1. Introduction
A lethal coronavirus known as SARS-CoV-2 (severe acute respiratory syndrome-coronavirus-2) first appeared in late 2019. It started the coronavirus disease 2019 (COVID-19) global pandemic, an acute respiratory infection that threatened human health globally [
1]. The COVID-19 pandemic has been in the spotlight of signalosome study following the outbreak in 2019 [
2], therapeutic interventions [
2] and possibly causing other diseases [
3,
4]. Millions of people who survived SARS-CoV-2 infection are now affected by a spectrum of post-COVID problems, including the so-called post-acute sequelae or long COVID-19 (PASC) or long-COVID. In previous articles of this series, we discussed the persistence of viral particles and/or one or more of its components in the recovered person's tissues [
5] circulated free and/or in ECVs (extracellular vesicles) [
6,
7], which is accompanied by the activated immune responses. The occurrence of the SARS-CoV-2 and/or some of its components in the ECVs has become established phenomenon in the virus biology, which plays a crucial role in its life cycle [
8,
9]. In the current report, we discuss the association of PASC with the continuity of the immune responses, which produces autoantibodies against different kinds of autoantigens, and the correlation between the autoimmune illnesses that follow SARS-CoV-2 infection and the PASC.
Long COVID (also known as PASC or protracted COVID (note, this is not an accurate expression, as it indicates that the COVID patient not recovered then (after months) have a symptom lookalike) is a multisystemic illness characterized by frequent serious manifestations that develops following an acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [
10]. At least 65 million persons globally have long COVID, according to a cautiously predicted prevalence of 10% of infected individuals with over 651 million recorded COVID-19 cases worldwide [
10]. Long COVID is expected to affect 10–30% of patients who are not hospitalized, 50–70% of patients who are hospitalized [
11], and 10-12% of people who have received vaccinations [
12]. People of all ages and acute disease phases have been associated with this condition; the age range between 36 and 50 years old has been associated with the highest number of cases, and non-hospitalized patients with mild disease account for almost all COVID-19 cases [
13]. Long-term COVID symptoms include brain fog, fatigue, sleeplessness, arthralgia, myalgia, pharyngitis, headaches, fever, digestive problems, skin rashes, and affective symptoms such as depression and anxiety, are comparable to those observed during the initial phase of infection [
14,
15,
16]. Patients with these symptoms exhibited multiple levels of long-term COVID-19 acuity. The more extreme degrees of COVID-19 infection, on the other hand, are connected to several early variables and the production of more severe extended COVID [
17].
The prevalence of autoimmune disorders (AID) is growing, with over 100 different medical conditions contributing to a significant disease impact globally. The key mechanisms that contribute to autoimmunity in COVID-19 encompass: (i) immune system hyper-activation, (ii) induced extreme neutrophil extracellular traps (NETs) formation, and (iii) interaction between SARS-CoV-2 and host self-components through cross-reaction. (NET) represents an advantageous antimicrobial mechanism of neutrophils, composed of a complex network of extracellular fibers, especially made of DNA and chromatin. Nevertheless, NETs can also function as a reservoir of self-antigens, leading to the development of autoimmune conditions such as Systemic Lupus Erythematosus (SLE), psoriasis, antiphospholipid syndrome, rheumatoid arthritis (RA), myositis, and multiple sclerosis (MS) [
18,
19,
20]). Zuo et al. [
21] showed that hospitalized patients with SARS-CoV-2 severe infection had increased levels of serum NETs. Additionally, they showed that serum from COVID-19 patients is a strong stimulator of NET formation when introduced to control neutrophils. Obtained results provide evidence that COVID-19, in hospitalized patients, can trigger the synthesis of new NETs which in turn could initiate autoimmune response [
21].
According to other reports, antigens from the gut, heart, kidney, lung, and brain may have cross-reactivity with SARS-CoV-2.Furthermore, SARS-CoV-2 proteins can exhibit homology to specific self-protein epitopes and initiate the molecular mimicry pathways [
22,
23].
The early diagnosis of AIDs is crucial for preventing consequences and tailoring appropriate therapy. Autoantibodies may be discovered in the serum of AIDS patients in the most cases, regardless of their presumed pathogenic activity, supporting clinicians in establishing a definite diagnosis and supplying screening methods for early and potential pre-clinical diagnosis [
24]. There are indications that SARS-CoV-2 infection is linked to the onset of autoimmunity worldwide [
25]. Antinuclear antibodies (ANAs; i.e., antibodies which bind to the cellular components in the nucleus, such as nuclear proteins, DNA, RNA, and nucleic acid-protein complexes, and which represent a defining feature of autoimmune connective tissue disease) were found in 35.6% of COVID-19 patients, anti-Sjögrens´s syndrome related antigen A (anti-Ro/SSA) in 25%, rheumatoid factor in 19%, lupus anticoagulant in 11%, and antibodies against interferon (IFN)-I in 10% [
26,
27].
Autoantibodies may remain after COVID-19, and latent PolyA levels in PCS (post COVID syndrome) rise with time [
28]. IgG autoantibodies to inflammatory cytokines (IL-2, CD8B, IFN) and thyroglobulin (as it is not cytokine) are present, and they correlate with the anti-SARS-CoV-2 antibodies [
29,
30]. Long-term COVID patients also have greater levels of autoantibodies to extractable and antinuclear antigens, which are linked to symptoms of fatigue and dyspnea [
31]. Particularly noteworthy are physiologically active autoantibodies against G protein-coupled receptors (GPCRs) that include nociception-like opioid receptor, angiotensin receptor, muscarinic M2-receptor, and 1- and 2-adrenoceptors (
Table 1) [
32].
Long COVID mechanisms are unknown. The most prominent hypotheses include immune response alterations, the continued presence of remnants viral elements causing continued inflammation or malfunction of endothelial cells, mitochondrial dysfunction, aberrant metabolites, micro-embolization, and reactivation of a previous chronic viral infection, microbiota imbalance, and unrepaired damage to the tissues [
46,
47]. However, these theories cross and overlap.
This review article aims to comprehensively investigate the emerging evidence and current understanding of the complicated relationship between autoimmunity and long-term COVID-19 manifestations, providing insight into the probable autoimmune mechanisms causing persistent symptoms. By merging prior study data, the article aims to improve knowledge of crucial immunological pathways underlying the prolonged and varied clinical symptoms seen present in people individuals with long-term COVID-19.
2. Molecular mechanisms underlying SARS-CoV-2 induced autoimmunity
Molecular mechanisms by which SARS-CoV-2 induces autoimmunity are not yet completely known. What we know is that SARS-CoV-2 spike protein binds to the Angiotensin 2 Converting Enzyme (ACE2) and downregulates ACE2 levels, leading to angiotensin II (Ang-II) increased levels. Ang-II is the principal bioactive peptide in the renin-angiotensin system (RAS), performing crucial roles in maintaining the physiological and pathophysiological balance of the body. Stegbauer et al. [
48] examined the role of RAS compounds in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis (MOG-EAE), a model of autoimmune inflammation of the central nervous system that parallels multiple sclerosis in several ways.
RAS blockade at the level of renin markedly improved the clinical course of experimental autoimmune encephalitis (EAE), implying that mainly Ang-II, rather than other Ang peptides, promotes the pathogenesis of MOG-EAE. These findings may support the hypothesis that SARS-CoV-2 could provoke autoimmunity by increasing the levels of Ang II through ACE2 downregulation. In addition to these findings, RT-PCR analysis showed that angiotensin II type 1 receptor (AT1R) mRNA was expressed in polymorphonuclear leukocytes (PMNs), monocytes, and B-lymphocyte, suggesting that elevation of Ang-II induced by SARS-CoV-2 may activate AT1R in anergic B lymphocytes reversing anergy and potentially leading to the development of autoimmunity [
49].
A subset of autoreactive anergic B cell population, referred to as "B
ND cells," was identified by Castleman et al. [
50]in human peripheral blood mononuclear cells (PBMCs) from healthy participants, people who had received an mRNA vaccine against SARS-CoV-2, as well as individuals who had minor and severe SARS-CoV-2 infection.
Expression markers associated with the loss of anergy in B
ND cells, such as CD86 and CD69, were elevated in the examined population with severe SARS-CoV-2 infection. Additionally, a decreased expression of inhibitory regulators, CD22 and CD27 which contribute to maintaining anergy in B cells, was discovered in individuals with severe infection compared to health controls or immunized individuals and those with non-severe infection. These phenotypic alterations in B
ND cell population during severe SARS-CoV-2 infection, support the hypothesis of inducing autoimmunity, as they actively contribute to the loss of cellular anergy [
50].
In their study, Lee et al. [
51] examined how COVID-19 affected the onset and course of RA by utilizing an animal model called collagen-induced arthritis (CIA). What they found was that an excessive expression of the SARS-CoV-2 spike protein markedly increased the mRNA and protein expression levels of the inflammatory cytokines, interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), and interferon gamma (IFN-γ), as well as the chemokine monocyte chemoattractant protein-1
(MCP-1). In addition, they observed that SARS-CoV-2 spike protein significantly increased the incidence and severeness of RA in the CIA mice. These findings implicate that SARS-CoV-2 accelerates RA by increasing inflammatory cytokine and chemokine expression. Since it is shown that
ACE2 reduces the expression of several proinflammatory cytokines, including Tumor Necrosis Factor (TNF-α) and Interleukin-6 (IL-6) [
52], inhibition of ACE2 by SARS-CoV-2 spike protein could partially explain the elevation of proinflammatory molecules during SARS-CoV-2 infection. Altogether, these findings support the hypothesis that overexpression of proinflammatory cytokines and chemokines during SARS-CoV-2 infections leads to hyper innate inflammatory response, potentially serving as a trigger for autoimmune response.
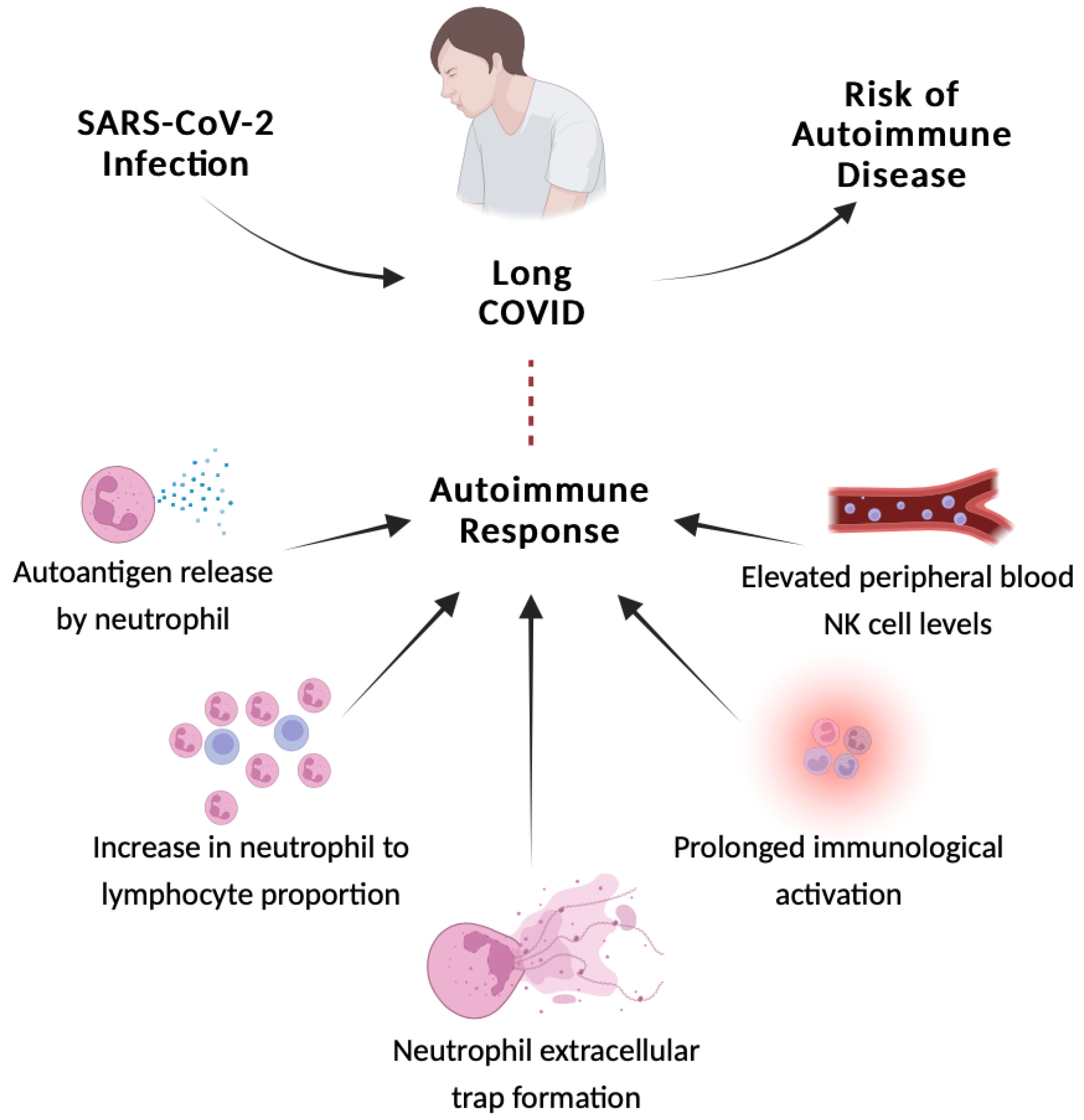
3. Autoimmune response
Individuals with long COVID have a hypervigilant immune system. Alteration in SARS-CoV-2-specific adaptive and unspecific innate immune responses have been reported in individuals with long COVID [
53]. Long-term COVID-19 infection is believed to be exacerbated by autoimmune reactive inflammation in addition to cytokines and abnormal cells of the immune system [
54]. The autoimmune response is linked to the production of autoantigens by both dead and functional neutrophils, an increase in the ratio of neutrophils to lymphocytes, and NETs. After the acute phase of SARS-CoV-2 infection, extracellular neutrophil traps and anticardiolipin autoantibodies were identified in patients [
55]. Wang et al. [
41] employed a high-throughput autoantibody discovery approach to search for autoantibodies against 2770 extracellular and secreted proteins (exoproteome) in a sample of 197 COVID-19 patients. Although the authors discovered that these individuals had remarkable elevations in a variety of autoantibody responses when compared to the uninfected controls, it does not necessarily reflect a link to the appearance of log-COVID symptoms, as the samples were collected during patient infection with SARS-CoV-2.
Schematic representation of the link between the autoimmune reactive inflammation and long COVID is shown in
Figure 1.
According to one research, COVID-19 post-acute symptoms are prevalent in COVID-19 individuals with rheumatic illness [
56]. Antinuclear antibody (ANA) titers of 1:160 were found in 43.6% of COVID-19 patients 12 months following symptom start, and these patients had substantially greater percentages of neuropsychological symptoms [
57]. Similarly, IFN specific autoantibodies have been identified in patients with severe COVID-19 [
58,
59] and have been hypothesized as a potential cause of the long COVID symptoms [
30].
Long COVID-19 has been linked to increased levels of autoantibodies against ACE2, muscarinic M2 receptor, β2-adrenoceptor, angiotensin II AT1 receptor, and the angiotensin 1-7 MAS receptor [
32,
40]. Some patients with COVID-19 have been reported to have autoantibodies against tissue, systemic organs (such as the central nervous system, skin, lung, and gastrointestinal tract), immune-modulating proteins (such as chemokines, cytokines, complement components, and cell-surface proteins), and connective tissue (such as extracellular matrix elements, vascular endothelium, coagulation factors, and platelets). All samples were collected from patients during SARS-CoV-2 infection [
41]) (
Table 1).
3.1 Adaptive immune cells
The SARS-CoV-2 adaptive humoral and cellular immune reaction aids in viral elimination. Immune memory remains post infection for the defense of the host, with virus-particular neutralizing antibodies and T-cell responses discovered up to 12 months after infection [
60]. A weakened immune response, on the other hand, may result in prolonged chronic immunological activation and perhaps long COVID. Low perforin (which is a protein that form pores) and which plays a crucial role in the granzyme-mediated programmed cell death and in the defense against the virus-infected or neoplastic cells [
33,
34,
35,
36,
37] expression in CD8
+T cells during the initial stages of severe SARS-CoV-2 infection has been reported to predict long COVID [
61]. Another research found that persons with long COVID-19 had more antiviral cytotoxicity in CD8
+ T cells and higher levels of the exhaustion marker PD-1 than patients who had fully recovered, indicating the presence of chronic inflammation [
62]. The depth of the spike-specific clonal CD4
+ T-cell receptor β was strongly related to both difficulty breathing and the frequency of symptoms at 12 months, indicating that infection-induced SARS-CoV-2-specific immune responses may alter long COVID [
63].
The immune system's adaptive response is also altered during healing from acute infection. T-cell subsets displayed variable seriousness- and time-dependent dynamics in a longitudinal investigation of COVID-19 patients [
64]. At 3-month follow-up after the recovery from severe illness, convalescent patients with long COVID had an exhausted (PD-1-expressing)/senescent (CD57-expressing) condition in CD4
+ and CD8
+ T cells, as well as a disruption in CD4
+ regulatory T cells. Up to 6 months after a severe infection, CD8
+ T cells remained in an exhausted/senescent state. This, in addition to a lower naive cell population and increased granzyme B and interferon gamma (IFN-γ) production, implies persistent inflammation during extended COVID [
64].
3.2 Innate immune cells
Long COVID is also linked with the number and operation of innate immune cells. Monocytes were observed to be substantially more common in the individuals with severe illness compared to those with mild-to-moderate infection 1-3 months after recovery, and they demonstrated greater response after
in vitro stimulation. Nevertheless, the human leukocyte antigen (HLA) class II marker HLA-DR proved to be considerably lowered in COVID-19 patients, indicating inhibited antigen-presenting function [
38].
Natural killer (NK) cells serve an important function in viral infection management, principally through cytotoxicity and IFN-
γ cytokine production. In patients with long COVID, the levels of the NK cells in the peripheral blood are considerably higher than those in the healthy controls [
38]. CD59 high NK cells are reduced in severe infection subcategories and are related to the elevated pro-inflammatory cytokines, particularly interleukin 6 (IL-6), which affects NK cell proliferation and function [
38]. Granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor levels were observed to be greater in patients with long COVID when compared to healthy controls. A cytokine profile of long COVID appears to consist of high serum levels of IL-17 and IL-2 (
Table 1) [
39], which were in 90 subjects without sequelae. The potential repetition of the cytokine pattern observed in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), wherein specific cytokine levels are elevated during the initial 2-3 years of the illness but subsequently decrease without a corresponding reduction in symptomatology is yet to be determined [
65].
4. Risk of autoimmune diseases
Although death from COVID-19 has decreased due to the acquisition of natural immunity and global immunization efforts, we continue to see significant morbidity and higher incidence of post-COVID-19 disorders, particularly sudden autoimmune and inflammatory illnesses in patients who have had COVID-19. Two large retrospective cohort research studies [
44,
66] have recently emphasized the extent and occurrence of these post-COVID-19 illnesses.
Pediatric patients with the multisystem inflammatory syndrome in children (MIS-C), which has similarities with other hyper-inflammatory syndromes, such as the Kawasaki disease, toxic-shock syndrome, and macrophage activation syndrome, providing several of the firsts pieces of evidence that SARS-CoV-2 infection causes dysfunctional immune responses [
67]. To learn more about the association, Chang et al. [
44] and Tesch et al. [
66]carried out retrospective cohort investigations.
Chang et al. [
44] selected a research population of nearly 5.9 million individuals from 48 worldwide healthcare institutions using the TriNetX network, which preserves the biggest global COVID-19 dataset. Propensity score matching was utilized to create two participant sets (COVID-19 and non-COVID-19) with a combined total of 887,455 individuals in order to identify the prevalence of autoimmune illnesses during the study period, which ran from January 1, 2020, to December 31, 2021. Only those who were not vaccinated were included in the research due to the possibility that SARS-CoV-2 immunization could introduce bias. At 6 months, the COVID-19 cohort had a considerably greater incidence of autoimmune disorders than the non-COVID-19 group [
44].
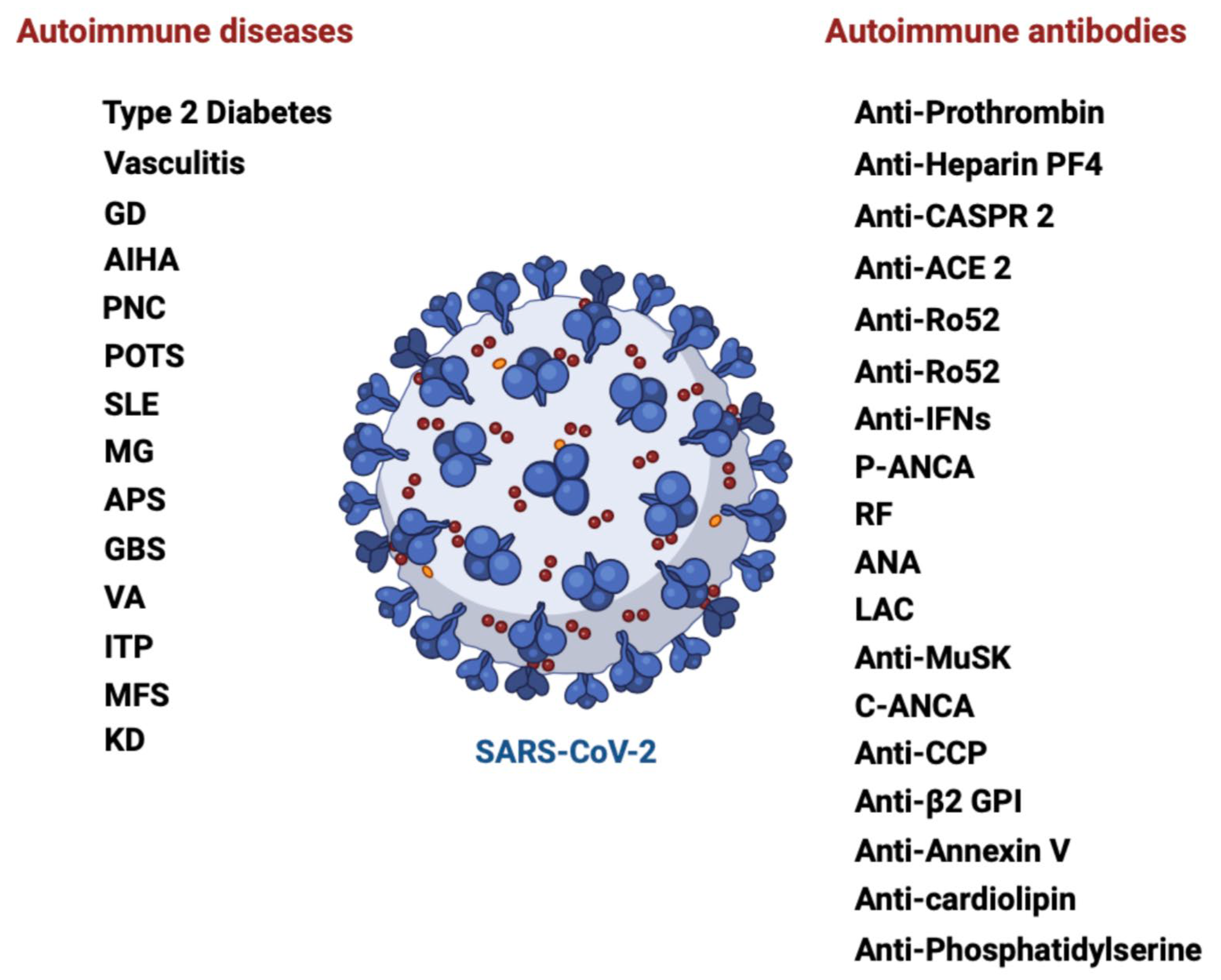
The breadth of disorders found following SARS-CoV-2 exposure distinguishes it from other viral infections (such as coronaviruses, coxsackie type 1, and Epstein-Barr virus). A wide range of autoimmune disorders was found in the COVID-19 cohort (
Figure 2) [
44]. According to the published research, COVID-19 patients can develop over 15 separate types of autoantibodies and more than 10 distinct AIDs [
22]. Among others, it included rheumatoid arthritis (adjusted hazard ratio (aHR) 2.98; 95% CI 2.78-3.20), systemic lupus erythematosus (SLE) (aHR 2.99; 95% CI 2.68-3.34), systemic sclerosis (aHR:2.58, 95% CI:2.02-3.28), vasculitis (aHR 1.96; 95% CI 1.74-2.20), inflammatory bowel disease (aHR 1.78; 95% CI 1.72-1.84) and type 1 diabetes mellitus (aHR 2.68; 95% CI 2.51–2.85) (
Table 1) [
44].
Tesch et al. [
66] conducted similar research in 2020 on 640,701 unvaccinated adults with a positive PCR testingCOVID-19 to assess the risk of autoimmune disorders. The scientists discovered a 42.6% increased risk of developing an autoimmune disorder 3-15 months post infection when compared to a non-COVID-19 cohort of 1,560,357 people who were paired for age, gender, and if they suffered a previous autoimmune disorder [
66].
Vasculitis illnesses, which are fairly uncommon autoimmune disorders, have the highest prevalence ratios. The findings further highlight that COVID-19 raised the likelihood of having another autoimmune illness by 23% in people who already had one. Because of the intrinsic characteristics of their methodology (retrospective cohort), both of these studies cannot demonstrate causality between SARS-CoV-2 and the occurrence of autoimmune diseases. Nevertheless, based on the spatial association with previous infections of COVID-19, they gave appealing and solid indications that SARS-CoV-2 infection is associated with a significantly higher likelihood of acquiring broad new-onset autoimmune diseases after the initial phase of SARS-CoV-2 infection [
66].
Chang et al. [
44] and Tesch et al. [
66] presented a thorough analysis of several new-onset autoimmune disorders after COVID-19. A significant characteristic of COVID-19 infection is a considerable rise in the total frequency and variety of autoimmune diseases in patients. Several hypotheses have been advanced to dilucidate the molecular foundation of COVID-19-related immune dysfunction, including viral protein mimicry, and multiorgan commitment of COVID-19 due to prevalent expression of the SARS-CoV-2 receptor ACE2, bystander triggering of immune cells, autoantigens release from virus-damaged tissue, lymphocyte activation triggered by superantigens and epitope spreading [
42,
43].
5. Elevated levels of autoantibodies and long COVID-19
Visvabharathy et al. investigated autoantibody responses in individuals with or without long COVID infection before and after a booster dose of mRNA vaccine [
45]. At V2, 15 (88%) of 17 Neuro-PASC patients had poly-autoantibody reactions (remarkable increases in more than two autoantibodies) associated with several autoimmune illnesses or disorders such asRA, systemic sclerosis, and autoimmune vasculitis. Unexpectedly, according to Visvabharathy et al., 12 of 14 (85%) of convalescent controls displayed a substantial rise in more than two autoantibodies with varied specificities at all-time points, in contrast to healthy controls [
45]. However, similar conclusions were not reported before [
68,
69].
Compared to healthy control participants, autoantibodies associated with the inflammatory myopathies and systemic lupus erythematosus (SLE) were increased among those raised in Neuro-PASC patients and to a lesser amount in convalescent controls. Furthermore, neuronal antigen specific GAD65 antibodies and anti-cytokines were significantly increased in Neuro-PASC patients. The greatest autoantibody titers were reported in individuals with past SARS-CoV-2 infection at V1 (3 weeks post-vaccination) or V2 (3.5 months post-vaccination), as opposed to baseline. Except for MDA5 (a RIG-I-like receptor dsRNA helicase), autoantibody titers were not substantially changed by age, gender, or time after acute infection in either group. Importantly, acute-phase inflammatory markers like C reactive protein (CRP) or acute phase COVID-19 disease intensity did not indicate if a patient will have high poly-autoantibody responses. Overall, these findings implied that even minor past SARS-CoV-2 infection might increase antibody auto-reactivity in long COVID patients more than in individuals lacking persistent symptoms [
45].
After autoantibodies against type I interferons (IFNs) were first found in patients with life-threatening COVID-19 [
27], there have been multiple studies that show elevated levels of autoantibodies against different additional cytokines and chemokines and their receptors [
41], G protein-coupled receptors (GPCR), molecules related to the renin-angiotensin system (RAS), and anti-cardiolipin [
70,
71], chromatin proteins, ribosomal P proteins, thyroid antigens [
72], anti-nuclear antigen (ANA) [
72], and anti-neutrophil cytoplasmic proteins (ANCA) [
73] in individuals with severe SARS-CoV-2 infections. Baiocchi et al. recently identified many autoantibodies associated with autoimmune disorders and COVID-19 severity [
74]. There is still a lack of research on autoantibodies' physiological role. Analysis of serum showed that autoantibodies are shared by healthy people [
75]. Autoantibodies have been demonstrated to have homeostatic functions by binding cellular antigens and assisting in the removal of apoptotic cells [
76]. According to these findings, Baiocchi et al. propose that cellular receptor expression may physically regulate autoantibody levels, suggesting a potential mechanism by which SARS-CoV-2 infection generates autoantibody profiles linked to the severity of COVID-19 [
74].
6. The roles of the microbiota in the immune system and COVID-19 severity
Disease prevention is significantly supported by a healthy microbiota [
77]. It has been frequently regarded as an organ of a person's body and is an integral part of the host [
78]. It plays a role in immune protection as well as participates in maintaining homeostasis, metabolic, and physiological activities. It also aids in the breakdown of complex nutrients like fats, complex carbohydrates, and fatty acids, fermentation of indigestible food residues, digestion, and the proliferation and differentiation of epithelial cells as well as the absorption of metal ions [
79]. It has been discovered to aid in the immune system's maturation in newborns [
80]. The microbiota that lives in the gastrointestinal tract, the body's largest immunological organ, is known to influence host immune responses [
81]
According to recent research, commensal microbiota regulates systemic inflammatory responses and is essential for preserving the equilibrium between pro- and anti-inflammatory cytokines [
82,
83]. Through their metabolites, they interact with the host's immune system and preserve this equilibrium [
82]. In an effort to comprehend the role of the microbiota in COVID-19 pathogenesis, numerous researchers worldwide are currently working on cutting-edge investigations on the oral, lung, brain, and gut microbiota [
84]. Numerous research endeavors have tackled this matter, demonstrating the gastrointestinal tract's role in the etiology of COVID-19 and establishing a connection between the disease's clinical result and the microbiota [
84]. As a result, it has been discovered that gut microbiome dysbiosis is linked to the intensity and course of the disease [
84,
85]. There is a positive correlation between the severity of COVID-19 and the reduction of commensals in the gut [
84].
This suggests that the disease has an impact on the structure of the gut microbiota, but it also highlights the importance of a healthy gut microbiota profile in delaying the start of the disease [
84]. Similarly, examination of the lung microbiome in COVID-19 patients showed dysbiosis and produced surprising results that suggested a major role for the microbiome in the onset of critical illness [
86,
87]
Beneficial bacteria have been observed to be diminished in the COVID-19 patients' microbiota [
84]. This reduced quantity of beneficial commensals could potentially account for some of the inflammation associated with COVID-19, which is manifested by elevated levels of IL-1β, IL-6, IFNγ, MCP1, and IP-10 [
88]. Higher blood plasma levels of IL-2, IL-7, IL-10, IP-10, MCP1, macrophage inflammatory protein 1α (MIP1α), IL-6 and tumor necrosis factor (TNF) are associated with more severe cases of cytokine storm [
88]. Patients with severe COVID-19 have bronchoalveolar lavage fluid that contains a population of monocyte-derived FCN1+ macrophages with an inflammatory role (Tay et al., 2020). Severe cases additionally have a greater percentage of CD14+ CD16+ inflammatory monocytes in peripheral blood [
88]. These cells cause the cytokine storm by secreting inflammatory cytokines and chemokines such as MCP1, IP-10, and MIP1α [
88]. It is established that these commensals possess immunomodulatory capabilities [
85]. Dysbiosis was linked to higher levels of inflammatory cytokines, aspartate aminotransferase, C-reactive protein, lactate dehydrogenase, and gamma-glutamyl transferase, as well as to blood indicators [
85].
Yeoh et colleagues. recently completed a two-hospital cohort research to better understand the role of the GI tract microbiota in COVID-19 patients and illness outcomes [
85]. The purpose of the study was to determine whether the gut microbiota of COVID-19 patients was related to the severity of the illness and whether microbiota dysbiosis improves after the virus is eradicated [
85]. 100 patients with SARS-CoV-2 infection provided blood and stool samples for the study, and serial stool samples were taken from 27 of these patients up to 30 days after viral clearance. Shotgun sequencing was used to examine the gut microbiome. Plasma was used to evaluate the concentration of blood indicators and inflammatory cytokines. The gut microbiota of patients and controls was observed to differ considerably by the authors [
85]. Patients had decreased levels of
Bifidobacteria,
Eubacterium rectale, and
Faecalibacterium prausnitzii, which persisted for up to 30 days following the resolution of the illness [
85,
89]. Other work found that critically ill individuals showed a complete depletion of
Bifidobacterium and
Clostridium genera. Additionally, there was a relative abundance of the
Pseudomonaceae family in these people, which is known to be linked to pathogenic diseases including severe acute respiratory syndromes [
90].
Additional studies showed that compared to patients treated in regular areas, COVID-19 patients needing intensive care unit (ICU) admission during hospitalization had a lower baseline gut microbiome diversity (Shannon index). The requirement for an ICU was linked to a dysbiosis index that showed the balance between enriched and reduced taxa in ICU patients as opposed to ward patients. This index included a reduction in the number of butyrate-producing bacteria and an enrichment of a partially oral bacterial flora. Following severe COVID-19, the composition of the gut microbiota during hospitalization was linked to 60-day mortality [
91]. Similarly, based on the gut metagenomic data derived from the population-based analysis of 2,871 adult subjects from 16 countries, it was concluded that altered gut microbiome composition and functions (e.g., lower abundance of
Eubacterium rectale and
Roseburia intestinalis in the gut) are associated with the COVID-19 mortality [
89].
7. Altered microbiota in long COVID and its connection with autoimmunity
Several studies have demonstrated that gut microbiota is also altered in long COVID. According to one study, the dysbiosis of the gut microbiota continued for up to 30 days after the illness resolved, which may be related to the enduring symptoms of PASC [
85]. Furthermore, it has been established that ACE2 affects the expression of neutral amino acid transporters in the gut [
92], which in turn controls the gut microbiota's composition, which in consequence affects both systemic and local immune responses [
93,
94]. In COVID-19 patients with pre-existing age-related comorbidities, ACE2 imbalance has been associated with poor outcomes (including increased disease severity and mortality rate) through its impact on intestinal dysbiosis [
95].
Liu et al. [
96] have verified in 6-month follow-up that long-COVID patients had distinct gut microbiota species compared to controls and a persistently lower α-diversity (Shannon and Chao-1 indexes). Interestingly, those individuals who had COVID-19 at the begining but did not have long COVID did not exhibit the same dysbiosis pattern. Increasing fecal relative abundance of opportunistic pathogens was positively associated with fatigue, respiratory, and neuropsychiatric symptoms in the long-COVID subgroup [
96]. Other work
demonstrated the presence of long COVID-19 which correlates with gut microbiota dysbiosis in recovered patients at one-year after discharge, indicating gut microbiota may play an important role in long COVID-19 [
97].
Remarkably, it was found that 76 % of patients had PACS at six months, with fatigue, memory loss, and hair loss being the most frequent symptoms [
96]. The composition of the gut microbiota at admission was linked to the presence of PACS. At six months, the gut microbiota profiles of patients without PACS had recovered to a level similar to non-COVID-19 controls. Patients diagnosed with PACS had a gut microbiota characterized by reduced levels
of Faecalibacterium prausnitzii and greater levels of
Bacteroides vulgatus and
Ruminococcus gnavus. At six months, bacteria that produce butyrate, such as
Faecalibacterium prausnitzii and
Bifidobacterium pseudocatenulatum, exhibited the strongest inverse relationships with PACS [
96].
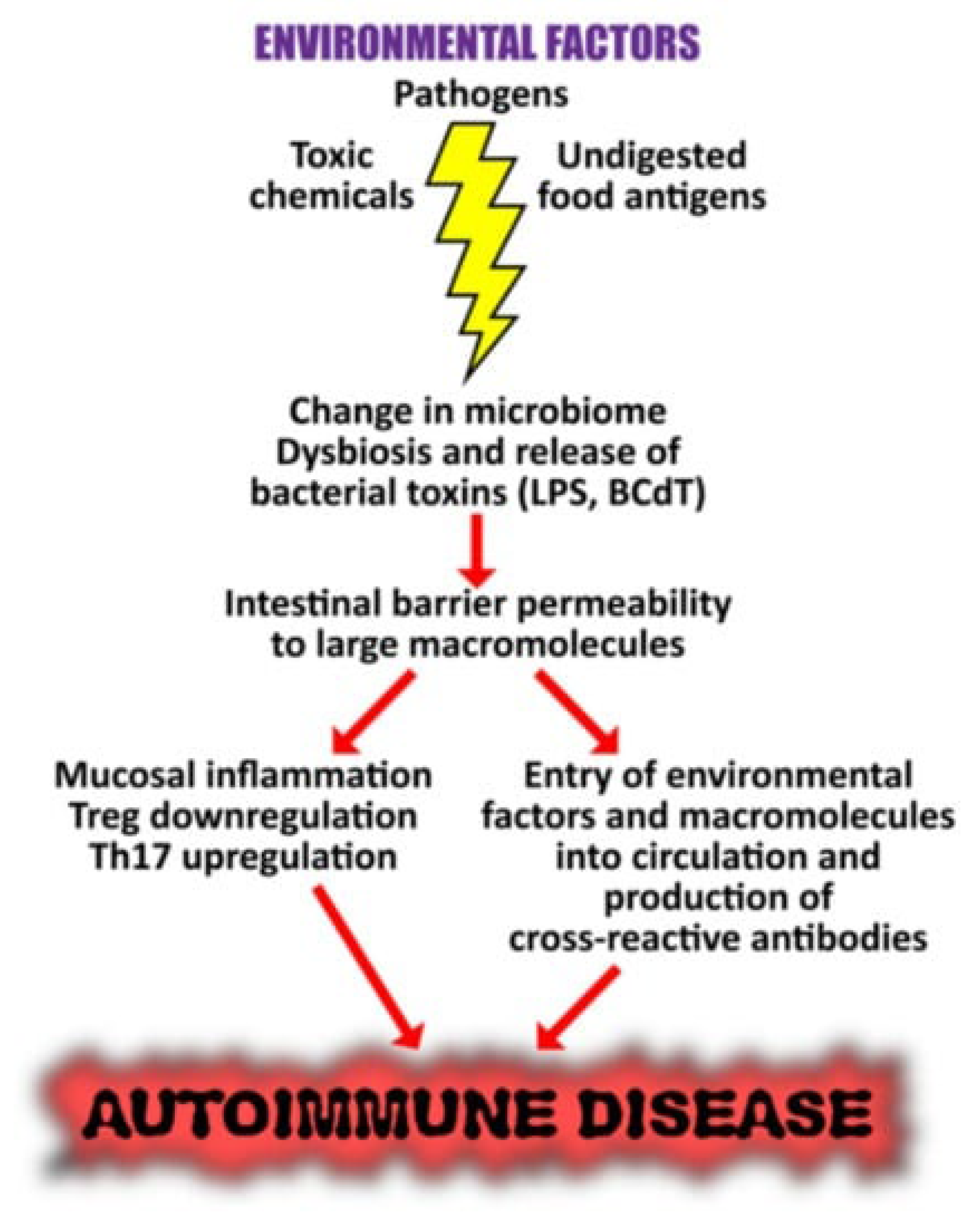
7.1. Connection between altered microbiome and the onset of autoimmunity
It is not surprising to learn that dysbiosis of the gut, oral, and skin microbiome has been linked to autoinflammation and tissue damage in susceptible or genetically predisposed individuals, given that environmental factors have the ability to trigger autoimmune responses and that they interact with different components of our microbiome. Changes in the human microbiome may potentially play a major role in autoimmunity, as a changed microbial composition can cause inflammation and a breakdown in immunological tolerance [
98]. This is due to the fact that a steady, healthy, and well-adjusted gut microbiota importantly contributes to immune system regulation in addition to facilitating the body's effective absorption of nutrients. Therefore, several autoimmune disorders may result from dysbiosis of the gut microbiota [
99,
100,
101,
102]. Furthermore, gut microbiota dysbiosis has been implicated in autoimmunity development, including disorders like systemic lupus erythematosus (SLE), rheumatoid arthritis, liver disease, type I diabetes, multiple sclerosis (MS), and Sjögren’s syndrome [
101,
103,
104,
105,
106,
107,
108,
109,
110].
For example, although the exact mechanisms underlying the onset of SLE, one of the most common autoimmune illnesses, are still unknown, hormonal, genetic, and environmental factors may have a role in the development of SLE flare-ups [
111]. According to recent research, there may be a correlation between the activity of SLE disease and changes in the balance and composition of the gut microbiota. It was noted that the abundance of several taxa and the Firmicutes/Bacteroides ratio were lower in SLE patients. Additionally, there were more Lachnospiraceae and less Lactobacillaceae in SLE patients [
105,
112,
113]. Serum sCD14 (a monocyte activation marker), fecal secretory IgA, calprotectin levels, and
Ruminococcus gnavus of the Lachnospiraceae family were all elevated in female SLE patients [
114]. Increased levels of endotoxin lipopolysaccharide (LPS) were also observed in SLE patients, maybe as a result of leaky gut. This finding raises the possibility that persistent microbial translocation plays a role in the development of SLE [
115,
116] . Bacterial amyloid/DNA complex has been demonstrated to trigger autoimmune responses in lupus-prone NZBxW/F1 mice, including type 1 IFN production and autoantibodies [
117]. When compared to age-matched healthy controls, young lupus-prone mice displayed a significant decrease in lactobacilli and an increase in Lachnospiraceae [
118].
Microbiota can be regarded as a risk environmental factor for rheumatoid arthritis (RA), an inflammatory autoimmune disease that is brought on by both genetic and environmental causes. In some proteins, citrullination is a post-translation modification (PMT) that changes the amino acid arginine into the amino acid citrulline. The immune system identifies these citrullinated proteins as foreign antigens, which causes an increase in inflammatory responses like those seen in RA. Based on available data, it appears that some oral and gut bacteria species, including
Porphyromonas gingivalis and
Prevotella copri, have a role in the pathogenesis of RA. These bacteria have the ability to facilitate the citrullination of bacterial or human proteins during dysbiosis, which sets off an immunological reaction that results in the production of autoantibodies [
110].
Inflammatory bowel disease (IBD) is hypothesized to arise from interactions between environmental, microbial, and immune-mediated factors in a genetically predisposed host. Research has demonstrated that the gut microbiota of individuals with IBD differs from that of healthy control people. Identification of gene alterations involved in microbiome-immune interactions among IBD patients provides additional evidence in favor of a fundamental function for the microbiome in these individuals [
119].
In a 2020 review paper, Boziki et al. showed that the gut microbiome also plays a role in MS. They emphasized the role of gut microbiota in both innate and adaptive immunity, as well as the gut-brain axis. In their conclusion, they suggested that altering the gut flora might result in potential MS treatment strategies [
120].
Figure 3 illustrates the suggested pathways, by which microbiome dysbiosis may result in trigerring the autoimmunity.
These reported changes in the microbiome driven by environmental variables are extremely important results that warrant additional exploration into their precise implications in the pathogenesis of autoimmune illness [
98]. Moreover, research indicates that lactobacilli can be restored and symptomatology improved by dietary intervention using retinoic acid, prebiotics, and probiotics that have the ability to modulate Tregs. The findings supported the use of retinoic acid as a dietary supplement to treat inflammatory flare-ups in lupus patients by showing significant alterations in the gut microbiota in mice with the disease [
121,
122]. Given this knowledge, the identification and modification of the microbiome's composition may offer therapeutic opportunities for the amelioration and potential full restoration of the immune system's malfunction in many autoimmune disorders [
98].
8. Reinstatement of microbiota as a potential treatment for long COVID-19
It is conceivable that using probiotics to restore intestinal dysbiosis could be one strategy for treating long-term COVID-19 [
123]. Viral infections are known to cause chronic fatigue syndrome (CFS), also known as myalgic encephalomyelitis (ME) in individuals, while data on COVID fatigue is still developing. Symptoms are usually used to make the diagnosis, as there are no particular biomarkers. Actually, a portion of COVID-19 patients met the requirements for the CFS/ME diagnosis category [
124]. Moreover, significant COVID-19 post-acute symptoms are similar to those of post-infectious ME/CFS [
125]. Fatigue is believed to be caused by alterations in neurotransmitter levels, inflammation, psychiatric disorders, stress, and cognitive dysfunction [
126]. Persistent inflammation and weariness are linked to elevated levels of pro-inflammatory cytokines and overexpression of IL-6 [
127]. Moreover, exhaustion following a viral infection is frequently caused by immunological dysregulation and mitochondrial dysfunction [
128]. Therefore, therapeutic strategies for post-COVID tiredness that target these several patho-physiologies can be assessed [
123]. For example, a randomized controlled trial incorporating probiotic supplementation was found to cause a larger reduction in fatigue compared to a placebo [
123]. These findings are encouraging, since probiotics have been demonstrated to enhance mood, improve cognitive function, and lessen fatigue in addition to their immunomodulatory [
129], anti-inflammatory [
130], antioxidant [
131], and antiviral [
132] properties. Probiotics have also shown to improve well-being as well as inflammatory and oxidative indexes in CFS/ME patients [
133], regulate brain health via the gut-brain axis [
134], and significantly improve mood and sleep quality as well as reduce fatigue, depression, and anger [
135,
136].
Currently, probiotics are being tested in some clinical trials (NCT05080244, NCT04813718, and NCT05227170) to determine their effectiveness in preventing and managing long COVID-19.
9. Conclusions
Researchers and clinicians alike face a difficult and complex problem when attempting to understand the complex link between autoimmunity and long-term symptoms of COVID-19. SARS-CoV-2 has not only caused a worldwide epidemic, but it has also revealed a range of immunological reactions and perhaps chronic side effects. Millions of people worldwide are impacted by extended COVID, which emphasizes how vital it is to comprehend its fundamental causes. Raised levels of autoantibodies against several cellular components, cytokines, and receptors indicate the autoimmune nature of the illness. The persistence of symptoms seen in long-term COVID patients may be attributed to these autoantibodies, which may last long after the original infection.
The precise molecular pathways responsible for SARS-CoV-2-induced autoimmunity remain unclear; nevertheless, it seems that the downregulation of ACE2 and the consequent elevation of Ang-II levels are crucial. Based on research using models of RA and experimental autoimmune encephalomyelitis, this imbalance can trigger pathways that lead to autoimmunity. Prolonged inflammation and changes in immune cell populations are features of both the innate and adaptive immune responses in long-term COVID-19 patients. The increased cytotoxicity, exhausted CD8+ T cells, and altered T-cell subsets might be factors in these people's extended immunological activation. Dysregulation of natural killer cells and monocytes, two types of innate immune cells, highlights the immune system's role in prolonged COVID-19.
The microbiota is vital for immunological defense, metabolic functions, and physiological activities, such as food breakdown and immune system maturation. Recent studies have demonstrated the important role that microbiota plays in the immune system and the course of diseases like COVID-19, especially when it comes to the gastrointestinal tract. Research has demonstrated that changes in the gut microbiota of COVID-19 patients may be responsible for the severity of the illness and chronic symptoms, sometimes referred to as long-term COVID or Post-Acute Sequelae of SARS-CoV-2 (PACS). Even after viral clearance, dysbiosis endures and may exacerbate COVID-19-related inflammation. Moreover, there is evidence connecting the development of autoimmunity and gut microbiota dysbiosis, highlighting the wider health implications of this condition. As an alternative for reducing long COVID symptoms, the potential therapeutic effect of restoring microbial balance—especially through probiotic supplementation—is receiving more attention from clinicians. Probiotics provide benefits for mood, cognitive function, and reducing fatigue in addition to immunomodulatory, anti-inflammatory, and antioxidant functions.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1: title; Table S1: title; Video S1: title.
Author Contributions
Conceptualization, A.H.-J., A.R.-C., V.N.U.; validation, A.H.-J., L.M., A.S., T.B., A.R.-C., E.M.R., and V.N.U.; formal analysis, A.H.-J., L.M., A.S., T.B., A.R.-C., E.M.R., and V.N.U.; investigation, A.H.-J., L.M., A.S., T.B., A.R.-C., E.M.R., and V.N.U.; writing – original draft preparation, A.H.-J., L.M., A.S., T.B., A.R.-C., E.M.R., and V.N.U.; writing – review and editing, A.R.-C., E.M.R., and V.N.U.; supervision, A.H.-J., A.R.-C., E.M.R., and V.N.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article as no new data was created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Lundstrom, K.; Hromić-Jahjefendić, A.; Bilajac, E.; Aljabali, A.A.A.; Baralić, K.; Sabri, N.A.; Shehata, E.M.; Raslan, M.; Ferreira, A.; Orlandi, L.; et al. COVID-19 signalome: Pathways for SARS-CoV-2 infection and impact on COVID-19 associated comorbidity. Cell Signal 2023, 101, 110495. [Google Scholar] [CrossRef] [PubMed]
- Hromić-Jahjefendić, A.; Barh, D.; Uversky, V.; Aljabali, A.A.; Tambuwala, M.M.; Alzahrani, K.J.; Alzahrani, F.M.; Alshammeri, S.; Lundstrom, K. Can COVID-19 Vaccines Induce Premature Non-Communicable Diseases: Where Are We Heading to? Vaccines (Basel) 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Hromić-Jahjefendić, A.; Barh, D.; Ramalho Pinto, C.H.; Gabriel Rodrigues Gomes, L.; Picanço Machado, J.L.; Afolabi, O.O.; Tiwari, S.; Aljabali, A.A.A.; Tambuwala, M.M.; Serrano-Aroca, Á.; et al. Associations and Disease-Disease Interactions of COVID-19 with Congenital and Genetic Disorders: A Comprehensive Review. Viruses 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- El-Baky, N.A.; Amara, A.A.; Uversky, V.N.; Redwan, E.M. Intrinsic factors behind long COVID: III. Persistence of SARS-CoV-2 and its components. J Cell Biochem 2024, 125, 22–44. [Google Scholar] [CrossRef]
- El-Maradny, Y.A.; Rubio-Casillas, A.; Mohamed, K.I.; Uversky, V.N.; Redwan, E.M. Intrinsic factors behind long-COVID: II. SARS-CoV-2, extracellular vesicles, and neurological disorders. J Cell Biochem 2023, 124, 1466–1485. [Google Scholar] [CrossRef] [PubMed]
- El-Maradny, Y.A.; Rubio-Casillas, A.; Uversky, V.N.; Redwan, E.M. Intrinsic factors behind long-COVID: I. Prevalence of the extracellular vesicles. J Cell Biochem 2023, 124, 656–673. [Google Scholar] [CrossRef]
- Saad, M.H.; Badierah, R.; Redwan, E.M.; El-Fakharany, E.M. A Comprehensive Insight into the Role of Exosomes in Viral Infection: Dual Faces Bearing Different Functions. Pharmaceutics 2021, 13. [Google Scholar] [CrossRef]
- Elrashdy, F.; Aljaddawi, A.A.; Redwan, E.M.; Uversky, V.N. On the potential role of exosomes in the COVID-19 reinfection/reactivation opportunity. J Biomol Struct Dyn 2021, 39, 5831–5842. [Google Scholar] [CrossRef]
- Ballering, A.V.; van Zon, S.K.R.; Olde Hartman, T.C.; Rosmalen, J.G.M. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet 2022, 400, 452–461. [Google Scholar] [CrossRef]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun 2022, 101, 93–135. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med 2022, 28, 1461–1467. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T.; Knight, M.; A'Court, C.; Buxton, M.; Husain, L. Management of post-acute covid-19 in primary care. Bmj 2020, 370, m3026. [Google Scholar] [CrossRef] [PubMed]
- Garrigues, E.; Janvier, P.; Kherabi, Y.; Le Bot, A.; Hamon, A.; Gouze, H.; Doucet, L.; Berkani, S.; Oliosi, E.; Mallart, E.; et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect 2020, 81, e4–e6. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Deeks, S.G. Early clues regarding the pathogenesis of long-COVID. Trends Immunol 2022, 43, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Al-Hadrawi, D.S.; Al-Rubaye, H.T.; Almulla, A.F.; Al-Hakeim, H.K.; Maes, M. Lowered oxygen saturation and increased body temperature in acute COVID-19 largely predict chronic fatigue syndrome and affective symptoms due to Long COVID: A precision nomothetic approach. Acta Neuropsychiatr 2023, 35, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Wigerblad, G.; Kaplan, M.J. NETs spread ever wider in rheumatic diseases. Nat Rev Rheumatol 2020, 16, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Radic, M. Oxidation and mitochondrial origin of NET DNA in the pathogenesis of lupus. Nat Med 2016, 22, 126–127. [Google Scholar] [CrossRef]
- Thierry, A.R.; Roch, B. Neutrophil Extracellular Traps and By-Products Play a Key Role in COVID-19: Pathogenesis, Risk Factors, and Therapy. J Clin Med 2020, 9. [Google Scholar] [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Dotan, A.; Muller, S.; Kanduc, D.; David, P.; Halpert, G.; Shoenfeld, Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev 2021, 20, 102792. [Google Scholar] [CrossRef] [PubMed]
- Ortona, E.; Malorni, W. Long COVID: to investigate immunological mechanisms and sex/gender related aspects as fundamental steps for tailored therapy. Eur Respir J 2022, 59. [Google Scholar] [CrossRef] [PubMed]
- Lenti, M.V.; Rossi, C.M.; Melazzini, F.; Gastaldi, M.; Bugatti, S.; Rotondi, M.; Bianchi, P.I.; Gentile, A.; Chiovato, L.; Montecucco, C.; et al. Seronegative autoimmune diseases: A challenging diagnosis. Autoimmun Rev 2022, 21, 103143. [Google Scholar] [CrossRef] [PubMed]
- Halpert, G.; Shoenfeld, Y. SARS-CoV-2, the autoimmune virus. Autoimmun Rev 2020, 19, 102695. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Han, T.; Chen, J.; Hou, C.; Hua, L.; He, S.; Guo, Y.; Zhang, S.; Wang, Y.; Yuan, J.; et al. Clinical and Autoimmune Characteristics of Severe and Critical Cases of COVID-19. Clin Transl Sci 2020, 13, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Ampudia, Y.; Monsalve, D.M.; Rojas, M.; Rodríguez, Y.; Zapata, E.; Ramírez-Santana, C.; Anaya, J.M. Persistent Autoimmune Activation and Proinflammatory State in Post-Coronavirus Disease 2019 Syndrome. J Infect Dis 2022, 225, 2155–2162. [Google Scholar] [CrossRef]
- Rojas, M.; Rodríguez, Y.; Acosta-Ampudia, Y.; Monsalve, D.M.; Zhu, C.; Li, Q.Z.; Ramírez-Santana, C.; Anaya, J.M. Autoimmunity is a hallmark of post-COVID syndrome. J Transl Med 2022, 20, 129. [Google Scholar] [CrossRef]
- Peluso, M.J.; Mitchell, A.; Wang, C.Y.; Takahashi, S.; Hoh, R.; Tai, V.; Durstenfeld, M.S.; Hsue, P.Y.; Kelly, J.D.; Martin, J.N.; et al. Low Prevalence of Interferon α Autoantibodies in People Experiencing Symptoms of Post-Coronavirus Disease 2019 (COVID-19) Conditions, or Long COVID. J Infect Dis 2023, 227, 246–250. [Google Scholar] [CrossRef]
- Son, K.; Jamil, R.; Chowdhury, A.; Mukherjee, M.; Venegas, C.; Miyasaki, K.; Zhang, K.; Patel, Z.; Salter, B.; Yuen, A.C.Y.; et al. Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long COVID symptoms. Eur Respir J 2023, 61. [Google Scholar] [CrossRef] [PubMed]
- Wallukat, G.; Hohberger, B.; Wenzel, K.; Fürst, J.; Schulze-Rothe, S.; Wallukat, A.; Hönicke, A.S.; Müller, J. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J Transl Autoimmun 2021, 4, 100100. [Google Scholar] [CrossRef]
- Vergelli, M.; Hemmer, B.; Muraro, P.A.; Tranquill, L.; Biddison, W.E.; Sarin, A.; McFarland, H.F.; Martin, R. Human autoreactive CD4+ T cell clones use perforin- or Fas/Fas ligand-mediated pathways for target cell lysis. J Immunol 1997, 158, 2756–2761. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Hiroishi, K.; Kaneko, T.; Moriyama, T.; Muto, Y.; Kayagaki, N.; Yagita, H.; Okumura, K.; Imawari, M. Perforin, Fas/Fas ligand, and TNF-alpha pathways as specific and bystander killing mechanisms of hepatitis C virus-specific human CTL. J Immunol 1997, 158, 5283–5291. [Google Scholar] [CrossRef] [PubMed]
- Praper, T.; Sonnen, A.; Viero, G.; Kladnik, A.; Froelich, C.J.; Anderluh, G.; Dalla Serra, M.; Gilbert, R.J. Human perforin employs different avenues to damage membranes. J Biol Chem 2011, 286, 2946–2955. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.E.; Kondos, S.C.; Matthews, A.Y.; D'Angelo, M.E.; Dunstone, M.A.; Whisstock, J.C.; Trapani, J.A.; Bird, P.I. The perforin pore facilitates the delivery of cationic cargos. J Biol Chem 2014, 289, 9172–9181. [Google Scholar] [CrossRef] [PubMed]
- Law, R.H.; Lukoyanova, N.; Voskoboinik, I.; Caradoc-Davies, T.T.; Baran, K.; Dunstone, M.A.; D'Angelo, M.E.; Orlova, E.V.; Coulibaly, F.; Verschoor, S.; et al. The structural basis for membrane binding and pore formation by lymphocyte perforin. Nature 2010, 468, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Ruenjaiman, V.; Sodsai, P.; Kueanjinda, P.; Bunrasmee, W.; Klinchanhom, S.; Reantragoon, R.; Tunvirachaisakul, C.; Manothummetha, K.; Mejun, N.; Liengswangwong, K.; et al. Impact of SARS-CoV-2 infection on the profiles and responses of innate immune cells after recovery. J Microbiol Immunol Infect 2022, 55, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.A.F.; Neves, P.; Lima, S.S.; Lopes, J.D.C.; Torres, M.; Vallinoto, I.; Bichara, C.D.A.; Dos Santos, E.F.; de Brito, M.; da Silva, A.L.S.; et al. Cytokine Profiles Associated With Acute COVID-19 and Long COVID-19 Syndrome. Front Cell Infect Microbiol 2022, 12, 922422. [Google Scholar] [CrossRef]
- Arthur, J.M.; Forrest, J.C.; Boehme, K.W.; Kennedy, J.L.; Owens, S.; Herzog, C.; Liu, J.; Harville, T.O. Development of ACE2 autoantibodies after SARS-CoV-2 infection. PLoS One 2021, 16, e0257016. [Google Scholar] [CrossRef]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.; Sarapultsev, A.; Solomatina, L.; Chereshnev, V. SARS-CoV-2-Specific Immune Response and the Pathogenesis of COVID-19. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Hammoudeh, S.M.; Hammoudeh, A.M.; Bhamidimarri, P.M.; Al Safar, H.; Mahboub, B.; Künstner, A.; Busch, H.; Halwani, R.; Hamid, Q.; Rahmani, M.; et al. Systems Immunology Analysis Reveals the Contribution of Pulmonary and Extrapulmonary Tissues to the Immunopathogenesis of Severe COVID-19 Patients. Front Immunol 2021, 12, 595150. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Yen-Ting Chen, T.; Wang, S.I.; Hung, Y.M.; Chen, H.Y.; Wei, C.J. Risk of autoimmune diseases in patients with COVID-19: A retrospective cohort study. EClinicalMedicine 2023, 56, 101783. [Google Scholar] [CrossRef] [PubMed]
- Visvabharathy, L.; Zhu, C.; Orban, Z.S.; Yarnoff, K.; Palacio, N.; Jimenez, M.; Lim, P.H.; Penaloza-MacMaster, P.; Koralnik, I.J. Autoantibody production is enhanced after mild SARS-CoV-2 infection despite vaccination in individuals with and without long COVID. medRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Deveau, T.M.; Munter, S.E.; Ryder, D.; Buck, A.; Beck-Engeser, G.; Chan, F.; Lu, S.; Goldberg, S.A.; Hoh, R.; et al. Chronic viral coinfections differentially affect the likelihood of developing long COVID. J Clin Invest 2023, 133. [Google Scholar] [CrossRef]
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The immunology and immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef]
- Stegbauer, J.; Lee, D.H.; Seubert, S.; Ellrichmann, G.; Manzel, A.; Kvakan, H.; Muller, D.N.; Gaupp, S.; Rump, L.C.; Gold, R.; et al. Role of the renin-angiotensin system in autoimmune inflammation of the central nervous system. Proc Natl Acad Sci U S A 2009, 106, 14942–14947. [Google Scholar] [CrossRef]
- Rasini, E.; Cosentino, M.; Marino, F.; Legnaro, M.; Ferrari, M.; Guasti, L.; Venco, A.; Lecchini, S. Angiotensin II type 1 receptor expression on human leukocyte subsets: a flow cytometric and RT-PCR study. Regul Pept 2006, 134, 69–74. [Google Scholar] [CrossRef]
- Castleman, M.J.; Stumpf, M.M.; Therrien, N.R.; Smith, M.J.; Lesteberg, K.E.; Palmer, B.E.; Maloney, J.P.; Janssen, W.J.; Mould, K.J.; Beckham, J.D.; et al. SARS-CoV-2 infection relaxes peripheral B cell tolerance. J Exp Med 2022, 219. [Google Scholar] [CrossRef]
- Lee, A.R.; Woo, J.S.; Lee, S.Y.; Lee, Y.S.; Jung, J.; Lee, C.R.; Park, S.H.; Cho, M.L. SARS-CoV-2 spike protein promotes inflammatory cytokine activation and aggravates rheumatoid arthritis. Cell Commun Signal 2023, 21, 44. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.B.; Mori, J.; McLean, B.A.; Basu, R.; Das, S.K.; Ramprasath, T.; Parajuli, N.; Penninger, J.M.; Grant, M.B.; Lopaschuk, G.D.; et al. ACE2 Deficiency Worsens Epicardial Adipose Tissue Inflammation and Cardiac Dysfunction in Response to Diet-Induced Obesity. Diabetes 2016, 65, 85–95. [Google Scholar] [CrossRef]
- Shah, W.; Hillman, T.; Playford, E.D.; Hishmeh, L. Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. Bmj 2021, 372, n136. [Google Scholar] [CrossRef] [PubMed]
- Gyöngyösi, M.; Alcaide, P.; Asselbergs, F.W.; Brundel, B.; Camici, G.G.; Martins, P.D.C.; Ferdinandy, P.; Fontana, M.; Girao, H.; Gnecchi, M.; et al. Long COVID and the cardiovascular system-elucidating causes and cellular mechanisms in order to develop targeted diagnostic and therapeutic strategies: a joint Scientific Statement of the ESC Working Groups on Cellular Biology of the Heart and Myocardial and Pericardial Diseases. Cardiovasc Res 2023, 119, 336–356. [Google Scholar] [CrossRef] [PubMed]
- Pisareva, E.; Badiou, S.; Mihalovičová, L.; Mirandola, A.; Pastor, B.; Kudriavtsev, A.; Berger, M.; Roubille, C.; Fesler, P.; Klouche, K.; et al. Persistence of neutrophil extracellular traps and anticardiolipin auto-antibodies in post-acute phase COVID-19 patients. J Med Virol 2023, 95, e28209. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, M.; Cook, C.E.; Vanni, K.M.M.; Patel, N.J.; D'Silva, K.M.; Fu, X.; Wang, J.; Prisco, L.C.; Kowalski, E.; Zaccardelli, A.; et al. DMARD disruption, rheumatic disease flare, and prolonged COVID-19 symptom duration after acute COVID-19 among patients with rheumatic disease: A prospective study. Semin Arthritis Rheum 2022, 55, 152025. [Google Scholar] [CrossRef]
- Seeßle, J.; Waterboer, T.; Hippchen, T.; Simon, J.; Kirchner, M.; Lim, A.; Müller, B.; Merle, U. Persistent Symptoms in Adult Patients 1 Year After Coronavirus Disease 2019 (COVID-19): A Prospective Cohort Study. Clin Infect Dis 2022, 74, 1191–1198. [Google Scholar] [CrossRef]
- Zhang, Q.; Bastard, P.; Cobat, A.; Casanova, J.L. Human genetic and immunological determinants of critical COVID-19 pneumonia. Nature 2022, 603, 587–598. [Google Scholar] [CrossRef]
- van der Wijst, M.G.P.; Vazquez, S.E.; Hartoularos, G.C.; Bastard, P.; Grant, T.; Bueno, R.; Lee, D.S.; Greenland, J.R.; Sun, Y.; Perez, R.; et al. Type I interferon autoantibodies are associated with systemic immune alterations in patients with COVID-19. Sci Transl Med 2021, 13, eabh2624. [Google Scholar] [CrossRef]
- Guo, L.; Wang, G.; Wang, Y.; Zhang, Q.; Ren, L.; Gu, X.; Huang, T.; Zhong, J.; Wang, Y.; Wang, X.; et al. SARS-CoV-2-specific antibody and T-cell responses 1 year after infection in people recovered from COVID-19: a longitudinal cohort study. Lancet Microbe 2022, 3, e348–e356. [Google Scholar] [CrossRef]
- Kundura, L.; Cezar, R.; André, S.; Campos-Mora, M.; Lozano, C.; Vincent, T.; Muller, L.; Lefrant, J.Y.; Roger, C.; Claret, P.G.; et al. Low perforin expression in CD8+ T lymphocytes during the acute phase of severe SARS-CoV-2 infection predicts long COVID. Front Immunol 2022, 13, 1029006. [Google Scholar] [CrossRef] [PubMed]
- Galán, M.; Vigón, L.; Fuertes, D.; Murciano-Antón, M.A.; Casado-Fernández, G.; Domínguez-Mateos, S.; Mateos, E.; Ramos-Martín, F.; Planelles, V.; Torres, M.; et al. Persistent Overactive Cytotoxic Immune Response in a Spanish Cohort of Individuals With Long-COVID: Identification of Diagnostic Biomarkers. Front Immunol 2022, 13, 848886. [Google Scholar] [CrossRef]
- Fjelltveit, E.B.; Blomberg, B.; Kuwelker, K.; Zhou, F.; Onyango, T.B.; Brokstad, K.A.; Elyanow, R.; Kaplan, I.M.; Tøndel, C.; Mohn, K.G.I.; et al. Symptom Burden and Immune Dynamics 6 to 18 Months Following Mild Severe Acute Respiratory Syndrome Coronavirus 2 Infection (SARS-CoV-2): A Case-control Study. Clin Infect Dis 2023, 76, e60–e70. [Google Scholar] [CrossRef]
- Wiech, M.; Chroscicki, P.; Swatler, J.; Stepnik, D.; De Biasi, S.; Hampel, M.; Brewinska-Olchowik, M.; Maliszewska, A.; Sklinda, K.; Durlik, M.; et al. Remodeling of T Cell Dynamics During Long COVID Is Dependent on Severity of SARS-CoV-2 Infection. Front Immunol 2022, 13, 886431. [Google Scholar] [CrossRef] [PubMed]
- Hornig, M.; Montoya, J.G.; Klimas, N.G.; Levine, S.; Felsenstein, D.; Bateman, L.; Peterson, D.L.; Gottschalk, C.G.; Schultz, A.F.; Che, X.; et al. Distinct plasma immune signatures in ME/CFS are present early in the course of illness. Sci Adv 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Tesch, F.; Ehm, F.; Vivirito, A.; Wende, D.; Batram, M.; Loser, F.; Menzer, S.; Jacob, J.; Roessler, M.; Seifert, M.; et al. Incident autoimmune diseases in association with SARS-CoV-2 infection: a matched cohort study. Clin Rheumatol 2023, 42, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Ganigara, M.; Galeotti, C.; Burns, J.; Berganza, F.M.; Hayes, D.A.; Singh-Grewal, D.; Bharath, S.; Sajjan, S.; Bayry, J. Multisystem inflammatory syndrome in children and Kawasaki disease: a critical comparison. Nat Rev Rheumatol 2021, 17, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Altmann, D.M.; Reynolds, C.J.; Joy, G.; Otter, A.D.; Gibbons, J.M.; Pade, C.; Swadling, L.; Maini, M.K.; Brooks, T.; Semper, A.; et al. Persistent symptoms after COVID-19 are not associated with differential SARS-CoV-2 antibody or T cell immunity. Nat Commun 2023, 14, 5139. [Google Scholar] [CrossRef]
- Peng, K.; Li, X.; Yang, D.; Chan, S.C.W.; Zhou, J.; Wan, E.Y.F.; Chui, C.S.L.; Lai, F.T.T.; Wong, C.K.H.; Chan, E.W.Y.; et al. Risk of autoimmune diseases following COVID-19 and the potential protective effect from vaccination: a population-based cohort study. EClinicalMedicine 2023, 63, 102154. [Google Scholar] [CrossRef]
- Cavalli, E.; Bramanti, A.; Ciurleo, R.; Tchorbanov, A.I.; Giordano, A.; Fagone, P.; Belizna, C.; Bramanti, P.; Shoenfeld, Y.; Nicoletti, F. Entangling COVID-19 associated thrombosis into a secondary antiphospholipid antibody syndrome: Diagnostic and therapeutic perspectives (Review). Int J Mol Med 2020, 46, 903–912. [Google Scholar] [CrossRef]
- Trahtemberg, U.; Rottapel, R.; Dos Santos, C.C.; Slutsky, A.S.; Baker, A.; Fritzler, M.J. Anticardiolipin and other antiphospholipid antibodies in critically ill COVID-19 positive and negative patients. Ann Rheum Dis 2021, 80, 1236–1240. [Google Scholar] [CrossRef]
- Juanes-Velasco, P.; Landeira-Viñuela, A.; García-Vaquero, M.L.; Lecrevisse, Q.; Herrero, R.; Ferruelo, A.; Góngora, R.; Corrales, F.; Rivas, J.L.; Lorente, J.A.; et al. SARS-CoV-2 Infection Triggers Auto-Immune Response in ARDS. Front Immunol 2022, 13, 732197. [Google Scholar] [CrossRef]
- Taeschler, P.; Cervia, C.; Zurbuchen, Y.; Hasler, S.; Pou, C.; Tan, Z.; Adamo, S.; Raeber, M.E.; Bächli, E.; Rudiger, A.; et al. Autoantibodies in COVID-19 correlate with antiviral humoral responses and distinct immune signatures. Allergy 2022, 77, 2415–2430. [Google Scholar] [CrossRef]
- Baiocchi, G.C.; Vojdani, A.; Rosenberg, A.Z.; Vojdani, E.; Halpert, G.; Ostrinski, Y.; Zyskind, I.; Filgueiras, I.S.; Schimke, L.F.; Marques, A.H.C.; et al. Cross-sectional analysis reveals autoantibody signatures associated with COVID-19 severity. J Med Virol 2023, 95, e28538. [Google Scholar] [CrossRef] [PubMed]
- Shome, M.; Chung, Y.; Chavan, R.; Park, J.G.; Qiu, J.; LaBaer, J. Serum autoantibodyome reveals that healthy individuals share common autoantibodies. Cell Rep 2022, 39, 110873. [Google Scholar] [CrossRef] [PubMed]
- Silverman, G.J.; Grönwall, C.; Vas, J.; Chen, Y. Natural autoantibodies to apoptotic cell membranes regulate fundamental innate immune functions and suppress inflammation. Discov Med 2009, 8, 151–156. [Google Scholar]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. New England journal of medicine 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Baquero, F.; Nombela, C. The microbiome as a human organ. 2012, <italic>18</italic>, 2-4.
- Guarner, F.; Malagelada, J.-R. Gut flora in health and disease. The lancet 2003, 361, 512–519. [Google Scholar] [CrossRef]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef]
- Schirmer, M.; Smeekens, S.P.; Vlamakis, H.; Jaeger, M.; Oosting, M.; Franzosa, E.A.; Ter Horst, R.; Jansen, T.; Jacobs, L.; Bonder, M.J. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell 2016, 167, 1125–1136. e1128. [Google Scholar] [CrossRef]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; Deroos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Weaver, L.K.; Minichino, D.; Biswas, C.; Chu, N.; Lee, J.-J.; Bittinger, K.; Albeituni, S.; Nichols, K.E.; Behrens, E.M. Microbiota-dependent signals are required to sustain TLR-mediated immune responses. JCI insight 2019, 4. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.; Yeoh, Y.K.; Li, A.Y.; Zhan, H.; Wan, Y.; Chung, A.C.; Cheung, C.P.; Chen, N. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology 2020, 159, 944–955. e948. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, H.H.; Fissel, J.A.; Fanelli, B.; Bergman, Y.; Gniazdowski, V.; Dadlani, M.; Carroll, K.C.; Colwell, R.R.; Simner, P.J. Metagenomic next-generation sequencing of nasopharyngeal specimens collected from confirmed and suspect COVID-19 patients. MBio 2020, 11, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Higginson, E.; Pereira-Dias, J.; Curran, M.D.; Parmar, S.; Khokhar, F.; Cuchet-Lourenço, D.; Lux, J.; Sharma-Hajela, S.; Ravenhill, B. Correction to: Ventilator-associated pneumonia in critically ill patients with COVID-19. Critical Care 2021, 25. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F. The trinity of COVID-19: immunity, inflammation and intervention. Nature Reviews Immunology 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Liu, Y.; Chan, M.T.; Chan, F.K.; Wu, W.K.; Ng, S.C.; Zhang, L. Lower gut abundance of Eubacterium rectale is linked to COVID-19 mortality. Frontiers in Cellular and Infection Microbiology 2023, 13. [Google Scholar] [CrossRef]
- Rueca, M.; Fontana, A.; Bartolini, B.; Piselli, P.; Mazzarelli, A.; Copetti, M.; Binda, E.; Perri, F.; Gruber, C.E.M.; Nicastri, E. Investigation of nasal/oropharyngeal microbial community of COVID-19 patients by 16S rDNA sequencing. International journal of environmental research and public health 2021, 18, 2174. [Google Scholar] [CrossRef]
- Trøseid, M.; Holter, J.C.; Holm, K.; Vestad, B.; Sazonova, T.; Granerud, B.K.; Dyrhol-Riise, A.M.; Holten, A.R.; Tonby, K.; Kildal, A.B. Gut microbiota composition during hospitalization is associated with 60-day mortality after severe COVID-19. Critical Care 2023, 27, 69. [Google Scholar] [CrossRef]
- Camargo, S.M.; Singer, D.; Makrides, V.; Huggel, K.; Pos, K.M.; Wagner, C.A.; Kuba, K.; Danilczyk, U.; Skovby, F.; Kleta, R. Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology 2009, 136, 872–882882. e873. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012, 487, 477–481. [Google Scholar] [CrossRef]
- Perlot, T.; Penninger, J.M. ACE2–From the renin–angiotensin system to gut microbiota and malnutrition. Microbes and infection 2013, 15, 866–873. [Google Scholar] [CrossRef]
- Viana, S.D.; Nunes, S.; Reis, F. ACE2 imbalance as a key player for the poor outcomes in COVID-19 patients with age-related comorbidities–role of gut microbiota dysbiosis. Ageing research reviews 2020, 62, 101123. [Google Scholar] [CrossRef]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.-Y.; Ng, S.S.S.; Zhang, F.; Li, A.Y.; Lu, W.; Hui, D.S.-C. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022, 71, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhou, Y.; Ma, Y.; Chen, P.; Tang, J.; Yang, B.; Li, H.; Liang, M.; Xue, Y.; Liu, Y. Gut microbiota dysbiosis correlates with long COVID-19 at one-year after discharge. Journal of Korean Medical Science 2023, 38. [Google Scholar] [CrossRef]
- Vojdani, A.; Vojdani, E.; Rosenberg, A.Z.; Shoenfeld, Y. The role of exposomes in the pathophysiology of autoimmune diseases II: Pathogens. Pathophysiology 2022, 29, 243–280. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Wang, G. Environmental agents, oxidative stress and autoimmunity. Current opinion in toxicology 2018, 7, 22–27. [Google Scholar] [CrossRef]
- Dehner, C.; Fine, R.; Kriegel, M.A. The microbiome in systemic autoimmune disease: mechanistic insights from recent studies. Current opinion in rheumatology 2019, 31, 201–207. [Google Scholar] [CrossRef]
- De Luca, F.; Shoenfeld, Y. The microbiome in autoimmune diseases. Clinical & Experimental Immunology 2019, 195, 74–85. [Google Scholar]
- Li, B.; Selmi, C.; Tang, R.; Gershwin, M.; Ma, X. The microbiome and autoimmunity: a paradigm from the gut–liver axis. Cellular & molecular immunology 2018, 15, 595–609. [Google Scholar]
- Abe, K.; Takahashi, A.; Fujita, M.; Imaizumi, H.; Hayashi, M.; Okai, K.; Ohira, H. Dysbiosis of oral microbiota and its association with salivary immunological biomarkers in autoimmune liver disease. PloS one 2018, 13, e0198757. [Google Scholar] [CrossRef]
- Choi, S.-C.; Brown, J.; Gong, M.; Ge, Y.; Zadeh, M.; Li, W.; Croker, B.P.; Michailidis, G.; Garrett, T.J.; Mohamadzadeh, M. Gut microbiota dysbiosis and altered tryptophan catabolism contribute to autoimmunity in lupus-susceptible mice. Science translational medicine 2020, 12, eaax2220. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, G.L.V.; Leite, A.Z.; Higuchi, B.S.; Gonzaga, M.I.; Mariano, V.S. Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology 2017, 152, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Harbison, J.E.; Roth-Schulze, A.J.; Giles, L.C.; Tran, C.D.; Ngui, K.M.; Penno, M.A.; Thomson, R.L.; Wentworth, J.M.; Colman, P.G.; Craig, M.E. Gut microbiome dysbiosis and increased intestinal permeability in children with islet autoimmunity and type 1 diabetes: A prospective cohort study. Pediatric diabetes 2019, 20, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Horta-Baas, G.; Romero-Figueroa, M.d.S.; Montiel-Jarquín, A.J.; Pizano-Zárate, M.L.; García-Mena, J.; Ramírez-Durán, N. Intestinal dysbiosis and rheumatoid arthritis: a link between gut microbiota and the pathogenesis of rheumatoid arthritis. Journal of immunology research 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Mousa, W.K.; Chehadeh, F.; Husband, S. Microbial dysbiosis in the gut drives systemic autoimmune diseases. Frontiers in Immunology 2022, 13, 906258. [Google Scholar] [CrossRef]
- Maeda, Y.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Ito, Y.; Gotoh, K.; Hirota, K.; Matsushita, M.; Furuta, Y.; Narazaki, M. Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis & rheumatology 2016, 68, 2646–2661. [Google Scholar]
- Alghamdi, M.A.; Redwan, E.M. Interplay of microbiota and citrullination in the immunopathogenesis of rheumatoid arthritis. Probiotics and antimicrobial proteins 2022, 14, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Moulton, V.R.; Suarez-Fueyo, A.; Meidan, E.; Li, H.; Mizui, M.; Tsokos, G.C. Pathogenesis of human systemic lupus erythematosus: a cellular perspective. Trends in molecular medicine 2017, 23, 615–635. [Google Scholar] [CrossRef]
- Mu, Q.; Zhang, H.; Liao, X.; Lin, K.; Liu, H.; Edwards, M.R.; Ahmed, S.A.; Yuan, R.; Li, L.; Cecere, T.E. Control of lupus nephritis by changes of gut microbiota. Microbiome 2017, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hevia, A.; Arboleya, S.; Gueimonde Fernández, M.; Margolles Barros, A. Intestinal dysbiosis associated with Systemic Lupus Erythematosus highlights microbial targets for intervention strategies. 2014.
- Azzouz, D.; Omarbekova, A.; Heguy, A.; Schwudke, D.; Gisch, N.; Rovin, B.H.; Caricchio, R.; Buyon, J.P.; Alekseyenko, A.V.; Silverman, G.J. Lupus nephritis is linked to disease-activity associated expansions and immunity to a gut commensal. Annals of the rheumatic diseases 2019, 78, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, Z.; Yu, A.M.; Wang, W.; Wei, Z.; Akhter, E.; Maurer, K.; Reis, P.C.; Song, L.; Petri, M. The SLE transcriptome exhibits evidence of chronic endotoxin exposure and has widespread dysregulation of non-coding and coding RNAs. PloS one 2014, 9, e93846. [Google Scholar] [CrossRef] [PubMed]
- Ogunrinde, E.; Zhou, Z.; Luo, Z.; Alekseyenko, A.; Li, Q.Z.; Macedo, D.; Kamen, D.L.; Oates, J.C.; Gilkeson, G.S.; Jiang, W. A link between plasma microbial translocation, microbiome, and autoantibody development in first-degree relatives of systemic lupus erythematosus patients. Arthritis & rheumatology 2019, 71, 1858–1868. [Google Scholar]
- Tursi, S.A.; Lee, E.Y.; Medeiros, N.J.; Lee, M.H.; Nicastro, L.K.; Buttaro, B.; Gallucci, S.; Wilson, R.P.; Wong, G.C.; Tükel, Ç. Bacterial amyloid curli acts as a carrier for DNA to elicit an autoimmune response via TLR2 and TLR9. PLoS pathogens 2017, 13, e1006315. [Google Scholar] [CrossRef] [PubMed]
- Gallo, P.M.; Rapsinski, G.J.; Wilson, R.P.; Oppong, G.O.; Sriram, U.; Goulian, M.; Buttaro, B.; Caricchio, R.; Gallucci, S.; Tükel, Ç. Amyloid-DNA composites of bacterial biofilms stimulate autoimmunity. Immunity 2015, 42, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M. The microbiome and inflammatory bowel disease. Journal of Allergy and Clinical Immunology 2020, 145, 16–27. [Google Scholar] [CrossRef]
- Boziki, M.K.; Kesidou, E.; Theotokis, P.; Mentis, A.-F.A.; Karafoulidou, E.; Melnikov, M.; Sviridova, A.; Rogovski, V.; Boyko, A.; Grigoriadis, N. Microbiome in multiple sclerosis: where are we, what we know and do not know. Brain sciences 2020, 10, 234. [Google Scholar] [CrossRef]
- Zhang, H.; Liao, X.; Sparks, J.B.; Luo, X.M. Dynamics of gut microbiota in autoimmune lupus. Applied and environmental microbiology 2014, 80, 7551–7560. [Google Scholar] [CrossRef]
- Abdelhamid, L.; Luo, X.M. Retinoic acid, leaky gut, and autoimmune diseases. Nutrients 2018, 10, 1016. [Google Scholar] [CrossRef]
- Rathi, A.; Jadhav, S.B.; Shah, N. A randomized controlled trial of the efficacy of systemic enzymes and probiotics in the resolution of post-COVID fatigue. Medicines 2021, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Kedor, C.; Freitag, H.; Meyer-Arndt, L.; Wittke, K.; Zoller, T.; Steinbeis, F.; Haffke, M.; Rudolf, G.; Heidecker, B.; Volk, H. Chronic COVID-19 Syndrome and Chronic Fatigue Syndrome (ME/CFS) following the first pandemic wave in Germany–a first analysis of a prospective observational study. <italic>MedRxiv </italic>2021, 2021.2002. 2006.21249256.
- Poenaru, S.; Abdallah, S.J.; Corrales-Medina, V.; Cowan, J. COVID-19 and post-infectious myalgic encephalomyelitis/chronic fatigue syndrome: a narrative review. Therapeutic advances in infectious disease 2021, 8, 20499361211009385. [Google Scholar] [CrossRef] [PubMed]
- Rudroff, T.; Fietsam, A.C.; Deters, J.R.; Bryant, A.D.; Kamholz, J. Post-COVID-19 fatigue: potential contributing factors. Brain sciences 2020, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- Perrin, R.; Riste, L.; Hann, M.; Walther, A.; Mukherjee, A.; Heald, A. Into the looking glass: Post-viral syndrome post COVID-19. Medical hypotheses 2020, 144, 110055. [Google Scholar] [CrossRef] [PubMed]
- Toogood, P.L.; Clauw, D.J.; Phadke, S.; Hoffman, D. Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): Where will the drugs come from? Pharmacological Research 2021, 165, 105465. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory effects of probiotics on cytokine profiles. BioMed research international 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Morshedi, M.; Hashemi, R.; Moazzen, S.; Sahebkar, A.; Hosseinifard, E.-S. Immunomodulatory and anti-inflammatory effects of probiotics in multiple sclerosis: a systematic review. Journal of neuroinflammation 2019, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Eguchi, K.; Fujitani, N.; Nakagawa, H.; Miyazaki, T. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Scientific reports 2019, 9, 4812. [Google Scholar] [CrossRef]
- Venturini, L.; Bacchi, S.; Capelli, E.; Lorusso, L.; Ricevuti, G.; Cusa, C. Modification of immunological parameters, oxidative stress markers, mood symptoms, and well-being status in CFS patients after probiotic intake: observations from a pilot study. Oxidative medicine and cellular longevity 2019, 2019. [Google Scholar] [CrossRef]
- Kim, N.; Yun, M.; Oh, Y.J.; Choi, H.-J. Mind-altering with the gut: Modulation of the gut-brain axis with probiotics. Journal of microbiology 2018, 56, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Marotta, A.; Sarno, E.; Del Casale, A.; Pane, M.; Mogna, L.; Amoruso, A.; Felis, G.E.; Fiorio, M. Effects of probiotics on cognitive reactivity, mood, and sleep quality. Frontiers in psychiatry 2019, 10, 427235. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Peters, C.; Ho, C.Y.X.; Lim, D.Y.; Yeo, W.-S. A meta-analysis of the use of probiotics to alleviate depressive symptoms. Journal of affective disorders 2018, 228, 13–19. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).