasc. Surg. 2018, 155, 75–76. [Google Scholar] [CrossRef]
- Nappi, F.; Acar, C. Monobloc or Separate Aortic and Mitral Homografts for Endocarditis of the Intervalvular Fibrosa? Ann. Thorac. Surg. 2021, 112, 1382–1383. [Google Scholar] [CrossRef]
- Nappi, F.; Nenna, A.; Petitti, T.; Spadaccio, C.; Gambardella, I.; Lusini, M.; Chello, M.; Acar, C. Long-term outcome of cryopreserved allograft for aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2018, 156, 1357–1365.e6. [Google Scholar] [CrossRef]
- Olivito, S.; Lalande, S.; Nappi, F.; Hammoudi, N.; D'Alessandro, C.; Fouret, P.; Acar, C. Structural deterioration of the cryopreserved mitral homograft valve. J. Thorac. Cardiovasc. Surg. 2012, 144, 313–320.e1. [Google Scholar] [CrossRef] [PubMed]
- Nappi, F.; Spadaccio, C.; Acar, C. Use of allogeneic tissue to treat infective valvular disease: Has everything been said? J. Thorac. Cardiovasc. Surg. 2017, 153, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Beganovic, M.; Luther, M.K.; Rice, L.B.; Arias, C.A.; Rybak, M.J.; LaPlante, K.L. A review of combination antimicrobial therapy for Enterococcus faecalis bloodstream infections and infective endocarditis. Clin Infect Dis 2018, 67, 30. [Google Scholar] [CrossRef] [PubMed]
- Iversen, K.; Ihlemann, N.; Gill, S.U.; et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N EnglJ Med 2019, 380, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, H.; Ihlemann, N.; Gill, S.U.; et al. Long-term outcomes of partial oral treatment of endocarditis. N Engl JMed 2019, 380, 1373–1374. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Chambers, H.F.; Musher, D.M.; Walsh, T.L.; Bayer, A.S. Evaluation of a paradigm shift from intravenous antibiotics to oral step-down therapy for the treatment of infective endocarditis: a narrative review. JAMA Intern Med 2020. [Google Scholar] [CrossRef] [PubMed]
- Venn, R.A.; Ning, M.; Vlahakes, G.J.; Wasfy, J.H. Surgical timing in infective endocarditis complicated by intracranial hemorrhage. Am Heart J 2019, 216, 102–112. [Google Scholar] [CrossRef]
- De Feo, M.; Cotrufo, M.; Carozza, A.; et al. The need for a specific risk prediction system in native valve infec- tive endocarditis surgery. Scientific World Journal. 2012, 2012, 307571. [Google Scholar] [CrossRef] [PubMed]
- Chu, V.H.; Park, L.P.; Athan, E.; et al. Association between surgical indications, operative risk, and clinical outcome in infective endocarditis: a prospective study from the International Collaboration on Endocarditis. Circulation 2015, 131, 131–140. [Google Scholar] [CrossRef]
- Okita, Y.; Minakata, K.; Yasuno, S.; et al. Optimal timing of surgery for active infective endocarditis with cerebral complications: a Japanese multicentre study. Eur J Cardiothorac Surg 2016, 50, 374–382. [Google Scholar] [CrossRef]
- Escolà-Vergé, L.; Peghin, M.; Givone, F.; et al. Prevalence of colorectal disease in Enterococcus faecalis: results of an observa- tional multicenter study. Rev Esp Cardiol (Engl Ed) 2019. [Google Scholar]
- Pericàs, J.M.; Corredoira, J.; Moreno, A.; et al. Relationship between Enterococcus faecalis infective endocarditis and colorec- tal neoplasm: preliminary results from a cohort of 154 patients. Rev Esp Cardiol (Engl Ed) 2017, 70, 451–458. [Google Scholar] [PubMed]
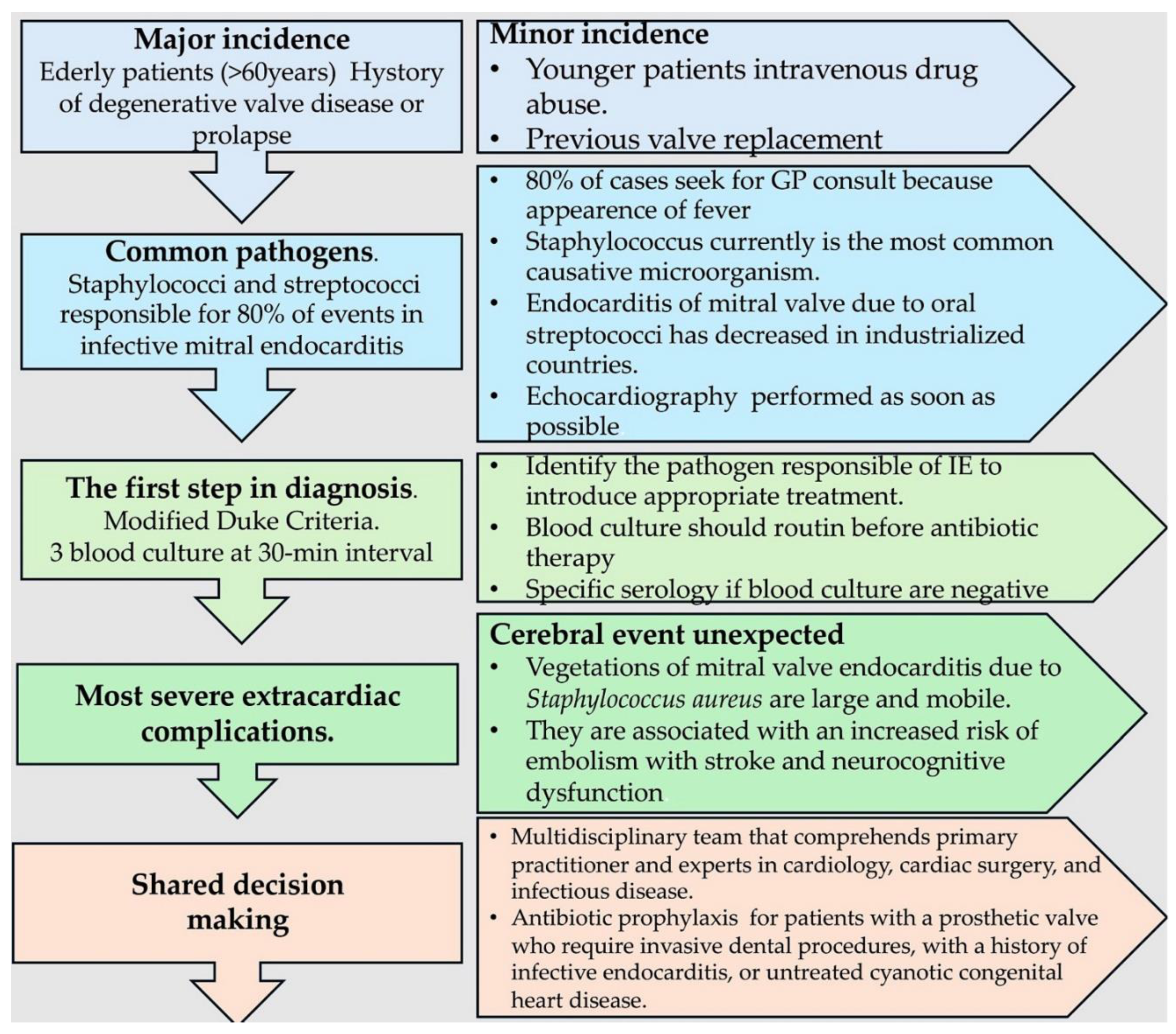
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





