Submitted:
07 June 2024
Posted:
11 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Deracemization Experiments
2.2. Analysis Techniques
3. Results
3.1. Isolated Mass Yield and Final c.e.e. from TCID Coupled with SOAT
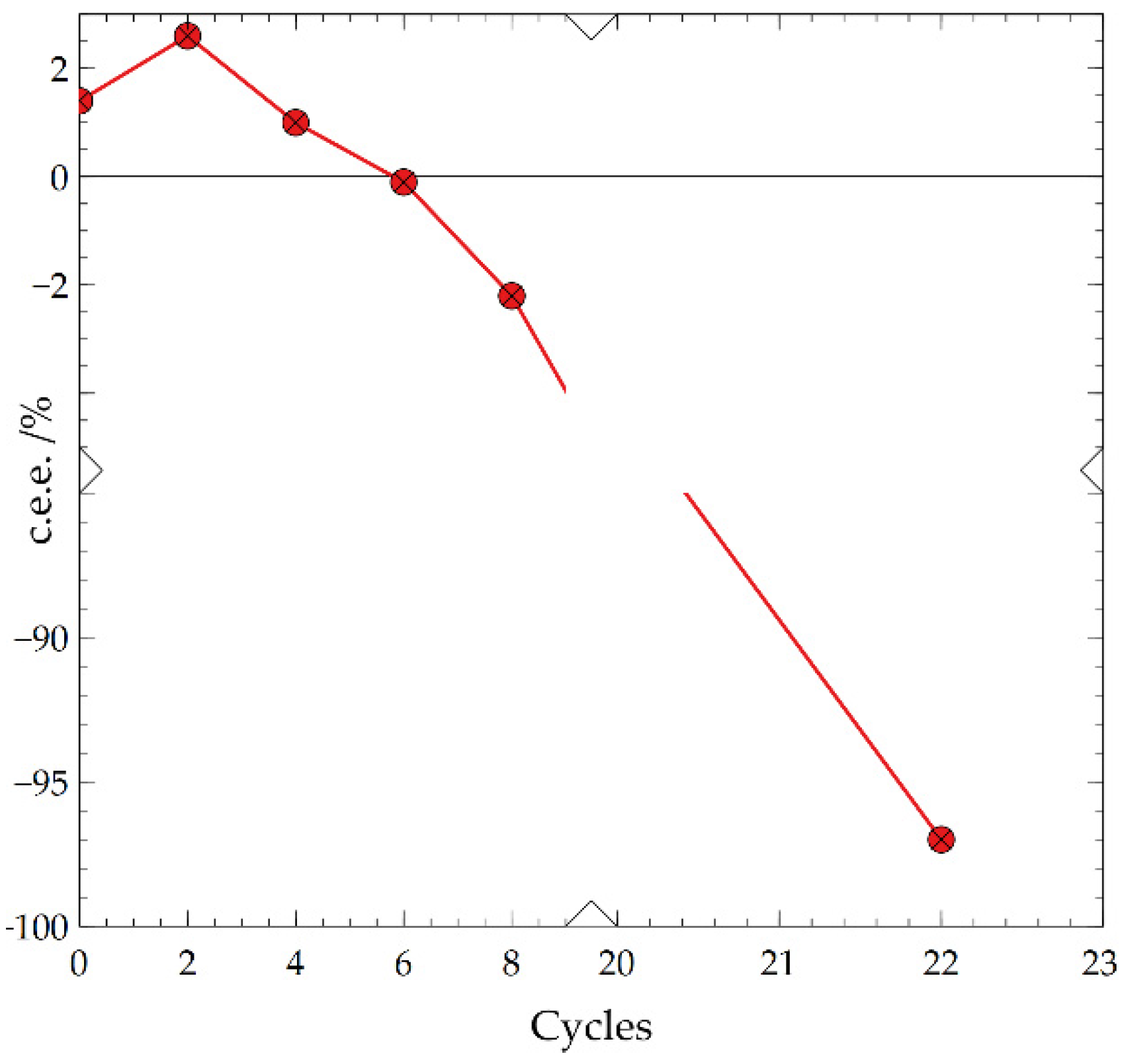
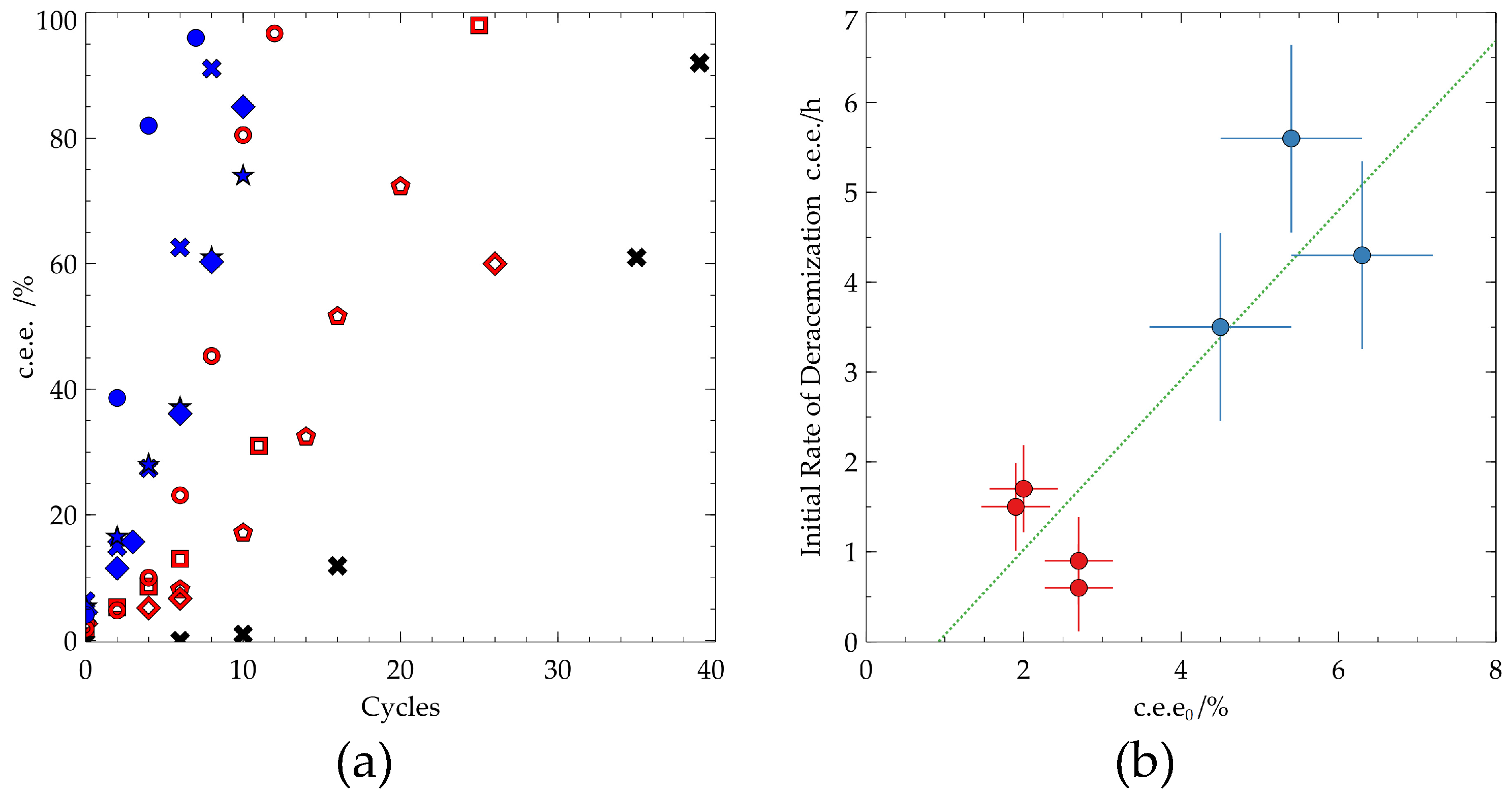
3.2. Kinetics of TCID with Low c.e.e0
4. Discussion
TCID Coupled with SOAT-Based Cooling
Minimizing c.e.e0 for More Efficient TCID
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rekoske, J.E. Chiral separations. AIChE J 2001, 47, 2. [Google Scholar] [CrossRef]
- Beesley, T.E.; Scott, R.P. Chiral chromatography; John Wiley & Sons: England, 1999. [Google Scholar]
- Fogassy, E.; Nógrádi, M.; Kozma, D.; Egri, G.; Pálovics, E.; Kiss, V. Optical resolution methods. Org. Biomol. Chem. 2006, 4, 3011–3030. [Google Scholar] [CrossRef] [PubMed]
- Suwannasang, K.; Flood, A.; Rougeot, C.; Coquerel, G. Using Programmed Heating–Cooling Cycles with Racemization in Solution for Complete Symmetry Breaking of a Conglomerate Forming System. Cryst. Growth. Des. 2013, 13, 3498–3504. [Google Scholar] [CrossRef]
- Breveglieri, F.; Maggioni, G.M.; Mazzotti, M. Deracemization of NMPA via Temperature Cycles. Cryst. Growth. Des. 2018, 18, 1873–1881. [Google Scholar] [CrossRef]
- Cameli, F.; Xiouras, C.; Stefanidis, G.D. Intensified deracemization via rapid microwave-assisted temperature cycling. CrystEngComm 2018, 20, 2897–2901. [Google Scholar] [CrossRef]
- Viedma, C. Chiral symmetry breaking and complete chiral purity by thermodynamic-kinetic feedback near equilibrium: implications for the origin of biochirality. Astrobiology 2007, 7, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Viedma, C. Chiral Symmetry Breaking During Crystallization: Complete Chiral Purity Induced by Nonlinear Autocatalysis and Recycling. Phys. Rev. Lett. 2005, 94, 065504. [Google Scholar] [CrossRef] [PubMed]
- Baglai, I.; Leeman, M.; Kellogg, R.M.; Noorduin, W.L. A Viedma ripening route to an enantiopure building block for Levetiracetam and Brivaracetam. Org. Biomol. Chem. 2019, 17, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, H.; Polenske, D.; Seidel-Morgenstern, A. Application of preferential crystallization to resolve racemic compounds in a hybrid process. Chirality 2006, 18, 828–840. [Google Scholar] [CrossRef]
- Levilain, G.; Coquerel, G. Pitfalls and rewards of preferential crystallization. CrystEngComm 2010, 12, 1983–1992. [Google Scholar] [CrossRef]
- Rougeot, C.; Hein, J.E. Application of continuous preferential crystallization to efficiently access enantiopure chemicals. Org. Process Res. Dev. 2015, 19, 1809–1819. [Google Scholar] [CrossRef]
- Dunn, A.S.; Svoboda, V.; Sefcik, J.; ter Horst, J.H. Resolution Control in a Continuous Preferential Crystallization Process. Org. Process Res. Dev. 2019, 23, 2031–2041. [Google Scholar] [CrossRef]
- Oketani, R.; Hoquante, M.; Brandel, C.; Cardinael, P.; Coquerel, G. Resolution of an Atropisomeric Naphthamide by Second-Order Asymmetric Transformation: A Highly Productive Technique. Org. Process Res. Dev. 2019, 23, 1197–1203. [Google Scholar] [CrossRef]
- Pálovics, E.; Bánhegyi, F.D.; Pataki, H.; Szilágyi, B. Enhancing temperature cycle-induced deracemization via combined cooling and antisolvent crystallization: A proof of concept study. Arab. J. Chem. 2024, 17, 105362. [Google Scholar] [CrossRef]
- Hosseinalipour, M.S.; Deck, L.-T.; Mazzotti, M. On Solute Recovery and Productivity in Chiral Resolution through Solid-State Deracemization by Temperature Cycling. Cryst. Growth. Des. 2024. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Oh, I.H.; Zhang, B.; Coquerel, G.; Kim, W.-S.; Park, B.J. Chiral Flipping in Viedma Deracemization. J. Phys. Chem. Lett. 2024, 15, 4367–4374. [Google Scholar] [CrossRef] [PubMed]
- Rougeot, C.; Guillen, F.; Plaquevent, J.-C.; Coquerel, G. Ultrasound-Enhanced Deracemization: Toward the Existence of Agonist Effects in the Interpretation of Spontaneous Symmetry Breaking. Cryst. Growth. Des. 2015, 15, 2151–2155. [Google Scholar] [CrossRef]
- Suwannasang, K.; Flood, A.E.; Rougeot, C.; Coquerel, G. Use of Programmed Damped Temperature Cycles for the Deracemization of a Racemic Suspension of a Conglomerate Forming System. Org. Process Res. Dev. 2017, 21, 623–630. [Google Scholar] [CrossRef]
- Lopes, C.; Cartigny, Y.; Brandel, C.; Dupray, V.; Body, C.; Shemchuk, O.; Leyssens, T. A Greener Pathway to Enantiopurity: Mechanochemical Deracemization through Abrasive Grinding. Chem. Eur. J. 2023, 29, e202300585. [Google Scholar] [CrossRef] [PubMed]
- Li, W.W.; Spix, L.; de Reus, S.C.A.; Meekes, H.; Kramer, H.J.M.; Vlieg, E.; ter Horst, J.H. Deracemization of a Racemic Compound via Its Conglomerate-Forming Salt Using Temperature Cycling. Cryst. Growth. Des. 2016, 16, 5563–5570. [Google Scholar] [CrossRef]
- Bodák, B.; Maggioni, G.M.; Mazzotti, M. Effect of Initial Conditions on Solid-State Deracemization via Temperature Cycles: A Model-Based Study. Cryst. Growth. Des. 2019, 19, 6552–6559. [Google Scholar] [CrossRef]
| Experiment Number |
Initial Mass of 1 /g |
c.e.e0 /% |
Final Mass /g | Final c.e.e. /% |
Yield /% |
|---|---|---|---|---|---|
| 1 | 2.44 | 4.0 | 2.25 | 99.9 | 92.2 |
| 2 | 2.51 | 5.0 | 2.29 | 99.9 | 91.2 |
| 3 | 2.46 | 4.1 | 2.24 | 99.6 | 91.1 |
| 4 | 2.56 | 4.5 | 2.31 | 99.4 | 90.3 |
| 5 | 2.50 | 1.6 | 2.32 | 99.9 | 92.8 |
| 6 | 8.76 | 4.2 | 7.86 | 99.1 | 89.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





