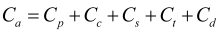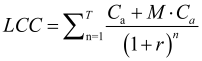1. Introduction
Faced with the urgent challenges of global climate change and the demand for clean energy, hydrogen energy is increasingly becoming a pivotal force in the energy transition due to its zero carbon emissions and high energy density. It is noteworthy that during the industrialization era, Europe accounting for 90% of global carbon emissions while America took over as the top carbon-emitting region in 1916. In the latter half of the 20th century, rapid economic growth in various countries drove up energy demand and consumption, leading to a significant increase in carbon emissions. Currently, China is the world’s largest carbon emitter, making it a crucial area of focus for research and development to reduce carbon emissions.
Throughout human history, our reliance on energy sources has evolved significantly, from the primitive use of firewood to the advent of coal and the steam engine, and later to the dominance of petroleum and the internal combustion engine. Yet, with escalating concerns over greenhouse gas (GHG) emissions and their impact on the climate, the quest for cleaner energy alternatives has led to a heightened focus on hydrogen—a plentiful, eco-friendly, and versatile form of secondary energy. Hydrogen boasts an impressive mass-energy density of 120 MJ/kg, despite a notably low volumetric energy density of 0.01079 MJ/L [
1]. In the current era, marked by a global surge in technological and industrial revolutions, cutting-edge energy technologies like hydrogen are witnessing rapid iterations like never before. Concurrently, the international political and economic arenas are experiencing profound shifts, which have catalyzed the momentum for energy diversification and decarbonization. This dynamic climate affords a substantial strategic opportunity for the advancement of hydrogen energy.
Globally, more than 20 countries and regions, including economic powerhouses such as South Korea, China, Germany, and the United States, have established national hydrogen energy strategies. These strategies are designed to stimulate research and development and to cultivate industrial expansion within the realms of hydrogen energy and fuel cell technology.
The United States has long acknowledged the strategic importance of hydrogen energy as a technology reserve. Through sustained research and development, the U.S. is working to drive down the costs of clean hydrogen and broaden its use across transportation, industrial, and power sectors.
European nations perceive hydrogen energy as a key agent for profound decarbonization and a transition to clean energy systems. Germany, for instance, has invested heavily in hydrogen infrastructure, fostering the generation of green hydrogen from renewable resources, and establishing hydrogen-centric industrial hubs.
South Korea envisions hydrogen energy as its third major pillar of industry with global strategic prowess, succeeding its display and semiconductor sectors. It prioritizes the development of hydrogen fuel cells in its research and development initiatives, aiming to boost its hydrogen power generation capacity to fuel economic advancement.
Traditional energy-exporting nations such as Australia, Russia, and Saudi Arabia are exploring economic diversification through hydrogen exports.
China is proactively establishing hydrogen energy demonstration projects, encouraging research and development, and fostering the integration of hydrogen energy technologies. China’s goal is to ramp up the production of green hydrogen as part of its efforts to reach peak carbon emissions and ultimately achieve carbon neutrality [
2].
Mastering the production of hydrogen is a prerequisite for its utilization. Over centuries of development, the processes for generating hydrogen have made significant strides. Current hydrogen production can be achieved through various methodologies and techniques:
Steam Methane Reforming (SMR) [
3]: This process entails the reaction of natural gas, or liquefied petroleum gas, with steam under high-temperature conditions to yield hydrogen and carbon dioxide. SMR stands as the most prevalent and conventional method of industrial hydrogen production.
Water Electrolysis [
4]: This technique employs electrolysis to decompose water into its constituent hydrogen and oxygen atoms. When powered by renewable energy sources such as solar or wind, this method facilitates the generation of green hydrogen.
Biomass Gasification [
5]: This method utilizes the thermochemical transformation of biomass materials, including wood and agricultural residues, at elevated temperatures to produce hydrogen alongside other gases. This technique allows for hydrogen production via renewable organic waste.
Methane Cracking [
6]: This approach involves the thermal decomposition of methane (natural gas) at high temperatures, resulting in hydrogen and solid carbon by-products. It leverages natural gas reserves for the generation of hydrogen.
The transition to hydrogen energy faces challenges including the high costs of production, storage, and transportation, as well as the need for new infrastructure. Cost competitiveness and the development of an extensive hydrogen infrastructure, including production facilities and refueling stations, are essential for its widespread adoption. The current scarcity of such infrastructure hampers the uptake of hydrogen energy. Additionally, hydrogen’s low energy density presents storage and transport difficulties, requiring efficient and safe solutions. As demand grows, it’s imperative to scale up production using renewable energy sources to provide green hydrogen. Enhancing public awareness, establishing clear regulations, and international standards are also critical to ensure safety and successful integration into energy systems.
Advancements in hydrogen storage and transportation technologies have been significant. High-pressure compression allows substantial hydrogen volumes to be stored compactly, and this technology has been widely adopted. Cooling hydrogen to cryogenic temperatures turns it into a liquid, increasing storage density, which is especially useful in aerospace and industry. Hydrogen pipelines provide a viable option for long distance transportation, linking production and consumption areas. Furthermore, research on materials like metal-organic frameworks (MOFs) and carbon nanotubes shows promise for future storage solutions with their exceptional hydrogen adsorption capabilities. This review synthesizes recent progress and identifies future research pathways in hydrogen storage and transportation, aiming to bolster academic and industrial efforts to overcome these challenges.
2. Storage of Hydrogen
2.1. Compressed Gaseous Hydrogen Storage
Compressed gaseous hydrogen storage is the most common hydrogen storage method in hydrogen fuel cell vehicles or hydrogen-powered vehicles [7]. According to several different standards or codes belong to different association comes from field related to vehicle cylinder, the hydrogen container and its related technologies are developing rapidly, and the application range of pressure is up to 70 Mpa [8]. With the rapid development of materials science, there are about 5 types of tanks for compressed gaseous hydrogen storage which are shown in Table 1 [9]. Type Ⅴ tanks are also under development [10], while type Ⅲ and type Ⅳ are primarily used currently.
Type Ⅲ and Ⅳ are now widely circulated in fuel cell vehicle (FCV) applications. Their inner surface consists of metal and polymer liner overwrapped by various layers of carbon fiber composites at different orientations to form stack [
11]. These stacks provide required strength and stiffness to the tank. Sapre [
11] et al. designed a Type IV filament-wound tank with fibers bearing the primary load and the matrix maintaining their position and orientation. High-density polyethylene (HDPE) was used as a liner to contain hydrogen. Finite element analysis confirmed that the simulated burst pressure aligns well with experimental data, indicating safety. HDPE is most used material of type Ⅳ tank. Except that, the South Korea based ILJIN Co., Ltd launched a type Ⅳ tank which used polythene-clay nano-composite liner. Toyota Motor Corporation adopted nylon-6 (PA6) as the liner material of 70 Mpa compressed gaseous hydrogen (CGH2) tank [
12].
As the most direct application of CGH2 storage, the refueling of FCVs is complex due to high pressures and variable temperatures, posing safety concerns and resulting in longer refueling times than traditional vehicles.
Maus [
13] et al. reviewed the CGH2 filling procedures used by 9 car manufacturers, noting a largely standardized process. Initially, the high-pressure tank is connected to the vehicle to equalize pressure. The vehicle tank’s residual pressure and volume are then assessed to calculate a heating curve and the necessary final pressure for full refueling.
Kim [14] et al. conducted numerical parametric studies on on a 175 L hydrogen tank’s refueling. They found that initial super capacitor state of charge (SOC) pressure condition and the maximum temperature rise of hydrogen are interconnected. Refueling hydrogen vehicles is complex due to the difficulty in precisely controlling pressure and flow rates in the absence of accurate, real-time data from onboard tanks, in contrast to the simpler and more efficient process for gasoline and diesel vehicles. In conventional refueling, an automatic mechanism in the nozzle shuts off fuel flow when the tank is full. Achieving a similar level of control in hydrogen refueling requires extensive monitoring equipment both inside and outside the tank, with real-time data sharing between the vehicle and refueling station to ensure safety, efficiency, and optimal tank utilization.
2.2. Cryogenic Liquid Hydrogen Storage
Liquid hydrogen (LH2) is an efficient and clean fuel predominantly used in aerospace applications. Beyond aerospace, LH2 has broad potential applications in civilian and industrial sectors, marking it as a significant direction for the development of new energy technologies.
Hydrogen liquefaction is achieved by cooling below its critical temperature of 33.2 K and compressing it.
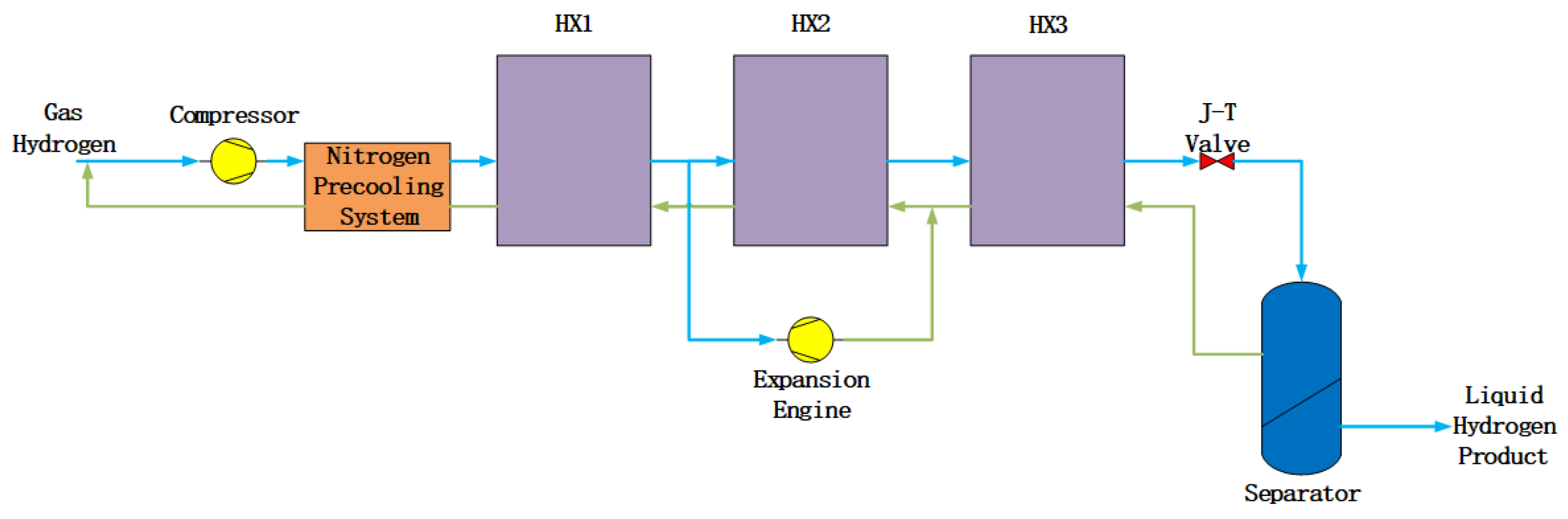
Claude cycles are widely adopted by large hydrogen liquefaction industry. Precooling systems are necessary, because single or muti-stage precooling can increase thermal efficiency and decrease energy consumption [15]. As showed in Figure 1, Nitrogen precooling Claude cycles have 4 main steps:
Compress hydrogen and remove compression heat
Precool with liquid nitrogen (80 K)
Cool gas hydrogen through the expansion engine (30 K)
Cool gas hydrogen again through the throttle valve to 20 K and finally liquefy
The CGH2 enters the nitrogen precooling system to nearly 80 K. Then it enters the cold box which contained 3 heat exchangers (HX) and an expansion engine to nearly 30 K. In order to guarantee the cooling efficiency, usually we will add an adsorption step between HXs to remove impurities, and o-p conversion sections will also be put in the HXs [
16]. Finally, it enters a Joule-Thompson valve (J-T valve) and undergoes an adiabatic expansion. The product temperature is between 20-23 K.
The storage of LH2 at cryogenic temperatures (20 K) imposes stringent requirements on the selection of materials for storage and transport containers, necessitating consideration of material adaptability in cryogenic environments, hydrogen embrittlement characteristics, mechanical properties, and thermal-physical performance—all critical for the safe and reliable design of LH2 containers.
Currently, stainless steel is the prevalent material choice for LH
2 storage and transportation due to its excellent low-temperature performance and resistance to hydrogen embrittlement [
17]. Different grades of stainless steel offer varying characteristics in terms of corrosion resistance, weldability, and cryogenic performance. For instance, the AISI 304 and 316 stainless steel are widely used in LH2 storage containers owing to their superior overall properties. However, material selection must be carefully evaluated based on the specific application requirements and cost-performance considerations.
With the growing demand for space LH2 storage and transportation, research into high specific strength cryogenic materials such as aluminum alloys, titanium alloys, and composite materials has been advancing [
18]. Aluminum alloys are especially favored in the aerospace industry for their light weight and satisfactory low-temperature properties. Titanium alloys are considered as one of the more promising materials for LH2 storage due to their superior strength-to-weight ratio and exceptional low-temperature mechanical properties. Composite materials, particularly carbon fiber-reinforced plastics (CFRP), have garnered widespread attention for their significant potential in weight reduction and efficiency enhancement. However, a primary drawback of composite materials is the issue of hydrogen permeation, which poses challenges for the sealing integrity and long-term stability of storage containers.
Although preliminary research has been conducted regarding the design of aerospace LH2 storage and transportation containers, as well as the mechanical properties and selection criteria of related materials, the civilian sector is nearly untouched, and the technological development is relatively behind. Therefore, engaging in systematic research to thoroughly understand the types and properties of cryogenic materials for LH2 storage and transportation containers is imperative to ensure the safety, reliability, and long-term operation of LH2 storage technologies.
2.3. Organic Liquids Hydrogen Storage
Organic liquids hydrogen storage involves two main steps: hydrogenation of hydrogen-lean molecules and dehydrogenation of hydrogen-rich molecules. There are two types of hydrogen-lean molecules: (1) Hydrogen-lean molecules that are extracted from atmosphere or exhaust gas mixtures, such as ammonia, methanol, and formic acid, which are also called ‘circular’ hydrogen carriers [
19]
. (2) Hydrogen-lean organic liquids, also called LOHCs [
20]
(Liquid Organic Hydrogen Carriers) are more potential for the safety and transport efficiency.
The development of organic liquids hydrogen storage is relatively late. It is characterized by its reversible hydrogenation reactions, high hydrogen storage density, and safety in storage and transport, rendering it advantageous for long distance transport. Additionally, LOHCs are compatible with existing hydrocarbon infrastructure, including pipelines and fuel stations.
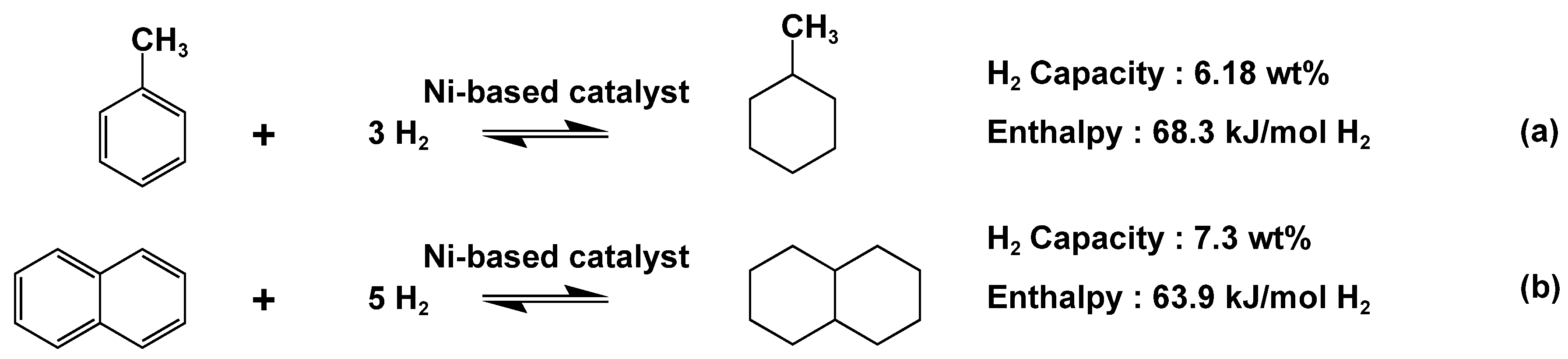
Toluene hydrogenation reaction is a widely used reversible reaction that involves reacting hydrogen gas with liquid toluene to produce liquid cyclohexane and hydrogen gas, thereby enabling the storage and hydrogen gas. The equation of Toluene hydrogenation reaction and Naphthalene hydrogenation reaction are shown in Figure 2. [
20]
Several challenges exist within the toluene hydrogenation process. High temperatures and pressures are requisite, complicating and elevating the system’s cost. Additionally, the reaction generates toxic by-products like benzene, raising concerns about sustainability and safety. A deeper grasp of the reaction’s kinetics and thermodynamics is essential. Some advancements on catalysts has been reported recently. Wang [
21] et al. reported a RuNi/TiO2 catalyst that effectively hydrogenates toluene to methylcyclohexane using crude hydrogen containing 1000-5000 ppm CO at approximately 180 ℃ and ambient pressure. The Ru-Ni interaction during the reduction process promoted the formation of highly dispersed Ni species, which is supposed to be the main active center for CO-tolerant hydrogenation action. The non-competitive adsorption of CO and toluene on Ru and Ni supports a new route for achieving the goal of crude hydrogen storage. Taiki Hashimoto [
22] et al. also demonstrated that LOHCs can potentially be used for H2 purification from CO- and CO2-rich crude H2 in addition to their well-established use in H2 storage.
Perhydro-dibenzyltoluene (18H-DBT) is considered one of the most popular LOHCs currently available. It exhibits low volatility and reactivity under ambient pressure and temperature conditions, and its dehydrogenation products pose relatively low environmental and health risks. 18H-DBT boasts a theoretical hydrogen storage capacity of 6.2 wt%, and its enthalpy of dehydrogenation (65.4 kJ/mol) is slightly lower than that of methylcyclohexane (
Figure 2(a)). However, both the hydrogenation and dehydrogenation processes of 18H-DBT tend to be incomplete. Currently, the most used catalysts for dibenzyltoluene are platinum-based catalysts adsorbed on a proper support which is mainly Al2O3 [
23].
Naphthalene (
Figure 2(b)) and N-ethylcarbazole have also been studied. Their primary issues revolve around safety and economic viability. However, if they are utilized as components of a mixed LOHC system, it may potentially mitigate these concerns to some extent and enhance hydrogen storage performance [
24]. Perhydro-N-ethyl carbazole (12H-NEC) offers unique advantages as an LOHC, particularly in terms of safety, environmental friendliness, and the utilization of existing infrastructure. However, challenges such as high-temperature and pressure dehydrogenation, catalyst costs, and system complexity need to be considered. As reported in this system, Pd supported on alumina was found to be effective as compared to Pt, Rh, and Ru [
25].
Zou [
26] et al. researched a hydrogen storage system based on ethylene glycol with a theoretic maximum content of 6.5 wt% hydrogen storage capacities, using a ruthenium pincer complex. Dong [
27] et al. investigated 2,3-dimethylindole (2,3-DMID), a candidate with a hydrogen storage capacity of 5.23 wt%. 2,3-DMID and the hydrogenated product 8H-2,3-DMID are not toxic to humans and environment, and the reaction conditions are relatively mild. 1,2,3,4-tetrahydroquinoline [
28] is also potential materials.
Future research in the field of LOHCs is driven by the pursuit of systems with enhanced hydrogen storage capacity. The aim is to identify materials that not only store hydrogen more densely but also exhibit improved kinetics for rapid hydrogenation/dehydrogenation cycles. Additionally, there is a push to develop advanced catalysts for LOHCs that facilitate hydrogen release at lower temperatures, thereby increasing energy efficiency. The longevity and reusability of LOHCs are also critical, necessitating compounds that remain stable over multiple cycles. Moreover, safety and minimal environmental impact are paramount, with a focus on non-toxic, non-flammable, and environmentally friendly LOHCs. The practical utility of these carriers hinges on their actual hydrogen storage capacity (wt%), which will likely be a focal point in optimizing the LOHC system’s properties for economic viability in commercial settings. Molecular simulations can be utilized to predict, design, and discover new MOFs. Additionally, high-throughput computational screening and machine learning are extensively applied, significantly aiding the development of hydrogen storage in MOFs [
29].
2.4. Solid Materials Hydrogen Storage
Currently, the most popular solid hydrogen storage materials include categories represented by magnesium hydride, sodium borohydride, and ammonia borane.
Since the 1960s, the development of metal hydride materials has diversified, encompassing elements such as titanium, zirconium, magnesium, and various rare earths. Selection criteria primarily include acquisition costs, hydrogen storage capacity, and hydrogen release temperature. Magnesium-based materials are particularly notable due to their low molecular weight, yielding a high gravimetric hydrogen storage capacity, and magnesium’s abundance in the earth’s crust, which potentially lowers material costs. However, a significant limitation of magnesium-based materials is their high hydrogen release temperature, typically exceeding 300 ℃.
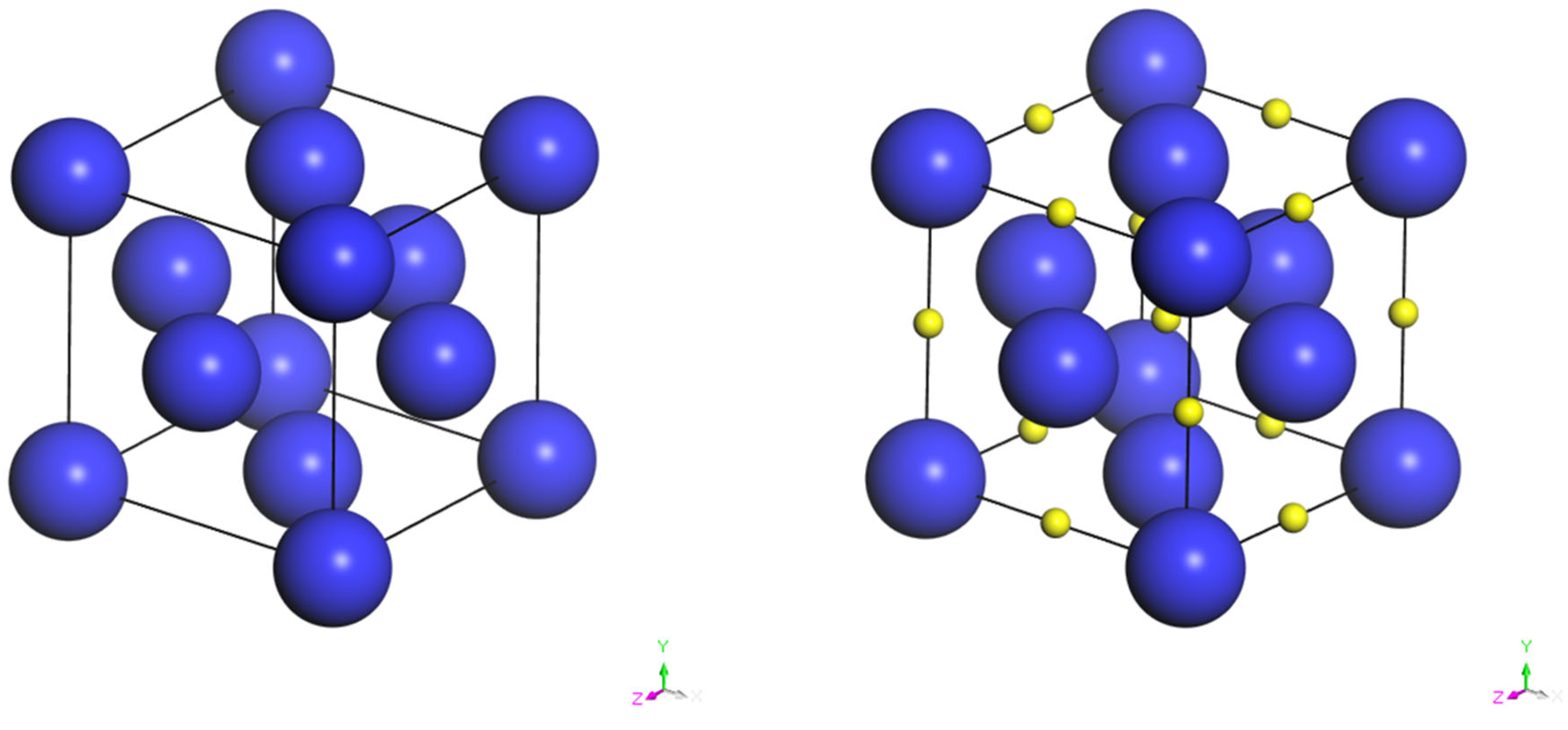
Figure 3 presents a simplified model of the Mg crystal structure to elucidate the hydrogen storage mechanism.
Table 2 presents the hydrogen desorption behavior of selected Mg-transition metal alloys [
30].Certainly, apart from magnesium, hydrides based on other metals also exhibit significant hydrogen storage capabilities under suitable conditions.
Table 2.
Hydrogen absorption and desorption behavior of partial Mg-transition metal alloys. [
30].
Table 2.
Hydrogen absorption and desorption behavior of partial Mg-transition metal alloys. [
30].
| Alloy composition (atomic%) |
Hydrogen content (wt%) |
Hydriding |
Hydrogen released (wt%) |
Dehydriding |
| |
|
Pr. (atm) |
Temp. (℃)
|
|
Pr. (atm) |
Temp. (℃)
|
| Mg2Ni |
3.6 |
25 |
300 |
- |
- |
- |
| Mg-Mg2Ni |
5.7 |
25 |
350 |
- |
- |
- |
| Mg2Cu |
2.7 |
30 |
300 |
- |
- |
- |
| Mg-Mg2Cu |
6.6 |
30 |
300 |
- |
- |
- |
| Mg2Fe |
5.4 |
20-120 |
450-520 |
- |
- |
- |
| Mg-Mg17Y13 |
5.0 |
10 |
400-450 |
4.5 |
3.0 |
320 |
| Mg-1Y |
4.5 |
56 |
400 |
4.0 |
1.0 |
300 |
| Mg-5Y |
7.0 |
56 |
400 |
3.4 |
1.0 |
300 |
| Mg-5Mn |
6.0 |
56 |
400 |
1.5 |
1.0 |
300 |
| Mg-5Co |
2.0 |
56 |
400 |
0.0 |
1.0 |
300 |
| Mg-1Ag |
5.7 |
56 |
400 |
2.0 |
1.0 |
300 |
| Mg-5Ag |
5.3 |
56 |
400 |
0.0 |
1.0 |
300 |
| Mg-1Ag-1Y |
6.0 |
56 |
400 |
- |
- |
- |
| Mg-1Ag-1Y |
6.3 |
56 |
400 |
- |
- |
- |
| Mg-5Ni-5Y |
5.2 |
56 |
400 |
3.1 |
1.0 |
300 |
| Mg-5Al-5Y |
5.0 |
56 |
400 |
3.1 |
1.0 |
300 |
| Mg-10Al-10Y |
4.1 |
43 |
400 |
- |
1.6 |
310 |
| Mg-34Al-10Y |
3.6 |
43 |
400 |
- |
2.2 |
310 |
| |
|
|
|
|
1.0 |
286 |
| Mg-10Cu-5Ni-0.5Y |
3.7 |
21 |
400 |
- |
2.0 |
310 |
| |
|
|
|
|
1.5 |
299 |
| Mg80Ag15Al5 |
1.7 |
|
|
|
2.2 |
300 |
| Mg85Ag5Al10 |
3.8 |
|
|
|
2.6 |
300 |
| Mg90Ag7.5Zn2.5 |
4.2 |
|
|
|
2.8 |
300 |
| Mg78Ag16.5Zn5.5 |
2.5 |
|
|
|
2.8 |
300 |
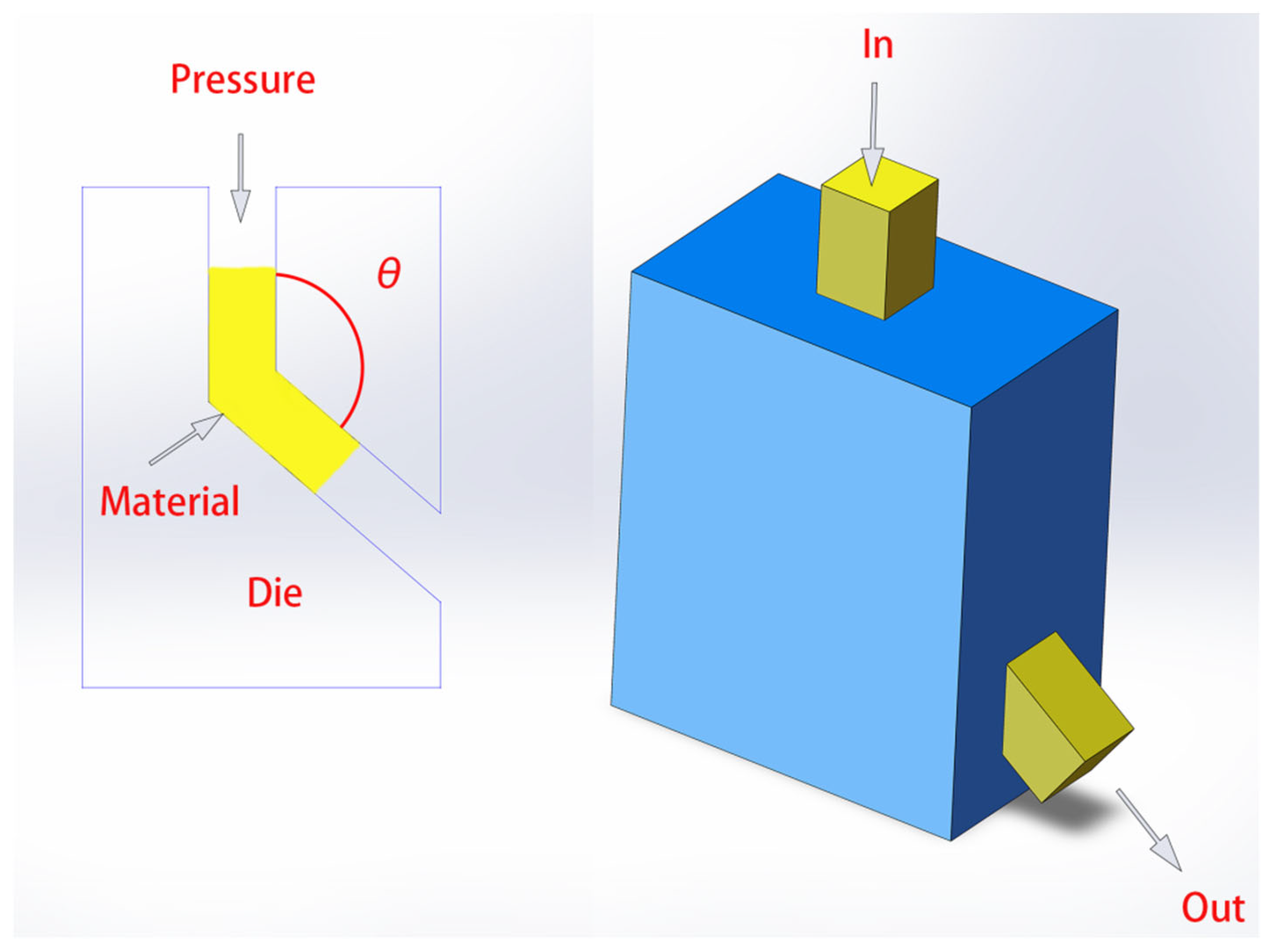
Variations in microstructure also have a significant impact on hydrogen storage performance. Consequently, technologies that can precisely control the microstructure of materials have become particularly important. The equal-channel angular pressing (ECAP) [
31,
32,
33] [
34] technique has long been applied in this field, as illustrated in
Figure 4. The surface area, grain size, and texture of the material have been effectively controlled. Furthermore, other methods like ball inert gas condensation, [
35], ball milling [
36], high-pressure torsion [
37], accumulative roll bonding [
38], crystallization [
39], and electrolytic deposition [
40] may also contribute to this field.
Sodium borohydride (NaBH4) is a relatively inexpensive hydrogen storage material that is stable at room temperature, which significantly facilitates storage and transportation. Its theoretical hydrogen storage capacity reaches 10.7 wt%, substantially exceeding that of most metal hydrides. However, the release of hydrogen from sodium borohydride is a multi-step process that requires high temperatures and pressures to liberate a significant portion of hydrogen. Additionally, the recycling and regeneration of its by-products are relatively complex. Currently, various new strategies such as nanoengineering, catalysis, destabilization additives, and chemical modifications are being employed to overcome the thermodynamic and kinetic limitations of NaBH4 thermal decomposition [
41,
42,
43]. Hydrolysis is an effective resolution, but self-hydrolysis of NaBH4 without any catalyst is very sluggish [
44]. In contrast, lithium borohydride (LiBH4) has a higher theoretical hydrogen storage capacity but its development and application are greatly limited by the high cost of lithium-based materials, poor thermal stability, and certain toxicity and corrosiveness.
Ammonia borane (NH3BH3, AB) possesses a notably high hydrogen content of approximately 19.6 wt%, and under dry and cool conditions, it remains relatively stable [
45,
46]. This stability makes it an exceptionally effective medium for hydrogen storage, suitable for long-term storage and transportation. However, the synthesis of AB involves relatively high costs and technical demands, making it unsuitable for cyclic processes. Neat AB decomposes more than dehydrogenates. It follows a complex mechanism, producing a variety of reaction intermediates, volatile products, and polymeric residues. Consequently, certain strategies are required to destabilize AB, thereby favoring and enhancing the selectivity of the dehydrogenation reaction. These strategies primarily include four categories: catalysis, doping, nanostructural adjustments, and solubilization in an aprotic solvent [
47].
The primary focus of current research is the identification of potential materials that offer both high hydrogen storage capacity and safety, with non-toxic characteristics. In this regard, metal hydrides and borohydrides, including their derivatives, which have lower atomic numbers, show considerable advantages. Subsequently, the reduction of desorption temperatures and the enhancement of decomposition rates are pursued through catalysis, doping, and microstructural modifications. Finally, the refinement of the recycling processes is essential for reducing costs. In fact, the theoretical maximum hydrogen storage capacity is almost never achieved, as the use of catalysts and dopants inevitably reduces the storage capacity, but these modifications are necessary for enhanced hydrogen storage performance. In some cases, to lower costs and improve the process cycle, it is not feasible to solely pursue high hydrogen storage capacities. For example, the complete dehydrogenation of AB results in the formation of stable boron nitride, which is highly detrimental to recovery.
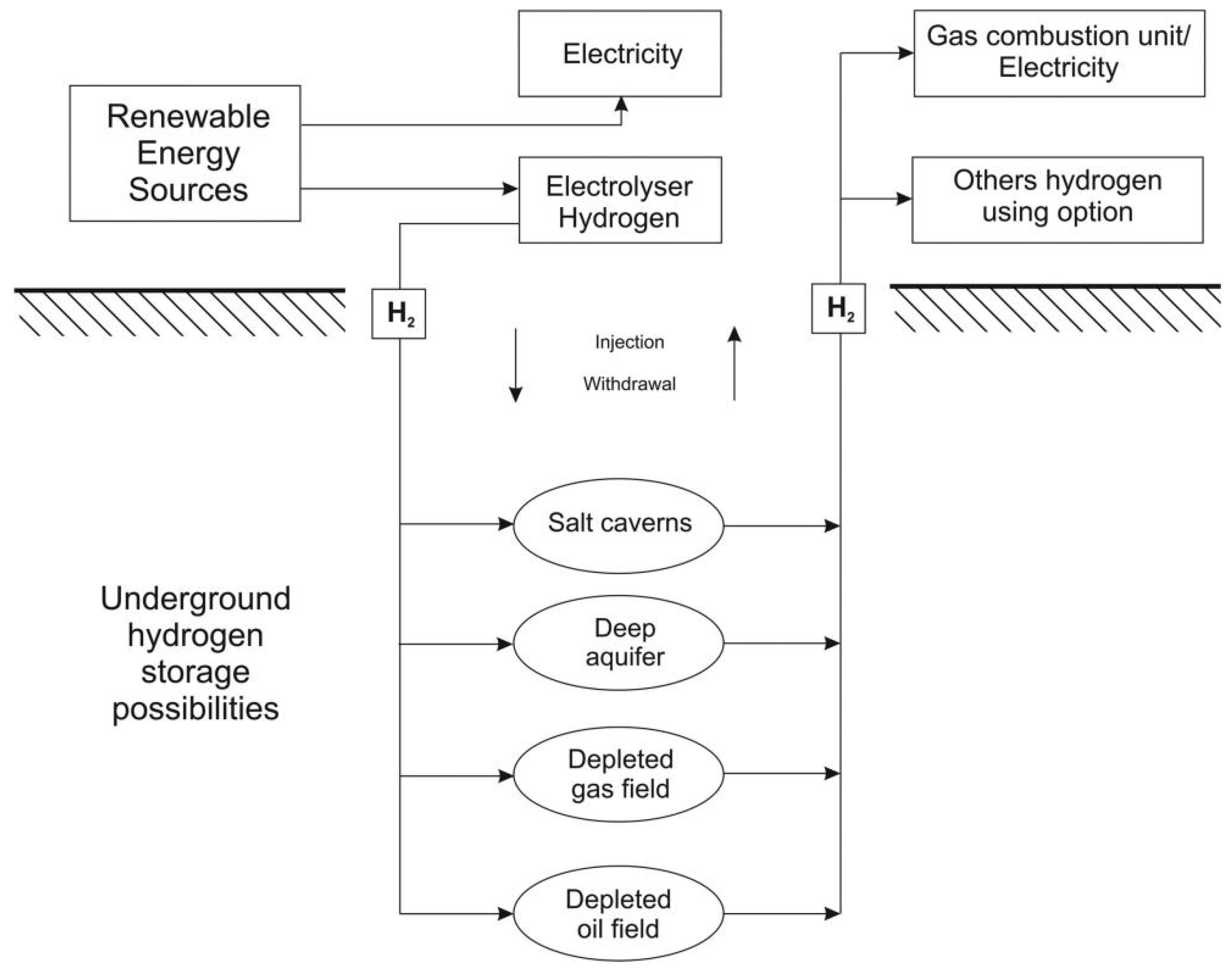
2.5. Underground Hydrogen Storage
Underground hydrogen storage (UHS) is a technique that involves storing hydrogen gas in underground reservoirs or salt caverns. It is considered a potential solution for hydrogen energy storage and dispatchability, as hydrogen gas has a large volume at ambient conditions and requires high-pressure or cryogenic storage to meet energy demands. Its primary goal is to store surplus electricity generated from renewable sources, converting it into hydrogen and releasing it for energy use when needed. UHS facilities are capital-intensive investments, so the economic viability will be affected by the cost of electricity for water electrolysis and expenditure on the construction of storage sites [
48,
49].
Currently, three primary UHS methods exist: storage in depleted gas/oil fields, aquifers, and salt caverns. Gas composition and hydrogen purity necessitate careful geological screening; for instance, depleted oil fields may be unsuitable for storing high-purity hydrogen due to potential reactions with residual oil. Storage in deep aquifers requires suitable reservoir properties and an impermeable cap rock [
50].
Figure 5 shows a typical UHS system model [
50]. Salt caverns offer a chemically stable environment with salinity that limits microbial hydrogen consumption. Storage capacity in these caverns increases with depth due to higher pressure, but this also escalates pipeline costs. At shallower depths, less buffer gas is needed [
51]. UHS primarily aims to minimize costs. Capital costs overall are mainly comprised of site selection, gas separation, risk assessment, exploration of bacterial activity, injection and withdrawal periods, infrastructural, and labor costs [
52]. Despite its advancement, UHS is not yet widely adopted and requires further technical and financial investment.
2.6. Metal-Organic Framework Hydrogen Storage
Metal-organic framework materials (MOFs) are porous materials with a periodic network structure formed by the assembly of metal ions and organic ligands through coordination interactions. In MOFs area, metal ions typically serve as nodes or central points, while organic ligands generally function as linkers. This possesses larger specific surface area (showed in
Table 3) and higher porosity compared to traditional porous materials. Additionally, the presence of organic components in these materials allows for designability, tailorable properties, adjustable pore sizes, and facile surface functionalization. In many domains including gas adsorption separation, water purification treatment [
53,
54], catalytic applications [
55,
56], electrode materials [
57,
58], and biomedical applications [
59], MOFs exhibits excellent performance.
Table 3.
Specific surface area of common adsorbent materials.
Table 3.
Specific surface area of common adsorbent materials.
| Materials |
Specific surface area (m2/g) |
Ref. |
| Activated carbon |
500-3000 |
[60,61,62]
|
| Carbon fibre |
50-1500 |
[62,63]
|
| Graphene |
400-2500 |
[64,65]
|
| MOFs |
1000-10000 |
[66,67]
|
While MOFs have been extensively studied for gas storage, their application in hydrogen storage still faces challenges. The principle behind using MOFs for hydrogen storage lies in their highly porous structure, which enables the adsorption and storage of hydrogen molecules. MOFs typically possess a large number of micropores and mesopores, providing a high specific surface area that enhances the contact between hydrogen molecules and the MOFs, facilitating hydrogen adsorption.
The hydrogen storage mechanism in MOFs can be categorized into physical adsorption and chemical adsorption. Physical adsorption primarily relies on intermolecular forces, and hydrogen molecules interact with the surface of MOFs materials through van der Waals forces, getting adsorbed and stored in the pores. Chemical adsorption is achieved through chemical bonding, which involves electron sharing or electron transfer similar to chemical reactions. Therefore, chemical adsorption generally exhibits stronger bonding forces compared to physical adsorption.
To enhance the hydrogen storage performance of MOFs, researchers have explored various improvements. Pressure and temperature have significant effects on MOFs, and within a certain range, lower temperatures and higher pressures can increase the hydrogen storage capacity [
68].
Table 4 shows Characteristics of different MOFs [
68]. Additionally, altering the choice of metal ions or organic ligands in MOFs can influence their hydrogen storage performance. Furthermore, researchers have also attempted surface modifications, doping, or structural designs to improve the hydrogen storage properties of MOF materials [
69]. Compared to other hydrogen storage methods, MOFs have also demonstrated their hydrogen storage capabilities. Jaramillo [
70] et al. achieved densities greater than that achievable with CGH2 at 700 bar using vanadium (II)-dihydrogen complexation in the framework V2Cl2.8 (btdd). The economic feasibility of utilizing MOFs in hydrogen fuel cells has also been verified [
71]. Generally, the application of moderate pressure and temperature conditions holds great promise for enhancing hydrogen storage in MOFs.
Table 4.
Characteristics of different MOFs [
68].
Table 4.
Characteristics of different MOFs [
68].
| Categories |
Advantages |
Disadvantages |
MOF catalysts
MOF hybrids
MOF with different metal centres
Metal ion doping in MOF |
High surface area, porosity and hydrogen storage capacity;
high stability;
tenabilty.
Enhanced surface area, porosity and hydrogen storage capacity;
enhanced loading capacity, high controllability.
Good binding energy; enhanced stability.
High hydrogen storage capacity. |
Low stability, low active metal centres.
Expensive linkers;
poor stability;
lack active centres.
Formation of interconnected pores;
low corrosion resistance.
Steric effect;
inert metals have inadequate stability. |
3. Transportation of Hydrogen
In addition to hydrogen storage, hydrogen transportation plays a crucial role in the entire hydrogen supply chain. Due to the complex physicochemical properties of hydrogen, any process involving hydrogen requires careful handling. Compared to storage, hydrogen transportation occurs in a less stable environment, necessitating prioritization of safety considerations. Furthermore, the cost of hydrogen transportation determines the feasibility of widespread hydrogen adoption.
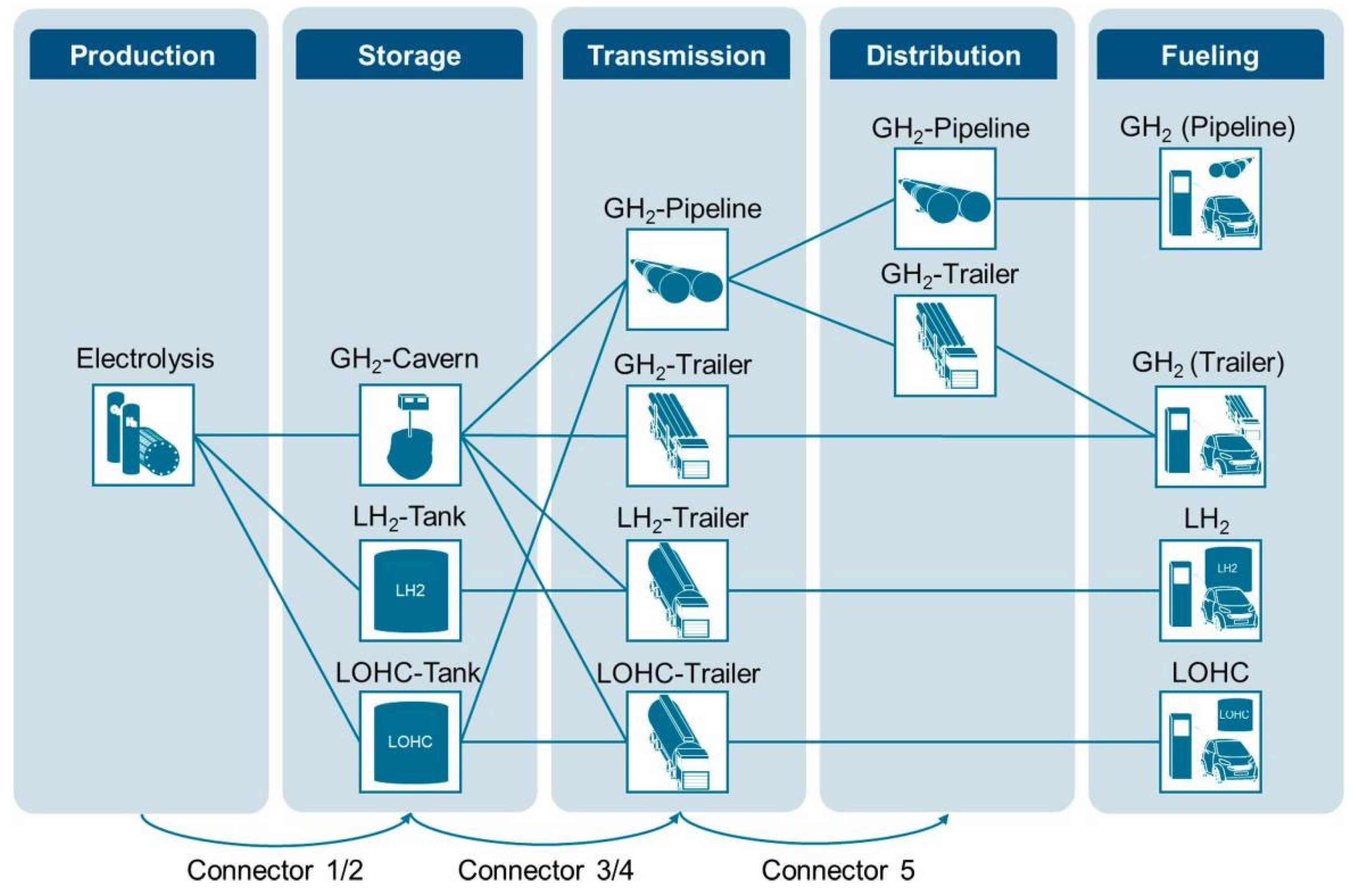
Given the diverse properties of hydrogen in different states, it is important to carefully select the appropriate transportation methods that align with these characteristics. In this regard, we can draw inspiration from hydrogen storage and make appropriate improvements to the transportation processes. Hydrogen is transported in two main ways, by road using trucks and trailers, and by pipeline. Rail transport is also considered a form of road transportation. However, due to its similarities with truck trailer transport in terms of hydrogen transportation principles and economic effects, it will not be separately discussed. Meanwhile, the transportation of MOFs will also not be discussed here because they are still at experimental stage. Different hydrogen transport modes and process models are shown in Figure 6 [72].
3.1. Road Transportation
Due to the limited load and capacity of trucks and trailers, two factors have a significant impact on the cost and energy consumption of transportation: the proportion of hydrogen in the total payload of a vehicle and the energy density. Our efforts are focused on maximizing the hydrogen storage capacity per unit volume and the weight of the container. However, these considerations are contingent upon the hydrogen storage method.
For high-pressure transportation, hydrogen is transported through trucks or trailers using gas cylinders under pressure in the range of 20 to 50 Mpa. It is more common to use Type I or Type II tanks (Table 1) with storage pressures below 30 Mpa. Horizontal hydrogen storage tanks are typically placed together on trucks or trailers, while vertical hydrogen storage tanks (hydrogen cylinders) are packaged and secured in metal frames before being placed on trucks or trailers. Figure 7 (a) shows the process of transporting CGH2 [
76].
In most cases, it is considered highly desirable for the weight of hydrogen to constitute 2% of the total payload weight [72]. To increase the amount of transported hydrogen, lighter tank materials that can operate at higher pressures should be manufactured. Type Ⅲ (30-70 Mpa) and type Ⅳ (>70 Mpa) will also be gradually applied in transportation. The filling and venting processes of high-pressure hydrogen tanks require special attention. Temperature and pressure variations should not occur too rapidly and should be controlled within appropriate ranges to prevent hazardous situations.
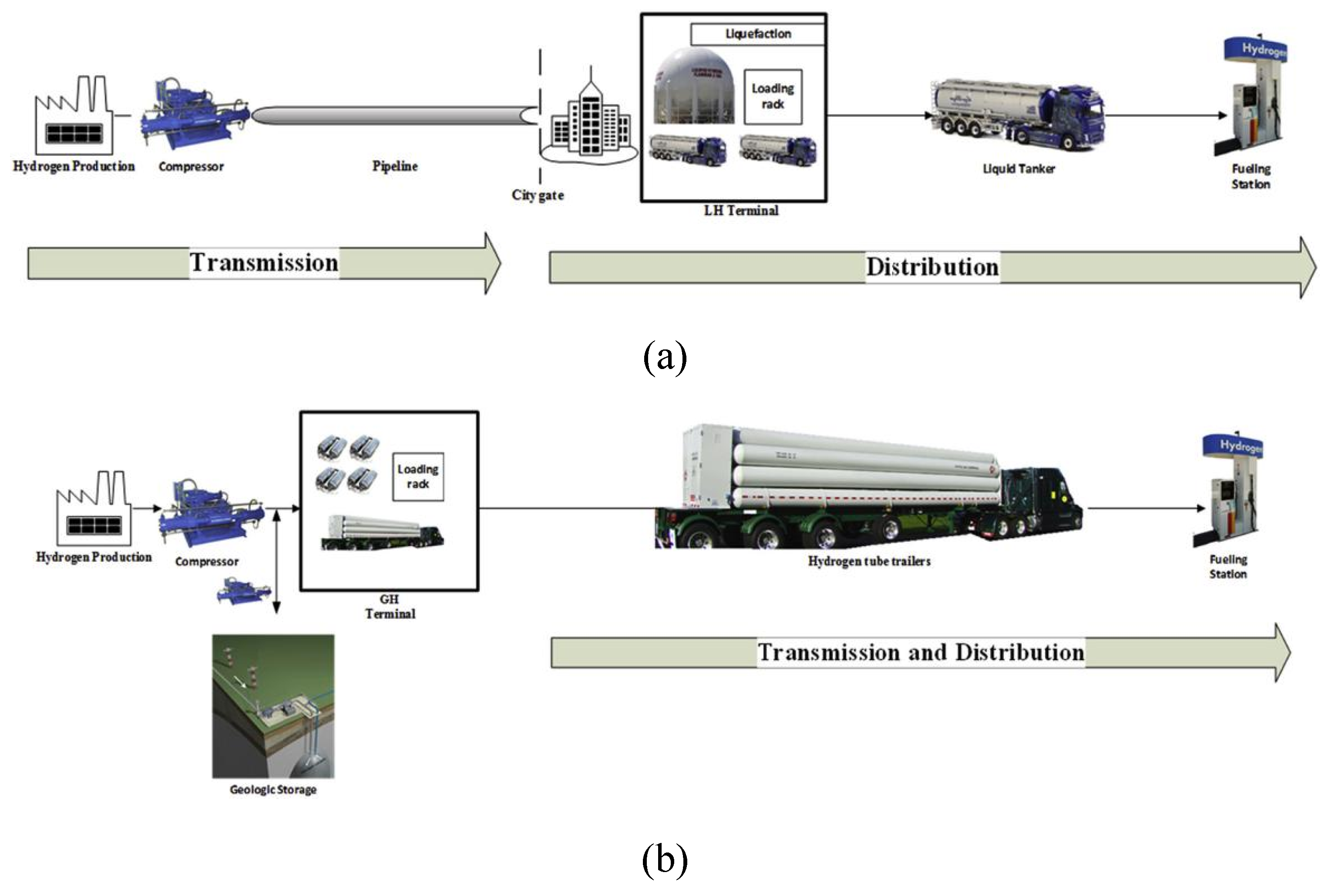
For cryogenic liquid hydrogen, the structure of the tank typically consists of a double-walled container, where the inner wall is used to store the LH2 and the outer wall provides insulation and protection. The inner wall is typically made of materials such as stainless steel or aluminum alloy to ensure the sealability and corrosion resistance of the LH2. The outer wall is composed of insulation materials and protective layers to minimize heat transfer and external environmental effects. When transporting LH2 tanks, it is essential to securely fasten them to prevent tank movement and collisions. Adequate protective measures, such as protective covers or structures, should be employed to safeguard the tanks from external damage. In the LH2 transportation mode shown in Figure 7 (b) [76], we can see a similar structure.
As previously mentioned, cryogenic liquid hydrogen can achieve a higher energy density, allowing for a greater amount of hydrogen to be transported compared to CGH2 trailers (approximately 10 times). A cryogenic LH2 trailer can carry 50000-60000 L, equivalent to 4000 kg of hydrogen in a single trip. Therefore, the cost of transporting LH2 is significantly lower than the cost of transporting CGH2. During the liquefying process, approximately 40% of the energy is lost [74], significantly increasing the overall cost. Additionally, the evaporation or boil-off of LH2 results in the loss of stored hydrogen. If the total transportation distance is long enough, LH2 transport can offer better economic benefits. On the other hand, if the total transportation distance is short, CGH2 would be more economical. 3.2. Pipeline Transportation
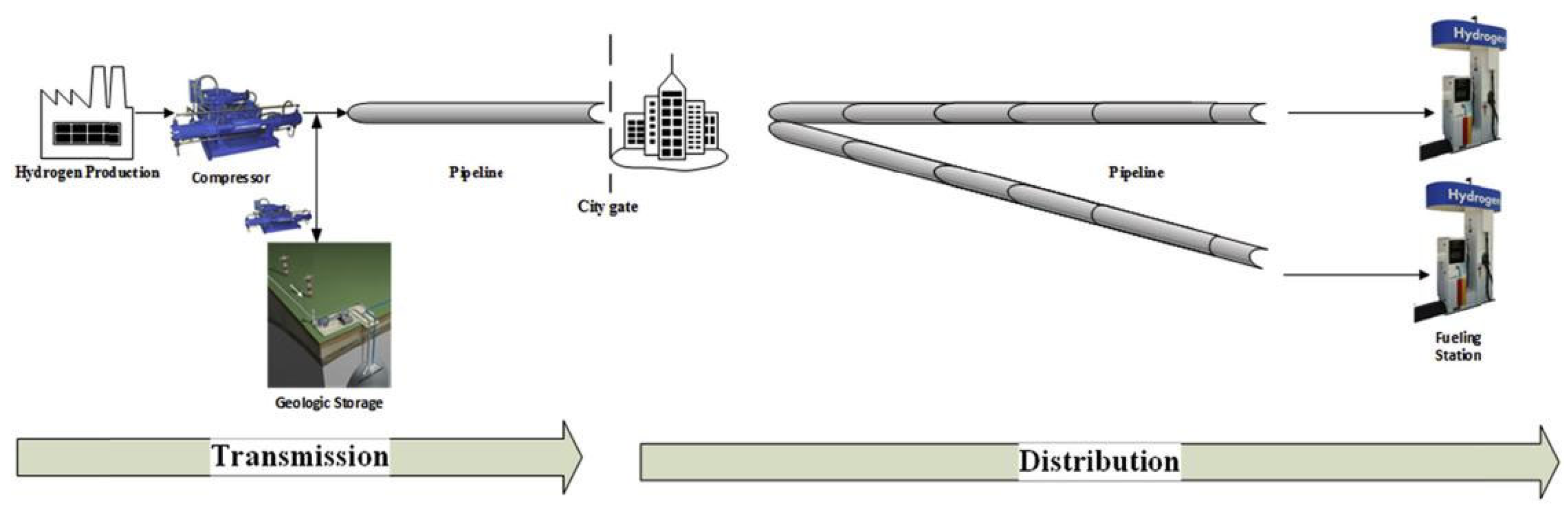
Compared to road transportation, pipeline transportation is a relatively low-cost option for conveying large quantities of hydrogen gas [75]. If hydrogen energy is to become a viable alternative to fossil fuels in the future, the construction of large-scale hydrogen pipeline infrastructure is essential [76]. However, the existing hydrogen pipeline infrastructure is relatively small in scale and primarily operated by large commercial hydrogen suppliers. These pipelines are predominantly dedicated industrial pipelines supplying hydrogen to refineries and chemical plants. Figure 8 shows the schematic diagram of hydrogen transportation by pipeline [76].
The proposal of blending hydrogen gas with natural gas and transporting it through natural gas pipelines has received significant attention in recent years [78]. Natural gas pipelines have been widely constructed and operated worldwide, with mature technology and broad coverage. Utilizing existing natural gas pipeline infrastructure for hydrogen transportation can reduce the cost and time of constructing new pipelines, improve the efficiency of hydrogen energy infrastructure development, and lower the cost. Technically, natural gas pipelines share similarities with hydrogen transportation, such as pipeline conveyance and high pressure. Although hydrogen has smaller molecular size, appropriate modifications and technological measures can enable natural gas pipelines to meet the requirements for hydrogen transportation.
Blending hydrogen into natural gas poses several challenges. Theoretically, natural gas and hydrogen should be in a completely blended state during transportation. However, in reality, significant variations occur due to the differences in their physical properties. Blended gas tends to stratify, particularly at lower speeds, higher pressures, lower temperatures, and higher hydrogen blending volume fractions. Additionally, stratification is more likely to occur in uphill sections of pipelines compared to downhill sections [79]. The concentration gradient resulting from stratification can lead to a dangerous concentration of hydrogen gas, accompanied by significant safety hazards. At the same time, the accumulation of hydrogen gas can intensify localized hydrogen embrittlement (HE) failure [80] and reduce the service life of pipelines.
The United States and Europe were the first to establish and deploy hydrogen pipeline networks. Currently, the total length of hydrogen transportation pipelines worldwide has exceeded 5,000 km, with the United States having the largest number of hydrogen pipelines, totaling over 2,700 km. Europe’s hydrogen transportation pipeline networks has also reached 1,770 km. China's total length of hydrogen transportation pipelines is approximately 400 km [81].
3.3. Seaborne Transportation
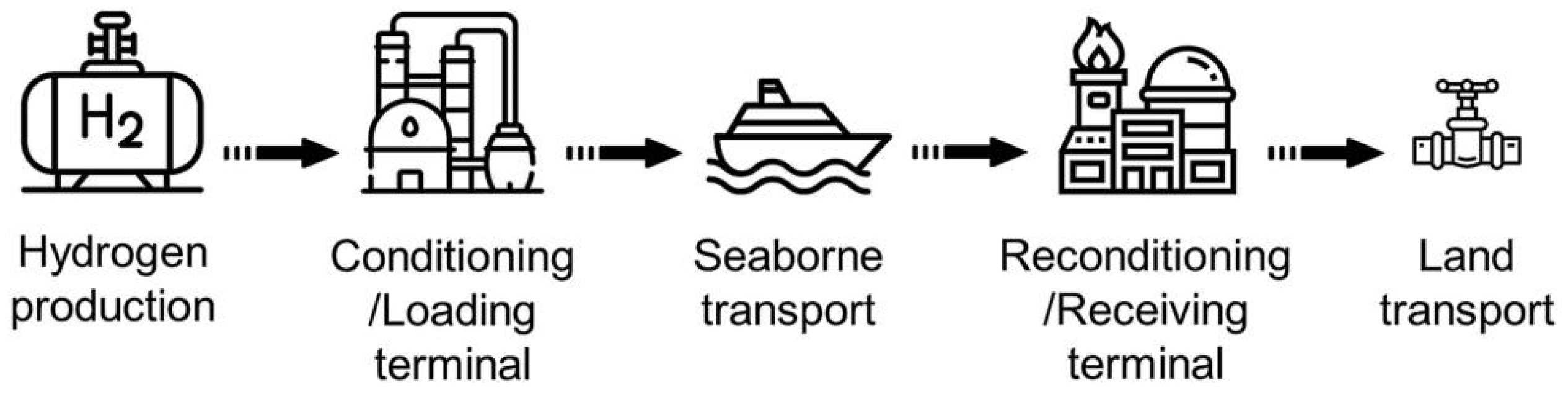
Generally, hydrogen is transported domestically through two mainstream methods: pipeline transportation and road transportation. However, long distance oversea transportation of hydrogen is usually carried out by ocean vessels. Similar to the common transportation of petroleum and liquefied natural gas, the physical and chemical storage of LH
2 has economic benefits for long distance oversea transportation due to its small footprint and the completeness of transportation infrastructure [
81]. In addition to traditional transportation of LH2, LOHCs and MOFs are also considered in oversea transportation for the future. For the current stage, the challenge of transporting LH2 lies not in the distance of transportation, but in the complexity of storage. Compared to CGH2, LH2 has a higher density of 71 kg/m3 and an energy content of up to 2.4 kWh/L. In addition to its high liquefaction energy, the main disadvantage is that LH2 is transported at 20 K, which requires special cryogenic containers to maintain low temperatures to minimize evaporation processes and ensure safety measures [
83].
For countries importing renewable energy, seaborne transportation is a very important option. Compared with long-term utilization and high dependence on natural gas pipeline networks, different countries seek more flexible options (e.g. Russia to Germany, Algeria to Spain and Portugal).
Figure 9 shows the schematic diagram of the utilization of hydrogen transported by sea to the land
[84]. In addition, due to safety concerns, the use of mixed hydrogen/natural gas pipeline transportation is limited. Different countries have different advantages and disadvantages. For example, in Asia and Europe, some countries have abundant energy resources and geographical advantages, while others have high technological and economic capabilities. The two can complement each other. Therefore, if an international hydrogen transportation system is established, it can solve the energy problem for both sides and even countries along the route [
85]. Roos [
86] studied the production, storage, and transportation of green hydrogen from South Africa to Japan and Europe, and calculated the possible roadmap scenarios for 2050.
4. Life-Cycle Cost (LCC) Assessment
Firstly, the hydrogen production from coal and natural gas contributed to approximately 93% of the Global output in 2020, with half being produced in China, United States, and Europe [
87]. Given the global landscape that hydrogen now faces, there is an urgent need for a detailed economic analysis of hydrogen systems, focusing on changing levelised costs and the impact of technological innovations.
The life-cycle cost (LCC) is used here to assess hydrogen transportation. In addition, the economic analysis used in this study is based on the LCC of hydrogen transport from a builder's perspective.
The overall LCC of hydrogen transport is measured as a net present value cost evenly distributed per kilometer (km), as shown in equation (1) and (2):
Where LCC, Cp, Cc, Cs, Ct, Cd, M and T represent transport cost per kilometer in the whole life cycle, sum of all costs, compression process cost, storage cost, transport cost and distribution terminal cost respectively. M is the proportion of annual maintenance cost to total cost; T is the life cycle of the project, in years; r is the discount rate, which calculates the present value of future costs. According to the above formulas, the present value of the total cost of hydrogen transportation can be obtained in this study, and we can use this result as an indicator to evaluate the economic feasibility of hydrogen transportation solutions.
Pipeline transportation is a good solution for long-distance H
2 transportation [
88]. One major advantage of pipeline transportation is the ability to refurbish existing natural gas pipelines to transport LOHC and even pure hydrogen gas [
89]. Generally speaking, the pressure of hydrogen gas decreases after passing through pipelines, and it needs to be re-compressed at regular intervals. The hypothetical calculations for the pipeline are shown in the
Table 5. Due to the continuous maintenance of compression devices and pipelines, it is assumed that 5% of the initial GHG emissions generated by pipeline construction each year will be used for maintenance work [
90].
Table 5.
Pipeline modeling assumptions.
Table 5.
Pipeline modeling assumptions.
| Parameter |
Assumption |
Unit |
| Pipeline maintenance |
5 |
%/annual |
| Hydrogen mass flow |
35 |
ton/day |
| Pipeline length |
100 |
km |
| Recompression |
0.02 |
kWh/kg |
Table 6 already lists assumptions about tank type, volume, weight, and storage capacity. Truck transportation of hydrogen gas is generally divided into two types: CGH
2 and LH
2, their common feature is that they all need to be compressed before loading. The energy consumed by compression mainly depends on the state of hydrogen gas, such as pressure, temperature, etc., among which the main factor is pressure. Generally speaking, compressing 1 kg of hydrogen gas to 200 bar requires 0.7 kWh of electricity, and compressing to 250 bar requires 0.9 kWh/kg of electricity [
87]. The electricity it requires mainly comes from the public power grid. In addition, when CGH2 is discharged from the steel cylinder, there is still a small amount of residual hydrogen gas in the transportation unit, accounting for approximately 5% [
91]. Therefore these can reduce the net content required for transporting hydrogen gas.
In summary, approximately 1000 kg of CGH2 can be transported to the refueling station during each transportation. If we assume a daily hydrogen demand of 1800 kg/day, then we need to transport hydrogen at least twice a day to ensure the smooth operation of the supply chain.
Table 6.
Tank modeling assumptions [
72].
Table 6.
Tank modeling assumptions [
72].
| Tank type |
Volume
(L) |
Pressure
(bar) |
H2-Capacity
(kg) |
Tank Tare Weight (kg) |
| Steel cylinder container (SC) |
23800 |
200 |
400 |
26298 |
| Steel tubes (ST) |
19292 |
200 |
324 |
27254 |
| Composite super light container |
45500 |
250 |
957 |
78854 |
| Composite (TITAN V) trailer |
44200 |
250 |
979 |
21810 |
There are two types: LH
2 and LOHCs. However, the use of LOHCs to transport hydrogen to the destination requires further processing, and it is nearly devoid of commercialization and application. The current international push for LOHC transport comes from Europe's Orion Project, which aims to achieve a huge annual capacity of 32 TWh blue/green H2 and has the longest project duration until 2050 and beyond, but it is somewhat doubtful whether the project will live up to its expectations [
92]. So it is not within the scope of this article’s discussion. The estimated LH2 quantity for each transportation supply task is approximately 4000 kg. The transportation capacity of a truck is 4500 kg of LH2, of which approximately 5% of the capacity cannot be fully utilized [
93]. A standard hydrogen refueling station needs to provide 1500 kg of hydrogen gas per day, approximately every two to three days. Set the distance from the hydrogen production plant to the hydrogen refueling station as 100 km. The truck left with a full load and when it returned, the fuel tank was empty. In each case, the vehicle under consideration is a 40 ton freight truck that meets Euro 5 emission standards [
72]. Calculate the transportation cost of LH2 based on the above assumptions. In this study, we assume a capacity of 1200 kg/day for all fueling stations. The amount of hydrogen that can be transported in a single trip by different means of transportation is listed in
Table 7.
Table 7.
Refueling station assumption [
94,
95,
96].
Table 7.
Refueling station assumption [
94,
95,
96].
| Parameter |
Assumption |
| Hydrogen volume of refueling station (2-950 bar) |
1200 kg/day |
| Pipeline transportation (40-950 bar) |
35000 kg/day |
| Compressed truck transportation (200-700 bar) |
42.2 kg-H2/m3
|
| Liquid truck transportation (250 bar) |
70.9 kg-H2/m3
|
In order to derive the cost of hydrogen transportation, this paper conducts a comprehensive analysis and comparison of the above transportation methods, and makes the following assumptions. According to the
current global market conditions, we have obtained the costs of different transportation modes of hydrogen as shown in
Table 8.
Table 8.
Transportation costing assumption.
Table 8.
Transportation costing assumption.
| Parameter |
Value (short distance < 200 km) |
Value (long distance > 200 km) |
| Pipeline transportation |
0.0048 $/kg/km |
0.0017 $/kg/km |
| Compressed truck transportation |
0.0151 $/kg/km |
0.0056 $/kg/km |
| Liquid truck transportation |
0.0375 $/kg/km |
0.0039 $/kg/km |
| Seaborne transportation |
|
|
0.0009 $/kg/km |
| |
|
5. Result Analysis and Discussion
Due to the different constraints of different modes of transportation, we divide the modes of transportation into two categories: short distance and long distance. At the same time, for the convenience of comparison, the hydrogen source is uniformly set as green hydrogen, and the average production cost is 4.36
$/kg, which is within the predicted range [
97].
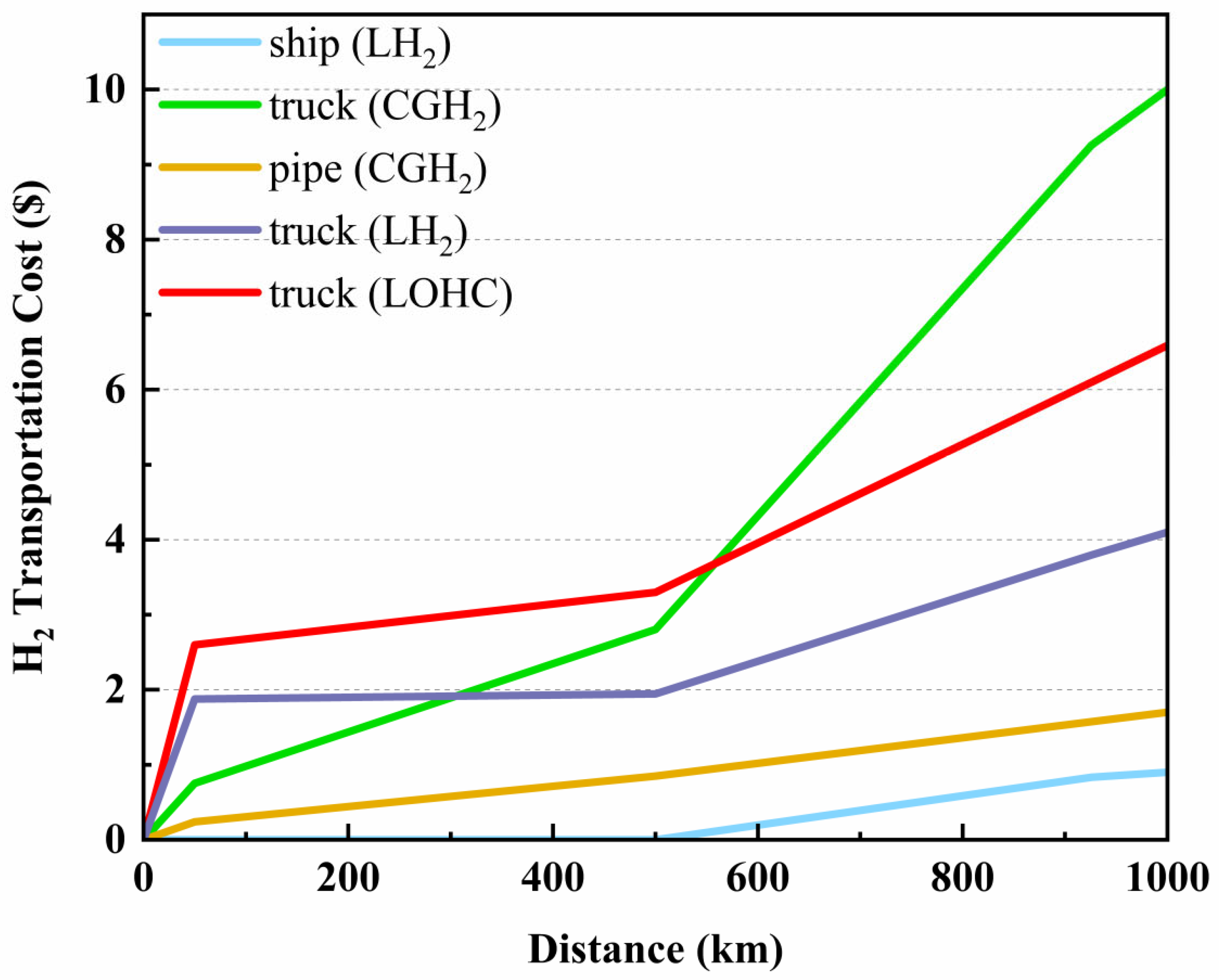
Firstly, the transportation of CGH2 uses trucks, and the cost of transportation increases significantly with distance. When the transportation distance is 50 km, the transportation cost of hydrogen is 0.7546 $/kg. As the transportation distance increases, the cost of long-tube trailer transportation gradually increases. When the distance is 500 km, the transportation cost reaches 2.8044 $/kg. Considering economic issues, long-tube trailer transportation of CGH2 is generally suitable for short distance transportation within 200 km.
Secondly, based on the above assumptions, it can be estimated that the cost of hydrogen transportation for a hydrogen refueling station with a capacity of 500 kg/d and a distance of 100 km from the hydrogen source point is 1.8858 $/kg. The cost changes of LH2 tankers are not sensitive to distance. When the distance between the hydrogen refueling station and the hydrogen source point is 50-500 km, the transportation price of LH2 tankers increases slightly within the range of 1.8774-1.9469 $/kg. Although transportation costs increase with distance, the increase is not significant. This is because the electricity consumption for liquefaction, which accounts for the largest proportion of the cost (about 60%), is only related to the hydrogen load, not the distance. The oil and road tolls that are positively correlated with distance account for a small proportion, so LH2 tankers have a cost advantage in long-distance transportation.
Finally, for the seaborne transportation, we will set the starting point in Shanghai, China, and the destinations as far away as Los Angeles, USA (20372 km) and as close as Seoul, South Korea (926 km). The final result is obvious. Seaborne transportation is the most ideal way to transport hydrogen, even for the recent trip to South Korea, the cost of transporting hydrogen is only 0.0009
$/kg/km. However, due to geographical factors, seaborne transportation can only play a significant role in transnational hydrogen transportation, while pipeline transportation is cheaper for domestic long distance hydrogen transportation (more than 200 km). Although our data shows that road transportation costs are higher than pipeline transportation, this is calculated by ignoring time and demand costs. In the short-term supply, truck transportation is the most economical choice in
Figure 10. Our final results are within the range of those obtained by the International Energy Agency using the levelized costs [
98]. Since there are no practical examples of commercial use of LOHC, we take the simulation results based on the latest development [
99].
6. Conclusions
As we survey the research progress and practical applications in the field of hydrogen storage and transportation globally, it is apparent that the potential of hydrogen as a clean energy carrier is being progressively unlocked, alongside technological advancements, innovations, and strategic collaborations and policy support among nations.
In the realm of hydrogen storage technologies, significant achievements have been made in experimental and industrial applications of methods such as compressed gaseous hydrogen storage, cryogenic liquid hydrogen storage, organic liquids hydrogen storage, solid materials hydrogen storage. However, these advancements are accompanied by challenges in efficiency, cost, safety, and regulatory framework. Moreover, the development of advanced materials like MOFs, nanomaterials, as well as the exploration of innovative concepts like UHS and LOHCs, are bringing revolutionary changes to the field of hydrogen storage.
In terms of hydrogen transportation, while certain progress has been made through various modes such as pipelines transportation, road transportation and seaborne transportation, issues such as leakage prevention, material compatibility, and infrastructure development still require in-depth research and solutions. We developed various types of hydrogen transportation models and estimated the transportation costs and unit prices of different models based on the current global market conditions. When transporting over long distance (926 km), the cost of seaborne transportation is the lowest (0.0009 $/kg/km). When transporting at short distance (200 km), although the pipeline transportation cost (0.0048 $/kg/km) is lower than that of truck transportation (0.0151 $/kg/km), considering the actual demand, truck transportation is generally adopted. The final LCC of H2 is calculated to be about 7.2-9.3 $/(kg·km).
We also recognize that despite the challenges faced by the widespread adoption of hydrogen energy, including cost, infrastructure, storage and transport efficiency, scalability of production, and public acceptance, these obstacles can be overcome through interdisciplinary collaboration, continuous research, and clear policy support. Future research directions should focus on developing more efficient, economical, and safer hydrogen storage and transportation technologies, as well as accelerating the scalability of green hydrogen production and the construction of a comprehensive hydrogen infrastructure network.
Finally, international harmonization and cooperation in standardization, regulatory framework, and certification systems will be key to facilitating the global deployment of hydrogen technologies. We have reason to believe that with collective efforts, hydrogen energy will become a vital force in supporting global energy transformation and addressing the challenges of climate change.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (Grant No. 52066002). The authors acknowledge the National Natural Science Foundation of China.
Author Contributions
Zongao Xie:Conceptualization, Methodology, Investigation, Data Analysis, Figures and Tables Editing, Writing - Original Draft, Draft Editing and Proofreading; Qihang Jin: Investigation, Data Analysis, Figures and Tables Editing, Draft Editing and Proofreading; Wei Lu: Conceptualization, Funding Acquisition, Resources, Supervision, Review.
Disclosure Statement
No potential conflict of interest was reported by the authors.
References
- Tarkowski, Radosław, Barbara Uliasz-Misiak, and Piotr Tarkowski. "Storage of hydrogen, natural gas, and carbon dioxide–Geological and legal conditions." International Journal of Hydrogen Energy 46.38 (2021): 20010-20022.
- Meng, Xiangyu, et al. "Status quo of China hydrogen strategy in the field of transportation and international comparisons." International Journal of Hydrogen Energy 46.57 (2021): 28887-28899. [CrossRef]
- Lu, Yi Ran, and Petr A. Nikrityuk. "Steam methane reforming driven by the Joule heating." Chemical Engineering Science 251 (2022): 117446. [CrossRef]
- Kumar, S. Shiva, and Hankwon Lim. "An overview of water electrolysis technologies for green hydrogen production." Energy Reports 8 (2022): 13793-13813. [CrossRef]
- Tezer, Özgün, et al. "Biomass gasification for sustainable energy production: A review." International Journal of Hydrogen Energy 47.34 (2022): 15419-15433. [CrossRef]
- Yousefi, Mahdi, and Scott Donne. "Technical challenges for developing thermal methane cracking in small or medium scales to produce pure hydrogen-A review." International Journal of Hydrogen Energy 47.2 (2022): 699-727. [CrossRef]
- Wang, Dongliang, et al. "Development of regulations, codes and standards on composite tanks for on-board gaseous hydrogen storage." International Journal of Hydrogen Energy 44.40 (2019): 22643-22653. [CrossRef]
- Du, Yan-Nan, et al. "Investigation on Standards on Hydrogen Cycle of Composite Tanks for Storage of High Pressure Hydrogen." Pressure Vessels and Piping Conference. Vol. 51593. American Society of Mechanical Engineers, 2018.
- Jian, L.I., et al. "High-pressure gaseous hydrogen storage vessels: Current status and prospects." Energy Storage Science and Technology 10.5 (2021): 1835.
- Landi, Daniele, et al. "A methodological approach for the design of composite tanks produced by filament winding." Comput. Des. Appl 17 (2020): 1229-1240. [CrossRef]
- Sapre, Shitanshu, Kapil Pareek, and Mayank Vyas. "Investigation of structural stability of type IV compressed hydrogen storage tank during refueling of fuel cell vehicle." Energy Storage 2.4 (2020): e150. [CrossRef]
- Fang, Qing, and Dongmei Ji. "Molecular simulation of hydrogen permeation behavior in liner polymer materials of Type Ⅳ hydrogen storage vessels." Mater Today Commun 35 (2023): 106302. [CrossRef]
- Maus, Steffen, et al. "Filling procedure for vehicles with compressed hydrogen tanks." International Journal of Hydrogen Energy 33.17 (2008): 4612-4621. [CrossRef]
- Kim, Moo-Sun, et al. "Analysis of hydrogen filling of 175 liter tank for large-sized hydrogen vehicle." Applied Sciences 12.10 (2022): 4856. [CrossRef]
- Yin, Liang, and Yonglin Ju. "Review on the design and optimization of hydrogen liquefaction processes." Frontiers in Energy 14 (2020): 530-544. [CrossRef]
- Al Ghafri, Saif ZS, et al. "Hydrogen liquefaction: a review of the fundamental physics, engineering practice and future opportunities." Energy & environmental science 15.7 (2022): 2690-2731.
- Aziz, Muhammad. "Liquid hydrogen: A review on liquefaction, storage, transportation, and safety." Energies 14.18 (2021): 5917.
- Abe, John O., et al. "Hydrogen energy, economy and storage: Review and recommendation." International journal of hydrogen energy 44.29 (2019): 15072-15086. [CrossRef]
- Abdin, Zainul, et al. "Large-scale stationary hydrogen storage via liquid organic hydrogen carriers." Iscience 24.9 (2021): 102966. [CrossRef]
- Rao, Purna Chandra, and Minyoung Yoon. "Potential liquid-organic hydrogen carrier (LOHC) systems: A review on recent progress." Energies 13.22 (2020): 6040. [CrossRef]
- Wang, Zhaohua, et al. "CO-tolerant RuNi/TiO2 catalyst for the storage and purification of crude hydrogen." Nature Communications 13.1 (2022): 4404. [CrossRef]
- Hashimoto, Taiki, et al. "Main group catalysis for H2 purification based on liquid organic hydrogen carriers." Science Advances 8.43 (2022): eade0189. [CrossRef]
- Sisakova, Katarina, et al. "Novel catalysts for dibenzyltoluene as a potential Liquid Organic Hydrogen Carrier use—A mini-review." Energy & Fuels 35.9 (2021): 7608-7623. [CrossRef]
- Díaz, Eva, Pablo Rapado-Gallego, and Salvador Ordóñez. "Effect of light alkanes and aromatics on decalin dehydrogenation over noble metal catalysts: A new strategy for the development of naphthalene-based LOHCs." Fuel 353 (2023): 129168. [CrossRef]
- Yang, Ming, et al. "A comparative study of catalytic dehydrogenation of perhydro-N-ethylcarbazole over noble metal catalysts." International journal of hydrogen energy 39.33 (2014): 18976-18983. [CrossRef]
- Zou, You-Quan, et al. "Ethylene glycol as an efficient and reversible liquid-organic hydrogen carrier." Nature catalysis 2.5 (2019): 415-422. [CrossRef]
- Dong, Yuan, et al. "Study of catalytic hydrogenation and dehydrogenation of 2, 3-dimethylindole for hydrogen storage application." RSC advances 11.26 (2021): 15729-15737.
- Ibrahim, Malek YS, Jeffrey A. Bennett, and Milad Abolhasani. "Continuous Room-Temperature Hydrogen Release from Liquid Organic Carriers in a Photocatalytic Packed-Bed Flow Reactor." ChemSusChem 15.14 (2022): e202200733.
- Moghadam, Peyman Z., Yongchul G. Chung, and Randall Q. Snurr. "Progress toward the computational discovery of new metal–organic framework adsorbents for energy applications." Nature Energy (2024): 1-13. [CrossRef]
- Ouyang, Liuzhang, et al. "Magnesium-based hydrogen storage compounds: A review." Journal of Alloys and Compounds 832 (2020): 154865. [CrossRef]
- Furukawa, M., et al. "Processing of metals by equal-channel angular pressing." Journal of materials science 36 (2001): 2835-2843. [CrossRef]
- Wang, Lisha, et al. "A critical review of Mg-based hydrogen storage materials processed by equal channel angular pressing." Metals 7.9 (2017): 324. [CrossRef]
- Jorge Jr, Alberto Moreira, et al. "An investigation of hydrogen storage in a magnesium-based alloy processed by equal-channel angular pressing." international journal of hydrogen energy 38.20 (2013): 8306-8312.
- Jinzhe, Lyu, Andrey M. Lider, and Viktor N. Kudiiarov. "An overview of progress in Mg-based hydrogen storage films." Chinese Physics B 28.9 (2019): 098801. [CrossRef]
- Saj, Alam, et al. "Production of Size-Controlled Gold Nanoclusters for Vapor–Liquid–Solid Method." Nanomaterials 12.5 (2022): 763.
- Linberg, Kevin, et al. "Controlling polymorphism in molecular cocrystals by variable temperature ball milling." Faraday Discussions 241 (2023): 178-193. [CrossRef]
- Dong, Yuanyuan, et al. "Effect of Annealing Temperature on the Microstructure and Mechanical Properties of High-Pressure Torsion-Produced 316LN Stainless Steel." Materials 15.1 (2021): 181. [CrossRef]
- Mroz, Sebastian, et al. "Effect of asymmetric accumulative roll-bonding process on the microstructure and strength evolution of the AA1050/AZ31/AA1050 multilayered composite materials." Materials 13.23 (2020): 5401.
- Spektor, Kristina, Andreas Fischer, and Ulrich Häussermann. "Crystallization of LiAlSiO4 Glass in Hydrothermal Environments at Gigapascal Pressures–Dense Hydrous Aluminosilicates." Inorganic Chemistry 55.16 (2016): 8048-8058. [CrossRef]
- Kalinina, Elena, and Elena Pikalova. "Opportunities, challenges and prospects for electrodeposition of thin-film functional layers in solid oxide fuel cell technology." Materials 14.19 (2021): 5584. [CrossRef]
- Salman, Muhammad Saad, et al. "Doping and Structure-Promoted Destabilization of NaBH4 Nanocubes for Hydrogen Storage." ACS Applied Nano Materials 6.6 (2023): 4178-4189. [CrossRef]
- Xu, Fengyan, et al. "A review of hydrogen production kinetics from the hydrolysis of NaBH4 solution catalyzed by Co-based catalysts." International Journal of Hydrogen Energy (2023). [CrossRef]
- Zhu, Yongyang, et al. "Closing the loop for hydrogen storage: facile regeneration of NaBH4 from its hydrolytic product." Angewandte Chemie 132.22 (2020): 8701-8707. [CrossRef]
- Balbay, Asım, and Cafer Saka. "Semi-methanolysis reaction of potassium borohydride with phosphoric acid for effective hydrogen production." International Journal of Hydrogen Energy 43.46 (2018): 21299-21306. [CrossRef]
- Huo, Jinrong, et al. "A review on hydrogen production from ammonia borane: Experimental and theoretical studies." Chinese Chemical Letters 34.12 (2023): 108280. [CrossRef]
- Castilla-Martinez, Carlos A., Romain Moury, and Umit B. Demirci. "Amidoboranes and hydrazinidoboranes: State of the art, potential for hydrogen storage, and other prospects." International Journal of Hydrogen Energy 45.55 (2020): 30731-30755.
- Demirci, Umit B. "Mechanistic insights into the thermal decomposition of ammonia borane, a material studied for chemical hydrogen storage." Inorganic Chemistry Frontiers 8.7 (2021): 1900-1930.
- Abdalla, Abdalla M., et al. "Hydrogen production, storage, transportation and key challenges with applications: A review." Energy conversion and management 165 (2018): 602-627. [CrossRef]
- Noussan, Michel, et al. "The role of green and blue hydrogen in the energy transition—A technological and geopolitical perspective." Sustainability 13.1 (2020): 298. [CrossRef]
- Tarkowski, Radoslaw. "Underground hydrogen storage: Characteristics and prospects." Renewable and Sustainable Energy Reviews 105 (2019): 86-94. [CrossRef]
- Raad, Seyed Mostafa Jafari, Yuri Leonenko, and Hassan Hassanzadeh. "Hydrogen storage in saline aquifers: Opportunities and challenges." Renewable and Sustainable Energy Reviews 168 (2022): 112846. [CrossRef]
- Navaid, Humza Bin, Hossein Emadi, and Marshall Watson. "A comprehensive literature review on the challenges associated with underground hydrogen storage." International Journal of Hydrogen Energy 48.28 (2023): 10603-10635. [CrossRef]
- Hu, Tong, et al. "Metal-organic frameworks (MOFs) and their derivatives as emerging catalysts for electro-Fenton process in water purification." Coordination Chemistry Reviews 451 (2022): 214277. [CrossRef]
- Ihsanullah, Ihsanullah. "Applications of MOFs as adsorbents in water purification: Progress, challenges and outlook." Current Opinion in Environmental Science & Health (2022): 100335.
- Jiao, Long, et al. "Metal–organic frameworks as platforms for catalytic applications." Advanced Materials 30.37 (2018): 1703663. [CrossRef]
- Liu, Jian, et al. "MOF-enabled confinement and related effects for chemical catalyst presentation and utilization." Chemical Society Reviews 51.3 (2022): 1045-1097. [CrossRef]
- Iqbal, Muhammad Zahir, et al. "Co-MOF/polyaniline-based electrode material for high performance supercapattery devices." Electrochimica Acta 346 (2020): 136039. [CrossRef]
- Chhetri, Kisan, et al. "Hollow carbon nanofibers with inside-outside decoration of bi-metallic MOF derived Ni-Fe phosphides as electrode materials for asymmetric supercapacitors." Chemical Engineering Journal 450 (2022): 138363. [CrossRef]
- Giliopoulos, Dimitrios, et al. "Polymer/metal organic framework (MOF) nanocomposites for biomedical applications." Molecules 25.1 (2020): 185. [CrossRef]
- Heidarinejad, Zoha, et al. "Methods for preparation and activation of activated carbon: a review." Environmental Chemistry Letters 18 (2020): 393-415. [CrossRef]
- Stoller, Meryl D., et al. "Graphene-based ultracapacitors." Nano letters 8.10 (2008): 3498-3502. [CrossRef]
- Wang, He, et al. "Micro-meso porous structured carbon nanofibers with ultra-high surface area and large supercapacitor electrode capacitance." Journal of Power Sources 482 (2021): 228986. [CrossRef]
- Huang, Xiaosong. "Fabrication and properties of carbon fibers." Materials 2.4 (2009): 2369-2403. [CrossRef]
- Zhang, Songdi, et al. "Measuring the specific surface area of monolayer graphene oxide in water." Materials Letters 261 (2020): 127098. [CrossRef]
- Stoller, Meryl D., et al. "Graphene-based ultracapacitors." Nano letters 8.10 (2008): 3498-3502. [CrossRef]
- Furukawa, Hiroyasu, et al. "The chemistry and applications of metal-organic frameworks." Science 341.6149 (2013): 1230444. [CrossRef]
- Pham, Hoai TB, et al. "Macrocyclic ligand-driven ion selectivity and high surface area in a 2D conductive MOF." Chem 10.1 (2024): 199-210. [CrossRef]
- Shet, Sachin P., et al. "A review on current trends in potential use of metal-organic framework for hydrogen storage." International Journal of Hydrogen Energy 46.21 (2021): 11782-11803. [CrossRef]
- Ren, Jianwei, et al. "Review on processing of metal–organic framework (MOF) materials towards system integration for hydrogen storage." International Journal of Energy Research 39.5 (2015): 607-620. [CrossRef]
- Jaramillo, David E., et al. "Ambient-temperature hydrogen storage via vanadium (II)-dihydrogen complexation in a metal–organic framework." Journal of the American Chemical Society 143.16 (2021): 6248-6256. [CrossRef]
- Peng, Peng, et al. "Cost and potential of metal–organic frameworks for hydrogen back-up power supply." Nature Energy 7.5 (2022): 448-458. [CrossRef]
- Reuß, Markus, et al. "A hydrogen supply chain with spatial resolution: Comparative analysis of infrastructure technologies in Germany." Applied Energy 247 (2019): 438-453. [CrossRef]
- Rödl, Anne, Christina Wulf, and Martin Kaltschmitt. "Assessment of selected hydrogen supply chains—factors determining the overall GHG emissions." Hydrogen supply chains. Academic Press, 2018. 81-109.
- Cardella, U., L. Decker, and H. Klein. "Roadmap to economically viable hydrogen liquefaction." International Journal of Hydrogen Energy 42.19 (2017): 13329-13338. [CrossRef]
- Fekete, James R., Jeffrey W. Sowards, and Robert L. Amaro. "Economic impact of applying high strength steels in hydrogen gas pipelines." international journal of hydrogen energy 40.33 (2015): 10547-10558. [CrossRef]
- Cerniauskas, Simonas, et al. "Options of natural gas pipeline reassignment for hydrogen: Cost assessment for a Germany case study." International Journal of Hydrogen Energy 45.21 (2020): 12095-12107. [CrossRef]
- Demir, Murat Emre, and Ibrahim Dincer. "Cost assessment and evaluation of various hydrogen delivery scenarios." International Journal of Hydrogen Energy 43.22 (2018): 10420-10430. [CrossRef]
- Di Lullo, Giovanni, Abayomi Olufemi Oni, and Amit Kumar. "Blending blue hydrogen with natural gas for direct consumption: Examining the effect of hydrogen concentration on transportation and well-to-combustion greenhouse gas emissions." International Journal of Hydrogen Energy 46.36 (2021): 19202-19216.
- Liu, Cuiwei, et al. "Study on the stratification of the blended gas in the pipeline with hydrogen into natural gas." International Journal of Hydrogen Energy 48.13 (2023): 5186-5196. [CrossRef]
- Wu, Xia, et al. "From the perspective of new technology of blending hydrogen into natural gas pipelines transmission: mechanism, experimental study, and suggestions for further work of hydrogen embrittlement in high-strength pipeline steels." International Journal of Hydrogen Energy (2022).
- Wu, Wei-Ping, et al. "Optimization of long-distance and large-scale transmission of renewable hydrogen in China: Pipelines vs. UHV." International Journal of Hydrogen Energy 47.58 (2022): 24635-24650. [CrossRef]
- Al-Breiki, Mohammed, and Yusuf Bicer. "Technical assessment of liquefied natural gas, ammonia and methanol for overseas energy transport based on energy and exergy analyses." International journal of hydrogen energy 45.60 (2020): 34927-34937. [CrossRef]
- Nath, Kaushik, and Debabrata Das. "Production and storage of hydrogen: Present scenario and future perspective." (2007).
- Lee, Ju-Sung, et al. "Large-scale overseas transportation of hydrogen: Comparative techno-economic and environmental investigation." Renewable and Sustainable Energy Reviews 165 (2022): 112556. [CrossRef]
- Aditiya, H.B., and Muhammad Aziz. "Prospect of hydrogen energy in Asia-Pacific: A perspective review on techno-socio-economy nexus." International Journal of Hydrogen Energy 46.71 (2021): 35027-35056. [CrossRef]
- Roos, Thomas H. "The cost of production and storage of renewable hydrogen in South Africa and transport to Japan and EU up to 2050 under different scenarios." International journal of hydrogen energy 46.72 (2021): 35814-35830. [CrossRef]
- FitzGerald, D., et al. "Documentation of changes implemented in the ecoinvent database v3. 10." database 3 (2023): 10.
- Miao, Bin, Lorenzo Giordano, and Siew Hwa Chan. "Long-distance renewable hydrogen transmission via cables and pipelines." International Journal of Hydrogen Energy 46.36 (2021): 18699-18718. [CrossRef]
- Cerniauskas, Simonas, et al. "Options of natural gas pipeline reassignment for hydrogen: Cost assessment for a Germany case study." International Journal of Hydrogen Energy 45.21 (2020): 12095-12107. [CrossRef]
- Krieg, Dennis. Konzept und Kosten eines Pipelinesystems zur Versorgung des deutschen Straßenverkehrs mit Wasserstoff. Vol. 144. Forschungszentrum Jülich, 2012.
- Bond, S. W., et al. “Hydrogen emissions to the atmosphere from industry and transportation.” Transition to hydrogen: Pathways toward clean transportation, Cambridge, United Kingdom: Cambridge University Press, submitted for publication (2011).
- Orion. Building a World-Leading Clean Energy Island. Available online: https://www.orioncleanenergy.com/ (accessed on 4 January 2024).
- Reuß, Markus, et al. "Seasonal storage and alternative carriers: A flexible hydrogen supply chain model." Applied energy 200 (2017): 290-302. [CrossRef]
- Reuß, Markus, et al. "Hydrogen road transport analysis in the energy system: A case study for Germany through 2050." Energies 14.11 (2021): 3166.
- Baldwin, Donald. Development of high pressure hydrogen storage tank for storage and gaseous truck delivery. No. DOE-HEXAGON-GO18062. Hexagon Lincoln LLC, Lincoln, NE (United States), 2017.
- Aziz, Muhammad. "Liquid hydrogen: A review on liquefaction, storage, transportation, and safety." Energies 14.18 (2021): 5917.
- Taibi, Emanuele, et al. "Green hydrogen cost reduction." (2020).
- International Energy Agency. (2023). Net zero by 2050 scenario—data product. International Energy Agency (IEA). https://www.iea.org/data-and-statistics/dataproduct/net-zero-by-2050-scenario.
- Lin, Andy, and Giuseppe Bagnato. "Revolutionising energy storage: The Latest Breakthrough in liquid organic hydrogen carriers." International Journal of Hydrogen Energy 63 (2024): 315-329.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).