1. Introduction
Fuel cell technology, which is now applicable to a wide range of technical areas, is becoming commercially viable. This includes stationary power supplies and electro-traction, among other things. The significant advancements in this field are the result of the dedicated efforts of numerous researchers over the past decades [
1]. The fuel cell (FC) is a device that has been extensively researched for its potential uses in various fields. It has shown promise as an alternative solution in the automotive industry, distributed power generation, and other industrial applications. The primary function of this device is to convert the chemical energy from a specific fuel into electrical energy [
2,
3]. One of the fuel cells that is widely used in the meantime is methanol. with progress in various areas, including catalysis, electrolytes, electrode structure, the theoretical understanding of gas diffusion, and fuel cell engineering. This progress also includes direct methanol fuel cells (DMFCs), which have long been viewed as the most challenging fuel cell technology due to issues with methanol crossover and catalytic inefficiency [
4]. In parallel, nanomaterials have been extensively utilized in various aspects of human life, such as reinforcing nanocomposites [
5,
6,
7,
8,
9,
10,
11,
12], and serving as adsorbents to eliminate hazardous substances [
13,
14,
15].
Among the available techniques, adsorption is the most appealing. In this method, two-dimensional materials with vast surface areas and numerous active sites are used as perfect adsorbents for environmental cleanup, similar to porous materials [
13,
14,
15]. Over the past two decades, researchers have been significantly interested in developing an appropriate adsorbent for the adsorbing desulfurization process [
16].
Boron nitride (BN) and aluminum nitride (AlN) are wideband gap III–V compounds known for their impressive physical properties and chemical stability. They consist of boron, aluminum, and nitrogen atoms arranged in a hexagonal pattern, similar to a honeycomb. This structure is made up of two-dimensional (2D) layers that are sp2-bonded, much like graphene, which is a flat monolayer of carbon atoms arranged in a 2D honeycomb lattice [
17]. Each layer of these hexagonal lattices is held together by strong covalent bonds between B, Al, and N atoms. However, the layers themselves are held together by weak van der Waals (vdW) forces, a characteristic they share with graphite [
18]. The intense study of graphene has generally sparked interest in other two-dimensional (2D) hexagonal networks, including h-BN [
19,
20] This material is particularly interesting as a topological insulator [
21]. Recently, the exfoliation of 2D BN nanosheets has been achieved. This is a necessary step to fully realize the potential of h-BN in various applications, from electronics to energy storage [
22]. This set of graphene materials exhibits significant potential as adsorbents for methanol. The process by which methanol is adsorbed onto the sides of these graphenes can be assessed using quantum mechanical computations, specifically those based on Density Functional Theory (DFT).
These calculations can provide valuable insights into the interactions between these molecules and the graphene surfaces, contributing to the development of more efficient adsorption processes. A variety of computational methods exist for studying the properties of nanomaterials prior to their experimental use in the lab. Given the high costs associated with experimental procedures, researchers often resort to highly accurate computational methods. From a molecular perspective, calculations based on quantum mechanics tend to align more closely with experimental results compared to other molecular simulations, such as molecular dynamics or Monte Carlo methods [
23,
24,
25,
26,
27,
28]. Density Functional Theory (DFT) as a robust computational tool is capable of handling the periodic boundary conditions required for simulating a graphene sheet. It holds the potential to compute the adsorption characteristics of an arbitrary molecule. Several computational atomistic calculations are used for simulating mechanical properties [
29], adsorption [
30], and electrical properties [
31,
32]. These include Classical Monte Carlo (MC), Classical Molecular Dynamics (MD), Semi-Empirical, and Ab-Initio Quantum Mechanics. Among these, the Ab-Initio Quantum Mechanics method is found to be more accurate and is among the most important of these methods [
33,
34]. This method can be further classified into Density Functional Theory (DFT) and Hartree-Fock (H–F) calculations. It is thought that DFT calculations could provide a good estimation of the interaction mechanism and mechanical behavior. Upon examining the results from these atomistic methods, it is observed that the results from DFT calculations for solid-state systems align best with experimental data [
35].
In this research, we investigate the adsorption properties of boron nitride, graphene, and aluminum nitride with methanol, utilizing density functional theory (DFT) simulations. The electronic structure of the molecules was calculated using SIESTA software. This study examined the adsorption of a single methanol molecule under various geometric conditions on these graphenes. The state with the most negative energy value was identified as the most stable possible state. In these most negative states, the amount of charge transferred, the results of state density, and electronic properties were analyzed. The investigation pinpoints the optimized structures and equilibrium bond lengths for both materials and probes the absorption energies for methanol at diverse sites on their surfaces. Aluminum nitride demonstrates a greater propensity for methanol than boron nitride and graphene, with the absorption described as weakly physical in nature. Variations in the electronic states of the materials were unveiled through Local Density of States (DOS) diagrams, signifying a more potent charge transfer from methanol to boron nitride. The research implies that the absorption properties could fluctuate under varying environmental conditions and that additional simulations with sophisticated software could yield more precise forecasts. Moreover, the examination of other nanoparticles might pinpoint materials with optimal absorption capacities for methanol.
2. Computational Method
We calculate the adsorbent energy between methanol atoms with boron nitride, graphene, and aluminum nitride was computed using the first-principle, Density Functional Theory (DFT). The calculations were performed using the Spanish Initiative for Electronic Simulations with Thousands of Atoms (SIESTA) code [
36]. The Local Density Approximation (LDA) has a notable limitation due to the overbinding of its extended solids. This often results in underpredicted lattice parameters, while cohesive energies, elastic moduli, and phonon frequencies are typically overpredicted. To address this limitation, we opted to use the Generalized Gradient Approximation (GGA) function to account for electronic exchange and correlation effects, as outlined by Perdew–Burke–Ernzerhof (PBE). The Perdew–Burke–Ernzerhof (PBE) function was utilized in conjunction with the Generalized Gradient Approximation (GGA) for the electronic exchange and correlation effects [
37]. Throughout the entire process, a split-valence double-zeta basis set of localized numerical atomic orbitals was employed, incorporating polarization functions (DZP) with a split norm of 0.25 and an energy shift of 50 meV [
36,
38]. This approach strikes a balance between accuracy and computational resources. An energy cutoff of 120 Ry was chosen for the grid integration to represent the charge density [
36].
The fdf file required the following information: system name, number of atoms in the structure, and the number of types of atoms. In the ChemicalSpecialLabel section, a unique counter was assigned to each atom. The LatticeConstant section determined the meshing scale in angstroms, and the LatticeVectors section accounted for the general meshing coordinates. The MeshCutoff section had a value of 120 Ry.
The kgrid-Monkhorst-Pack matrix is related to alternating movements in the xyz direction. To expedite calculations, a ‘#’ sign was placed in front of the propositions. For DM.MixingWeight, a value of 0.08 was considered, and the number of DM.NumberPulay was set to 8. The diagon method was used for solving the equations, and an electric temperature of 25 millielectron volts was used. The DZP mode was used to approximate the super electron layer.
In the Atomic Coordinates and Atomic Species section, the coordinates of all the atoms in the structure were entered based on the type of atoms, the number of atoms, and the numbers assigned to them above in a matrix with six columns. The Atomic coordinates Format section specified the unit of abbreviated numbers as angstroms.
The adsorption energy between the methanol molecule and the adsorbents was computed using the fundamental principles of density functional theory. The local density approximation, despite its limitations due to the excessive connections of its solids, allows for a general prediction of network fronts. Throughout the process, a series of established numerical atomic orbitals with a double axis and split power layer were utilized. This approach ensured compatibility between accuracy and the reduction of computational fluid energy, which was set equal to Ry for the integration of the night to distribute the energy density. It also facilitated the determination of the most stable state of the atom coordinates relative to each other in the system, through a run with the Siesta software. This operation can be time-consuming, potentially requiring several weeks of calculations depending on the number of atoms in the system.
To achieve adsorption at each active center, three runs are necessary: one for the adsorber alone, one for the adsorbate alone, and one for the entire system with both adsorber and adsorbate. The adsorption energy (
Eads) is then determined according to the formula by following the equation (eq. 1)
Where E(total) represents the total energy of the methanol molecule and adsorbent (system energy), E(methanol) is the energy of the methanol molecule, and E(adsorbent)is the monolayer adsorption energy of AlN, BN, and graphene. After calculating the adsorption energy at different sites, the site with the lowest (more negative) adsorption energy (negative binding energy presents stable adsorption construction) is selected for further calculations such as topical density or DOS or CT load calculations.
3. Results and Discussions
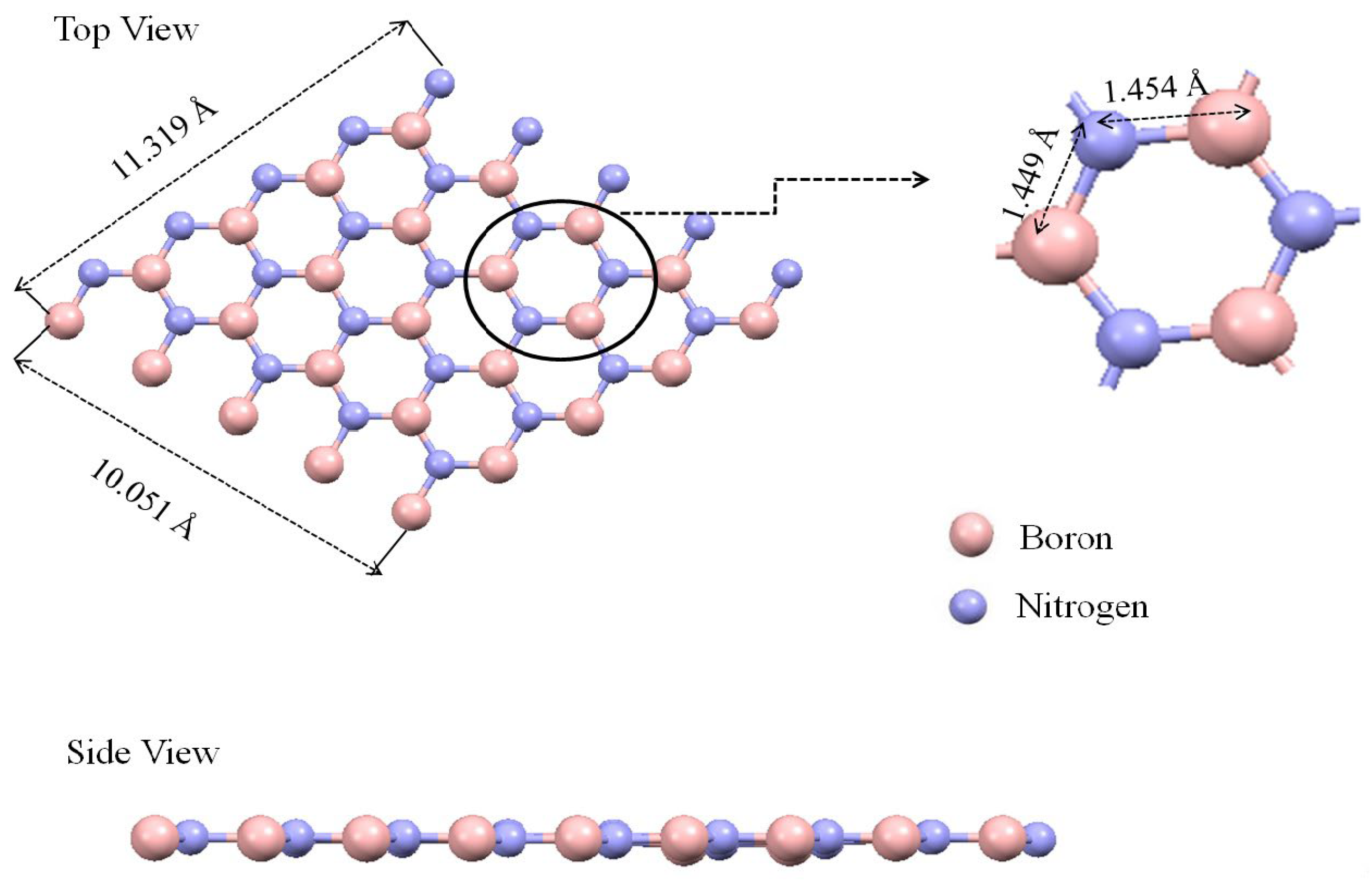
The geometrical structure of monolayer AlN, BN, and graphene was optimized using the Density Functional Theory (DFT) method. The optimized structure of the bare monolayer BN, as shown in
Figure 1, has dimensions of 11.319 Å × 10.051 Å. The equilibrium length of the boron and nitrogen atoms is approximately 1.454 Å. As shown in
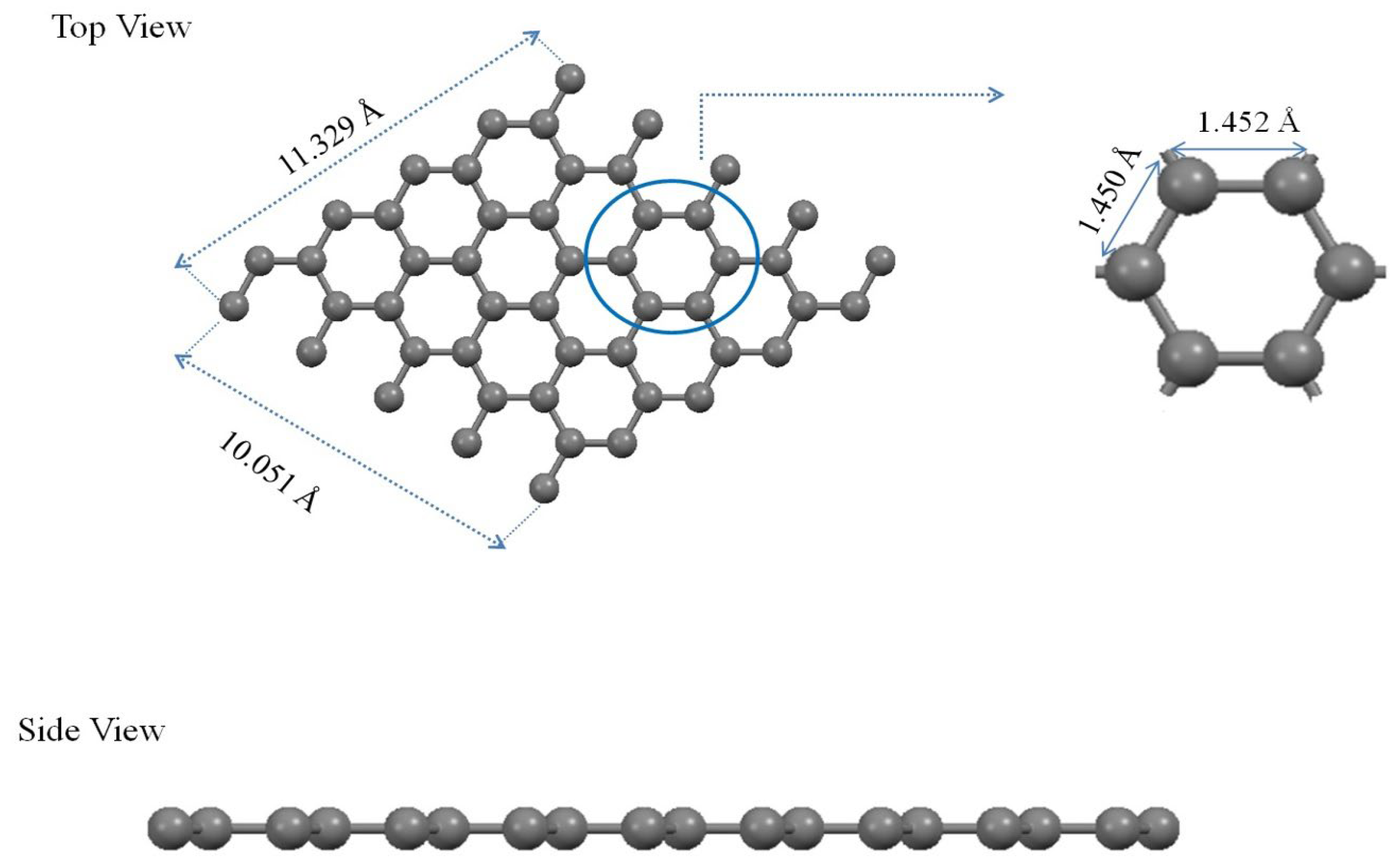
Figure 2, The optimized structure of the bare monolayer graphene has dimensions of 11.329 Å × 10.051 Å. The equilibrium length of the C-C bond is approximately 1.452 Å.
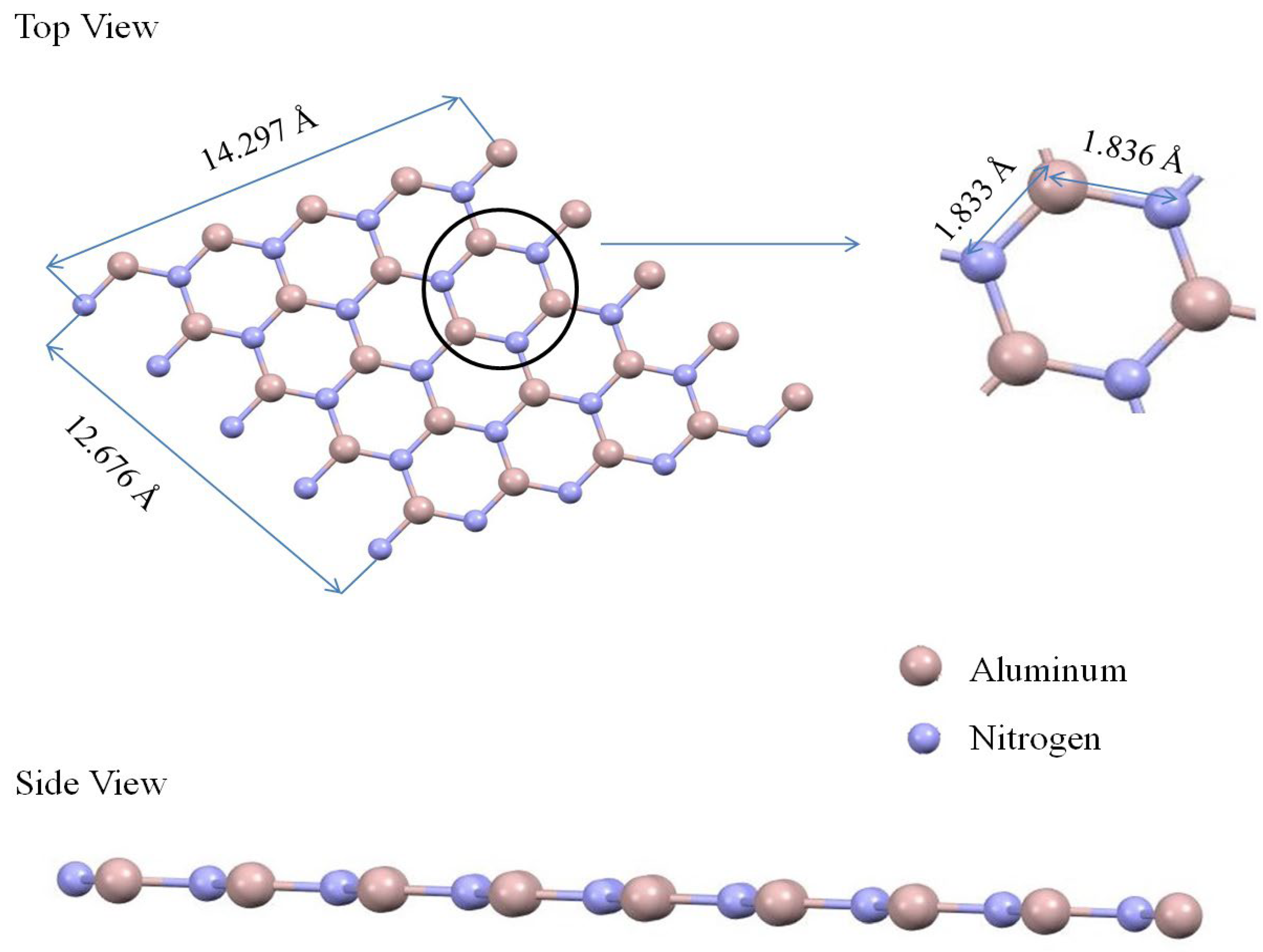
Figure 3 shows the structure of aluminum nitride graphene with 14.297 Å × 12.676 Å dimensions. The equilibrium length of aluminum and nitrogen atoms is approximately 1.836 Å. The study examined the adsorption of methanol on the surfaces of AlN, BN, and graphene, considering various adsorption sites. Eight adsorption sites for BN were considered. For methanol on the graphene surface, six adsorption sites were considered. In the case of AlN, the adsorption behavior of methanol was examined at eight different sites, as indicated in
Figure 4,
Figure 5 and
Figure 6. It’s important to note that the initial distance between the methanol atom and the surfaces of AlN, graphene, and BN was set to 2.30 Å for each site under consideration.
Memarian et al. [
36] employed molecular mechanics and the DFT method to calculate the mechanical properties of graphene. They used DFT calculations, such as the Generalized Gradient Approximation (GGA) and the Local Density Approximation (LDA), to estimate the optimized bond length between two carbon atoms. They found that the lengths were about 1.426 Å and 1.414 Å, respectively. They also reported that the bond length between two carbon atoms in graphene, obtained from AIREBO potential, was about 1.41 Å, from Tersoff potential was 1.464 Å, and from EDIP-Marks potential was 1.42 Å.
Ghorbanzadeh Ahangari in This study [
39] focuses on evaluating the mechanical properties of various monolayer sheets, both carbon-based and non-carbon-based, to identify the most suitable nanofiller for enhancing polypropylene. The materials examined include graphene, SiC-m, BN-m, AlN-m, GaN-m, and silicene. The results suggest that silicene, despite being less stiff, may form a stronger composite with polypropylene due to its superior interaction energy, making it a promising candidate for nanofiller applications. The team calculated the mechanical properties of these monolayer sheets and studied their interaction with propylene. By using Density Functional Theory (DFT) calculations, they optimized the geometry for monolayer sheets of the materials mentioned. The resulting bond lengths were found to be: Graphene: 1.42 Å - SiC-m: 1.81 Å - BN-m: 1.45 Å - AlN-m: 1.83 Å - GaN-m: 1.86 Å - Silicene: 2.27 Å
Our measurements align well with previous theoretical studies as well as previous study measurements [
39,
40,
41,
42,
43].
Figure 1 shows two main views: an expanded view and a side view. In the expanded view, atoms are represented by spheres. Boron atoms are shown in pink and nitrogen atoms are shown in blue, as indicated by the legend. There are measurements provided for specific distances between atoms. For instance, the distance between adjacent boron and nitrogen atoms is 1.454 Å. A specific section of the expanded view is circled to emphasize a particular arrangement of atoms. In the side view, the tubular structure of the boron nitride nanotube is shown. The alternating pattern of pink (boron) and gray (nitrogen) spheres represents their periodic arrangement in this compound.
Figure 2 shows a complex molecule made up entirely of carbon atoms. Each carbon atom is represented by a gray sphere. The bonds between these carbon atoms are shown as sticks connecting the spheres. There are measurements in angstroms (Å) indicating specific distances between certain groups of atoms within the molecule. A blue circle highlights a particular ring structure within this larger molecule, with distances marked from this ring to other parts of the molecule. On the right side, there’s an isolated view of a ring structure similar to that highlighted in blue in the main molecular structure, with its diameter measured. Section A of the Figure presents a two-dimensional lattice of the molecular structure. The atoms within this structure are symbolized by spheres and are interconnected by lines, which represent bonds. The bond lengths are specifically indicated, with measurements such as 11.329 Å, 10.051 Å, 1.450 Å, and 1.452 Å. A hexagonal section within the structure is encircled and magnified, providing a detailed perspective on the atomic spacing and arrangement. This expanded view offers an in-depth look at the atomic structure, allowing us to understand the intricate details of the molecular configuration. Moving on to Section B, we see a linear array of atoms, suggesting a one-dimensional material or molecule. This section provides a clear view of the linear arrangement of atoms, represented by spheres of different colors. This side view complements the expanded view in Section A, offering a broader perspective on the overall structure of the molecule.
Figure 3 presents two distinct perspectives: a magnified view and a lateral view. In the magnified view, atoms are depicted as spheres, with aluminum atoms represented in pink and nitrogen atoms in gray, as denoted by the legend. Specific interatomic distances are provided, such as the 1.836 Å distance between neighboring aluminum and nitrogen atoms. A particular atomic arrangement is highlighted in a circled section of the magnified view. The lateral view, on the other hand, illustrates the tubular formation of the boron nitride nanotube. The periodic pattern of pink (aluminum) and gray (nitrogen) spheres signifies their alternating arrangement in this compound. It illustrates a complex molecular structure composed of two types of atoms. Each atom is represented by a sphere, with aluminum atoms depicted in pink and nitrogen atoms in gray. The bonds between these atoms are shown as lines connecting the spheres. There are measurements in angstroms (Å) indicating specific distances between certain groups of atoms within the molecule. A blue circle highlights a particular hexagonal structure within this larger molecule, with distances marked from this hexagon to other parts of the molecule. On the right side, there’s an isolated view of a linear arrangement of atoms, similar to the periodic arrangement highlighted in the main molecular structure, with its length measured. The image provided offers a comprehensive view of a complex molecular structure, divided into two main sections, labeled (Top View) and (Side View). Section Top View presents a two-dimensional lattice of a molecular structure. The atoms are symbolized by spheres and are interconnected by lines representing bonds. The bond lengths are specifically indicated, with measurements such as 14.297 Å, 12.676 Å, and 1.836 Å. A hexagonal section within the structure is encircled and magnified, providing a detailed perspective on the atomic spacing and arrangement. Section Side View, on the other hand, shows cases of a linear array of atoms, suggesting a one-dimensional material or molecule. This section provides a clear view of the linear arrangement of atoms, represented by spheres of different colors.
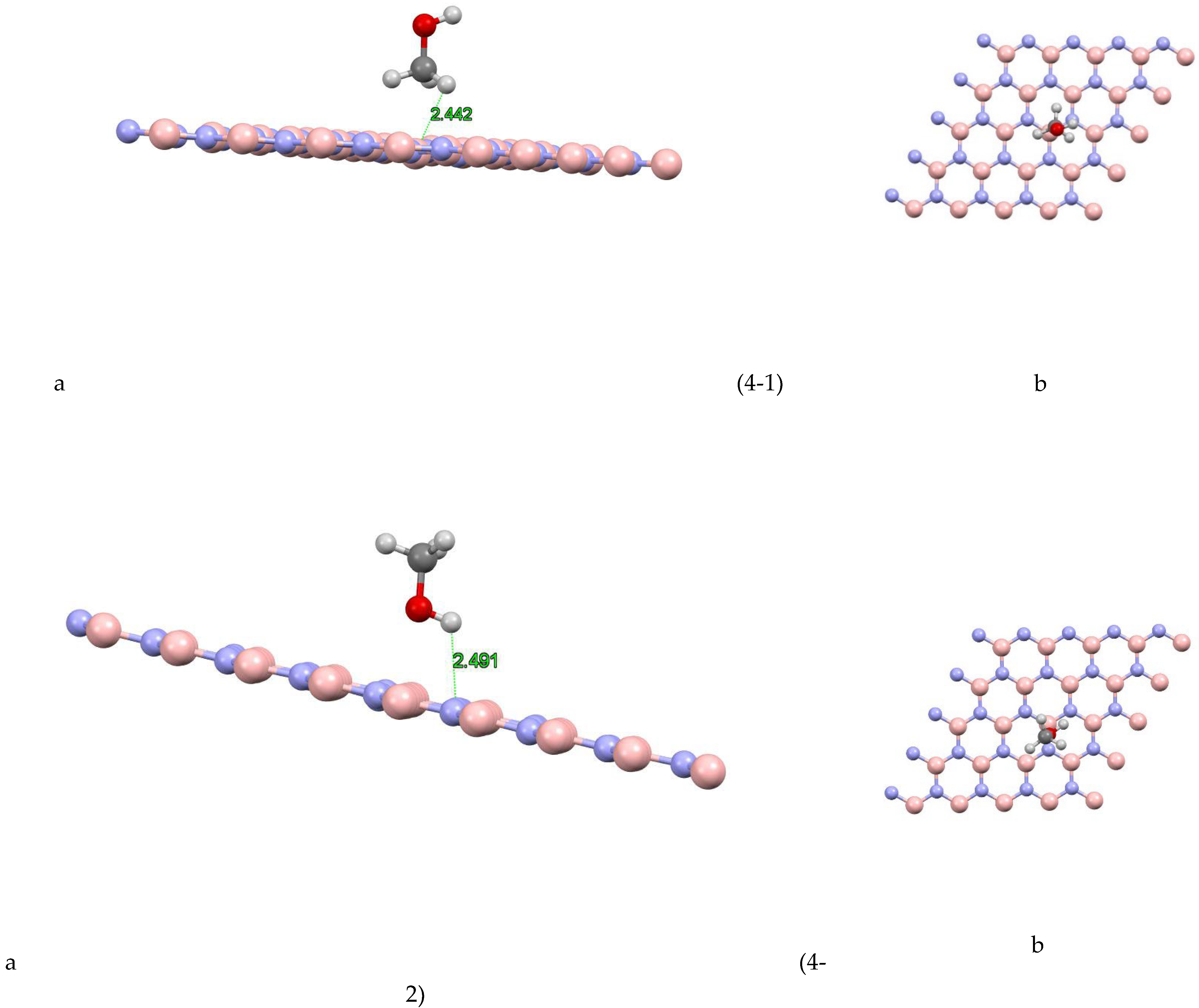
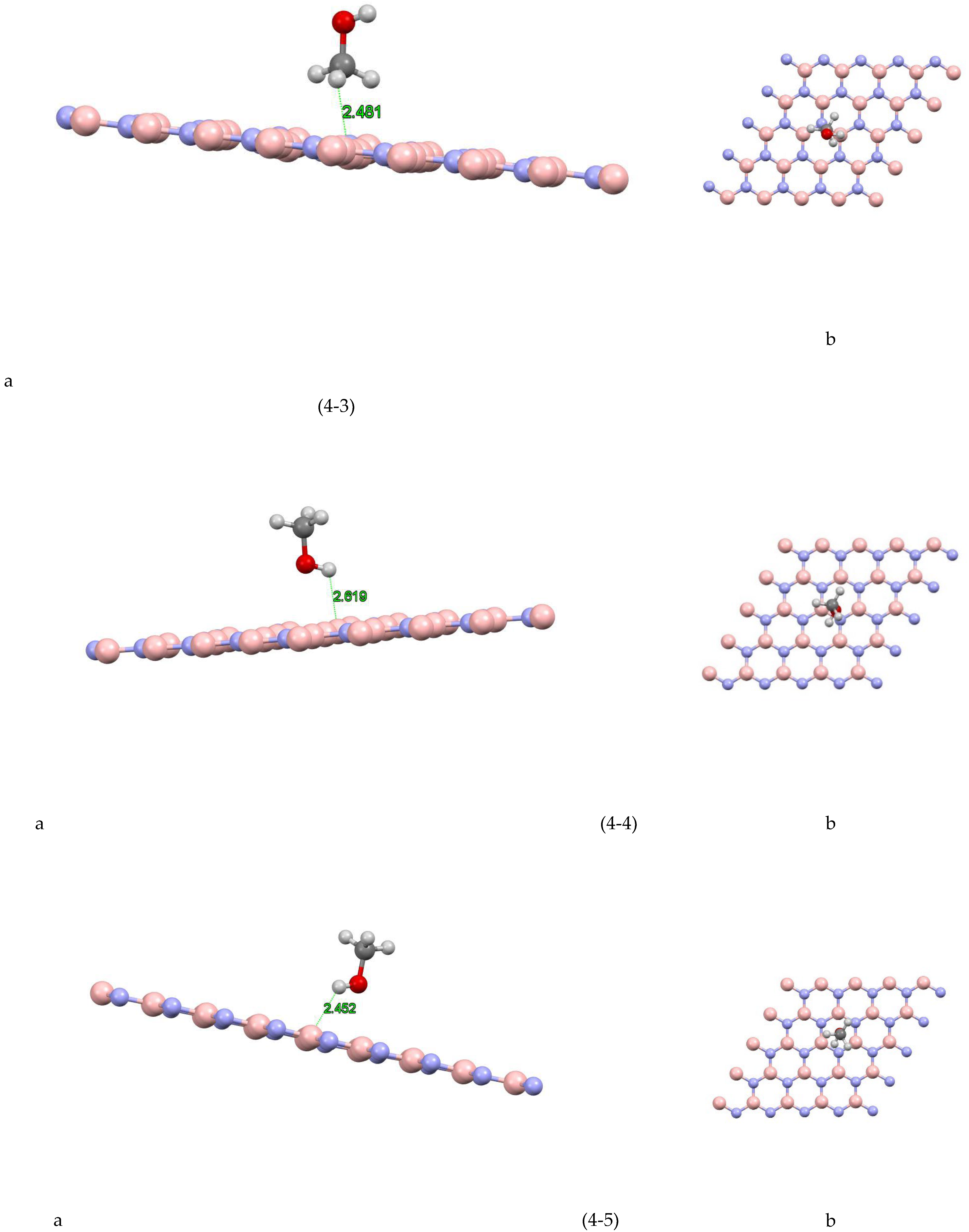
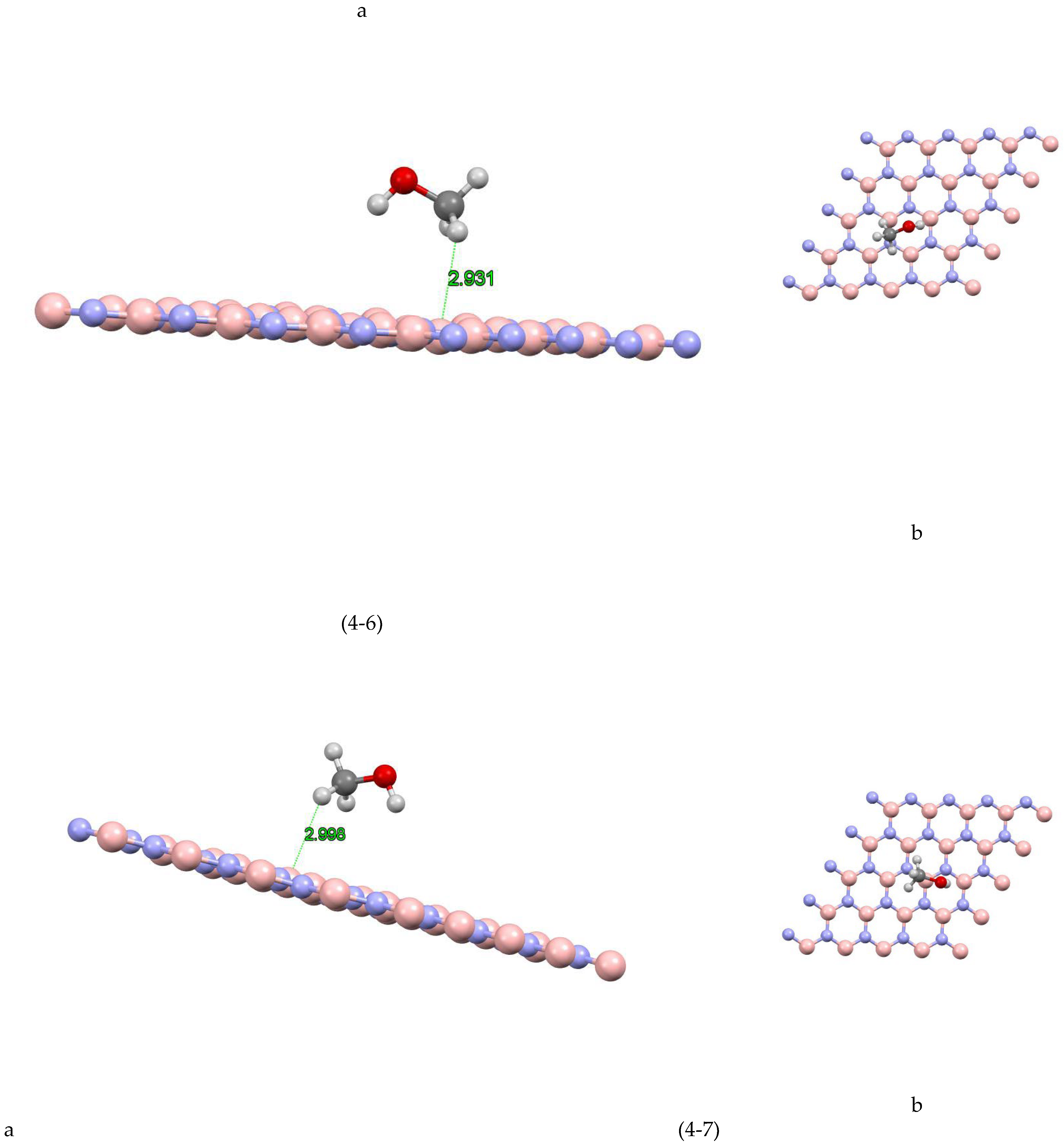
In Figure (4-1) the coordinates of the most stable state are shown for the boron nitride-methanol site 1 system. The closest atom to the boron nitride plane is the hydrogen atom with a distance of 2.442 Å and the absorption energy of this system is equal to 2742/0+(eV). Figure (4-2) the coordinates of the most stable state are shown for the boron nitride-methanol site 2 system. The closest atom to the boron nitride plane is a hydrogen atom with a distance of 2.491 Å and the absorption energy of this system is equal to -0.1198 (eV). Figure (4-3) shows the coordinates of the most stable state for the boron nitride-methanol site 3 system. The closest atom to the boron nitride plane is the hydrogen atom with a distance of 2.481 Å and the absorption energy of this system is equal to +0.0242 (eV). Figure (4-4) shows the coordinates of the most stable state for the boron nitride-methanol site 4 system. The closest atom to the boron nitride plane is the hydrogen atom with a distance of 2.619 Å. The absorption energy of this system is -0.1351 (eV). Also, the value of Mulliken charge is equal to 0.022, which has been transferred from methanol to boron nitride. Figure (4-5) shows the coordinates of the most stable state for the boron nitride-methanol site 5 system. The closest atom to the boron nitride plane is the hydrogen atom with a distance of 2.492 Å and the absorption energy of this system is equal to -0.1093 (eV). Figure (4-6) shows the coordinates of the most stable state for boron nitride-methanol site 6 system. The closest atom to the boron nitride plane is the hydrogen atom with a distance of 2.931 Å and the absorption energy of this system is equal to -0.0922 (eV). Figure (4-7) the coordinates of the most stable state are shown for the boron nitride-methanol site 7 system. The closest atom to the boron nitride plane is a hydrogen atom with a distance of 2.998 Å and the absorption energy of this system is equal to -0.1219 (eV).
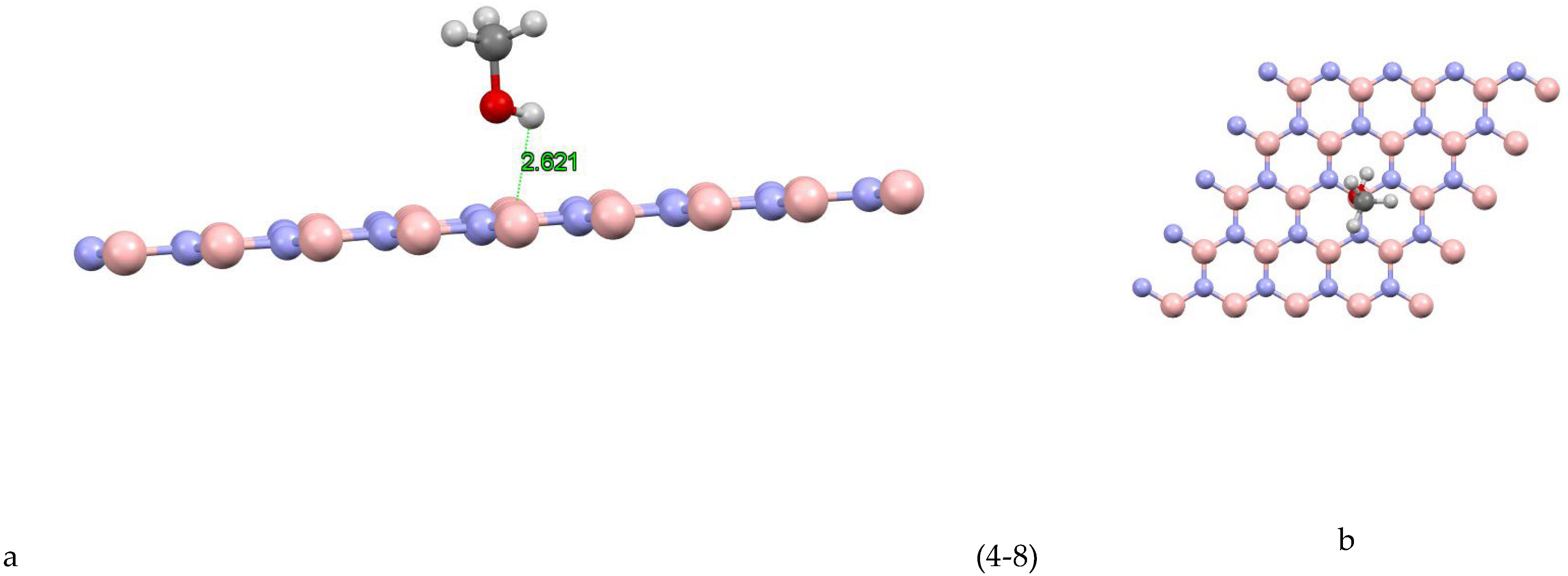
Figure 4(-8) shows the coordinates of the most stable state for the boron nitride-methanol site 8 system. The closest atom to the boron nitride plane is the hydrogen atom with a distance of 2.621 Å and the absorption energy of this system is equal to -0.1096 (eV).
Table 1 includes the closest atom to the boron nitride plane, the distance of this atom from the boron nitride plane, the absorption energy of the system, and the Mulliken charge (where provided). This should make it easier to compare the properties of the system at different sites, (Mulliken charge is only provided for site 4).
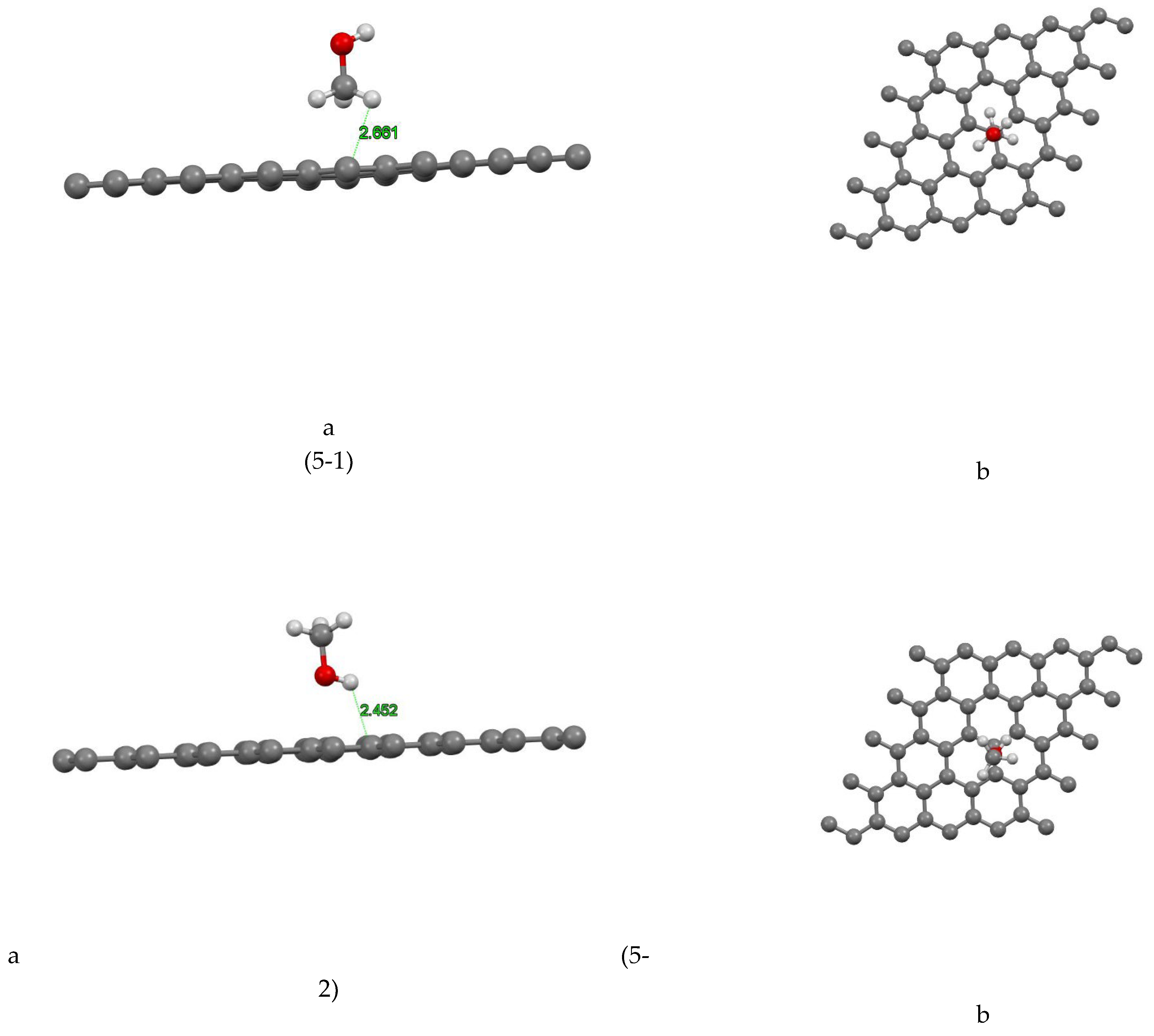
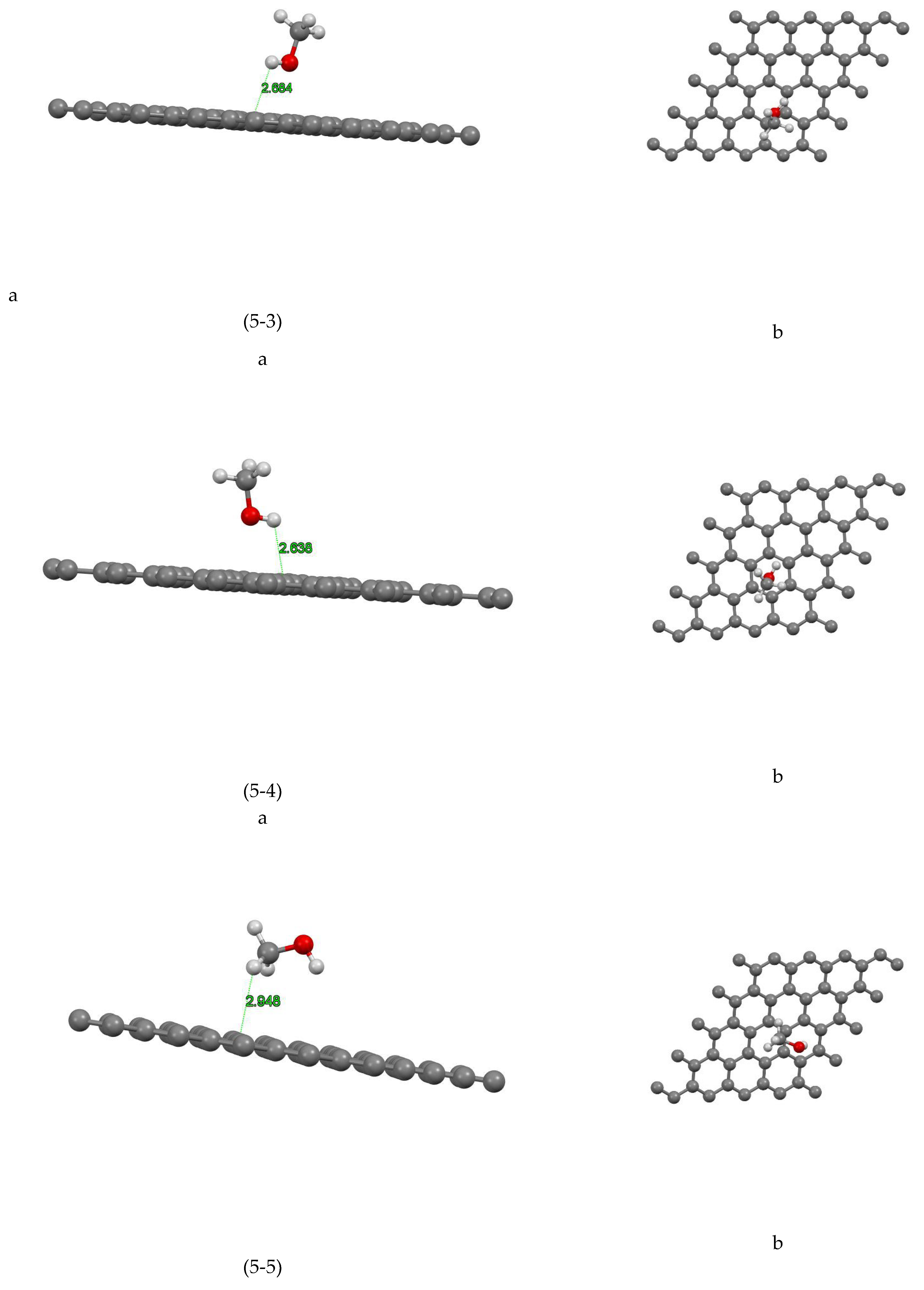
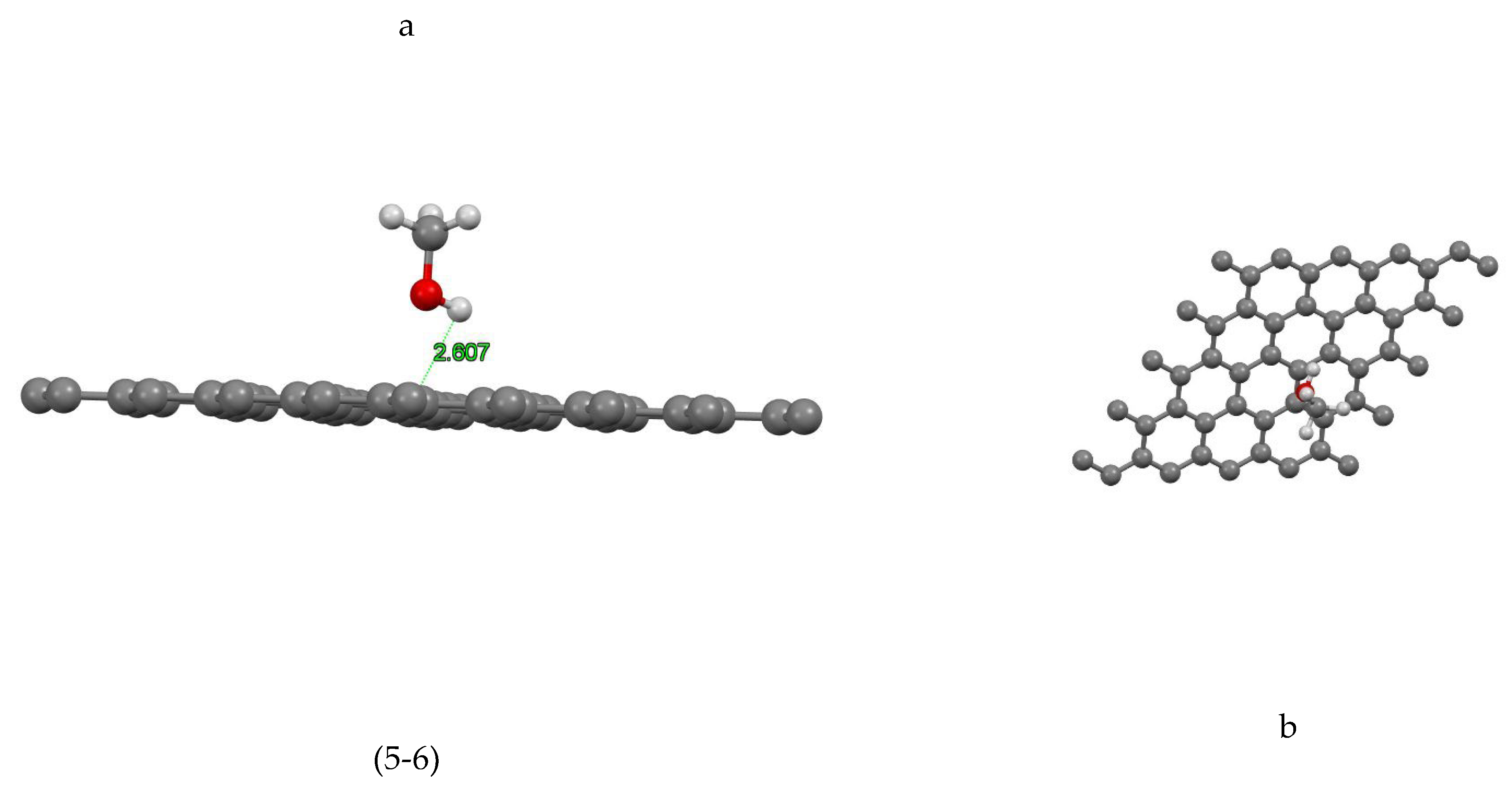
Figure (5-1) shows the coordinates of the most stable state for the graphene-methanol site 1 system. The closest atom to the graphene plane is the hydrogen atom with a distance of 2.661Å and the absorption energy of this system is equal to +0.122 (eV). In Figure( 5-2) the coordinates of the most stable state are shown for the graphene-methanol system site 2. The closest atom to the graphene plane is the hydrogen atom with a distance of 2.452Å and the absorption energy of this system is equal to -0.004 (eV). In Figure (5-3) the coordinates of the most stable state are shown for the graphene-methanol system site 3. The closest atom to the graphene plane is the hydrogen atom with a distance of 2.684Å and the absorption energy of this system is equal to -0.039 (eV). Figure (5-4) shows the coordinates of the most stable state for the graphene-methanol site 4 system. The closest atom to the graphene plane is the hydrogen atom with a distance of 2.638Å. The absorption energy of this system is equal to -0.033(eV) and also the amount of mulliken charge is equal to +0.004, which has been transferred from methanol to graphene. In Figure (5-5) the coordinates of the most stable state are shown for the graphene-methanol system site 5. The closest atom to the graphene plane is the hydrogen atom with a distance of 2.948Å and the absorption energy of this system is equal to -0.073 (eV), In Figure (5-6) the coordinates of the most stable state are shown for the graphene-methanol system site 6. The closest atom to the graphene plane is the hydrogen atom with a distance of 2.607Å and the absorption energy of this system is equal to +0.01 (eV).
Table 2 includes the closest atom to the graphene plane, the distance of this atom from the graphene plane, the absorption energy of the system, and the Mulliken charge (where provided). This should make it easier to compare the properties of the system at different sites, (Mulliken charge is only provided for site 5).
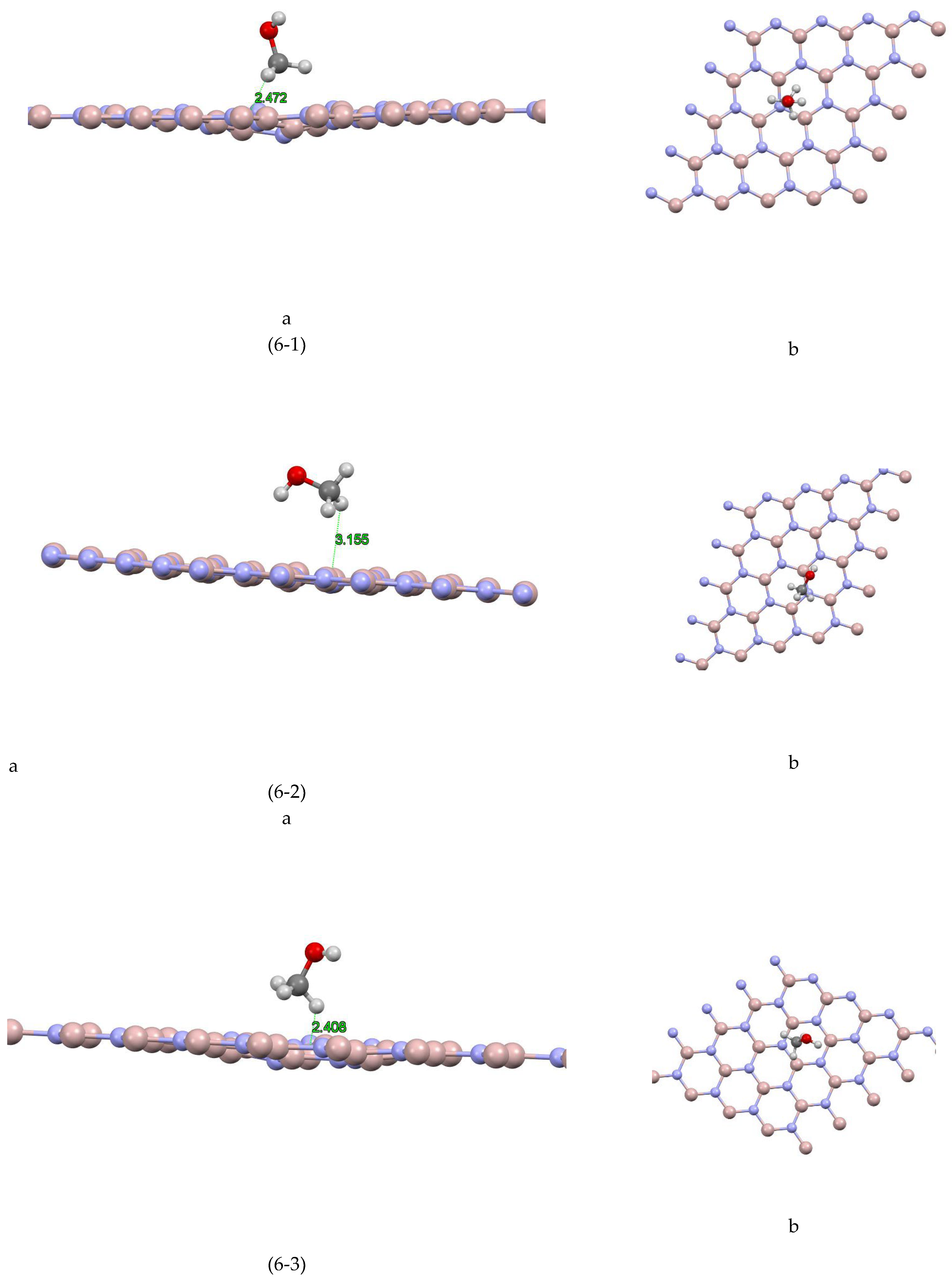
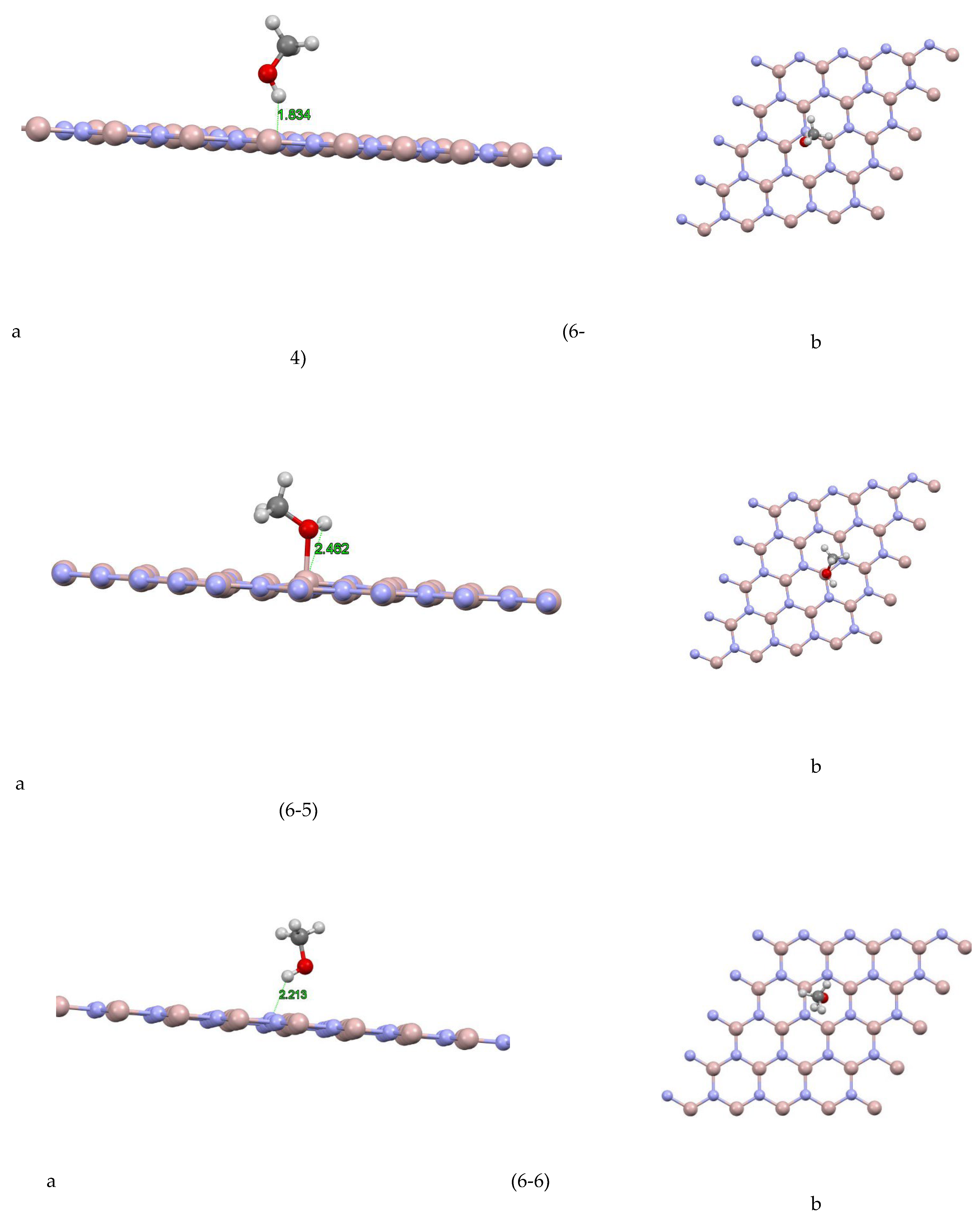
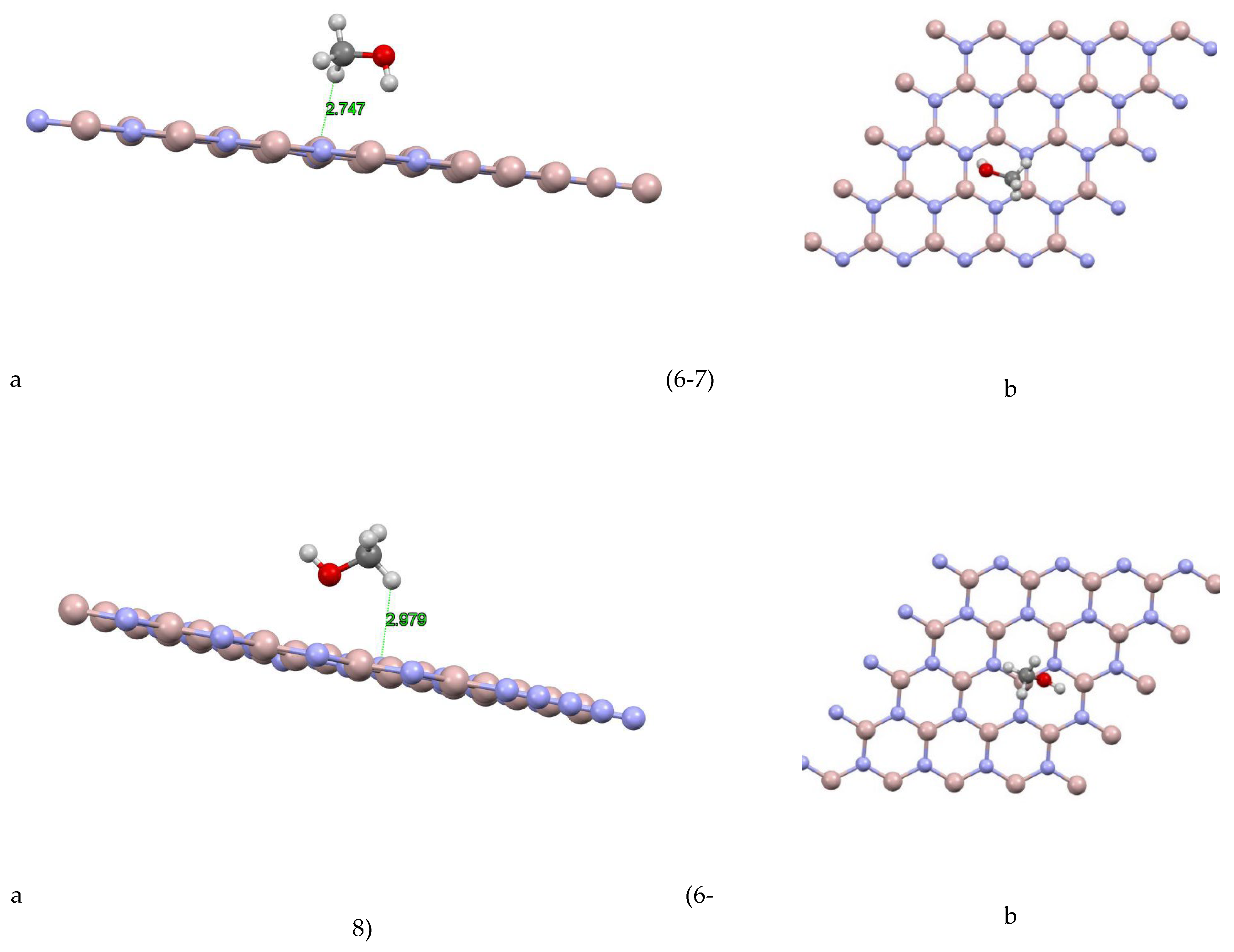
The aluminum nitride-methanol system presents a fascinating case study. This system comprises several sites, each exhibiting unique characteristics. For instance, as depicted in Figure (6-1) For site 1, the closest atom to the aluminum nitride plane is a hydrogen atom at a distance of 2.472 Å, and the absorption energy of this system is +0.4532 eV. For site 2 Figure (6-2), the closest atom is also a hydrogen atom, but at a slightly greater distance of 3.155 Å, and the absorption energy is +0.0272 eV. Site 3 Figure (6-3) shows a similar pattern, with the closest atom being a hydrogen atom at a distance of 2.408 Å, and an absorption energy of +0.3175 eV. In site 4 Figure (6-4), the closest atom to the aluminum nitride plane is a hydrogen atom at a distance of 1.834 Å, and the absorption energy is -0.226 eV. For site 5 Figure (6-5), the closest atom is a hydrogen atom at a distance of 2.462 Å, and the absorption energy is -0.5532 eV. Additionally, a Mulliken charge of +0.075 has been transferred from methanol to aluminum nitride. Site 6 Figure (6-6) shows the closest atom to the aluminum nitride plane as a hydrogen atom at a distance of 2.213 Å, and the absorption energy of this system is -0.2275 eV. For site 7 Figure (6-7), the closest atom is a hydrogen atom at a distance of 2.747 Å, and the absorption energy is -0.0877 eV. Finally, for site 8 Figure (6-8), the closest atom to the aluminum nitride plane is a hydrogen atom at a distance of 2.979 Å, and the absorption energy of this system is -0.1393 eV.
Table 3 includes the closest atom to the aluminum nitride plane, the distance of this atom from the aluminum nitride plane, the absorption energy of the system, and the Mulliken charge (where provided). This should make it easier to compare the properties of the system at different sites. the Mulliken charge is only provided for site 5.
These findings provide a comprehensive understanding of the most stable states for the aluminum nitride-methanol system across different sites, highlighting the variations in absorption energy and the closest atom to the aluminum nitride plane.
In conclusion, these Figures provide a detailed insight into the interactions between boron nitride and methanol across different sites. Each site presents a unique interaction, with varying distances and absorption energies, contributing to our understanding of these complex molecular systems.
Table 4 shows the results of energy calculations for methanol on boron nitride absorber in eight sites as follows. As can be seen from the above table, the best active site to continue calculations is site 4. According to the amount of absorption energy, the absorption of methanol on boron nitride is a physical absorption type with an energy of -0.1351(eV), which is a good value. It presents a detailed analysis of the adsorption energy, system energy, methanol energy, and boron nitride energy at different sites on the surface of boron nitride. The adsorption energy, measured in electron volts (eV), indicates the energy change when a methanol molecule is adsorbed onto the surface. Positive values represent endothermic reactions where energy is absorbed, while negative values represent exothermic reactions where energy is released. The system energy represents the total energy of the boron nitride with the adsorbed methanol molecule. The methanol energy and boron nitride energy columns represent the energy of the isolated methanol molecule and the bare boron nitride surface, respectively. The data suggests that the adsorption process is exothermic at sites 2, 4, 5, 6, 7, and 8, as indicated by the negative adsorption energy values. Conversely, the process is endothermic at sites 1 and 3, where the adsorption energy values are positive.
Table 5: shows the result of energy calculations for methanol on graphene absorber in six sites
Table 5 presents a detailed analysis of the adsorption energy, system energy, methanol energy, and graphene energy at different sites on the surface of graphene. The adsorption energy, measured in electron volts (eV), indicates the energy change when a methanol molecule is adsorbed onto the surface. Positive values represent endothermic reactions where energy is absorbed, while negative values represent exothermic reactions where energy is released. The system energy represents the total energy of the graphene with the adsorbed methanol molecule. The methanol energy and graphene energy columns represent the energy of the isolated methanol molecule and the bare graphene surface, respectively. The data suggests that the adsorption process is exothermic at sites 2, 3, 4, and 5, as indicated by the negative adsorption energy values. Conversely, the process is endothermic at sites 1 and 6, where the adsorption energy values are positive. This information is crucial for understanding the interaction between methanol and graphene, which could have significant implications for its use in various applications. Further experimental studies would be beneficial to validate these computational results and explore their implications in real-world applications. the best active site to continue calculations is the site 5. According to the amount of absorption energy, the absorption of methanol on graphene is a weak physical absorption type with an absorption energy of -0.073(eV).
Table 6: shows the result of energy calculations for methanol on aluminum nitride absorber in six sites
Table 6 presents a detailed analysis of the adsorption energy, system energy, methanol energy, and aluminum nitride energy at different sites on the surface of aluminum nitride. The adsorption energy, measured in electron volts (eV), indicates the energy change when a methanol molecule is adsorbed onto the surface. Positive values represent endothermic reactions where energy is absorbed, while negative values represent exothermic reactions where energy is released. The system energy represents the total energy of the aluminum nitride with the adsorbed methanol molecule. The methanol energy and aluminum nitride energy columns represent the energy of the isolated methanol molecule and the bare aluminum nitride surface, respectively. The data suggests that the adsorption process is exothermic at sites 4, 5, 6, 7, and 8, as indicated by the negative adsorption energy values. Conversely, the process is endothermic at sites 1, 2, and 3, where the adsorption energy values are positive. the best active site to continue calculations is site 5. According to the amount of absorption energy, the absorption energy is -0.5532 eV.
This information is crucial for understanding the interaction between methanol and AlN, graphene, and BN which could have significant implications for its use in various applications.
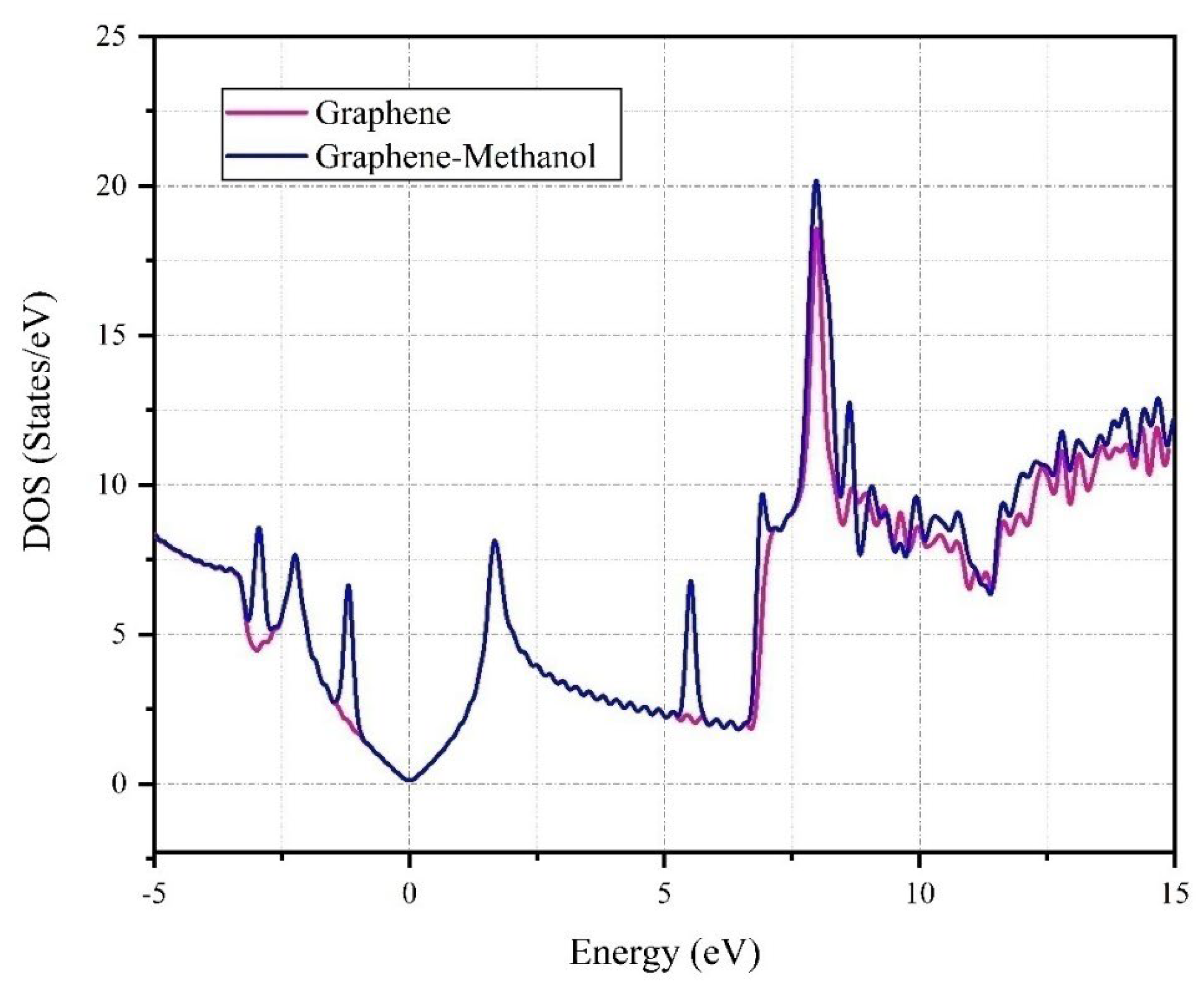
Our study into the interaction between graphene and methanol, as depicted in
Figure 7, unveiled significant modifications in the density of states (DOS). The DOS for pristine graphene, denoted by the red line, showcases distinct peaks at specific energy levels, underscoring its unique electronic properties. However, the incorporation of methanol, represented by the blue line, results in a noticeable shift and modulation in these peaks, suggesting a redistribution of electronic states. This could potentially modify the electronic and chemical properties of graphene. Notably, the Graphene-Methanol system exhibits more pronounced peaks around 10 eV, indicating a higher density of states at this energy level compared to pristine graphene.
Figure 7 provides a comparative analysis of the DOS for Graphene and Graphene-Methanol. The x-axis represents energy levels in electron volts (eV), while the y-axis denotes DOS in states/eV. The graph features two distinct plots: the red line for pure Graphene and the blue line for Graphene-Methanol. The latter exhibits more pronounced peaks and troughs, suggesting a significant alteration in the electronic structure upon the introduction of methanol.
Interestingly, there is no Fermi peak near zero, and the band gap of graphene and graphene-methanol remains unchanged at 3.9 eV. No shift is observed, and there is no electron transfer in this region. Based on the density of the state diagram, the absorption energy, and the Mulliken charge, it can be inferred that the absorption process is of a weak physical type. These findings provide valuable insights into the electronic structure of graphene and its interaction with methanol, paving the way for further research in this area.
The graph in the provided image illustrates the Density of States (DOS) for Graphene and Graphene-Methanol. The DOS for Graphene, represented by the red line, exhibits distinct peaks at specific energy levels, indicative of its unique electronic properties. However, upon interaction with methanol, represented by the purple line, there is a noticeable shift and modulation in these peaks. This suggests that the incorporation of methanol leads to a redistribution of electronic states, potentially offering modified electronic and chemical properties.
The pronounced peaks in the DOS for Graphene-Methanol, particularly around 2 eV and between 10 to 15 eV, suggest a significant redistribution of electronic states. This could be indicative of changes in the electronic structure of Graphene upon the introduction of methanol. The changes in the electronic structure could potentially alter the chemical reactivity and electronic conductivity of Graphene, thereby influencing its performance in various applications.
For instance, in the field of catalysis, the altered electronic structure could potentially enhance the catalytic activity of Graphene. The pronounced peaks in the DOS might correspond to the availability of more electronic states at specific energy levels, which could facilitate electron transfer during catalytic reactions. Similarly, in sensor development, the changes in the DOS could influence the sensitivity and selectivity of Graphene-based sensors.
Moreover, the absence of a Fermi peak near zero and the unchanged band gap of 3.9 eV suggest that the interaction between Graphene and methanol is likely of a weak physical nature, rather than a strong chemical interaction. This could have implications for the stability and recyclability of Graphene in practical applications.
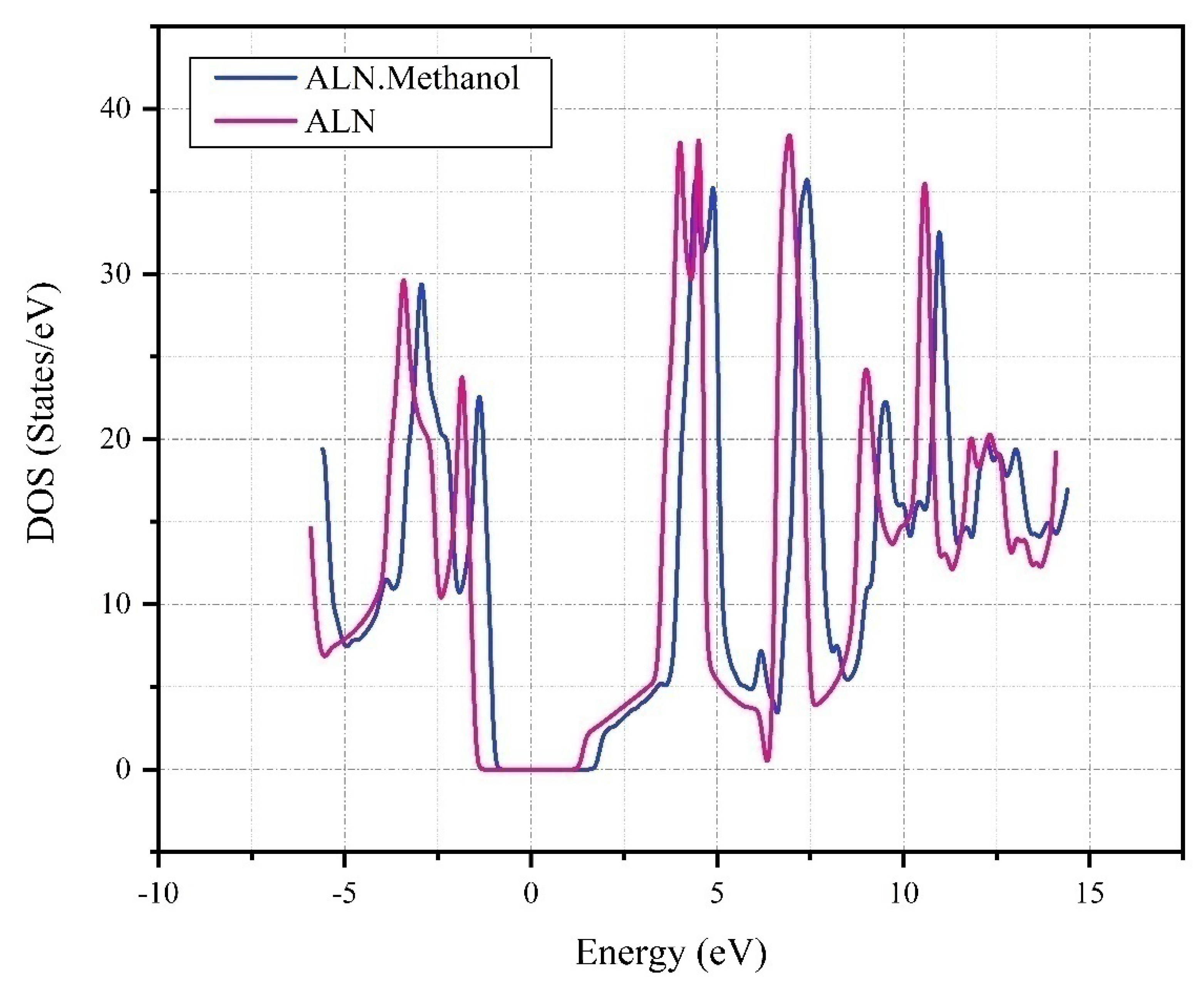
Figure 8 provides a comprehensive illustration of the Density of States (DOS) for Aluminum Nitride (AlN) and its interaction with Methanol (AlN-Methanol). The graph plots energy levels in electron volts (eV) against DOS in states/eV. The DOS for pure AlN, depicted by the red line, presents a distinct pattern of peaks and troughs at specific energy levels, a testament to its unique electronic properties. However, the introduction of methanol, represented by the blue line, noticeably alters this pattern. The peaks and troughs become more pronounced, especially around -5, 0, and 5 eV, suggesting a significant redistribution of electronic states. This implies that methanol's incorporation could potentially modify the electronic and chemical properties of AlN.
The pronounced peaks and troughs in the DOS for AlN-Methanol, particularly around -5, 0, and 5 eV, indicate changes in the electronic structure of AlN upon methanol's introduction. These changes could potentially influence the chemical reactivity and electronic conductivity of AlN, thereby affecting its performance in various applications. For instance, in catalysis, the altered electronic structure could enhance AlN's catalytic activity. The pronounced peaks in the DOS might correspond to the availability of more electronic states at specific energy levels, facilitating electron transfer during catalytic reactions. Similarly, in sensor development, the changes in the DOS could affect the sensitivity and selectivity of AlN-based sensors.
Interestingly, the absence of a Fermi peak near zero and the unchanged band gap of 3.9 eV suggest that the interaction between AlN and methanol is likely of a weak physical nature, rather than a strong chemical interaction. This could have implications for the stability and recyclability of AlN in practical applications.
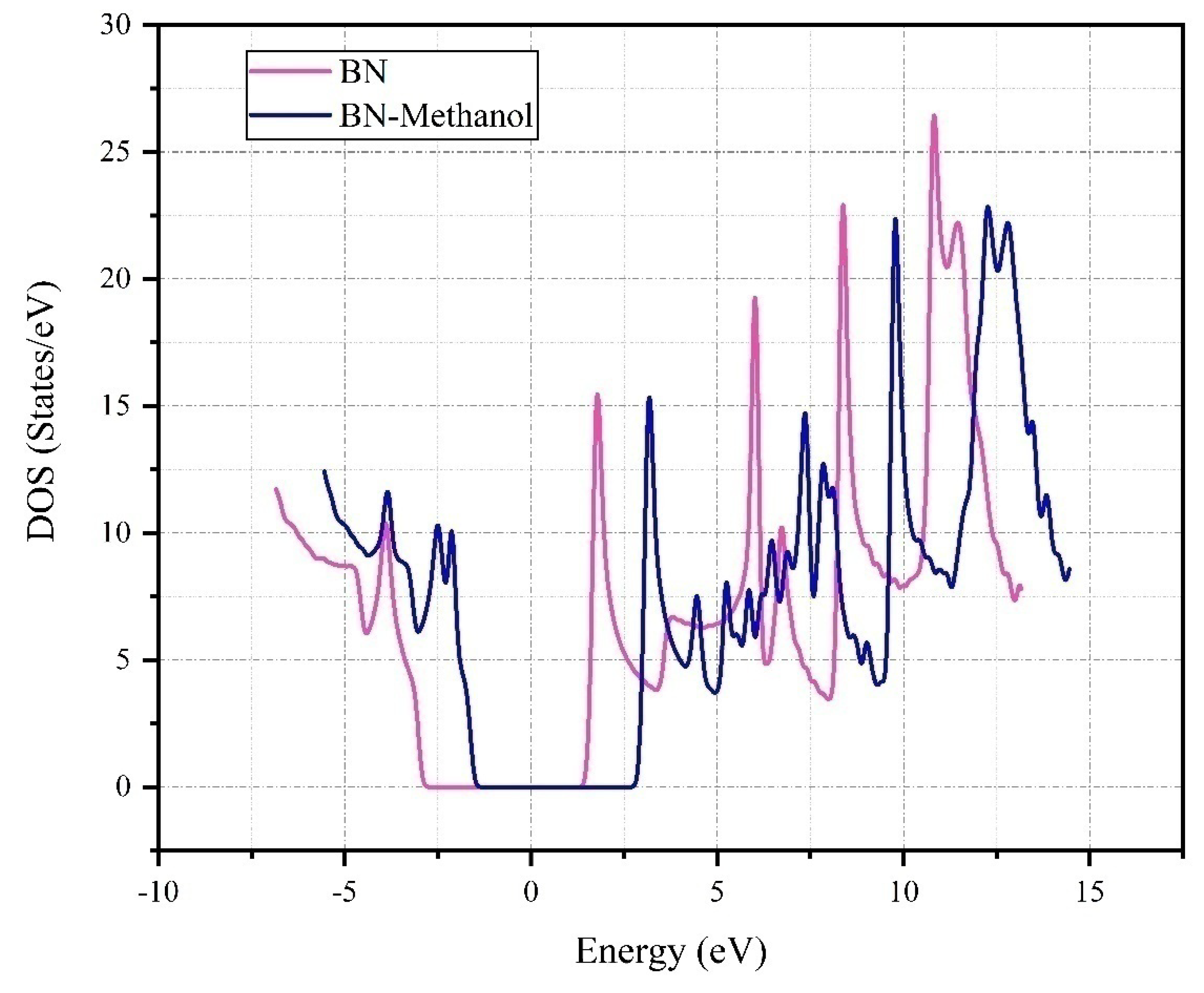
Figure 9 provides a comprehensive illustration of the Density of States (DOS) for Boron Nitride (BN) and its interaction with Methanol (BN-Methanol). The graph plots energy levels in electron volts (eV) against DOS in states/eV. The DOS for pure BN, depicted by the blue line, presents a distinct pattern of peaks and troughs at specific energy levels, a testament to its unique electronic properties. However, the introduction of methanol, represented by the pink line, noticeably alters this pattern. The peaks and troughs become more pronounced, especially around -5, 0, and 5 eV, suggesting a significant redistribution of electronic states. This implies that methanol’s incorporation could potentially modify the electronic and chemical properties of BN.
It is observed that there are no significant changes in the surface states around the Fermi level of zero. The band gap is seen to reduce from 5.7 eV to 5.3 eV, indicating a shift of 1.4 eV to the right. This shift could be attributed to the electron transfer in the system. Considering the absorption energy and the equilibrium distance of the methanol molecule from boron nitride, along with the local density diagram, it can be inferred that the adsorption process is a physical process. This means that the methanol molecule is physically adsorbed onto the BN surface, without any significant alteration or disruption to the electronic structure of the BN. This observation is crucial as it provides insights into the interaction between methanol and BN, which could have significant implications for its use in various applications.
The pronounced peaks in the DOS for BN-Methanol, particularly around -5, 0, and 5 eV, suggest a significant redistribution of electronic states. This could be indicative of changes in the electronic structure of BN upon the introduction of methanol. The changes in the electronic structure could potentially alter the chemical reactivity and electronic conductivity of BN, thereby influencing its performance in various applications. For instance, in the field of catalysis, the altered electronic structure could potentially enhance the catalytic activity of BN. The pronounced peaks in the DOS might correspond to the availability of more electronic states at specific energy levels, which could facilitate electron transfer during catalytic reactions. Similarly, in sensor development, the changes in the DOS could influence the sensitivity and selectivity of BN-based sensors.
Moreover, the absence of a Fermi peak near zero and the unchanged band gap of 3.9 eV suggest that the interaction between BN and methanol is likely of a weak physical nature, rather than a strong chemical interaction. This could have implications for the stability and recyclability of BN in practical applications.