Submitted:
08 June 2024
Posted:
10 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cell Culture, THC Exposure, and Sample Collection
2.2. Cell Viability Analysis
2.3. RNA/DNA Extraction and cDNA Synthesis
2.4. Library Prep and RNA Sequencing
2.5. Pathway Analysis
2.6. Gene Validation by Real-time Quantitative PCR (qPCR)
2.7. Role of Ferroptosis in Cell Viability Test
2.8. Statistical analysis
3. Results
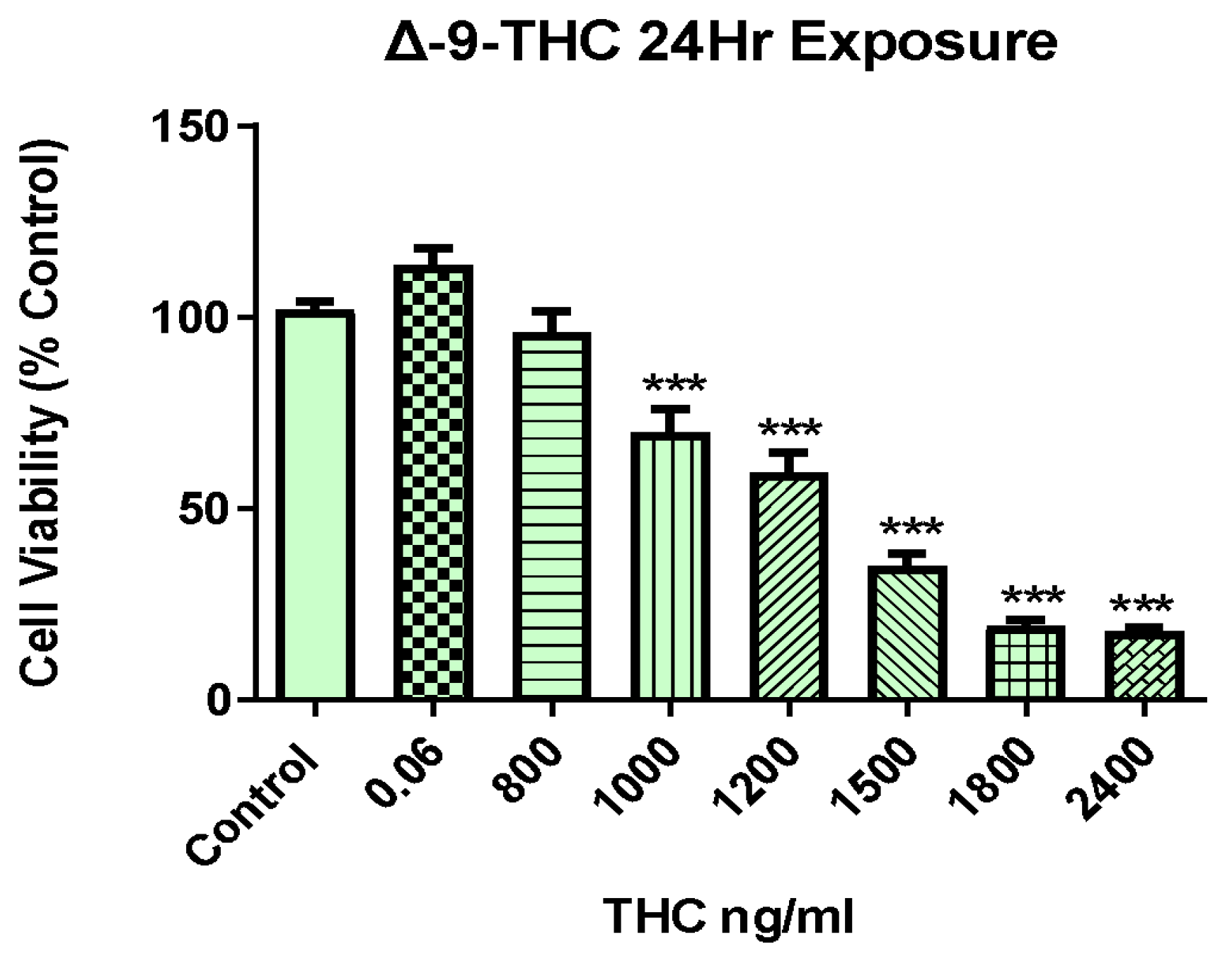
3.1. Δ-9-THC Dose-Dependent Viability of BEAS-2B Cells
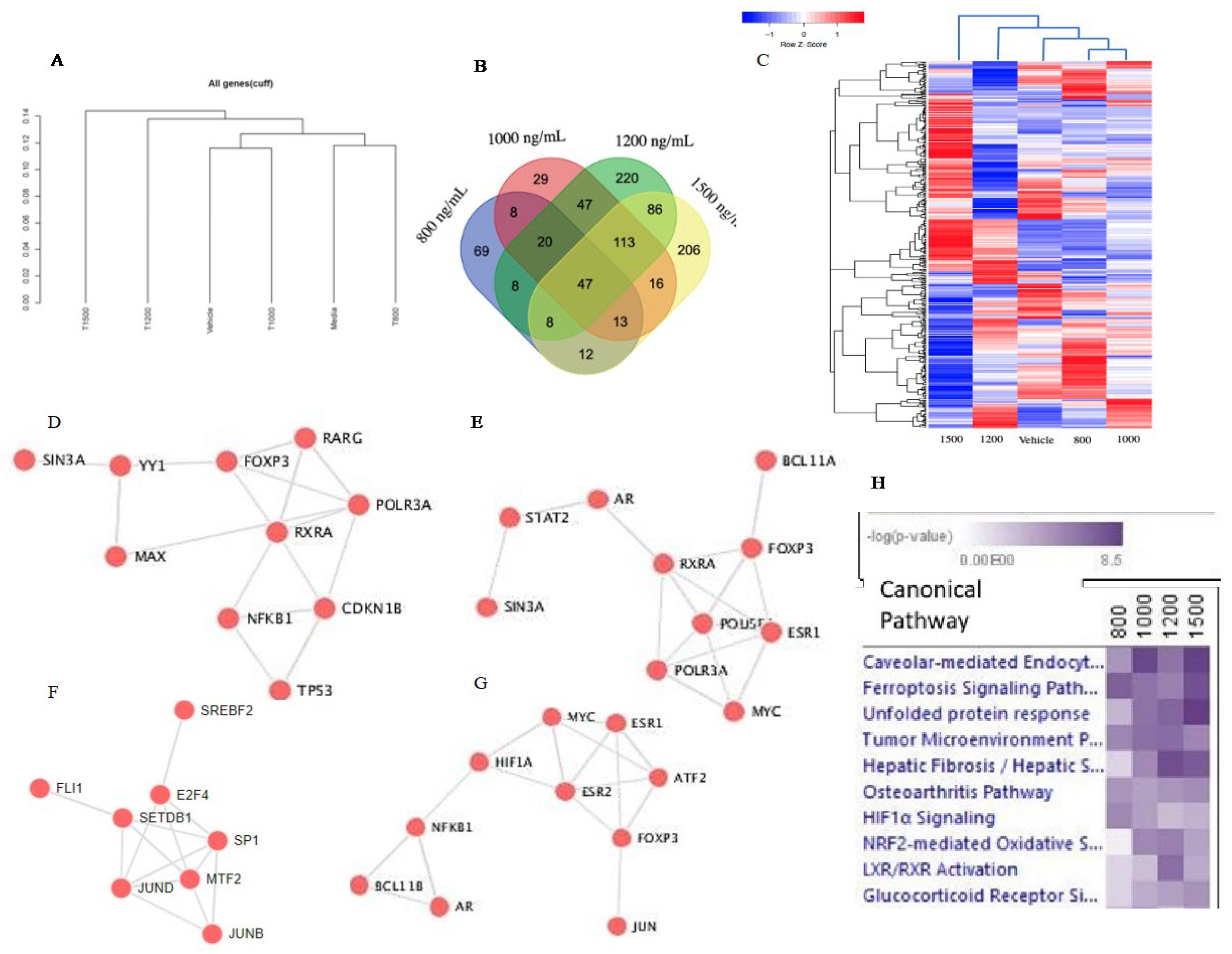
3.2. Δ-9-THC Dose-Mediated Global Alterations in Gene Expression Profile, Biomarkers, Transcription Factors, and Canonical Pathways
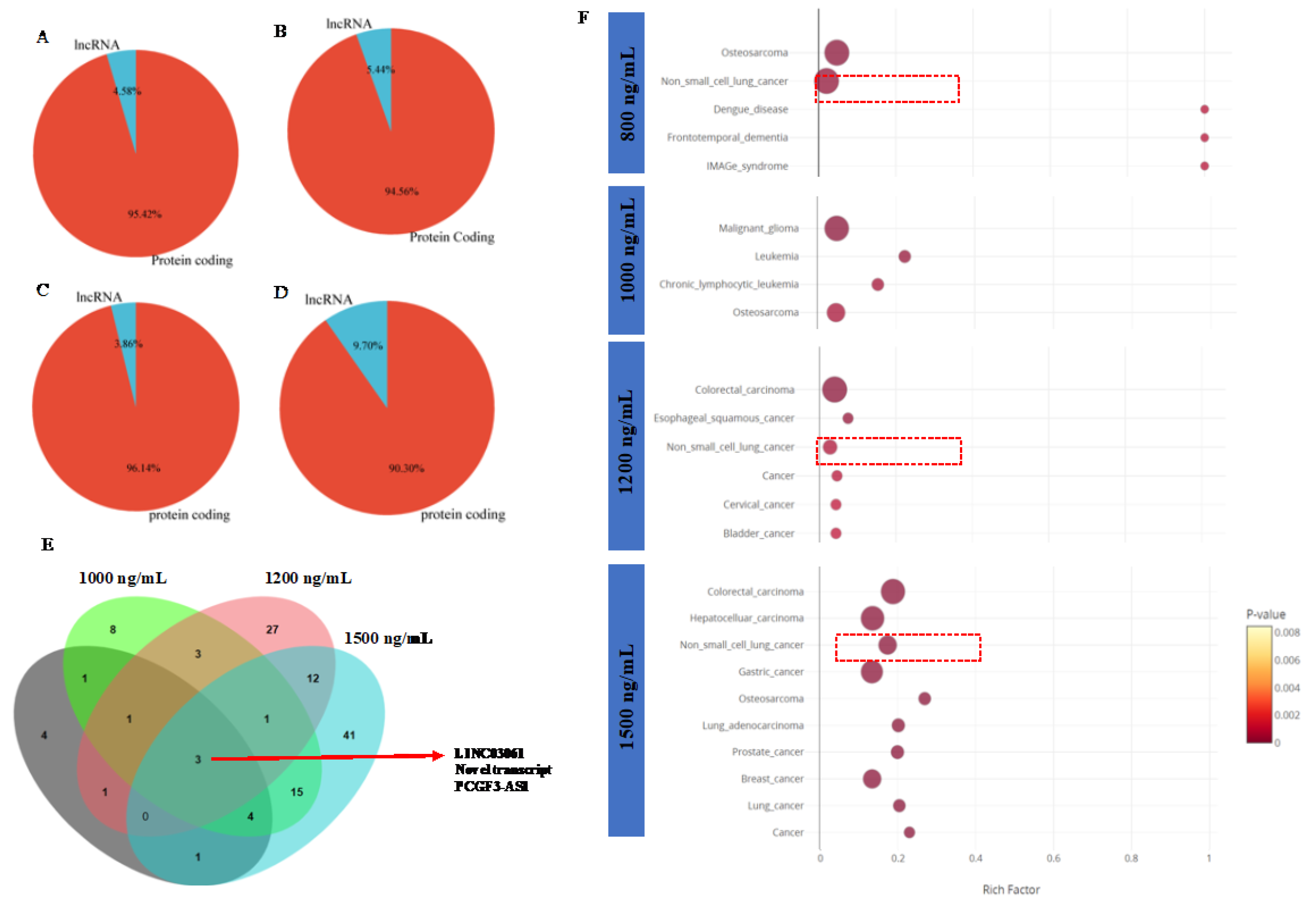
3.3. Δ-9-THC Mediated Enrichment of Long Non-Coding RNA (lncRNA) in BEAS-2B Cells Associated in Activation of Lung Cancerous Pathways
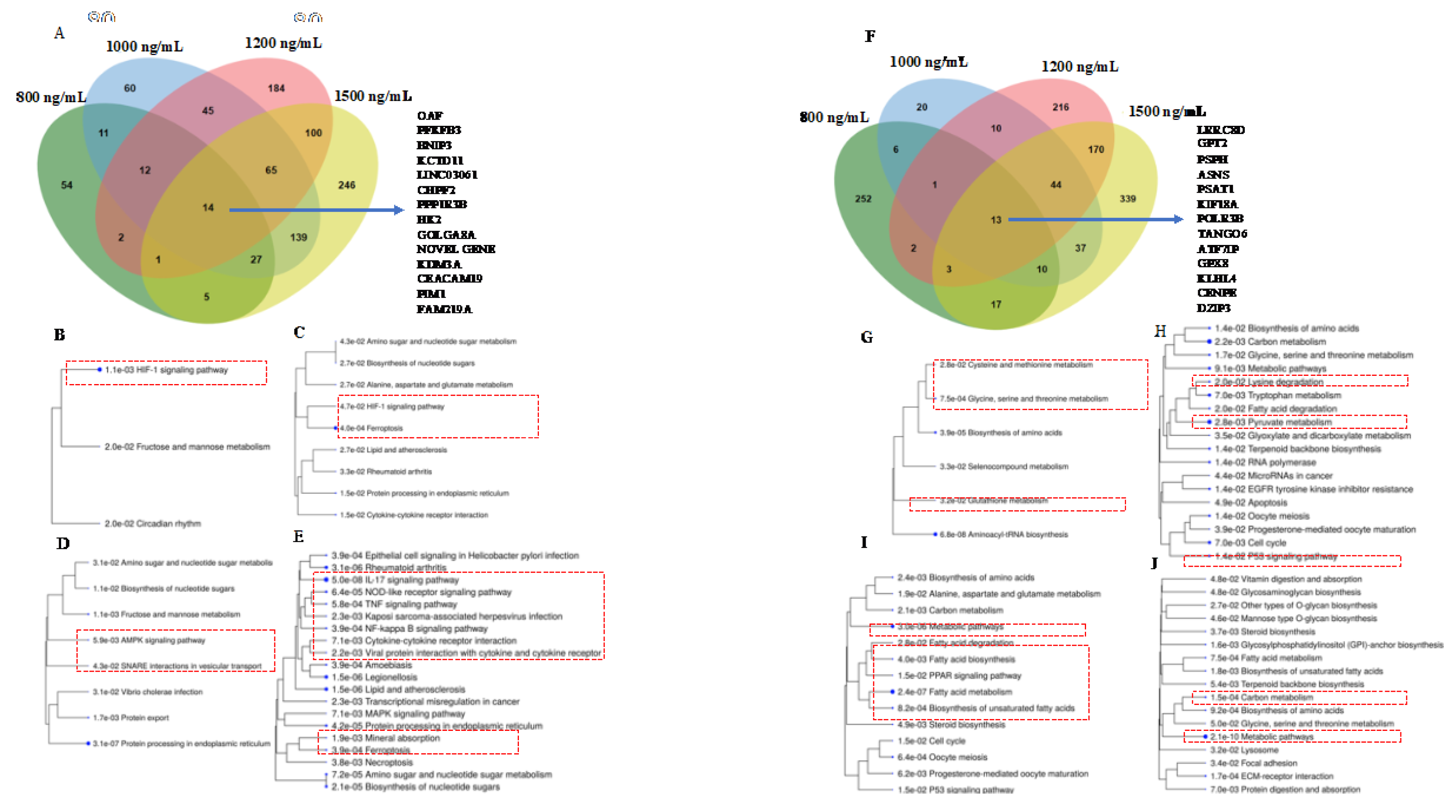
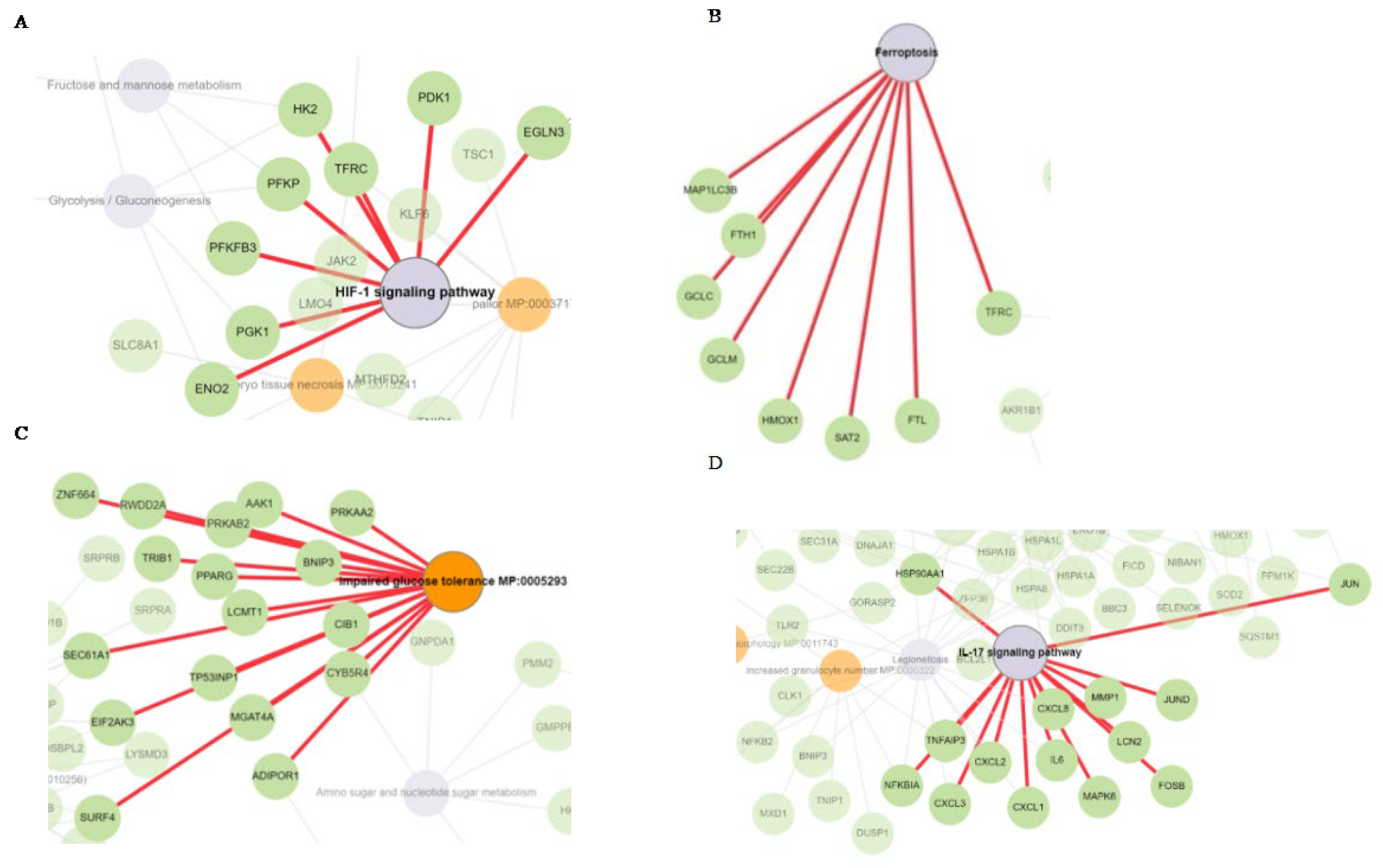
3.4. HIF-1 Signaling, Ferroptosis, AMPK Signaling, and Immunogenic Pathways were Triggered by Δ-9-THC Dose-Dependent Upregulated Genes in BEAS-2B Cells
3.5. Cysteine-Methionine Metabolism, Glutathione Metabolism, Amino Acid Metabolism, and Fatty Acid Metabolism were Significantly Enriched by THC-Mediated Downregulated Genes in Human Lung Cells
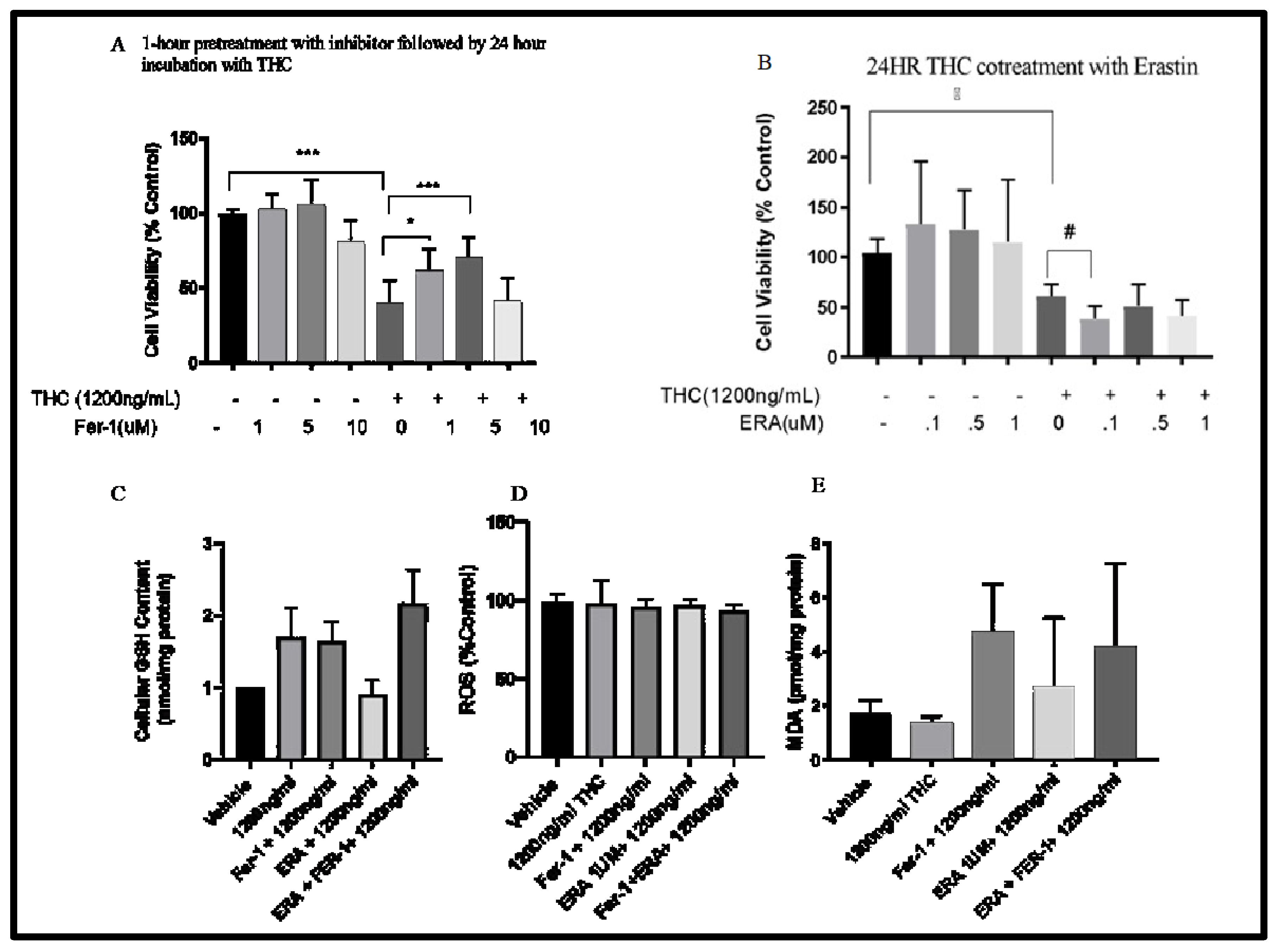
3.6. Cell Viability- Ferroptosis
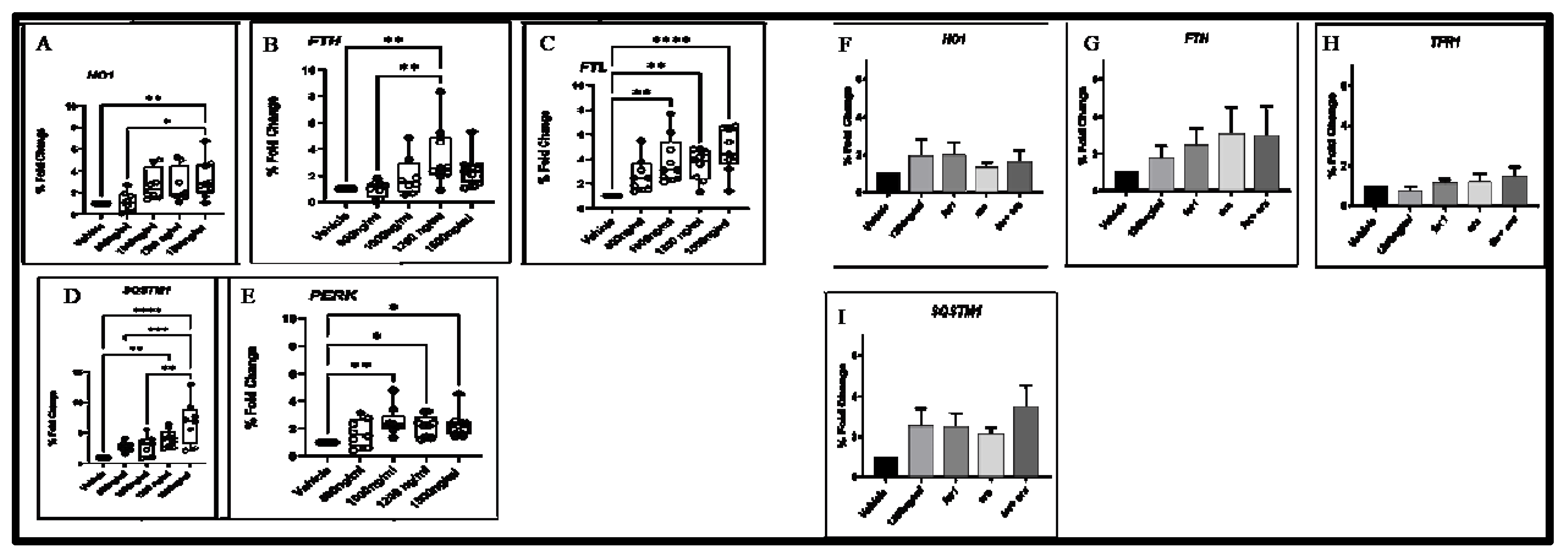
3.7. Aberrant Expression Of Ferroptosis, Autophagy, and ER Response Gene
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgements
References
- Bose, J.; Hedden, S.L.; Lipari, R.N.; Park-Lee, E. Key substance use and mental health indicators in the United States: Results from the 2017 National Survey on Drug Use and Health (HHS Publication No. SMA 2018: 17-5044, NSDUH Series H-52.
- Holland, J. The pot book: A complete guide to cannabis: Simon and Schuster; 2010.
- Lukhele, S.T.; Motadi, L.R. Cannabidiol rather than Cannabis sativa extracts inhibit cell growth and induce apoptosis in cervical cancer cells. BMC Complement Altern Med 2016, 16, 335. [Google Scholar] [CrossRef]
- Mucke, M.; Weier, M.; Carter, C.; Copeland, J.; Degenhardt, L.; Cuhls, H.; Radbruch, L.; Hauser, W.; Conrad, R. Systematic review and meta-analysis of cannabinoids in palliative medicine. J Cachexia Sarcopenia Muscle 2018, 9, 220–234. [Google Scholar] [CrossRef]
- Sarafian, T.A.; Magallanes, J.A.M.; Shau, H.; Tashkin, D.; Roth, M.D. Oxidative stress produced by marijuana smoke: An adverse effect enhanced by cannabinoids. Am J Respir Cell Mol Biol 1999, 20, 1286–1293. [Google Scholar] [CrossRef]
- Baron, E.P. Comprehensive review of medicinal marijuana, cannabinoids, and therapeutic implications in medicine and headache: What a long, strange trip it’s been. Headache: J Head and Face Pain. 2015, 55, 885–916. [Google Scholar] [CrossRef]
- Cooper, Z.D.; Haney, M. Actions of delta-9-tetrahydrocannabinol in cannabis: Relation to use, abuse, dependence. Int Rev Psychiatry 2009, 21, 104–112. [Google Scholar] [CrossRef]
- Goode, E. Marijuana: Routledge; NY, USA 2017.
- Lu, H.-C.; Mackie, K. An introduction to the endogenous cannabinoid system. Biol Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef]
- Bouaboula, M.; Rinaldi, M.; Carayon, P.; Carillon, C.; Delpech, B.; Shire, D.; Le Fur, G.; Casellas, P. Cannabinoid-receptor expression in human leukocytes. Eur J Biochem 1993, 214, 173–180. [Google Scholar] [CrossRef]
- Galiègue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carrière, D.; Carayon, P.; Bouaboula, M.; Shire, D.; LE Fur, G.; Casellas, P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J biochem 1995, 232, 54–61. [Google Scholar] [CrossRef]
- Turcotte, C.; Blanchet, M.-R.; Laviolette, M.; Flamand, N. Impact of cannabis, cannabinoids, and endocannabinoids in the lungs. Front Pharmacol. 2016, 7, 317. [Google Scholar] [CrossRef]
- Aldington, S.; Williams, M.; Nowitz, M.; Weatherall, M.; Pritchard, A.; McNaughton, A.; Robinson, G.; Beasley, R. The effects of cannabis on pulmonary structure, function and symptoms. Thorax. 2007. 62, 1058–1063. [CrossRef]
- Hancox, R.J.; Shin, H.H.; Gray, A.R.; Poulton, R.; Sears, M.R. Effects of quitting cannabis on respiratory symptoms. Eur Respir J 2015, 46, 80–87. [Google Scholar] [CrossRef]
- Macleod, J.; Robertson, R.; Copeland, L.; McKenzie, J.; Elton, R.; Reid, P. Cannabis, tobacco smoking, and lung function: A cross-sectional observational study in a general practice population. Br J Gen Pract 2015, 65, e89–e95. [Google Scholar] [CrossRef]
- Mégarbane, B.; Chevillard, L. The large spectrum of pulmonary complications following illicit drug use: Features and mechanisms. Chem Biol Interact 2013, 206, 444–451. [Google Scholar] [CrossRef]
- Owen, K.P.; Sutter, M.E.; Albertson, T.E. Marijuana: Respiratory tract effects. Clin Rev Allergy Immunol 2014, 46, 65–81. [Google Scholar] [CrossRef]
- Tan, W.C.; Lo, C.; Jong, A.; Xing, L.; FitzGerald, M.J.; Vollmer, W.M.; Buist, S.A.; Sin, D.D. Marijuana and chronic obstructive lung disease: A population-based study. CMAJ 2009, 180, 814–820. [Google Scholar] [CrossRef]
- Bayazit, H.; Selek, S.; Karababa, I.F.; Cicek, E.; Aksoy, N. Evaluation of oxidant/antioxidant status and cytokine levels in patients with cannabis use disorder. Clin Psychopharmacol Neurosci 2017, 15, 237. [Google Scholar] [CrossRef]
- Wolff, V.; Schlagowski, A.-I.; Rouyer, O.; Charles, A.-L.; Singh, F.; Auger, C.; Schini-Kerth, V.; Marescaux, C.; Raul, J.-S.; Zoll, J. Tetrahydrocannabinol induces brain mitochondrial respiratory chain dysfunction and increases oxidative stress: A potential mechanism involved in cannabis-related stroke. BioMed Res Int 2015, 2015, 323706. [Google Scholar] [CrossRef]
- Zamberletti, E.; Gabaglio, M.; Prini, P.; Rubino, T.; Parolaro, D. Cortical neuroinflammation contributes to long-term cognitive dysfunctions following adolescent delta-9-tetrahydrocannabinol treatment in female rats. European Neuropsychopharmacology 2015, 25, 2404–2415. [Google Scholar] [CrossRef]
- Campbell, V.A. Tetrahydrocannabinol-induced apoptosis of cultured cortical neurones is associated with cytochrome c release and caspase-3 activation. Neuropharmacol 2001, 40, 702–709. [Google Scholar] [CrossRef]
- Park, E.-J.; Park, Y.-J.; Lee, S.J.; Lee, K.; Yoon, C. Whole cigarette smoke condensates induce ferroptosis in human bronchial epithelial cells. Toxicol Lett 2019, 303, 55–66. [Google Scholar] [CrossRef]
- Lemecha, M.; Chalise, J.P.; Takamuku, Y.; Zhang, G.; Yamakawa, T.; Larson, G.; Itakura, K. Lcn2 mediates adipocyte-muscle-tumor communication and hypothermia in pancreatic cancer cachexia. Molecular Metabolism. 2022, 66, 101612. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome research. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Wang, Y.; Lin, H.; Wu, B. TLSEA: A tool for lncRNA set enrichment analysis based on multi-source heterogeneous information fusion. Frontiers in Genetics. 2023, 14, 1181391. [Google Scholar] [CrossRef]
- Adan, A.; Kiraz, Y.; Baran, Y. Cell proliferation and cytotoxicity assays. Current pharmaceutical biotechnology. 2016, 17, 1213–1221. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Xie, F.; Liu, K.; Liu, H.; Xie, J. Evaluation of whole cigarette smoke induced oxidative stress in A549 and BEAS–2B cells. Environmental toxicology and pharmacology. 2017, 54, 40–7. [Google Scholar] [CrossRef]
- Hoffmann, R.F.; Zarrintan, S.; Brandenburg, S.M.; Kol, A.; de Bruin, H.G.; Jafari, S.; Dijk, F.; Kalicharan, D.; Kelders, M.; Gosker, H.R.; et al. Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respir Res 2013, 14, 97. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, X.; Liu, J.; Zhou, D.B.; Kuang, X.; Xiao, J.; Yu, Q.; Lu, X.; Li, W.; Xie, B.; et al. Serum IL-1β and IL-17 levels in patients with COPD: Associations with clinical parameters. International journal of chronic obstructive pulmonary disease. 2017 24, 1247–54.
- Lai, H.; Rogers, D.F. New pharmacotherapy for airway mucus hypersecretion in asthma and COPD: Targeting intracellular signaling pathways. Journal of aerosol medicine and pulmonary drug delivery. 2010, 23, 219–231. [Google Scholar] [CrossRef]
- Webster, K.A. Mitochondrial membrane permeabilization and cell death during myocardial infarction: Roles of calcium and reactive oxygen species. Future Cardiol 2012, 8, 863–884. [Google Scholar] [CrossRef]
- Campbell, V.A. Tetrahydrocannabinol-induced apoptosis of cultured cortical neurones is associated with cytochrome c release and caspase-3 activation. Neuropharmacol 2001, 40, 702–709. [Google Scholar] [CrossRef]
- Sarafian, T.A.; Tashkin, D.P.; Roth, M.D. Marijuana smoke and Δ9-tetrahydrocannabinol promote necrotic cell death but inhibit Fas-mediated apoptosis. Toxicol Appl Pharmacol 2001, 174, 264–272. [Google Scholar] [CrossRef]
- Zeissler, I.; Weiner, L.; Vogel, Z. Δ9-tetrahydrocannabinol increases C6 glioma cell death produced by oxidative stress. Neurosci 2005, 134, 567–574. [Google Scholar] [CrossRef]
- Semlali, A.; Beji, S.; Ajala, I.; Rouabhia, M. Effects of tetrahydrocannabinols on human oral cancer cell proliferation, apoptosis, autophagy, oxidative stress, and DNA damage. Arch Oral Biol 2021, 105200. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by lipid peroxidation. Trends Cell Biol 2016, 26, 165–176. [Google Scholar] [CrossRef]
- Sarafian, T.A.; Kouyoumjian, S.; Khoshaghideh, F.; Tashkin, D.P.; Roth, M.D. Δ9-Tetrahydrocannabinol disrupts mitochondrial function and cell energetics. Am J Physiol-Lung Cell Mol Physiol 2003, 284, L298–L306. [Google Scholar] [CrossRef]
- Yoshida, M.; Minagawa, S.; Araya, J.; Sakamoto, T.; Hara, H.; Tsubouchi, K.; Hosaka, Y.; Ichikawa, A.; Saito, N.; Kadota, T. Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nat Commun 2019, 10, 1–14. [Google Scholar]
- Park, E.-J.; Park, Y.-J.; Lee, S.J.; Lee, K.; Yoon, C. Whole cigarette smoke condensates induce ferroptosis in human bronchial epithelial cells. Toxicol Lett 2019, 303, 55–66. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X. Ferroptosis as a target for protection against cardiomyopathy. Proc Nat Acad Sci USA. 2019, 116, 2672–2680. [Google Scholar] [CrossRef]
- Lin, H.; Chen, X.; Zhang, C.; Yang, T.; Deng, Z.; Song, Y.; Huang, L.; Li, F.; Li, Q.; Lin, S. EF24 induces ferroptosis in osteosarcoma cells through HMOX1. Biomed Pharmacother. 2021, 136, 111202. [Google Scholar] [CrossRef]
- Hao, S.; Liang, B.; Huang, Q.; Dong, S.; Wu, Z.; He, W.; Shi, M. Metabolic networks in ferroptosis. Oncol Lett 2018, 15, 5405–5411. [Google Scholar]
- Du, J.; Wang, T.; Li, Y.; Zhou, Y.; Wang, X.; Yu, X.; Ren, X.; An, Y.; Wu, Y.; Sun, W. DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Rad Biol Med 2019, 131, 356–69. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, J.; Cai, L.; Wang, S.; Liu, C.; Zhang, Y.; You, L.; Fu, Y.; Shi, Z.; Yin, Z. The effect of anti-inflammatory properties of ferritin light chain on lipopolysaccharide-induced inflammatory response in murine macrophages. Biochim Biophys Acta Mol Cell Res 2014, 1843, 2775–2783. [Google Scholar] [CrossRef]
- Cocco, E.; Porrini, V.; Derosas, M.; Nardi, V.; Biasiotto, G.; Maccarinelli, F.; Zanella, I. Protective effect of mitochondrial ferritin on cytosolic iron dysregulation induced by doxorubicin in HeLa cells. Mol Bio Rep 2013, 40, 6757–6764. [Google Scholar] [CrossRef]
- Sarafian, T.; Habib, N.; Mao, J.T.; Tsu, I.H.; Yamamoto, M.L.; Hsu, E.; Tashkin, D.P.; Roth, M.D. Gene expression changes in human small airway epithelial cells exposed to Δ9-tetrahydrocannabinol. Toxicol Lett 2005, 158, 95–107. [Google Scholar] [CrossRef]
- Aguiar, J.A.; Huff, R.D.; Tse, W.; Stämpfli, M.R.; McConkey, B.J.; Doxey, A.C.; Hirota, J.A. Transcriptomic and barrier responses of human airway epithelial cells exposed to cannabis smoke. Physiol Rep 2019, 7, e14249. [Google Scholar] [CrossRef]
- Hu, B.; Liu, Y.; Chen, X.; Zhao, J.; Han, J.; Dong, H.; Zheng, Q.; Nie, G. Ferrostatin-1 protects auditory hair cells from cisplatin-induced ototoxicity in vitro and in vivo. Biochem Biophys Res Commun 2020, 533, 1442–1448. [Google Scholar] [CrossRef]
- Li, Q.; Han, X.; Lan, X.; Gao, Y.; Wan, J.; Durham, F.; Cheng, T.; Yang, J.; Wang, Z.; Jiang, C.; et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight 2017, 2, e90777. [Google Scholar] [CrossRef]
- Liu, P.; Feng, Y.; Li, H.; Chen, X.; Wang, G.; Xu, S.; Li, Y.; Zhao, L. Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cell Mol Biol Lett 2020, 25, 1–14. [Google Scholar] [CrossRef]
- Miotto, G.; et al. Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biol 2020, 28, 101328. [Google Scholar] [CrossRef]
- Seibt, T.M.; Proneth, B.; Conrad, M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic Biol Med 2019, 133, 144–152. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic Biol Med 2020, 152, 175–185. [Google Scholar] [CrossRef]
- Constantin-Teodosiu, D.; Constantin, D. Molecular mechanisms of muscle fatigue. International Journal of Molecular Sciences. 2021, 22, 11587. [Google Scholar] [CrossRef]
- Mallia, P.; Webber, J.; Gill, S.K.; Trujillo-Torralbo, M.B.; Calderazzo, M.A.; Finney, L.; Bakhsoliani, E.; Farne, H.; Singanayagam, A.; Footitt, J.; et al. Role of airway glucose in bacterial infections in patients with chronic obstructive pulmonary disease. Journal of Allergy and Clinical Immunology. 2018, 142, 815–823. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Lee, D.-H.; Choudry, H.A.; Bartlett, D.L.; Lee, Y.J. Ferroptosis-induced endoplasmic reticulum stress: Cross-talk between ferroptosis and apoptosis. Mol Cancer Res 2018, 16, 1073–1076. [Google Scholar] [CrossRef]
- Wei, R.; Zhao, Y.; Wang, J.; Yang, X.; Li, S.; Wang, Y.; Yang, X.; Fei, J.; Hao, X.; Zhao, Y. Tagitinin C induces ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal cancer cells. Int J Biol Sci 2021, 17, 2703. [Google Scholar] [CrossRef]
- Ramirez, M.U.; Hernandez, S.R.; Soto-Pantoja, D.R.; Cook, K.L. Endoplasmic reticulum stress pathway, the unfolded protein response, modulates immune function in the tumor microenvironment to impact tumor progression and therapeutic response. Int J Mol Sci 2020, 21, 169. [Google Scholar] [CrossRef]
- Whiteside, T. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Diehl, J.A. Coordination of ER and oxidative stress signaling: The PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol 2006, 38, 317–332. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J Cell Sci 2012, 125, 5591–5596. [Google Scholar] [CrossRef]
- Domingo-Vidal, M.; Whitaker-Menezes, D.; Martos-Rus, C.; Tassone, P.; Snyder, C.M.; Tuluc, M.; Philp, N.; Curry, J.; Martinez-Outschoorn, U. Cigarette smoke induces metabolic reprogramming of the tumor stroma in head and neck squamous cell carcinoma. Mol Cancer Res 2019, 17, 1893–1909. [Google Scholar] [CrossRef]
- Blanco, F.J.; Guitian, R.; Vázquez-Martul, E.; de Toro, F.J.; Galdo, F. Osteoarthritis chondrocytes die by apoptosis: A possible pathway for osteoarthritis pathology. Arthritis Rheum 1998, 41, 284–289. [Google Scholar] [CrossRef]
- Zahan, O.-M.; Serban, O.; Gherman, C.; Fodor, D. The evaluation of oxidative stress in osteoarthritis. Med Pharm Reports. 2020, 93, 12. [Google Scholar] [CrossRef]
- Rigoglou, S.; Papavassiliou, A.G. The NF-κB signalling pathway in osteoarthritis. Int J Biochem Cell Biol 2013, 45, 2580–2584. [Google Scholar] [CrossRef]
- Khan, N.M.; Ahmad, I.; Haqqi, T.M. Nrf2/ARE pathway attenuates oxidative and apoptotic response in human osteoarthritis chondrocytes by activating ERK1/2/ELK1-P70S6K-P90RSK signaling axis. Free Radic Biol Med 2018, 116, 159–71. [Google Scholar] [CrossRef]
- Ankita Bansal, M. Celeste Simon; Glutathione metabolism in cancer progression and treatment resistance. J Cell Biol 2018, 217, 2291–2298. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Chen, Y.; Zhou, B.; Shan, X.; Yang, G. Eriodictyol inhibits IL-1β-induced inflammatory response in human osteoarthritis chondrocytes. Biomed Pharmacother 2018, 107, 1128–1134. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Goncharov, I.; Weiner, L.; Vogel, Z. Δ9-tetrahydrocannabinol increases C6 glioma cell death produced by oxidative stress. Neurosci 2005, 134, 567–574. [Google Scholar] [CrossRef]
- Zeissler, M.-L.; Eastwood, J.; McCorry, K.; Hanemann, C.O.; Zajicek, J.P.; Carroll, C.B. Delta-9-tetrahydrocannabinol protects against MPP+ toxicity in SH-SY5Y cells by restoring proteins involved in mitochondrial biogenesis. Oncotarget 2016, 7, 46603. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol 2019, 23, 101107. [Google Scholar] [CrossRef]
- Fan, Z.; Wirth, A.; Chen, D.; Wruck, C.; Rauh, M.; Buchfelder, M.; Savaskan, N. Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis. 2017, 6, e371. [Google Scholar] [CrossRef]
- Lu, C.; Xu, W.; Zhang, F.; Shao, J.; Zheng, S. Nrf2 knockdown attenuates the ameliorative effects of ligustrazine on hepatic fibrosis by targeting hepatic stellate cell transdifferentiation. Toxicol 2016, 365, 35–47. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Cullinan, S.B.; Diehl, J.A. Coordination of ER and oxidative stress signaling: The PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol 2006, 38, 317–332. [Google Scholar] [CrossRef]
- Zimmermann, K.; Baldinger, J.; Mayerhofer, B.; Atanasov, A.G.; Dirsch, V.M.; Heiss, E.H. Activated AMPK boosts the Nrf2/HO-1 signaling axis—A role for the unfolded protein response. Free Radic Biol Med 2015, 88, 417–426. [Google Scholar] [CrossRef]
- Hou, H.H.; Pan, H.J.; Liao, W.Y.; Lee, C.H.; Yu, C.J. Autophagy in fibroblasts induced by cigarette smoke extract promotes invasion in lung cancer cells. Int J Cancer 2020, 147, 2587–2596. [Google Scholar] [CrossRef]
- Dai, E.; Han, L.; Liu, J.; Xie, Y.; Kroemer, G.; Klionsky, D.J.; Zeh, H.J.; Kang, R.; Wang, J.; Tang, D. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy 2020, 16, 2069–2083. [Google Scholar] [CrossRef]
- Kiss, A.L.; Botos, E. Endocytosis via caveolae: Alternative pathway with distinct cellular compartments to avoid lysosomal degradation? J Cell Mol Med 2009, 13, 1228–1237. [Google Scholar] [CrossRef]
- Duffney, P.F.; Embong, A.K.; McGuire, C.C.; Thatcher, T.H.; Phipps, R.P.; Sime, P.J. Cigarette smoke increases susceptibility to infection in lung epithelial cells by upregulating caveolin-dependent endocytosis. PLoS ONE 2020, 15, e0232102. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Koukourakis, M.; Sivridis, E.; Turley, H.; Talks, K.; Pezzella, F.; Gatter, K.; Harris, A. Relation of hypoxia inducible factor 1 α and 2 α in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer 2001, 85, 881–890. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Q.; Song, Y.; Li, X.; Wang, Y.; Wan, S.; Zhang, Z.; Su, H. Polymorphisms of HIF1A gene are associated with prognosis of early stage non-small-cell lung cancer patients after surgery. Med Oncol 2014, 31, 1–9. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, F. Role of the HIF-1 signaling pathway in chronic obstructive pulmonary disease. Exp Ther Med 2018, 16, 4553–4561. [Google Scholar] [CrossRef]
- Ulven, S.M.; Dalen, K.T.; Gustafsson, J.-Å.; Nebb, H.I. LXR is crucial in lipid metabolism. Prostaglandins Leukot Essent Fatty Acids 2005, 73, 59–63. [Google Scholar] [CrossRef]
- Cha, J.-Y.; Repa, J.J. The liver X receptor (LXR) and hepatic lipogenesis: The carbohydrate-response element-binding protein is a target gene of LXR. J Biol Chem 2007, 282, 743–751. [Google Scholar] [CrossRef]
- Larrede, S.; Quinn, C.M.; Jessup, W.; Frisdal, E.; Olivier, M.; Hsieh, V.; Kim, M.-J.; Van Eck, M.; Couvert, P.; Carrie, A. Stimulation of cholesterol efflux by LXR agonists in cholesterol-loaded human macrophages is ABCA1-dependent but ABCG1-independent. Arterioscler Thromb Vasc Biol 2009, 29, 1930–1936. [Google Scholar] [CrossRef]
- Morichika, D.; Miyahara, N.; Fujii, U.; Taniguchi, A.; Oda, N.; Senoo, S.; Kataoka, M.; Tanimoto, M.; Kakuta, H.; Kiura, K. A retinoid X receptor partial agonist attenuates pulmonary emphysema and airway inflammation. Respir Res 2019, 20, 1–14. [Google Scholar] [CrossRef]
- Fujii, U.; Miyahara, N.; Taniguchi, A.; Oda, N.; Morichika, D.; Murakami, E.; Nakayama, H.; Waseda, K.; Kataoka, M.; Kakuta, H. Effect of a retinoid X receptor partial agonist on airway inflammation and hyperresponsiveness in a murine model of asthma. Respir Res 2017, 18, 1–10. [Google Scholar] [CrossRef]
- Adcock, I.M.; Mumby, S. Glucocorticoids. Handb Exp Pharmacol 2017, 237, 171–196. [Google Scholar]
- Kadmiel, M.; Cidlowski, J.A. Glucocorticoid receptor signaling in health and disease. Trends Pharmacol Sci 2013, 34, 518–530. [Google Scholar] [CrossRef]
- Palumbo, M.L.; Prochnik, A.; Wald, M.R.; Genaro, A.M. Chronic stress and glucocorticoid receptor resistance in asthma. Clin Ther 2020, 42, 993–1006. [Google Scholar] [CrossRef]
- Enweasor, C.; Flayer, C.H.; Haczku, A. Ozone-Induced Oxidative Stress, Neutrophilic Airway Inflammation, and Glucocorticoid Resistance in Asthma. Front Immunol 2021, 12, 192. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, H.; Xuan, N.; Zhou, L.; Wu, Y.; Zhu, C.; Li, M.; Weng, Q.; Shen, J.; Zhang, H. Induction of ferroptosis-like cell death of eosinophils exerts synergistic effects with glucocorticoids in allergic airway inflammation. Thorax. 2020, 75, 918–927. [Google Scholar] [CrossRef]
- Vassall, M.; Chakraborty, S.; Feng, Y.; Faheem, M.; Wang, X.; Bhandari, R.K. Transcriptional alterations induced by delta-9 tetrahydrocannabinol in the brain and gonads of adult medaka. Journal of Xenobiotics. 2023, 13, 237–251. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




