Submitted:
08 June 2024
Posted:
10 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
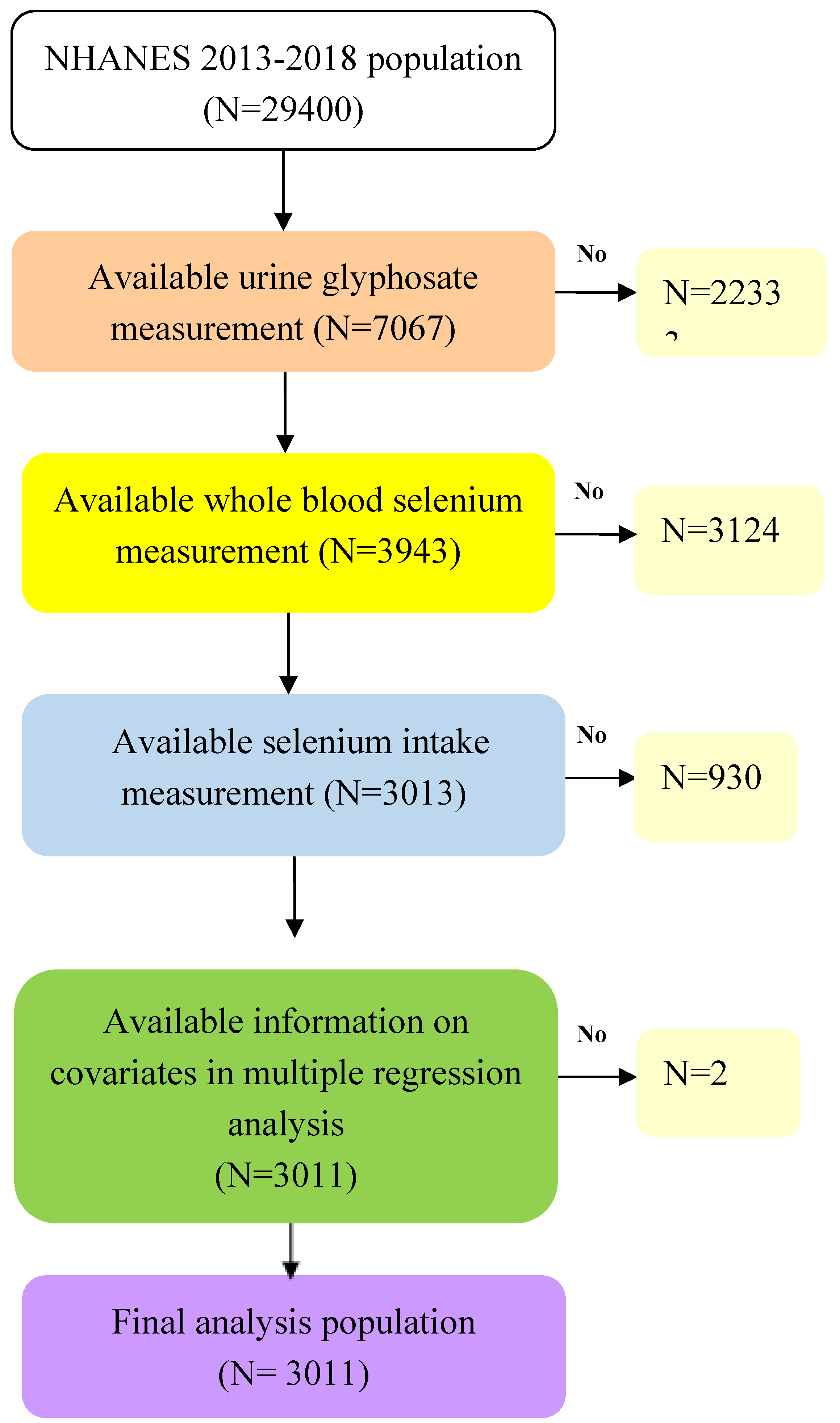
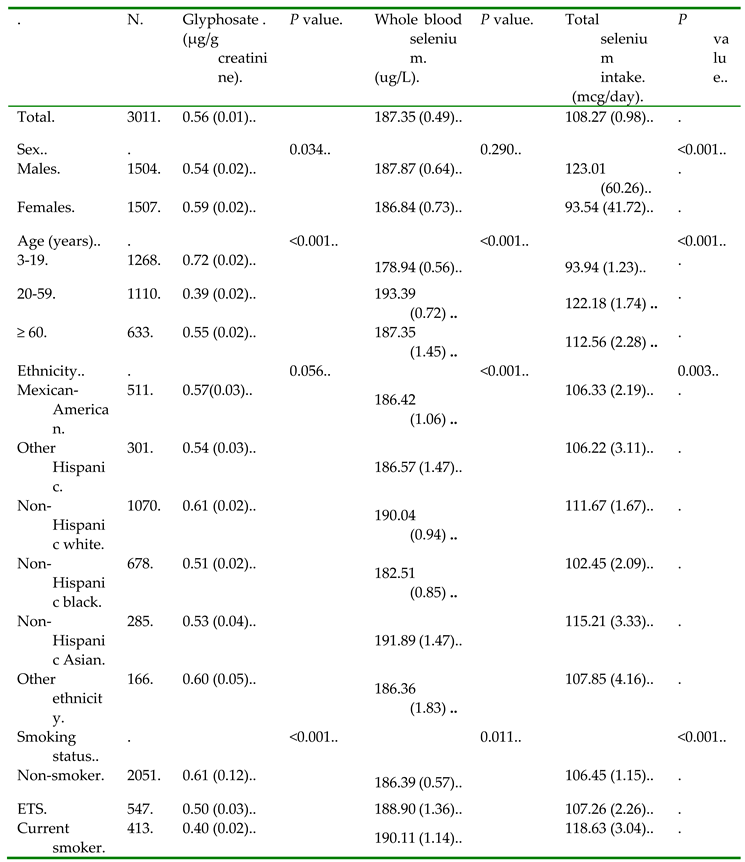
2.1. Study Population
2.2. Measurement of Urinary Glyphosate Levels
2.3. Measurement of Whole Blood Selenium
2.4. Covariates
2.3. Statistics
3. Results
4. Discussion
4. Conclusions
Funding
Author’s contributions
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Competing interests
References
- Wang, J.; Yang, L.; Li, H.; Li, Y.; Wei, B. Dietary selenium intake based on the Chinese Food Pagoda: The influence of dietary patterns on selenium intake. Nutrition journal 2018, 17, 50. [Google Scholar] [CrossRef] [PubMed]
- Combs, G.F., Jr. Biomarkers of selenium status. Nutrients 2015, 7, 2209–2236. [Google Scholar] [CrossRef]
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. The British journal of nutrition 2008, 100, 254–268. [Google Scholar] [CrossRef]
- Lacroix, R.; Kurrasch, D.M. Glyphosate toxicity: In vivo, in vitro, and epidemiological evidence. Toxicological Sciences 2023, 192, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.J.; Kania-Korwel, I.; Fagan, J.; McEvoy, L.K.; Laughlin, G.A.; Barrett-Connor, E. Excretion of the Herbicide Glyphosate in Older Adults Between 1993 and 2016. Jama 2017, 318, 1610–1611. [Google Scholar] [CrossRef]
- Hsiao, C.C.; Yang, A.M.; Wang, C.; Lin, C.Y. Association between glyphosate exposure and cognitive function, depression, and neurological diseases in a representative sample of US adults: NHANES 2013-2014 analysis. Environ Res 2023, 237, 116860. [Google Scholar] [CrossRef]
- Barnor, K.; Caton, J.; Miljkovic, D. The Role of Funding on Research and Science: The Impact of Glyphosate Herbicides on Health and the Environment. Journal of Policy Modeling 2023. [Google Scholar] [CrossRef]
- Fang, Y.W.; Wang, C.; Lin, C.Y. Association between urinary glyphosate levels and hand grip strength in a representative sample of US adults: NHANES 2013-2014. Front Public Health 2024, 12, 1352570. [Google Scholar] [CrossRef]
- Qi, X.; Huang, Q.; Chen, X.; Qiu, L.; Wang, S.; Ouyang, K.; Chen, Y. Associations between urinary glyphosate and diabetes mellitus in the US general adult: A cross-sectional study from NHANES 2013-2016. Environ Sci Pollut Res Int 2023, 30, 124195–124203. [Google Scholar] [CrossRef]
- Chang, M.H.; Chu, P.L.; Wang, C.; Lin, C.Y. Association between Glyphosate Exposure and Erythrograms in a Representative Sample of US Adults: NHANES 2013-2014. Environ Sci Pollut Res Int 2023, 30, 91207–91215. [Google Scholar] [CrossRef]
- Mertens, M.; Höss, S.; Neumann, G.; Afzal, J.; Reichenbecher, W. Glyphosate, a chelating agent—Relevant for ecological risk assessment? Environmental Science and Pollution Research 2018, 25, 5298–5317. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Lydon, J.; Koskinen, W.C.; Moorman, T.B.; Chaney, R.L.; Hammerschmidt, R. Glyphosate effects on plant mineral nutrition, crop rhizosphere microbiota, and plant disease in glyphosate-resistant crops. J Agric Food Chem 2012, 60, 10375–10397. [Google Scholar] [CrossRef] [PubMed]
- Tsui, M.T.K.; Wang, W.-X.; Chu, L.M. Influence of glyphosate and its formulation (Roundup®) on the toxicity and bioavailability of metals to Ceriodaphnia dubia. Environmental Pollution 2005, 138, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.M.; de Oliveira, J.M.; de Oliveira, V.M.; de Oliveira, I.M.; Torres, Y.R.; Bargi-Souza, P.; Martino Andrade, A.J.; Romano, M.A. Could Glyphosate and Glyphosate-Based Herbicides Be Associated With Increased Thyroid Diseases Worldwide? Front Endocrinol (Lausanne) 2021, 12, 627167. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.A.; Schrödl, W.; Aldin, A.A.; Hafez, H.M.; Krüger, M. The effect of glyphosate on potential pathogens and beneficial members of poultry microbiota in vitro. Curr Microbiol 2013, 66, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Pessione, E. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front Cell Infect Microbiol 2012, 2, 86. [Google Scholar] [CrossRef] [PubMed]
- Jakob, F.; Becker, K.; Paar, E.; Ebert-Duemig, R.; Schütze, N. Expression and regulation of thioredoxin reductases and other selenoproteins in bone. Methods in enzymology 2002, 347, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Cao, J.J.; Combs, G.F., Jr. Selenium in bone health: Roles in antioxidant protection and cell proliferation. Nutrients 2013, 5, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, M.; Huras, B.; Bukowska, B. The effect of metabolites and impurities of glyphosate on human erythrocytes (in vitro). Pestic Biochem Physiol 2014, 109, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Bali, Y.A.; Kaikai, N.E.; Ba-M'hamed, S.; Bennis, M. Learning and memory impairments associated to acetylcholinesterase inhibition and oxidative stress following glyphosate based-herbicide exposure in mice. Toxicology 2019, 415, 18–25. [Google Scholar] [CrossRef]
- Arrigo, E.; Gilardi, S.; Muratori, L.; Raimondo, S.; Mancardi, D. Biological effects of sub-lethal doses of glyphosate and AMPA on cardiac myoblasts. Frontiers in Physiology 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- CDC. NHANES 2017-2018. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2017 (accessed on May 18).
- Schütze, A.; Morales-Agudelo, P.; Vidal, M.; Calafat, A.M.; Ospina, M. Quantification of glyphosate and other organophosphorus compounds in human urine via ion chromatography isotope dilution tandem mass spectrometry. Chemosphere 2021, 274, 129427. [Google Scholar] [CrossRef] [PubMed]
- CDC. 2017-2018 Data Documentation, Codebook, and Frequencies: Glyphosate (GLYP). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/SSGLYP_J.htm (accessed on May 18).
- CDC. Lead, Cadmium, Total Mercury, Selenium, & Manganese - Blood. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/PBCD_J.htm (accessed on June 5).
- CDC. 2017-2018 Data Documentation, Codebook, and Frequencies. Smoking - Cigarette Use. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/SMQ_J.htm (accessed on May 18).
- CDC. 2017-2018 Dietary Data - Continuous NHANES. Available online: https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Dietary&CycleBeginYear=2017 (accessed on May 18).
- CDC. ANALYTIC AND REPORTING GUIDELINES: The National Health and Nutrition Examination Survey (NHANES). Available online: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf (accessed on.
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. Jama 2008, 300, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Chen, P.C.; Lin, Y.C.; Lin, L.Y. Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults. Diabetes care 2009, 32, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Kandhro, F.; Kazi, T.G.; Afridi, H.I.; Baig, J.A. Compare the nutritional status of essential minerals in milk of different cattle and humans: Estimated daily intake for children. Journal of Food Composition and Analysis 2022, 105, 104214. [Google Scholar] [CrossRef]
- NIH. Selenium. Available online: https://ods.od.nih.gov/factsheets/Selenium-Consumer/ (accessed on June 2).
- Gillezeau, C.; van Gerwen, M.; Shaffer, R.M.; Rana, I.; Zhang, L.; Sheppard, L.; Taioli, E. The evidence of human exposure to glyphosate: A review. Environmental Health 2019, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Duarte, S.C.; Costa, E.; Pereira, A.; Silva, L.J.G.; Almeida, A.; Lino, C.; Pena, A. Urine biomonitoring of glyphosate in children: Exposure and risk assessment. Environ Res 2021, 198, 111294. [Google Scholar] [CrossRef] [PubMed]
- Trasande, L.; Aldana, S.I.; Trachtman, H.; Kannan, K.; Morrison, D.; Christakis, D.A.; Whitlock, K.; Messito, M.J.; Gross, R.S.; Karthikraj, R.; et al. Glyphosate exposures and kidney injury biomarkers in infants and young children. Environ Pollut 2020, 256, 113334. [Google Scholar] [CrossRef]
- Eskenazi, B.; Gunier, R.B.; Rauch, S.; Kogut, K.; Perito, E.R.; Mendez, X.; Limbach, C.; Holland, N.; Bradman, A.; Harley, K.G.; et al. Association of Lifetime Exposure to Glyphosate and Aminomethylphosphonic Acid (AMPA) with Liver Inflammation and Metabolic Syndrome at Young Adulthood: Findings from the CHAMACOS Study. Environ Health Perspect 2023, 131, 37001. [Google Scholar] [CrossRef]
- Combs, G.F., Jr.; Watts, J.C.; Jackson, M.I.; Johnson, L.K.; Zeng, H.; Scheett, A.J.; Uthus, E.O.; Schomburg, L.; Hoeg, A.; Hoefig, C.S.; et al. Determinants of selenium status in healthy adults. Nutrition journal 2011, 10, 75. [Google Scholar] [CrossRef]
- Burk, R.F.; Norsworthy, B.K.; Hill, K.E.; Motley, A.K.; Byrne, D.W. Effects of chemical form of selenium on plasma biomarkers in a high-dose human supplementation trial. Cancer epidemiology, biomarkers & prevention : A publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2006, 15, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Longnecker, M.P.; Stram, D.O.; Taylor, P.R.; Levander, O.A.; Howe, M.; Veillon, C.; McAdam, P.A.; Patterson, K.Y.; Holden, J.M.; Morris, J.S.; et al. Use of selenium concentration in whole blood, serum, toenails, or urine as a surrogate measure of selenium intake. Epidemiology (Cambridge, Mass.) 1996, 7, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Clausen, J.; Nielsen, S.A. Comparison of whole blood selenium values and erythrocyte glutathione peroxidase activities of normal individuals on supplementation with selenate, selenite, L-selenomethionine, and high selenium yeast. Biological trace element research 1988, 15, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Sempértegui, F.; Estrella, B.; Vallejo, W.; Tapia, L.; Herrera, D.; Moscoso, F.; Cerón, G.; Griffiths, J.K.; Hamer, D.H. Selenium serum concentrations in malnourished Ecuadorian children: A case-control study. International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. Journal international de vitaminologie et de nutrition 2003, 73, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Rose, A.H.; Hoffmann, P.R. The role of selenium in inflammation and immunity: From molecular mechanisms to therapeutic opportunities. Antioxidants & redox signaling 2012, 16, 705–743. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, N.; kelishadi, M.R.; Asbaghi, O.; Naeini, F.; Afsharfar, M.; Mirzadeh, E.; Naserizadeh, S.k. Selenium supplementation and oxidative stress: A review. PharmaNutrition 2021, 17, 100263. [Google Scholar] [CrossRef]
- Sidthilaw, S.; Sapbamrer, R.; Pothirat, C.; Wunnapuk, K.; Khacha-ananda, S. Effects of exposure to glyphosate on oxidative stress, inflammation, and lung function in maize farmers, Northern Thailand. BMC Public Health 2022, 22, 1343. [Google Scholar] [CrossRef] [PubMed]
- Chang, V.C.; Andreotti, G.; Ospina, M.; Parks, C.G.; Liu, D.; Shearer, J.J.; Rothman, N.; Silverman, D.T.; Sandler, D.P.; Calafat, A.M.; et al. Glyphosate exposure and urinary oxidative stress biomarkers in the Agricultural Health Study. J Natl Cancer Inst 2023, 115, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Eaton, J.L.; Cathey, A.L.; Fernandez, J.A.; Watkins, D.J.; Silver, M.K.; Milne, G.L.; Velez-Vega, C.; Rosario, Z.; Cordero, J.; Alshawabkeh, A.; et al. The association between urinary glyphosate and aminomethyl phosphonic acid with biomarkers of oxidative stress among pregnant women in the PROTECT birth cohort study. Ecotoxicol Environ Saf 2022, 233, 113300. [Google Scholar] [CrossRef]
- Vasseur, C.; Serra, L.; El Balkhi, S.; Lefort, G.; Ramé, C.; Froment, P.; Dupont, J. Glyphosate presence in human sperm: First report and positive correlation with oxidative stress in an infertile French population. Ecotoxicology and Environmental Safety 2024, 278, 116410. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Ima-Nirwana, S.; Chin, K.Y. Are Oxidative Stress and Inflammation Mediators of Bone Loss Due to Estrogen Deficiency? A Review of Current Evidence. Endocr Metab Immune Disord Drug Targets 2020, 20, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Kaboli Kafshgiri, S.; Farkhondeh, T.; Miri-Moghaddam, E. Glyphosate effects on the female reproductive systems: A systematic review. Rev Environ Health 2022, 37, 487–500. [Google Scholar] [CrossRef] [PubMed]
| Selenium intake (mcg/day) .. | ||||
| Food intake | Total intake | |||
| Continuous variables | Adjusted β (SE) | P value | Adjusted β (SE) | P value |
| Ln-glyphosate (µg/L) | -1.626 (1.609) | 0.318 | -2.567 (1.973) | 0.200 |
| Age (years) | 0.171 (0.054) | 0.003 | 0.366 (0.063) | <0.001 |
| Urinary creatinine (g/L) | -0.545 (1.584) | 0.732 | -1.651 (2.006) | 0.415 |
| Whole blood selenium (ug/L) | ||||
| Model 1 | Model 2 | |||
| Continuous variables | Adjusted β (SE) | P value | Adjusted β (SE) | P value |
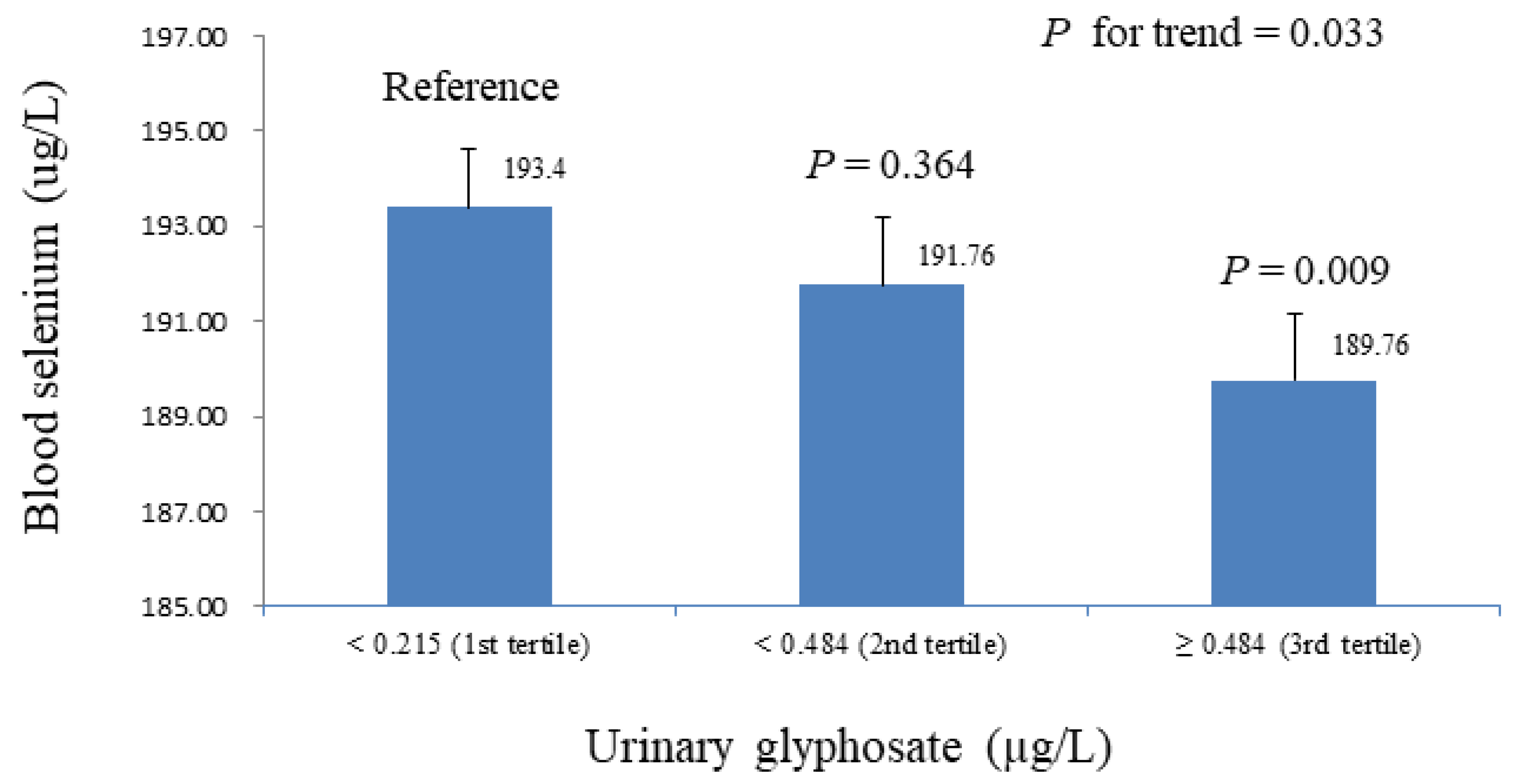
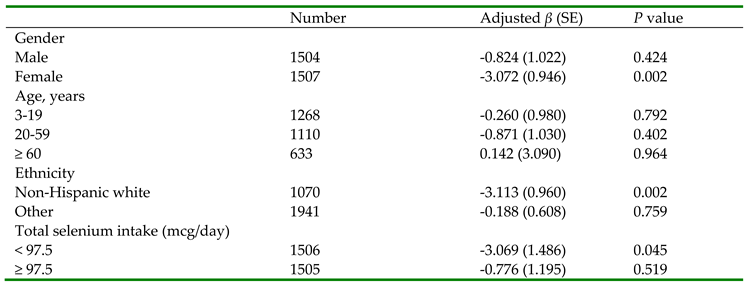
| Ln-glyphosate (µg/L) | -2.163 (0.618) | 0.001 | -1.984 (0.639) | 0.003 |
| Age (years) | 0.221(0.043) | <0.001 | 0.196 (0.045) | <0.001 |
| Urinary creatinine (g/L) | 0.605 (1.077) | 0.577 | 0.721 (1.046) | 0.494 |
| Total selenium intake (mcg/day) | 0.070 (0.025) | 0.008 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





