Submitted:
07 June 2024
Posted:
11 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Mushroom Materials and Treatments
2.2. Measurements of Browning Index, Firmness and Weight Loss
2.3. Determinations of Polyphenol Oxidase Activity, Total Phenolic Content, Malondialdehyde (MDA) and Electrolyte Leakage
2.4. Ultrastructural Observation of Cells
2.5. Transcriptome Sequencing and Data Analysis
2.6. Widely Targeted Metabolomics Profiling and Analysis
2.7. RNA Extraction and Real-Time Quantitative PCR (qPCR)
2.8. Statistical Analysis
3. Results
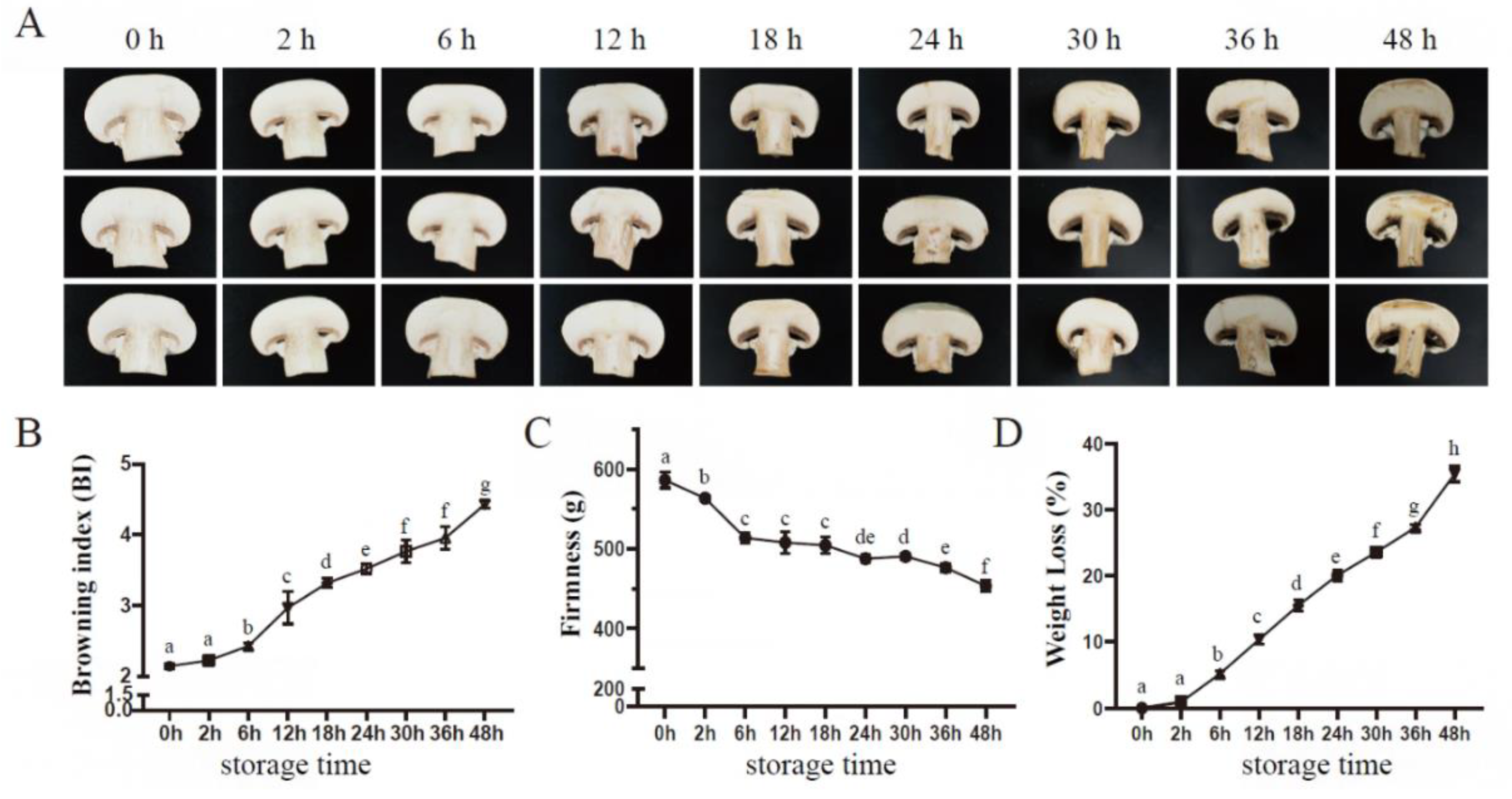
3.1. Browning Processof Postharvest A. bisporus Storage at Room Temperature
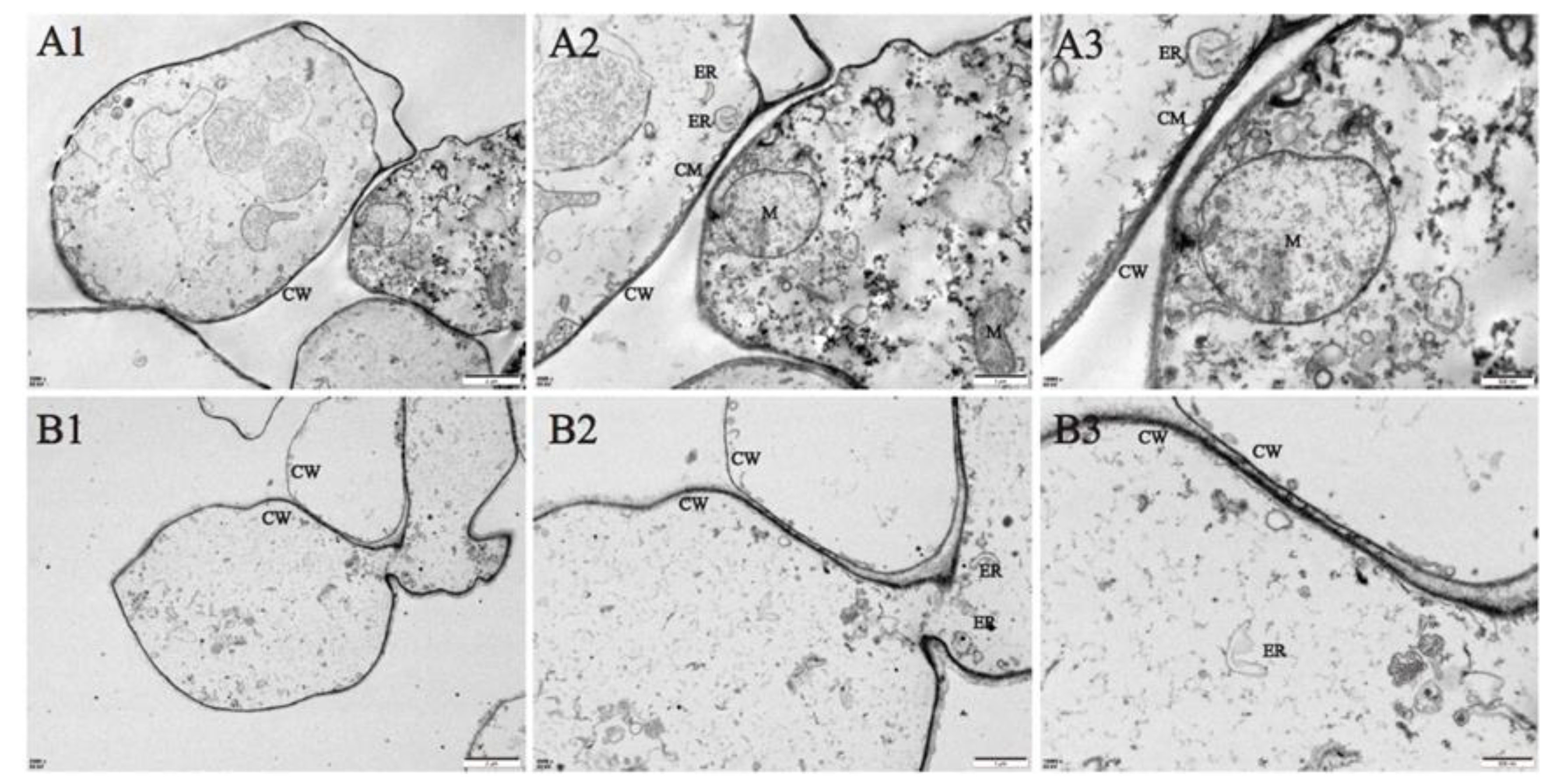
3.2. Lipid Metabolites Associated Ultrastructural Changes of Cell Membrane Degradation during Ambient Storage of A. bisporus
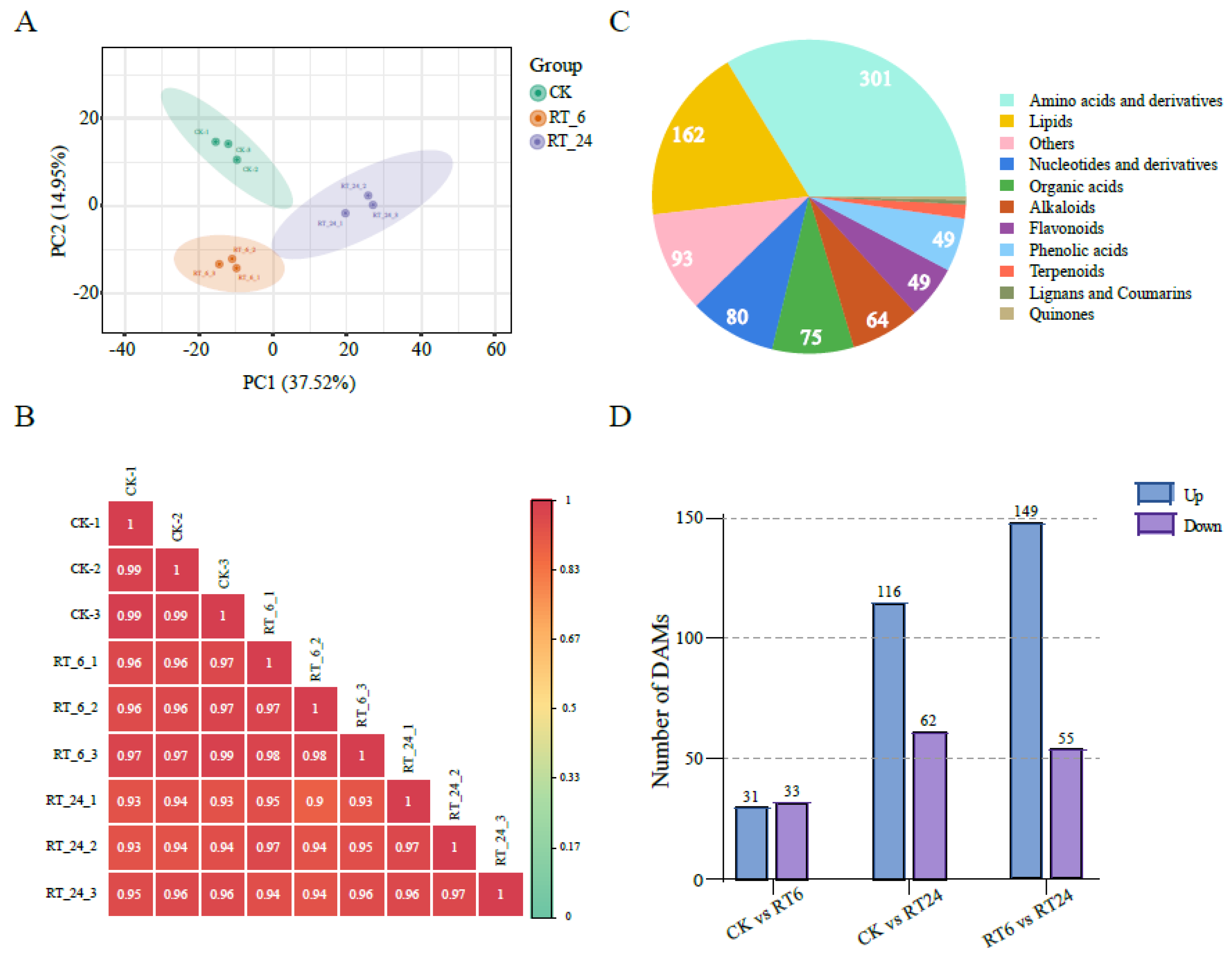
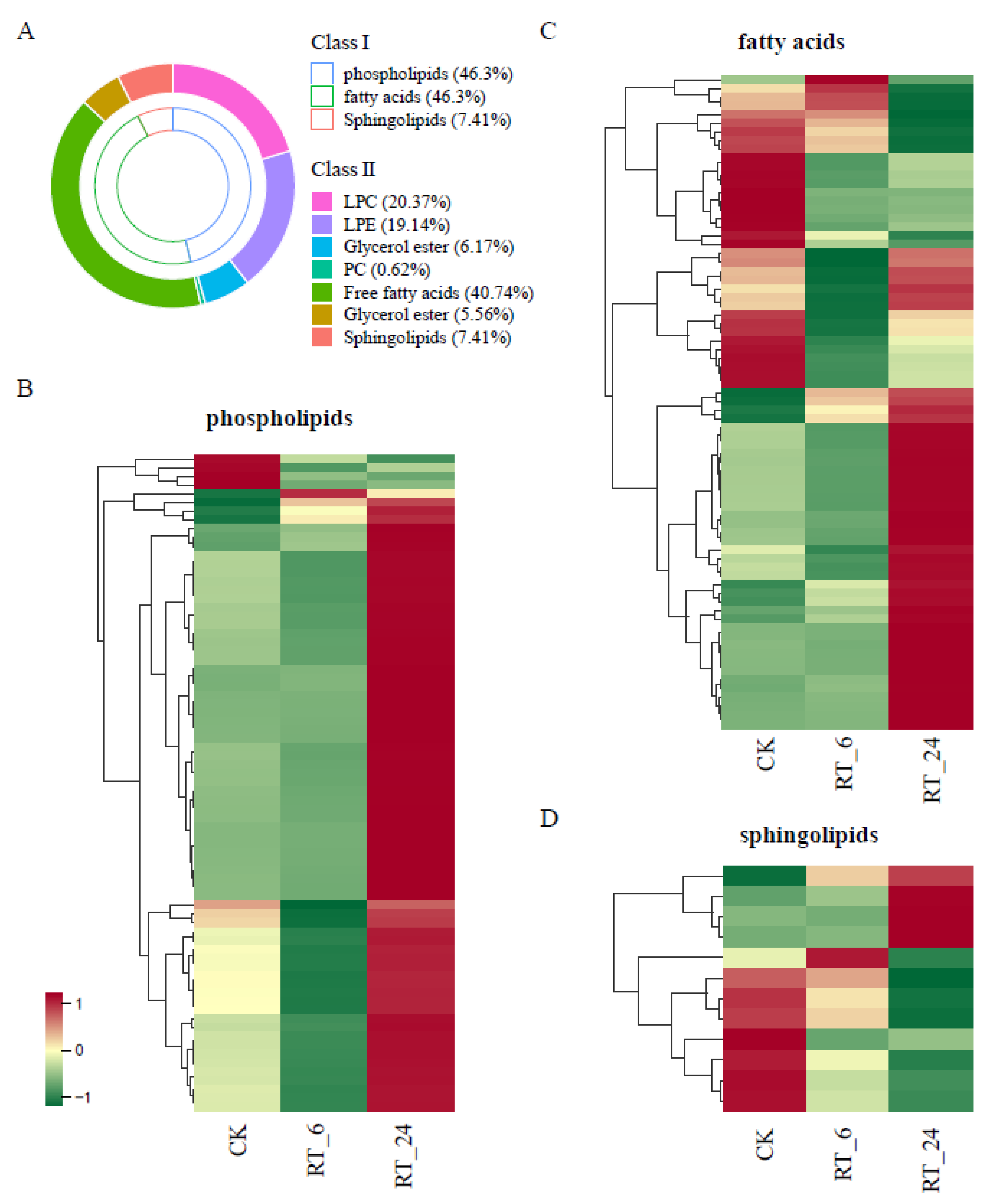
3.3. Differentially Accumulated Lipidmetabolites Analysis of Flesh under Different Storage Times
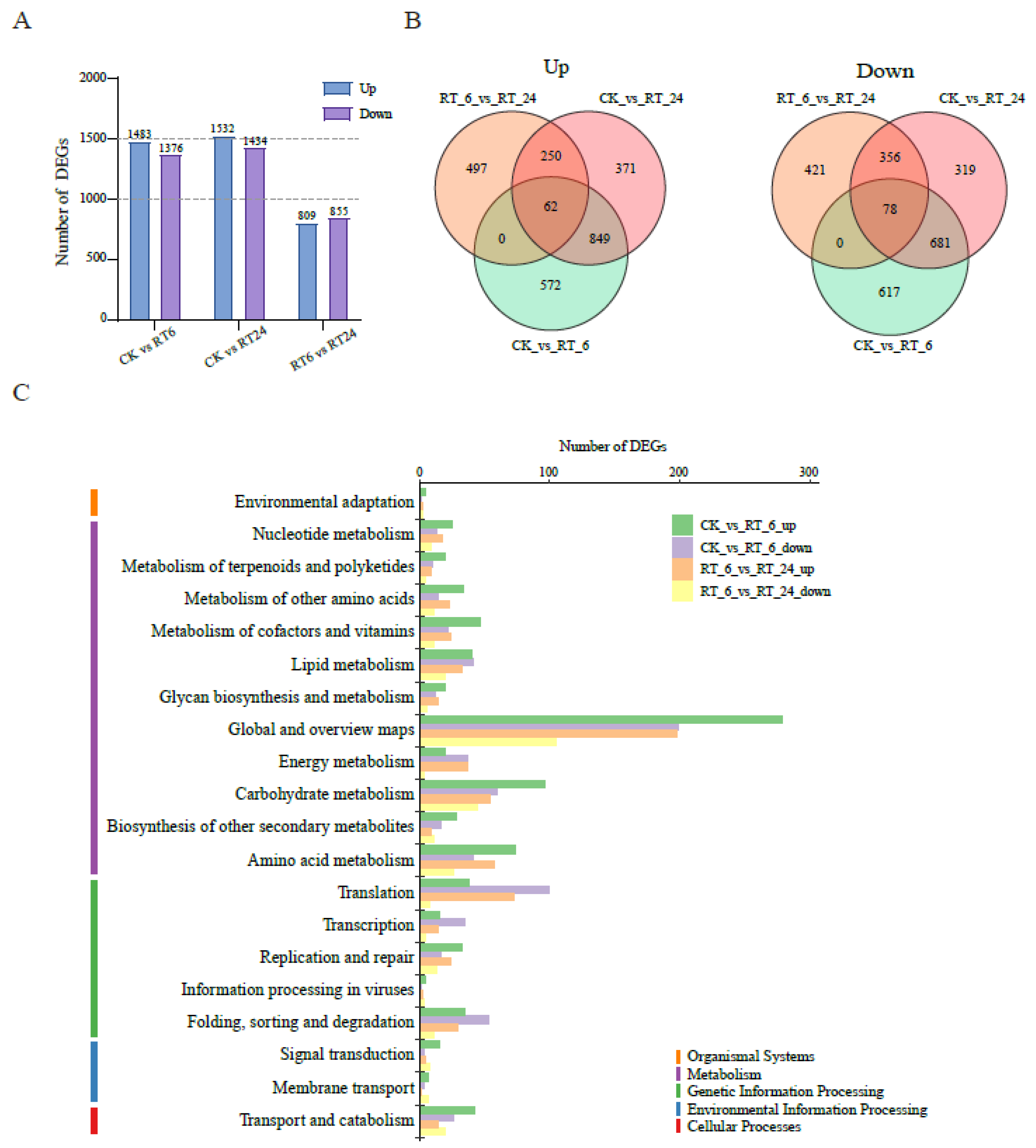
3.4. Transcriptomic Analysis of Flesh under Different Storage Times
3.5. Alterations of Lipid Metabolism Pathway Genes in the Flesh under Different Storage Times
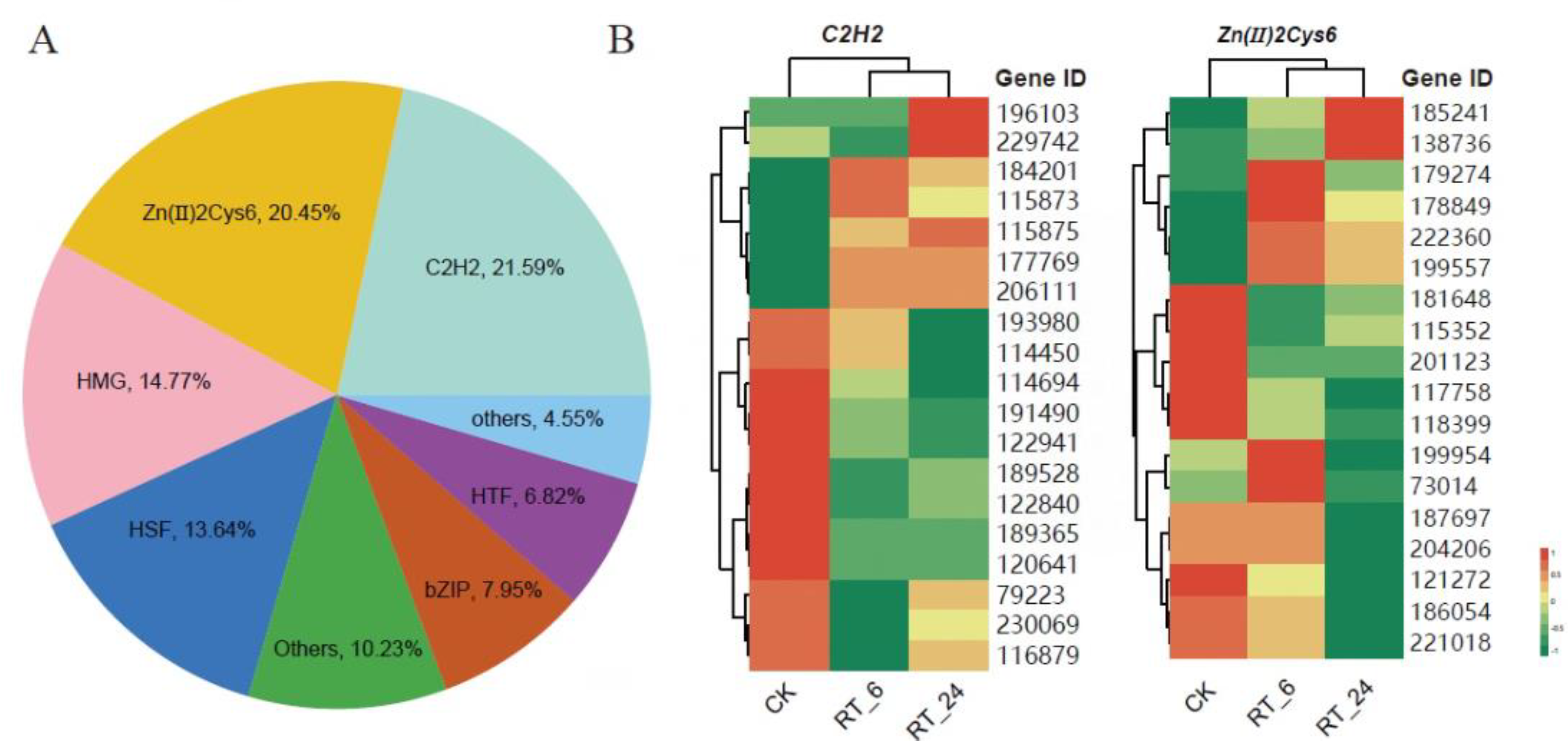
3.6. Transcription Factors Are Correlated with the Differential Accumulation of Metabolites in A. bisporus
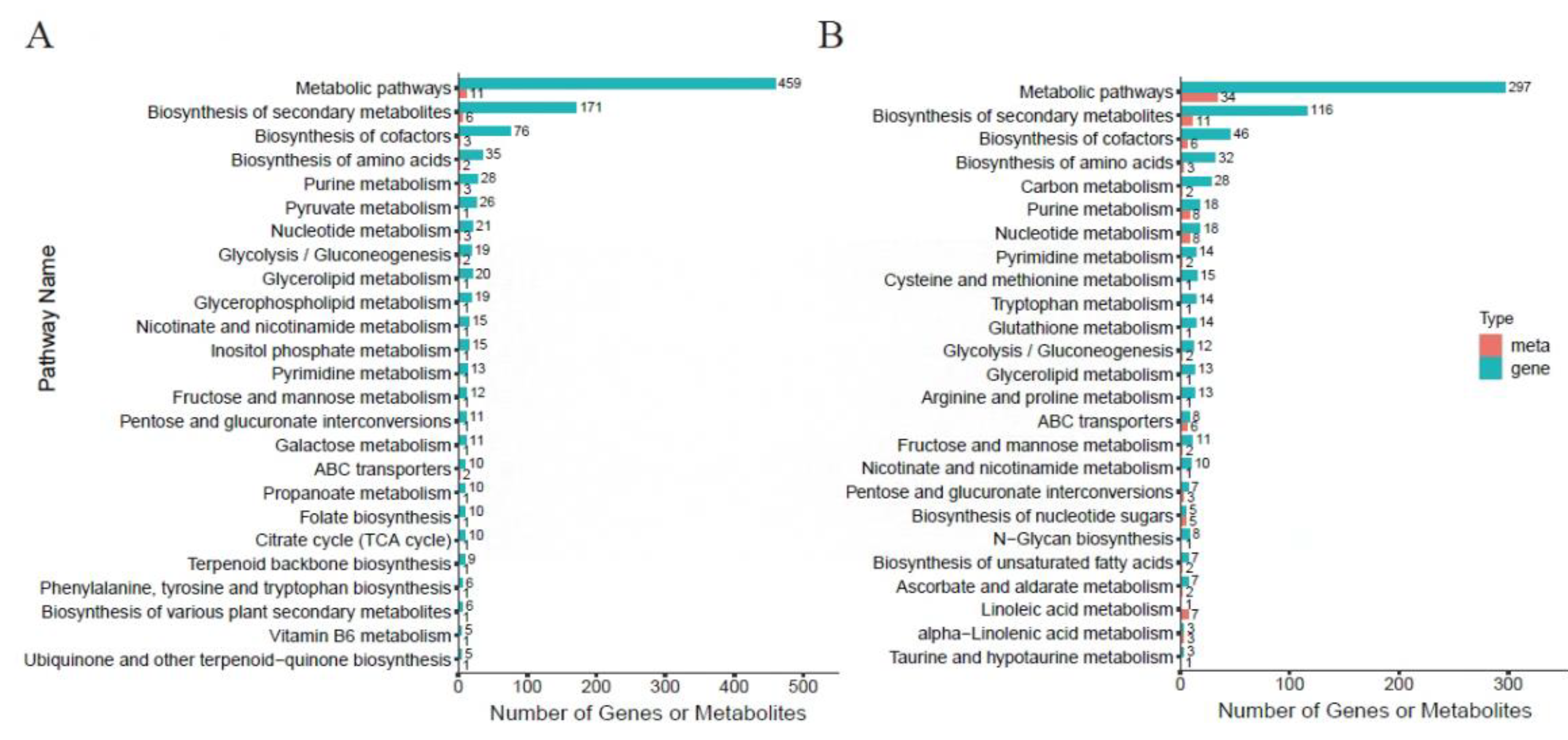
3.7. Integrated Transcriptomic and Metabolomic Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sen, S.; Chakraborty, R. Herbal Medicine in India: Indigenous Knowledge, Practice, Innovation and Its Value; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal mushrooms: bioactive compounds, use, and clinical trials. Int J Mol Sci. 2021, 22, 634. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Murtaza, G.; Ditta, A. Nutritional, medicinal, and cosmetic value of bioactive compounds in button mushroom (Agaricus bisporus): A review. Appl Sci. 2021, 11, 5943. [Google Scholar] [CrossRef]
- Chaitanya, M.V.N.L.; Jose, A.; Ramalingam, P.; Mandal, S.C.; Kumar, P.N. Multi-targeting cytotoxic drug leads from mushrooms. Asian Pacific J. Trop. Med. 2019, 12, 531. [Google Scholar] [CrossRef]
- Tirta Ismaya, W.; Tjandrawinata, R.R.; Rachmawati, H. Lectins from the Edible Mushroom Agaricus bisporus and Their Therapeutic Potentials. Molecules 2020, 25, 2368. [Google Scholar] [CrossRef] [PubMed]
- Predanócyová, K.; Árvay, J.; Šnirc, M. Exploring consumer behavior and preferences towards edible mushrooms in Slovakia. Foods. 2023, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Â.; Antonio, A.L.; Oliveira, M.B.P.P.; Martins, A.; Ferreira, I.C.F.R. Effect of gamma and electron beam irradiation on the physico-chemical and nutritional properties of mushrooms: A review. Food Chem. 2012, 135, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Pu, Y.Y.; Sun, D.W. Recent advances in quality preservation of postharvest mushrooms (Agaricus bisporus): A review. Trends Food Sci. Technol. 2018, 78, 72–82. [Google Scholar] [CrossRef]
- Qu, H.; Zhou, H.B.; Ma, T.; Zheng, Z.H.; Zheng, E.P.; Yang, H.L.; Gao, H.Y. TMT-based quantitative proteomic analysis of postharvest Coprinus comatus fruiting body during storage. Postharvest Biol. Technol. 2022, 185, 111786. [Google Scholar] [CrossRef]
- Yurttas, Z.S.; Moreira, R.G.; Castell-Perez, E. Combined vacuum impregnation and electron-beam irradiation treatment to extend the storage life of sliced white button mushrooms (Agaricus bisporus). J. Food Sci. 2014, 79, E39–E46. [Google Scholar] [CrossRef]
- Gantner, M.; Guzek, D.; Pogorzelska, E.; Brodowska, M.; Wojtasik-Kalinowska, I.; Godziszewska, J. The Effect of Film Type and Modified Atmosphere Packaging with Different Initial GAS Composition on the Shelf Life of White Mushrooms (Agaricus bisporus L.). J. Food Process. Preserv. 2016, 41, e13083. [Google Scholar] [CrossRef]
- Dellarosa, N.; Frontuto, D.; Laghi, L.; Dalla Rosa, M.; Lyng, J.G. The impact of pulsed electric fields and ultrasound on water distribution and loss in mushrooms stalks. Food Chem. 2017, 236, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Fan, X.; Yan, R. Effect of combination of ultraviolet light and hydrogen peroxide on inactivation of Escherichia coli O157: H7, native microbial loads, and quality of button mushrooms. Food Control. 2013, 34, 554–559. [Google Scholar] [CrossRef]
- Aday, M.S. Application of electrolyzed water for improving postharvest quality of mushroom. LWT Food Sci. Technol. 2016, 68, 44–51. [Google Scholar] [CrossRef]
- Nasiri, M.; Barzegar, M.; Sahari, M.A.; Niakousari, M. Tragacanth gum containing Zataria multiflora Boiss. essential oil as a natural preservative for the storage of button mushrooms (Agaricus bisporus). Food Hydrocoll. 2017, 72, 202–209.
- Wang, T.; Yun, J.; Zhang, Y.; Bi, Y.; Zhao, F.; Niu, Y. Effects of ozone fumigation combined with nano-film packaging on the postharvest storage quality and antioxidant capacity of button mushrooms (Agaricus bisporus). Postharvest Biol. Technol. 2021, 176, 111501. [Google Scholar] [CrossRef]
- Azevedo, S.; Cunha, L.M.; Oliveira, J.C.; Mahajan, P.V.; Fonseca, S.C. Modelling the influence of time, temperature and relative humidity conditions on the mass loss rate of fresh oyster mushrooms. J. Food Eng. 2017, 212, 108–112. [Google Scholar] [CrossRef]
- Singh, P.; Langowski, H.C.; Wani, A.A.; Saengerlaub, S. Recent advances in extending the shelf life of fresh Agaricus mushrooms: A review. J. Sci. Food Agric. 2010, 90, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Guan, W.; Yan, R.; Lei, J.; Xu, L.; Wang, Z. Effects of UV-C on antioxidant activity, total phenolics and main phenolic compounds of the melanin biosynthesis pathway in different tissues of button mushroom. Postharvest Biol. Technol. 2016, 118, 51–58. [Google Scholar] [CrossRef]
- Gao, X. ; Wu,W. ; Chen,H.; Niu,B.;Han,Y.; Fang,X.;Chen,H.; Liu,R.;Gao, H. Nitric oxide treatment delays quality deterioration and enzymatic browning of Agaricus bisporus via reactive oxygen metabolism regulation, Food Front. 2023, 4, 447–458. [Google Scholar]
- Ding, Y.; Zhu, Z.; Zhao, J.; Nie, Y.; Zhang, Y.; Sheng, J.; Meng, D.; Mao, H.; Tang, X. Effects of postharvest brassinolide treatment on the metabolism of white button mushroom (Agaricus bisporus) in relation to development of browning during storage. Food Bioprocess Technol. 2016, 9, 1327–1334. [Google Scholar] [CrossRef]
- Toivonen, P.M.A.; Brummell, D.A. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol. Technol. 2008, 48, 1–14. [Google Scholar] [CrossRef]
- Wrona, M.; Bentayeb, K.; Nerín, C. A novel active packaging for extending the shelf-life of fresh mushrooms (Agaricus bisporus). Food Control 2015, 54, 200–207. [Google Scholar] [CrossRef]
- Ghasemi-Varnamkhasti, M.; Mohammad-Razdari, A.; Yoosefian, S.H.; Izadi, Z. Effects of the combination of gamma irradiation and Ag nanoparticles polyethylene films on the quality of fresh bottom mushroom (Agaricus bisporus L.). J. Food Processing Preserv. 2018, 42, e13652. [Google Scholar] [CrossRef]
- Joshi, K.; Warby, J.; Velverde, J.; Tiwari, B.; Cullen, P.J.; Frias, J.M. Impact of cold chain and product variability on quality attributes of modified atmosphere packed mushrooms (Agaricus bisporus) throughout distribution. J. Food Eng. 2018, 232, 44–55. [Google Scholar] [CrossRef]
- Lei, J.; Li, B.; Zhang, N.; Yan, R.; Guan, W.; Brennan, C.S.; Gao, H.; Peng, B. Effects of UV-C treatment on browning and the expression of polyphenol oxidase (PPO) genes in different tissues of Agaricus bisporus during cold storage. Postharvest Biol. Technol. 2018, 139, 99–105. [Google Scholar] [CrossRef]
- Cai, Z.-X.; Chen, M.-Y.; Lu, Y.-P.; Guo, Z.-J.; Zeng, Z.-H.; Liao, J.-H.; Zeng, H. Metabolomics and transcriptomics unravel the mechanism of browning resistance in Agaricus bisporus. PLoS ONE 2022, 17, e0255765. [Google Scholar] [CrossRef]
- Lin,Y. ;Zhan,L.;Shao,P.; Sun, P. Phase-change materials and exogenous melatonin treatment alleviated postharvest senescence of Agaricus bisporus by inhibiting browning and maintaining cell membrane integrity. Postharvest Biol. Technol. 2022, 192, 112009. [Google Scholar]
- [Li, L.; Kitazawa, H.; Zhang, X.H.; Zhang, L.M.; Sun, Y.; Wang, X.Y.; Liu, Z.L.; Guo, Y.Y.; Yu, S.X. Melatonin retards senescence via regulation of the electron leakage of postharvest white mushroom (Agaricus bisporus). Food Chem. 2021, 340, 127833. [Google Scholar]
- Zhang, Y.; Zhao, X.; Ma, Y.; Zhang, L.; Jiang, Y.; Liang, H.; Wang, D. Transcriptome and metabolome profiling to elucidate mechanisms underlying the blue discoloration of radish roots during storage. Food Chem. 2021, 362, 130076. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, B.; Huang, H.; Huang, W.; Zhang, Z.; Wang, Q.; Luo, H.; An, B. Metabolomic and Transcriptomic Analyses Provide Insights into Metabolic Networks during Cashew Fruit Development and Ripening. Food Chem. 2023, 404, 134765. [Google Scholar] [CrossRef]
- Li, T.; Zhang, J.; Gao, X.Y.; Chen, J.F.; Zheng, Y.F.; Gao, Y.Q.; Qiu, L.Y. The molecular mechanism for the ethylene regulation of postharvest button mushrooms maturation and senescence. Postharvest Biol. Technol. 2019, 156, 110930. [Google Scholar] [CrossRef]
- Nazir, A.; AlDhaheri, M.; Mudgil, P.; Marpu, P.; Kamal-Eldin, A. Hyperspectral imaging based kinetic approach to assess quality deterioration in fresh mushrooms (Agaricus bisporus) during postharvest storage. Food Control 2021, 131, 108298. [Google Scholar] [CrossRef]
- Meng, D.; Song, T.; Shen, L.; Zhang, X.; Sheng, J. Postharvest application of methyl jasmonate for improving quality retention of Agaricus bisporusfruit bodies. J. Agric. Food Chem. 2012, 60, 6056–6062. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tian, S.; Meng, X.; Xu, Y. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biol. Technol. 2007, 44, 300–306. [Google Scholar] [CrossRef]
- Plesoianu, A.M.; Nour, V. Effect of Some Polysaccharide-Based Edible Coatings on Fresh White Button Mushroom (Agaricus bisporus) Quality during Cold Storage. Agriculture. 2022, 12, 1491. [Google Scholar] [CrossRef]
- Yan, X.; Cheng, M.; Wang, Y.; Zhao, P.; Wang, K.; Wang, Y.; Wang, X.; Wang, J. Evaluation of film packaging containing mesoporous nanosilica and oregano essential oil for postharvest preservation of mushrooms (Agaricus bisporus). Postharvest Biol. Technol. 2023, 198, 112263. [Google Scholar] [CrossRef]
- Choi,Y. J.; Eom, H.;Yang,S.-H.;Nandre,R.;Kim,S.;Kim,M.;Oh,Y.-L.;Nakazawa,T.;Honda,Y.;Ro,H.-S. Heterokaryosis, the main obstacle in the generation of PPO1-edited Agaricus bisporus by CRISPR/Cas9 system. Sci. Hortic. 2023, 318, 112095. [Google Scholar] [CrossRef]
- Hammond, J.B.W.; Nichols, R. Changes in respiration and soluble carbohydrates during the post-harvest storage of mushrooms (Agaricus bisporus). J. Sci. Food Agric. 1975, 26, 835–842. [Google Scholar] [CrossRef]
- Mohebbi, M.; Ansarifar, E.; Hasanpour, N.; Amiryousefi, M.R. Suitability of Aloe vera and Gum Tragacanth as Edible Coatings for Extending the Shelf Life of Button Mushroom. Food Bioprocess Technol. 2012, 5, 3193–3202. [Google Scholar] [CrossRef]
- Thakur, R.R.; Shahi, N.C.; Mangaraj, S.; Lohani, U.C.; Chand, K. Development of an organic coating powder and optimization of process parameters for shelf life enhancement of button mushrooms (Agaricus bisporus). J. Food Process. Preserv. 2011, 45, e15306. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Liu, Z.; Zhao, Z.; Zhao, J.; Wang, Z.; Zhou, G.; Liu, P.; Liu, M. Transcriptome and Metabolome Profiling Unveil the Mechanisms of Ziziphus jujuba Mill. Peel Coloration. Food Chem. 2019, 312, 125903. [Google Scholar] [CrossRef] [PubMed]
- Mou, Z.L.; Wang, L.; Zeng, Z.X.; Su, X.G.; Ji, S.J.; Shan, W.; Kuang, J.; Lu, W. ; Chen,Y. ;Zhao,Y.;Chen,J. Metabolomics integrated with transcriptomics unveil the regulatory pathways of modified atmosphere packaging–maintained leaf quality of Chinese flowering cabbage, Food Chem. 2023, 405, 134910. [Google Scholar]
- Hou, J.; Yan, D.; Liu, Y.; Wang, W.; Hong, M.; He, M.; Yang,X. ; Zeng, K.;Yao, S. Global changes in metabolic pathways in endocarp of ‘Dayagan’ hybrid citrus fruit during segment drying revealed by widely targeted metabolomics and transcriptomics analysis. Postharvest Biol. Technol. 2023, 198, 112255. [Google Scholar] [CrossRef]
- Li, L.; Kiroaki, H.; Zhang, R.; Wang, X.; Zhang, L.; Yu, S.; Li, Y. New insights into the chilling injury of postharvest white mushroom (Agaricus bisporus) related to mitochondria and electron transport pathway under high O2/CO2 controlled atmospheres. Postharvest Biol. Technol. 2019, 152, 45–53. [Google Scholar] [CrossRef]
- Subramaniam, S.; Jiao, S.; Zhang, Z.; Jing, P. Impact of post-harvest processing or thermal dehydration on physiochemical, nutritional and sensory quality of shiitake mushrooms. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2560–2595. [Google Scholar] [CrossRef] [PubMed]
- [Tao, L.; Long, H.; Zhang, J.; Qi, L.; Zhang, S.; Li, T.; Li, S. Preparation and coating application of γ-polyglutamic acid hydrogel to improve storage life and quality of shiitake mushrooms. Food Control. 2021, 130, 108404. [Google Scholar]
- D. Mohapatra, Z.M. Bira, J.P. Kerry, J.M. Frías, F.A. Rodrigues, Postharvest Hardness and Color Evolution of White Button Mushrooms (Agaricus bisporus), J. Food Sci. 2010, 75, E146–E152.
- Mohapatra, D.; Bira, Z.M.; Kerry, J.P.; Frías, J.M.; Rodrigues, F.A. Postharvest hardness and color evolution of white button mushrooms (Agaricus bisporus). J. Food Sci. 2010, 75, 146–152. [Google Scholar] [CrossRef]
- Qu, T.; Li, B.; Huang, X.; Li, X.; Ding, Y.; Chen, J.; Tang, X. Effect of Peppermint Oil on the Storage Quality of White Button Mushrooms (Agaricus Bisporus). Food Bioprocess Technol. 2020, 13, 404–418. [Google Scholar] [CrossRef]
- Zhang, L.M.; Liu, Z.L.; Sun, Y.; Wang, X.M.; Li, L. Combined antioxidant and sensory effects of active chitosan/zein film containing a-tocopherol on Agaricus bisporus. Food Packag. Shelf Life 2020, 24, 100470. [Google Scholar] [CrossRef]
- Gao, M.S.; Feng, L.F.; Jiang, T.J. Browning inhibition and quality preservation of button mushroom (Agaricus bisporus) by essential oils fumigation treatment. Food Chem. 2014, 149, 107–113. [Google Scholar] [CrossRef]
- Kong, X.; Wei, B.; Gao, Z.; Zhou, Y.; Shi, F.; Zhou, X.; Zhou, Q.; Ji, S. Changes in membrane lipid composition and function accompanying chilling injury in bell peppers. Plant Cell Physiol. 2017, 59, 167–178. [Google Scholar] [CrossRef]
- Hu, Y.-H.; Chen, C.-M.; Xu, L.; Cui, Y.; Yu, X.-Y.; Gao, H.-J.; Wang, Q.; Liu, K.; Shi, Y.; Chen, Q.-X. Postharvest application of 4-methoxy cinnamic acid for extending the shelf life of mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2015, 104, 33–41. [Google Scholar] [CrossRef]
- Huang, Q.; Qian, X.; Jiang, T.; Zheng, X. Effect of chitosan and guar gum based composite edible coating on quality of mushroom (Lentinus edodes) during postharvest storage. Sci. Hortic. 2019, 253, 382–389. [Google Scholar] [CrossRef]
- [Van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The Critical Role of Phosphatidylcholine and Phosphatidylethanolamine Metabolism in Health and Disease. Biochim. Biophys. Acta (BBA)—Biomembr. 2017, 1859, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Kuk, A.C.Y.; Ding, M.; Chin, C.F.; Galam, D.L.A.; Nah, J.M.; Tan, B.C.; Yeo, H.L.; Chua, G.L.; Benke, P.I.; et al. Spns1 is a lysophospholipid transporter mediating lysosomal phospholipid salvage. Proc. Natl. Acad. Sci.USA 2022, 119, e2210353119. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-E.; Chen, W.-J.; Chang, C.-K.; Li, P.-H.; Lu, P.-L.; Hsieh, C.-W. Effect of a high voltage electrostatic field (HVEF) on the shelf life of persimmons (Diospyros kaki). LWT. 2017, 75, 236–242. [Google Scholar] [CrossRef]
- Petriccione, M.; Pagano, L.; Forniti, R.; Zampella, L.; Mastrobuoni, F.; Scortichini, M.; Mencarelli, F. Postharvest treatment with chitosan affects the antioxidant metabolism and quality of wine grape during partial dehydration. Postharvest Biol. Technol. 2018, 137, 38–45. [Google Scholar] [CrossRef]
- Yan, M.; Yuan, B.; Xie, Y.; Cheng, S.J.; Huang, H.D.; Zhang, W.; Chen, J.Q.; Cao, C.J. Improvement of postharvest quality, enzymes activity and polyphenoloxidase structure of postharvest Agaricus bisporus in response to high voltage electric field. Postharvest Biol. Technol. 2020, 166, 111230. [Google Scholar] [CrossRef]
- Cheng, R.; Li, W.; Wang, Y.; Cheng, F.; Wu, H.; Sun, Y. Low voltage electrostatic field treatment of fresh-cut pineapples with slightly acidic electrolytic water: Influence on physicochemical changes and membrane stability. Sci. Hortic. 2023, 308, 111602. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Khan, Z.U.; Mao, L.; Yin, T. Effect of nitric oxide on energy metabolism in postharvest banana fruit in response to chilling stress. Postharvest Biol. Technol. 2015, 108, 21–27. [Google Scholar] [CrossRef]
- Luo, S.; Hu, H.; Wang, Y.; Zhou, H.; Zhang, Y.; Zhang, L.; Li, P. The role of melatonin in alleviating the postharvest browning of lotus seeds through energy metabolism and membrane lipid metabolism. Postharvest Biol. Technol. 2020, 167, 111243. [Google Scholar] [CrossRef]
- Yan,Z. ; Wang, H.;Kou,X.;Wu,C.;Fan,G.;Li,T.;Zhou,D. Metabolomics analysis reveals that MeJA treatment induces postharvest blueberry resistance to Botrytis cinerea. Postharvest Biol. Technol. 2022, 194, 112075. [Google Scholar]
- Wang, K.; Yin, X.-R.; Zhang, B.; Grierson, D.; Xu, C.-J.; Chen, K.-S. Transcriptomic and metabolic analyses provide new insights into chilling injury in peach fruit. Plant Cell Environ. 2017, 40, 1531–1551. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Wang, K.; Wu, C.X.; Zhao, Y.; Yin, X.R.; Zhang, B.; Grierson, D.; Chen, K.S.; Xu, C.J. Effect of Ethylene on Cell Wall and Lipid Metabolism during Alleviation of Postharvest Chilling Injury in Peach. Cells. 2019, 8, 1612. [Google Scholar] [CrossRef]
- Gutterson, N.; Reuber, T.L. Regulation of disease resistance pathways by AP2/ERF transcription factors. Curr. Opin. Plant Biol. 2004, 7, 465–471. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




