Submitted:
07 June 2024
Posted:
10 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Culture Medium and Preparation
2.2. Substrate Utilization Spectrum
2.3. NMR Experiments
2.4. Genome Search
2.5. Analytical Methods
3. Results and Discussion
3.1. Enzymatic Activity
3.2. Degradation of Amino Acids
3.3. Degradation of Proteins
3.4. Conversion of Fatty Acids to Alcohols
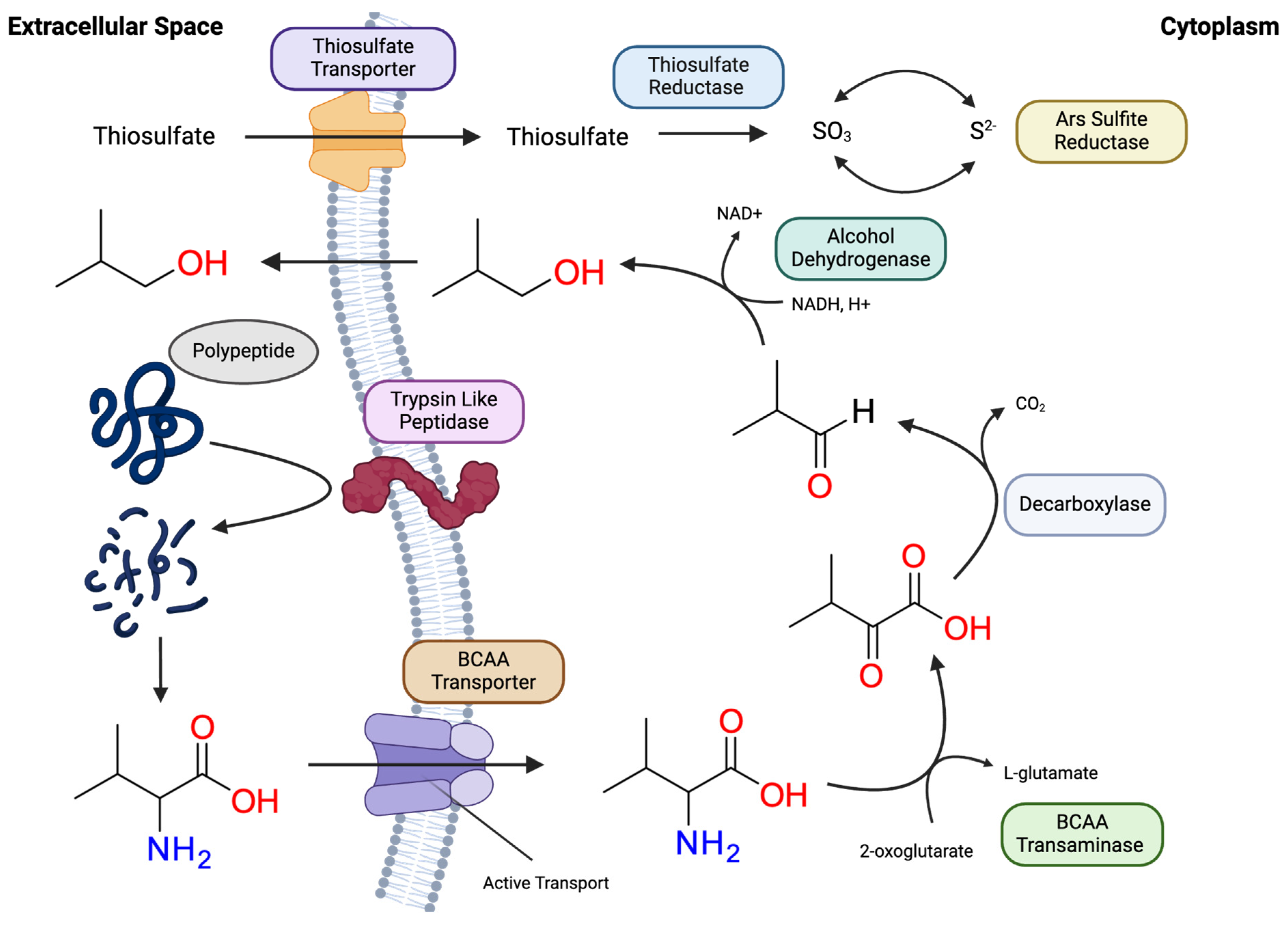
3.5. Genome and Pathway of Amino Acid Metabolism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stackebrandt, E. The Family Thermoanaerobacteraceae. In The Prokaryotes; Springer Berlin Heidelberg: Berlin, Heidelberg, 2014; pp. 413–419. [Google Scholar]
- Wiegel, J.; Tanner, R.; Rainey, R. An Introduction to the Family Clostridiaceae. In Prokaryotes; 2006; pp. 654–678.
- Collins, M.D.; Lawson, P.A.; Willems, A.; Cordoba, J.J.; Fernandez-Garayzabal, J.; Garcia, P.; Cai, J.; Hippe, H.; Farrow, J.A. The Phylogeny of the Genus Clostridium: Proposal of Five New Genera and Eleven New Species Combinations. Int J Syst Bacteriol 1994, 44, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.; Nielsen, P.; Ahring, B.K. Thermoanaerobacter mathranii Sp. Nov., an Ethanol-Producing, Extremely Thermophilic Anaerobic Bacterium from a Hot Spring in Iceland. Arch Microbiol 1997, 168, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-E.; Jain, M.K.; Lee, C.; Zeikus, J.G. Taxonomic Distinction of Saccharolytic Thermophilic Anaerobes: Description of Thermoanaerobacterium xylanolyticum Gen. Nov., Sp. Nov., and Thermoanaerobacterium saccharolyticum Gen. Nov., Sp. Nov.; Reclassification of Thermoanaerobium. Int J Syst Bacteriol 1993, 43, 41–51. [Google Scholar] [CrossRef]
- Wagner, I.D.; Wiegel, J. Diversity of Thermophilic Anaerobes. Ann N Y Acad Sci 2008, 1125, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Slobodkin, A.I.; Tourova, T.P.; Kuznetsov, B.B.; Kostrikina, N.A.; Chernyh, N.A.; Bonch-Osmolovskaya, E.A. Thermoanaerobacter siderophilus Sp. Nov., a Novel Dissimilatory Fe(III)-Reducing, Anaerobic, Thermophilic Bacterium. Int J Syst Bacteriol 1999, 49, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Kozianowski, G.; Canganella, F.; Rainey, F.A.; Hippe, H.; Antranikian, G. Purification and Characterization of Thermostable Pectate-Lyases from a Newly Isolated Thermophilic Bacterium, Thermoanaerobacter italicus Sp. Nov. Extremophiles 1997, 1, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Kublanov, I. V; Tsiroulnikov, K.B.; Kaliberda, E.N.; Rumsh, L.D.; Haertle, T.; Bonch-Osmolovskaya, E.A. Keratinase of an Anaerobic Thermophilic Bacterium Thermoanaerobacter Sp. Strain 1004-09 Isolated from a Hot Spring in the Baikal Rift Zone. Microbiology (N Y) 2009, 78, 67–75. [Google Scholar] [CrossRef]
- Bonch-Osmolovskaya, E.A.; Miroshichenko, L.M.; Chernykh, N.A.; Kostrikina, N.A.; Pikuta, E. V; Rainey, F.A. Reduction of Elemental Sulfur by Moderately Thermophilic Organotrophic Bacteria and the Description of Thermoanaerobacter sulfurophilus Sp. Nov. Mikrobiologiya 1997, 66, 581–587. [Google Scholar]
- Jessen, J.E.J.; Orlygsson, J. Production of Ethanol from Sugars and Lignocellulosic Biomass by Thermoanaerobacter J1 Isolated from a Hot Spring in Iceland. J Biomed Biotechnol 2012, 1869–1882. [Google Scholar] [CrossRef]
- Onyenwoke, R.U.; Kevbrin, V. V.; Lysenko, A.M.; Wiegel, J. Thermoanaerobacter pseudethanolicus Sp. Nov., a Thermophilic Heterotrophic Anaerobe from Yellowstone National Park. Int J Syst Evol Microbiol 2007, 57, 2191–2193. [Google Scholar] [CrossRef]
- Wiegel, J.; Ljungdahl, L.G. Thermoanaerobacter ethanolicus Gen. Nov., Spec. Nov., a New, Extreme Thermophilic, Anaerobic Bacterium. Arch Microbiol 1981, 128, 343–348. [Google Scholar] [CrossRef]
- Orlygsson, J.; Scully, S.M. Biotechnological Prospects of Thermoanerobacter AK15: End-Product Formation from Carbohydrates, Amino Acids, and Lignocellulosic and Macroalgae Hydrolysates. Int J Mol Sci 2024, 25, 3490. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, S.M.; Hamilton-Brehm, S.D.; Giannone, R.J.; Engle, N.L.; Tschaplinski, T.J.; Hettich, R.L.; Elkins, J.G. A Comparative Multidimensional LC-MS Proteomic Analysis Reveals Mechanisms for Furan Aldehyde Detoxification in Thermoanaerobacter pseudethanolicus 39E. Biotechnol Biofuels 2014, 7, 165. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, T.I.; Ahring, B.K. Evaluation of Continuous Ethanol Fermentation of Dilute-Acid Corn Stover Hydrolysate Using Thermophilic Anaerobic Bacterium Thermoanaerobacter BG1L1. Appl Microbiol Biotechnol 2007, 77, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, T.I.; Mikkelsen, M.J.; Ahring, B.K. High Ethanol Tolerance of the Thermophilic Anaerobic Ethanol Producer Thermoanaerobacter BG1L1. Cent Eur J Biol 2007, 2, 364–377. [Google Scholar] [CrossRef]
- Tomás, A.F.; Karakashev, D.; Angelidaki, I. Thermoanaerobacter pentosaceus Sp. Nov., an Anaerobic, Extreme Thermophilic, High Ethanol-Yielding Bacterium Isolated from Household Waste. Int J Syst Evol Microbiol 2013, 63, 2396–2404. [Google Scholar] [CrossRef]
- Tomás, A.F.; Karagöz, P.; Karakashev, D.; Angelidaki, I. Extreme Thermophilic Ethanol Production from Rapeseed Straw: Using the Newly Isolated Thermoanaerobacter pentosaceus and Combining It with Saccharomyces Cerevisiae in a Two-Step Process. Biotechnol Bioeng 2013, 110, 1574–1582. [Google Scholar] [CrossRef]
- Sittijunda, S.; Tomas, A.F.; Reungsang, A.; O-Thong, S.; Angelidaki, I. Ethanol Production from Glucose and Xylose by Immobilized Thermoanaerobacter pentosaceus at 70°C in an up-Flow Anaerobic Sludge Blanket (UASB) Reactor. Bioresour Technol 2013, 143, 598–607. [Google Scholar] [CrossRef] [PubMed]
- McInerney, M.J. Anaerobic Hydrolysis and Fermentation of Fats and Proteins. In Biology of Anaerobic Microorganisms; Zehnder, A.J.B., Ed.; John Wiley & Sons, Ltd.: New York, 1988; pp. 373–415.
- Andreesen, J.R.; Bahl, H.; Gottschalk, G. Introduction to the Physiology and Biochemistry of the Genus Clostridium. In Clostridia; Minton, N.P., Clarke, D.J., Eds.; Plenum Press: New York, 1989; pp. 27–62.
- Reid, S.J.; Stutz, H.E. Nitrogen Assimilation in Clostridia. In Handbook of Clostridia; Durre, P., Ed.; CRC Press: Boca Raton, 2005; pp. 239–260.
- Mitruka, B.M.; Costilow, R.N. Arginine and Ornithine Catabolism by Clostridium botulinum. J Bacteriol 1967, 93, 295–301. [Google Scholar] [CrossRef]
- Tjaberg, T.B. Proteases of Clostridium botulinum II. The Relationship Between Growth Medium and the Production of Proteases by Clostridium botulinum Types A, B, C, D, E and F. Acta Vet Scand 1973, 14, 193–200. [Google Scholar] [CrossRef]
- Tjaberg, T.B. Proteases of Clostridium botulinum I. Classification of Proteases and Literature Survey. Acta Vet Scand 1973, 14, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, B.R.; Sugiyama, H. Isolation and Characterization of Protease from Clostridium botulinum Type B. Biochim Biophys Acta 1972, 268, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Lynt, R.K.; Kautter, D.A.; Solomon, H.M. Differences and Similarities Among Proteolytic and Nonproteolytic Strains of Clostridium botulinum Types A, B, E and F: A Review. J Food Prot 1982, 45, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Hapchuk, L.T.; Perason, A.M.; Price, J.F. Effect of Proteolytic Enzyme Production by Clostridium perfringens upon Porcine Muscle. Food Chem 1979, 4, 213–223. [Google Scholar] [CrossRef]
- Laanbroek, H.J.; Smit, A.J.; Nulend, G.K.; Veldkamp, H. Competition for L-Glutamate Between Specialised and Versatile Clostridium Species. Arch Microbiol 1979, 66, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Deklevat, M.L.; Dasgupta, B.R. Purification and Characterization of a Protease from Clostridium botulinum Type A That Nicks Single-Chain Type A Botulinum Neurotoxin into the Di-Chain Form. J Bacteriol 1990, 172, 2498–2503. [Google Scholar] [CrossRef] [PubMed]
- Elsden, S.R.; Hilton, M.G. Volatile Acid Production from Threonine, Valine, Leucine and Isoleucine by Clostridia. Arch Microbiol 1978, 117, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Elsden, S.R.; Hilton, M.G. Amino Acid Utalization Patterns in Clostridial Taxonomy. Arch Microbiol 1979, 123, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Elsden, S.R.; Hilton, M.G.; Waller, J.M. The End Products of the Metabolism of Aromatic Amino Acids by Clostridia. Arch Microbiol 1976, 107, 283–288. [Google Scholar] [CrossRef]
- Schwartz, A.C.; Schäfer, R. New Amino Acids, and Heterocyclic Compounds Participating in the Stickland Reaction of Clostridium sticklandii. Archives of Mcrobiology 1973, 276, 267–276. [Google Scholar] [CrossRef]
- Stickland, L.H. Studies in the Metabolism of the Strict Anaerobes (Genus Clostridium). The Oxidation of Alanine by Cl. sporogenes. IV. The Reduction of Glycine by Cl. sporogenes. Biochemical Journal 1935, 29, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Stickland, L.H. CCXXXII. Studies in the Metabolism of the Strict Anaerobes (Genus Clostridium). I. The Chemical Reactions by Which Cl. sporogenes Obtains Its Energy. Biochemical Journal 1934, 28, 1746–1759. [Google Scholar] [CrossRef] [PubMed]
- Tarlera, S.; Stams, A.J.M. Degradation of Proteins and Amino Acids by Caloramator proteoclasticus in Pure Culture and in Coculture with Methanobacterium Thermoformicicum Z245. Appl Microbiol Biotechnol 1999, 53, 133–138. [Google Scholar] [CrossRef]
- Chrisostomos, S.; Patel, B.K.C.; Dwivedi, P.P.; Denman, S.E. Caloramator indicus Sp. Nov., a New Thermophilic Anaerobic Bacterium Isolated from the Deep-Seated Nonvolcanically Heated Waters of an Indian Artesian Aquifer. Int J Syst Bacteriol 1996, 46, 497–501. [Google Scholar] [CrossRef]
- Riessen, S.; Antranikian, G. Isolation of Thermoanaerobacter keratinophilus Sp. Nov., a Novel Thermophilic, Anaerobic Bacterium with Keratinolytic Activity. Extremophiles 2001, 5, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Fardeau, M.L.; Patel, B.K.C.; Magot, M.; Ollivier, B. Utilization of Serine, Leucine, Isoleucine, and Valine by Thermoanaerobacter Brockii in the Presence of Thiosulfate or Methanobacterium Sp. as Electron Accepters. Anaerobe 1997, 3, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Faudon, C.; Fardeau, M.L.; Heim, J.; Patel, B.; Magot, M.; Ollivier, B.; Orstom, D.M.; Provence, U. De; Hugo, V.; Cedex, M.; et al. Peptide and Amino Acid Oxidation in the Presence of Thiosulfate by Members of the Genus Thermoanaerobacter. Curr Microbiol 1995, 31, 152–157. [Google Scholar] [CrossRef]
- Örlygsson, J. The Role of Interspecies Hydrogen Transfer on Thermophilic Protein and Amino Acid Metabolism. Ph.D., Swedish University of Agricultural Sciences, 1994.
- Leigh, J.A.; Wolfe, R.S. Acetogenium kivui Gen. Nov., Sp. Nov., a Thermophilic Acetogenic Bacterium. Int J Syst Bacteriol 1983, 33, 866. [Google Scholar] [CrossRef]
- Ollivier, B.M.; Mah, R.A.; Ferguson, T.J.; Boone, D.R.; Garcia, J.L.; Robinson, R. Emendation of the Genus Thermobacteroides: Thermobacteroides proteolyticus Sp. Nov. a Proteolytic Acetogen from a Methanogenic Enrichment. International Journal of Systematic and Evolutionary Bacteriology 1985, 35, 425–428. [Google Scholar] [CrossRef]
- Scully, S.M.; Orlygsson, J. Branched-Chain Amino Acid Catabolism of Thermoanaerobacter Strain AK85 and the Influence of Culture Conditions on Branched-Chain Alcohol Formation. Amino Acids 2019, 51. [Google Scholar] [CrossRef]
- Scully, S.M.; Orlygsson, J. Branched-Chain Amino Acid Catabolism of Thermoanaerobacter pseudoethanolicus Reveals Potential Route to Branched-Chain Alcohol Formation. Extremophiles 2020, 24, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Scully, S.M.; Orlygsson, J. Branched-Chain Alcohol Formation from Branched-Chain Amino Acids by Thermoanaerobacter brockii and Thermoanaerobacter yonseiensis. Anaerobe 2014, 30, 82–84. [Google Scholar] [CrossRef]
- Scully, S.M.; Orlygsson, J. Amino Acid Metabolism of Thermoanaerobacter Strain AK90: The Role of Electron-Scavenging Systems in End Product Formation. J Amino Acids 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Sonne-Hansen, J.; Mathrani, M.; Ahring, B.K. Xylanolytic Anaerobic Thermophiles from Icelandic Hot-Springs. Appl Microbiol Biotechnol 1993, 38, 537–541. [Google Scholar] [CrossRef]
- Carlier, J.P.; Bonne, I.; Bedora-Faure, M. Isolation from Canned Foods of a Novel Thermoanaerobacter Species Phylogenetically Related to Thermoanaerobacter mathranii (Larsen 1997): Emendation of the Species Description and Proposal of Thermoanaerobacter mathranii Subsp. Alimentarus Su. Anaerobe 2006, 12, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Sveinsdottir, M.; Beck, S.R.; Orlygsson, J. Ethanol Production from Monosugars and Lignocellulosic Biomass by Thermophilic Bacteria Isolated from Icelandic Hot Springs. Icelandic Agricultural Science 2009, 22, 45–58. [Google Scholar]
- Hungate, R.E. A Roll Tube Method for Cultivation of Strict Anaerobes. In Methods in Microbiology; Norris, J.R., Ribbons, Eds.; Academic Press: New York, 1969; pp. 117–132. [Google Scholar]
- Miller, T.L.; Wolin, M.J. A Serum Bottle Modification of the Hungate Technique for Cultivating Obligate Anaerobes. Appl Microbiol 1974, 27, 985–987. [Google Scholar] [CrossRef]
- Scully, S.M. Amino Acid and Related Catabolism of Thermoanaerobacter Species. Ph.D., University of Iceland, 2019.
- Orlygsson, J.; Baldursson, S.R.B. Phylogenetic and Physiological Studies of Four Hydrogen-Producing Thermoanareobes from Icelandic Geothermal Areas. Icelandic Agricultural Science 2007, 93–105. [Google Scholar]
- Cline, J.D. Spectrophotometric Determination of Hydrogen Sulfide in Natural Waters. Limnol Oceanogr 1969, 14, 454–458. [Google Scholar] [CrossRef]
- Scully, S.M.; Orlygsson, J. Branched-Chain Amino Acid Catabolism of Thermoanaerobacter Strain AK85 and the Influence of Culture Conditions on Branched-Chain Alcohol Formation. Amino Acids 2019, Accepted. [Google Scholar] [CrossRef]
- Seddon, S. V; Hemingway, I.; Borriello, S.P. Hydrolytic Enzyme Production by Clostridium Difficile and Its Relationship to Toxin Production and Virulence in the Hamster Model. Journal of Medicinal Microbiology 1990, 31, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Hitschler, L.; Kuntz, M.; Langschied, F.; Basen, M. Thermoanaerobacter Species Differ in Their Potential to Reduce Organic Acids to Their Corresponding Alcohols. Appl Microbiol Biotechnol 2018, 102, 8465–8476. [Google Scholar] [CrossRef] [PubMed]
- Scully, S.M.; Brown, A.; Ross, A.B.; Orlygsson, J. Biotransformation of Organic Acids to Their Corresponding Alcohols by Thermoanaerobacter pseudoethanolicus. Anaerobe 2019, 57. [Google Scholar] [CrossRef] [PubMed]
- Scully, S.M.; Brown, A.E.; Mueller-Hilger, Y.; Ross, A.B.; Örlygsson, J. Influence of Culture Conditions on the Bioreduction of Organic Acids to Alcohols by Thermoanaerobacter pseudoethanolicus. Microorganisms 2021, 9, 1–24. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Dashti, M.; Prange, A.; Rainey, F.A.; Rohde, M.; Whitman, W.B.; Wiegel, J. Thermoanaerobacter sulfurigignens Sp. Nov., an Anaerobic Thermophilic Bacterium That Reduces 1 M Thiosulfate to Elemental Sulfur and Tolerates 90 MM Sulfite. Int J Syst Evol Microbiol 2007, 57, 1429–1434. [Google Scholar] [CrossRef]
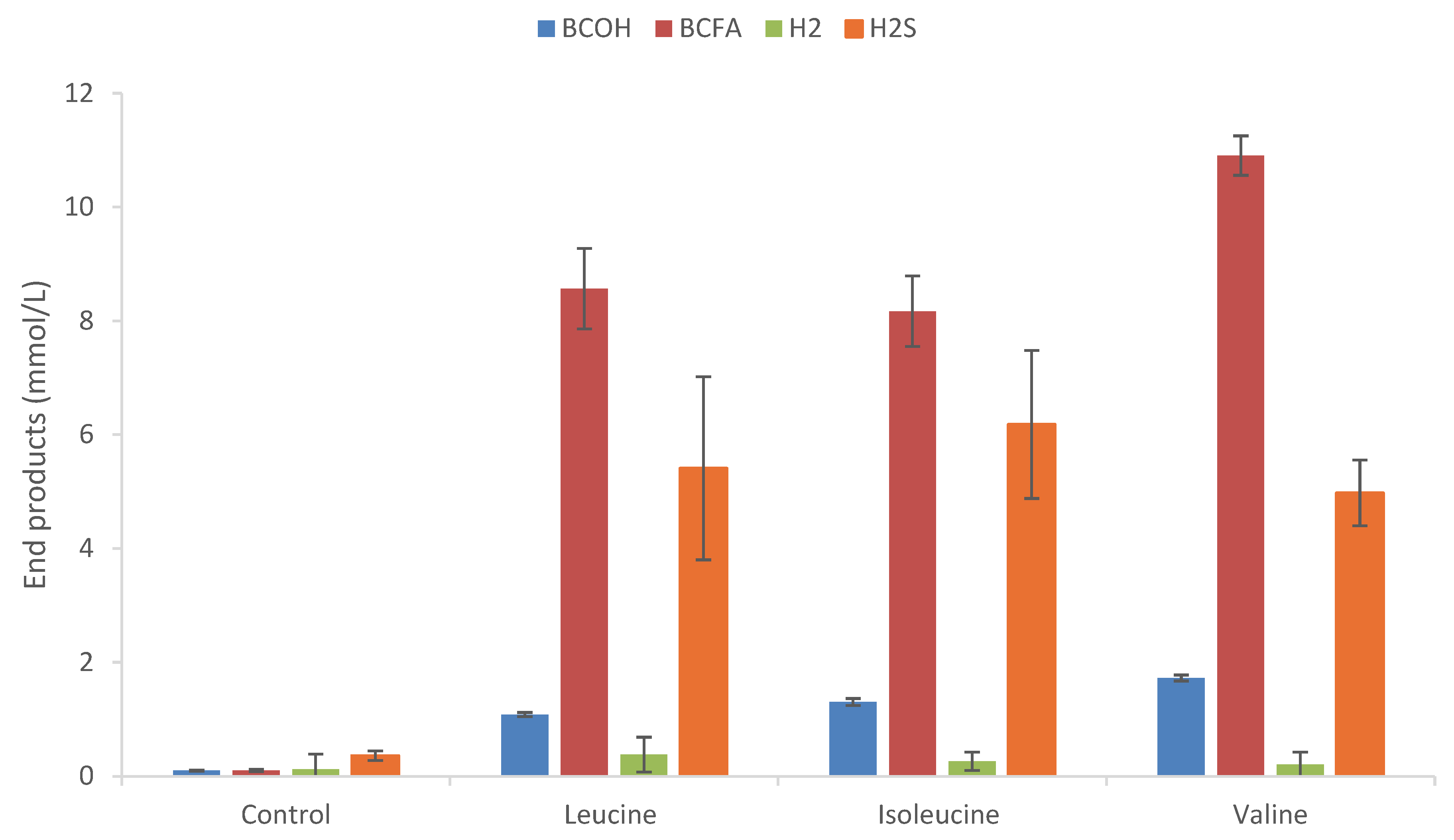
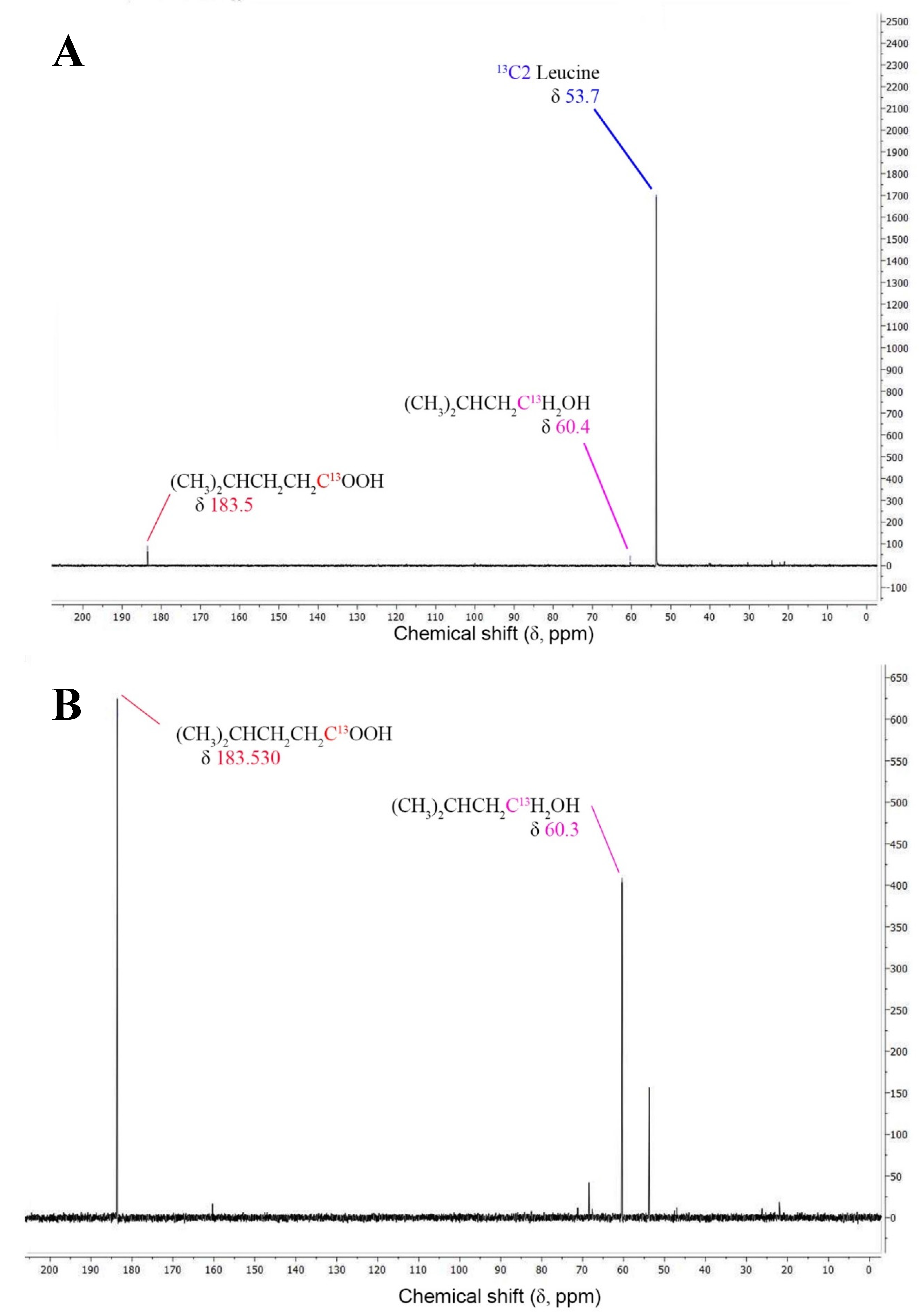
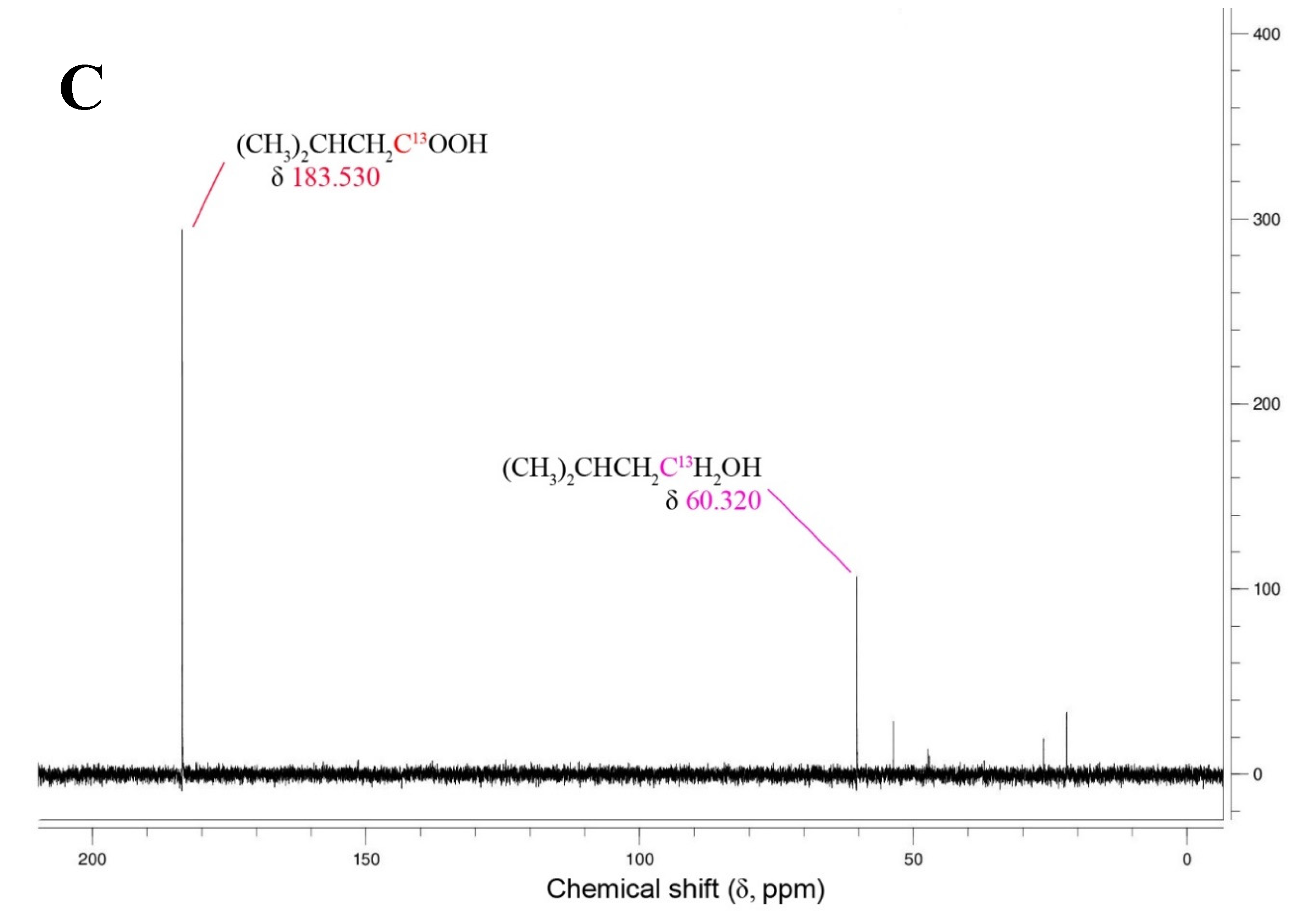
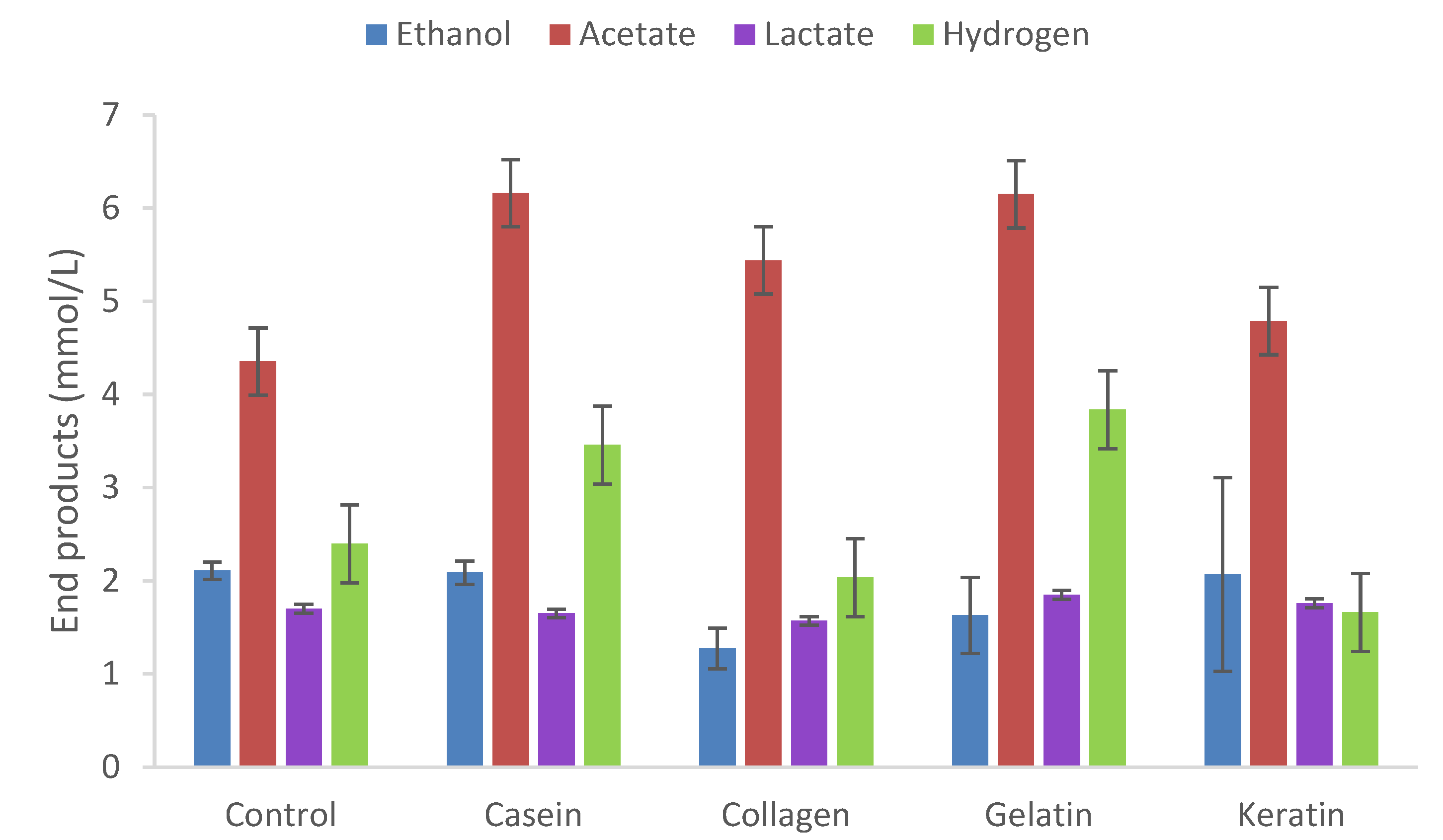
| Strain | Substrate | RCOOH (mM) | ROH (mM) | ROH/RCOOH ratio | Reference |
| T. mathranii (DSM 11426) | Leucine + S2O3 | 8.6 | 2.8 | 0.33 | This study |
| T. pseudethanolicus (DSM 2355) | Leucine + S2O3 | 10.3 | 1.2 | 0.12 | (Scully & Orlygsson, 2020) |
| Thermoanaerobacter brockii (DSM 1457) | Leucine + S2O3 | 13.6 | 1.5 | 0.11 | (Scully & Orlygsson, 2014) |
| T. ethanolicus (DSM 2246) | Leucine + S2O3 | 5.1 | 0.6 | 0.12 | (Scully et al., 2015) |
| Thermoanaerobacter strain AK68 | Leucine + S2O3 | 13.0 | 2.5 | 0.15 | (Scully et al., 2015) |
| Thermoanaerobacter strain AK85 | Leucine + S2O3 | 10.6 | 1.4 | 0.13 | (Scully & Orlygsson, 2019) |
| Thermoanaerobacter strain AK90 | Leucine + S2O3 | 9.7 | 3.5 | 0.36 | (Scully & Orlygsson, 2015) |
| Thermoanaerobacter strain AK152 | Leucine + S2O3 | 8.8 | 2.3 | 0.26 | (Scully et al., 2015) |
| Gene | Pathway(s) | T. mathranii | T. pseudethanolicus |
| Bifunctional aldehyde alcohol dehydrogenase | BCAA fermenation RCOOH reduction Ethanol fermentation |
Y | Y |
| Aldehyde ferredoxin oxidoreductase | RCOOH reduction Ethanol fermentation |
N | Y |
| Alcohol dehydrogenase | BCAA fermenation RCOOH reduction Ethanol fermentation |
Y [4] | Y [4] |
| BCAA transaminase | BCAA fermentation | Y | Y |
| BCAA ABC transporter permease | BCAA fermentation | Y | N |
| Oxaloacetate decarboxylase subunit alpha | BCAA fermentation | Y | Y |
| Butyrate kinase | RCOOH reduction | Y [2] | Y [2] |
| Butyryl phosphotransferase | RCOOH reduction | Y | Y |
| Pyruvate:ferredoxin oxidoreductase | Pyruvate fermenation | Y [2] | Y [2] |
| Acetate kinase | Pyruvate fermenation | Y | Y |
| Acetyl phosphotransferase | Pyruvate fermenation | Y | Y |
| YeeE/YedE thiosulfate transporter family protein | Thiosulfate utilization | Y [2] | Y [3] |
| Thiosulfate reductase | Thiosulfate utilization | NA | NA |
| Ars sulfite reductase* | Thiosulfate utilization | Y | Y |
| Trypsin-like peptidase | Protease | Y [2] | Y [2] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





