1. Introduction
The corpus luteum (CL) is a transient gland resulting from the transformation of the postovulatory ovarian follicle that is vital in vertebrate reproduction. It constitutes the primary source of progesterone, a hormone essential to sustain eutherian pregnancy. The CL’s lifespan determines the length of the luteal phase of the ovarian cycle, characterized as a progesterone-dominated phase following an estrogen-dominated follicular phase. The loss of the CL’s secretory activity due to regression at the end of each cycle enables the initiation of a new cycle. Therefore, CL’s lifespan is a major determining factor of the pace of vertebrate life history. Placental mammals exhibit high interspecific variation in mechanisms of CL lifespan regulation, namely the mechanisms that prolong its lifespan during pregnancy, ensuring a stable progesterone supply, or those that shorten it during the non-pregnant cycle.
Most well-known regulators of CL lifespan are extra-ovarian, deriving from the maternal pituitary, the uterus, or the fetal placenta. Mechanisms that extend CL lifespan by preventing regression (“rescue”) after fertilization are known as mechanisms for maternal recognition of pregnancy (MRP). Although these exogenous sources of CL regulation seem unique to Placental mammals, the CL itself is shared among vertebrates. The origin of this gland in early chordates added a phase to the pre-existing ovarian cycle, which at the root of vertebrates consisted of follicle maturation, ovulation, and expelling the eggs from the body. Before the multiple origins of internal fertilization in vertebrates (
Figure 1), the CL might have initially enabled the short internal embryo retention associated with internal fertilization. Upon the transition to viviparity, however, it acquired a more distinct supportive role: in non-mammalian vertebrates, despite the independent origins of viviparity, a clear trend points to the CL lifespan of viviparous species being longer than in the oviparous species of each class.
In this review, we identify the key physiological innovations in CL regulation and elaborate on the context and the role that these innovations may have played in the evolution of eutherian pregnancy. To achieve this, we synthesize the literature on CL regulation in a broad vertebrate phylogenetic context, starting with a section on the origin of the CL as an endocrine gland in early vertebrates, followed by review of available knowledge about CL’s regulation in nonmammalian and mammalian vertebrates. We pay special attention to the distinct roles of CL in independent origins of vertebrate viviparity. Eutherians are the only taxon in which full gestation is invariably dependent on progesterone, and this requirement has had enormous consequences for the evolution of mammalian traits. Eutherian pregnancy occurs in the time-limited luteal phase of the ovarian cycle, imposing a physiological constraint on gestation length. Decoupling the lengths of the homologous pregnant and non-pregnant cycles (so-called maternal recognition of pregnancy; MRP) thus played a key role in the evolution of gestational length. As we will show, the mechanisms underlying MRP are diverse, suggesting independent origins in major mammalian lineages. This eutherian key innovation furthermore enabled prolonged gestation, likely driven by selection on increased body size, response to which was enabled by MRP.
In short, we show that mammalian gestation is best understood if considered in the context of the female cycle and its evolution, rather than as an independently evolving trait.
Early Research into the Endocrine Function of the Corpus Luteum
The beginnings of corpus luteum research can be traced to Regnier de Graaf (1641–73), who first provided a detailed description and illustrations of the structures present in the ovaries of pregnant rabbits (Asdell 1928). In 1681, Marcello Malpighi named the structure corpus luteum (yellow body, hereafter CL). Gustav Born (1851-1900) first explicitly theorized about its possible function in pregnancy (Simmer 1971), but it wasn’t until the early twentieth century when Ludwig Fraenkel demonstrated that rabbits could not maintain pregnancy after removal of all corpora lutea (Fraenkel 1903). Not much later, Leo Loeb showed for the first time that the CL enables implantation by inducing changes in the endometrial tissue (Loeb 1907), and soon after it was demonstrated that luteal extracts could rescue early pregnancy in rabbits after ovariectomy (Allen and Corner 1930). Apart from preparing the endometrium for implantation, it was also discovered that the CL functions to inhibit ovulation. Loeb’s interpretation that removing the corpus luteum hastened ovulation in guinea pigs (Loeb 1911) confirmed another long-standing intuition: the gland possessed an anti-ovulatory effect, an action first speculated by John Beard (1858-1924) and Auguste Prenant (1861-1927) (Simmer 1971). Following this idea, Ludwig Haberlandt first demonstrated the suppression of ovulation in mating rabbits after the injection of ovarian extracts (Haberlandt 1922). At the beginning of the 20th century, the research devoted to this gland diversified; with related, if sometimes opposite motivations: from exploring its role in pregnancy, through emphasizing its role on sexual plasticity (Steinach and Kun 1931), to postulating its use in hormonal sterilization and later, contraception (Simmer 1970).
In 1934, several independent groups successfully isolated the gland’s active substance, progesterone (Allen and Windersteiner 1934; Butenandt and Westphal 1934, Hartmann and Wettstein 1934), which received its name from a combination of “progestin” and “luteosterone” (Allen et al. 1935). Early comparative research on the endocrine role of the CL briefly challenged the view of CL-progesterone as the mammal-wide pregnancy maintenance hormone. Researchers working on species where the placenta produces progesterone later in gestation, what we know today as the “luteal-placental shift”, soon discovered that ovarian progesterone was not equally necessary for pregnancy in all mammalian species, and, among these, the lack of definitive evidence in humans weighed strongly (Reynolds 1939). Csapo and Pulkkinen (1978) reestablished the widespread requirement for progesterone by showing that the corpus luteum was indispensable for early human pregnancy. While they acknowledged species-specific differences in endocrine regulation, such as the presence and timings of a luteal-placental shift, they confirmed the indispensability of the CL in implantation and of progesterone for gestation (Csapo and Pulkkinen 1978).
Various other organs were shown to regulate corpus luteum lifespan and function. Hypophysectomy experiments revealed the supportive function of the anterior pituitary during pregnancy (Pencharz and Long 1931), and further research identified the trophoblast as an important endocrine player supporting CL in a number of species (Rowson and Moor 1967). Hysterectomies, in contrast, revealed that the uterus is primarily luteolytic, rather than luteotrophic: a signal from the uterus triggers luteal regression in many species (e.g., hysterectomy in nonpregnant guinea pig allows the persistence of the functional CL for up to three months: Loeb 1923, 1927, Rowlands and Short 1959). This interplay between luteolytic and luteotrophic factors was soon recognized to be a decisive factor determining the length of eutherian pregnancy. It was in this context that Short (1969) first defined maternal recognition of pregnancy as those ways in which the embryo first signals its presence to the uterus, which result in a CL of pregnancy protected from luteolytic signals (Short 1969).
2. Evolutionary Origin of the CL and Its Endocrine Function
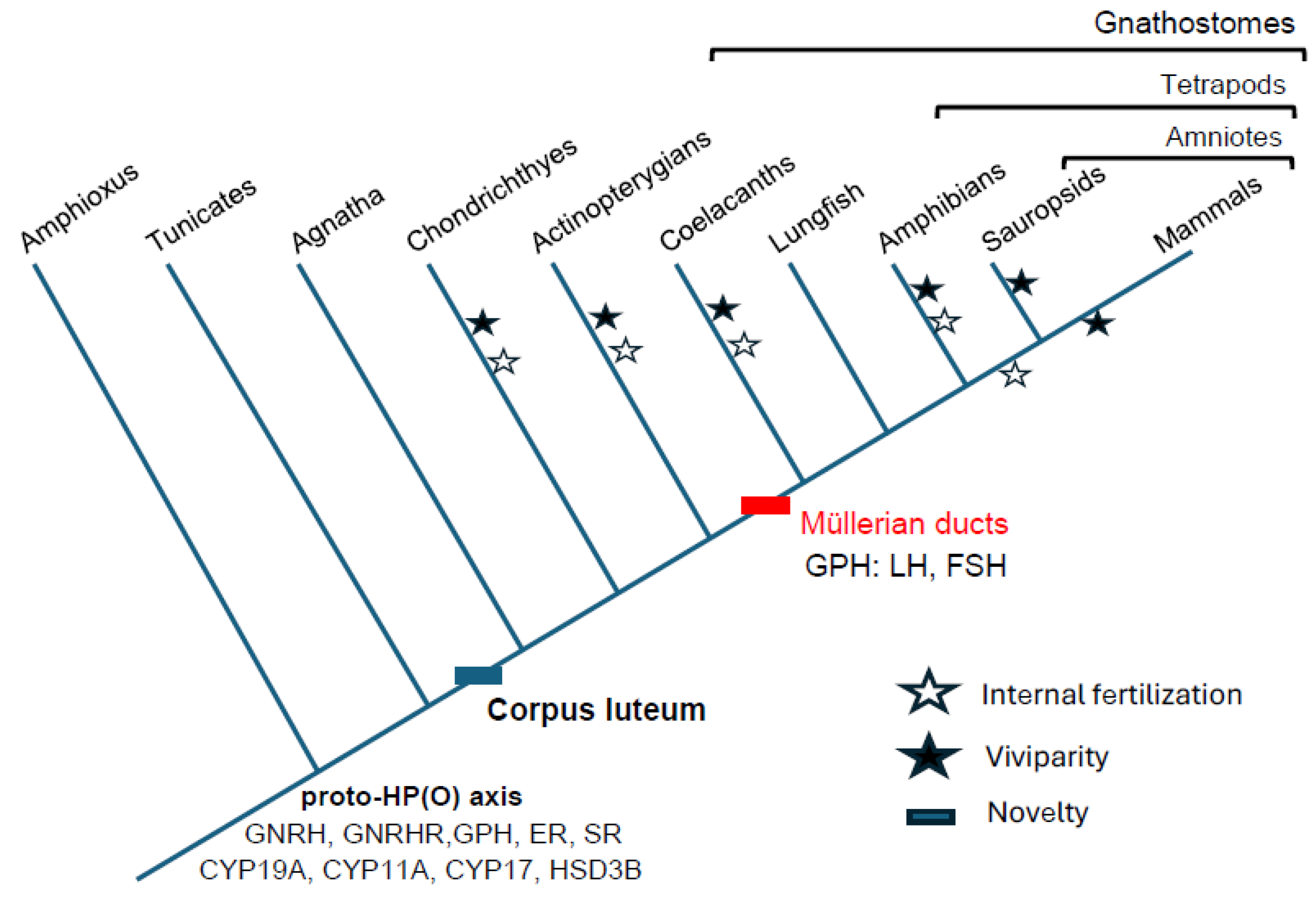
The corpus luteum is embedded within a larger reproductive organ system known as the hypothalamic-pituitary-ovarian axis (HPO axis). The corpus luteum evolved early in the evolution of vertebrates (
Figure 1). Corpora lutea are absent in tunicates and cephalochordates (ciona and amphioxus) but present in hagfish ovaries (jawless vertebrates, Agnatha), where they are also likely functional (Gorbman 1983, Powell et al., 2006). Evidence indicates that a CL does not form in the lamprey ovary (Dodd, 1986), which may be attributed to the fact that lampreys die soon after spawning. To understand whether steroidogenic functions originated with the CL itself, we will summarize the evolution of the vertebrate female reproductive system and the context in which the CL was first integrated.
2.1. The Evolution of the HPO Axis
Hypothalamic gonadotropin-releasing hormones (GnRH) are the hormones by which eutherian hypothalamus regulates the pituitary release of follicular stimulating hormone (FSH) and luteal hormone (LH). GnRH-like peptides have been detected immunochemically or in genome sequence in a broad range of species, including bivalves and cnidarians (Guilgur et al. 2006). The neurosecreted GnRH family of peptides is thus shared among chordates, and several of its receptors are found in ascidians and amphioxus (Kah et al. 2007, Adams et al. 2003, Chambery et al. 2009, Tello and Sherwood 2009, Roch et al. 2014). The specific amphioxus GnRH, ortholog of mammalian hypothalamic GnRH-I, is expressed broadly across the nervous system, albeit with 10-fold higher concentration in the anterior part of the body, peaking before the gonadal maturation (Chambery et al. 2009) and influencing gonadal development and function (Fang 1989). GnRH injection in ascidians (Ciona intestinalis) induces spawning (Terakado 2001, Adams et al. 2003, Sherwood et al. 2006), and the incubation of rat pituitary with amphioxus GnRH induces a luteinizing hormone (LH) release comparable to that following the incubation with mammalian GnRH, suggesting wide conservation of reproductive functionality (Chambery et al. 2009).
Early chordates lack the pituitary gland, but the Hatschek’s pit in amphioxus is considered the homolog of the vertebrate anterior pituitary (Gorbman 1983, Gorbman and Tamarin 1985, Wang et al. 2018; but see G. Schlosser 2021, pp. 177-180). The Hatschek’s pit is an oral cavity organ composed of ciliated cells exposed to external stimuli, with likely sensory function (Schlosser 2021). This organ secretes a single heterodimeric glycoprotein hormone (thyrostimulin) and a cytokine that may be related to prolactin (Li et al. 2014). A reproductive role of Hatschek’s pit is supported by the fact that its damage prevents gonadal development and ovulation in Brachiostoma belcheri (Fang et al. 2001). A homolog of the pituitary gland in ascidians remains controversial, but an organ called the neural gland complex has been proposed (Ruppert 1990, Kano 2010).
The amphioxus ovarian follicular cells show morphological signs of steroidogenesis (round mitochondria, large lipid droplets, large golgi complex, ER etc., Welsch and Fang 1997), and genomic alignment and functional studies confirm the presence of the major steroidogenic enzymes (CYP19A, CYP11A, CYP17, HSD3B) (Kubokawa et al. 2010, Markov et al., 2017, Mizuta and Kubokawa 2007, Callard et al. 2011; Zhang et al., 1984). However, the production of sex steroids does not necessitate signaling via their cognate receptors. We will address their possible early functions below.
The most phylogenetically distant, fully functional vertebrate HPO axis likely occurs in hagfishes (Agnatha), which already have a two-lobed pituitary. Immunoreactive GnRH has been identified in the Atlantic hagfish Myxine glutinosa, where it is associated with the seasonal reproductive state (Kavanaugh et al. 2005, Powell et al. 2004). Hypophysectomies in the inshore hagfish (Eptatretus burgeri) affect gonadal development, confirming pituitary regulation of ovarian function (Patzner 1977). Agnathans have only one pituitary glycoprotein hormone, GPH (homologous to the ancestral thyrostimulin: Uchida et al. 2013). This heterodimeric hormone has diversified by duplications of the alpha and beta subunits in the stem lineage of Gnathostomes. Thereby the ancestral GPH, which in cephalochordates and agnathans was already involved in gonadal development and spawning, gave rise to the family of heterodimeric pituitary gonadotroph GPH (including TSH, LH, and FSH; Kawauchi and Sower 2006).
Sex steroids by which the ovary signals also diversified early in vertebrates (Markov et al. 2009). The order in which ovarian steroids’ signaling functions originated is determined by the evolutionary expansion of sex steroid receptors (Baker 2003). The ancestral estrogen receptor (ER) resulted from a gene duplication of the ancestral steroid receptor (SR) in basal chordates, as both are present in amphioxus (
Figure 1). Ovarian estradiol is reported to signal back to the hypothalamus-pituitary level, which implies that estrogen signaling is in place in agnathans (Nozaki 2013). The ancestral progesterone receptor (PR), on the other hand, arose through a 3-ketosteroid receptor gene duplication prior to the divergence of agnathans (Baker 2019), which would mark the earliest possible origin of progesterone signaling function. Finally, early gnathostome genome duplication resulted in subfunctionalization of the ancestral ER into ERa and ERb, shared between Chondrichthyes and mammals (Baker 2019).
Taken together, comparative evidence suggests that the predecessors of all segments of the HPO axis (GnRH, a pituitary-like and steroidogenic signals) were present and had reproductive functions in stem chordates, considerably predating the origin of the corpus luteum. However, these reproductive functions were likely less entrenched and specific. Broad expression of GnRH across tissues in Amphioxus (Tsai and Zhang 2008, Schlosser 2021) suggests that GnRH in early chordates might have served a more general regulatory function than female reproduction and later became reproduction-specific. Photoperiod-sensing, including the synchronization of spawning with environmental light conditions, could be one such function. Indeed, spawning in Ciona is regulated by photoperiod and can be induced by GnRH (Sherwood et al. 2006), suggesting that the latter mediates the reproductive effects of seasonal and light changes (as is the case in many mammals).
Upon the origin of the HPO axis, the integration of a further chemosensory input from a pituitary-like Hatschek’s pit with a photosensor controlling GnRH expression would have resulted in the coupling of spawning to increasingly specific conditions, leading to the reproductive specialization of vertebrate HPO axis. The reproduction-specificity of this signaling axis became further entrenched following gnathostome-specific genome duplication, which resulted in pituitary glycoprotein hormones and steroid hormone receptor diversification. Moreover, this diversification happened concurrently in Gnathostomes with the appearance of the Müllerian ducts and their reproductive tract derivatives, which eventually became integrated as targets of the HPO signals. This ensuing entrenched complexity of a network of interdependent processes, may have, in turn, prevented subsequent substantial evolutionary change of that network, resulting in a structurally conserved gnathostome HPO axis.
2.2. The Origin of Luteal Phase
The development of the corpus luteum initiates the luteal phase; the phase of the ovarian cycle after the release of ova during which the postovulatory follicle produces progesterone. The origin of the luteal phase involves a switch from the estrogen production of the maturing follicle to progesterone production of the corpus luteum.
As shown above, the ability to synthesize ovarian sex steroids and the origin of some steroid receptors predated the origin of the corpus luteum in stem gnathostomes (
Figure 1). Browning (1973) proposed that ovarian steroid synthesis may have evolved to metabolize and remove the surplus cholesterol of the yolk (Browning 1973). According to this hypothesis, the ovarian steroids themselves would be a byproduct, initially lacking an endocrine role. Indeed, the enzymes involved in steroidogenesis most likely evolved from xenobiotic catabolic pathways (Markov et al. 2009). Furthermore, the steroid derivatives of cholesterol are increasingly more hydrophilic and thus easier to transport passively and dispose of, which further supports Browning’s scenario. Given the evolution of the appropriate receptors transducing the signal to the nucleus, this hydrophilic nature might have enabled the co-option of steroids into the role of long-distance signaling molecules. The most ancient metabolic byproduct of this pathway likely was estrogen (Browning 1973), possibly because of its greater stability than the 3-keto steroids. Progesterone is an intermediate of estrogen biosynthesis; therefore, its synthesis came “for free” with the presence of estrogen, before its co-option into a signaling role and before the interaction with its receptor evolved (Thornton 2001, 2003).
In the hagfish (Agnatha), estrogen has a signaling function, enhancing hepatic vitellogenesis and signaling to the pituitary (Nozaki 2013). Vitellogenesis is the process of yolk formation in the maturing ooctyte, hence entailing the accumulation of lipid yolk. Cholesterol catabolism leading to estrogen would be counterproductive in this context, given its acquired pro-vitellogenic role. In this situation, reducing the conversion of cholesterol into estrogen would lead instead to the accumulating progesterone-related molecules in the post-ovulatory follicle or CL. It is thus conceivable that postovulatory progesterone accumulation might have evolved even prior to progesterone’s PR-mediated functions, and could have constituted an alternative cholesterol metabolite. In the same vein, decreased conversion into estrogen would have provided a way to delay vitellogenesis and impose cycle control. Stem vertebrates already possess a form of PR of uncertain functional role, so it is not clear to us whether progesterone already had signaling functions in this group. The above scenario could explain cooptions of both sex steroids in the reproductive system, prior to the evolution of their signaling roles.
In summary, the corpus luteum originated in the stem lineage of vertebrates, after the precursor of the HPO axis had already been established. The origin of CL predates the diversification of the pituitary gonadotrop hormone family, the origin of the Müllerian duct and likely predates the origin of progesterone’s signaling functions, based on our reading of the structure and evolution of steroid metabolism. Consequently the CL originated in the context of external fertilization and external embryo development, and therefore certainly not driven by the roles it plays in extant mammals. If the CL’s ability to accumulate progesterone derived from the need to replace estrogen in yolk lipid disposal (Browning 1973), CL’s ancestral lifespan was presumably positively correlated with the amount of yolk in the follicle, and thereby intrinsic to the ovary rather than regulated by the extra-ovarian mechanisms. These amounts probably increased in early vertebrates with yolk-rich eggs and the origin of the liver. Regulation of progesterone conversion into estrogen could have provided a way to regulate the length of CL lifespan and the dynamics of the cycle as a whole by generating the estrogen-progesterone switch.
It is an open question how precisely progesterone was co-opted into its reproductive and specifically its signaling roles. However, the origin of the progestogenic CL generated a context in which these new functions could evolve, eventually leading to new reproductive modes. With the origin of Müllerian ducts as an oviductal structure in gnathostomes (
Figure 1), the temporal coordination of the ovarian cycle with the activity of the reproductive duct likely was of considerable advantage. Further functional differentiation of the CL signaling thus likely occurred in coevolution with an increasing regionalization of the Müllerian duct, allowing the latter to specifically respond to ovarian steroid dynamics by the generation of secretions and controlled muscular contractions. This initial coordination between the ovary and the reproductive tract became ever more complex with the evolution of internal fertilization, when the simple expelling of eggs was replaced by prolonged internal embryonic development and the production of histotroph, albumen and eggshell. To understand the origin of the highly derived mammalian CL functions, in the next section, we first review non-mammalian lineages.
3. CL Endocrine Function and Its Regulation in Non-Mammalian Vertebrates
The comparative morphology of the non-mammalian corpus luteum is well described, and steroidogenesis is confirmed. Nevertheless, the mechanisms of non-mammalian CL formation and maintenance and their functional significance remain poorly understood. Vertebrates display a high diversity of reproductive modes, including multiple origins of internal fertilization, ovoviviparity (egg retention), and viviparity. In this section, we summarize what is known about the role of the CL and progesterone in non-mammalian vertebrate reproductive modes. Despite the interesting diversity of teleost reproduction, we avoid this group, as it has undergone additional genome duplications and extensive modifications of the female reproductive tract anatomy, complicating comparison with the sarcopterygian/tetrapod lineage.
Relative to the ancestrally symmetrical situation between males and females, consisting of expulsion of gametes, the asymmetry between sexes of investment in post-gametogenic reproductive processes between sexes increased in vertebrates. Internal fertilization independently arose in amniotes, elasmobranchs, some subgroups of teleosts, and amphibians. Increased female internalization of the reproductive events (internal fertilization and at least a degree of internal development) not only intensified the maternal investment in reproduction in the clades in which it evolved, but also augmented the cost of an unfertilized cycle. In species with spontaneous ovulation, the association between ovulation and fertilization is not guaranteed. Unfertilized cycles not only imply the loss of eggs and delay in reproduction but also the loss of investment in physiological preparation for fertilization and/or pregnancy. This is less prominent in species with induced ovulation but can occur if the ovulatory trigger is not the mating itself, but e.g., photoperiod.
Consequently, the species with potential for an unfertilized (or sterile) cycle show two distinct contexts: the CL of the fertile cycle (passing or incubating a fertilized egg and a fetus) and that of the sterile cycle (unfertilized egg or nonpregnant luteal phase). Although the sterile cycle may not often occur in the wild (Conaway 1971), its length nevertheless indicates a periodicity of the corpus luteum lifespan that may constrain or drive the evolution of gestation length and breeding seasonality.
Internal fertilization is necessary for the origin of viviparity. Remarkably, viviparity arose independently in at least some species of every major clade capable of internal fertilization, except for birds (Blackburn 2015): teleosts, chondrichthyes, amphibians, reptiles, and therian mammals, but only in the latter it involves stabilized long-term implantation and is present in the whole clade. The length of CL lifespan is affected by reproductive mode. Most instances of the evolution of viviparity involve a longer CL lifespan. Yet, we will show that, contrary to eutherian mammals, the necessity for the corpus luteum does not encompass the entire pregnancy in most nonmammalian vertebrates.
In the most basally branching gnathostomes, chondrichthyans, the GnRH and two anterior pituitary gonadotropins regulate follicular development and the ovarian estradiol levels in viviparous and oviparous species, confirming a functional HPO axis (Awruch 2013). Many studies note the variable steroid hormone dynamics and its association with the temporal separation of the follicular and luteal phases. Estrogen always increases during folliculogenesis and is vitellogenic. When estrogen levels remain elevated after ovulation, folliculogenesis continues, and new mature preovulatory follicles are ready soon after oviposition. If, in contrast, folliculogenesis is suppressed after ovulation, estrogen peaks shortly before ovulation and decreases thereafter. The first scenario characterizes many oviparous and non seasonal breeders (e.g., the Little skate, Leucoraja erinacea, Koob and Callard 1999; or the Draughtboard shark, C. laticeps, Awruch et al. 2008). The second scenario applies to the case of viviparous species such as the aplacental Atlantic stingray (Dasyatis subina; Tricas et al. 2000). This suggests that in viviparous species, by counteracting vitellogenesis or by direct suppression of folliculogenesis, ovarian progesterone effects the introduction of a new phase of the ovarian cycle, a distinct non-folliculogenic luteal phase. It has also been shown that hepatic vitellogenesis is less sensitive to estrogen during chondrichthyan pregnancy (Callard et al. 1990).
Luteal progesterone in elasmobranchs is restricted to the reproductive season and low or absent when they do not cycle (Lutton 2011). In oviparous species, progesterone is reported to peak even shortly before ovulation (e.g., in the Little skate, L. erinacea; Koob and Callard 1999), decreases before egg encapsulation, and is low during egg passage and oviposition. It thus could have a role during the periovulatory phase (Koob et al. 1986). In viviparous elasmobranchs, the decrease of progesterone levels is postponed, and progesterone remains high for at least the first part of pregnancy and in some species up until parturition (e.g., the Round stingray, U. halleri; Mull et al. 2010). Luteal progesterone thus appears to be associated with egg retention and pregnancy in elasmobranchs, possibly complementing lecithotrophic provisioning by enhancing uterine histotroph secretion or inhibiting myometrial contractions. We found no report on CL lifespan in infertile cycles and no indication of embryonic or placental control of CL lifespan in this group (but see Hamlett 1999, Callard and Koob 1993). The data from the viviparous dogfish shark M.canis suggest that while the pituitary is necessary for folliculogenesis, it is not required for CL formation or maintenance (Dodd 1983), implying that the lifespan of elasmobranch CL may be inherently determined, potentially by the amount of cholesterol remaining in the CL, possibly proportional with the amount of yolk.
The CL has also been described in oviparous and viviparous amphibians. While most anurans have external fertilization, most salamanders and all caecilians fertilize internally, and many of them evolved viviparity (Wake 1980, Wake and Dickie 1998). One anuran exception is the toads of the genus Nectophrynoides. The Nimba toad (N. occidentalis) ovulates yolk-poor eggs, and the internally fertilized embryos are nourished by extensive secretions of the female reproductive tract during a long pregnancy, resulting in the birth of metamorphosed young (Xavier 1978, reviewed in Sandberger-Loua et al. 2017). This species is an example of an independently evolved prolonged luteal phase involving the same upstream factors but different downstream mechanisms and consequences. The Nimba toad has distinct estrogen-rich follicular and progesterone-dominated luteal phases, accompanied by extensive remodeling of the gestational part of the reproductive tract. The progesterone-secreting CLs persist for the entire gestation length. Remarkably, this species manifests pseudopregnancy without mating, maintaining extensive remodeling of the reproductive tract. Xavier (1978) demonstrated that progesterone is necessary for early pregnancy but is not crucial for maintaining gestation thereafter. The effect of initially high levels of progesterone is to slow embryonic development in early pregnancy, which occurs during a dry season with toads burrowed and inactive. After emerging from the ground, decreasing progesterone levels accelerates development, and progesterone becomes dispensable. In addition to growth regulation, progesterone is crucial for reproductive tract remodeling, particularly in primiparous females. The female tract supports the CL in early pregnancy, as hysterectomy before emergence from the ground leads to luteolysis. There is ovary-pituitary feedback, as ovarian signaling affects the cell composition of the pituitary during vitellogenesis and early gestation.
Viviparity originated multiple times in salamanders, yet it is rare and variable in extent, from birthing aquatic larvae to fully metamorphosed young. It mainly entails less yolk than ovoviviparous populations, even within the same species (Buckley et al. 2007), and additional provisioning by maternal secretions. Steroidogenic corpora lutea are present in salamanders; however, the plasma progesterone, as estrogen, stays relatively low, whereas testosterone levels increase after ovulation. High progesterone is limited to the ovary during pregnancy but is low in plasma. Progesterone has been associated with vitellogenesis and epithelial and vascular remodeling of the reproductive tract to accommodate pregnancy but is not required to maintain pregnancy.
The amphibian clade with most species with internal fertilization is Gymnophiona (caecilians), which also evolved aplacental viviparity. In well-studied viviparous caecilians, ovulation is triggered by endogenous and exogenous cues (e.g., Typhlonectes compressicauda, Brun et al. 2020). The progesterone-producing CL of fertilized females is maintained throughout pregnancy, degenerating only at parturition. The physiological relevance of progesterone in gestation is unknown but it could enhance secretions after hatching. The fetuses hatch from the egg membrane after the abundant yolk has been absorbed, and it has been reported that fetal teeth are used to ingest endometrial secretions (Wake 1977a, 1977b). Wake (1977a) described the distinct remodeling of the reproductive tract in viviparous caecilians during pregnancy, with increased epithelial proliferation and the forming of gland-like secretory pockets. It is unknown whether this remodeling is induced by fetal presence and thus limited to pregnancy or maternally induced, in which case it would be present in unfertilized females.
Overall, the corpus luteum of oviparous amphibians disintegrates soon after ovulation but is maintained longer in the fertilized ovoviviparous and viviparous amphibians (with the exemptions of salamanders), in some viviparous species up to complete gestation. A shared role of progesterone is in remodeling the reproductive tract for the internal part of development in ovoviviparity and viviparity. In addition, progesterone has acquired species-specific roles, such as slowing the embryonal development of Nimba toad or enhancing endometrial secretions and epithelial hyperplasia in caecilians. Together, this suggests that the evolution of ovoviviparity and viviparity in amphibians is associated with a prolonged persistence of postovulatory corpus luteum. Whether this CL prolongation is due to maternal (as in Nimba toad) or fetal signals remains an open question.
All amniotes practice internal fertilization and, thus, at least a minimal degree of internal embryo support, even in egg-laying species. Reptile CL lifespan across species correlates with the time the embryo is retained in the female tract, and CL is the main source of progesterone in most reptilian species (Bragdon 1952, Highfill and Mead 1975). In oviparous species, progesterone levels decrease soon after ovulation, but the CL is maintained until egg-laying. In fact, the surgical removal of the corpora lutea causes premature oviposition in all oviparous lizard species examined (Guillette 1987, Jones and Baxter 1991), implying that some other product of the CL rather than progesterone is required for the length of egg retention.
The corpus luteum of viviparous lizards persists for a variable portion of gestational time (Cieslak 1954, Weekes 1934), and progesterone may remain elevated during this time; however, the degradation of the CL is observable well before parturition, even in those species in which CL is histologically recognizable until after (Weeks 1934, Bennett and Jones 2002). Ovariectomies do not cause a shortening of internal development in viviparous or ovoviviparous lizards or snakes (Amoroso and Finn 1962), except if performed early in pregnancy in some species, in which case it can impact either pregnancy length or parturition (Clausen 1940, Fraenkel et al. 1940, Yaron 1972, Guillette 1987). Intriguingly, squamate reptiles (but not turtles and crocodiles) use a single pituitary gonadotropin in ovarian regulation (Licht 1983). While the orthologs of mammalian FSH and LH -specific ß-chains have been found in the genome, it is unclear whether they produce one or two different proteins (Bluhm et al. 2004). Hypophysectomy followed by injection of heterologous mammalian FSH or LH hormones did not affect Thamnophis sirtalis and Lacerta vivipara (reviewed in Norris and Jones 2012, p.263). Bragdon (1951) found that hypophysectomy in Natrix sipedon and T. sirtalis did not interrupt pregnancy at any stage (but see Clausen 1940), including the first week after ovulation. In contrast, ovariectomies did not interfere with gestation only from the second trimester on (Bragdon 1951), implying that in these species, the ovary (likely the CL) is crucial for the first trimester of pregnancy. In contrast, pituitary hormones are not required to maintain CL or any other pregnancy support. Hence, whereas squamate folliculogenesis is under pituitary control, luteal development and maintenance is not (Yaron 1972).
Finally, the avian post-ovulatory follicle regresses and is phagocytosed rapidly after ovulation, and their granulosa and theca cells do not undergo proliferation, luteinization or hypertrophy. Based on this, to name avian post-ovulatory follicle corpus luteum appears a misnomer (Davis 1942, Payne 1966). However, the postovulatory follicle still appears to have a function in determining the interval between ovulations: it was shown that removing the post-ovulatory follicle delays egg-laying, although the possible mechanism behind it is unclear (Rothchild and Fraps 1944).
In summary, across most nonmammalian vertebrates studied, the CL lifespan in the fertile cycle of (ovo)viviparous species is longer than that of the closely related oviparous species (Gemmell 1995). Scarce information on non the presence or length of sterile cycles, however, does not allow a general conclusion on whether this length implies a mechanism for the maternal recognition of pregnancy. One of the first functions of progesterone likely entailed inhibiting vitellogenesis in the liver and preparing the reproductive tract for egg formation or internal development. Different degrees of vitellogenesis inhibition were observed during pregnancy in viviparous elasmobranchs (Teshima and Mizue 1972, Hisaw and Abramowitz 1973, Tsang and Callard 1987) and are also encountered in amphibians and reptiles (Callard et al. 1985). Inhibition of vitellogenesis prevents the subsequent ovarian follicular growth and ovulation during a fertile cycle, thus spacing the follicular phases and individuating the luteal phase - aspects of the cycle widely shared among nonmammals and mammals. In addition, progesterone obtained functions in the early luteal remodeling of the reproductive tract, such as preparing the reproductive tract or enhancing eggshell production or nutritive secretions. While the role in the reproductive tract is generally widely present, its exact realization, i.e., which aspects of internal development are enhanced, appears species-specific. We suggest that these additional functions explain the variation observed in progesterone dependence after performing ovariectomies at different gestation times in non-mammalian species. In striking contrast to placental mammals, in most viviparous nonmammalian vertebrates, progesterone is not essential for an entire gestation, and its withdrawal, particularly in the later stages, is not a ubiquitous trigger of parturition.
The preexistence of the CL relative to internal fertilization and internal development, and the fact that only the formation, but not the lifespan of CL, is regulated by ovary-external maternal signals in at least early branching vertebrate lineages, suggests that the lifespan of CL is ancestrally inherent to the ovary (see below), and only subsequently became responsive to signals from the endometrium and trophoblast, as already suggested by Rothchild (1981). The process of evolution of the role of the pituitary in maintaining the steroidogenic postovulatory CL, or extending its lifespan beyond the luteal length of the sterile cycle in nonmammalian vertebrates, is not yet understood.
4. CL Endocrine Function and Its Regulation in Mammals
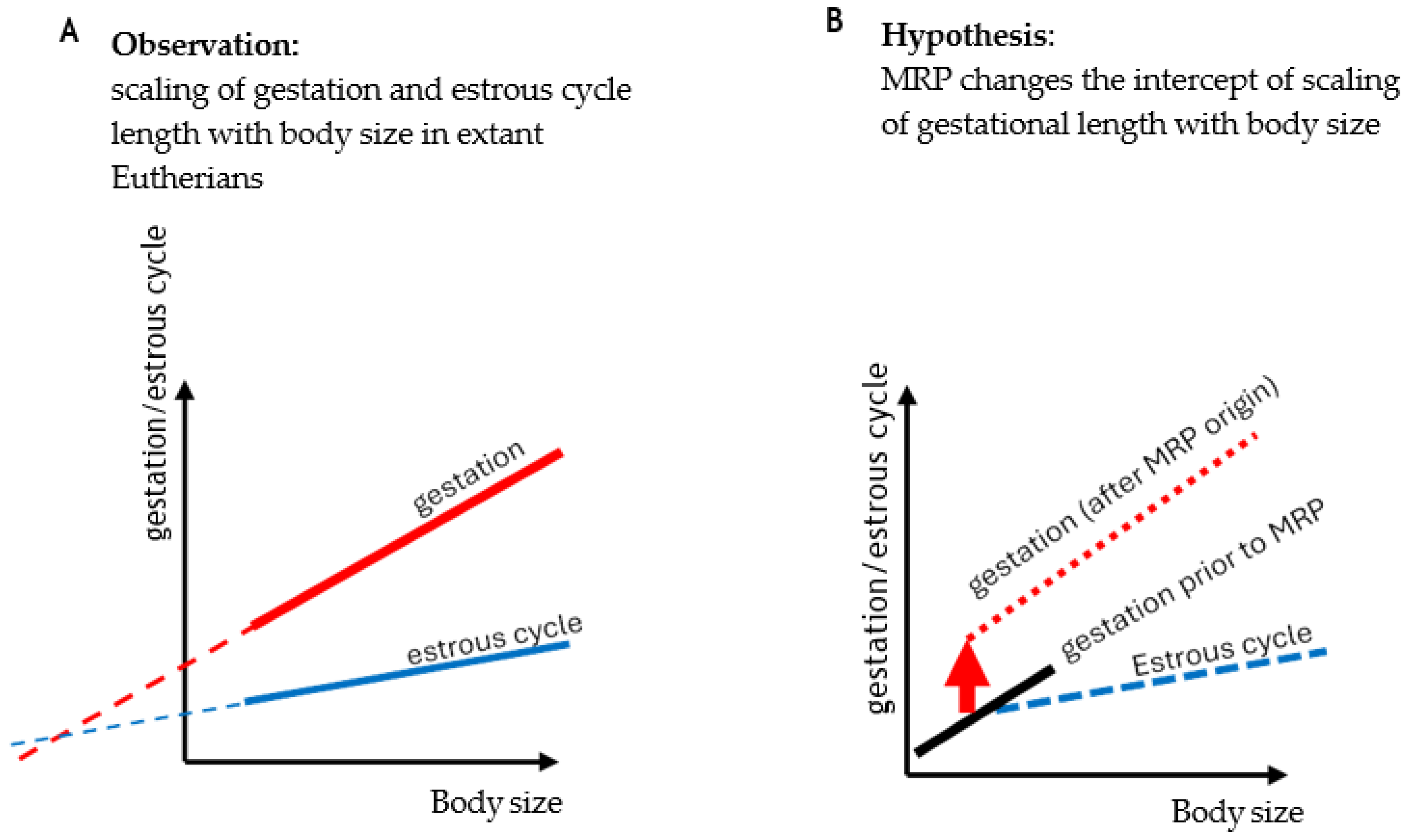
Therian pregnancy originated independently of nonmammalian occurrences. Whereas ancestral marsupials have superficial and short pregnancies, the ancestral eutherian pregnancy is characterized by implantation and a highly invasive placentation. In eutherian mammals, the full length of pregnancy depends on elevated progesterone levels, which in most species are secreted by the CL. Thus, only in this group do we find a consistent role of progesterone in pregnancy maintenance, which may be related to the unique presence of invasive embryo implantation, or also the lack of yolk (Rothchild 2003).
In this section, we will consider this novel maternal-fetal relationship as a potential driving factor for the further evolution of CL regulation. In mammals, the endocrine function, lifespan, and other properties of the CL often differ between CL during pregnancy (pCL) and those during a nonpregnant cycle (npCL). This could have resulted from a selective pressure to decouple the two instances, so that the modification of the pregnant luteal phase does not at the same time apply to the nonpregnant luteal phase. The sterile CL lifespan may not impose a cost for those species in which internal development is shorter than the interval between ovulations, as in these cases the CL does not interfere with the subsequent cycle (i.e., in rare, induced or seasonal ovulation). In these cases, the npCL lifespan would be neutral with respect to fitness. However, in species where the ovulations re-occurs in shorter intervals, mechanisms to shorten the CL lifespan in sterile cycle, or prolong it only when fertilized, would be predicted to increase reproductive output and thus fitness. CL regulatory mechanisms are revealed when comparing the CL of pregnancy (pCL) to that of the nonpregnancy (npCL). However, the latter is rarely reported, and, even if physiologically possible, are probably only rarely realized in nature.
In most eutherians where known, the nonpregnant cycle’s luteal phase is shorter than the length of intrauterine fetal development. In contrast, pregnancy is confined within the length of the npCL lifespan in most marsupials; such as in gray-tailed opossum (Monodelphis domestica), where pregnancy occurs within the length of sterile luteal phase. This supports the idea that the pCL and npCL lifespans diverged early in the evolution of eutherian pregnancy. In some eutherian species (e.g., the dog), the sterile and pregnant luteal phase are of equal length, but this is an exception and not the rule, as will be discussed below. In such species, only a few changes are associated with the pregnant cycle. In canids, for example, the latter includes a pregnancy-specific prolactin elevation and delayed oocyte maturation (Papa and Mariusz 2020).
Eutherians manifest a wide range of regulatory mechanisms for either prolonging the progesterone secretion by CL or inducing it from other (fetal or maternal) sources during the pregnant cycle, or shortening the npCL by luteolytic factors, thus accelerating the transition into the next, potentially fertile cycle. Most of the known eutherian luteotrophic or luteolytic mechanisms are ovary-extrinsic. Consistent evidence suggests that ancestral CL lifespan regulation was intrinsic to the ovary. To understand how the intrinsic regulation of the CL may have become modified to decouple pCL and npCL, we will next review the intrinsic regulation of this endocrine body.
4.1. Ovary-Intrinsic Regulation of CL Lifespan
In contrast to the oscillatory activity of other endocrine glands that are regulated by the negative feedback from the pituitary (e.g., the thyroid), the CL lifespan has an intrinsic regulatory component (Rothchild 1981). The primary endogenous mechanism of CL regulation consists of a balance between steroidogenesis and reactive oxygen species (ROS) production, the latter resulting primarily from the ovary’s local cellular respiration and mitochondrial steroidogenesis (Bose et al. 2002, Stocco 2000 and 2001, Chapman et al. 2005). The CL undergoes structural and functional regression when the balance of these processes tilts towards ROS accumulation. ROS induce apoptotic cell death (Kato et al. 1997, Behrman et al. 2001, Garrel et al. 2007) and interfere at several levels with progesterone synthesis, either by impairing LH signaling, inhibiting the translocation of cholesterol to the mitochondria, or interrupting the activity of the rate-limiting enzyme CYP11A1 (Behrman et al. 2001). Balancing this destructive process, ROS production is inhibited by steroid hormones and luteal antioxidants, with superoxide dismutases and small antioxidants constituting the primary defense (Hanukoglu 2006). While progesterone directly counteracts ROS production, luteotrophins increase antioxidants, therefore decreasing ROS activity. Given this endogenous lifespan control mechanism, luteotrophic or luteolytic factors converge on modifying the production or activity of ROS and antioxidants (Al-Gubory et al. 2005, Sugino 2006).
Rothschild’s suggestion that the CL lifespan is determined by the intrinsic balance of progesterone and prostaglandin production complements the evolutionary reconstruction of Browning (1973), according to which prostaglandins first served follicle’s fast post-ovulatory regression, and the origin of progesterone signaling allowed a scenario in which this regression could be delayed. Prostaglandins F2α and E2 exert autocrine, paracrine, and endocrine effects that depend on the receptors they bind to (Narumiya et al. 1999; Narumiya and FitzGerald 2001). By binding to FP and EP1 receptors, PGF2α activates the phospholipase C-protein kinase pathway, decreasing the translation of steroidogenic enzymes and steroidogenic acute regulatory protein (StAR) (Sandhoff and McLean 1996, Niswender et al. 2000). This common luteolytic factor, prostaglandin F2α, facilitates ROS accumulation by decreasing the expression of ROS scavenger proteins (Foyouzi et al. 2005). Likewise, ROS enhances PGF2α’s abundance, leading to an accelerating process of luteolysis, and the locally produced O2, NO, and H2O2 activate the enzymes involved in the synthesis of prostaglandins, further enhancing luteal production of PGF2α (Arosh et al. 2004). In contrast, PGE2 binding to EP2 and EP4 receptors, activates protein kinase A (PKA), inducing the transcription of genes involved in steroidogenesis, angiogenesis, and cell survival (Ziecik et al. 2018). PKA is also phosphorylated in response to external luteotrophic factors such as LH, indicating that endogenous and exogenous factors have synergistic effects. The decrease in the PGE2:PGF2α ratio restricts luteal blood flow and inhibits steroidogenesis, as well as decreases StAR expression while increases prostaglandin G/H synthase and the release of oxytocin by the CL (Milvae et al. 1996, 2000). The PGE2 to PGF2α ratio is thus essential to CL’s lifespan (Blitek and Ziecik 2005; Breuiller-Fouché et al. 2010).
Although it is clear that CL endocrine functionality results from a balance between luteotrophic and luteolytic factors and that, for almost all mammalian species studied so far, ovary-intrinsic or extrinsic PGF2α is the primary luteolysin, the exact mechanisms by which prostaglandin F2α initiates luteal regression are not entirely known. This difficulty comes in part from the fact that PGF2α works along other factors and seems to lose its luteolytic effect when acting upon isolated steroidogenic cells (Hansel et al. 1991). One example of a mechanism of action of PGF2α is the activation of endothelin 1 (ET-1), which binds to receptors in the steroidogenic cells and decreases LH stimulation of progesterone and blood supply to the CL (Milvae 2000). The importance of angiogenesis and the role of the luteal microvasculature have been extensively revisited (Reynolds et al. 2000, Davis et al. 2003, Tamanini and De Ambrogi 2004). While follicle luteinization requires the action of angiogenic factors that allow LDL cholesterol to reach the luteal cells and be converted into progesterone. Endothelial cells, possibly via secretion of the luteotrophic prostaglandin PGI2, can enhance P4 secretion in the luteal cells (Grish et al. 1995); endothelial factors also play a role in luteal maintenance, as well as regression.
Besides the positive feedback between PGF2α and ROS production, immune cells and cytokines are also involved in CL cell death. In turn, the expression of proinflammatory cytokines by the immune cells recruited into the CL from the systemic circulation constitutes an early signal of CL regression. The luteotrophic and luteolytic nature of immune factors depends on the luteal environment, particularly on the absence of immunosuppressive factors such as progesterone (Walusimbi and Pate 2013). Macrophages likely play a crucial role in the resolution of the luteolytic process and avoidance of an inflammatory response (Pate and Keyes 2001), whereas T lymphocytes enhance progesterone production in the CL of several species (Emi et al. 1991, Hughes et al. 1990 and 1991). On the other hand, progesterone inhibits lymphocyte proliferation and interferes with the cytokine action on the luteal cells (reviewed by Siiteri and Stities 1982, Grossmanm 1984, Kelly 1994). These protective effects weaken as progesterone concentration declines, which eventually precipitates CL regression (extensively reviewed by Pate and Keyes 2001, Davis and Rueda 2002, Cannon and Pate 2003, Bornstein et al. 2004).
Much of the research on luteolysis is conducted on mammals, and it is unclear whether these mechanisms apply to non-mammals. ROS production in basic cellular biochemical processes, such as steroidogenesis, respiration or hydroxylation is conserved across vertebrates. It is therefore likely that the ROS and progesterone - mediated intrinsic CL lifespan control is shared across vertebrate CL. The ancestral CL possibly expressed the key enzyme involved in prostaglandin biosynthesis, the cyclooxygenase (in vertebrates isozymes COX-1 and COX-2), as the origins of these enzymes predate vertebrates (Knight et al. 1999, Kolijak et al. 2001, Valmsen et al. 2001). Similarly, prostaglandin F has been associated with spawning and hatching processes in marine invertebrates (Ruggeri and Thoroughgood 1985), suggesting broad phylogenetic distribution.
Lastly, part of the intrinsic mechanisms for CL maintenance could derive from the mechanisms underlying the resolution of inflammation after follicular injury of ovulation (Espey et al. 2004). Inflammation precipitates angiogenesis, resulting in the infiltration of leukocytes into the preovulatory follicle and the development of new vessels during CL formation. Although the immune dynamics and cell composition over the ovarian cycle follow complicated patterns, it has been suggested that the immune response shifts toward a type-2 response, dominated by T-helper 2 cells and involved in wound repair and tissue regeneration after infection or injury, in the luteal phase (Bouman et al. 2001, Faas et al. 2000).
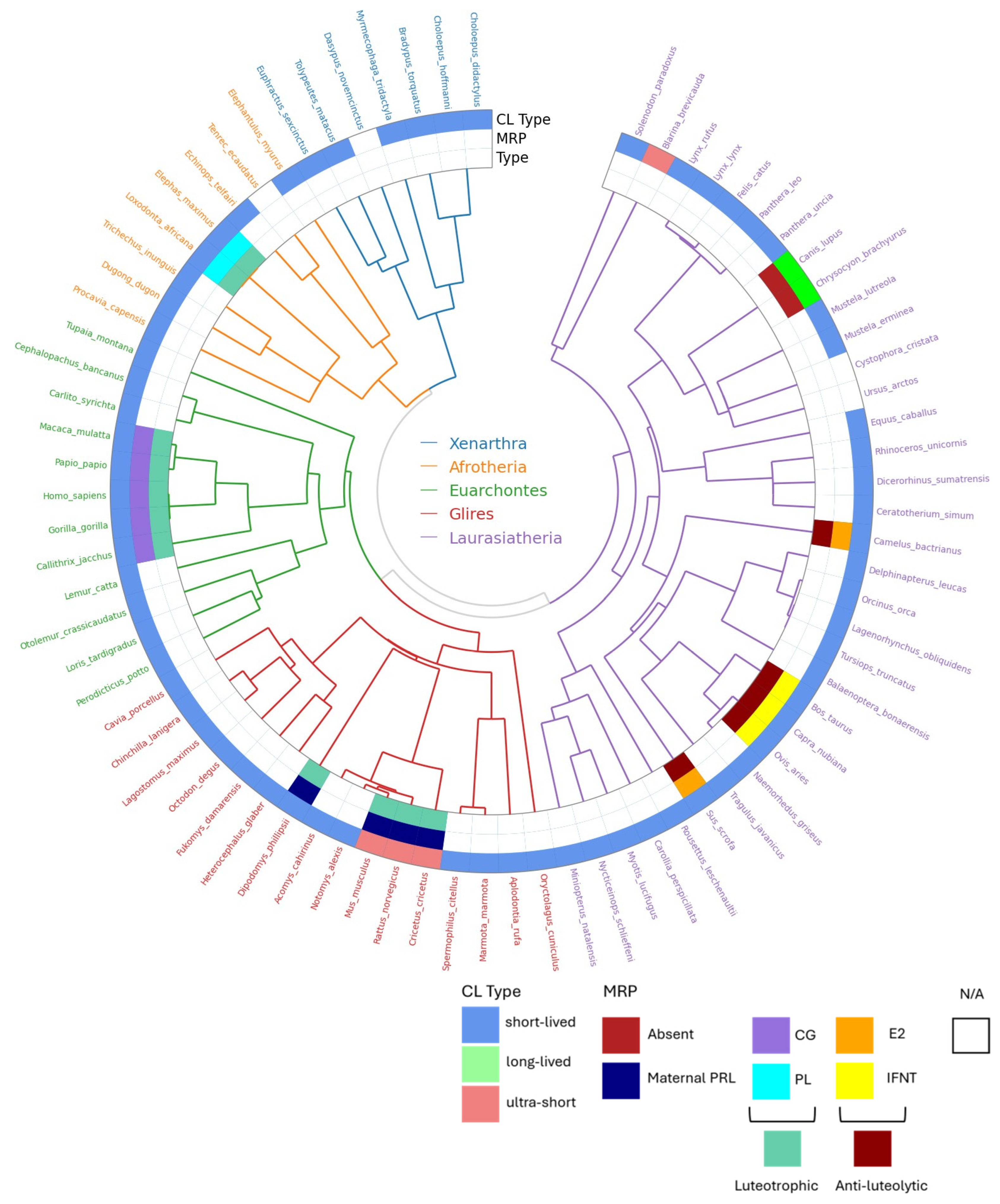
Regarding this last point and connecting with the next section, the evolution of eutherian non-ovarian sources of CL regulation could have been connected to other significant innovations characterizing eutherian reproductive biology, such as the decidual cell. The decidual cell and the functional CL are transient structural entities necessary for successful embryo implantation that result from a transformation of the stress response. The emergence of decidualization might have promoted the new signals for prolonging the CL cycle, as the evolutionary origin of the decidual cell was probably related to a stress reaction at the molecular and cellular levels (Erkenbrack et al. 2018, Wagner et al. 2019). Many signals for MRP, like CG, PGE2, or PRL, have anti-apoptotic roles on both the CL and stromal fibroblasts, and early embryonic signals work through similar mechanisms in the luteal and decidual cells to prevent cell death and resolve the stress response. Furthermore, luteal progesterone has protective anti-ROS effects on both the CL and the decidual cell. For instance, progesterone increases superoxide dismutase activity in the endometrium, decreasing ROS and prostaglandin F2α (Sugino et al. 1996, Sugino et al. 2000, Sugino 2006). Finally, many of the molecules released by the blastocyst to ensure uterine receptivity, such as PGE2, play luteotrophic roles as well (Psychoyos and Gravanis 1995).
In summary, the intrinsic regulation of CL lifespan thus results primarily from a balance between steroidogenesis, ROS and prostaglandin production, and luteotrophic and luteolytic ovary-extrinsic signals affect CL lifespan by modifying this balance. CL’s lifespan susceptibility to exogenous regulation by hormones of various origins, namely the maternal pituitary, the endometrium, or the placenta, makes the CL a unique gland and regulatory hub that integrates input from multiple sources (Stormshak et al. 1987). When this susceptibility first evolved, remains to be elucidated.
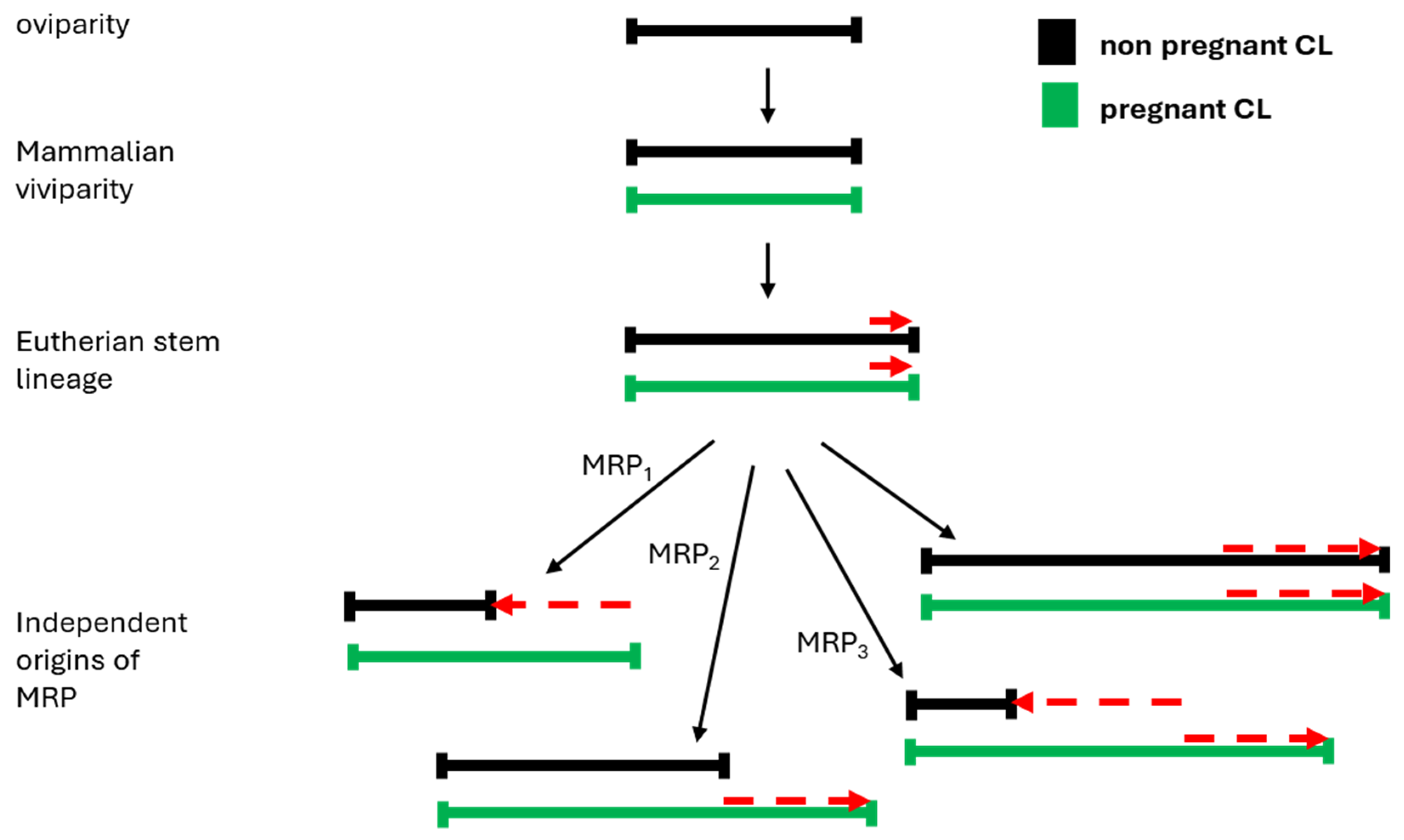
4.2. Ovary-Extrinsic Regulation of CL Lifespan (Maternal Recognition of Pregnancy)
As previously mentioned, maternal recognition of pregnancy (MRP) is conventionally defined as the mechanism that prolongs the luteal progesterone production in pregnancy (Short 1969) beyond that of nonpregnancy, i.e., “rescues” the CL from luteolysis. MRP occurs soon after blastocyst formation in eutherians, with the trophoblast inducing changes in CL as well as in the uterine endometrium. Accordingly, most accounts consider MRP a unique eutherian trait (Flint et al. 1990). Recently, authors have applied the MRP concept more broadly to designate any alteration in the maternal physiology during pregnancy that distinguishes it from the nonpregnant condition. For example, this broader notion, applicable in particular to marsupials, may encompass the difference between the pregnant and non-pregnant uterus, also referred to as endometrial recognition of pregnancy (Laird et al. 2017, Griffith et al. 2019).
A CL “rescue” function of placental signals does not seem to be present in marsupials. Although blastocyst-mediated CL reactivation following hypophysectomy occurs in marsupial embryonic diapause (Tyndale-Biscoe 1979), early research showed that the blastocyst does not extend CL lifespan (Hinds and Tyndale-Biscoe 1982). Likewise, the differences in progesterone levels in pregnant versus non-pregnant cycles are caused directly by a placental signal, as it has been shown for the koala (Johnston et al. 2000) or in the quokka (Cake et al. 1980) and tammar (Hinds and Tyndale-Biscoe 1982) early gestation. With exceptions, female marsupials have pregnant cycles shorter than their oestrus cycle (Tyndale and Renfree 1974, Merchant 1979). This observation led to the hypothesis that early progesterone falls at the end of the pregnant cycle could result from the luteolytic effect or a luteolytic signal from the fetus (Hinds and Tyndale-Biscoe 1982b). In this regard, Hinds proposed a model according to which the release of endometrial prostaglandin triggered by fetal glucocorticoids causes pulses of pituitary prolactin (Hinds 1990). The latter would be responsible for shortening CL lifespan only during pregnant cycle. Based on this evidence, Renfree (2000, 2010) considers that while endometrial recognition of pregnancy is ancestral in marsupials (present in basal branching Monodelphis domestica), maternal recognition of pregnancy, defined in this context as as any type of influence through systemic signals of the fetal-placental unit on CL lifespan, is likely a derived trait in Macropodidae. While these results suggest that the embryo of at least some marsupial species has the ability to signal its presence and influence ovarian function, the signal, as opposed to the ones involved in eutherian MRP (reviewed in Bazer 2015), reduces CL lifespan (Bradshaw and Bradshaw 2001).
If we follow Short’s classical definition of MRP as a mechanism that prolongs the luteal progesterone production, we find that the phenomena is not ubiquitous even among eutherians, and only if understood more broadly (as endometrial recognition) can it be considered a universal eutherian feature. In fact, some eutherians do not prolong the CL function in pregnancy - these are primarily species with long non-pregnant luteal phases to encompass pregnancy (e.g., dog), thus lacking typical mechanisms for maternal recognition of pregnancy. These cases could resemble viviparity in non-mammalian vertebrates or gestation in marsupials, and result from gestation lengths short enough to be encompassed by the ancestral luteal phase in the lineage. Alternatively, such long non-pregnant luteal phases might have evolved secondarily, if the extended pCL lifespan resulted in a prolonged npCL too, particularly in cases in which selective pressures to shorten the npCL may be missing, such as in seasonal reproduction or synchronized breeding in the group.
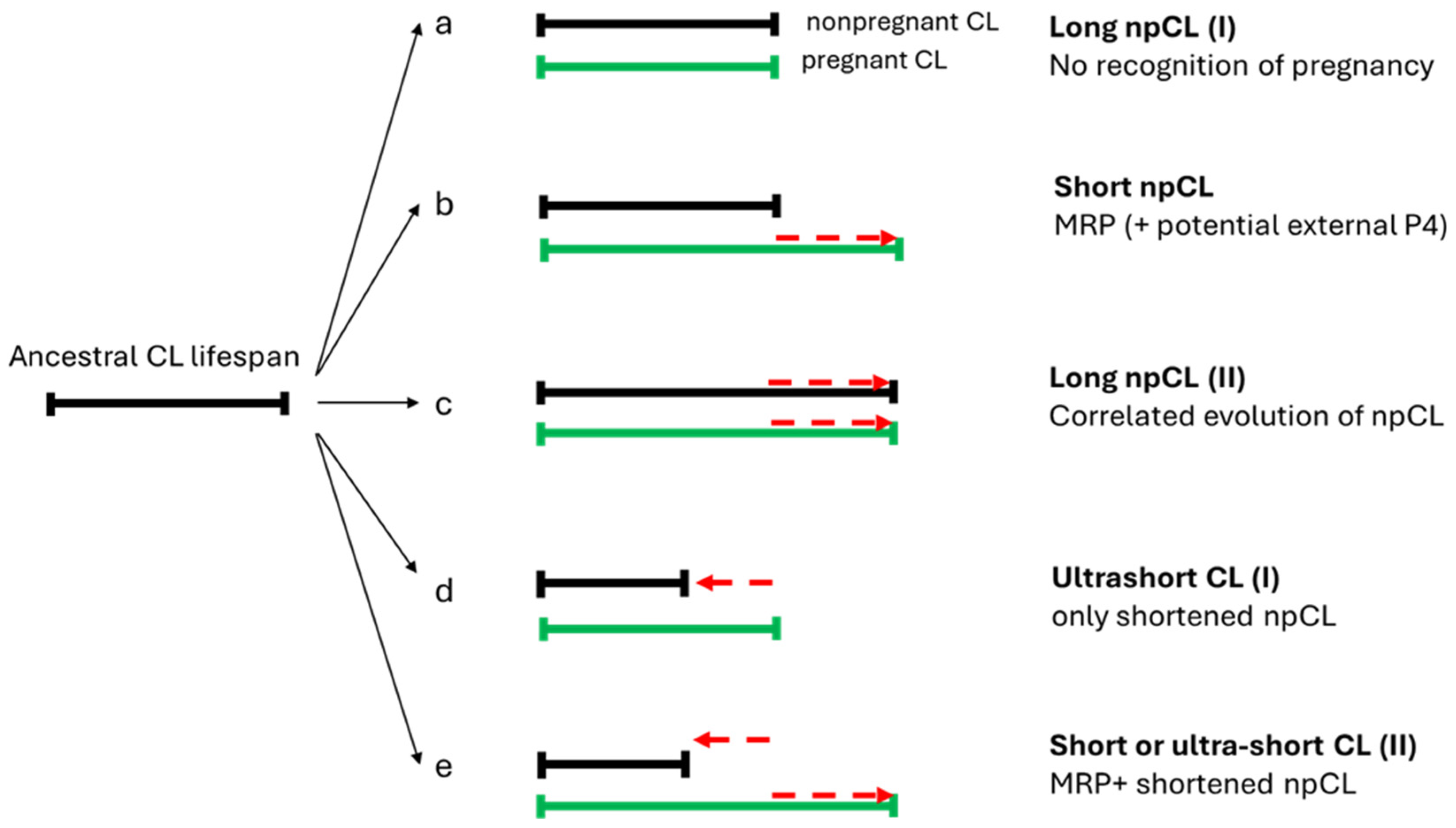
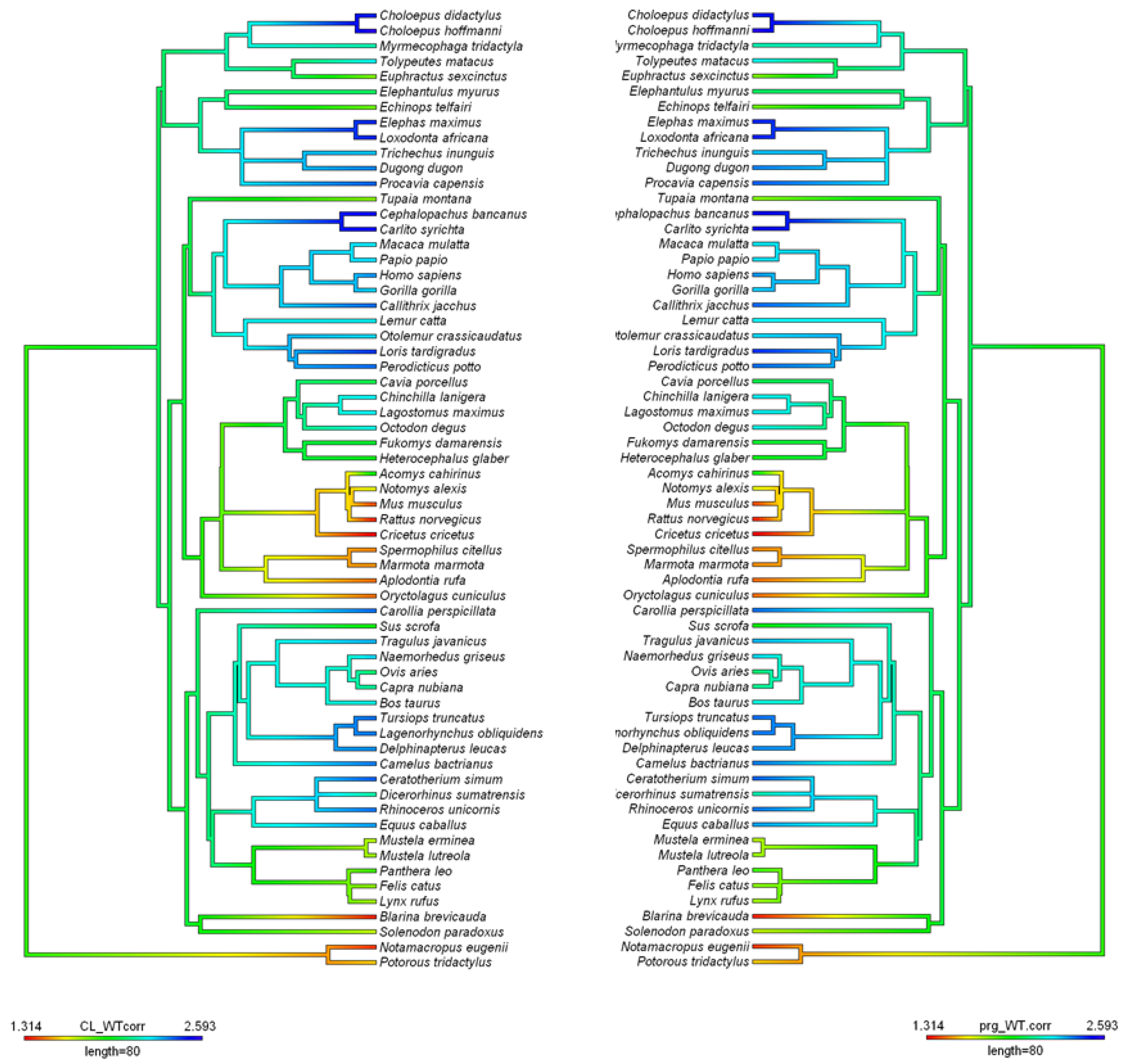
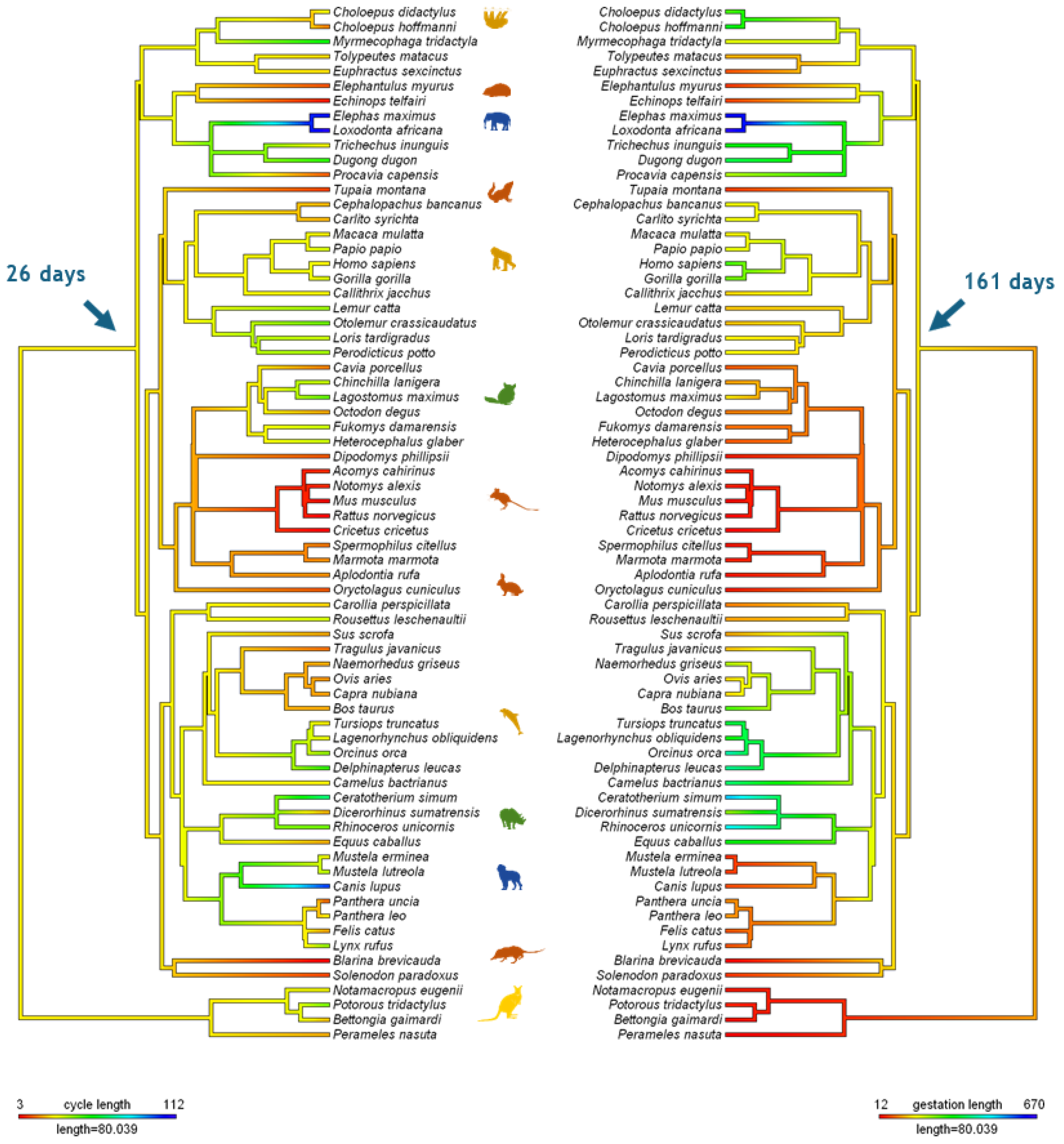
A distinction can be made between three patterns of npCL lifespan: long-lived, short-lived, and ultra-short lived (Hennebold 2018;
Figure 2, adapted). As said, species with long-lived CL show no substantial differences between the pregnant and non-pregnant states, a situation also known as
physiological or
covert pseudopregnancy. In the short-lived corpora lutea, the CL lifespan is prolonged in response to conceptus, compared to the non-pregnant cycle. This type is found conspicuously in most eutherian species and every superorder. On the other side of the spectrum are many rodents, in which the non-pregnant luteal phase is very short and likely secondarily reduced for fast reproduction. In species with an ultra-short luteal phase, a fully functional npCL does not fully develop, and a functional luteal phase can only be induced, mostly by mating. In case of sterile mating a
true pseudopregnancy develops, meaning a pregnancy-like luteal phase that can be induced but is not a part of the regularly occurring cycle. Finally, in species with reflex ovulation, ovulation is also induced by mating, and hence, the CL of pregnancy or nonpregnancy (if unfertilized), only develop after mating. Induced ovulation does not show specificity for any order, as it is found in Lagomorpha, Carnivora, Artiodactyla, Perissodactyla, and Eulipotyphla, and might be ancestral (Pavlicev and Wagner 2016). Here, we review the types of ovarian cycles based on luteal CL lifespan across eutherians, as well as important information on their physiology of reproduction.
4.3. Review of the Luteal Phase and MRP across Eutherians
Xenarthra
Although most xenarthran species prolong the lifespan of the CL in pregnancy, to our knowledge, the mechanisms for maternal recognition of pregnancy in Cingulata and Pilosa are unknown.
The xenarthtran order Cingulata include the nine-banded armadillo (Dasypus novemcinctus), with an invasive, villous hemochorial placentation (Enders and Carter 2012), a delayed implantation and, intriguingly, polyembryony (Hamlett 1933, 1935). The nine-banded female armadillo has a single annual ovulation, and fertilization is followed by an embryonal diapause, delaying implantation by 3 to 5 months (Talmage and Buchanan 1954). Progesterone levels during embryonic diapause do not differ from those of non-pregnant animals (Labhsetwar and Enders 1968), but removing both ovaries during the diapause period precipitates implantation via unknown underlying mechanisms (Buchanan 1956). The pCL shows signs of regression in the latter part of gestation, and progesterone is supplemented by the fetal adrenal gland, whereas the trophoblast is only weakly steroidogenic (Nakakura et al. 1982). Authors have suggested that armadillo’s reproductive features hint that the ancestral ovarian cycle entailed a long, intrinsically regulated luteal phase, into which the invasive implantation and placentation in eutherians have evolved (Hennebold 2018). However, studies on the ovarian cycle of different armadillo species have reported short-lived, functional corpora lutea. For example, authors have indicated that the oestrus cycle of the six-banded armadillo (Euphractus sexcinctus) is around 26 days (Campos et al. 2016) and about 25 for the three-banded armadillo (Tolypeutes matacus). The dissociation of these traits could explain the observed plasticity in type of ovulation (induced vs. spontaneous) and number of yearly cycles (monoestrous vs. polyestrous) in response to environmental cues without implying a difference in the presence/absence of MRP (Stephens et al. 2013).
Representatives of the other xenarthran order, Pilosa (anteaters and sloths), also show a cyclicity and a short-lived, functional npCL. For example, Giant anteaters (Myrmecophaga tridactyla) are polyestrous, nonseasonal breeders with villous hemochorial placentas and the gestation period of around 6 months. Their regular ovarian cycles are around 53 days long, with the luteal phase of about 2-3 weeks and regular preovulatory estrogen peaks followed by increasing progesterone levels (Knott et al. 2013, Patzl 1998). Similarly, the two-toed sloth Choloepus didactylus (suborder Folivora) has regular nonseasonal cycles of around 30 days, with short-lived corpus luteum (luteal phase of 11-16d) and pregnancy of 10 months (Troll 2013, Taube 2008). Shorter cycles of around 16 days have been suggested for the Hoffmann’s two-toed Sloth (Choloepus hoffmanni; Calvo-Fernandez et al. 2024), with the same gestation length. Gestation length of the sloth genus Bradypus with more emphasized seasonality, the gestation length is around 6 months (Taube et al. 2008). Sloths also possess labyrinthine endotheliochorial placentas (Carter and Moss 2012). Although most xenarthran species must possess ways of prolonging the lifespan of the CL in pregnancy, to our knowledge, the mechanisms for maternal recognition of pregnancy in Cingulata and Pilosa are unknown. Therefore, the available evidence on early Eutherian CL regulation and whether there are xenarthran species with long-lived CL is still inconclusive. On the other hand, the extragonadal supplementation of progesterone through the co-option of different steroidogenic tissues in different species may be an early trait of eutherians.
Afrotheria
Within Afrotheria, the species within the clade Paenungulata (Loxodonta africana, Elphas maximus, Dugong dugon, Tricheus inuguis, or Procavia capensis) show similar reproductive traits. Elephants (order Proboscidea) have particularly long infertile ovarian cycles as well as gestational lengths. African elephants prolong their pregnant progesterone levels by accumulating up to 42 CL in the ovary despite uniparity (Perry 1953; Smith et al. 1969), as they produce accessory CLs (acCLs) from non-ovulated, luteinized follicles that develop during pregnancy (Laws 1970). The accessory corpora lutea are essential for maintaining the long elephant pregnancies of around 22 months, during which the ovary is the only source of progesterone (5α-reduced progesterone metabolites) (Lueders et al. 2012). In the absence of an embryo, the elephant CL begins to regress after six weeks, constituting a conspicuously long luteal phase. A luteotropic placental lactogen is the main candidate for the mechanism for maternal recognition of pregnancy in the African elephant (Yakamoto et al. 2011). Interestingly, elephant CL’s rescue only occurs once a viable blastocyst attaches to the endometrium, instead of during the preimplantation phase, possibly to ensure the presence of a healthy embryo, and is characterized as a last-minute mechanism for pregnancy recognition (Lueders et al. 2012).
Species of the closely related order Sirenia (dugong, manatee) show similar reproductive patterns: accessory corpora lutea, uniparity, relatively long ovarian cycle (46-64 days in dugong; Wakai et al. 2002)) long gestational length of 12-13 months, and endotheliochorial placentation (Marsh 1984, Rodrigues et al. 2008, Carter et al. 2008). In contrast, the Rock hyrax (Procavia capensis, order Hyracoidea) lacks accessory CL; instead, the number of CL matches the number of conceptuses, and the CL forms only from ovulated follicles. Based on vaginal smear composition, their cycles are estimated to be of around 14 days (Gombe 1983). Hyracoidea species show remarkably long pregnancies (around seven months) for their small size and have hemochorial placentation (Kayanja 1973). It is unclear whether sources other than the CL provide progesterone for the full length of pregnancy. However, early reports indicated the presence of chorionic gonadotropin hormone in the hyrax placenta (Charanjit 1980).
Less is known about the oestrus cycle in the order Afrosoricida. Small African mammals of the family Tenrecidae breed seasonally (Gould and Eisenberg 1966; Nicoll and Racey 1985) and have long gestations (50-70 days). For example, small madagascar hedgehog Tenrec Echinops telfairi is seasonally polyestrous, with very short recurring oestrus cycles of six days (Godfrey and Oliver 1978) and long gestations of 62-65 days (Eisenberg and Muckenhirn 1968). Details of potential pseudopregnancy or the progesterone sources are unknown.
Lastly, the best-studied species of order Macroscelidea is the eastern rock elephant-shrew (Elephantulus myurus), owing to its spontaneous decidualization, menstruation, and invasive placentation (Carter 2018). The estrous cycle of the elephant shrews is about 12 days, with a short functional luteal phase that is extended in the pregnant cycle to a gestation length of 24 - 75 days (51 in E. myurus) (Tripp 1972). Aardvark, belonging to Tubulidentidae, has a long gestation (7 months) and seasonal reproduction (Van Aarde 1984). To our knowledge, the mechanisms for MRP or placental steroidogenesis have not been studied in elephant shrews or other African insectivores.
No reported cases of luteal-placental shift in Afrotherian species were found. Overall, the most common pattern in the Afrotherian orders is that of a short, functional npCL and a long gestation.
Laurasiatheria
Ruminants display a short-lived corpus luteum that is extended in pregnancy. In the well-studied cattle and sheep, interferon tau (IFNT) has been identified as the primary signal for MRP. This cytokine, produced by the trophectoderm (TE) cells during the peri-implantation period, rescues the CL via multiple mechanisms (Roberts et al. 1992). The best-known mechanism is by silencing the estrogen receptor alpha (ESR1), and thereby downregulating oxytocin receptors in the endometrial epithelium and disrupting OXT-induced release of endometrial PGF2α (Bazer et al. 2008). Less explored mechanisms by which IFNT extends CL lifespan include inhibiting lymphocyte proliferation, modifying the PGE2:PGF2α ratio by prioritizing PGE2 biosynthesis, or altering prostaglandin transport to the CL (Arosh et al., 2004, 2016). The bovine placenta contributes to progesterone production towards late pregnancy (Shenavai et al., 2010). In sheep, the expression of endogenous Jaagsiekte sheep retroviruses (EnJSRVs) in the endometrial luminal and glandular epithelium is enhanced by luteal progesterone and stimulates trophoblast proliferation and production of IFNT (Spencer et al. 2006, Spencer and Bazer 2002). The ewe presents a luteal-placental shift at around 55 days of gestation (Casida and Warwick 1945). IFNT is also likely involved in the primary MRP mechanism in other Artiodactyla species.
The pig ovarian cycle lasts 18-21 days, with the CL forming 4-5 days post ovulation, and peaking in progesterone production at day 12-14 in nonpregnancy. Hysterectomies in the mid-luteal non-pregnant phase result in a CL lifespan of 114 days or more (Anderson 1973), implicating uterine production of a luteolytic factor. Bazer and Thatcher (1977) proposed that in the pig, the endocrine secretion of endometrial PGF2α in the non-pregnant cycle is replaced by the exocrine, secreted in the uterine lumen, in pregnancy (the endocrine-exocrine theory of MRP). The substance secreted by the pig conceptus that causes this change from endometrial PGF2α endocrine secretion to exocrine secretion is estradiol-17 β (E2) (Bazer et al. 2008, 2013). The pig conceptus also increases the PGE2/PGF2α ratio by increasing PGE2 levels and releases cytokines and interferon-γ (IFNG) and IFN-δ (IFND) into the uterine lumen at the time of implantation (Ka et al. 2018). However, these have no anti-luteolytic effect but rather prime the endometrium for implantation. Finally, studies have reported increasing levels of placental progesterone production throughout gestation (Knight 1994), suggesting, at least, a partial luteal-placental shift.
The Camelidae family possesses several unique features for Artiodactyla, such as induced ovulation followed by the development of a short-lived CL. In the induced nonpregnant cycle (i.e., pseudopregnancy), progesterone peaks ten days after ovulation, with endometrial luteolytic pulses of PGF2α starting to peak a day later. If fertilized, MRP in the dromedary (with an ovarian cycle of about 28 days; Musa 1979) occurs between days 8 and 12 after mating, and the CL is the only source of progesterone throughout 15-month-gestation (Skidmore 2018). Although the exact mechanism of MRP is not established, it is suggested to also involve blastocyst estrogen release and endometrial estrogen receptors (Bianchi 2022).
Slightly longer ovarian cycles have been reported for Cetaceans. All the studied Cetaceans are spontaneous ovulators with a short, functional npCL and MRP, the mechanism of which is unknown (Fukui 2007, Pomeroy 2011, Robeck and O’Brien 2017). While the oestrus cycle in the atlantic bottlenose dolphin (Tursiops truncatus) and pacific white-sided dolphin (Lagenorhynchus obliquiden) is of around 27 days, respectively 31 days (Robeck et al. 2005; Robeck et al. 2009), with gestational lengths around 12 months. Longer cycles of about 40 and 50 days have been described in the orca (Robeck and O’Brien 2017) and beluga whale (Steinman et al. 2012), which also have longer gestations (17, respectively 14 months). The steroidogenic capacity of the cetacean placenta has been shown in single species, such as in the Minke Whale (Balaenoptera bonaerensis, Sasaki et al. 2013) or Killer Whale (Orcinus orca, Robeck et al. 2018). To summarize, the Cetartiodactyla ovarian cycles outlined in here entail a relatively short, functional npCL, with oestrus cycles ranging from 15 (e.g., lesser mouse deer Tragulus javanicus, Kusuda et al. 2013) to around 50 days in some cetaceans.
Perissodactyls (horses, rhinoceros, tapirs) manifest seasonal spontaneous polyestrous nonpregnant cycles of 21-22 days and a progesterone peak around 6-18 days post-estrus. The mare conceptus releases an independently evolved chorionic gonadotropin, equine chorionic gonadotropin (eCG) (Carter 2022) that binds to LH receptors. eCG appears only around day 35 in pregnancy, prolonging the pregnancy by inducing additional ovulations and promoting the formation of accessory corpora lutea (Murphy 2018), being a late mechanism of pregnancy recognition, likely evolving in addition to an earlier mechanism. In the mare, the conceptus inhibits the endometrial release of PGF2α by an unknown underlying mechanism (Klein and Troedsson 2011), for which several possibilities have been suggested (Lawson 2022, Smits 2020). By week 16, the mare presents a luteal-placental shift (Conley 2016). The sumatran rhinoceros (Dicerorhinus sumatrensis), on the other hand, is the only perissodactylan induced ovulator reported (Roth et al. 2001). Rhinos possess a short-lived functional CL, but exact length of the ovarian cycles can vary widely across species, ranging from 21 days in Asian rhinos, 43 days in Indian rhinos or up to 70 days in white rhinos (Roth and Brown 1999, Schwarzenberg et al. 1998, 2000). Lastly, a luteal-placental shift after the third month of gestation has been reported for several rhinoceros species, with placental progesterone until the end of 16-17-month pregnancy (Schwarzenberger and Hermes 2023).
The speciose order Chiroptera manifests broad variation in ovarian cycles: in particular in temperate zones, many species are monoestrous (e.g., the Schlieffen’s bat, Van der Merwe and Rautenbach 1987; Common bent-wing bat, Bernard et al. 1991), whereas polyestrous cycles are more common among the tropical species (e.g., wild fulvous fruit bat, Zhang et al. 2007; Cave nectar bat, Krutzsch 2005). Several families include species with spontaneous decidualization in nonpregnancy and menstrual-like bleeding, among them the basal branching Pteropodidae (e.g., the short-tailed fruit bat Carollia; Rassweiler and Bonilla 1992), Molossidae (Rassweiler 1991), and Phyllostomidae (Hamlett 1934). These species have ovarian cycle lengths of around thirty days (Rasweiler and Bonilla 1992, Zhang et al. 2007). Chiropterans can modify their reproductive cycle through delayed ovulation, implantation or embryonal development, and during the latter two the activity of CL is reported to be reduced (Crichton et al. 1989), which complicates determining the length of pregnancy and pregnant CL. Progesterone concentration peaks close to parturition in most species examined, such as in the big brown bat (Eptesicus fuscus, Greville et al. 2022), or in the little brown bat (Myotis lucifugus, Currie et. al. 1988). Placental progesterone synthesis is known in several species, such as in the Natal clinging bat Micropterus schreibersii natalensis (Van Aarde et al. 1994) and in the little brown bat (Currie et. al. 1988). Signs of structural luteolysis are reported to significantly precede parturition in many bat species, which is in itself not surprising given placental progesterone synthesis. Intriguingly, in some species, progesterone levels are reported to start decreasing in mid pregnancy (Burns and Easley 1977), but this may be related to a developmental delay. Overall, Chiropterans manifest a short npCL, which is prolonged in pregnancy, and additional progesterone supplied by the placenta. Chiroptera also presents an extensive range of invasiveness, from endotheliochorial to invasive hemochorial placentation (Gopalakrishna and Karim 1979).
Among Carnivora, the families of the suborder Caniformia differ substantially in the length of npCL, yet all species studied present endotheliochorial placentas (Enders and Carter 2012). The reproductive biology of the Mustelidae family is known for its widespread obligate embryonic diapause. Ferret (Mustela putorius), for example, is seasonally polyestrous with copulation-induced ovulation leading to pregnancy or pseudopregnancy of 39-42 days (Deanesly 1967), and the luteal progesterone levels that start to decline midpregnancy, much before structural luteolysis occurs. The European mink (Mustela lutreola) is seasonally polyestrous (Amstislavsky et al. 2009 a,b) and has a breeding season with oestrus lasting from 1 to 12 days and, when not mated, can re-enter new cycles after 2 to 4 weeks. As is in other Carnivorans, there is no evidence of steroidogenesis in the mink or ferret placentae (Douglas et al. 1998, Blatchley and Donovan 1976). Most species of the Ursuidea family are seasonally polyestrous, induced ovulators (Spady et al. 2007, Boone 2004). Most bears have embryonic diapause, with the CL and the embryo entering a dormant phase after fertilization, enabling the female to enter a new cycle and mate repeatedly, potentially accumulating a litter with different fathers. The litter implants simultaneously after the diapause is finished. The long CL has been reported in seasonally spontaneously ovulating black bears (Tsubota et al. 1987) if no matings occurred, similar to Canids. No placental progesterone synthesis has been found in bears (tested in Japanese black bear, Sato et al. 2001), and MRP is unknown. Seasonal monoestrous cycles with a long npCL are common also in seals (Pinnipedia superfamily; Boyd et al. 1991), with exemptions (e.g., the polyestrous Hawaiian monk seal Monachus schauinslandi; Iwasa and Atkinson 1997). No placental progesterone synthesis was found in the seal species examined (Ishinazaka et al. 2001). As ursids and mustelids, most species of Pinnipedia have embryonic diapause (Renfree and Calaby 1981).
The family Canidae presents some unique characteristics, such as obligatory periods of ovarian inactivity or anestrus (Nagashima and Songsasen 2021). Most species are spontaneous ovulators, with some exceptions (e.g., the maned wolf, Chrysocyon brachyurus; Johnson et al. 2014). Canids have a long monoestrous cycle, with a luteal phase of around two months, irrespective of pregnancy (Concannon 2018). Although the uterus does not shorten or lengthen the sterile ovarian cycle, as shown by hysterectomies, PGF2α does cause a prepartum luteolysis in pregnancy, frequently leading to the pregnancy being somewhat shorter than the sterile luteal phase (Kowalewski 2014). In contrast, in Felids, the pregnant and nonpregnant luteal phases diverge, displaying differences in progesterone patterns. This suggests a role for the luteotrophic and luteolytic factors such as relaxin, prolactin, and prostaglandin F2α (Banks et al. 1983, Tsutsui et al. 1993, Siemieniuch et al. 2010). Felids are mostly induced ovulators but also include species such as lynx, reported to ovulate spontaneously under natural conditions (Brown 2006; Painer et al. 2014). Many felids display polyestrous cycles (Wildt et al. 1981). The domestic cat (Felis catus) has induced ovulation and can enter a new oestrus cycle after 2 to 3 weeks if nonpregnant. After a fertile mating, pregnancy lasts 58 - 65 days. Other felids have slightly longer cycles and gestation periods, like the Bengal tiger (Panthera tigris) with over 100 days gestation (Brown 2011). The Eurasian and Iberian Lynx are interesting exceptions, with a long monoestrous cycle and a CL life span of up to two years or more, with elevated progesterone levels (Painer et al. 2014; Göritz et al. 2009). Oestrus and pregnant cycles (44, respectively about 50 days) are close in length also in the bobcat (Lynx rufus; Göritz et al. 2009). Studies have reported the presence of steroidogenic enzymes and detectable levels of progesterone in the felid placentae for domestic cat and Iberian lynx (Malassiné et al. 1979, Braun et al. 2012), but the details of the luteal-placental shift are not well-known.
Finally, within the order Eulipotyphla, a very short estrous cycle (2-4 days) has been reported for the seasonally polyestrous short-tailed shrew (Blarina brevicauda; Nowak 1999), with gestation averages of about 21 days, and for the Hispaniolan solenodon (Solenodon paradoxus), with a 9-13 days estrous cycle and gestation average of 50 days (Eisenberg and Gould 1966).
In summary, the nonpregnant luteal phase of Laurasiatherians encompasses all types of cycles, including the ultra-short, short and long-lived npCL. In some of the species with short-lived npCL, above all Cetardiodactyla and Perissodactyla, anti-luteolytic mechanisms for maternal recognition of pregnancy have been identified, but in most orders they remain unknown.
Euarchontoglires
Euarchontoglires is the mammal clade including the relatives of Euarchonta, the primates and their relatives, as well as Glires, rodents and lagomorphs. Reproduction of the rabbit (Oryctolagus cuniculus) is best investigated of the lagomorphs. Rabbits undergo mating-induced ovulation with the subsequent formation of a CL of pregnancy (30 days) or pseudopregnancy (16-18 days; Bazer 2015). The CL persists as the primary source of progesterone throughout pregnancy. When and how the function of the CL in pregnancy is rescued beyond the life span in pseudopregnancy is not known (Browning and Wolf 1981, Marcinkiewicz et al. 1992, Gadsby et al. 1983), as no significant differences between pregnant and pseudopregnant females until days after implantation have been found. Estrogen has been suggested as the main luteotrophic factor (Keyes and Gadsby 1987).
Rodents are the largest mammalian group, with a highly diverse reproductive biology. In the following, we follow the phylogeny of Swanson et al. (2019), staying at the suborder level. The basally-branching suborder of Ctenohystrica (Guinea pig, chinchilla, viscacha, degu, porcupine, etc.) is characterized by spontaneous ovulation, a functional nonpregnant CL, and a long gestation. For example, the Guinea pig (Cavia porcellus) has a 16-day cycle, 12 of which are the luteal phase, and a gestation length of 70 days. In the guinea pig, a release of a luteotropic substance three days after ovulation induces the formation of functional CL, while the second release depends on embryo implantation between days 4 and 6 of gestation, maintaining the CL until mid-gestation, when luteal-placental shift occurs (Aldred et al. 1961). The maintenance of the CL beyond the luteal phase of the nonpregnancy has been suggested to be related to the presence of a chorionic gonadotropin-like hormone (Sherman et al. 2001, Carter 2022) or a prolactin-like hormone secreted by the spongiotrophoblast (Alam et al. 2010); however, the exact mechanism of maternal recognition of pregnancy remains unknown. Chinchilla has a long non-pregnant cycle of 30-40 days, and 105-115 days gestation, similar to plains viscacha (Lagostomus maximus), with a 45 days estrous cycle and a 154 days gestation (Weir 1971). In Degu (Octodon degus), the cycle is of 17-21 days, while gestation lasts 90-95 days (Reynolds and Wright 1979). Naked mole rats (Heterocephalus glaber) have a gestation length of 70 days.
Similar gestation lengths can be found in the sister-clade Sciuromorpha, for instance, in the Castor Canadensis gestation lasts 100-130 days (refs). In other species, like the Arena Mountain Beaver (Aplodontia rufa nigra) shorter gestation lengths (up to a month) have been reported, although they probably constitute an underestimation (for a discussion see Zielinski and Mazurek 2016). Similar is the case with the monoestrous Alpine marmot (Marmota marmota; 33-35 days) or Woodchuck (Marmota monax; 34 days), and similar gestation lengths are reported for ground squirrels (28-34 days), and somewhat longer in squirrels (up to 42 days). The length of the estrous cycle or luteal phase is not reported in most members of Sciuromorpha. In the suborder Castorimorpha, the nonpregnant cycle suggests short, functional npCL, and these species also primarily manifest a long pregnancy: Beaver (Castor sp.), a seasonally polyestrous species with a cycle of ca. two weeks, and a pregnancy of 100-110 days or the Kangaroo rat (Dipodomys sp.), with a cycle of 12-13d and a gestation length of ca. 31days.
Notably, many of the species with long nonpregnant cycles breed seasonally in nature- possibly indicating that their npCL is under less selection for rapid succession of estrous phases under natural conditions- even if they may be physiologically able to breed continuously (e.g., in captivity).
Classical rodent model species belong to the superfamily Muroidea in the suborder Myomorpha: rats, hamsters, deer mice, gerbils and mice. These species are characterized by a short (4-5d) nonpregnant cycle with an ultra-short nonfunctional npCL, and lack chorionic gonadotrophins and placental progesterone production. In the paradigmatic house mice (Mus musculus), the progesterone from the postovulatory follicle is low, and the follicle soon degenerates. Mating is necessary in mice for a functional CL with sufficient progesterone for implantation. Mating induces the release of prolactin (PRL) from the anterior pituitary, the initial luteotrophic signal enhancing LH receptors and inhibiting the activity of progesterone-catabolizing enzyme 2-hydroxysteroid dehydrogenase (Soares 2004). If mating is sterile, this results in a 12-14 days long pseudopregnancy. If mating is fertile, lactogenic hormones from the conceptus and the uterine decidua maintain the luteal cells and progesterone secretion after day 12, shifting the dependency of pregnancy to placental luteotrophins (Choundary and Greenwald 1969). While its function in lactation is shared among eutherians, prolactin (PRL) has a broad species-specific array of additional functions (Soares at el. 2007), and the involvement in pregnancy of PRL gene family members have been reported in rodents, primates, and ruminants (Soares 2004), even if their exact role is not fully understood (Ben-Jonathan et al. 2008). In most species, PRL production is restricted to the pituitary and the decidua; however, in mice, trophoblasts also express PRL genes (Rawn and Cross 2008). Pituitary PRL plays a similar, if not identical, role as the pregnant luteotrophic hormone in hamsters and rats. Cricetidae hamsters and rats also have ultra-short npCL, with short estrous cycles of four days and gestation periods of 16 and 22 days, respectively. They have induced pulses of prolactin from the maternal anterior pituitary gland and lactogenic hormones from the uterine decidua and placenta. While shared among (most efficiently reproducing) model species, this type of cycle is not representative of all Myomorpha. Indeed even within the Muridae family, several species show long nonpregnant cycles with functional luteal phase, such as the Spinifex hopping mouse (Notomys alexis) with a 8 days cycle (Breed 1975, Breed et al. 2011), or the African spiny mice (Acomys cahirinus), with 6 - 10 days, and a menstruation of ca. three days at the end of the luteal phase (Peitz 1981, Bellofiore et al. 2018). A functional npCL also exists among Critecidae, e.g., the Cotton rat (Sigmodon hispidus) has a 7-9 day cycle (Faith et al. 1997). Interestingly, these species also have a longer gestation than the species with ultra-short CL (38-41 days in N. alexis, 38-45 days in A. cahirinus, 27-28 days in S. hispidus). The presence of a luteal-placental shift or mechanisms of MRP have not been reported for these species.
Primates are characterized by a short-lived functional npCL which is extended during pregnancy. The syncytiotrophoblast of the blastocyst adhering to the maternal endometrium secretes chorionic gonadotropin (CG) in New World monkeys, Old World monkeys, apes, and humans. The gene for its β-subunit arose by the duplication of the β-subunit gene of the luteinizing hormone (present in all mammals) in the lineage of anthropoid primates (Maston and Ruvolo 2002). Its action in humans is well understood: human CG (hCG) signaling constitutes a classical mechanism for maternal recognition of pregnancy, with the gene transcribed at the eight-cell stage before implantation. hCG takes over the direct luteotrophic effect of LH on the corpus luteum, signaling via the same receptor (LHCGR), (Sugino et al. 2000). Besides hCG, additional factors could be involved in promoting human CL’s steroidogenesis after implantation (Oon and Johnson 2000). Placental progesterone production (i.e., luteo-placental shift) sets in at about seven weeks into human pregnancy (Fazleabas et al. 2004).
Although with some specializations (which may or may not have a parallel in Old World primate adaptations), such as more variation in litter size, New World monkeys exhibit typical primate cycles. For instance, the Marmoset (Callitrhix jacchus) has cycles of around 28-30 days (but lacks menstruation) and gestation lengths of around 144 days (Tardif et al. 2003). It was reported early on that the duration of CL’s progesterone secretion is longer in the Marmoset than other primates, indicating that the luteal-placental shift occurs at a later stage of pregnancy (Hodges et al. 1983). (Hodges et al. 1983). Interestingly, it has been proposed that a higher number of fetuses (twins in nature) may extend the period of elevated chorionic gonadotropin, resulting in a delayed luteal-placental shift (Ziegler et al. 2023).
Prosimian primates (lemurs and their relatives) do not produce chorionic gonadotropin. In addition, strepsirrhine primates possess epitheliochorial placentas and non-invasive implantations (Mossman 1987, Carter and Enders 2003). Prosimians have nonseasonal spontaneous ovarian cycles, as well as long gestations, like other primates do (reviewed in Van Horn and Eaton 1979). For instance, the mean ovarian cycle length of the ring-tailed lemur (Lemur catta) is around 39 days, with gestations of about 136 days (Evans and Goy 1968). On the shorter end, the Philippines tarsier (Carlito syrichta) has cycles of about 23 days and gestations of about six months (Catchpole and Fulton 1943). Some prosimians, such as the brown greater galago (Galago crassicaudatus), have conditional seasonality: this nocturnal primate is polyestrous in captive conditions while remaining monoestrous in the wild (Von Horn and Eaton 1979). Their oestrus cycle length, however, is within the range for primates (about 44 days) (Eaton 1973), with a gestation of 133 days, illustrating that short-lived npCL can coincide with monoestrous reproductive modes. To our knowledge, nothing is known about the prosimian luteo-placental shift, nor about the mechanisms for MRP that extend CL’s lifespan in prosimians.
In summary, most Euarchontoglires manifest a short-lived functional npCL. A subset of rodents, including several animal models, have a likely derived ultra-short npCL with an induced luteal phase. No cases of long-lived corpora lutea have been reported in this superorder. Prolactin and chorionic gonadotropin are the most widespread signals for MRP in this superorder, but they are also the only ones studied to date. The MRP signals that play a role in most rodents with a short functional npCL, or in prosimians, are unknown.
4.4. Evolutionary Trends in Mammalian CL Lifespan and Life History Traits
The endocrine participation of the fetus, first through signals for MRP and then through steroid synthesis, introduced a shift in the control of pregnancy support from the ovary to the blastocyst and then to the placenta. These two mechanisms are two steps of a crucial event, namely the decoupling of the pregnant and nonpregnant cycle, and constitute essential novelties in neuroendocrine control that paved the way for the longer gestation lengths characteristic of eutherians. Still, understanding the evolution of gestation and its decoupling from the homologous luteal phase requires considering what may have driven the evolution of eutherian gestation length before and after the appearance of these signaling events.
First, the phylogenetic co-distribution of reproductive traits shows that the maternal recognition of pregnancy is widely distributed, regardless of the type of placentation or the presence of the luteo-placental shift. This observation challenges the notion that the underlying driving force for the evolution of MRP is the maternal-offspring conflict (McCoy and Haig 2020), which would be particularly manifested in invasive placentation, in which fetal signaling to the mother is most direct. In contrast, while the mechanisms of decoupling, such as endocrine activity of the blastocyst (MRP) and the placenta (luteo-placental shift) are present in species with invasive placentation, they are equally present in the lineages with noninvasive placentation, such as ungulates (
Figure 3). Therefore, the different types of fetal over maternal control are not correlated, as might be expected when following the notion of maternal-fetal conflict. A short-lived, functional npCL is the most widely distributed type of cycle among extant eutherians, co-occurring with all placentation types, regardless of the presence of luteal-placental shift, pseudopregnancy and the type of ovulation (
Figure 3). The long-lived npCL in long monoestrous cycle, in contrast, is rare and coincides with an endotheliochorial placentation in carnivores. Similarly lineage-specific is the ultra-short-lived npCL and induced-luteal phases (Muridae, Cricetidae, Eulipotyphla, Afrosoricida and Macroscelidea), which only seem to have evolved in species with hemochorial placentas with no gonadotropin or progesterone expression. From the nestedness of the species with exceptionally long or ultra-short npCL in clades with short-lived npCL, we suggest that the two former are derived cycles, whereas the latter is the shared condition for most major groups, following the origin of MRP.
Second, it has long been appreciated that mammalian evolution involves increases in body size (Cope 1887, Alroy 1998) and that the gestation length scales allometrically with body size in species giving birth to precocial as well as altricial young, albeit to a different extent (Martin and MacLarnon 1985). These relationships were also found in our dataset (
Figure 4A). Moreover, we found that the length of the nonpregnant cycle is also positively correlated with body size (
Figure 4B). The latter implies that the length of the nonpregnant cycle coevolved with the gestation length as their correlation is maintained even when accounting for body size (by regression on log-log scale;
Figure 4C). We anticipate an even more pronounced correlation between the actual homologs, namely the lifespans of corpora lutea of pregnancy and nonpregnancy, than is seen between the gestation length and cycle length. We expect this because in many precocial species, gestation is prolonged by non-ovarian sources of progesterone (or accessory corpora lutea), and because our data on nonpregnant cycle refers to the whole cycle rather than just to the luteal phase. Assuming that a sufficient amount of data on the pCL and npCL lifespans can be generated, testing this relationship in the future will inform us whether an evolutionary constraint to gestation length increase due to a coupling between pCL and npCL persists.
To avoid the effects of the bias towards larger body size in extant species, we removed the effect of body size (using regression on the log-log scale) before conducting the ancestral state reconstruction of ovarian cycle and gestation length (
Figure 5). Even so, the inference suggests that the ancestral length of the nonpregnant cycle was substantially shorter than the ancestral gestational length (26 days vs. 161 days, respectively,
Figure 6), implying that a mechanism for decoupling and thus maternal recognition of pregnancy existed in the eutherian ancestor. This seemingly contradicts the empirical evidence of different MRP mechanisms in major lineages (
Figure 7), which strongly suggests that these have originated independently after the lineages separated. We can think of two potential solutions to this conundrum, both pointing to problems in ancestral state inference when the traits do not change continuously.
One possibility is that the eutherian ancestor lacked regular, endogenous nonpregnant cycles. It has been suggested that the ancestral type of ovulation is induced, whereas spontaneous ovulation evolved repeatedly within eutherian lineages (Pavlicev and Wagner 2016). In this case, the prolongation of the pregnant cycle would have been possible at no cost of the prolonged nonpregnant cycle, as the latter (i.e., pseudopregnancy) in most cases does not occur, and thus does not carry a fitness cost. Assuming this situation, MRP would only become necessary once spontaneous ovulation evolved in major lineages, consistent both with early prolongation of pregnancy, as well as with the evidence for the independent origins of MRP.
Another possibility is that the ancestral pregnancy length has been systematically overestimated in phylogenetic inference because the origin of MRP involves a discontinuous change in gestation length and hence extrapolation from species with MRP is not permissible (
Figure 8A,B). Several phylogenetic reconstructions and the fossil data suggest that the ancestral eutherian was of the size of a small rat, but there is less consensus on whether its neonates were altricial or precocial, although recent evidence is tilting towards the latter (altricial: Werneburg et al. 2016, Ferner et al. 2017, precocial: Funston et al. 2022, White et al. 2023). The independent extensions of gestation would postdate the independent origins of MRP in major lineages if we assume altriciality in the eutherian ancestor. Assuming precociality, on the other hand, entails that an extended gestation length would have occurred already in stem eutherians, possibly in close succession with the origin of implantation. This extension may not have amounted to much, as small body size would require relatively short gestation length even for precocial young. If the pregnant and nonpregnant cycles became decoupled only much later, this extension in the stem lineage of Eutheria would have involved a prolongation of the nonpregnant luteal phase as well, due to correlated evolution (
Figure 9). This scenario involves two stages: the first stage consists of an initial extension of gestation in small-bodied ancestral eutherians that likely affected the length of the non-pregnant luteal phase. The second stage, driven by an independent selection on body size in all major lineages (Cope 1887, Alroy 1998, Baker et al. 2015), entails the further prolongation of gestation length. In this second stage, the increases in body size intensified the selection for a decoupling between pregnant and non-pregnant cycles. It is in this second stage when the diverse lineage-specific mechanisms for maternal recognition of pregnancy evolved, as well as non-luteal sources of progesterone. Our proposed scenario suggests that the increase in gestation length was not mainly driven by the maternal-fetal conflict but rather by the increase in body size.
To end with, our scenario implies that the extant derived cases of long-lived npCL (e.g., dog) could have resulted from the extension of the nonpregnant cycle as a consequence of two possible causes (
Figure 9). One is a further extension of the pCL, if MRP is lost secondarily due to absence of selection for short npCL. The second could be the advantage of long npCL for pack breeding. In both cases a long-lived npCL could potentially represent an atavistic regulation. Similarly, ultra-short cycles are likely due to a secondary shortening of the npCL, without changes to gestation length (
Figure 9). The length of mouse pseudopregnancy would thus reveal the secondarily concealed ancestral luteal phase length. Both derived cases also reveal a persistent underlying correlation between the pregnant and nonpregnant cycle.
5. Conclusions and Prospects
Our aim was to understand when and how the innovations of the CL regulation, critical for female reproduction, originated in vertebrate evolution, what physiological role they may have played, and what evolutionary consequences they had for female reproduction.
Despite variation in its formation, cell type composition, or the roles of luteal progesterone, the corpus luteum is a homologous vertebrate endocrine structure (Gemmell 1995). Present already in hagfishes, the CL evolved into the context of an existing HP(O) axis, the predecessor of which can be recognized in amphioxus. Early vertebrate genome duplications set the stage for diversifying the factors involved in HPO signaling, specifically pituitary gonadotrophs and steroid sex hormone receptors. Therefore, the origin of the corpus luteum cannot be explained by its current role in eutherian gestation; instead, it predates internal fertilization, and thus any form of ovoviviparity or viviparity. The origin of the gland was associated with the ancestral role of progesterone, likely before progesterone evolved a signaling role via the evolution of its nuclear receptor. We thus suggest that the most plausible early effect of progesterone was to postpone folliculogenesis and delay subsequent ovulations.
We want to emphasize that the evolution of internal fertilization was the critical transition in the evolution of vertebrate reproductive modes. With the exception of Amniotes, which share a single origin of internal fertilization, all other instances of viviparity origination (elasmobranchs, teleosts, amphibians) are nested in lineages with independently evolved internal fertilization. Consequently, the origins of different instances of viviparity are rooted in different coordination regimes between the ovary and the reproductive tract, and are cases of convergence or parallelism rather than homology, implying that the role of progesterone in these processes likely evolved independently in each group. For these reasons, the inference of mechanisms involved in viviparity across different lineages should be done with much caution (Blackburn 2006). Internal fertilization was the setting in which the new reproductive functions of progesterone first arose. While progesterone is necessary in non-mammalian species for early developmental processes, it is not generally essential for maintaining full-length internal development. The roles that progesterone acquired in non-mammalian reproduction include the ones shared with eutherians, such as the coordination of the ovary with the oviduct and/or the endometrium, but also species-specific ones, like delaying embryonic development or complementing maternal provisioning. Overall, whether progesterone is essential in certain stages of non-mammalian gestation depends on which of these additional, species-specific roles are realized. The increasing regulation of the CL by non-ovarian sources, together with the functional divergence of the post-ovulatory follicle, is a hallmark of eutherian reproduction. We have found no conclusive evidence reported for the existence of such an exogenous regulation extending the pCL lifespan outside placental mammals.
The key innovation in eutherian reproduction is the accommodation of invasive implantation. All eutherians require progesterone from implantation to parturition, even if the serum levels and temporal profiles of progesterone vary widely across species (e.g., from 10 ng/ml in Japanese macaques (Shimizu 2008), >100 ng/ml in mice (Murr et al. 1974), to >200 ng/ml in human). Likely fueled by this requirement, eutherians have evolved multiple but non-redundant ways of controlling CL lifespan or maintaining high progesterone by other means, including blastocyst and decidual signals, accessory corpora lutea, or placental steroidogenesis.
We have argued that a unique characteristic trait in eutherians is the ability to decouple the pregnant and nonpregnant cycles by rescuing the CL only during pregnancy. However, even if maternal recognition of pregnancy (in terms of CL length) is an exclusively eutherian characteristic, it is not a homologous trait among major lineages (mechanisms for MRP differ across the eutherian lineages), and is not universally present across Eutheria. For instance, eutherian species with a long npCL or induced ovulation do not experience the cost of the long nonpregnant cycle. The ancestral reconstruction of the eutherian gestation length likely points to the limits of the phylogenetic method for inferring ancestral traits in isolation from their biological context. The method assumes a continuous change of the trait, without substantial punctuated change or novelties. A crucial aspect of our work has been to consider gestation length in the context of its ancestral coupling with the nonpregnant cycle and its coevolution with the body size. This enables us to see that the evolution of the extant gestational lengths involved novelties, namely overcoming the coupling of pregnancy to the nonpregnant cycle (i.e., the origin of MRP), which in addition we argue occurred independently in most major lineages. This decoupling could have led to an abrupt change in the pregnancy length, with little effect on the nonpregnant cycle. In such a situation, the phylogenetic comparative method will overestimate the ancestral pregnancy length. Another aspect of gestational length that makes it a problematic trait to trace, is that it is a composite trait. While implantation is likely homologous, the segments of pregnancy in different eutherian species are likely not, and are instead based on independently evolved mechanisms (e.g., a luteal-placental shift in progesterone production at a given gestational point).
An interesting corollary of the proposed model for the evolution of gestation length concerns the evolution of adult mammalian body size. While selection for increased body size has likely driven the extension of gestational length needed to develop a large neonate, the instances of decoupling of the pregnant from nonpregnant cycles were likely the key events enabling the body size increase we see in all major lineages. As pregnancy length must increase with the body size, the lack of decoupling of the nonpregnant luteal phase would mean that in case of a sterile cycle, the next pregnancy would be substantially delayed, which would be deleterious in most polyestrous species as it lowers the overall reproductive success. The co-distribution of reproductive traits across eutherians does not support conflict narratives depicting a progression towards more fetal control over maternal resources. MRP co-exists with secondarily non-invasive placentations, such as in ungulates, or with the lack of a luteal-placental shift, such as in proboscidea species, where the accessory CL prolonging pregnancy are in fact maternal structures. Rather than by mother-offspring conflict, the origin of MRP and extended pregnancy is likely driven by evolution of an increased body size.
Finally, we would like to note that there are many aspects that remain unaddressed in our scenario, in part due to the lack of data for many species in the wild, and in part due to the lack of attention to the nonpregnant cycles, or to the lifespan of the CL in particular. In addition, gestational length is certainly affected by further factors that we did not consider here, such as litter size. Another important aspect that remains unaddressed in this review, but may reveal further, past or present, constraints, are the roles that reproductive factors played in the development and function of the male reproductive system. As male and female reproductive functions may often be coupled in the evolutionary process (e.g., pituitary hormones also regulate the function of male gonads), evolutionary explanations may require considering the biology of both sexes.
Acknowledgments
The authors thank Günter Wagner and the members of Pavlicev lab for their valuable feedback. This work was funded by the Austrian Science Foundation FWF (grant #P33540 to MP).
References
- Adams, B.A.; Tello, J.A.; Erchegyi, J.; Warby, C.; Hong, D.J.; Akinsanya, K.O.; Mackie, G.O.; Vale, W.; Rivier, J.E.; Sherwood, N.M. Six Novel Gonadotropin-Releasing Hormones Are Encoded as Triplets on Each of Two Genes in the Protochordate, Ciona intestinalis. Endocrinology 2003, 144, 1907–1919. [Google Scholar] [CrossRef] [PubMed]
- Alam S., K., Konno T., Rumi M. K., Dong Y., Weiner C. P. and Soares M. J. 2010. Prolactin family of the guinea pig, Cavia porcellus. Endocrinology, 151(8), 3918-3928.
- Aldred, J.P.; Sammelwitz, P.H.; Nalbandov, A.V. MECHANISM OF FORMATION OF CORPORA LUTEA IN GUINEA-PIGS. Reproduction 1961, 2, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Al-Gubory, K.H.; Ceballos-Picot, I.; Nicole, A.; Bolifraud, P.; Germain, G.; Michaud, M.; Mayeur, C.; Blachier, F. Changes in activities of superoxide dismutase, nitric oxide synthase, glutathione-dependent enzymes and the incidence of apoptosis in sheep corpus luteum during the estrous cycle. Biochim. et Biophys. Acta (BBA) - Gen. Subj. 2005, 1725, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Allen W., M. and Corner G. W. 1930. Physiology of corpus luteum VII. Maintenance of pregnancy in rabbit after very early castration, by corpus luteum extracts. Proceedings of the Society for Experimental Biology and Medicine, 27(5), 403-405.
- Allen W., M. and Wintersteiner O. 1934. Crystalline progestin. Science, 80(2069), 190-191.
- Allen, W.M.; Butenandt, A.; Corner, G.W.; Slotta, K.H. Nomenclature of Corpus Luteum Hormone. Nature 1935, 136, 303–303. [Google Scholar] [CrossRef]
- Alroy, J. Cope’s Rule and the Dynamics of Body Mass Evolution in North American Fossil Mammals. Science 1998, 280, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Amoroso E., C. and Finn C. A. 1962. Ovarian activity during gestation, ovum transport and implantation. The ovary, 1, 451-537.
- Amstislavsky, S. 2009a. Reproductive biology and embryo technology in Mustelidae. Kuopion yliopisto.
- Amstislavsky, S., Lindeberg H., Ternovskaya Y., Zavjalov E., Zudova G., Klochkov D. and Gerlinskaya L. 2009b. Reproduction in the European mink, Mustela lutreola: oestrous cyclicity and early pregnancy. Reproduction in domestic animals, 44(3), 489-498.
- Anderson L., L., Ford J. J., Melampy R. M. and Cox, D. F. 1973. Relaxin in porcine corpora lutea during pregnancy and after hysterectomy. American Journal of Physiology-Legacy Content, 225(5), 1215-1219.
- Awruch, C.A.; Pankhurst, N.W.; Frusher, S.D.; Stevens, J.D. Endocrine and morphological correlates of reproduction in the draughtboard shark Cephaloscyllium laticeps (Elasmobranchii: Scyliorhinidae). J. Exp. Zool. Part A: Ecol. Integr. Physiol. 2008, 309A, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Awruch, C. Reproductive endocrinology in chondrichthyans: The present and the future. Gen. Comp. Endocrinol. 2013, 192, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Arosh, J.A.; Banu, S.K.; Kimmins, S.; Chapdelaine, P.; MacLaren, L.A.; Fortier, M.A. Effect of Interferon-τ on Prostaglandin Biosynthesis, Transport, and Signaling at the Time of Maternal Recognition of Pregnancy in Cattle: Evidence of Polycrine Actions of Prostaglandin E2. Endocrinology 2004, 145, 5280–5293. [Google Scholar] [CrossRef] [PubMed]
- Arosh, J.A.; Banu, S.K.; McCracken, J.A. Novel concepts on the role of prostaglandins on luteal maintenance and maternal recognition and establishment of pregnancy in ruminants. J. Dairy Sci. 2016, 99, 5926–5940. [Google Scholar] [CrossRef]
- Asdell, S.A. THE GROWTH AND FUNCTION OF THE CORPUS LUTEUM. Physiol. Rev. 1928, 8, 313–345. [Google Scholar] [CrossRef]
- Banks, D.R.; Paape, S.R.; Stabenfeldt, G.H. Prolactin in the Cat: I. Pseudopregnancy, Pregnancy and Lactation 1. Biol. Reprod. 1983, 28, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.E. Evolution of adrenal and sex steroid action in vertebrates: a ligand-based mechanism for complexity. BioEssays 2003, 25, 396–400. [Google Scholar] [CrossRef]
- Baker, M.E. Steroid receptors and vertebrate evolution. Mol. Cell. Endocrinol. 2019, 496, 110526. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Meade, A.; Pagel, M.; Venditti, C. Adaptive evolution toward larger size in mammals. Proc. Natl. Acad. Sci. 2015, 112, 5093–5098. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Thatcher, W.W. Theory of maternal recognition of pregnancy in swine based on estrogen controlled endocrine versus exocrine secretion of prostaglandin F2α by the uterine endometrium. Prostaglandins 1977, 14, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Bazer F., W., Spencer T. E., Ott T. and Johnson G. A. 2008. Maternal recognition of pregnancy. Pages 260-285 in The Endometrium: Molecular, Cellular and Clinical Perspectives, Second Edition. CRC Press.
- Bazer, F.W. Pregnancy recognition signaling mechanisms in ruminants and pigs. J. Anim. Sci. Biotechnol. 2013, 4, 23–23. [Google Scholar] [CrossRef] [PubMed]
- Bazer F., W. 2015. History of maternal recognition of pregnancy. Pages 5-25 in Regulation of Implantation and Establishment of Pregnancy in Mammals. Springer, Cham.
- Beard, J. 1897. The span of gestation and the cause of birth: a study of the critical period and its effects in mammalia. Jena, German: Fischer.
- Behrman H., R., Kodaman P. H., Preston S. L. and Gao S. 2001. Oxidative stress and the ovary. Journal of the Society for Gynecologic Investigation, 8 (1_suppl), S40-S42.
- Bellofiore, N.; Cousins, F.; Temple-Smith, P.; Dickinson, H.; Evans, J. A missing piece: the spiny mouse and the puzzle of menstruating species. J. Mol. Endocrinol. 2018, 61, R25–R41. [Google Scholar] [CrossRef] [PubMed]
- Ben-Jonathan, N., LaPensee C. R. and LaPensee E. W. 2008. What can we learn from rodents about prolactin in humans? Endocrine reviews, 29(1), 1-41.
- Bennett, E.J.; Jones, S.M. Interrelationships among plasma progesterone concentrations, luteal anatomy and function, and placental ontogeny during gestation in a viviparous lizard (Niveoscincus metallicus: Scincidae). Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2002, 131, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Bernard, R.T.F.; Bojarski, C.; Millar, R.P. Plasma progesterone and luteinizing hormone concentrations and the role of the corpus luteum and LH gonadotrophs in the control of delayed implantation in Schreibers’ long-fingered bat (Miniopterus schreibersii). Reproduction 1991, 93, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.P.; Gallelli, M.F.; Herrera, J.M.; Benavente, M.A.; Rossetto, L.; Aba, M.A. Current knowledge about the processes of luteolysis and maternal recognition of pregnancy in camelids. Reprod. Domest. Anim. 2022, 58, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, D.G. SQUAMATE REPTILES AS MODEL ORGANISMS FOR THE EVOLUTION OF VIVIPARITY. Herpetol. Monogr. 2006, 20. [Google Scholar] [CrossRef]
- Blackburn, D.G. Evolution of vertebrate viviparity and specializations for fetal nutrition: A quantitative and qualitative analysis. J. Morphol. 2014, 276, 961–990. [Google Scholar] [CrossRef] [PubMed]
- Blatchley, F.R.; Donovan, B.T. Progesterone secretion during pregnancy and pseudopregnancy in the ferret. Reproduction 1976, 46, 455–456. [Google Scholar] [CrossRef] [PubMed]
- Blitek, A.; Ziecik, A.J. Effect of LH on prostaglandin F2α and prostaglandin E2 secretion by cultured porcine endometrial cells. Reproduction 2005, 130, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Boone, W.R.; Keck, B.B.; Catlin, J.C.; Casey, K.J.; Boone, E.T.; Dye, P.S.; Schuett, R.J.; Tsubota, T.; Bahr, J.C. Evidence that bears are induced ovulators. Theriogenology 2004, 61, 1163–1169. [Google Scholar] [CrossRef]
- Bradshaw, F.J.; Bradshaw, D. Progesterone and reproduction in marsupials: A review. Gen. Comp. Endocrinol. 2011, 170, 18–40. [Google Scholar] [CrossRef] [PubMed]
- Bragdon, D.E. The non-essentiality of the corpora lutea for the maintenance of gestation in certain live-bearing snakes. J. Exp. Zool. 1951, 118, 419–435. [Google Scholar] [CrossRef]
- Bragdon, D.E. Corpus luteum formation and follicular atresia in the common garter snake, Thamnophis sirtalis. J. Morphol. 1952, 91, 413–445. [Google Scholar] [CrossRef]
- Braun, B.C.; Vargas, A.; Jewgenow, K. The molecular detection of relaxin and its receptor RXFP1 in reproductive tissue of Felis catus and Lynx pardinus during pregnancy. Reproduction 2012, 143, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Breed, W.G. ENVIRONMENTAL FACTORS AND REPRODUCTION IN THE FEMALE HOPPING MOUSE, NOTOMYS ALEXIS. Reproduction 1975, 45, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Breed, W.G.; Leigh, C.M. Reproductive biology of an old endemic murid rodent of Australia, the Spinifex hopping mouse, Notomys alexis: adaptations for life in the arid zone. Integr. Zool. 2011, 6, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Breuiller-Fouché, M.; Leroy, M.-J.; Dubois, O.; Reinaud, P.; Chissey, A.; Qi, H.; Germain, G.; Fortier, M.A.; Charpigny, G. Differential Expression of the Enzymatic System Controlling Synthesis, Metabolism, and Transport of PGF2 Alpha in Human Fetal Membranes1. Biol. Reprod. 2010, 83, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L. Comparative endocrinology of domestic and nondomestic felids. Theriogenology 2006, 66, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L. Female reproductive cycles of wild female felids. Anim. Reprod. Sci. 2011, 124, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Browning, H.C. The Evolutionary History of the Corpus Luteum. Biol. Reprod. 1973, 8, 128–157. [Google Scholar] [CrossRef] [PubMed]
- Browning J., Y. and Wolf R. C. 1981. Maternal recognition of pregnancy in the rabbit: effect of conceptus removal. Biology of Reproduction, 24(2), 293-297.
- Bluhm, A.P.; A Toledo, R.; Mesquita, F.M.; Pimenta, M.T.; Fernandes, F.M.; Ribela, M.T.C.; Lazari, M.F.M. Molecular cloning, sequence analysis and expression of the snake follicle-stimulating hormone receptor. Gen. Comp. Endocrinol. 2004, 137, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Brun, C.; Exbrayat, J.-M.; Raquet, M. Localization of Receptors for Sex Steroids and Pituitary Hormones in the Female Genital Duct throughout the Reproductive Cycle of a Viviparous Gymnophiona Amphibian, Typhlonectes compressicauda. Animals 2020, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Bornstein S., R., Rutkowski H. and Vrezas, I. 2004. Cytokines and steroidogenesis. Molecular and cellular endocrinology, 215(1-2), 135-141.
- Bose, H.S.; Lingappa, V.R.; Miller, W.L. Rapid regulation of steroidogenesis by mitochondrial protein import. Nature 2002, 417, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Boyd I., L. 1991. Environmental and physiological factors controlling the reproductive cycles of pinnipeds. Canadian Journal of Zoology, 69(5), 1135-1148.
- Bouman, A.; Moes, H.; Heineman, M.; DE Leij, L.; Faas, M. Cytokine Production by Natural Killer Lymphocytes in Follicular and Luteal Phase of the Ovarian Cycle in Humans. Am. J. Reprod. Immunol. 2001, 45, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, G.D.; Enders, A.C.; Talmage, R.V. IMPLANTATION IN ARMADILLOS OVARIECTOMIZED DURING THE PERIOD OF DELAYED IMPLANTATION. J. Endocrinol. 1956, 14, 121–8. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.M.; Easley, R.G. Hormonal control of delayed development in the California leaf-nosed bat, Macrotus californicus: III. Changes in plasma progesterone during pregnancy. Gen. Comp. Endocrinol. 1977, 32, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Buckley, D., Alcobendas M., García-París M. and Wake M. H. 2007. Heterochrony, cannibalism, and the evolution of viviparity in Salamandra salamandra. Evolution & development, 9(1), 105-115.
- Butenandt, A. and Westphal U. 1934. Zur Isolierung und Charakterisierung des Corpus-luteum-Hormons. Berichte der deutschen chemischen Gesellschaft (A and B Series), 67(8), 1440-1442.
- Cake, M.H., Owen F.J., Bradshaw S.D., 1980. Difference in concentration of progesterone in plasma between pregnant and non-pregnant quokkas (Setonix brachyurus). J. Endocrinol. 84, 153–158.
- Callard I., P., Ho S. M., Gapp D. A., Kleis S., Heisermann G., Lofts B. and Holmes W. N. 1985. Regulation of vitellogenesis in reptiles: correlations with oviparity and viviparity. In pages 359-361 Proceedings of the 9th International Symposium on Comparative Endocrinology.
- Callard, I.P.; Riley, D.; Perez, L. Vitellogenesis in reptiles as a model for mammalian sex-differentiated hepatic protein synthesis. J. Exp. Zool. 1990, 256, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Callard I., P. and Koob T. J. 1993. Endocrine regulation of the elasmobranch reproductive tract. Journal of Experimental Zoology, 266(5), 368-377.
- Callard, G.; Tarrant, A.; Novillo, A.; Yacci, P.; Ciaccia, L.; Vajda, S.; Chuang, G.-Y.; Kozakov, D.; Greytak, S.; Sawyer, S.; et al. Evolutionary origins of the estrogen signaling system: Insights from amphioxus. J. Steroid Biochem. Mol. Biol. 2011, 127, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Fernandez, C.; Such, R.; Gutiérrez-Cepeda, L.; Gómez-Redondo, I.; García-Vila, E.; Cerdeira, J.; Mayenco-Aguirre, A.M.; Santiago-Moreno, J.; Hernández, L.; Sánchez-Calabuig, M.-J. REPRODUCTIVE CYCLE STAGE ASSESSMENT IN THE TWO-TOED SLOTH (CHOLOEPUS HOFFMANNI), PRELIMINARY RESULTS. J. Zoo Wildl. Med. 2023, 54, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Campos L., B., Peixoto G. C. X., Lima G. L., Castelo T. S., Silva A. M., Freitas C. I. A. and Silva A. R. 2016. Monitoring the reproductive physiology of six-banded armadillos (Euphractus sexcinctus, Linnaeus, 1758) through different techniques. Reproduction in Domestic Animals, 51(5), 736-742.
- Cannon M., J. and Pate J. L. 2003. The role of major histocompatibility complex molecules in luteal function. Reproductive Biology and Endocrinology, 1(1), 93.
- Carter, A.M.; Enders, A.C. The Evolution of Epitheliochorial Placentation. Annu. Rev. Anim. Biosci. 2013, 1, 443–467. [Google Scholar] [CrossRef] [PubMed]
- Carter A., M. and Mess A. 2007. Evolution of the placenta in eutherian mammals. Placenta, 28(4), 259-262.
- Carter, A.M.; Miglino, M.A.; Ambrosio, C.E.; Santos, T.C.; Rosas, F.C.W.; Neto, J.A.D.; Lazzarini, S.M.; Carvalho, A.F.; da Silva, V.M.F. Placentation in the Amazonian manatee (Trichechus inunguis). Reprod. Fertil. Dev. 2008, 20, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Carter A., M. 2018. Classics revisited: CJ van der Horst on pregnancy and menstruation in elephant shrews. Placenta, 67, 24-30.
- Carter, A.M. Evolution of Placental Hormones: Implications for Animal Models. Front. Endocrinol. 2022, 13, 891927. [Google Scholar] [CrossRef] [PubMed]
- Casida, L.E.; Warwick, E.J. The Necessity of the Corpus Luteum for Maintenance of Pregnancy in the Ewe. J. Anim. Sci. 1945, 4, 34–36. [Google Scholar] [CrossRef]
- Catchpole, H.R.; Fulton, J.F. The Oestrus Cycle in Tarsius: Observations on a Captive Pair. J. Mammal. 1943, 24, 90–93. [Google Scholar] [CrossRef]
- Chambery, A.; Parente, A.; Topo, E.; Garcia-Fernàndez, J.; D’Aniello, S. Characterization and Putative Role of a Type I Gonadotropin-Releasing Hormone in the Cephalochordate Amphioxus. Endocrinology 2009, 150, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.C.; Polanco, J.R.; Min, S.; Michael, S.D. Mitochondrial 3 beta-hydroxysteroid dehydrogenase (HSD) is essential for the synthesis of progesterone by corpora lutea: An hypothesis. Reprod. Biol. Endocrinol. 2005, 3, 11–11. [Google Scholar] [CrossRef] [PubMed]
- Charanjit S., B. 1980. Maternal Acceptance Of The Foetal Allograft: Role Of Placental Gonadotrophins (Doctoral dissertation, University of Nairobi).
- Choudary, J.B.; Greenwald, G.S. Luteotropic complex of the mouse. Anat. Rec. 1969, 163, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Cieslak, E.S. Relations between the Reproductive Cycle and the Pituitary Gland in the Snake Thamnophis radix. Physiol. Zool. 1945, 18, 299–329. [Google Scholar] [CrossRef]
- Clausen, H.J. STUDIES ON THE EFFECT OF OVARIOTOMY AND HYPOPHYSECTOMY ON GESTATION IN SNAKES1. Endocrinology 1940, 27, 700–704. [Google Scholar] [CrossRef]
- Conaway, C.H. Ecological Adaptation and Mammalian Reproduction. Biol. Reprod. 1971, 4, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Concannon P., W., Castracane V. D., Temple M. and Montanez A. 2018. Endocrine control of ovarian function in dogs and other carnivores. Animal Reproduction 6(1), 172-193.
- Conley, A. Review of the reproductive endocrinology of the pregnant and parturient mare. Theriogenology 2016, 86, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Cope E., D. 1887. The origin of the fittest: essays on evolution. New York, Appleton.
- Crichton, E.G.; Seamark, R.F.; Krutzsch, P.H. The status of the corpus luteum during pregnancy in Miniopterus schreibersii (Chiroptera: Vespertilionidae) with emphasis on its role in developmental delay. Cell Tissue Res. 1989, 258, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Csapo, A.I.; Pulkkinen, M. INDISPENSABILITY OF THE HUMAN CORPUS LUTEUM IN THE MAINTENANCE OF EARLY PREGNANCY LUTEECTOMY EVIDENCE. Obstet. Gynecol. Surv. 1978, 33, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Currie, W.B.; Blake, M.; Wimsatt, W.A. Fetal development, and placental and maternal plasma concentrations of progesterone in the little brown bat (Myotis lucifugus). Reproduction 1988, 82, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.E. The regression of the avian post-ovulatory follicle. Anat. Rec. 1942, 82, 297–307. [Google Scholar] [CrossRef]
- Davies I., J. and Ryan K. J. 1972. Comparative endocrinology of gestation. Vitamins & Hormones, 30, 223-279.
- Davis, J.S.; Rueda, B.R. The corpus luteum: an ovarian structure with maternal instincts and suicidal tendencies. Front. Biosci. 2002, 7, d1949–78. [Google Scholar] [CrossRef] [PubMed]
- Davis J., S., RuedaB. R. and Spanel-Borowski, K. 2003. Microvascular endothelial cells of the corpus luteum. Reproductive Biology and Endocrinology, 1(1), 89.
- de Magalhães, J.P.; Abidi, Z.; dos Santos, G.A.; A Avelar, R.; Barardo, D.; Chatsirisupachai, K.; Clark, P.; A De-Souza, E.; Johnson, E.J.; Lopes, I.; et al. Human Ageing Genomic Resources: updates on key databases in ageing research. Nucleic Acids Res. 2023, 52, D900–D908. [Google Scholar] [CrossRef] [PubMed]
- Deanesly, R. EXPERIMENTAL OBSERVATIONS ON THE FERRET CORPUS LUTEUM OF PREGNANCY. Reproduction 1967, 13, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Dodd J., M. 1983. 2 Reproduction in Cartilaginous Fishes (Chondrichthyes). Pages 31 - 95 in Fish physiology (Vol. 9). Academic Press.
- Douglas, D.A.; Song, J.-H.; Moreau, G.M.; Murphy, B.D. Differentiation of the Corpus Luteum of the Mink (Mustela vison): Mitogenic and Steroidogenic Potential of Luteal Cells from Embryonic Diapause and Postimplantation Gestation. Biol. Reprod. 1998, 58, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Eaton, G.; Slob, A.; Resko, J. Cycles of mating behaviour, oestrogen and progesterone in the thick-tailed bushbaby (Galago crassicaudatus crassicaudatus) under laboratory conditions. Anim. Behav. 1973, 21, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, J.F.; Gould, E. The behavior of Solenodon paradoxus in captivity with comments in the behavior of other insectivora. Zool. Sci. Contrib. New York Zool. Soc. 1966, 51, 49–58. [Google Scholar] [CrossRef]
- Eisenberg, J. and Muckerhirn N. 1968. The reproduction and rearing of tenrecoid insectivores in captivity. International Zoo Yearbook, 8(1), 106-110.
- Emi, N., Kanzaki H., Yoshida M., Takakura K., Kariya M., Okamoto N., Imai K. and Mori, T. 1991. Lymphocytes stimulate progesterone production by cultured human granulosa luteal cells. American journal of obstetrics and gynecology, 165(5), 1469-1474.
- Enders, A.; Carter, A. The evolving placenta: Convergent evolution of variations in the endotheliochorial relationship. Placenta 2012, 33, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Erkenbrack, E.M.; Maziarz, J.D.; Griffith, O.W.; Liang, C.; Chavan, A.R.; Nnamani, M.C.; Wagner, G.P. The mammalian decidual cell evolved from a cellular stress response. PLOS Biol. 2018, 16, e2005594. [Google Scholar] [CrossRef] [PubMed]
- Espey L., L., Bellinger A. S. and Healy J. A. 2004. Ovulation: an inflammatory cascade of gene expression. The ovary, 2, 145-165.
- Evans C., S. and Goy R. W. 1968. Social behaviour and reproductive cycles in captive ring-tailed lemurs (Lemur catta). Journal of Zoology, 156(2), 181-197.
- Faas, M.; Bouman, A.; Moesa, H.; Heineman, M.J.; de Leij, L.; Schuiling, G. The immune response during the luteal phase of the ovarian cycle: a Th2-type response? Fertil. Steril. 2000, 74, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- E Faith, R.; A Montgomery, C.; Durfee, W.J.; Aguilar-Cordova, E.; Wyde, P.R. The cotton rat in biomedical research. Laboratory animal science 1997, 47, 337–45. [Google Scholar]
- Fang, Y. Position in the evolution of reproductive endocrine of amphioxus, Branchiostoma belcheri. Chin. Sci. Bull. 1998, 43, 177–184. [Google Scholar] [CrossRef]
- Fang, Y., Weng Y. and Chen L. 2001. Effects of damage of Hatschek’s pit of amphioxus on its structure and function. Chinese Science Bulletin, 46, 1528-1533.
- Fazleabas, A.; Kim, J.; Strakova, Z. Implantation: Embryonic Signals and the Modulation of the Uterine Environment—A Review. Placenta 2004, 25, S26–S31. [Google Scholar] [CrossRef] [PubMed]
- Ferner, K.; Schultz, J.A.; Zeller, U. Comparative anatomy of neonates of the three major mammalian groups (monotremes, marsupials, placentals) and implications for the ancestral mammalian neonate morphotype. J. Anat. 2017, 231, 798–822. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.P.F.; Hearn, J.P.; Michael, A.E. The maternal recognition of pregnancy in mammals. J. Zool. 1990, 221, 327–341. [Google Scholar] [CrossRef]
- Foyouzi N., Cai Z., Sugimoto Y. and Stocco C. 2005. Changes in the expression of steroidogenic and antioxidant genes in the mouse corpus luteum during luteolysis. Biology of reproduction, 72(5), 1134-1141.
- Fraenkel, L. 1903. Die Funktion des corpus luteum. Archiv für Gynäkologie, 68(2), 438-545.
- Fraenkel, L.; Martins, T.; Mello, R.F. STUDIES ON THE PREGNANCY OF VIVIPAROUS SNAKES. Endocrinology 1940, 27, 836–837. [Google Scholar] [CrossRef]
- Fukui, Y.; Iwayama, H.; Matsuoka, T.; Nagai, H.; Koma, N.; Mogoe, T.; Ishikawa, H.; Fujise, Y.; Hirabayashi, M.; Hochi, S.; et al. Attempt at Intracytoplasmic Sperm Injection of In Vitro Matured Oocytes in Common Minke Whales (Balaenoptera acutorostrata) Captured During the Kushiro Coast Survey. J. Reprod. Dev. 2007, 53, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Fukui, Y. 2016. Ovary, Oogenesis, and Ovarian Cycle. Pages 205-226 in Reproductive Biology and Phylogeny of Cetacea: Whales, Porpoises and Dolphins. CRC Press.
- Funston, G.F.; Depolo, P.E.; Sliwinski, J.T.; Dumont, M.; Shelley, S.L.; Pichevin, L.E.; Cayzer, N.J.; Wible, J.R.; Williamson, T.E.; Rae, J.W.B.; et al. The origin of placental mammal life histories. Nature 2022, 610, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Gadsby, J.E.; Keyes, P.L.; Bill, C.H. Control of Corpus Luteum Function in the Pregnant Rabbit: Role of Estrogen and Lack of a Direct Luteotropic Role of the Placenta*. Endocrinology 1983, 113, 2255–2262. [Google Scholar] [CrossRef]
- Garrel, C., Ceballos-Picot I., Germain G. and Al-Gubory K. H. 2007. Oxidative stress-inducible antioxidant adaptive response during prostaglandin F2α-induced luteal cell death in vivo. Free radical research, 41(3), 251-259.
- Gemmell R., T. 1995. A comparative study of the corpus luteum. Reproduction, Fertility and Development, 7(3), 303-312.
- Godfrey G., K. and Oliver W. L. R. 1978. The reproduction and development of the pigmy hedgehog tenrec, Echinops telfairi. Dodo J Jersey Wildl Preserv Trust, 15, 38-51.
- Gopalakrishna, A.; Karim, K.B. Fetal membranes and placentation in Chiroptera. Reproduction 1979, 56, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Gorbman, A. Early Development of the Hagfish Pituitary Gland: Evidence for the Endodermal Origin of the Adenohypophysis. Am. Zool. 1983, 23, 639–654. [Google Scholar] [CrossRef]
- Gorbman, A. and Tamarin A. 1985. Early development of oral, olfactory and adenohypophyseal structures of agnathans and its evolutionary implications. Pages 165-185 in Evolutionary biology of primitive fishes. Boston, MA: Springer US.
- Gopalakrishna, A.; Karim, K.B. Fetal membranes and placentation in Chiroptera. Reproduction 1979, 56, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Göritz, F.; Dehnhard, M.; Hildebrandt, T.; Naidenko, S.; Vargas, A.; Martinez, F.; López-Bao, J.; Palomares, F.; Jewgenow, K. Non Cat-Like Ovarian Cycle in the Eurasian and the Iberian Lynx – Ultrasonographical and Endocrinological Analysis. Reprod. Domest. Anim. 2009, 44, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Gould, E.; Eisenberg, J.F. Notes on The Biology of The Tenrecidae. J. Mammal. 1966, 47, 660–686. [Google Scholar] [CrossRef]
- Girsh, E.; Greber, Y.; Meidan, R. Luteotrophic and Luteolytic Interactions between Bovine Small and Large Luteal-Like Cells and Endothelial Cells1. Biol. Reprod. 1995, 52, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Greville, L.J.S.; Bueno, L.M.; Pollock, T.; Faure, P.A. Quantification of Urinary Sex Steroids in the Big Brown Bat (Eptesicus fuscus). Physiol. Biochem. Zool. 2022, 95, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W.; Chavan, A.R.; Pavlicev, M.; Protopapas, S.; Callahan, R.; Maziarz, J.; Wagner, G.P. Endometrial recognition of pregnancy occurs in the grey short-tailed opossum (Monodelphis domestica). Proc. R. Soc. B: Biol. Sci. 2019, 286, 20190691. [Google Scholar] [CrossRef] [PubMed]
- Grossman, C.J. Regulation of the Immune System by Sex Steroids*. Endocr. Rev. 1984, 5, 435–455. [Google Scholar] [CrossRef]
- Guillette L., J. 1987. The evolution of viviparity in fishes, amphibians and reptiles: An endocrine approach. Pages 523-562 in Hormones and reproduction in fishes, amphibians, and reptiles. Springer, Boston, MA.
- Guilgur L., G., Moncaut N. P., Canário A. V. and Somoza G. M. 2006. Evolution of GnRH ligands and receptors in gnathostomata. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 144(3), 272-283.
- Haberlandt, L. Über hormonale Sterilisierung weiblicher Tiere durch subcutane Transplantation von Ovarien trächtiger Weibchen. Pfl?gers Arch. Eur. J. Physiol. 1922, 194, 235–270. [Google Scholar] [CrossRef]
- Hamlett G. W., D. 1933. Polyembryony in the armadillo: genetic or physiological?. The Quarterly Review of Biology, 8(3), 348-358.
- Hamlett G. W., D. 1934. Uterine bleeding in a bat, Glossophaga soricina. The Anatomical Record, 60(1), 9-17.
- Hamlett, G.W.D. Delayed Implantation and Discontinuous Development in the Mammals. Q. Rev. Biol. 1935, 10, 432–447. [Google Scholar] [CrossRef]
- Hamlett W., C. 1999. Placenta and placental analogs in elasmobranchs. Encyclopedia of reproduction, 3, 831-840.
- Hansel W, Alila HW, Dowd JP and Milvae RA. 1991. Differential origin and control mechanisms in small and large bovine luteal cells. Journal of Reproduction and Fertility Supplement 43, 77–89.
- Hartmann, M. and Wettstein A. 1934. Zur Kenntnis der Corpus luteum-Hormone (2. Mitteilung.). Helvetica Chimica Acta, 17(1), 1365-1372.
- Hanukoglu, I. Antioxidant Protective Mechanisms against Reactive Oxygen Species (ROS) Generated by Mitochondrial P450 Systems in Steroidogenic Cells. Drug Metab. Rev. 2006, 38, 171–196. [Google Scholar] [CrossRef] [PubMed]
- Hennebold J., D. 2018. Corpus luteum. Pages 99-105 in Encyclopedia of Reproduction. Elsevier.
- Hinds, L.A.; Tyndale-Biscoe, C.H. Plasma progesterone levels in the pregnant and non-pregnant tammar, Macropus eugenii. J. Endocrinol. 1982, 93, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Hinds, L.A.; Tyndale-Biscoe, C.H. Prolactin in the Marsupial Macropus Eugenii, During the Estrous Cycle, Pregnancy and Lactation. Biol. Reprod. 1982, 26, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Hinds L., A. 1990. Control of pregnancy, parturition and luteolysis in marsupials. Reproduction, Fertility and Development, 2(5), 535-552.
- Highfill D., R. and Mead R. A. 1975. Sources and levels of progesterone during pregnancy in the garter snake, Thamnophis elegans. General and Comparative Endocrinology, 27(3), 389-400.
- Hodges, J.K.; Henderson, C.; Hearn, J.P. Relationship between ovarian and placental steroid production during early pregnancy in the marmoset monkey (Callithrix jacchus). Reproduction 1983, 69, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Howell-Stephens, J.; Bernier, D.; Brown, J.; Mulkerin, D.; Santymire, R. Using non-invasive methods to characterize gonadal hormonal patterns of southern three-banded armadillos (Tolypeutes matacus) housed in North American zoos. Anim. Reprod. Sci. 2013, 138, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Hisaw F., L. and Abramowitz A. A., 1937. The physiology of reproduction in the dogfish, Mustelus canis. Report. Woods Hole Oceanographic Institution, 1937, pp. 21–2.
- Hughes Jr., F. M., Lane T. A., Chen T. T. and Gorospe W. C. 1990. Effects of cytokines on porcine granulosa cell steroidogenesis in vitro. Biology of reproduction, 43(5), 812-817.
- Hughes Jr., F. M., Pringle C. M. and Gorospe W. C. 1991. Production of progestin-stimulatory factors (s) by enriched populations of rat T and B lymphocytes. Biology of reproduction, 44(5), 922-926.
- Ishinazaka T., Suzuki M., Yamamoto Y., Isono T., Harada N., Mason J. I., Watabe M., Tsunokawa M. and Ohtaishi N. 2001. Immunohistochemical localization of steroidogenic enzymes in the corpus luteum and the placenta of the ribbon seal (Phoca fasciata) and Steller sea lion (Eumetopias jubatus). Journal of Veterinary Medical Science, 63(9), 955-959.
- Iwasa, M.; Atkinson, S. ANALYSIS OF CORPORA LUTEA TO ESTIMATE REPRODUCTIVE CYCLES OF WILD HAWAIIAN MONK SEALS (MONACHUS SCHAUINSLANDI). Mar. Mammal Sci. 1996, 12, 182–198. [Google Scholar] [CrossRef]
- Johnson, A.E.; Freeman, E.W.; Colgin, M.; McDonough, C.; Songsasen, N. Induction of ovarian activity and ovulation in an induced ovulator, the maned wolf (Chrysocyon brachyurus), using GnRH agonist and recombinant LH. Theriogenology 2014, 82, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Johnston S., D., McGowan M. R., O’callaghan P., Cox R. and Nicolson V. 2000. Studies of the oestrous cycle, oestrus and pregnancy in the koala (Phascolarctos cinereus). Journal of Reproduction and Fertility, 120(1), 49-58.
- Jones R., E. and Baxter D. C. 1991. Gestation, with emphasis on corpus luteum biology, placentation, and parturition. Vertebrate endocrinology: fundamentals and biomedical implications, 4(Part A), 205-302.
- Ka, H., Seo H., Choi Y., Yoo I. and Han, J. 2018. Endometrial response to conceptus-derived estrogen and interleukin-1β at the time of implantation in pigs. Journal of animal science and biotechnology, 9(1), 1-17.
- Kah, O.; Lethimonier, C.; Somoza, G.; Guilgur, L.; Vaillant, C.; Lareyre, J. GnRH and GnRH receptors in metazoa: A historical, comparative, and evolutive perspective. Gen. Comp. Endocrinol. 2007, 153, 346–364. [Google Scholar] [CrossRef] [PubMed]
- Kano, S. 2010. Genomics and developmental approaches to an acidian adenohypophysis primordium. Integrative Comparative Biology 50(1), 35–52.
- Kavanaugh, S.I.; Root, A.R.; Sower, S.A. Distribution of gonadotropin-releasing hormone (GnRH) by in situ hybridization in the tunicate Ciona intestinalis. Gen. Comp. Endocrinol. 2005, 141, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, H.; Sower, S.A. The dawn and evolution of hormones in the adenohypophysis. Gen. Comp. Endocrinol. 2006, 148, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Sugino, N.; Takiguchi, S.; Kashida, S.; Nakamura, Y. Roles of reactive oxygen species in the regulation of luteal function. Rev. Reprod. 1997, 2, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Kayanja F. I., B. and Sale J. B. 1973. The ovary of rock hyrax of the genus Procavia. Reproduction, 33(2), 223-230.
- Kelly R., W. 1994. Pregnancy maintenance and parturition: the role of prostaglandin in manipulating the immune and inflammatory response. Endocrine reviews, 15(5), 684-706.
- Keyes P., L. and Gadsby J. E. 1987. Role of estrogen and the placenta in the maintenance of the rabbit corpus luteum. Pages 361-378 in Regulation of Ovarian and Testicular Function. Boston, MA: Springer US.
- Klein, C. and Troedsson M. H. T. 2011. Maternal recognition of pregnancy in the horse: a mystery still to be solved. Reproduction, Fertility and Development, 23(8), 952-963.
- Knight, J.W. Aspects of placental estrogen synthesis in the pig. Exp. Clin. Endocrinol. Diabetes 1994, 102, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Knight, J., Taylor G. W., Wright P., Clare A. S. and Rowley A. F. 1999. Eicosanoid biosynthesis in an advanced deuterostomate invertebrate, the sea squirt (Ciona intestinalis). Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1436(3), 467-478.
- Knott, K.K.; Roberts, B.M.; A Maly, M.; Vance, C.K.; DeBeachaump, J.; Majors, J.; Riger, P.; DeCaluwe, H.; Kouba, A.J. Fecal estrogen, progestagen and glucocorticoid metabolites during the estrous cycle and pregnancy in the giant anteater (Myrmecophaga tridactyla): evidence for delayed implantation. Reprod. Biol. Endocrinol. 2013, 11, 83–83. [Google Scholar] [CrossRef] [PubMed]
- Koljak, R., Järving I., Kurg R., Boeglin W. E., Varvas K., Valmsen K., Ustav M., Brash R.A. and Samel, N. 2001. The basis of prostaglandin synthesis in coral: molecular cloning and expression of a cyclooxygenase from the Arctic soft coral Gersemia fruticosa. Journal of Biological Chemistry, 276(10), 7033-7040.
- Koob, T.J.; Tsang, P.; Callard, I.P. Plasma Estradiol, Testosterone, and Progesterone Levels during the Ovulatory Cycle of the Skate (Raja Erinacea)1. Biol. Reprod. 1986, 35, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Koob T., J. and Callard I. P. 1999. Reproductive endocrinology of female elasmobranchs: lessons from the little skate (Raja erinacea) and spiny dogfish (Squalus acanthias). Journal of Experimental Zoology, 284(5), 557-574.
- Kowalewski, M.P. Luteal regression vs. prepartum luteolysis: Regulatory mechanisms governing canine corpus luteum function. Reprod. Biol. 2014, 14, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Suleski, M.; Craig, J.M.; E Kasprowicz, A.; Sanderford, M.; Li, M.; Stecher, G.; Hedges, S.B. TimeTree 5: An Expanded Resource for Species Divergence Times. Mol. Biol. Evol. 2022, 39. [Google Scholar] [CrossRef] [PubMed]
- Krutzsch, P.H. Reproductive anatomy and cyclicity of the bat Eonycteris spelaea Dobson (Chiroptera: Pteropodidae) in West Malaysia. Acta Chiropterologica 2005, 7, 51–64. [Google Scholar] [CrossRef]
- Kubokawa, K.; Tando, Y.; Roy, S. Evolution of the Reproductive Endocrine System in Chordates. Integr. Comp. Biol. 2010, 50, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Kusuda, S.; Adachi, I.; Fujioka, K.; Nakamura, M.; Amano-Hanzawa, N.; Goto, N.; Furuhashi, S.; Doi, O. Reproductive characteristics of female lesser mouse deers (Tragulus javanicus) based on fecal progestagens and breeding records. Anim. Reprod. Sci. 2013, 137, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Labhsetwar, A.P.; Enders, A.C. PROGESTERONE IN THE CORPUS LUTEUM AND PLACENTA OF THE ARMADILLO, DASYPUS NOVEMCINCTUS. Reproduction 1968, 16, 381–387. [Google Scholar] [CrossRef]
- Laird, M.K.; McShea, H.; McAllan, B.M.; Murphy, C.R.; Thompson, M.B. Uterine remodelling during pregnancy and pseudopregnancy in the brushtail possum (Trichosurus vulpecula; Phalangeridae). J. Anat. 2017, 231, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Laws R., M. 1970. The Tsavo research project. Oryx, 10(6), 355-361.
- Lawson, E.F.; Grupen, C.G.; A Baker, M.; Aitken, R.J.; Swegen, A.; Pollard, C.-L.; Gibb, Z. Conception and early pregnancy in the mare: lipidomics the unexplored frontier. Reprod. Fertil. 2022, 3, R1–R18. [Google Scholar] [CrossRef] [PubMed]
- Li K., L., Lu T. M. and Yu J. K. 2014. Genome-wide survey and expression analysis of the bHLH-PAS genes in the amphioxus Branchiostoma floridae reveal both conserved and diverged expression patterns between cephalochordates and vertebrates. Evodevo, 5(1), 1-20.
- Licht, P. Evolutionary Divergence in the Structure and Function of Pituitary Gonadotropins of Tetrapod Vertebrates. Am. Zool. 1983, 23, 673–683. [Google Scholar] [CrossRef]
- Loeb, L. 1907. Über die experimentelle Erzeugung von Knoten von Deciduagewebe in dem Uterus des Meerschweinchens nach stattgefundener Copulation. Copulation Zentralbl AlIg Pathol Pathol Anat. 18, 563-565.
- Loeb, L. 19111. Über die Bedeutung des Corpus luteum für die Periodizität des sexuellen Zyklus beim weiblichen Säugetierorganismu. Deutsche Medizinische Wochenschrift. 37, 17.
- Loeb, L. The effect of extirpation of the uterus on the life and function of the corpus luteum in the guinea pig. Exp. Biol. Med. 1923, 20, 441–443. [Google Scholar] [CrossRef]
- Loeb, L.; McCracken, J.A.; Custer, E.E.; Lamsa, J.C. THE EFFECTS OF HYSTERECTOMY ON THE SYSTEM OF SEX ORGANS AND ON THE PERIODICITY OF THE SEXUAL CYCLE IN THE GUINEA PIG. Am. J. Physiol. Content 1927, 83, 202–224. [Google Scholar] [CrossRef]
- Lueders, I.; Niemuller, C.; Rich, P.; Gray, C.; Hermes, R.; Goeritz, F.; Hildebrandt, T.B. Gestating for 22 months: luteal development and pregnancy maintenance in elephants. Proc. R. Soc. B: Biol. Sci. 2012, 279, 3687–3696. [Google Scholar] [CrossRef] [PubMed]
- Lutton B., V. 2011. The elasmobranch ovary. Pages 247 - 276 in Reproductive biology and phylogeny of Chondrichthyes. CRC Press.
- Malassiné, A. and Ferré F. 1979. Δ5, 3 β Hydroxysteroid Dehydrogenase Activity in Cat Placental Labyrinth: Evolution during Pregnancy, Subcellular Distribution. Biology of Reproduction, 21(4), 965-971.
- Marcinkiewicz J., L., Moy E. S. and Bahr J. M. 1992. Change in responsiveness of rabbit corpus luteum to prostaglandin F-2α during pregnancy and pseudopregnancy. Reproduction, 94(2), 305-310.
- Markov, G.V.; Tavares, R.; Dauphin-Villemant, C.; Demeneix, B.A.; Baker, M.E.; Laudet, V. Independent elaboration of steroid hormone signaling pathways in metazoans. Proc. Natl. Acad. Sci. 2009, 106, 11913–11918. [Google Scholar] [CrossRef] [PubMed]
- Markov, G.V.; Gutierrez-Mazariegos, J.; Pitrat, D.; Billas, I.M.L.; Bonneton, F.; Moras, D.; Hasserodt, J.; Lecointre, G.; Laudet, V. Origin of an ancient hormone/receptor couple revealed by resurrection of an ancestral estrogen. Sci. Adv. 2017, 3, e1601778–1601778. [Google Scholar] [CrossRef] [PubMed]
- Marsh, H.; Heinsohn, G.; Channells, P. Changes in the Ovaries and Uterus of the Dugong, Dugong dugon (Sirenia:Dugongidae), with Age and Reproductive Activity. Aust. J. Zool. 1984, 32, 743–766. [Google Scholar] [CrossRef]
- Martin, R.D.; MacLarnon, A.M. Gestation period, neonatal size and maternal investment in placental mammals. Nature 1985, 313, 220–223. [Google Scholar] [CrossRef]
- Maston, G.A.; Ruvolo, M. Chorionic Gonadotropin Has a Recent Origin Within Primates and an Evolutionary History of Selection. Mol. Biol. Evol. 2002, 19, 320–335. [Google Scholar] [CrossRef]
- McCoy D., E. and Haig D. 2020. Embryo selection and mate choice: can ‘honest signals’ be trusted?. Trends in Ecology & Evolution, 35(4), 308-318.
- Merchant, J.C. The effect of pregnancy on the interval between one oestrus and the next in the tammar wallaby, Macropus eugenii. Reproduction 1979, 56, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Millesi, E.; Strauss, A.; Burger, T.; E Hoffmann, I.; Walzl, M. Follicular development in European ground squirrels (Spermophilus citellus) in different phases of the annual cycle. Reproduction 2008, 136, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Milvae, R.; Hinckley, S.; Carlson, J. Luteotropic and luteolytic mechanisms in the bovine corpus luteum. Theriogenology 1996, 45, 1327–1349. [Google Scholar] [CrossRef] [PubMed]
- Milvae, R.A. 2000. Inter-relationships between endothelin and prostaglandin F2alpha in corpus luteum function. Reviews of Reproduction, 5(1), 1-5.
- Mizuta, T.; Kubokawa, K. Presence of Sex Steroids and Cytochrome P450 Genes in Amphioxus. Endocrinology 2007, 148, 3554–3565. [Google Scholar] [CrossRef] [PubMed]
- Mossman, H.W. 1987. Vertebrate Fetal Membranes:Comparative Ontogeny and Morphology; Evolution; Phylogenetic Significance; Basic Functions; Research Opportunities. London: Macmillan.
- Mull C., G., Lowe C. G. and Young K. A. 2010. Seasonal reproduction of female round stingrays (Urobatis halleri): steroid hormone profiles and assessing reproductive state. General and Comparative Endocrinology, 166(2), 379-387.
- Murr S., M., Stabenfedlt G. H., Bradford G. E. and Geschwing I. I. 1974. Plasma progesterone during pregnancy in the mouse. Endocrinology, 94(4), 1209-1211.
- Murphy B., D. 2018. Equine chorionic gonadotropin: an enigmatic but essential tool. Animal Reproduction (AR), 9(3), 223-230.
- Musa B., E. 1979. Studies on the ovary of the camel (Camelus dromedarius). Sudan journal of veterinary science and animal husbandry 20, 51-64.
- Nagashima, J.B.; Songsasen, N. Canid Reproductive Biology: Norm and Unique Aspects in Strategies and Mechanisms. Animals 2021, 11, 653. [Google Scholar] [CrossRef] [PubMed]
- Nakakura, K.; Czekala, N.M.; Lasley, B.L.; Benirschke, K. Fetal--maternal gradients of steroid hormones in the nine-banded armadillo (Dasypus novemcinctus). Reproduction 1982, 66, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Narumiya, S.; Sugimoto, Y.; Ushikubi, F. Prostanoid Receptors: Structures, Properties, and Functions. Physiol. Rev. 1999, 79, 1193–1226. [Google Scholar] [CrossRef] [PubMed]
- Narumiya, S. and FitzGerald G. A. 2001. Genetic and pharmacological analysis of prostanoid receptor function. The Journal of clinical investigation, 108(1), 25-30.
- Nicoll, M.E.; Racey, P.A. Follicular development, ovulation, fertilization and fetal development in tenrecs (Tenrec ecaudatus). Reproduction 1985, 74, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Niswender, G.D.; Juengel, J.L.; Silva, P.J.; Rollyson, M.K.; McIntush, E.W. Mechanisms Controlling the Function and Life Span of the Corpus Luteum. Physiol. Rev. 2000, 80, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Norris D. O. and Jones R. E. (Eds.). 2012. Hormones and reproduction in fishes, amphibians, and reptiles. Springer Science & Business Media.
- Nowak, R. 1999. Walker’s Mammals of the World, Fourth Edition. Baltimore and London: The Johns Hopkins University Press.
- Nozaki, M. Hypothalamic-Pituitary-Gonadal Endocrine System in the Hagfish. Front. Endocrinol. 2013, 4, 200. [Google Scholar] [CrossRef] [PubMed]
- Oon V. J., H. and Johnson M. R. 2000. The regulation of the human corpus luteum steroidogenesis: a hypothesis? Human reproduction update, 6(5), 519-529.
- Papa P., C. and Kowalewski M. P. 2020. Factors affecting the fate of the canine corpus luteum: Potential contributors to pregnancy and non-pregnancy. Theriogenology, 150, 339-346.
- Painer, J.; Goeritz, F.; Dehnhard, M.; Hildebrandt, T.B.; Naidenko, S.V.; Sánchez, I.; Muñoz, M.A.Q.; Jewgenow, K. Hormone-induced luteolysis on physiologically persisting corpora lutea in Eurasian and Iberian lynx (Lynx lynx and Lynx pardinus). Theriogenology 2014, 82, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Pate J., L. and Keyes P. L. 2001. Immune cells in the corpus luteum: friends or foes? Reproduction, 122(5), 665-76.
- Patzl, M.; Schwarzenberger, F.; Osmann, C.; Bamberg, E.; Bartmann, W. Monitoring ovarian cycle and pregnancy in the giant anteater (Myrmecophaga tridactyla) by faecal progestagen and oestrogen analysis. Anim. Reprod. Sci. 1998, 53, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Patzner R., A. 1977. Effects of hypophysectomy on the testis of the hagfish, Eptatretus burgeri Girard (Cyclostomata). Zoologischer Anzeiger, 199, 371-380.
- Pavlicev, M. and Wagner G. P. 2016. The evolutionary origin of female orgasm. J. Exp. Zool. (Mol. Dev. Evol.) 326B: 326–337.
- Payne, R.B. The post-ovulatory follicles of blackbirds (Agelaius). J. Morphol. 1966, 118, 331–351. [Google Scholar] [CrossRef] [PubMed]
- Peitz, B. The oestrous cycle of the spiny mouse (Acomys cahirinus). Reproduction 1981, 61, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Pencharz R., I. and Long J. A. 1931. The effect of hypophysectomy on gestation in the rat. Science, 74(1912), 206-206.
- Perry J., S. 1953. The reproduction of the African elephant, Loxodonta africana. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 93-149.
- Pomeroy, P. Reproductive cycles of marine mammals. Anim. Reprod. Sci. 2011, 124, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.L.; Kavanaugh, S.I.; Sower, S.A. Seasonal concentrations of reproductive steroids in the gonads of the atlantic hagfish, Myxine glutinosa. J. Exp. Zool. 2004, 301, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.L.; Kavanaugh, S.; Sower, S.A. Identification of a functional corpus luteum in the Atlantic hagfish, Myxine glutinosa. Gen. Comp. Endocrinol. 2006, 148, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Psychoyos, A.; Nikas, G.; Gravanis, A. The role of prostaglandins in blastocyst implantation. Hum. Reprod. 1995, 10, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Rasweiler IV J., J. 1991. Spontaneous decidual reactions and menstruation in the black mastiff bat, Molossus ater. American journal of anatomy, 191(1), 1-22.
- Rasweiler, J.J.; de Bonilla, H. Menstruation in short-tailed fruit bats (Carollia spp.). Reproduction 1992, 95, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Rawn S., M. and Cross J. C. 2008. The evolution, regulation, and function of placenta-specific genes. Annual review of cell and developmental biology, 24, 159-181.
- Renfree, M.B.; Calaby, J.H. Background to delayed implantation and embryonic diapause. J. Reprod. Fertil. 1981, 29, 1–9. [Google Scholar]
- Renfree M., B. 2000. Maternal recognition of pregnancy in marsupials. Reviews of Reproduction, 5(1), 6-11.
- Renfree, M.B. Review: Marsupials: Placental Mammals with a Difference. Placenta 2010, 31, S21–S26. [Google Scholar] [CrossRef] [PubMed]
- Revell, L.J. phytools 2.0: an updated R ecosystem for phylogenetic comparative methods (and other things). PeerJ 2024, 12, e16505. [Google Scholar] [CrossRef] [PubMed]
- Reynolds S. R., M. 1939. Physiology of the Uterus. Paul B. Hoeber.
- Reynolds, L.P.; Grazul-Bilska, A.T.; Redmer, D.A. Angiogenesis in the Corpus Luteum. Endocrine 2000, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, T.J.; Wright, J.W. Early postnatal physical and behavioural development of degus (Octodon degus). Lab. Anim. 1979, 13, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Robeck, T.R.; Blum, J.L.; Steinman, K.J.; Ratner, J.R.; Bergfelt, D.R.; O’Brien, J.K. Longitudinal profiles of relaxin and progestagens during pregnancy, pregnancy loss and false pregnancy in the killer whale (Orcinus orca). Gen. Comp. Endocrinol. 2018, 267, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Robeck, T.R.; Steinman, K.J.; O’Brien, J.K. Characterization and longitudinal monitoring of serum androgens and glucocorticoids during normal pregnancy in the killer whale (Orcinus orca). Gen. Comp. Endocrinol. 2017, 247, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Robeck T., R., Steinman K. J., Yoshioka M., Jensen E., O’Brien J. K., Katsumata E., Gili G., McBain J.F., Sweeney J. and Monfort S. L. 2005. Estrous cycle characterisation and artificial insemination using frozen–thawed spermatozoa in the bottlenose dolphin (Tursiops truncatus). Reproduction, 129(5), 659-674.
- Roberts, R.M.; Cross, J.C.; Leaman, D.W. Interferons as Hormones of Pregnancy*. Endocr. Rev. 1992, 13, 432–452. [Google Scholar] [CrossRef] [PubMed]
- Roch, G.J.; Tello, J.A.; Sherwood, N.M. At the Transition from Invertebrates to Vertebrates, a Novel GnRH-Like Peptide Emerges in Amphioxus. Mol. Biol. Evol. 2013, 31, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues F., R., Da Silva V. M. F., Barcellos J. F. M. and Lazzarini S. M. 2008. Reproductive anatomy of the female Amazonian manatee Trichechus inunguis Natterer, 1883 (Mammalia: Sirenia). The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology: Advances in Integrative Anatomy and Evolutionary Biology, 291(5), 557-564.
- Roth T., L. and Brown J. L. 1999. Is there any rhyme or reason to rhino reproduction? A summary of reproductive characteristics, species specificities and challenges for the future. In American Association of Zoo Veterinarians (pp. 97-99).
- Roth T., L., O’Brien J. K., McRae M. A., Bellem A. C., Romo S. J., Kroll J. L., and Brown J. L. 2001. Ultrasound and endocrine evaluation of the ovarian cycle and early pregnancy in the Sumatran rhinoceros (Dicerorhinus sumtrensis). Reproduction, 121(1):139-49.
- Rothchild, I.; Fraps, R.M. On the function of the Ruptured Ovarian Follicle of the Domestic Fowl. Exp. Biol. Med. 1944, 56, 79–82. [Google Scholar] [CrossRef]
- Rothchild, I. 1981. The regulation of the mammalian corpus luteum. Pages 183-393 in Proceedings of the 1980 Laurentian Hormone Conference. Academic Press.
- Rothchild, I. The Yolkless Egg and the Evolution of Eutherian Viviparity. Biol. Reprod. 2003, 68, 337–357. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, I.W.; Short, R.V. THE PROGESTERONE CONTENT OF THE GUINEA-PIG CORPUS LUTEUM DURING THE REPRODUCTIVE CYCLE AND AFTER HYSTERECTOMY. J. Endocrinol. 1959, 19, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Rowson L. E., A. and Moor R. M. 1967. The influence of embryonic tissue homogenate infused into the uterus, on the life-span of the corpus luteum in the sheep. Reproduction, 13(3), 511-516.
- Ruggeri, B. and Thoroughgood C. A. 1985. Prostaglandins in aquatic fauna: a comprehensive. MARINE ECOLOGY-PROGRESS SERIES, 23, 301-306.
- Ruppert, E.E. Structure, Ultrastructure and Function of the Neural Gland Complex of Ascidia interrupta (Chordata, Ascidiacea): Clarification of Hypotheses Regarding the Evolution of the Vertebrate Anterior Pituitary. Acta Zool. 1990, 71, 135–149. [Google Scholar] [CrossRef]
- Sandberger-Loua, L.; Müller, H.; Rödel, M.-O. A review of the reproductive biology of the only known matrotrophic viviparous anuran, the West African Nimba toad, Nimbaphrynoides occidentalis. Zoosystematics Evol. 2017, 93, 105–133. [Google Scholar] [CrossRef]
- Sandhoff, T.W.; McLean, M.P. Prostaglandin F2α reduces steroidogenic acute regulatory (StAR) protein messenger ribonucleic acid expression in the rat ovary. Endocrine 1996, 5, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Amano, Y.; Hayakawa, D.; Tsubota, T.; Ishikawa, H.; Mogoe, T.; Ohsumi, S.; Tetsuka, M.; Miyamoto, A.; Fukui, Y.; et al. Structure and Steroidogenesis of the Placenta in the Antarctic Minke Whale (Balaenoptera bonaerensis). J. Reprod. Dev. 2013, 59, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Sato, M., Tsubota T., Komatsu T., Watanabe G., Taya K., Murase T., Kita I. and Kudo T. 2001. Changes in sex steroids, gonadotropins, prolactin, and inhibin in pregnant and nonpregnant Japanese black bears (Ursus thibetanus japonicus). Biology of Reproduction, 65(4), 1006-1013.
- Schlosser, G. 2021. Evolutionary Origin of Sensory and Neurosecretory Cell Types: Vertebrate Cranial Placodes, volume 2. CRC Press.
- Schwarzenberger, F.; Walzer, C.; Tomasova, K.; Vahala, J.; Meister, J.; Goodrowe, K.; Zima, J.; Strauß, G.; Lynch, M. Faecal progesterone metabolite analysis for non-invasive monitoring of reproductive function in the white rhinoceros (Ceratotherium simum). Anim. Reprod. Sci. 1998, 53, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberger, F., Rietschel W., Vahala J., Holeckova D., Thomas P., Maltzan J., Baumgartner K. and Schaftenaar W. 2000. Fecal progesterone, estrogen, and androgen metabolites for noninvasive monitoring of reproductive function in the female Indian rhinoceros, Rhinoceros unicornis. General and Comparative Endocrinology, 119(3), 300-307.
- Schwarzenberger, F.; Hermes, R. Comparative analysis of gestation in three rhinoceros species (Diceros bicornis; Ceratotherium simum; Rhinoceros unicornis). Gen. Comp. Endocrinol. 2023, 334, 114214. [Google Scholar] [CrossRef] [PubMed]
- Shenavai, S.; Hoffmann, B.; Dilly, M.; Pfarrer, C.; Özalp, G.R.; Caliskan, C.; Seyrek-Intas, K.; Schuler, G. Use of the progesterone (P4) receptor antagonist aglepristone to characterize the role of P4 withdrawal for parturition and placental release in cows. Reproduction 2010, 140, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Sherman G., B., Heilman D. F., Hoss A. J., Bunick D. and Lund L. A. 2001. Messenger RNAs encoding the beta subunits of guinea pig (Cavia porcellus) luteinizing hormone (gpLH) and putative chorionic gonadotropin (gpCG) are transcribed from a single-copy gpLH/CGbeta gene. Journal of Molecular Endocrinology, 26(3), 267-280.
- Sherwood N., M., Tello J. A. and Roch G. J. 2006. Neuroendocrinology of protochordates: insights from Ciona genomics. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 144(3), 254-271.
- Shimizu, K. Reproductive Hormones and the Ovarian Cycle in Macaques. J. Mamm. Ova Res. 2008, 25, 122–126. [Google Scholar] [CrossRef]
- Short R., V. 1969. Implantation and the maternal recognition of pregnancy. Foetal Autonomy, 2, 31.
- Siemieniuch, M.J.; Bowolaksono, A.; Skarzynski, D.J.; Okuda, K. Ovarian steroids regulate prostaglandin secretion in the feline endometrium. Anim. Reprod. Sci. 2010, 120, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Siiteri, P.K.; Stites, D.P. Immunologic and Endocrine Interrelationships in Pregnancy. Biol. Reprod. 1982, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Simmer H., H. 1970. On the history of hormonal contraception I. Ludwig Haberlandt (1885–1932) and his concept of “hormonal sterilization”. Contraception, 1(1), 3-27.
- Simmer H., H. 1971. The first experiments to demonstrate an endocrine function of the corpus luteum on the occasion of the 100. birthday of Ludwig Fraenkel (1870-1951). Sudhoffs Archiv, (H. 4), 392-417.
- Skidmore J., A. 2018. Reproduction in dromedary camels: an update. Animal Reproduction (AR), 2(3), 161-171.
- Smits, K.; Willems, S.; Van Steendam, K.; Van De Velde, M.; De Lange, V.; Ververs, C.; Roels, K.; Govaere, J.; Van Nieuwerburgh, F.; Peelman, L.; et al. Proteins involved in embryo-maternal interaction around the signalling of maternal recognition of pregnancy in the horse. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.G.; Hanks, J.; Short, R.V. BIOCHEMICAL OBSERVATIONS ON THE CORPORA LUTEA OF THE AFRICAN ELEPHANT, LOXODONTA AFRICANA. Reproduction 1969, 20, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.J. The prolactin and growth hormone families: Pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod. Biol. Endocrinol. 2004, 2, 51–51. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.J.; Konno, T.; Alam, S.K. The prolactin family: effectors of pregnancy-dependent adaptations. Trends Endocrinol. Metab. 2007, 18, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Spady, T.J.; Lindburg, D.G.; Durrant, B.S. Evolution of reproductive seasonality in bears. Mammal Rev. 2007, 37, 21–53. [Google Scholar] [CrossRef]
- Spencer T., E. and Bazer F. W. 2002. Biology of progesterone action during pregnancy recognition and maintenance of pregnancy. Front Biosci, 7(7), d1879-d1898.
- Spencer, T.E.; Johnson, G.A.; Bazer, F.W.; Burghardt, R.C.; Palmarini, M. Pregnancy recognition and conceptus implantation in domestic ruminants: roles of progesterone, interferons and endogenous retroviruses. Reprod. Fertil. Dev. 2007, 19, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Steinach, E. and Kun H. 1931. Luteingewebe und männliche Greschlechtscharaktere. Pflüger’s Archiv für die gesamte Physiologie des Menschen und der Tiere, 227(1), 266-278.
- Steinman, K.; O’brien, J.; Monfort, S.; Robeck, T. Characterization of the estrous cycle in female beluga (Delphinapterus leucas) using urinary endocrine monitoring and transabdominal ultrasound: Evidence of facultative induced ovulation. Gen. Comp. Endocrinol. 2012, 175, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Stocco D., M. 2000. The role of the StAR protein in steroidogenesis: challenges for the future. The Journal of endocrinology, 164(3), 247-253.
- Stocco, D.M. StAR Protein and the Regulation of Steroid Hormone Biosynthesis. Annu. Rev. Physiol. 2001, 63, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Stormshak, F., Zelinski-Wooten M. B. and Abdelgadir S. E. 1987. Comparative aspects of the regulation of corpus luteum function in various species. Regulation of Ovarian and Testicular Function, 327-360.
- Sugino, N.; Shimamura, K.; Takiguchi, S.; Tamura, H.; Ono, M.; Nakata, M.; Nakamura, Y.; Ogino, K.; Uda, T.; Kato, H. Changes in activity of superoxide dismutase in the human endometrium throughout the menstrual cycle and in early pregnancy. Hum. Reprod. 1996, 11, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Sugino, N., Nakata M., Kashida S., Karube A., Takiguchi S. and Kato H. 2000. Decreased superoxide dismutase expression and increased concentrations of lipid peroxide and prostaglandin F2α in the decidua of failed pregnancy. Molecular human reproduction, 6(7), 642-647.
- Sugino, N. Roles of reactive oxygen species in the corpus luteum. Anim. Sci. J. 2006, 77, 556–565. [Google Scholar] [CrossRef]
- Swanson, M.T.; Oliveros, C.H.; Esselstyn, J.A. A phylogenomic rodent tree reveals the repeated evolution of masseter architectures. Proc. R. Soc. B: Biol. Sci. 2019, 286, 20190672. [Google Scholar] [CrossRef] [PubMed]
- Talmage, R.V. and Buchannan G.D. 1954. The armadillo (Dasypus novemcinctus): a review of its natural history, ecology, anatomy and reproductive physiology. Pages 1-135 in The Rice Institute Monograph in Biology XLI(2), Houston.
- Tamanini, C.; De Ambrogi, M. Angiogenesis in Developing Follicle and Corpus Luteum. Reprod. Domest. Anim. 2004, 39, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Tardif, S.D.; A Smucny, D.; Abbott, D.H.; Mansfield, K.; Schultz-Darken, N.; Yamamoto, M.E. Reproduction in captive common marmosets (Callithrix jacchus). Comp. Med. 2003, 53, 364–8. [Google Scholar] [PubMed]
- Taube, E.; Keravec, J.; Vie, J.-C.; Duplantier, J.-M. Reproductive biology and postnatal development in sloths, Bradypus and Choloepus: review with original data from the field (French Guiana) and from captivity. Mammal Rev. 2001, 31, 173–188. [Google Scholar] [CrossRef]
- Terakado, K. Induction of Gamete Release by Gonadotropin-Releasing Hormone in a Protochordate, Ciona intestinalis. Gen. Comp. Endocrinol. 2001, 124, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Tello, J.A.; Sherwood, N.M. Amphioxus: Beginning of Vertebrate and End of Invertebrate Type GnRH Receptor Lineage. Endocrinology 2009, 150, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Thornton, J.W. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc. Natl. Acad. Sci. 2001, 98, 5671–5676. [Google Scholar] [CrossRef] [PubMed]
- Thornton, J.W.; Need, E.; Crews, D. Resurrecting the Ancestral Steroid Receptor: Ancient Origin of Estrogen Signaling. Science 2003, 301, 1714–1717. [Google Scholar] [CrossRef] [PubMed]
- Teshima, K.; Mizue, K. Studies on sharks. I. Reproduction in the female sumitsuki shark Carcharhinus dussumieri. Mar. Biol. 1972, 14, 222–231. [Google Scholar] [CrossRef]
- Tripp, H.R.H. Capture, laboratory care and breeding of elephant-shrews (Macroscelididae). Lab. Anim. 1972, 6, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Tsai P., S. and Zhang L. 2008. The emergence and loss of gonadotropin-releasing hormone in protostomes: orthology, phylogeny, structure, and function. Biology of reproduction, 79(5), 798-805.
- Tsang, P.C.; Callard, I.P. Morphological and endocrine correlates of the reproductive cycle of the aplacental viviparous dogfish, Squalus acanthias. Gen. Comp. Endocrinol. 1987, 66, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Tyndale-Biscoe C., H. 1979. Hormonal control of embryonic diapause and reactivation in the tammar wallaby. Pages 173-190 in Ciba Foundation Symposium 64-Maternal Recognition of Pregnancy.Chichester, UK: John Wiley & Sons, Ltd.
- Tricas, T.C.; Maruska, K.P.; Rasmussen, L. Annual Cycles of Steroid Hormone Production, Gonad Development, and Reproductive Behavior in the Atlantic Stingray. Gen. Comp. Endocrinol. 2000, 118, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Troll, S.; Gottschalk, J.; Seeburger, J.; Ziemssen, E.; Häfner, M.; Thielebein, J.; Einspanier, A. Characterization of the ovarian cycle in the two-toed sloths (Choloepus didactylus): An innovative, reliable, and noninvasive method using fecal hormone analyses. Theriogenology 2013, 80, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, T.; Takahashi, Y.; Kanagawa, H. Changes in Serum Progesterone Levels and Growth of Fetuses in Hokkaido Brown Bears. Bears: Their Biol. Manag. 1987, 7, 355. [Google Scholar] [CrossRef]
- Tsutsui, T.; Stabenfeldt, G.H. Biology of ovarian cycles, pregnancy and pseudopregnancy in the domestic cat. J. Reprod. Fertil. 1993, 47, 29–35. [Google Scholar]
- Uchida, K.; Moriyama, S.; Sower, S.A.; Nozaki, M. Glycoprotein hormone in the pituitary of hagfish and its evolutionary implications. Fish Physiol. Biochem. 2012, 39, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Valmsen, K.; Järving, I.; Boeglin, W.E.; Varvas, K.; Koljak, R.; Pehk, T.; Brash, A.R.; Samel, N. The origin of 15 R -prostaglandins in the Caribbean coral Plexaura homomalla: Molecular cloning and expression of a novel cyclooxygenase. Proc. Natl. Acad. Sci. 2001, 98, 7700–7705. [Google Scholar] [CrossRef] [PubMed]
- Van Aarde R.J. 1984. Aardvark. in The Encyclopedia of Mammals, edited by Macdonald D. New York, NY: Facts on File Publications. ISBN 978-0-87196-871-5.
- Van Aarde R., J., Van Der Merwe M. and Skinner D. C. 1994. Progesterone concentrations and contents in the plasma, ovary, adrenal gland and placenta of the pregnant Natal clinging bat Miniopterus schreibersii natalensis. Journal of Zoology, 232(3), 457-464.
- Van Horn R., N. and G. Gray Eaton. 1979. Reproductive physiology and behavior in prosimians. Pages 79-122 in The study of prosimian behavior, edited by Doyle G.A. Elsevier.
- Van der Merwe, M. and Rautenbach I. L. 1987. Reproduction in Schlieffen’s bat, Nycticeius schlieffenii, in the eastern Transvaal lowveld, South Africa. Reproduction, 81(1), 41-50.
- Walusimbi S., S. and Pate J. L. 2013. Physiology and Endocrinology Symposium: role of immune cells in the corpus luteum. Journal of animal science, 91(4), 1650-1659.
- Wagner, G.P.; Erkenbrack, E.M.; Love, A.C. Stress-Induced Evolutionary Innovation: A Mechanism for the Origin of Cell Types. BioEssays 2019, 41, e1800188–e1800188. [Google Scholar] [CrossRef] [PubMed]
- Wakai, Y. Hasegawa K. Sakamoto S. Asano S. Watanabe G. and Taya K. 2002. Annual changes of urinary progesterone and estradiol-17β of the dugong (Dugong dugon) in captivity. Zoological science, 19(6), 679-682.
- Wake M., H. 1977a. Fetal maintenance and its evolutionary significance in the Amphibia: Gymnophiona. Journal of Herpetology, 379-386.
- Wake M., H. 1977b. The reproductive biology of caecilians: an evolutionary perspective. Pages 73-101 in The reproductive biology of amphibians. Boston, MA: Springer US.
- Wake M., H. 1980. The reproductive biology of Nectophrynoides malcolmi (Amphibia: Bufonidae), with comments on the evolution of reproductive modes in the genus Nectophrynoides. Copeia, 193-209.
- Wake M., H. and Dickie R. 1998. Oviduct structure and function and reproductive modes in amphibians. Journal of Experimental Zoology, 282(4-5), 477-506.
- Wang P., Liu S., Yang Q., Liu Z. and Zhang S. 2018. Functional characterization of thyrostimulin in amphioxus suggests an ancestral origin of the TH signaling pathway. Endocrinology, 159(10), 3536-3548.
- Weekes, H.C. 1934. The corpus luteum in certain oviparous and viviparous reptiles. In Proceedings of the Linnean Society of New South Wales 59, 380-391.
- Weir B., J. 1971. The reproductive organs of the female plains viscacha, Lagostomus maximus. Reproduction, 25(3), 365-373.
- Werneburg, I.; Laurin, M.; Koyabu, D.; Sánchez-Villagra, M.R. Evolution of organogenesis and the origin of altriciality in mammals. Evol. Dev. 2016, 18, 229–244. [Google Scholar] [CrossRef] [PubMed]
- White, H.E.; Tucker, A.S.; Fernandez, V.; Miguez, R.P.; Hautier, L.; Herrel, A.; Urban, D.J.; Sears, K.E.; Goswami, A. Pedomorphosis in the ancestry of marsupial mammals. Curr. Biol. 2023, 33, 2136–2150.e4. [Google Scholar] [CrossRef] [PubMed]
- Welsch, U. and Fang Y. 1997. Ultrastructure and function of follicle cell in the ovary of Branchiostoma belcheri. Science in China Series C: Life Sciences, 40, 60-70.
- Wildt D. E., Chan S. Y. W., Seager S. W. J. and Chakraborty P. K. 1981. Ovarian activity, circulating hormones, and sexual behavior in the cat. I. Relationships during the coitus-induced luteal phase and the estrous period without mating. Biology of reproduction, 25(1), 15-28.
- Xavier, F. 1978. Une espèce nouvelle de Nectophrynoides (Anura, Bufonidae) des Monts Nimba, N. liberiensis n.sp. I –description de l’espèce. Bulletin de la Société Zoologique de France, 103, 431–441.
- Yamamoto, Y.; Yamamoto, T.; Taya, K.; Watanabe, G.; Stansfield, F.; Allen, W. Placentation in the African elephant (Loxodonta africana). V. The trophoblast secretes placental lactogen. Placenta 2011, 32, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Yaron, Z. Endocrine aspects of gestation in viviparous reptiles. Gen. Comp. Endocrinol. 1972, 3, 663–674. [Google Scholar] [CrossRef]
- Zhang, X., Zhu C., Lin H., Yang Q., Ou Q., Li Y., Chen Z., Racey P., Zhang S. and Wang, H. 2007. Wild fulvous fruit bats (Rousettus leschenaulti) exhibit human-like menstrual cycle. Biology of reproduction, 77(2), 358-364.
- Ziecik, A.J.; Przygrodzka, E.; Jalali, B.M.; Kaczmarek, M.M. Regulation of the porcine corpus luteum during pregnancy. Reproduction 2018, 156, R57–R67. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, T.E.; Tardif, S.D.; Ross, C.N.; Snowdon, C.T.; Kapoor, A.; Rutherford, J.N. Timing of the luteal-placental shift is delayed with additional fetuses in litter-bearing callitrichid monkeys, Saguinus oedipus and Callithrix jacchus. Gen. Comp. Endocrinol. 2023, 333, 114195–114195. [Google Scholar] [CrossRef]
- Zielinski, W.J.; Mazurek, M.J. Reproductive Characteristics of the Point Arena Mountain Beaver (Aplodontia rufa nigra). Northwest Sci. 2016, 90, 136–145. [Google Scholar] [CrossRef]
Figure 1.
Summary of the early events in vertebrate HPO axis evolution. Note that the CL predates any of the origins of viviparity. Internal fertilization originated several times independently and was followed in these taxa by the origin of viviparity. Only in Amniotes did viviparity evolve several times on the basis of shared internal fertilization. The asterisks denote that internal fertilization and viviparity originated in these clades, but do imply that all species of the clade share it. CYP11A, CYP17, HSD3B: enzymes in the steroidogenic pathway; GNRH, GNRHR: gonadotropin releasing hormone and ist receptor; HPO: hypothalamic-pituitary-ovarian, GPH: gonadotropin-releasing hormone; ER, SR: estrogen and steroid receptor; LH: luteinizing hormone; FSH: folicle stimulating hormone.
Figure 1.
Summary of the early events in vertebrate HPO axis evolution. Note that the CL predates any of the origins of viviparity. Internal fertilization originated several times independently and was followed in these taxa by the origin of viviparity. Only in Amniotes did viviparity evolve several times on the basis of shared internal fertilization. The asterisks denote that internal fertilization and viviparity originated in these clades, but do imply that all species of the clade share it. CYP11A, CYP17, HSD3B: enzymes in the steroidogenic pathway; GNRH, GNRHR: gonadotropin releasing hormone and ist receptor; HPO: hypothalamic-pituitary-ovarian, GPH: gonadotropin-releasing hormone; ER, SR: estrogen and steroid receptor; LH: luteinizing hormone; FSH: folicle stimulating hormone.
Figure 2.
Scheme with the plausible modifications of the lifespan of the corpus luteum of pregnancy relative to the nonpregnant CL.
Figure 2.
Scheme with the plausible modifications of the lifespan of the corpus luteum of pregnancy relative to the nonpregnant CL.
Figure 3.
Character state matrix for five reproductive characters (CL type, placental progesterone, ovulation type, altriciality and degreee of invasiveness) plotted along the phylogenetic tree of placental mammals (timetree: Kumar et al. 2022). Branches are coloured according to superorders (center key).
Figure 3.
Character state matrix for five reproductive characters (CL type, placental progesterone, ovulation type, altriciality and degreee of invasiveness) plotted along the phylogenetic tree of placental mammals (timetree: Kumar et al. 2022). Branches are coloured according to superorders (center key).
Figure 4.
Strong effect of body size on the length of nonpregnant and pregnant cycles in precocial and altricial mammals. Relationship between body size and gestation length (A) and body size and estrous cycle length (B) on a log scale across mammalian taxa (see data in supplementary material). Relationship between the residual pregnant and nonpregnant cycle lengths after regression on the body weight (C). Note that the nonpregnant cycle scales equally strongly with body size in altricial and precocial species. In contrast, gestation length scaling with body size is stronger in precocial species than in altricial species, explaining much of the variation in the former and implying that body size might drive the divergence between precocial and altricial species. After accounting for body size, gestation length correlates well with the nonpregnant cycle length in both cases. All regressions p<0.05.
Figure 4.
Strong effect of body size on the length of nonpregnant and pregnant cycles in precocial and altricial mammals. Relationship between body size and gestation length (A) and body size and estrous cycle length (B) on a log scale across mammalian taxa (see data in supplementary material). Relationship between the residual pregnant and nonpregnant cycle lengths after regression on the body weight (C). Note that the nonpregnant cycle scales equally strongly with body size in altricial and precocial species. In contrast, gestation length scaling with body size is stronger in precocial species than in altricial species, explaining much of the variation in the former and implying that body size might drive the divergence between precocial and altricial species. After accounting for body size, gestation length correlates well with the nonpregnant cycle length in both cases. All regressions p<0.05.
Figure 5.
Maximum likelihood ancestral state reconstruction of ovarian cycle and pregnancy lengths. Plots were generated using the contMap() function in phytools (Revell 2024) and using the phylogenetic tree of timetree (Kumar et al. 2022). Values plotted are residuals after regression for body mass (WTcorr). Pregnancy length and body mass data were sourced from AnAge database (de Magalhães et al. 2024). Ovarian cycles lengths were compiled from several sources (references in the review of the luteal phase and MRP across eutherians).
Figure 5.
Maximum likelihood ancestral state reconstruction of ovarian cycle and pregnancy lengths. Plots were generated using the contMap() function in phytools (Revell 2024) and using the phylogenetic tree of timetree (Kumar et al. 2022). Values plotted are residuals after regression for body mass (WTcorr). Pregnancy length and body mass data were sourced from AnAge database (de Magalhães et al. 2024). Ovarian cycles lengths were compiled from several sources (references in the review of the luteal phase and MRP across eutherians).
Figure 6.
The estimation of the ovarian cycle and gestation length (in days) ancestral to eutherians suggest an early decoupling of the cycles. Values were plotted using the same approach as in
Figure 5 but without regression to remove body mass. In blue the reconstructed cycle and gestation length for the eutherian common ancestor.
Figure 6.
The estimation of the ovarian cycle and gestation length (in days) ancestral to eutherians suggest an early decoupling of the cycles. Values were plotted using the same approach as in
Figure 5 but without regression to remove body mass. In blue the reconstructed cycle and gestation length for the eutherian common ancestor.
Figure 7.
No single control mechanism can be used to explain corpus luteum lifespan across eutherians. Character state matrix for the CL type along with mechanisms for MRP plotted along the phylogenetic tree of placental mammals. Although unknown for the vast majority of species, there is no universal factor that rescues the CL. Signals can have local or systemics effects and be luteolytic or anti-luteolytic. Note that MRP is absent in those species long enough to encompass pregnancy and that in species with very short cycles it may be governed by maternal hypophysial prolactin (PRL). CG: chorionic-gonadotropin; PL: placental lactogen; E2: estrogen; IFNT: interferon-tau.
Figure 7.
No single control mechanism can be used to explain corpus luteum lifespan across eutherians. Character state matrix for the CL type along with mechanisms for MRP plotted along the phylogenetic tree of placental mammals. Although unknown for the vast majority of species, there is no universal factor that rescues the CL. Signals can have local or systemics effects and be luteolytic or anti-luteolytic. Note that MRP is absent in those species long enough to encompass pregnancy and that in species with very short cycles it may be governed by maternal hypophysial prolactin (PRL). CG: chorionic-gonadotropin; PL: placental lactogen; E2: estrogen; IFNT: interferon-tau.
Figure 8.
A proposed explanation for the inference of relatively long gestational lengths in ancestral eutherian due to the puctuated divergence of gestation from estrous cycle length. Extrapolation is illustrated here relative to body size, as the latter appears to drive gestational length evolution. A: The scaling observed in extant eutherians implies a difference in pregnant and nonpregnant cycle lengths at all body sizes. B: Hypothetical change of the intercept of gestation length after the origin of MRP (red arrow). The gestation length of an ancestor lacking MRP would be shorter than in extant species with a comparable size. All axes are on log scale. MRP: maternal recognition of pregnancy.
Figure 8.
A proposed explanation for the inference of relatively long gestational lengths in ancestral eutherian due to the puctuated divergence of gestation from estrous cycle length. Extrapolation is illustrated here relative to body size, as the latter appears to drive gestational length evolution. A: The scaling observed in extant eutherians implies a difference in pregnant and nonpregnant cycle lengths at all body sizes. B: Hypothetical change of the intercept of gestation length after the origin of MRP (red arrow). The gestation length of an ancestor lacking MRP would be shorter than in extant species with a comparable size. All axes are on log scale. MRP: maternal recognition of pregnancy.
Figure 9.
A proposed model for the evolution of the lifespans of pregnant and nonpregnant corpus luteum in eutherians. The model proposes an early co-extension of pregnant and nonpregnant cycle, to be followed by their independent decoupling in major eutherian lineages, by the independent origins of the maternal recognition of pregnancy mechanisms. No claims are made about the sequence of MRP origins here but note that some regimes likely derive from others (for example, MRP3 may arise from MRP1 or MRP2). MRP: maternal recognition of pregnancy, CL: corpus luteum.
Figure 9.
A proposed model for the evolution of the lifespans of pregnant and nonpregnant corpus luteum in eutherians. The model proposes an early co-extension of pregnant and nonpregnant cycle, to be followed by their independent decoupling in major eutherian lineages, by the independent origins of the maternal recognition of pregnancy mechanisms. No claims are made about the sequence of MRP origins here but note that some regimes likely derive from others (for example, MRP3 may arise from MRP1 or MRP2). MRP: maternal recognition of pregnancy, CL: corpus luteum.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).