1. Introduction
Transport and binding of individual molecules to specific targets appear to be involved in the assembly and function of supramolecular structures in living cells. There is broad consensus that the analysis of these processes in vitro and vivo is of central importance in life sciences. In cellular compartments transport is often driven by diffusion, which tends to equalize local concentration gradients. Diffusion behavior has been studied in recent decades using various experimental technologies, mainly based on fluorescence techniques such as confocal or far-field optics including nanoscopy [
1]. In order to dentify localization-specific dynamics and interactions of fluorescently labeled species, time-resolved protein mobility data are linked to images of cellular structures measured by fluorescence laser scanning microscopy [
2]. The spatial resolution of mobility and interaction is no longer limited by the diffraction-limited size of the excitation volume (observation/detection volume), as it is the case in confocal fluorescence microscopy/spectroscopy setups. Green fluorescent protein (GFP) and its spectral relatives are selected for many of these approaches due to their high fluorescence yield and photobleaching properties in the intracellular environment [
3], and they can be fused to proteins in vivo [
4,
5]. As a result of these technological and biotechnological developments, single-molecule studies in liquids and live cells have emerged without immobilization or without hydrodynamics.. Such studies refer to measurements of individual molecules (individual particles), but cannot be equated with them [
6]. In the present research article, significant progress has been made in the biophysics and biochemistry of single molecules/particles by demonstrating localized Brownian motion and fractal diffusion in crowded living cell with computer simulation experiments. The “Introduction” and “Methods” sections provide comprehensive background information and a detailed description of the methodology used. For free diffusion-controlled reactions or systems, there are physical criteria to make a clear decision, whether a single molecule/a single particle (an individul molecule/an individula particel) is detected or not as summarized in Table 1.
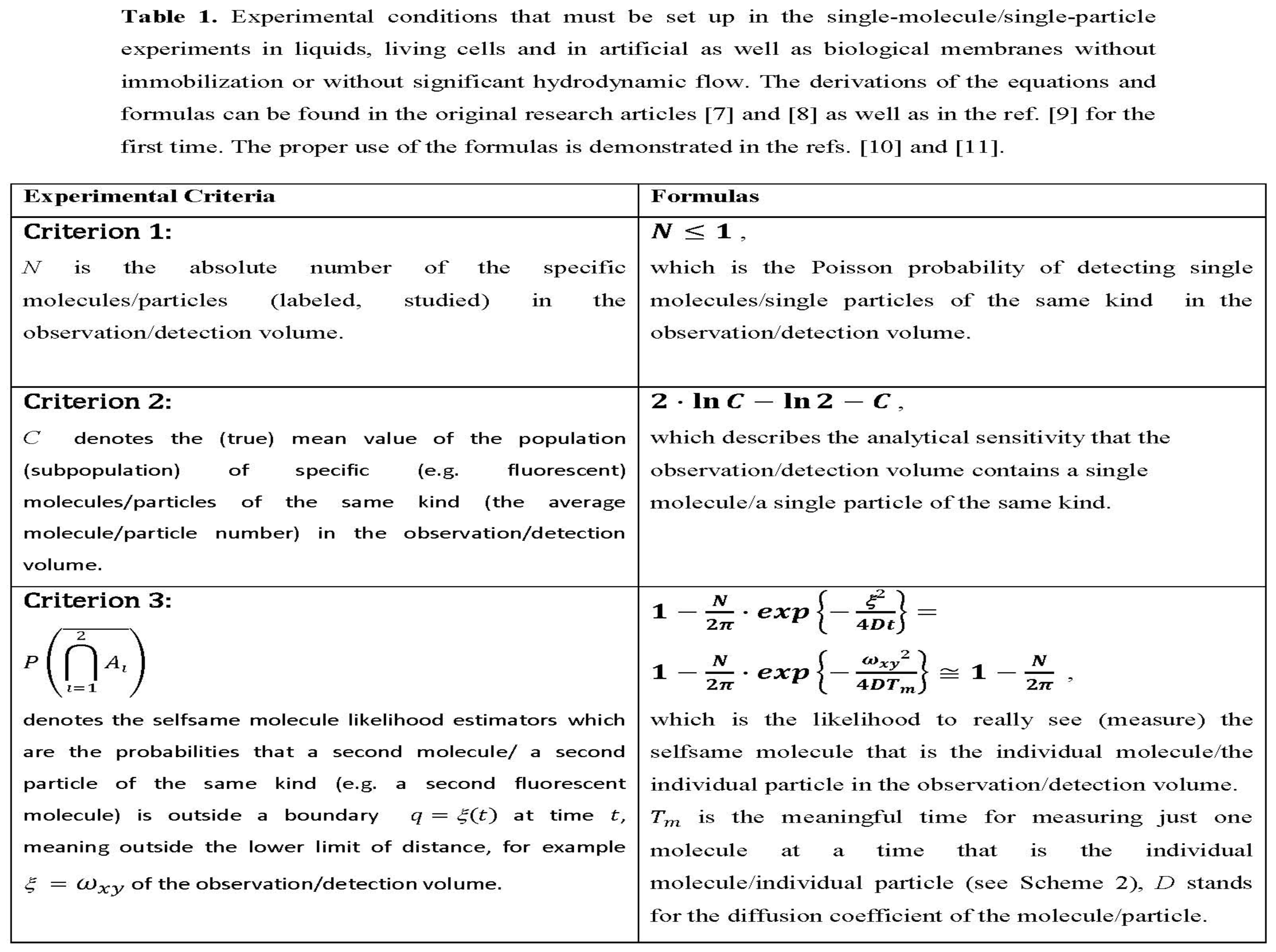
Molecule number fluctuations are currently of interest. The fundamental results are due to Markov processes which are the gateway to dynamical systems whose number of molecules fluctuates. The probability of separating two individual molecules or two individual particles as independent molecular entities during the measurement time is the criterion 3 as exemplified in ref. [
11] (see Table 1 contained therein for various experimental conditions) and Table 1 of this article.
The
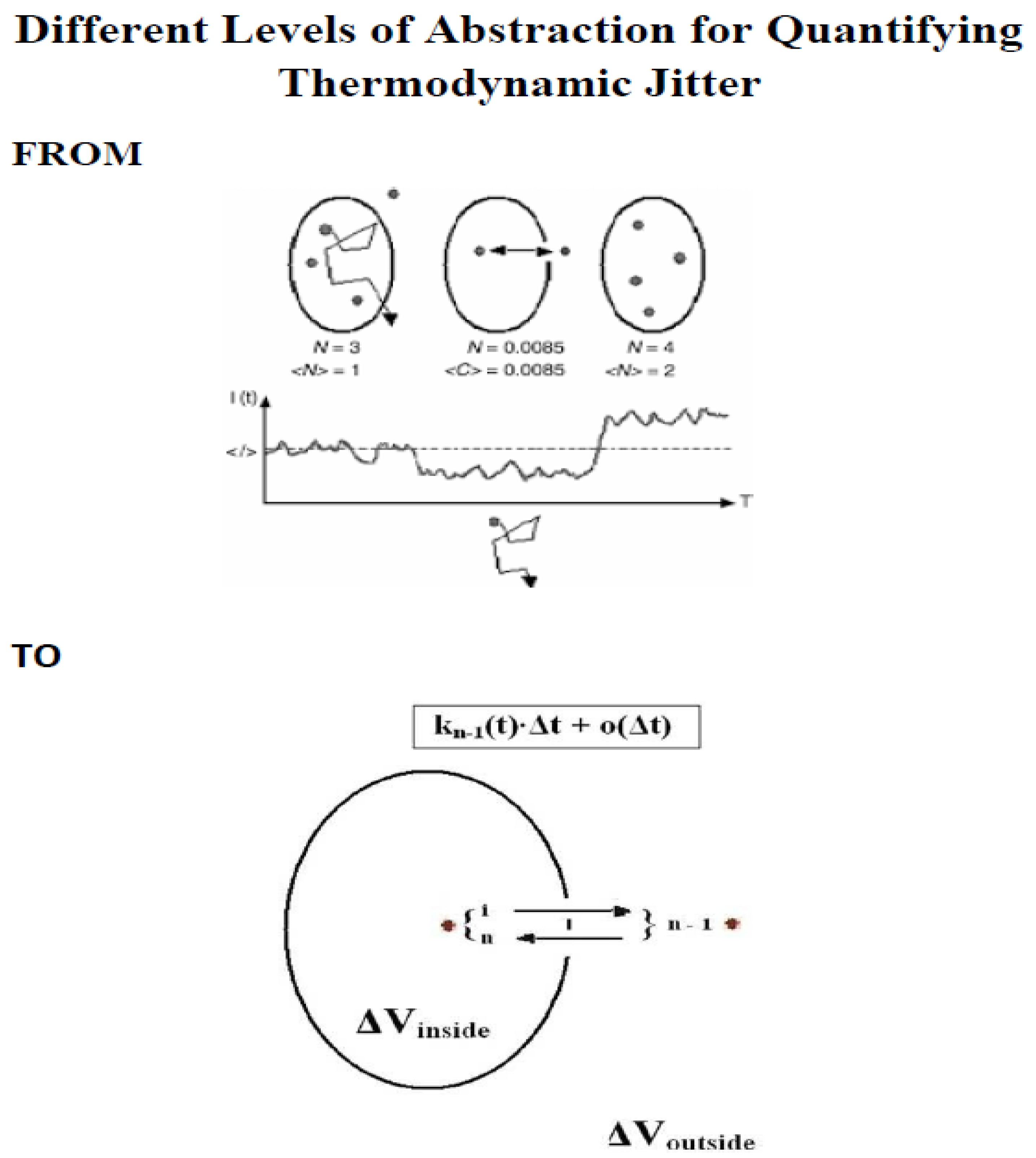
Scheme 1 and the
Scheme 2 summarize important theoretical results for the measurement of just one molecule at a time, e.g. in liquids at room temperature or in living cells under physiological conditions, without immobilization or without significant hydrodynamic flow. We have already gained extensive experience with single-molecule detection in liquids and living cells (see subsection
3.1. Evaluation of single-molecule detection: A brief Narrative o
n the motivation of the simulation experiments and their design as experimetnal starting point for the obtained results). Here, we want to make a contribution in the field of ‘single-molecule tracking’ for the benefit of researchers, for example in molecular sciences such as biophysics, physical chemistry and biochemistry. We provide an analysis of the new findings by connecting them to broader theoretical concepts. The importance of thermodynamic jitter and implications for single-molecule studies are well established.
2. Methods
Our simulations presented in this research article are based on the generator g24 from ref. [
15], which simulates the fractal structure in a crowded biological cell, e. g. in its cytoplasm [
15]. A limited continuous time random walk (LCTRW) is then performed on this structure (generalized 3D Sierpinski carpet) to assess the statistical properties of the diffusion process. The continuous time random walk (on this 3D geometric structure) is based on a distribution function that is freely configurable. Theoretical considerations for simulation and analyzing mobility data in this way for live cells and their compartments such as the cytoplasm are given in ref. [
15].
The theoretical and simulation details from Ref. [
15] clearly showed that randomness in cellular systems can be either spatial (anomalous) or temporal (heterogeneous). We limited our analyses to generalized Sierpinski carpets (GSC), which eliminate no more than 50% of the generator’s components in d dimensions. We created a generator in this manner, designating it with 24 sub-cubes in three dimensions, which we indicated with g
24 also called fractal generator g24. The GSC design specifications are contained in [
15]. The collection of these GSCs may be seen as idealized representations of a region with a wide range of sizes of diffusion barriers. Molecular crowding represented by geometric spatial constraints of the fractal support may impede the movements of proteins. Widely used experimental methods such as fluorescence fluctuation spectroscopy like fluorescence correlation spectroscopy (FCS) reveal apparent values of the diffusion coefficients and the scaling exponents. In the examples chosen so far in ref. [
15], a good agreement between the measured apparent diffusion coefficients in the cytoplasm of a living cell and the known molecular weights showed that our predictions are reasonable, for example, on the 3D fractal support indicated with „g24 generator“. Here, we used an apparent diffusion coefficient of
Dapp ≈ 0,1 x 10
-10 [m
2/s] given in the Figure 9 of ref. [
15]. The Figure 9 in ref. [
15] showed three examples for simulated data of the selfsame molecule from which MSD (mean square displacement) and the power law behavior can be evaluated.The distribution function of a CTRW running on the fractal support, for example the g24 generator, may be chosen to imitate energetic landscapes. In the present article, we thus take a closer look at the motion as a set of tracks performed on a fractal support. Examples of three-dimensional random walk tracks with Gaussian (normal) and gamma distributions in the CTRW are shown in the
Figure 1 and
Figure 2 of the present article. These two distributions represent two distinct processes. Clarifying the behavior of random walks on fractal supports is of great interest (see
3. Results section).
The distribution function of a CTRW may be chosen to simulate the differences between Brownian and anomalous diffusion, which makes the investigated tracks typical (see 3. Results section); this means that the examined distribution functions characterize the tracks. A conceptual framework for crowded and heterogeneous environments like biological cells and their cellular compartments is translational anomalous, sub-diffusive motions of bio-macromolecules due to geometric and/or energetic disorder [
15].
To highlight the disparities in the distribution function on the fractal structure, we investigated two scenarios in this original research article, each consisting of typical tracks. All pathways have one thing in common: they are placed in the coordinate origin that we have chosen for utilization, which is within the femtoliter measurement volume (detection/observation volume, ’chamber’) that is the focus of a laser used for excitation and detection of fluorescent molecules (for further theoretical and simulation details see ref. [
15]).
3. Results
3.1. Evaluation of Single-Molecule Detection: A Brief Narrative on the Motivation of the Simulation Experiments and Their Design as Experimetnal Starting Point for the Obtained Results
The basic idea of single molecule/single particle detection is simple. A single molecule/single particle experiment is, strictly speaking, an experiment that studies the properties of an individual molecule/particle that is one and the same molecule/particle (selfsame molecule/selfsame particle) only. Two facts are of great importance. First, only one probe molecule/probe particle should diffuse into the excited detection volume, and second, the light emitted from this individual molecule/individual particle should be distinguishable from the experimental noise. So far so simple, but it becomes much more difficult when the scenario is quantified based on the temporal movement (time resolution) of individual molecules/individual particles. The thermodynamic concept of diffusion fluctuations also called thermodynamic jitter due to the individual molecule/individual particle (selfsame molecule, selfsame particle) was developed in refs. [
7,
8,
9,
10,
12]. The way from the mathematical core to the physical Theory of Single-Molecule/Single-Particle Biophysics & Biochemistry based on the stochastic nature of diffusion is exemplified in refs. [
11,
16,
17]. Particle means physically each molecule. The conceptual and theoretical basis of thermodynamic jitter has been established in these series of publications and it was considered pioneering work [
18]. In the present study, the concept of thermodynamic jitter was utilized to analyze the motion of single molecules within the cytoplasm of live cells. By simulating these movements and observing the resulting tracks, we were able to demonstrate that individual molecules exhibit localized Brownian motion and fanning out patterns typical of fractal diffusion (see below in the subsection
3.2. Single-Molecule Tracking).
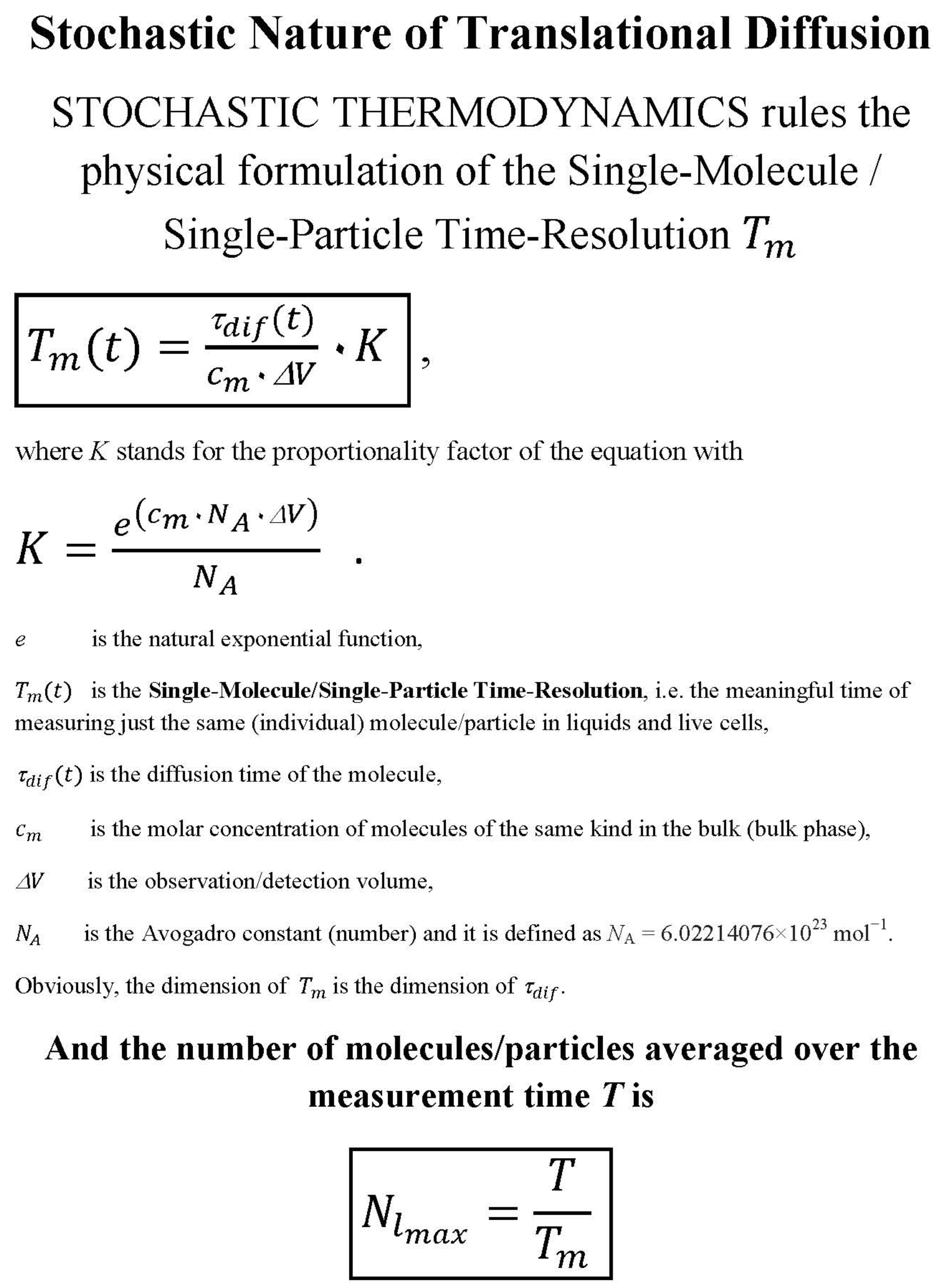
To decide whether there is a single molecule/particle (an individual molecule/individual particle) or not, physical criteria are required, which we summarized as compact and short introduction in
Scheme 1 for interested readers who want to get the mathematical details and the original derivations of these formulas. These criteria 1 to 3 then result in the time resolution of a single molecule/single particle (individual molecule/individual particle), which represents the criterion 4 in optical microscopy/nanoscopy and spectroscopy shown in
Scheme 2, which covers the most important families of dynamic systems with randomness.
The main question addressed by the research article is well defined in the title. Special attention deserve the potential applicability of the study.
The measurement of the individual molecule over several milliseconds to seconds and even minutes is considered one of the most demanding research trends in spectroscopy, microscopy and nanoscopy. The way to understand what occurs during diffusion of an individual molecule/individual particle in the observation/detection volume embedded into a bulk phase without immobilization or without significant hydrodynamics (hydrodynamic flow) is looking deeper into this theory of single-molecule/single-particle detection of one and the same molecule/particle (the individual molecule/individual particle) [
15,
19]. And that is exactly what the short introductory presentation in
Scheme 1 and
Scheme 2 is for. They outline the challenge of measuring single molecules/particles at room temperature or under physiological conditions without immobilization or significant hydrodynamics by mentioning the Theory of Single Molecule/Single Particle Biophysics and Biochemistry based on the stochastic nature of diffusion in liquids and live cells without immobilization on artificial or biological surfaces/membranes or without significant hydrodynamic flow (hydrodynamics).
Next, let us now shed some light in tracking of single molecules (individual molecules) for live cells by simulations. In live cells or their compartments such as the cytoplasm or membranes as well as the nucleus, the movements of molecules are complicated due to the large crowding and expected heterogeneity of the intracellular environment compared to liquids. The simulation approaches that we use are continuous time random walks (CTRW) on a fractal support of a generalized 3D Sierpinski carpet representing a live cell, e. g. its cytoplasm (see
Section 2.
Methods).
3.2. Single- Molecule Tracking
Let us say, we have diffused to about 1 nanometer from another protein. Our encounter has begun, and in roughly a nanosecond we will either touch the surface of the other protein, or be repelled by it. For the first time, we studied the effects of randomly distributed diffusivities using two distribution models that run on the fractal structure representing the cytosol in a living cell (see
Section 2 Methods). The more widely spread Gaussian (normal) model was compared with the gamma model. The Gaussian distribution is a parametric distribution that depends on two variables, the mean and the standard deviation of the diffusion coefficient. The main motivation for using the gamma distribution is that the gamma distribution may be potentially more informative than the Gaussian distribution due to an additional parameter and the representation of time intervals between events (see
Section 2. Methods).
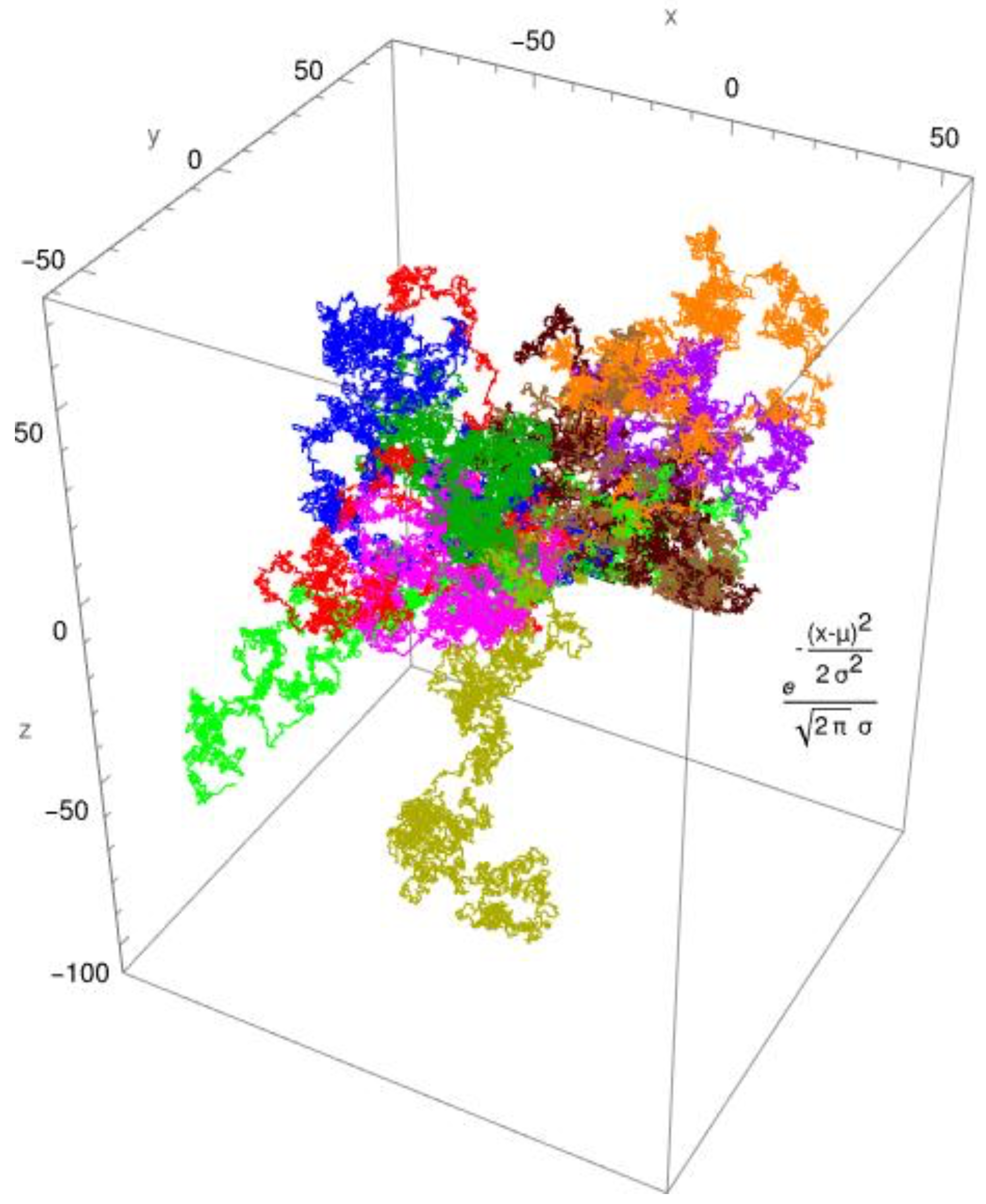
In
Figure 1, the simulated diffusion of a protein with a diffusion coefficient of
Dapp ≈ 0,1 x 10
-10 [m
2/s] is shown for a Brownian motion. In
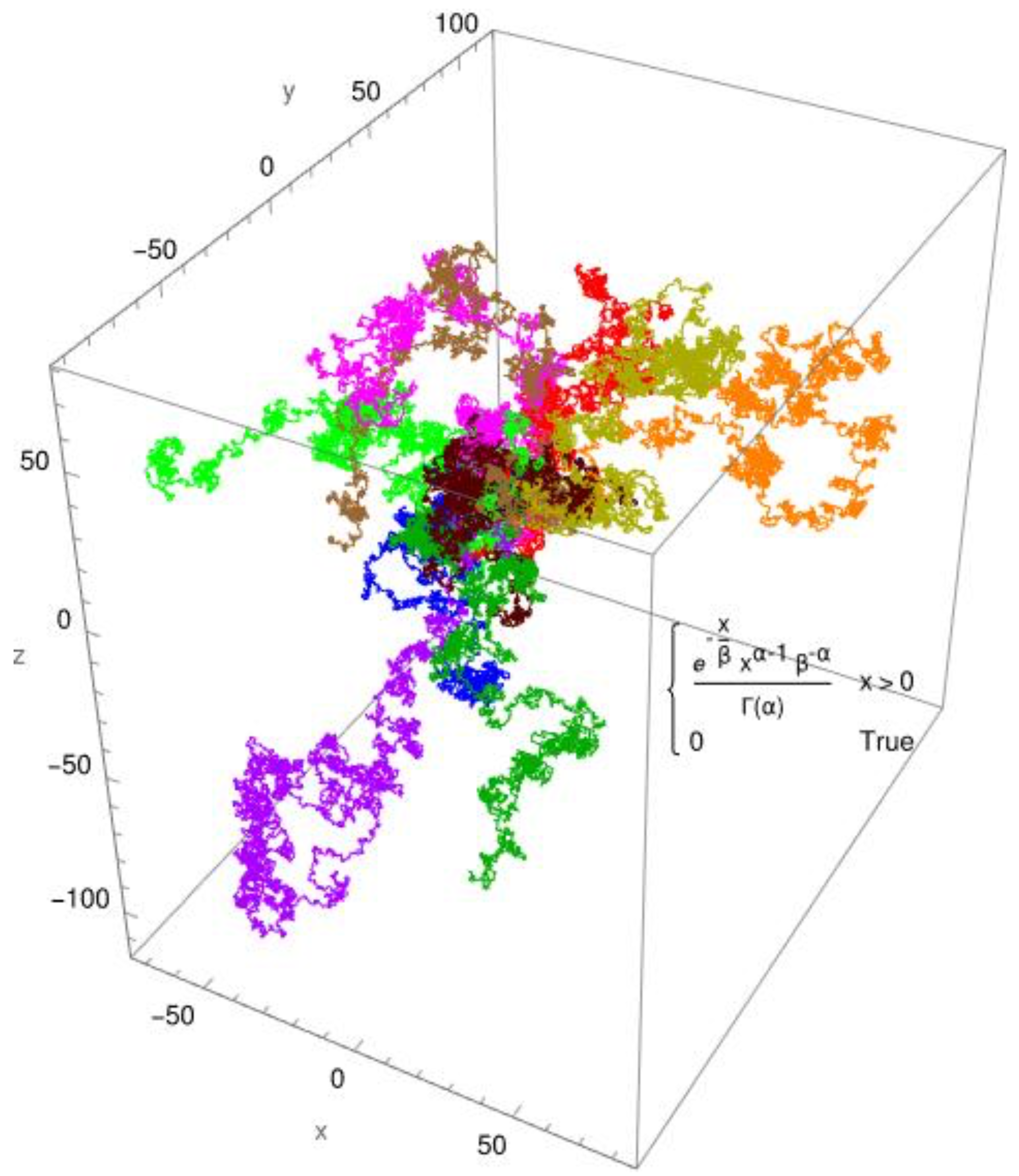
Figure 2, the simulated diffusion of a protein with a diffusion coefficient of
Dapp ≈ 0,1 x 10
-10 [m
2/s] is shown for an anomalous diffusion. The colors indicate 10 different tracks for individual molecules/individual particles. The CTRW steps (
continuous-time random walk steps) employed the two distribution functions: the standard Gaussian distribution for Brownian diffusion (
Figure 1) and, as an example, the gamma distribution for anomalous diffusion processes (
Figure 2). The created pathways look similar, yet they differ in significant aspects which are depicted in
Figure 1 and
Figure 2. It is evident that the Gaussian distribution yields pathways that are quite similar to each other. The gamma function, which produces a fractional diffusion, provides pathways that are quite diverse from one another and diverge significantly from those of the Gaussian distribution. However, the most remarkable feature is that the Gaussian distribution generates pathways that are close to the measurement origin and concentrated around it. The situation with the gamma distribution is fundamentally different; here, paths emerge that have a certain localization around the measurement origin but are also delocalized in more than 60%. In other words, the pathways become looser and have large leaps, which are typical of fractional diffusion. This form of unwinding is typical of fractional diffusion.
4. Discussion
The concept of thermodynamic jitter was applied to analyze the motion of single molecules (individual molecules) within the croweded cytoplasm (see
Section 2. Methods). The simulations of the movements and the resulting tracks showed that individual molecules can carry out localized Brownian movements and fanning patterns typical of fractal diffusion in live cells. What theoretically happens occupied several publications. We got into the ergodic hypothesis where it was clear ‘single molecule’/’single particle’ behavior should not be extrapolated to all molecule/particle behavior [
19].
The minimum variation occurs if the number of randomly selected single molecule traces is
Nℓmax = 32 as found by computer simualtions. All other values of
Nℓ deliver only a local minimum of a global minimum [
19]. The plots also showed (see
Figure 1 in Ref. [
19]) that the variation approaches a stable value as
Nℓ approaches large values, i.e. only a small subpopulation of individual molecules provides minimal variation. In other words, the most striking feature of ensemble averaging in sparse subpopulations of single molecules is the same average obtained in an ergodic system composed of many molecules when the number of randomly selected single molecule traces is
Nℓmax = 32 [
19]. Therefore, broken ergodicity and unbroken ergodicity can no longer be distinguished. Furthermore, if averaging procedures are performed without knowing whether the underlying molecular system behaves ergodic or non-ergodic, any measurement may be associated with ergodic or non-ergodic behavior unless one can identify the individual molecule/ individual particle (single molecule/single particle) fingerprint of non-ergodicity [
19].
The single-molecule/single-particle fingerprint of non-ergodicity is the thermodynamic fingerprint (signature of thermodynamic jitter) of a single molecule/single particle in liquids or living cells without immobilization or without hydrodynamic flow [
14]: The novel physical theory offers a new way to understand the molecular behavior when individual biomacromolecules/molecules in living cells are trapped in interactions with their neighboring ligands or reactants in a crowded environment at room temperature or in physiological conditions and their cellular compartments [
13]. Therefore, single-molecule/single-particle studies may be contrasted with measurements on an ensemble or bulk collection of molecules. It clearly follows from these results that the biggest breakthrough in microscopy/nanoscopy and spectroscopy would be a breakthrough in sensitivity in the time domain for measuring an individual single molecule over several milliseconds to seconds and even minutes (up to hour) without immobilization an artificial surfaces or biological structures (membranes) as well as without significant hydrodynamics.
The meaningful time is the measurement time for measuring just one single molecule (individual molecule, selfsame molecule) or one single particle (individual particle, selfsame particle) in contrast to the measurement times for the many-molecule measurements. We simplify its discussion by focusing on the most critical points. We distinguish three types of meaningful points in time:
- (i)
the meaningful time as single molecule/single particle time resolution is discussed here (
for mathematical details see: ref. [
12]),
- (ii)
the meaningful times as mathematical (physical) limits of the measurement time which should not be exceeded in order to track the same individual molecule/particles with high probability in one, two or three dimensions (
for mathematical details see: the Table 1 in ref. [
11]) and
- (iii)
the meaningful time as a quantitative measure of the meaningful re-entries of the same individual molecule/same individual particles into the observation/detection volume ((
for mathematical details see: equation (12) in ref. [
7] and equations (2a) – (2c) and (3) in ref. [
8]).
The dimensions of the meaningful times are the dimensions of the diffusion times.
The measurement of the individual molecule or particle is one of the most demanding research trends in spectroscopy, microscopy and nanoscopy. This is not least the case because individual molecules (oligonucleotides and single-stranded DNA sequences) were first theoretically described in chemical oligonucleotide syntheses on solid supports after their release from the solid phase as early as 1994 [
20], but corresponding measurements are not yet possible today. A head start is the mathematically proven theory that turns knowledge into strength, even at odds with the mediocre mainstream, for example ref. [
21] and examples can also be found in ref. [
11]. What new insights can be gained from the foundation of the theory on ‘Single-Molecule/Single Particle Biophysics & Biochemistry Based On the Stochastic Nature of Diffusion’, that is the new physical theory for quantifying the thermodynamic jitter of molecules/particles without immobilization on an artificial surface or on a biological structure or without significant hydrodynamic flow? For example, the thermodynamic signatures of single molecules and single particles in liquids and live cells (cytoplasm, membranes, nucleus) without immobilization or without hydrodynamic flow were found in ref. [
14] and shown by simulations in the
Figure 1 and the
Figure 2 of the present article. There may be too much thermodynamic jitter in the experiments with live cells and in dilute liquids without immobilization or without significant hydrodynamic flow under physiological conditions or at room temperature [
22].
Taken together, this article presents a significant advancement in single-molecule/particle biophysics and biochemistry through simulations demonstrating localized Brownian motion and fractal diffusion. The introduction and methods sections provide a thorough background and detailed methodology. Of course, there are many types of live cells. The study does not differentiate between them, but this could be achieved by using more specific values for underlying fractal structures and distributions functions.
5. Conclusions
The formulas and relationships specified in this article apply. They are simple and safe for determining how many molecules are averaged during measurement times.They must be used to validate that only a single molecule/a single particle (an individual molecule/an individual particle, the (self)-same molecule/the (self)-same particle) is measured with high probability during the measurement times. The obtained results are directly linked to the call for increased sensitivity in the time domain of measurements.
6. Addendum
Due to plagiarism and illegal publications of an earlier manuscript draft by the open access journal ‘Systematic Reviews in Pharmacy’, which contained wrongly typeset, incorrect mathematical-physical formulas and relationships as well as a fake author (as the main author), we changed the content and made linguistic changes and improvements in this original research article. Now, all formulas and physical relationships are correct and confirmed by both authors. We are very grateful for the support and help by the US Prepint.org team in this very unpleasant situation caused by the open access journal ‘Systematic Reviews in Pharmacy’. Preprint.org supported us by uncovering this copyright scandal from an ethical perspective and Preprint.org was very helpful in dealing with it correctly (email correspondences).
Funding
This research received no external funding.
Data Availability Statement
All data generated and analyzed in this study that supports the findings are included in this published article were not published before.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- DʼEste, E.; Lukinavičius, G.; Lincoln, R.; Opazo, F.; Fornasiero, E.F. Advancing cell biology with nanoscale fluorescence imaging: essential practical considerations. Trends Cell Biol. 2024, Jan 5, S0962-8924(23)00239-8. Epub ahead of print. [CrossRef] [PubMed]
- Loidolt-Krueger, M. New Confocal Microscope for quantitative fluorescence lifetime imaging with improved reproducibility. Microscopy Today. 2023, 31, 24–31. [Google Scholar]
- Harms, G.S.; Cognet, l..; Lommerse, P.H.; Blab, G.A;, Schmidt, T. Autofluorescent proteins in single-molecule research: applications to live cell imaging microscopy. Biophys. J. 2001, 80, 2396–2408.
- White, J.; Stelzer, E. Photobleaching GFP reveals protein dynamics inside live cells. Trends Cell Biol. 1999, 9, 61–65. [Google Scholar] [PubMed]
- Lin, Y.; Exell, J.; Lin, H.; Zhang, C.; Welsher, K.D. Hour-long, kilohertz sampling Rate three-dimensional single-virus tracking in live cells enabled by StayGold fluorescent protein fusions. J. Phys. Chem. B 2024, online publication date May 29. [CrossRef]
- Foote, A.K.; Ishii, K.; Cullinane, B.; Tahara, T.; Goldsmith, R.H. Quantifying microsecond solution-phase conformational dynamics of a DNA hairpin at the single-molecule level. ACS Phys. Chem. 2024, online publication date May 29. [CrossRef]
- Földes-Papp, Z. Fluorescence fluctuation spectroscopic approaches to the study of a single molecule diffusing in solution and a live cell without systemic drift or convection: a theoretical study. Curr. Pharm. Biotechnol. 2007, 8, 261–273. [Google Scholar] [PubMed]
- Földes-Papp, Z. ‘True’ single-molecule molecule observations by fluorescence correlation spectroscopy and two-color fluorescence cross-correlation spectroscopy. Exp. Mol. Pathol. 2007, 82, 147–155. [Google Scholar] [PubMed]
- Földes-Papp, Z.; Baumann, G.; Kinjo, M.; Tamura, M. Single-Phase Single-Molecule Fluorescence Correlation Spectroscopy (SPSM-FCS). In Encyclopedia of Medical Genomics & Proteomics; Fuchs, J., Podda, M., Eds.; Taylor & Francis: New York, USA, 2005; pp. 1–7. [Google Scholar]
- Földes-Papp, Z. What it means to measure a single molecule in a solution by fluorescence fluctuation spectroscopy. Exp. Mol. Pathol. 2006, 80, 209–218. [Google Scholar] [PubMed]
- Földes-Papp, Z. Single-molecule time resolution in dilute liquids and live cells at the molecular scale: Constraints on the measurement time. Am. J. Transl. Med. 2021, 5, 154–165. [Google Scholar]
- Földes-Papp, Z. Measurements of Single Molecules in Solution and Live Cells Over Longer Observation Times Than Those Currently Possible: The Meaningful Time. Curr. Pharm Biotechnol. 2013, 14, 441–444. [Google Scholar]
- Baumann, G.; Földes-Papp, Z. Study on Single-Molecule Biophysics and Biochemistry in dilute liquids and live cells without immobilization or significant hydrodynamic flow: The thermodynamic Single-Molecule DEMON. Curr. Pharm. Biotechnol. 2022, 23, 1750–1757. [Google Scholar] [PubMed]
- Földes-Papp, Z. The thermodynamic signature of a single molecule or a single particle in dilute liquids and live cells: Single-Molecule Biophysics & Biochemistry based on the stochastic nature of diffusion. Am. J. Transl. Med. 2023, 7, 74–77. [Google Scholar]
- Baumann, G.; Place, R.F.; Földes-Papp, Z. Meaningful interpretation of subdiffusive measurements in living cells (crowded environment) by fluorescence fluctuation microscopy. Curr. Pharm. Biotechnol. 2010, 11, 527–543. [Google Scholar] [PubMed]
- Földes-Papp, Z. Individual macromolecule motion in a crowded living cell. Curr. Pharm. Biotechnol. 2015, 16, 1–2. [Google Scholar]
- Földes-Papp, Z.; Baumann, G.; Li, L.-C. Visualization of subdiffusive sites in a live single cell. J. Biol. Methods 2021, 8, e142. [Google Scholar]
- Digman, M.A.; Gratton, E. Lessons in fluctuation correlation spectroscopy Annu. Rev. Phys. Chem. 2011, 62, 645–668. [Google Scholar]
- Földes-Papp, Z.; Baumann, G. Fluorescence molecule counting for single-molecule studies in crowded environment of living cells without and with broken ergodicity. Curr. Pharm. Biotechnol. 2011, 12, 824–833. [Google Scholar] [PubMed]
- Földes-Papp, Z.; Herold, A.; Seliger, H.; Kleinschmidt, A.K. Error propagation theory of chemically solid phase synthesized oligonucleotides and DNA sequences for biomedical application. In Fractals in Biology and Medicine; Nonnenmacher, T.F., Losa, G.A., Weibel, E.R., Eds.; Springer: Boston, USA, 1994; Volume 1, pp. 165–173. [Google Scholar]
- Gaire, S.K.; Daneshkhah, A.; Flowerday, E.; Gong, R.; Frederick, J.; Backman, V. Deep learning-based spectroscopic single-molecule localization microscopy. J. Biomedical Optics 2024, 29, 1–19. [Google Scholar]
- Xu, X.; Gao, C.; Emusani, R.; Jia, C.; Xiang, D. Toward Practical Single-Molecule/Atom Switches. Adv. Sci. (Weinh.), 2024, online publication day May 29, e2400877. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).