Submitted:
07 June 2024
Posted:
11 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Background Information
2.3. Periodontal Examination
2.4. Sample Collection
2.5. Medical Examination
2.6. Assay of IgA Concentration in Saliva
2.7. DNA Preparation and Microbiota Analysis
2.8. Bayesian Network Analysis and Classification Trees
2.9. Statistical Analysis
3. Results
3.1. Participant's Information, Periodontal and Medical Examinations, and Salivary IgA Levels
3.2. Diversity and Composition of Bacterial Flora in the Saliva
3.3. Diversity and Composition of Bacterial Flora in the Feces
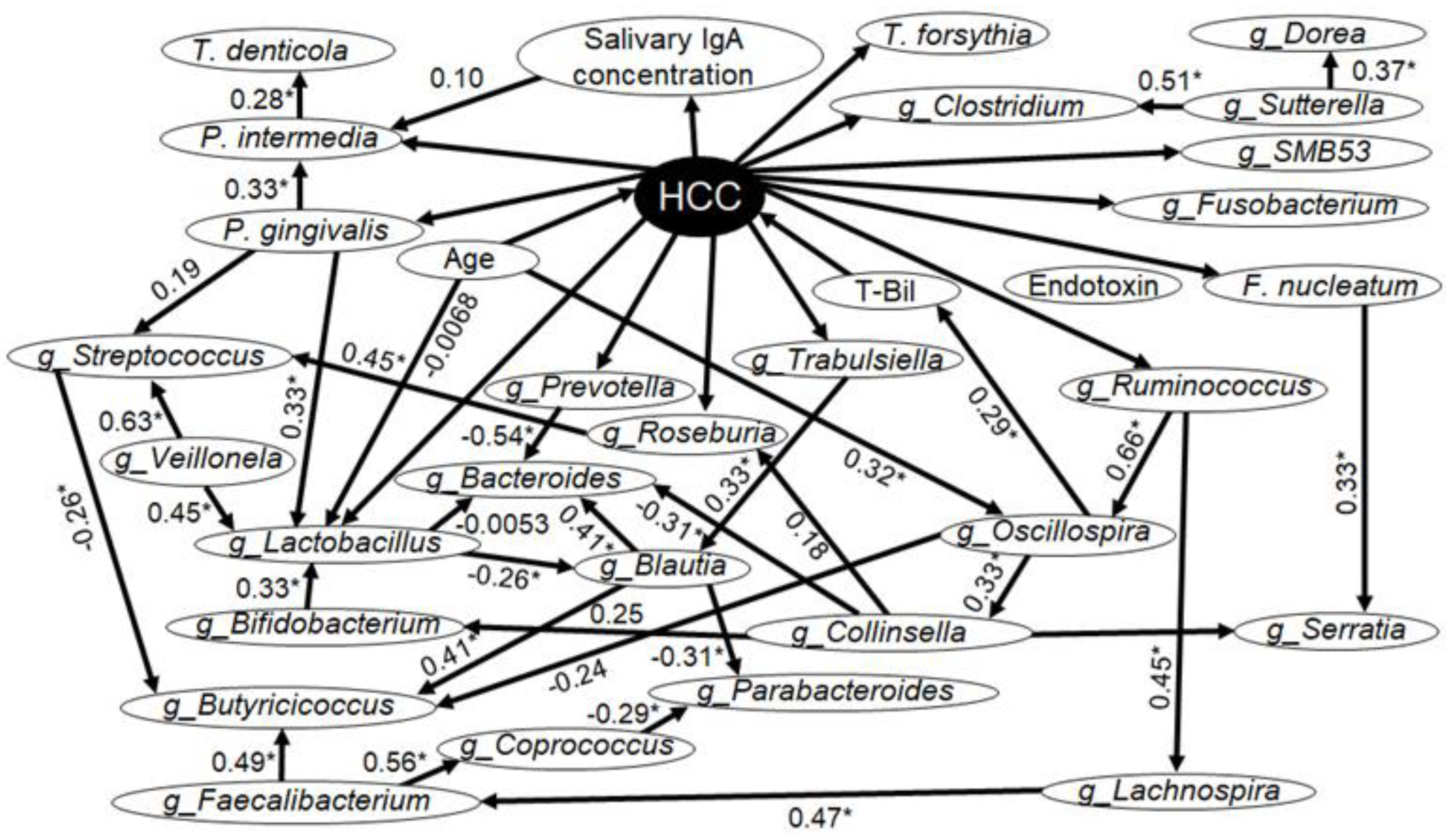
3.4. Determination of Causal Effects Using Bayesian Network Analysis
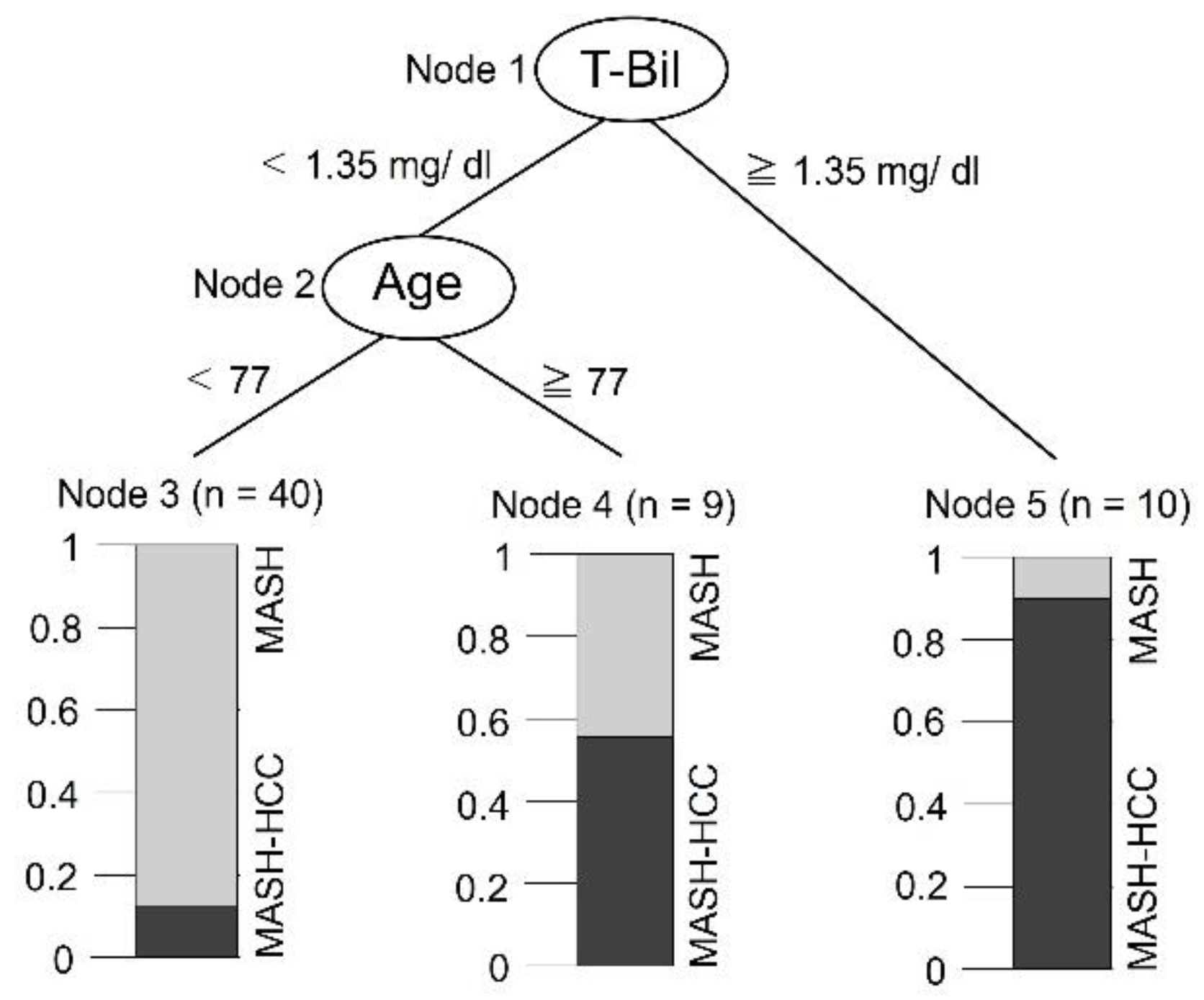
3.5. Classification Tree to Assess Disease Type
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Zopf, S.; Kremer, A.E.; Neurath, M.F.; Siebler, J. Advances in hepatitis C therapy: What is the current state—What come’s next? World J. Hepatol. 2016, 8, 139–147. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Whittle, E.; Leonard, M.O.; Harrison, R.; Gant, T.W.; Tonge, D.P. Multi-method characterization of the human circulating microbiome. Front. Microbiol. 2018, 9, 3266. [Google Scholar] [CrossRef]
- Emery, D.C.; Cerajewska, T.L.; Seong, J.; Davies, M.; Paterson, A.; Allen-Birt, S.J.; West, N.X. Comparison of blood bacterial communities in periodontal health and periodontal disease. Front. Cell. Infect. Microbiol. 2020, 10, 577485. [Google Scholar] [CrossRef] [PubMed]
- Arimatsu, K.; Yamada, H.; Miyazawa, H.; Minagawa, T.; Nakajima, M.; Ryder, M.I.; Gotoh, K.; Motooka, D.; Nakamura, S.; Iida, T.; et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci. Rep. 2014, 4, 4828. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.; Kamada, N. Exploring the oral-gut linkage: Interrelationship between oral and systemic diseases. Mucosal Immunol. <italic>in press</italic>. 2023. [Google Scholar] [CrossRef]
- Nwizu, N.; Wactawski-Wende, J.; Genco, R.J. Periodontal disease and cancer: Epidemiologic studies and possible mechanisms. Periodontol. 2000 2020, 83, 213–233. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tabernero, J.; et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012, 22, 292–298. [Google Scholar] [CrossRef]
- Boehm, E.T.; Thon, C.; Kupcinskas, J.; Steponaitiene, R.; Skieceviciene, J.; Canbay, A.; Malfertheiner, P.; Link, A. Fusobacterium nucleatum is associated with worse prognosis in Lauren’s diffuse type gastric cancer patients. Sci. Rep. 2020, 10, 16240. [Google Scholar] [CrossRef] [PubMed]
- Parhi, L.; Alon-Maimon, T.; Sol, A.; Nejman, D.; Shhadeh, A.; Fainsod-Levi, T.; Yajuk, O.; Isaacson, B.; Abed, J.; Maalouf, N.; et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 2020, 11, 3259. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Li, S.; Ma, Z.; Liang, S.; Shan, T.; Zhang, M.; Zhu, X.; Zhang, P.; Liu, G.; Zhou, F.; et al. Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect. Agent Cancer 2016, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Takaki, A.; Tomofuji, T.; Endo, Y.; Kasuyama, K.; Ekuni, D.; Yasunaka, T.; Yamamoto, K.; Morita, M. Stage of hepatocellular carcinoma is associated with periodontitis. J. Clin. Periodontol. 2011, 38, 1015–1020. [Google Scholar] [CrossRef]
- Takuma, R.; Morozumi, T.; Yamamoto, Y.; Kobayashi, T.; Matsui, T.; Yoneda, M.; Kessoku, T.; Nogami, A.; Tamura, M.; Kamata, Y.; et al. Association between non-alcoholic steatohepatitis-related hepatocellular carcinoma and periodontopathic bacteria: A cross-sectional pilot study. Appl. Sci. 2023, 13, 3893. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Watanabe, S.; Kameoka, S.; Shinozaki, NO.; Kubo, R.; Nishida, A.; Kuriyama, M.; Takeda, AK. A cross-sectional analysis from the Mykinso Cohort Study: establishing reference ranges for Japanese gut microbial indices. Biosci Microbiota Food Health. 2021, 40, 123–134. [Google Scholar] [CrossRef]
- Fujihara, H.; Matsunaga, M.; Ueda, E.; Kajiwara, T.; Takeda, AK.; Watanabe, S.; Baba, K.; Hagihara, K.; Myowa, M. Altered Gut Microbiota Composition Is Associated with Difficulty in Explicit Emotion Regulation in Young Children. Microorganisms. 2023, 11, 2245. [Google Scholar] [CrossRef] [PubMed]
- Maglogiannis, I.; Zafiropoulos, E.; Platis, A.; Lambrinoudakis, C. Risk analysis of a patient monitoring system using Bayesian Network modeling. J. Biomed. Inform. 2006, 39, 637–647. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Morozumi, T.; Hirata, T.; Takahashi, T.; Fuchida, S.; Toyoda, M.; Nakajima, S.; Minabe, M. Effect of periodontal disease on diabetic retinopathy in type 2 diabetic patients: A cross-sectional pilot study. J. Clin. Med. 2020, 9, 3234. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Nishijima, S.; Kojima, Y.; Hisada, Y.; Imbe, K.; Miyoshi-Akiyama, T.; Suda, W.; Kimura, M.; Aoki, R.; Sekine, K.; et al. Metagenomic identification of microbial signatures predicting pancreatic cancer from a multinational study. Gastroenterology 2022, 163, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Lu, J.; Beck, J.D.; Moss, K.L.; Prizment, A.E.; Demmer, R.T.; Porosnicu Rodriguez, K.A.; Joshu, C.E.; Michaud, D.S.; Platz, E.A. Periodontal and other oral bacteria and risk of lung cancer in the atherosclerosis risk in communities (ARIC) study. Cancer Epidemiol. Biomarkers Prev. 2023, 32, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Tortora, S.C.; Bodiwala, V.M.; Quinn, A.; Martello, L.A.; Vignesh, S. Microbiome and colorectal carcinogenesis: Linked mechanisms and racial differences. World J. Gastrointest. Oncol. 2022, 14, 375–395. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Witkowski, J.M.; Kotb, R.; Hirokawa, K.; Pawelec, G. Immunosenescence and cancer. Crit. Rev. Oncog. 2013, 18, 489–513. [Google Scholar] [CrossRef]
- Sheng, C.C.; Han, F.Y. Immunoregulation effects of TIM-3 on tumors. Neoplasma 2019, 66, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, G.; Stähli, A.; Zhu, X.; Auber Alberi, L.; Sculean, A.; Eick, S. Periodontal microorganisms and Alzheimer disease – A causative relationship? Periodontol. 2000 2022, 89, 59–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, T.; Li, Y.; Huang, L.; Yin, D. Fusobacterium nucleatum: The opportunistic pathogen of periodontal and peri-Implant diseases. Front. Microbiol. 2022, 13, 860149. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.E.; Shetty, K.; Fidel, P.L. Jr. Oral opportunistic infections in HIV-positive individuals: Review and role of mucosal immunity. AIDS Patient Care STDs 2004, 18, 443–456. [Google Scholar] [CrossRef]
- Borges-Canha, M.; Portela-Cidade, J.P.; Dinis-Ribeiro, M.; Leite-Moreira, A.F.; Pimentel-Nunes, P. Role of colonic microbiota in colorectal carcinogenesis: A systematic review. Rev. Esp. Enferm. Dig. 2015, 107, 659–671. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, D.; Li, Z.; Jiang, H.; Li, J.; Ren, R.; Gao, X.; Li, J.; Wang, X.; Wang, W.; et al. The fecal microbiota of patients with pancreatic ductal adenocarcinoma and autoimmune pancreatitis characterized by metagenomic sequencing. J. Transl. Med. 2021, 19, 215. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Kawaguchi, M.; Mikami, H.; Diao, P.; Zhang, X.; Zhang, Z.; Nakajima, T.; Iwadare, T.; Kimura, T.; Nakayama, J.; et al. Establishment of novel mouse model of dietary NASH rapidly progressing into liver cirrhosis and tumors. Cancers 2023, 15, 3744. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, A.; Ejtahed, H.S.; Ettehad Marvasti, F.; Taghavi, M.; Pakmehr, A.; Hajipour, F.; Larijani, B. The critical role of gut microbiota dysbiosis in skeletal muscle wasting: A systematic review. J. Appl. Microbiol. 2023, 134, lxac014. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Yang, X.; Zhang, R.; Li, J.; Xiao, X.; Hu, Y.; Chen, Y.; Yang, F.; Lu, N.; Wang, Z.; et al. Dysbiosis signature of fecal microbiota in colorectal cancer patients. Microb. Ecol. 2013, 66, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Tesolato, S.; Ortega-Hernández, A.; Gómez-Garre, D.; Claver, P.; De Juan, C.; De la Serna, S.; Paz, M.; Domínguez-Serrano, I.; Dziakova, J.; Rivera, D.; et al. Gut microbiota profiles in feces and paired tumor and non-tumor tissues from colorectal cancer patients. Relationship to the body mass index. PLOS ONE 2023, 18, e0292551. [Google Scholar] [CrossRef]
- Liu, P.; Yang, J.; Chen, Y.; Zhu, Y.; Tang, Y.; Xu, X.; He, H. Alterations of gut microbiota and metabolome in early chronic kidney disease patients complicated with hyperuricemia. Heliyon 2023, 9, e20328. [Google Scholar] [CrossRef]
- Rajapakse, J.; Khatiwada, S.; Akon, A.C.; Yu, K.L.; Shen, S.; Zekry, A. Unveiling the complex relationship between gut microbiota and liver cancer: Opportunities for novel therapeutic interventions. Gut Microbes 2023, 15, 2240031. [Google Scholar] [CrossRef]
- Huang, C.; Mei, S.; Zhang, X.; Tian, X. Inflammatory milieu related to dysbiotic gut microbiota promotes tumorigenesis of hepatocellular carcinoma. J. Clin. Gastroenterol. 2023, 57, 782–788. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, D.; Ann, H.W.; Han, M.; Lee, J.A.; Lee, Y.; Ahn, S.; Seo, H.W.; Kim, J.H.; Ahn, J.Y.; et al. Deciphering gut microbiota in patients with severe sepsis and septic shock. Shock 2024, 61, 28–33. [Google Scholar] [CrossRef]
- Hymel, E.; Vlock, E.; Fisher, K.W.; Farazi, P.A. Differential progression of unhealthy diet-induced hepatocellular carcinoma in obese and non-obese mice. PLOS ONE 2022, 17, e0272623. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Wang, S.; Bian, X.; Jiang, X.; Wu, J.; Wang, K.; Wang, Q.; Xia, J.; Jiang, S.; et al. Western diet aggravated carbon tetrachloride-induced chronic liver injury by disturbing gut microbiota and bile acid metabolism. Mol. Nutr. Food Res. 2021, 65, e2000811. [Google Scholar] [CrossRef]
- Guo, S.; Chen, F.; Li, L.; Dou, S.; Li, Q.; Huang, Y.; Li, Z.; Liu, W.; Zhang, G. Intracellular Fusobacterium nucleatum infection increases METTL3-mediated m6A methylation to promote the metastasis of esophageal squamous cell carcinoma. J. Adv. Res. in press. 2023. [Google Scholar] [CrossRef] [PubMed]
- Sydor, S.; Best, J.; Messerschmidt, I.; Manka, P.; Vilchez-Vargas, R.; Brodesser, S.; Lucas, C.; Wegehaupt, A.; Wenning, C.; Aßmuth, S.; et al. Altered microbiota diversity and bile acid signaling in cirrhotic and noncirrhotic NASH-HCC. Clin. Transl. Gastroenterol. 2020, 11, e00131. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, K.; Amiri Moghaddam, M.; Etajuri, E.A.; Badkoobeh, A.; Tavakol, O.; Rafinejad, M.; Forutan Mirhosseini, A.; Fathi, A. Periodontitis and progression of gastrointestinal cancer: Current knowledge and future perspective. Clin. Transl. Oncol. 2023, 25, 2801–2811. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, I.; Han, S.J.; Kwon, S.; Min, E.J.; Cho, W.; Koh, H.; Koo, B.N.; Lee, J.S.; Kwon, J.S.; et al. Oral Porphyromonas gingivalis infection affects intestinal microbiota and promotes atherosclerosis. J. Clin. Periodontol. 2023, 50, 1553–1567. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Arimatsu, K.; Kato, T.; Matsuda, Y.; Minagawa, T.; Takahashi, N.; Ohno, H.; Yamazaki, K. Oral administration of P. gingivalis induces dysbiosis of gut microbiota and impaired barrier function leading to dissemination of enterobacteria to the liver. PLOS ONE 2015, 10, e0134234. [Google Scholar] [CrossRef] [PubMed]
- Conti, L.; Borro, M.; Milani, C.; Simmaco, M.; Esposito, G.; Canali, G.; Pilozzi, E.; Ventura, M.; Annibale, B.; Lahner, E. Gastric microbiota composition in patients with corpus atrophic gastritis. Dig. Liver Dis. 2021, 53, 1580–1587. [Google Scholar] [CrossRef]
- Qi, Z.; Zhibo, Z.; Jing, Z.; Zhanbo, Q.; Shugao, H.; Weili, J.; Jiang, L.; Shuwen, H. Prediction model of poorly differentiated colorectal cancer (CRC) based on gut bacteria. BMC Microbiol. 2022, 22, 312. [Google Scholar] [CrossRef]
- Chen, T.; Ding, R.; Chen, X.; Lu, Y.; Shi, J.; Lü, Y.; Tang, B.; Zhang, W.; Ye, C.; Yuan, M.; et al. Firmicutes and Blautia in gut microbiota lessened in chronic liver diseases and hepatocellular carcinoma patients: A pilot study. Bioengineered 2021, 12, 8233–8246. [Google Scholar] [CrossRef]
- Zhang, X.; Coker, O.O.; Chu, E.S.; Fu, K.; Lau, H.C.H.; Wang, Y.X.; Chan, A.W.H.; Wei, H.; Yang, X.; Sung, J.J.Y.; et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 2021, 70, 761–774. [Google Scholar] [CrossRef]
- Cheung, M.K.; Yue, G.G.L.; Lauw, S.; Li, C.S.Y.; Yung, M.Y.; Ng, S.C.; Yip, H.C.; Kwan, H.S.; Chiu, P.W.Y.; Lau, C.B.S. Alterations in gut microbiota of esophageal squamous cell carcinoma patients. J. Gastroenterol. Hepatol. 2022, 37, 1919–1927. [Google Scholar] [CrossRef]
- Rodríguez-García, A.; Arroyo, A.; García-Vicente, R.; Morales, M.L.; Gómez-Gordo, R.; Justo, P.; Cuéllar, C.; Sánchez-Pina, J.; López, N.; Alonso, R.; et al. Short-chain fatty acid production by gut microbiota predicts treatment response in multiple myeloma. Clin. Cancer Res. in press. 2023. [Google Scholar] [CrossRef]
- Minnebo, Y.; Delbaere, K.; Goethals, V.; Raes, J.; Van de Wiele, T.; De Paepe, K. Gut microbiota response to in vitro transit time variation is mediated by microbial growth rates, nutrient use efficiency and adaptation to in vivo transit time. Microbiome 2023, 11, 240. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, B.; Zhao, X.; Zhang, P.; Guo, J.; Zhuang, Y.; Wang, S. Coffee leaf tea extracts improve hyperuricemia nephropathy and its associated negative effect in gut microbiota and amino acid metabolism in rats. J. Agric. Food Chem. 2023, 71, 17775–17787. [Google Scholar] [CrossRef]
- McBrearty, N.; Arzumanyan, A.; Bichenkov, E.; Merali, S.; Merali, C.; Feitelson, M. Short chain fatty acids delay the development of hepatocellular carcinoma in HBx transgenic mice. Neoplasia 2021, 23, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Fazio, G.; Galioto, F.; Ferlito, A.; Coronella, M.; Palmucci, S.; Basile, A. Cavitated pulmonary nodules in a female patient with breast cancer: Keep in mind Serratia marcescens’ infections. Respir. Med. Case Rep. 2021, 33, 101441. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhang, X.; Zhu, X.; Jiao, J.; Wu, Y.; Li, Y.; Zhao, L. Fusobacterium nucleatum aggravates ulcerative colitis through promoting gut microbiota dysbiosis and dysmetabolism. J. Periodontol. 2023, 94, 405–418. [Google Scholar] [CrossRef]
- Hägewald, S.; Bernimoulin, J.P.; Köttgen, E.; Kage, A. Salivary IgA subclasses and bacteria-reactive IgA in patients with aggressive periodontitis. J. Periodont. Res. 2002, 37, 333–339. [Google Scholar] [CrossRef]
- Yu, F.; Xu, Q.A.; Chen, W. A targeted fimA DNA vaccine prevents alveolar bone loss in mice after intra-nasal administration. J. Clin. Periodontol. 2011, 38, 334–340. [Google Scholar] [CrossRef]
- Chang, E.; Kobayashi, R.; Fujihashi, K.; Komiya, M.; Kurita-Ochiai, T. Impaired salivary SIgA antibodies elicit oral dysbiosis and subsequent induction of alveolar bone loss. Inflamm. Res. 2021, 70, 151–158. [Google Scholar] [CrossRef]
- Zhang, T.; Cheng, S.; Li, J.; Shang, Y.; Zheng, M. Evaluation of the effect of ultrasound interventional injection of cisplatin in the treatment of liver cancer. Am. J. Transl. Res. 2021, 13, 5603–5609. [Google Scholar] [PubMed]
- Li, T.; Yang, G.; Hao, Q.; Zhang, X.; Zhang, X. Daphnetin ameliorates the expansion of chemically induced hepatocellular carcinoma via reduction of inflammation and oxidative stress. J. Oleo Sci. 2022, 71, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, H.; Bukhari, I.; Zhao, Y.; Huang, H.; Yu, Y.; Sun, X.; Mi, Y.; Mei, L.; Zheng, P. Effects of cholesterol-lowering probiotics on non-alcoholic fatty liver disease in FXR gene knockout mice. Front. Nutr. 2023, 10, 1121203. [Google Scholar] [CrossRef]
- Chen, W.; Wei, Y.; Xiong, A.; Li, Y.; Guan, H.; Wang, Q.; Miao, Q.; Bian, Z.; Xiao, X.; Lian, M.; et al. Comprehensive analysis of serum and fecal bile acid profiles and interaction with gut microbiota in primary biliary cholangitis. Clin. Rev. Allergy Immunol. 2020, 58, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Takaki, A.; Wada, N.; Yasunaka, T.; Ikeda, F.; Maruyama, T.; Tamaki, N.; Uchida, D.; Onishi, H.; Kuwaki, K.; et al. The serum oxidative/anti-oxidative stress balance becomes dysregulated in patients with non-alcoholic steatohepatitis associated with hepatocellular carcinoma. Intern. Med. 2017, 56, 243–251. [Google Scholar] [CrossRef]
- Defaye, M.; Nourrisson, C.; Baudu, E.; Lashermes, A.; Meynier, M.; Meleine, M.; Wawrzyniak, I.; Bonnin, V.; Barbier, J.; Chassaing, B.; et al. Fecal dysbiosis associated with colonic hypersensitivity and behavioral alterations in chronically Blastocystis-infected rats. Sci. Rep. 2020, 10, 9146. [Google Scholar] [CrossRef]
| Parameter | MASH Group (N = 41) |
MASH-HCC Group (N = 19) |
p-Value |
|---|---|---|---|
| Gender (men/women) | 25/16 | 13/6 | 0.578 |
| Smoking status (+/−) | 9/32 | 4/15 | 0.973 |
| Age (years) | 59 (55–70) | 79 (64–82) | 0.0004* |
| BMI | 26.2 (22.3–31.5) | 27.7 (25.9–30.8) | 0.503 |
| Number of remaining teeth | 26 (21–27) | 25 (21–27) | 0.598 |
| PD (mm) | 2.8 (2.6–3.2) | 2.9 (2.6–3.2) | 0.653 |
| BOP (%) | 14.9 (10.7–24.1) | 15.4 (7.3–31.4) | 0.799 |
| Tooth mobility | 0 (0–0.1) | 0 (0–0.1) | 0.703 |
| PlI | 0.9 (0.8–1.4) | 0.9 (0.7–1.4) | 0.619 |
| Salivary IgA (ug/mL) | 231.7 (146.5–482.8) | 102.7 (85.8–168.9) | <0.001* |
| Endotoxin (EU) | 0.13 (0.08–0.17) | 0.22 (0.15–0.28) | <0.0001* |
| CRP (mg/dL) | 0.14 (0.09–0.48) | 0.13 (0.07–0.34) | 0.487 |
| AST (U/L) | 51 (27–62) | 38 (29–58) | 0.546 |
| ALT (U/L) | 53 (26–71) | 29 (23–44) | 0.094 |
| T-Bil (mg/dL) | 0.8 (0.6–1) | 1.3 (0.7–1.7) | 0.014* |
| Parameter | MASH Group (N = 41) |
MASH-HCC Group (N = 19) |
p-Value |
|---|---|---|---|
| Diversity of bacterial flora | |||
| Shannon index | 6.72 (6.45–7.13) | 6.51 (6.05–6.80) | 0.03* |
| Phylum | |||
| Actinomycetota (%) | 11.7 (9.56–15.19) | 13.15 (10.38–14.25) | 0.661 |
| Bacillota (%) | 39.5 (36.94–43.06) | 42.7 (35.56–45.30) | 0.604 |
| Bacteroidota (%) | 24.94 (21.61–28.02) | 23.45 (20.54–30.08) | 0.450 |
| Campylobacterota (%) | 0.976 (0.618–1.50) | 1.13(0.806–1.57) | 0.418 |
| Cyanobacteria (%) | 0 (0–0) | 0 (0–0) | 0.928 |
| Desulfobacterota (%) | 0 (0–0.0346) | 0.0135 (0–0.0208) | 0.945 |
| Fusobacteriota (%) | 8.12 (3.94–9.81) | 8.23 (5.42–9.61) | 0.335 |
| Patescibacteria (%) | 1.83 (0.463–2.54) | 1.32 (0.337–2.01) | 0.167 |
| Pseudomonadota (%) | 13.93 (6.79–16.71) | 13.17 (3.01–17.39) | 0.727 |
| Spirochaetota (%) | 0.269 (0.025–0.638) | 0.061 (0.029–0.386) | 0.185 |
| Synergistetes (%) | 0.040 (0–0.103) | 0.020 (0.013–0.025) | 0.228 |
| Genus | |||
| Actinomyces (%) | 6.27 (3.62–8.05) | 5.39 (3.84–6.72) | 0.418 |
| Alloprevotella (%) | 1.54 (0.788–3.26) | 1.16 (0.691–1.91) | 0.348 |
| Atopobium (%) | 0.337 (0.165–0.774) | 0.495 (0.147–0.768) | 0.962 |
| Campylobacter (%) | 1.02 (0.650–1.50) | 1.13 (0.806–1.57) | 0.525 |
| Capnocytophaga (%) | 0.897 (0.282–1.85) | 0.976 (0.524–1.70) | 0.391 |
| Corynebacterium (%) | 0.306 (0.191–0.804) | 0.484 (0.146–0.931) | 0.340 |
| Fusobacterium (%) | 3.25 (1.84–5.07) | 4.48 (2.24–5.58) | 0.266 |
| Gemella (%) | 2.15 (1.14–3.07) | 2.04 (1.17–3.29) | 0.949 |
| Granulicatella (%) | 2.73 (1.86–3.73) | 2.39 (1.60–3.16) | 0.221 |
| Haemophilus (%) | 3.36 (1.70–5.34) | 3.96 (1.10–5.77) | 0.836 |
| Lactobacillus (%) | 0.00620 (0–0.239) | 0.122 (0–1.02) | 0.247 |
| Lautropia (%) | 0.141 (0–0.633) | 0.138 (0–0.831) | 0.572 |
| Leptotrichia (%) | 2.83 (1.36–4.58) | 2.23 (1.53–3.84) | 0.567 |
| Megasphaera (%) | 0.413 (0.151–0.873) | 0.536 (0.106–1.03) | 0.861 |
| Neisseria (%) | 5.20 (1.14–8.72) | 1.25 (1.03–9.20) | 0.505 |
| Peptostreptococcus (%) | 0.256 (0.0694–0.643) | 0.183 (0–0.664) | 0.262 |
| Porphyromonas (%) | 3.71 (1.72–7.14) | 3.98 (0.900–6.49) | 0.589 |
| Prevotella (%) | 16.1 (11.8–19.3) | 15.9 (11.6–20.6) | 0.799 |
| Rothia (%) | 3.09 (1.53–5.53) | 4.42 (2.38–5.42) | 0.409 |
| Streptococcus (%) | 20.3 (16.1–24.6) | 22.2 (16.0–27.0) | 0.340 |
| TM7x (%) | 0.729 (0.221–1.509) | 0.463 (0.0127–1.04) | 0.249 |
| Treponema (%) | 0.292 (0.0331–0.561) | 0.0611 (0.0302–0.225) | 0.0768 |
| Veillonella (%) | 7.98 (6.07–10.7) | 9.68 (6.30–12.4) | 0.204 |
| Species | |||
| Actinomyces israelii (%) | 0 (0–0) | 0 (0–0.0256) | 0.101 |
| Bifidobacterium dentium (%) | 0 (0–0.040) | 0 (0–0.0748) | 0.620 |
| Capnocytophaga gingivalis (%) | 0.345 (0.130–0.691) | 0.427 (0.154–1.25) | 0.193 |
| Dialister pneumosintes (%) | 0 (0–0.0821) | 0 (0–0.0587) | 0.917 |
| Fusobacterium nucleatum (%) | 0.189 (0.0200–0.443) | 0.362 (0.170–0.928) | 0.014* |
| Lactobacillus crispatus (%) | 0 (0–0) | 0 (0–0.00302) | 0.544 |
| Lactobacillus fermentum (%) | 0 (0–0.287) | 0 (0–0.150) | 0.661 |
| Lactobacillus gasseri (%) | 0 (0–0.0257) | 0 (0–0.122) | 0.165 |
| Lactobacillus salivarius (%) | 0 (0–0.00405) | 0 (0–0.0874) | 0.363 |
| Metamycoplasma hyosynoviae (%) | 0 (0–0.0576) | 0 (0–0.0599) | 1.000 |
| Porphyromonas endodontalis (%) | 0.457(0.138–0.982) | 0.195(0–0.377) | 0.0923 |
| Porphyromonas gingivalis (%) | 0.138 (0–1.26) | 0.400 (0–0.725) | 0.520 |
| Prevotella denticola (%) | 0.459 (0.0750–0.948) | 0.429 (0.0683–0.924) | 0.905 |
| Prevotella enoeca (%) | 0 (0–0.00780) | 0 (0–0) | 0.149 |
| Prevotella intermedia (%) | 0 (0–0.334) | 0 (0–0.158) | 0.532 |
| Prevotella nigrescens (%) | 0 (0–0.0243) | 0 (0–0) | 0.175 |
| Streptococcus anginosus (%) | 0.124 (0.0379–0.247) | 0.165 (0.0126–0.373) | 0.937 |
| Streptococcus mutans (%) | 0.0110 (0–0.0608) | 0.0685 (0–0.118) | 0.159 |
| Streptococcus pneumoniae (%) | 0.557 (0.277–0.998) | 0.376(0–1.43) | 0.260 |
| Streptococcus sobrinus (%) | 0 (0–0.0183) | 0(0–0.00838) | 0.919 |
| Tannerella forsythia (%) | 0.146 (0.0612–0.287) | 0.0827 (0.0200–0.328) | 0.661 |
| Treponema denticola (%) | 0.0638 (0–0.195) | 0 (0–0.0325) | 0.0223* |
| Parameter | MASH Group (N = 41) |
MASH-HCC Group (N = 19) |
p-Value |
|---|---|---|---|
| Diversity of bacterial flora | |||
| Shannon index | 5.79 (5.54–6.17) | 5.43 (4.92–5.56) | <0.001* |
| Phylum | |||
| Actinomycetota (%) | 5.92 (2.54–9.96) | 4.62 (1.91–9.57) | 0.515 |
| Bacillota (%) | 46.8 (42.1–51.1) | 45.9 (42.1–51.7) | 0.793 |
| Bacteroidota (%) | 37.3(31.5-42.6) | 35.7(31.2-41.2) | 0.634 |
| Campylobacterota (%) | 0(0-0) | 0(0-0) | 0.307 |
| Desulfobacterota (%) | 0.210(0.0138-0.687) | 0.203(0-0.753) | 0.719 |
| Fusobacteriota (%) | 0.0172 (0–0.452) | 1.11 (0.263–2.32) | 0.002* |
| Patescibacteria (%) | 0(0-0) | 0(0-0) | 0.580 |
| Pseudomonadota (%) | 4.98 (3.99–10.6) | 6.11 (3.46–12.3) | 0.861 |
| Spirochaetota (%) | 0 (0–0) | 0 (0–0) | 0.197 |
| Synergistetes (%) | 0 (0–0) | 0 (0–0) | 1.000 |
| Verrucomicrobia (%) | 0 (0–0) | 0 (0–0) | 0.776 |
| Genus | |||
| Bacteroides (%) | 29.8 (19.0–34.5) | 25.6 (19.4–29.4) | 0.360 |
| Bifidobacterium (%) | 3.84 (0.39–9.48) | 1.87 (1.02–7.72) | 0.836 |
| Blautia (%) | 7.16 (4.66–10.8) | 8.11 (1.31–10.4) | 0.424 |
| Butyricicoccus (%) | 0.540 (0.195–0.825) | 0.170 (0.029–0.370) | 0.022* |
| Clostridium (%) | 0.310 (0.165–0.820) | 0.270 (0.130–0.770) | 0.594 |
| Collinsella (%) | 1.83 (0.0100–2.98) | 1.20 (0.0100–3.00) | 0.930 |
| Coprococcus (%) | 1.51 (0.375–4.17) | 0.830 (0.140–2.00) | 0.133 |
| Dorea (%) | 2.70 (1.71–4.62) | 2.68 (0.460–4.05) | 0.259 |
| Faecalibacterium (%) | 1.68 (0–6.16) | 0.119 (0–3.45) | 0.282 |
| Fusobacterium (%) | 0.0100 (0–0.550) | 1.11 (0.260–2.00) | 0.002* |
| Lachnospira (%) | 0.479 (0.0150–1.65) | 0.070 (0.0100–1.00) | 0.292 |
| Lactobacillus (%) | 0.0200 (0–0.505) | 0.220 (0–8.54) | 0.104 |
| Oscillospira (%) | 0.610 (0.205–1.43) | 1.19 (0.290–1.75) | 0.500 |
| Parabacteroides (%) | 2.51 (1.26–5.93) | 3.42 (1.73–5.83) | 0.490 |
| Prevotella (%) | 0.0100 (0–0.0300) | 0.0200 (0.0100–0.850) | 0.074 |
| Roseburia (%) | 0.970 (0.380–1.76) | 0.360 (0.150–1.29) | <0.05* |
| Ruminococcus (%) | 1.38 (0.105–4.00) | 0.239 (0.110–1.80) | 0.294 |
| Serratia (%) | 0.0200 (0–0.560) | 0.0100 (0–4.36) | 0.562 |
| SMB53 (%) | 0.110 (0.0200–0.265) | 0.239 (0–0.650) | 0.707 |
| Streptococcus (%) | 0.790 (0.190–3.45) | 1.56 (0.210–4.42) | 0.634 |
| Sutterella (%) | 3.20 (0.985–4.12) | 2.13 (0.580–3.87) | 0.259 |
| Trabulsiella (%) | 0.270 (0.040–0.920) | 0.440 (0.090–2.39) | 0.499 |
| Veillonela (%) | 0.0500 (0–1.23) | 0.050 (0.0100–0.650) | 0.797 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





