1. Introduction
The type 2 coronavirus that causes severe acute respiratory syndrome (SARS-CoV-2) is an obligatory intracellular pathogen that kidnaps the cell machinery that it needed for its replicative cycle. Lipidic interactions are essential during the entrance of this virus to the host cell, and afterwards for the budding process of new virions [
1]. The first step of the viral infection is the union of the spike protein (S) of the virus with (subunit S1) the angiotensin converting enzyme 2 (ACE2) which contributes to a conformational change in the S2 subunit of the S protein resulting in the binding of the virus with the trans membrane protein serine protease 2 (TMPRSS2). Both the TMPRSS2 and ACE2 are present in the lipid rafts that are microdomains in the cell membrane of the host [
2]. SARS-CoV-2 may use two mechanisms for its internalization: 1) fusion and 2) endocytosis. When fusion occurs the viral glycoprotein S interact with surface cell receptors ACE2 as already mentioned, and then there is fusion of the membranes of the virus and the host. Endocytosis happens after fusion, and it is accompanied by invagination and transport. In this step, a diminished pH is required which favors the union between the endosomal membrane and the viral envelope and the subsequent liberation of the nucleocapsid to the cytoplasm [
2]. However, the degree of virulence also depends on the pH of the extracellular environment, the membrane rigidity, the density of the receptors and the amount of the viral spike proteins [
3].
Lipids including phospholipids (PLs), fatty acids (FA), and cholesterol (CT) are essential nutrients that play important roles in anatomical and physiological functions such as cell signaling, death, and survival pathways in all cells. Furthermore, they are components of the bilipid plasma membrane and of the membranes of the organelles [
4]. Bilipid membranes are conformed by CT and PLs molecules which constitute 50 %of the total mass of the membrane and the rest is constituted by structural proteins [
4]. In turn, PLs are made up of two FAs and a phosphate molecule. The SARS-CoV-2 can reprogram the metabolism of the FA in the host cell, to obtain them for the synthesis of the PLs necessary for the formation of the viral membrane of new virions. In this sense, the isomers of the transcription factor sterol regulatory element binding protein (SREBP) are involved [
5]. The viral infection also stimulates the production the bioactive FA mediators that participate in the host immune response such as prostaglandins (PGs), thromboxanes (TXs) and leukotrienes (LK) which are metabolites of the arachidonic acid (AA) pathway which are formed by ciclooxigenases (COXs), thromboxane synthase, lipo-oxygenase (LOX) and cytochrome P450 (CYP). These mediators participate as pro- and anti-inflammatory molecules in the infection process [
1]. Other FA that may also play a pivotal role in the replication of the SARS-CoV-2 are; palmitic (PA), oleic (OA), linoleic (LA), stearic and dihomo-γ-linoleic (D-γ-LA) acids. These FA are very important for the formation and function of the viral membrane [
1,
4]. In this sense, the SARS-CoV-2 may contribute to increase of the activity of the FA synthase (FAS) in the host cell. This enzyme is key in the synthesis of PA which is then used as raw materials for the synthesis of other FA which become part of the lipid bilayer of membranes of the virus and facilitate the virus propagation [
6].
In addition to FA, other lipids such as CT, which are abundant in the lipid rafts of the membrane where the ACE2 receptor is anchored, are important in viral pathways such as its biosynthesis and assembly [
7]. Therefore, an intricate disturbance in lipid metabolism might happen in the process of infection and replication of the SARS-CoV-2 for the benefit of the virus. Nevertheless, this leads to a decompensation of the pathways for lipid synthesis in the host that contributes to the clinical picture present in the patient, with a probable fatal outcome. The breakage of this intricate balance during the infection process is essential to reduce the clinical-pathological picture present in the patient. In this context, the regulation of the lipid metabolism can be used as an adjuvant therapeutic target in patients infected by SARS-CoV-2 and as an addition to other strategies including the battery of vaccines against this virus. In this sense, it has been demonstrated that supplementation with omega-3 FA (eicosapentaenoic acid EPA and docosahexanoic DHA acid) or PLs enriched with EPA and DHA could reduce viral entrance to the host cells, ameliorate immune function, and diminishe the severity of some COVID-19 complications [
8].
On the other hand, tocopherol also known as vitamin E (Vit E) is an essential micronutrient and fat-soluble antioxidant in mammals that prevents the oxidation of FA of the cell membranes by radical oxygen species (ROS), thus contributing to an increased fluidity of membranes and to a decrease in lipid peroxidation (LPO) associated to the Haber-Weiss reaction [
9]. The rate of tocopherol decay is α > β > γ > δ, in association with the biological potencies of these forms of Vit E [
9]. Even though deficiencies in Vit E are rare in humans, they may occur by intestinal malabsorptive disorders. Moreover, Vit E acts synergistically with Vit C, through the reduction of tocopheroxyl radicals [
10].
Vit E deficiency compromises both cell-mediated and humoral immune function in animal trials and the supplementation with Vit E strengthens immunity and increases resistance against several pathogens [
11]. Vit E supplementation (60 mg/7day) decreased LPO index in blood plasma, and in the lung and liver in male mice with influenza [
12]. However, only a limited number of human trials have shown the effects of Vit E on resistance against infectious diseases such as in sepsis compared to those performed in animals [
13]. The focus of these human trials was aimed at the elderly, who frequently contract respiratory diseases. The treatment with Vit E as a mono-therapy has been poorly studied in COVID-19 despite the proposed beneficial effect of this treatment in various review manuscript in the literature [
14,
15]. Moreover, the adjuvant therapy with Vit E supplementation could decrease the production of ROS and restore the balance in the alteration of the metabolism of PLs in the patients with COVID-19 thus contributing to decrease the clinical manifestation in the progression of the infection [
16]. Therefore, the aim of this study was to demonstrate if the treatment with Vit E is capable to restore the modified of the FA in PLs profile in serum from patients with COVID-19.
2. Materials and Methods
2.1. Population of the Study
This was a prospective, analytical, open and longitudinal (before-after) study run in 22 patients with COVID-19 who received Vit E treatment. The group before treatment with Vit E is compared with 23 HS which constitute the control group. Inclusion criteria: COVID-19 patients were 18 years old or more, subjects admitted to the intensive care unit (ICU) of the CITIBANAMEX Center and that developed or not septic shock, secondary to moderate or severe pneumonia by SARS-CoV-2 infection. The treatment with Vit E was applied during the 2020 pandemic between the months of August and September [
17]. The patients were not vaccinated against SARS-CoV-2 because a vaccine had not yet been approved at the time this study was carried out. The Sepsis-3 consent was used for diagnostic criteria of septic shock [
18]. An informed consent written for recruitment and the use of patient data was obtained from each patient or their legal representative, in accordance with the Helsinki declaration. COVID-19 patients were considered to have septic shock when there was an acute increase of at least 2 points in the Sequential Organ Failure Assessment (SOFA) score [
18] which is the scale for assessing the condition of the patients during their stay in the ICU and that includes the stage of neurological, respiratory, hemodynamic, hepatic, and hematologic conditions, with lactate levels ≥ 2 mmol/L, and considering if the patients were dependent on a vasopressor for at least 2 hours before recruitment. The SOFA score was evaluated at admission and during the days of the treatment to determine organ dysfunction [
19]. Hospitalized patients with COVID-19 were classified as severe or moderate according to their ventilatory status. COVID-19 patients with the severe condition required invasive mechanical intubation according to the Berlin criteria for acute respiratory distress syndrome (ARDS) [
18]. Exclusion criteria were: patients that were under chronic use (last 6 months) or recent use of Vit E, antioxidants such as Vit C, statins and steroids; patients that were not able to grant an informed consent, or who refused to be included, pregnant women or women that were breast feeding. Patients were given individualized management according to an algorithm suggested by Soto et al. and they were not given hydroxychloroquine or antivirals [
20]. Some results related to this study were previously reported by Chavarría et al. during the before and after treatment with Vit E treatment evaluation [
17].
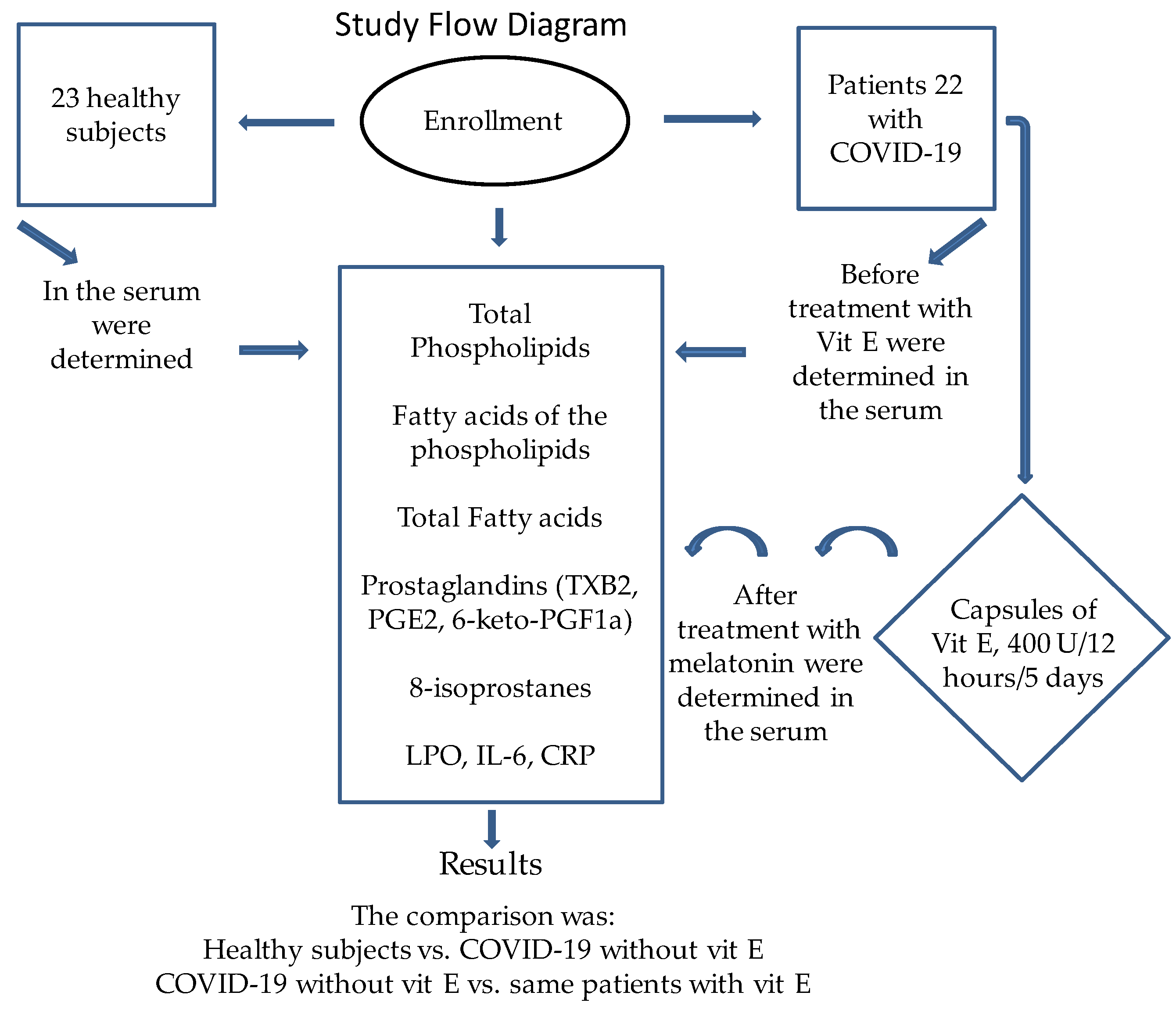
Figure 5 shows the flow chart of the recruitment of the patients and of the treatment with Vit E in the patients with COVID-19.
2.2. Detection of SARS-CoV-2 by Real-Time Reverse Transcriptase Polymerase Chain Reaction
Swab samples were collected from COVID-19 patients to apply the Paired Technique (nasopharyngeal and saliva). Samples were considered positive for SARS-CoV-2 when both the N1 and N2 protein primer-presets were detected. Specific probes to detect the virus and the real-time reverse transcriptase polymerase chain reaction technique (qRT-PCR) were employed to determine the presence of the SARS-CoV-2 virus.
2.3. Healthy Subjects
15 men and 8 women matched by age and gender and negative for the SARS-CoV-2 infection were included in the group of HS which served as controls. Autoimmune diseases, thyroid disease, obesity, overweight, dyslipidemia, arterial hypertension, diabetes mellitus, inflammatory, and degenerative diseases were not present in the HS. The intake of antioxidant and non-steroidal anti-inflammatory drugs in HS that could interfere with the results of the study were suspended 48 hours before the sample was obtained. Biochemical variables such as glucose, calcium (Ca2+), creatinine, uric acid, CT, TG, HDL, LDL, and C reactive protein (CRP) were determined.
2.4. Therapeutic Management
During hospitalization the treatment for COVID-19 was chosen according to standard maneuvers and the requirements of each individual patient; the electrolyte demands, hemodynamic, and the ventilator demands were considered. Treatment was begun before the recognition of the presence or absence of septic shock and during the first hour after admission. Depending on the hemodynamic status, management with crystalloid solutions and/or albumin was taken into account, by means of dynamic indicators. If necessary, vasopressors were given to keep a mean arterial pressure (MAP) ≥65 mmHg. Inotropic drugs (dobutamine) were administered when myocardial dysfunction was present. Norepinephrine (NE) was the first option and/or vasopressin was used when there was a need to increase the MAP or reduce the NE dose. When there was a decrease in hemoglobin (<7.0 g/dL) in the absence of severe hypoxemia, myocardial ischemia, or severe bleeding, transfusion of blood packs were used. Mechanical ventilation with initial volumes of 6 mL/kg was used in ARDS patients [
21]. Plateau pressure was maintained at ≤30 cm H
2O, and alveolar conduction pressure of ≤13 cm H
2O. Positive end expiration pressure titration was managed by the use of the fraction of inspired oxygen/positive end expiration pressure (FiO
2/PEEP). The treatment with anticoagulants was based on the Thatched guidelines [
60]. The management with the prone position was necessary in patients with PaO
2/FiO
2 of ≤150 mmHg [
21].The standard therapeutic management with dexamethasone 8 mg i.v., every 24 hours for 7 days in all patients, between 1 and 21 days of the onset of symptoms when not counter indicated. Pentoxifylline tablets of 400 mg were applied every 12 hours by oral route or nasal-enteral tube for 5 days [
17]. It was counter indicated when there was a requirement of O
2 >3L, progressive requirement of =2, PaO
2/FiO
2 ≤250 mmHg, O
2 use plus bilateral infiltrates in the radiography, O
2 use plus DHL ≤250 U/L or ferritin ≥300 or DD ≥1000 ng/mL, CPK ≥2 times the upper normal value. The following conditions were not considered as counter indications or relative counter indications: glucose >250 mg/dL with hypoglycemia, hypokalemia <3.3 meq, blood pressure >155/95 mmHg with antihypertensive treatment, glaucoma, triglycerides >500 mg/dL (start treatment), history of known peptic ulcer or bleeding from recent gastro intestinal tract, untreated or decompensated dementia or psychiatric illness, use of non-potassium sparing diuretics or use of inhaled B
2 agonists. The next conditions were monitored at follow-up: pre-prandial capillary glucose (7–13–1 hours.) for 10 days, even in fasting patients, MAP per shift and basal potassium every 72 hours. The presences of comorbidities or of potential allergies or heart rhythm disorders due to each individual history were considered before the treatment with Vit E [
20]. All data entry was monitored at the coordinating center for patient management, with site visits for source data verification. The dose of the Vit E (α-tocopheryl acetate) capsules of 400 IU equivalent to 800 mg were administered every 12 h for 5 days.
2.5. Peripheral Blood Samples
10 mL of peripheral blood per patient were collected by venopuncture. The blood was centrifuged for 20 min at 936 g and 4 °C. The red blood cell pellet was discarded; the serum was recovered and collected in polypropylene tubes of 400 μL and stored at−30 °C until use.Interleukin-6 (IL-6), CRP, TG, HDL, LDL, glucose, the urea nitrogen, creatinine, CT, Ca2+, hemoglobin, leukocytes, lymphocytes, platelets, albumin, D-dimer, fibrinogen, and ferritin were determined in the serum from the COVID-19 patients. Data from the patient’s medical history including demographic data, prior illnesses to SARS-CoV-2 infection, COVID-19 test result, whether mechanical ventilation was used, and type of treatment given were used for the analysis of the results.
2.6. Ethical Considerations
Ethical approval was obtained on 19th August 2020 (Control-9867/2020, register REG. CONBIOETICA-09-CEI-01120160627). The protocol was registered (TRIAL REGISTRATION: ClinicalTrials.gov Identifier: NCT04570254).The Research and Ethics Committee of our institution approved the research protocol (Institutional protocol number: 22-1289). The study was carried out according to the international ethical standards and the General Health Law, as well as according to the Helsinki declaration, modified at the Congress of Tokyo, Japan. An informed consent written for recruitment and the use of patient data was obtained from each patient or control subjects or their legal representative.
2.7. Determination of the Marker of Lipid Peroxidation
The LPO index was measured indirectly through malondialdehyde MDA and was read spectrophotometrically at 532 nm. 100 μL of serum were used for this determination. Methanol with BHT at 4% (100 μL) and KH2PO4 buffer pH 7.4 (1 mL) was added to the sample, and incubated at 37 °C for 30 min. Then, 2-thiobarbituric acid at 0.8 M (1.5 mL) was added, and incubated at 90 °C for 1 h. Afterwards, KCl at 5% (1 mL) plus n-butanol (4 mL) were added and the sample was shaken for 30 sec, and centrifuged at 4000 rpm for 2 min. The organic phase was extracted, and the absorbance was measured.
2.8. Determinations of 8-Isoprostane, TXB2, PGE2, 6-keto-PGF1α and IL-6
50 μL of serum were used for these determinations. The quantification of 8-isoprostane (#516351 ELISA Kit), TXB2 (#501020 ELISA Kit), PGE2 (#514531 ELISA Kit), 6-keto-PGF1α (#515211 ELISA Kit) were provided by Cayman chemical (1180 E. Ellsworth Rd. Ann Arbor. MI, USA) and IL-6 (#D6050B) was provide by R&D systems a biotechne, and were measured at a wavelength range of 450-492 nm, using a visible light micro plate reader (Stat Fax 3200 Awareness Technology Palm City, FL, USA), according at the manufacturer’s specifications.
2.9. Vitamin E Quantification in Serum
For the extraction and derivatization of Vit E, to 100 µl of serum diethyl ether, saline solution 0.09% (1mL respectively) and hexane (3 mL), were added and the samples were shaken with a vortex for 30 sec, and centrifuged at 3000 rpm for 4 min. The organic phase was recovered and evaporated under a stream of nitrogen. The residue was suspended with pyridine (200 mL) hexamethyldesilazane (100 mL), shaken in a vortex for 30 sec. Then, chlorotrimethylsilane (50 mL) was added and derivatized at 60 °C for 20 min, and then hexane (3 mL) was added. The organic phase was recovered, filtered and evaporated with a gentle stream of nitrogen [
22]. The calibration curve was carried out with 400 U α- tocopherol as standard a 12.5, 25, 50,100 and 200 μL correspondent to 0.04, 0.08, 0.16, and 0.32 activity units. Vit E was identified by gas chromatography-FID in a Carlo Erba Fratovap 2300 chromatograph equipped with a capillary column packed with the stationary phase: HP-FFAP (Description: 30 m length × 0.320 mm diameter x 0.25 µm film) and fitted with a flame ionization detector at 240°C, with helium as the carrier gas at a flow rate of 1.2 ml/min. The areas under of the peak were calculated using the Cromatograf software version 1.1 coupled to gas chromatograph.
2.10. Total Fatty Acid (TFA) Determination
For the extraction and derivatization of the TFA, 100 µl of serum were used according to the method described by Folch et al. [
23], in the presence of 50 µg of margaric acid (C17:0) as an internal standard. Then, 1 mL of saline solution (0.09%) was added and mixed for 15 sec, and then 2 mL of methanol chloroform mixture (2:1 vol/vol) plus 0.002% BHT was added and centrifuged at 3000 rpm by 5 min. This step was repeated twice and the organic phase was recovered and evaporated under a gentle current of nitrogen (N
2). TFA were trans-esterified to their FA methyl esters by heating them at 90°C for 2 hours with 2 ml of methanol plus 0.002% BHT, 40 µl of H
2SO
4 and 100 µl toluene. Afterwards 1 mL of saline solution and 4 mL the hexane were added, and the mixture was centrifuged at 3000 rpm by 5 min. The hexane phase was recovered and evaporated under a gentle current of N
2. The evaporated residue containing FA was suspended in 100 µL of hexane and 4 µL was injected into the chromatograph. The TFA methyl esters were separated and identified by gas chromatography-FID in a Carlo Erba Fratovap 2300 chromatograph equipped with a capillary column packed with the stationary phase: HP-FFAP (Description: 30 m length × 0.320 mm diameter x 0.25 µm film) and fitted with a flame ionization detector at 210°C, with helium as the carrier gas at a flow rate of 1.2 ml/min. The areas under of the peaks were calculated using the Cromatograf software version 1.1 coupled to the gas chromatograph. The identification of each FA methyl ester was made comparing their retention time with their corresponding standard [
1].
2.11. Fatty Acid Analysis in a Acetone Insoluble Phospholipid Fraction (FAAIPF) Determination in Serum
For fatty acid analysis in a acetone insoluble phospholipid fraction (FAAIPF) extraction, 200 μL of serum were used in the presence of 50 μg of L-γ-phosphatidylcholine-di-heptadecanoyl acid as internal standard plus acetone (1 mL) and 1 mL of saline solution (0.09%). The mixture was shaken 30 sec and centrifuged at 1145g, at room temperature for 4 min. The supernatant was removed, and the button was suspended with 1 mL of saline solution (0.09%) and mixed for 15 sec, Then, a mixture of the chloroform-methanol plus with BHT (0.002%) was added (2:1, vol/vol, 4 mL according to the method described previously [
64] FAAIPF were trans-esterified to their FAPL methyl esters and separated as described above [
1]. The acetone insoluble phospholipid fraction are phosphatidyl choline, phosphatidyl ethanolamine, phosphatidyl inositol and phosphatidic acid [
24].
2.12. Total Phospholipids (TPL)
The TPL determination in serum was made with 100 µl of serum by a colorimetric assay through an enzymatic method utilizing N-ethyl-N-(2,hydroxy-3-sulfopropyl)-3,5-domethoxyaniline, according to the manufacturer’s recommendations (Fujifilm (Phospholipids C, Code No. 997-01801, FUJIFLIM Wako Diagnostic U.S.A. Corporation). The absorbance was measured at 600 nm. This method is able to detect lecithin, lysolecithin and sphingomyelin of phospholipids but phosphatidyl ethanolamine are not measured in the sample.
2.13. Statistical Analysis
TFA and PLs are expressed as a percentage. Categorical variables were expressed as frequencies and percentages. Continuous variables were compared with the Mann–Whitney U rank sum test followed by the normality test (Shapiro–Wilk) between HS vs. before the treatment with Vit E in the COVID-19 patients, and by Kruskall–Wallis test before vs. after the treatment with Vit E in the COVID-19 patients. The sample size was calculated by paired test of two correlated means, specifying the standard error of the differences. The calculation was taken according to the data found in our study and previously reported in the article by Chavarría et al. [
17]. The calculation was based on two forms: The first with the percentage of cases with elevated LPO pretreatment and post-treatment, which ranged between 1.75 before and 1.05 with an estimated Delta of 0.80 and with an α error of 0.05 and 0.01 as well as a power of 0.84 and 0.99 respectively. We decided to take the calculation with an alpha error of 0.05 and a power of 84 and we included 22 patients even if only 13 were required. SigmaPlot
® version 15. (Systat Software Inc., SanJose, CA 95131, USA, EE.UU, North First Street, Suite 360, Jandel Corporation, San Jose, CA, USA) was used to generate the analysis and graphs. Differences were considered statistically significant when p ≤0.05.
4. Discussion
The SARS-CoV-2 virus responsible for the COVID-19 pandemic that devastated the world between 2020-2021, continues to mutate and still claims the lives of millions of people around the world. Although there are already different vaccines provided by different pharmaceutical companies against this virus (13.590 million vaccine doses have been administered and a complete vaccination charts has been given to around 37% of the population and total population vaccinated with at least one booster dose of a COVID-19 vaccine is of 32%), recent data from the WHO Coronavirus (COVID-19) Dashboard, show that the deaths have reached 7,044,637 people in the world. For this reason, it is of vital importance to continue understanding both the metabolic pathways compromised by this virus in the patients, as well as the possible use of adjuvant therapeutic agents that may contribute to combat it. Adjuvant agents lead to lesser manifestations of the clinical data in patients and help in reducing the number of deaths. In this sense, Vit E at a dose of 400 IU/day has been utilized as an important anti-inflammatory and anti-OS therapy in humans with immunodeficiency and positive to the presence of the virus with promising results. This treatment decreases the viral load [
25]. In addition, it has been demonstrated that a decrease the Vit E is present in the plasma from the patients with COVID-19 [
26]. Another study showed that in 49 patients diagnosed with COVID-19 a Vit E deficiency of 7% was present [
27]. Moreover, Vit E levels were lower in pregnant women with COVID-19 [
28]. Patients with COVID-19 also show an alteration in lipid metabolism characterized in part by a decrease in plasma PLs [
29] that has been associated with Vit E deficiency. In this sense, the aim this study was to demonstrate if the treatment with Vit E restores the modification of the profile of the FA in PLs in serum from patients with COVID-19.
Our results show a decrease in Vit E levels in serum of patients with COVID-19,which confirms the previously mentioned reports [
26] and suggests that in the infectious process caused by SARS-CoV-2, there is depletion of molecules with antioxidant capacity such Vit E which contributes to the progression of the infection which is associated with the inflammatory degree [
30]. In this sense, IL-6 and CRP, which are pro-inflammatory molecules, were elevated in the serum of the COVID-19 patients in our study. The rising viral load in COVID-19 results in a fast increase in inflammatory monocytes and neutrophils in the lungs in an effort to try to counteract the infection. These molecules further activate IL and PGs that continuously and irreversibly affect the lung tissue [
31]. However, the treatment with Vit E decreased the IL-6, CRP, PGE
2 and TXB
2 levels but it increased the 6-keto-PGF
1α which is an anti-inflammatory molecule. These results suggest that it is necessary to maintain and restore the levels of Vit E in the serum during the infection to reduce or control the progression of inflammation. A previous study evaluated the association between Vit E and COVID-19, and hypothesized that Vit E could amplify the immune system due to its antioxidant properties and its roles in maintaining the integrity of the T-cell membranes thus reducing the duration of the infection [
32].Therefore our results suggest that the treatment with Vit E is an effectively regulate the immune activity that confers protection against SARS-CoV-2 by decreasing IL-6 and CRP [
33]. In this regard, it has been reported that the most important effects of Vit E supplementation on immune system activities include; post-mitogenic stimulation, lymphocyte proliferation and an increase of delayed type hypersensitivity [
34]. Studies in vitro and in vivo have demonstrated that Vit E treatment improves naive T-lymphocytes, natural-killer, and dendritic cell activities, to promote the initiation of T-cell activation. It also inhibits the production of pro-inflammatory cytokines, such as IL-1, -6, -8 and TNF-α but stimulate the IFN system, which exerts antiviral activities [
34].
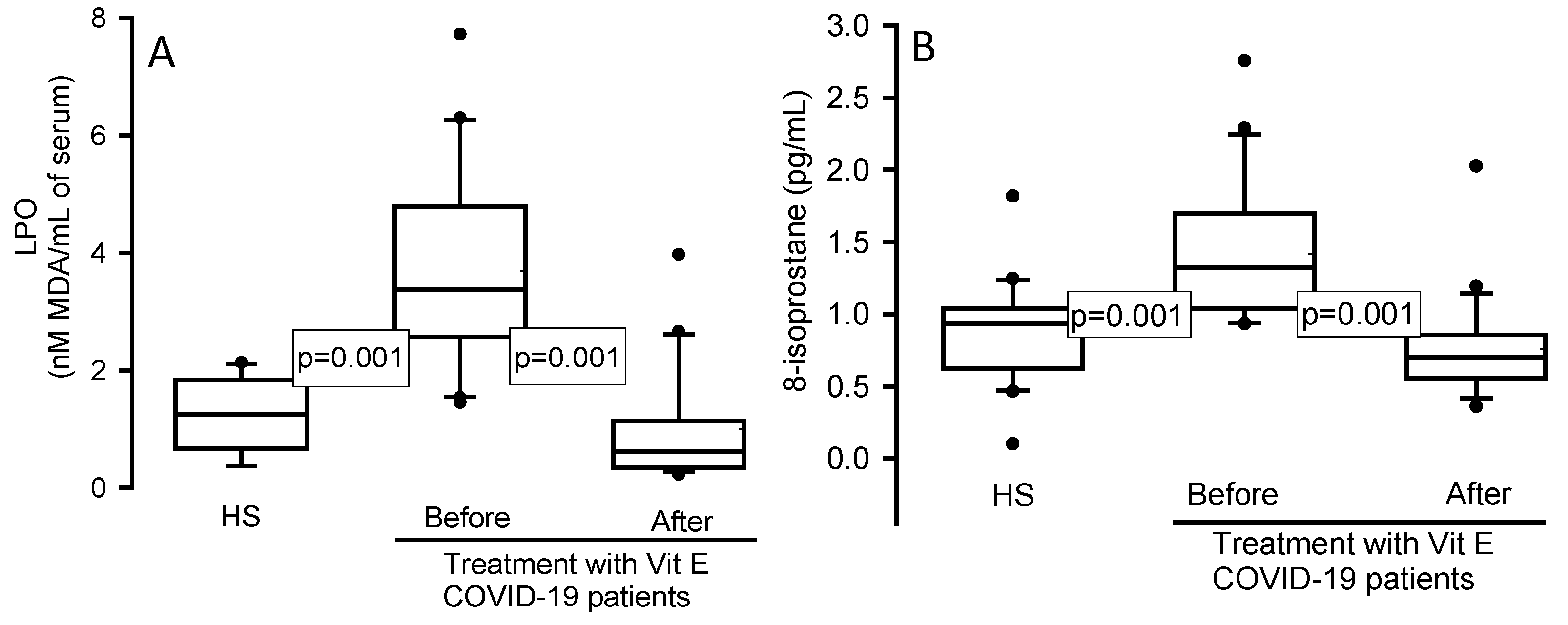
Moreover, high levels the LPO are present in the inflammatory process, and these are in part the result of the ferroptosis present in these patients [
35]. Our results showed an increase in the LPO index in patients with COVID-19 in comparison to HS. Moreover, the treatment with Vit E favored a decrease. This suggests that Vit E supplementation is capable of decreasing the LPO index associated to the increase in ferroptosis [
36]. In this regard, Vit E reacts with peroxyl radicals to prevent the formation of lipid hydroperoxides associated with the FA oxidation of the cell membrane. Afterward, glutathione (GSH) and enzymes that employ GSH may detoxify oxidized lipids [
37]. Furthermore, Vit E deficiency is related to ferroptosis and other studies suggest that Vit E supplementation at the high dose of 500 mg/kg may act as a treatment to inhibit ferroptosis in COVID-19 patients and decrease the damages caused by ferroptosis to multiple organs, such as lung, kidney, liver, gut, heart, and nervous system [
38]. Our results show an increase in the ferroptosis in COVID-19 before of the treatment with Vit E. However, we could not validate whether ferroptosis decreased in these patients after treatment with Vit E, and therefore, more studies are required to confirm this. Nevertheless there was a significant decrease the LPO.
The increase in LPO in COVID-19 suggests an oxidizing background in the SARS-CoV-2 infection as has already been documented [
37]. This situation favors the oxidation of AA to 8-isoprostanes which are molecules considered as markers of LPO and oxidative injury. For this reason, this molecule has been considered an ideal marker in the broncho-alveolar lavage fluid of patients with interstitial lung disease [
39]. Our results revealed a statistically significant elevation of 8-isoprotane in COVID-19 patients and there was decrease in its level after of the treatment Vit E. This suggests that 8-isoprostane may be a predictive marker against the OS present in these patients and reinforces that the Vit E supplementation contributes to decrease the oxidizing background. In patients co-infected with malaria and COVID-19, the treatment with Vit E decreases 8-isoprostane levels [
40].
Regarding the results of the TPLs fraction obtained (lecithin, lysolecithin and sphingomyelin), the patients with COVID-19 showed a significant decrease in serum. Different studies in COVID-19 have demonstrated an alteration in the profile of the lipid metabolism in this disease [
1,
4,
8]. Therefore, the rescheduling of the FA metabolism in the host seems to be essential [
41]. The decrease in the mass fraction in TPLs of the lecithin, lysolecithin and sphingomyelin quantified could be due in part by their use in the formation new viral membranes, the reparation of the membrane of the host cell after the budding process and to restore the high oxidation by free radicals that results in oxidized PLs which contribute to the LPO index. However, the treatment with Vit E was capable of diminishing this [
42] as the results demonstrate, the administration of 500 mg/d of Vit E for 12 weeks in patients with hepatitis C virus showed an increase in total PUFAS in red blood cells of the PLs [
43], since it has been described that the Vit E can exert its maximum protective effect in concentration the one molecule for every 2,000 PLs molecules [
44]. In addition, the analysis of the FAAIPF, showed an increased in PA and stearic acid and a decrease in the LA in this study. However, in TFA the PA, palmitoliec and stearic acids showed a decrease. These results might seem paradoxical; however, these alterations may be due in part to the need of host membranes to be repaired as the budding process of new virions occurs. In this sense, our results show that the TFA in the serum tend to increase in COVID-19 patients. At this stage there is a greater demand in the synthesis of FA. There is also a great need of these molecules for the formation of the viral membrane and because they are necessary for the binding to the receptor-binding domain such as LA [
45]. However, the treatment with Vit E may restore these requirements [
46]. In this sense, has been demonstrated that the FA of the PLs in the serum reflect FA from PLs in the membranes of erythrocytes [
47].
In addition, the desaturation process constitutes an important step in the metabolism of FA and desaturases are key enzymes in the biosynthesis of the MUFA from SFA. These enzymes introduce a cis-double bond into SFA, being very important in the control of the structural, fluidity and disorders of the membrane [
48]. Our results show significant changes in the activities of ∆
9–, ∆
6– and ∆
5– desaturases in the serum of the patients with COVID-19, but the treatment with Vit E restored the activities of these enzymes, both in FA and TPL. These results suggest that there is an alteration in the activity of desaturases in the SARS-CoV-2 infection, but the supplementation with Vit E restores them. The disorders associated with an imbalance in the activity of desaturases include modifications in membrane ionic transport, receptor accessibility and cellular enzymatic activities [
49]. Our results evidenced the modification in the proportions of SFA, MUFA, TFA, SFA and PUFA n-3 of the FAAIPF. However, treatment with Vit E restored them, and even increased PUFAs n-6 in both in TFA and FAAIPF. This result suggests that there exists an alteration in the proportion of SFA and MUFA in the SARS-CoV-2 infection but that the supplementation with Vit E restores it. The increase in PUFA n-6 or the tendency in PUFA n-3 in both TFA and FAAIPF is important because, even though we did not provide a diet with -3 or -6, these molecules were increased. These FA influence the immune response. They are essential elements since there is a need to consume them in the diet because they cannot be endogenously synthesized. The PUFA-3 fatty acids are largely comprised of α-linolenic acid, DHA and EPA. These FA may trigger anti-inflammatory reactions in the body. Also both PUFA-3 and -6 can change the composition of the PLs bilayer of the host cell membrane, thereby preventing viral entry. When incorporated into the plasma membrane, they can affect the clumping of toll-like receptors associated with the prevention of signals that activate NF-κB, production or other pro-inflammatory mediators and the reduction in clinical complications in COVID-19 patients [
50]. Furthermore, DHA and EPA also serve as precursors of resolvins which reduce the production of pro-inflammatory mediators eventually resulting in a decrease in systemic inflammation [
51]. In this sense, there exists an interrelation between Vit E and PUFAn-3 and -6. For example, the supplementation with Vit E increased the levels of PUFA n-3 and decreased the levels of SFA in hypertriglyceridemic rats [
52]. In zebra fish, a deficiency of Vit E leads to a depletion of PUFA [
53], and the amount of vit E present in tissues is related to the PUFA concentration, especially in membranes [
54].
Furthermore, the increase of the PUFA could contribute to decrease the PGE
2, TXB
2 and favor the 6-keto-PGF1
α increase that is observed in our results and this may in part decrease the inflammatory state and thrombosis in the patients with COVID-19. Thus, Vit E may interact with different enzymes, such as protein kinases, protein phosphatases, lipid kinases, lipid phosphatases, lipid metabolic enzymes and enzymes involved in cyclic adenosine monophosphate metabolism [
55]. It regulates a wide spectrum of key cellular processes, including many enzymes which are involved in pro-inflammatory events like COX
2, phospholipase A
2, 5-, 12-, and 15-LOX [
56].
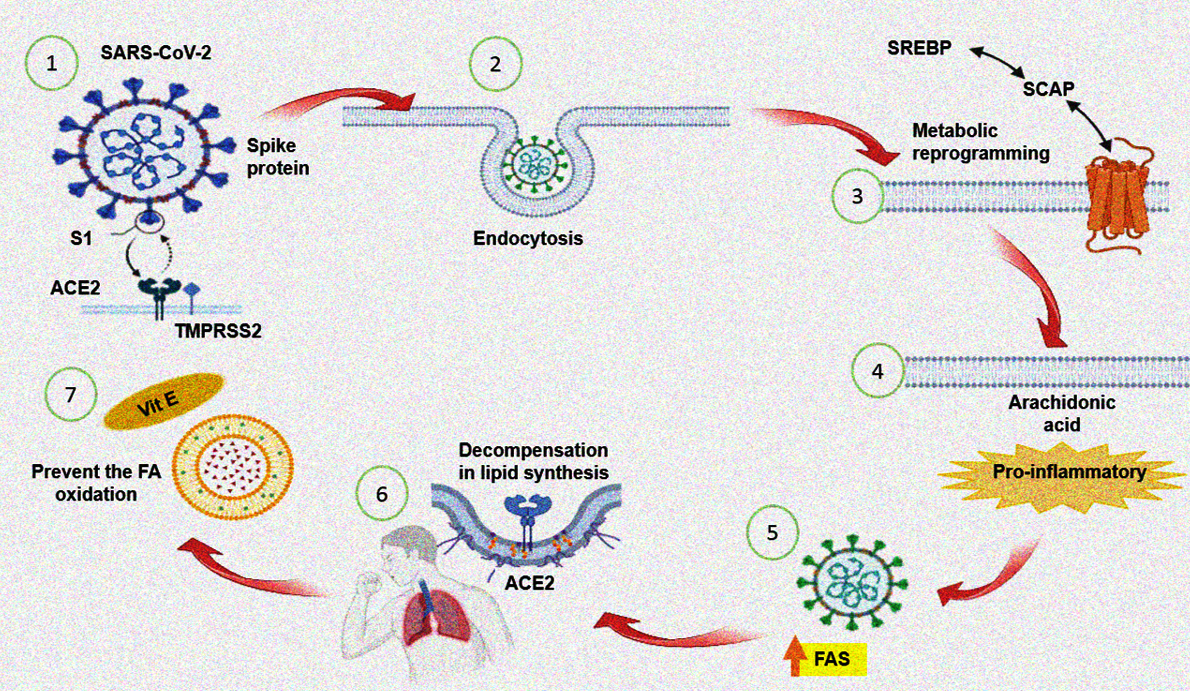
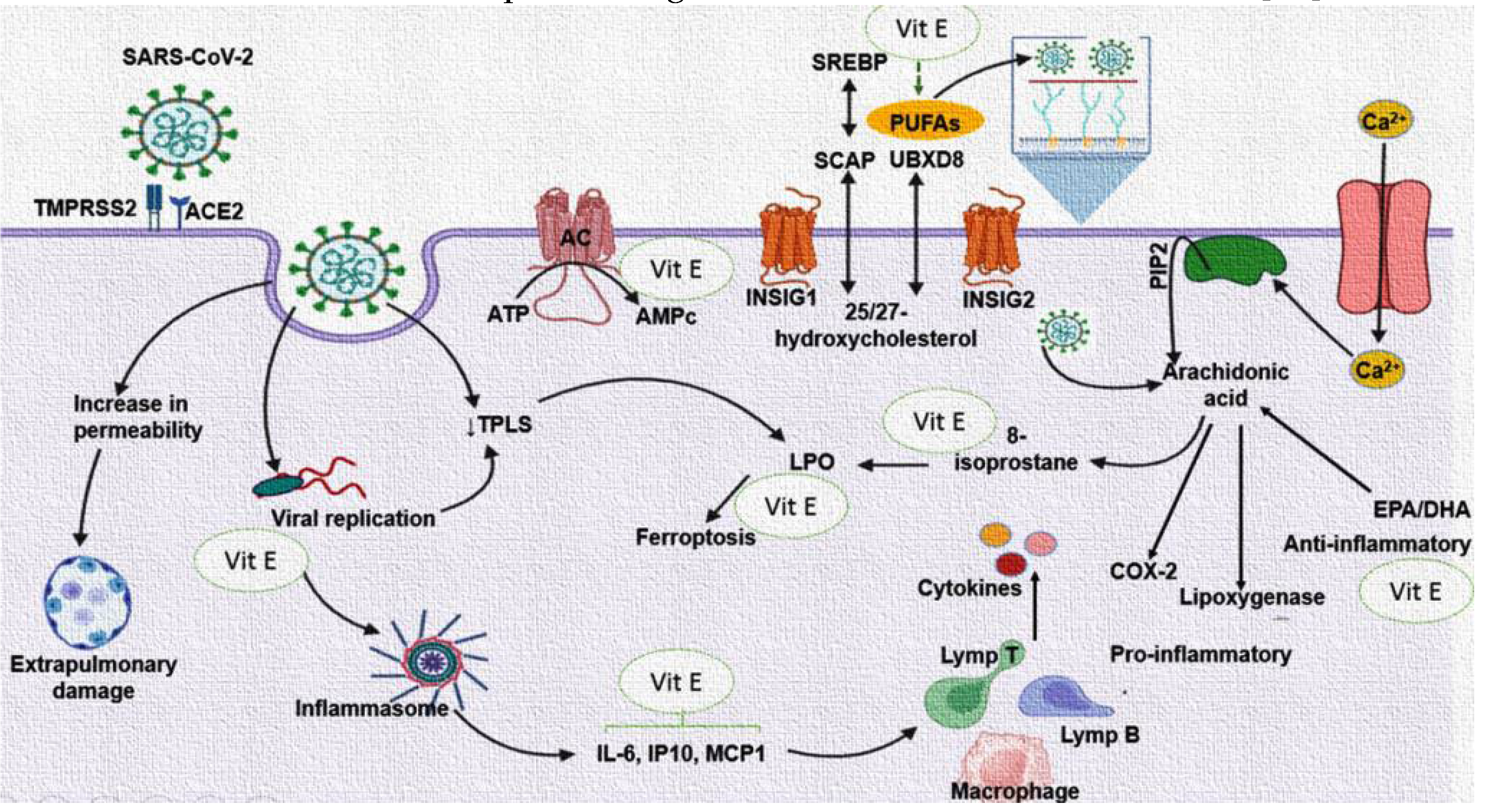
Figure 4 summarizes the beneficial effect of the treatment with Vit E on the reduction of the FA in the PLs fraction oxidized in the SARS-CoV-2 infection.
On the other hand, Vit E deficiency is related to a greater synthesis of platelet activating factor (PAF) which is produced by perivascular mast cells. The increase of the PAF leads to inflammation and high immunothrombosis in COVID-19 patients [
57]. This has been associated with the antiphospholipid antibody syndrome (aPLs), which is an autoimmune thrombophilia mediated by autoantibodies directed against plasma phospholipid-binding proteins, mainly β2 glycoprotein I and prothrombin. [
58]. The exact mechanism by which these aPLs induce thrombosis is still not well understood in the SARS-CoV-2 infection. In this sense, the results in different studies are controversial; for example, some papers report an increase while others a decrease [
59,
60]. However, there is evidence of an increase in aPLs antibodies in SARS-CoV-2 infection [
61]. In this regard, a study that measured the concentration of aCL IgG antibodies in the positive serum of COVID-19 before and after vaccination with different vaccines or following SARS-CoV-2 infection, were not clinically pathogenic for the risk of thrombosis [
62]. However, another study demonstrated that vaccination did not trigger early autoantibody production [
63]. Despite the above, treatment with Vit E has shown that it may inhibit the synthesis of the PAF that induced platelet aggregation [
64]. Although the relationship between aPLs and SARS-CoV-2 infection is not solid, a healthy diet containing PAF inhibitors such Vit E and flavonoids could have an effect not only on inflammation but also in the reduction of OS thus preventing the harmful effects of thrombosis [
65].
Figure 5.
Flow diagram used during the study.
Figure 5.
Flow diagram used during the study.
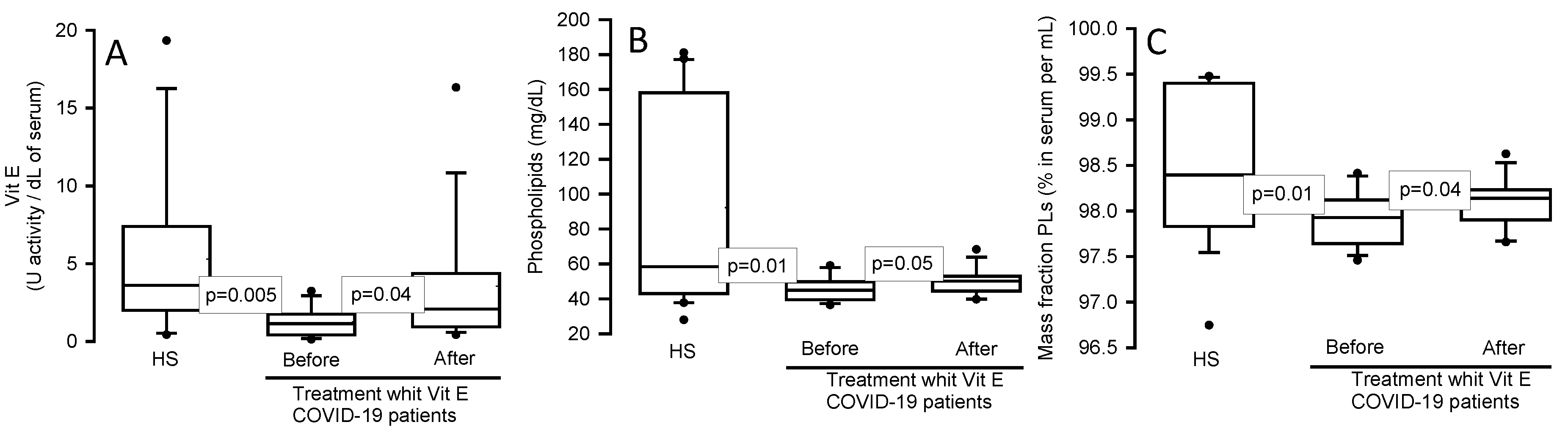
Figure 1.
Vit E levels, TPLs and mass fraction of the PLs in the serum of COVID-19 patients and HS before and after of the treatment with Vit E. Vit E, TPLs and mass fraction of the PLs showed a significant decrease respectively in COVID-19 patients when compared to HS, but after treatment with Vit E the levels were restored. Panel (A) Vit E, Panel (B) TPLs and Panel (C) mass fraction of the PLs. The dark circles that stand out from each bar are the outliers. The results are shown such as median, first quartile, third quartile, and half dotted line. Abbreviations: Vit= vitamin, TPLs= total phospholipids.
Figure 1.
Vit E levels, TPLs and mass fraction of the PLs in the serum of COVID-19 patients and HS before and after of the treatment with Vit E. Vit E, TPLs and mass fraction of the PLs showed a significant decrease respectively in COVID-19 patients when compared to HS, but after treatment with Vit E the levels were restored. Panel (A) Vit E, Panel (B) TPLs and Panel (C) mass fraction of the PLs. The dark circles that stand out from each bar are the outliers. The results are shown such as median, first quartile, third quartile, and half dotted line. Abbreviations: Vit= vitamin, TPLs= total phospholipids.
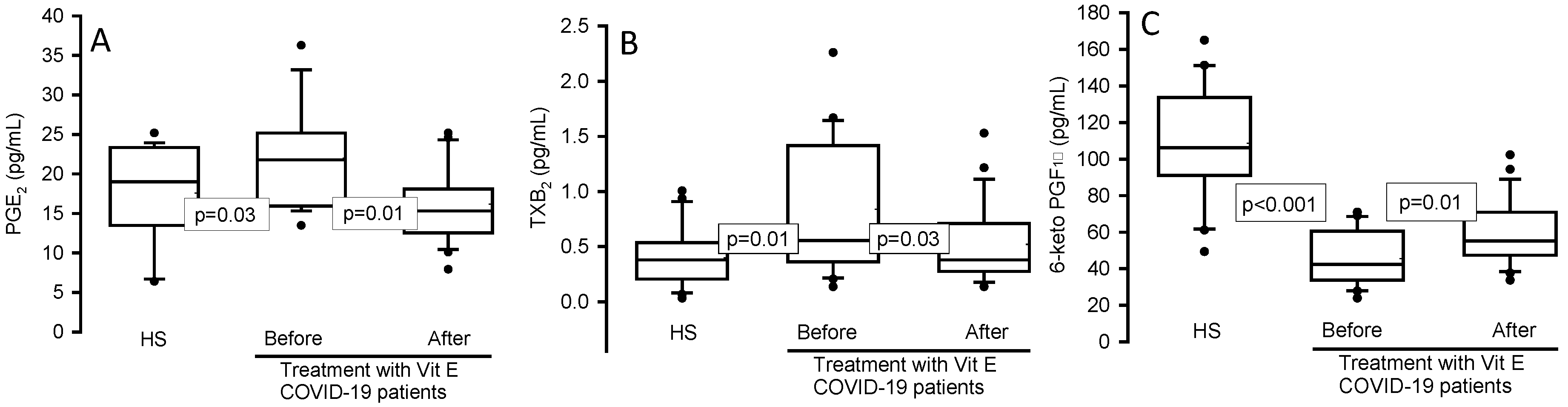
Figure 2.
Prostaglandin concentrations in the serum of HS and the COVID-19 patients before and after of the treatment with Vit E. PGE2 (Panel A), TXB2 (Panel B) and 6-keto PGF1α (Panel C). PGE2 and TXB2 showed a significant increase in COVID-19 patients compared with the HS, but after treatment with Vit E a decrease was observed. However, the contrary effect was present in the 6-keto PGF1α concentration. The dark circles that stand out from each bar are the outliers. The results are shown such as median, first quartile, third quartile, and half dotted line. Abbreviations: Vit= vitamin, HS= healthy subjects, PGE2= prostraglandin E2, TXB2= thromboxane B2, 6-keto PGF1α= 6- keto prostaglandin F 1 alpha.
Figure 2.
Prostaglandin concentrations in the serum of HS and the COVID-19 patients before and after of the treatment with Vit E. PGE2 (Panel A), TXB2 (Panel B) and 6-keto PGF1α (Panel C). PGE2 and TXB2 showed a significant increase in COVID-19 patients compared with the HS, but after treatment with Vit E a decrease was observed. However, the contrary effect was present in the 6-keto PGF1α concentration. The dark circles that stand out from each bar are the outliers. The results are shown such as median, first quartile, third quartile, and half dotted line. Abbreviations: Vit= vitamin, HS= healthy subjects, PGE2= prostraglandin E2, TXB2= thromboxane B2, 6-keto PGF1α= 6- keto prostaglandin F 1 alpha.
Figure 3.
LPO (Panel A) and 8-isoprostane (Panel B) concentration in the serum from HS and COVID-19 patients before and after of the treatment with Vit E. LPO and 8-isoprostane showed a significant increase in COVID-19 patients compared with the HS, but after treatment with Vit E they were decreased. The dark circles that stand out from each bar are the outliers. The results are shown such as median, first quartile, third quartile, and half dotted line. Abbreviations: Vit= vitamin, HS= healthy subjects, LPO= lipid peroxidation.
Figure 3.
LPO (Panel A) and 8-isoprostane (Panel B) concentration in the serum from HS and COVID-19 patients before and after of the treatment with Vit E. LPO and 8-isoprostane showed a significant increase in COVID-19 patients compared with the HS, but after treatment with Vit E they were decreased. The dark circles that stand out from each bar are the outliers. The results are shown such as median, first quartile, third quartile, and half dotted line. Abbreviations: Vit= vitamin, HS= healthy subjects, LPO= lipid peroxidation.
Figure 4.
A decrease in the Vit E and alteration in the PLs of the plasma of the patients with COVID-19 has been observed and is associated with the increase the IL-6, CRP, PGE
2, TXB
2, LPO index, ferroptosis and 8-isoprotane. However, the Vit E supplementation may decrease IL-6, CRP, PGE
2, LPO, ferroptosis, TXB
2 and 8-isoprotane levels and increased the 6-keto-PGF
1α. This could contribute to amplify the immune system and decrease the viral load. The treatment can also decrease the oxidation PLs. These are used in the formation new viral membranes, the reparation of the membrane of the host cell after the budding process, and this effect is attributed to Vit E which can exert its maximum protective effect on approximately 2,000 PLs molecules [
44].
Figure 4.
A decrease in the Vit E and alteration in the PLs of the plasma of the patients with COVID-19 has been observed and is associated with the increase the IL-6, CRP, PGE
2, TXB
2, LPO index, ferroptosis and 8-isoprotane. However, the Vit E supplementation may decrease IL-6, CRP, PGE
2, LPO, ferroptosis, TXB
2 and 8-isoprotane levels and increased the 6-keto-PGF
1α. This could contribute to amplify the immune system and decrease the viral load. The treatment can also decrease the oxidation PLs. These are used in the formation new viral membranes, the reparation of the membrane of the host cell after the budding process, and this effect is attributed to Vit E which can exert its maximum protective effect on approximately 2,000 PLs molecules [
44].
Table 1.
Demographic characteristics at admission in patients infected with COVID-19 before of the treatment with Vit E.
Table 1.
Demographic characteristics at admission in patients infected with COVID-19 before of the treatment with Vit E.
| Variables |
Median and Min–Max range |
| Age |
55 (31 – 88) |
| Gender |
3 Women and 19 Men |
| BMI |
28 (20 – 38) |
| Comorbidities number and (%) |
| Diabetes Mellitus |
6 (27) |
| Hypertension |
3 (14) |
| Dyslipidemia |
10 (46) |
| Normal Weight |
5 (23) |
| Overweight |
9 (41) |
| Morbid Obesity |
8 (36) |
| Gasometry and blood biochemistry median and min–max range |
| PaO2
|
72 (47 – 233) |
| PCO2
|
32 (26 – 60) |
| PaO2/FIO2 (mmHg) |
126 (50 – 318) |
| SPO2/FIO2 (mmHg) |
144 (67 – 320) |
| HR bpm |
85 (20 – 115) |
| MAP (mmHg) |
74 (61 – 99) |
| Temperature |
36 (35 – 38) |
| BUN |
18.4 (8.6 – 40.9) |
| Leukocytes 103/µL |
9.5 (4.4 – 17.3) |
| Lymphocytes 103/µL |
6.5 (0.20 – 1.1) |
| Platelets 103/µL |
226 (97 – 529) |
| Ferritin ng/m |
586 (189 – 1443) |
| D-dimer ng/dL |
1040 (440 – 13100) |
| N/L |
12 (4 – 50) |
| Procalcitonin ng/dL |
0.10 (0.02 – 10.7) |
| Median and Min–Max range |
| SOFA |
1 (0 – 8) |
| APACHE II |
5.5 (3 – 8) |
| SAPS |
28 (15 – 32) |
| Glasgow |
15 (12 – 15) |
| CORADS |
4 (3 – 5) |
| COVID GRAM |
119 (76 – 175) |
Table 2.
Gender, age, and serum biochemical determinations in HS and patients with COVID 19 before of the treatment with Vit E.
Table 2.
Gender, age, and serum biochemical determinations in HS and patients with COVID 19 before of the treatment with Vit E.
| |
HS (n=23)
Median
Min-Max range |
COVID-19 (n=22)
Median
Min-Max range |
P |
| Age |
60 (27 – 87) |
55 (31–88) |
NS |
| Women |
8 (35) |
3 (14) |
NS |
| Men |
15 (55) |
19 (86) |
NS |
| Blood biochemical (mg/dL) |
| Glucose |
99 (32 – 199) |
120 (81 – 330) |
0.006 |
| C2+
|
9,1 (8.4 – 9.7) |
9,2 (7.9 – 10.10) |
NS |
| Uric acid |
6.6 (2.9 – 9.6) |
4.7 (1.5 – 8.07) |
0.01 |
| Creatinine |
0.83 (0.60 – 1.20) |
0.90 (0.50 – 1.70) |
NS |
| CT |
165 (97 – 276) |
134 (94 – 189) |
0.01 |
| HDL |
37 (20 – 54) |
34.7 (22.3 – 47.9) |
NS |
| LDL |
92 (30 – 148) |
70.3 (38.9 – 129.7) |
0.01 |
| TG |
115 (67 – 389) |
122 (70 – 249) |
NS |
Table 3.
Inflammation markers in patients with COVID-19 patients before and after of the treatment with Vit E.
Table 3.
Inflammation markers in patients with COVID-19 patients before and after of the treatment with Vit E.
| Variables |
HS |
Before of the vit E treatment
in COVID-19 patients
Median (Min–Max) |
After of the vit E treatment
in COVID-19 patients
Median (Min–Max) |
| IL-6 (pg/mL) |
0.9 (0.4 –1.8) |
98.5 (7.8 – 637.6)*** |
7.8 (7.8 – 547.7)* |
| CRP (mg/dL) |
1.8 (0.2 –35.0) |
177 (4.1 – 384.7)*** |
49 (1.8 – 173.5)* |
Table 4.
Total fatty acid compositions in the serum from the HS and COVID-19 patients before and after of the treatment with Vit E.
Table 4.
Total fatty acid compositions in the serum from the HS and COVID-19 patients before and after of the treatment with Vit E.
| Fatty Acid (%) |
HS |
COVID-19 patients
before of the Vit E |
COVID-19 patients after of the Vit E |
| C16:0 |
28.8 ± 1.5 |
24.9 ± 1.4* |
20.9 ± 1.3 |
| C16:1 |
4.2 ± 0.3 |
3.1 ± 0.2*** |
4.0 ± 0.3* |
| C18:0 |
12.0 ± 1.3 |
8.4 ± 0.4* |
9.1 ± 0.7 |
| C18:1n-9 |
20.8 ± 0.8 |
26.9 ± 1.0*** |
24.9 ± 1.4 |
| C18:2n-6 |
23.1 ± 1.3 |
22.8 ± 1.1 |
21.3 ± 2.0 |
| γ-C18:3n-6 |
1.1 ± 0.3 |
1.4 ± 0.5 |
3.9 ± 1.9** |
| α-C18:3n-6 |
1.0 ± 0.2 |
1.5 ± 0.3 |
3.2 ± 0.6* |
| C20:3n-6 |
1.8 ± 0.6 |
1.8 ± 0.5 |
2.8 ± 0.4** |
| C20:4n-6 |
3.5 ± 0.4 |
4.2 ± 0.5 |
5.8 ± 0.8 |
| C20:5n-3 |
2.5 ± 0.5 |
2.2 ± 0.5 |
3.3 ± 0.4§
|
| C22:6n-3 |
1.2 ± 0.3 |
1.6 ± 0.5 |
3.0 ± 0.6** |
| Total fatty acid mg/mL the serum |
| |
2.8 ± 0.2 |
3.2 ± 0.4§
|
2.0 ± 0.3§
|
Table 5.
Total fatty acid compositions of saturated monounsaturated and polyunsaturated in the serum from HS and COVID-19 patients before and after of the treatment with Vit E.
Table 5.
Total fatty acid compositions of saturated monounsaturated and polyunsaturated in the serum from HS and COVID-19 patients before and after of the treatment with Vit E.
| Fatty acid (%) |
HS |
COVID-19 patients
before of the Vit E |
COVID-19 patients after of the Vit E |
| SFA |
39.11 ± 2.20 |
32.69 ± 1.38** |
29.52 ± 1.45 |
| MUFA |
24.61 ± 0.76 |
30.04 ± 1.10** |
28.89 ± 1.25 |
| PUFA (n-3) |
30.27 ± 1.93 |
30.19 ± 1.12 |
33.82 ± 2.82§
|
| PUFA (n-6) |
5.77 ± 1.16 |
5.36 ± 1.06 |
9.52 ± 1.07* |
Table 6.
Indirect desaturation indexes of the desaturases in the total fatty acids from HS and COVID-19 patients before and after of the treatment with Vit E.
Table 6.
Indirect desaturation indexes of the desaturases in the total fatty acids from HS and COVID-19 patients before and after of the treatment with Vit E.
| Dasaturation index |
HS |
COVID-19 patients
before of the Vit E |
COVID-19 patients after of the Vit E |
| C16:1n-7/C16:0 (∆9) |
0.15 ± 0.008 |
0.13 ± 0.01* |
0.19 ± 0.01 |
| C18:1n-9/C18:0 (∆9) |
1.90 ± 0.10 |
3.36 ± 0.23*** |
2.97 ± 0.23 |
| γ-C18:3n-6/C18:2n-6 (∆6) |
3.02 ± 0.37 |
7.16 ± 2.01** |
2.29 ± 0.30* |
| C20:4n-6/C20:3n-6 (∆5) |
0.43 ± 0.09 |
0.62 ± 0.12 |
1.04 ± 0.19** |
| C22:6n-3/C20:5n-3 (∆5) |
0.05 ± 0.01 |
0.06 ± 0.02 |
0.33 ± 0.18§
|
Table 7.
Fatty acid compositions of the FAAIPF in the serum from the HS, before and after of the treatment with Vit E in COVID-19 patients.
Table 7.
Fatty acid compositions of the FAAIPF in the serum from the HS, before and after of the treatment with Vit E in COVID-19 patients.
| Fatty Acid (%) |
HS |
COVID-19 patients
before of the Vit E |
COVID-19 patients after of the Vit E |
| C16:0 |
21.5 ± 1.2 |
28.4 ± 1.8*** |
23.6 ± 1.1** |
| C16:1 |
3.3 ± 0.2 |
4.1 ± 0.5 |
4.5 ± 0.4 |
| C18:0 |
7.8 ± 0.6 |
10.0 ± 0.6* |
9.8 ± 0.4 |
| C18:1n-9 |
23.1 ± 1.3 |
20.0 ± 1.0§
|
17.7 ± 0.8 |
| C18:2n-6 |
31.2 ± 1.2 |
20.4 ± 1.1*** |
21.9 ± 0.9 |
| γ-C18:3n-6 |
0.9 ± 0.2 |
1.0 ± 0.2 |
1.4 ± 0.2 |
| α-C18:3n-3 |
1.8 ± 0.6 |
1.2 ± 0.2 |
2.3 ± 0.9 |
| C20:3n-6 |
2.7 ± 0.7 |
2.5 ± 0.2§
|
2.0 ± 0.6* |
| C20:4n-6 |
4.7 ± 0.7 |
7.8 ± 1.5 |
7.4 ± 1.8 |
| C20:5n-3 |
2.2 ± 0.8 |
2.4 ± 0.8 |
4.7 ± 0.9* |
| C22:6n-3 |
0.9 ± 0.4 |
1.5 ± 0.5 |
4.4 ± 1.0* |
Table 8.
Fatty acid compositions of the phospholipids; SFA, MUFA and PUFA in the serum from the HS and COVID-19 patients before and after of the treatment with Vit E.
Table 8.
Fatty acid compositions of the phospholipids; SFA, MUFA and PUFA in the serum from the HS and COVID-19 patients before and after of the treatment with Vit E.
| Fatty acid (%) |
HS |
COVID-19 patients
before of the Vit E |
COVID-19 patients after of the Vit E |
| SFA |
28.9 ± 0.9 |
38.4 ± 1.8*** |
33.4 ± 1.4§
|
| MUFA |
26.5 ± 1.2 |
24.2 ± 1.0 |
22.2 ± 0.8 |
| PUFA (n-3) |
39.6 ± 1.0 |
31.8 ± 1.3*** |
32.8 ± 1.4§
|
| PUFA (n-6) |
4.9 ± 1.5 |
5.6 ± 1.4 |
11.5 ± 2.0* |
Table 9.
Indirect desaturation indexes of the desaturases in the fatty acids of the FAAIPF from HS and COVID-19 patients.
Table 9.
Indirect desaturation indexes of the desaturases in the fatty acids of the FAAIPF from HS and COVID-19 patients.
| Dasaturation index |
HS |
COVID-19 patients
before of the Vit E |
COVID-19 patients after of the Vit E |
| C16:1n-7/C16:0 (∆9) |
0.17 ± 0.02 |
0.16 ± 0.02 |
0.19 ± 0.01 |
| C18:1n-9/C18:0 (∆9) |
3.43 ± 0.37 |
2.13 ± 0.17* |
1.85 ± 0.12 |
| γ-C18:3n-6/C18:2n-6 (∆6) |
3.17 ± 0.72 |
4.00 ± 0.85 |
4.44 ± 0.89 |
| C20:4n-6/C20:3n-6 (∆5) |
0.28 ± 0.10 |
0.57 ± 0.24 |
0.68 ± 0.19§
|
| C22:6n-3/C20:5n-3 (∆5) |
0.03 ± 0.007 |
0.05 ± 0.01 |
0.07 ± 0.01* |