1. Introduction
With the development of livestock and poultry farming and the increased demand for feed resources, traditional silage cannot fully meet the needs of the farming industry.Therefore, the pursuit of reasonably priced, highly productive and efficient novel protein feeds to completely or partially replace traditional protein feeds is of great significance in improving the quality of animal products [
1]. In such context, amaranth, as a plant-based protein feed with rich nutrients and unique properties, is considered as a feed resource with great potential.Amaranth can be grown in many areas of China, and the yield is generally 75,000-150,000 kg per hectare of fresh weight, with some varieties yielding as much as 225,000 kg, which has been considered to be a promising feed resource [
2].
Amaranth belongs to the genus Amaranthus of the family Amaranthaceae and is used as both human food and animal feed. It is rich in vitamins and minerals, with high protein content, high resistance and high yield, making it a high-quality feed resource for ruminants [
3,
4]. In addition, the dry matter of amaranth has good degradability and fermentation properties which can add value to ruminant feed. The addition of amaranth silage has been reported to contribute to increase body weight gain and reduce rumen methane emissions in male lambs [5-8]. However, amaranth cannot be preserved for a long period of time by conventional method due to its high protein content, high water content and thick stalks that cannot be easily dried into hay [
3,
4]. Therefore, silage is a good way to improve the utilization of amaranth, which can not only prolong the preservation time, but also improve the palatability of the feed. Amaranth is characterized by high moisture and low soluble carbohydrate content.Thus, preserving amaranth directly though conventional silage methods are considered difficult. The soluble carbohydrate content of amaranth was determined to be only 50.46 g kg
-1 DM, which only meets the requirement of the 50 g kg
-1 soluble carbohydrate content threshold for the preparation of superior silage [
9]. It has been reported that the addition of 10% cornmeal improves the fermentation quality and apparent digestibility of silage [
10]. Moreover corn meal, is characterized by high contents of soluble carbohydrates (WSC) and dry matter, is a good mixed silage auxiliary, which can directly increase the fermentation substrate, make up for the lack of fermentable carbohydrates in amaranth, and reduce the water content to improve the fermentation success of silage. However,to the best of our knowledge, few studies had been conducted to detect the effect of mixed amaranth silage with corn meal during ensiling.
The success of silage also depends on appropriate biological and chemical conditions that allow a rapid and sufficient decrease in the pH of the silage. Therefore, silage additives are recommended to manipulate fermentation and prolong aerobic stability [
3]. Microbial additives such as lactic acid bacteria and cellulase can lead to a rapid drop in pH, facilitating the silage process and improving the fermentation quality [
11].
Therefore, the aim of this experiment was to explore the effects of moisture and additives on the fermentation quality, aerobic stability and in vitro digestibility of mixed silage of amaranth and corn meal by adding lactobacilli and cellulase individually or in a mixture. This study can provide more feed choices and ways to utilize the resources in the farming industry, and promote the sustainable development of the livestock and poultry farming industry.
2. Materials and Methods
2.1. Experimental Materials and Design
The cultivation experiment was conducted in the experimental field of Jilin University (123.3°E, 44.1°N), China. Planting was carried out in June 2020, and harvest was in September.Amaranth is in full maturity at the time of harvesting.
Amaranth was chopped into approximately 1 to 2 cm lengths before ensiling. The mixing ratios (w/w) of amaranth and corn meal ingredients were 69:31, 76:24, and 84:16 with the moisture content of the silage ingredients was 60%, 65%, and 70%, respectively. For each silage moisture, the silage treatment was designed as follows: no additive (U), lactic acid bacteria inoculant (L), cellulase (E), and a mixed preparation of lactic acid bacteria and cellulase (M). For the lactic acid bacteria inoculant, Chikusou-1 (Lactobacillus plantarum) was obtained from Snow Brand Seed Co. Ltd. (Tokyo, Japan). Cellulase, Acremonium cellulase, was provided by Meiji Seika Pharma Co. Ltd., Tokyo, Japan (Lot No.: ACCF-6940). The dosage of lactic acid bacteria was 4.7 × 106 colony-forming units (cfu) per gram of fresh weight (FW). Meanwhile, Acremonium cellulase was added at a dosage of 50 mg kg−1 FW, with a measurable activity level of 4.2 × 10−3 U per gram of FW. Then, the mixed silage was loaded into a 5-litre plastic silo. The silage density was 550.1 ± 20.0 kg m-3 FW, and the silo was kept at room temperature (21°C-25°C) for anaerobic fermentation. After 60 days of fermentation, three repeating silos were opened for the determination of silage’s chemical composition, fermentation quality and in vitro digestibility. The remaining silage was repeatedly mixed for the aerobic stability tests.
2.2. Fermentation Quality Analysis
Once opened, silage samples were taken by the "tetrad" method. Subsequently, thoroughly mixed 20 g of silage with 180 mL of distilled water, and homogenized in a polyethylene vacuum bags for 1 minute. The sample was then extracted in a refrigerator at a constant temperature of 4°C for 24 hours, filtered through 4 layers of gauze and qualitative filter paper [
15]. A portion of the resulting extract was used to measure pH using a pH meter (PHSJ-4F, Yidian Scientific Instruments Co., Ltd., Shanghai, China). The other part was frozen and stored at -20°C for the determination of organic acids and ammonia nitrogen (NH
3-N) contents. NH
3-Ncontentwas determined by the Robinson method [
16]. Lactic acid (LA), acetic acid (AA), propionic acid (PA), and butyric acid (BA) were determined by using high-performance liquid chromatography(column: ShodexRspak KC-811s-DVB gel column, Japan; detector: SPD-M10AVP; mobile phase: 3 mmol L
−1 perchloric acid; flow rate: 1 mL min
−1; column temperature: 50°C; detection wavelength: 210 nm; injection volume: 5 µL).
2.3. Chemical Composition, Energy, and In Vitro DegradabilityAnalysis
The dry matter (DM) content of fresh samples and silages were determined in a 65°C oven for 48 hours. The dried samples were grounded and passed through a 1.0 mm sieve for chemical analysis. The contents of organic matter (OM) and crude protein (CP)were determined by the method of Official Association of Analytical Chemists [
16]. The neutral detergent fibre (NDF), acid detergent fibre (ADF), and acid detergent lignin (ADL) contents were determined ac-cording to the methods reported by Van et al. [
18]. The water-soluble carbohydrate (WSC) was measured using the anthrone-sulfate colorimetric method [
19]. Buffering capacity (BC) was measured using the method of Playne and McDonald [
20]. The gross energy (GE) content was determined by an oxygen cartridge calorimeter (SDAC1000, Sundy, Changsha, China).
In vitro degradability experiment was conducted according to the principles of the Laboratory Animal Guidelines for Ethical Review of Animal Welfare. The protocol of the study was reviewed and approved by the Animal Ethics and Welfare Committee of Jilin University (Jilin, China; Approval Number: SY202009600). The dried silage sample (0.5 g) was placed in a filter bag (ANKOM F57; diameter of hole 25 µm; Ankang Technology; Macedon, NY, USA) and sealed by hand pressure sealing machine (PFS-400; Zhejiang dongfeng packing machine co.ltd. Wenzhou, China) for later
in vitro incubation. Before placing the samples, the fibre bags were rinsed with acetone and then thoroughly air-dried for 5 hours at 105°C in a forced convection drying oven (VL 115, VWR, Shanghai, China). A total of 196 fibre bags were prepared (48 silage silos × 4 parallel samples + 4 blank controls). The filter bag was processed and then loaded into a 130mL serum bottle. Four little-tailed billy goats were fed a mixture consisting of 40% corn silage, 20% alfalfa hay, and 40% concentrate (DM-based) twice daily. Ruminal fluid was collected from these animals. The rumen fluid was maintained at a temperature of 39°C under an atmosphere of carbon dioxide. The medium was filtered through four layers ofcoarse cotton cloth, and then the filtrate was mixed with McDougall artificial saliva at a 1:4 (v/v) ratio. Each serum vial was supplemented with a 60 ml mixture followed by incubation in a CO
2 atmosphere at 39°C. The incubation was performed in a water bath. After 72 hours of incubation, the filter bag was re-moved from the serum vial and gently rinsed with cold distilled water until the water becomes clear. The fibre bags are gently squeezed to remove excess moisture and then dried in a forced convection oven at 100°C for 24 hours. The residue is then weighed and measured for
in vitro dry matter digestibility (
ivDMD) [
22].
In vitro neutral deter-gent fiber digestibility (
ivNDFD) was determined by analyzing the residual NDF [
23]. The formulas for
ivDMD,
ivNDFD,
in vitro crude protein digestibility (
ivCPD), and
in vitro organic matter digestibility (
ivOMD) are as follows: (respective weights of DM, NDF, CP, and OM before digestion-respective residual weight of DM, NDF, CP, and OM)/(respective weights of DM, NDF, CP, and OM before digestion).
2.4. Microbiological Analysis
Fresh samples (20 g) were mixed uniformly with 180 mL of a sterile saline solution (0.85% NaCl), shaken on a shaker for 30 min. Then, 1 mL of the homogenate was taken for 10 × serial dilutions. Each gradient was prepared as 3 parallel replicates and poured into dishes. Finally, 100 µL of the dilutions at various concentrations were evenly applied on an agar medium as described below with coated rods. Lactobacillus bacteria were cultivated using De Man, Rogosa and Sharp agar media (Budweiser Technology Co, Ltd, Shanghai, China) via incubation for 48 hours under anaerobic conditions at 37°C. Aerobic bacteria were cultivated using nutrient agar medium via incubation at 37°C (Hope Biotechnology Co., Ltd., Qingdao, China). Yeast and moulds were grown using potato glucose agar medium at 28°C for 48 hours (Budweiser Technology Co., Ltd., Shanghai, China). The numbers of microorganisms were counted on plates of 20-200 cfu. All microbial data were converted to log10 cfu g−1. The results are reported as fresh weight basis.
2.5. Aerobic Stability Analysis
Amaranth silage from each treatment was placed in a clean 1 L plastic bucket. A thermocouple wire was placed in the centre of the amaranth silage, and the ambient temperature was recorded by the thermocouple line in the empty bucket using a data recorder (OHR-G100T; Hongrun Company, Ltd., Fujian, China). Silage temperature was recorded at 1-hour intervals. Ambient temperature was also recorded every hour as a blank. Aerobic stability is the time taken for silage to reach a temperature 2°C higher than the ambient temperature.
2.6. Statistical Analysis
The data were analysed usingthe GLM program of SPSS statistical software (version 26; International Business Machine Corporation; Armonk, NY, USA)for each indicator according to the model:
In the above model, Yijk is the response variable, a is the overall mean, and Wi is the fixed effect of the moisture content of silage material i (i= 1, 2, 3), Ejis the fixed effect of cellulase j (j = 1, 2), Lk is the fixed effect of lactic acid bacteria k (k = 1, 2). (W × E)ij is the interaction of silage feedstock moisture content i and cellulase j. (W × L)ik is the interaction of silage feedstock moisture content i and lactic acid bacteria k. (L × E)jk is the interaction of cellulase j and lactic acid bacteria k. (W × E × L)ijk is the interaction of moisture i, cellulase j, and lactic acid bacteria k. bijk is the residual error.
Multiple comparisons were made using Tukey's test based on the results of significance tests for water content, enzyme treatment, bacterial treatment and interaction.
3. Results
3.1. Chemical Composition and Microbial Counts of Fresh Materials
The chemical composition, gross energy, buffering capacity, and microbial counts of amaranth and corn meal mixed silage are shown in
Table 1. The DM, CP, and WSC contents of amaranth were 185 g kg
-1, 124 g kg
-1 DM, and 50.46 g kg
-1 DM, respectively. The bufferingcapacity value of amaranth was 340 mEq kg
-1 DM, which was 4.0 times higher than corn meal (mEq kg
-1 DM). The number of lactic acid bacteria, yeast and mould adhering to the surface of amaranth was 2.42 log
10 cfu
-1, 2.00 log
10 cfu
-1, and 0.41 log
10 cfu
-1, respectively.
3.2. Fermentation Quality of AC-silage
Table 2 showed the fermentation quality of AC-silage. All treatment groups had pH values less than 4.0 after 60 days of silage. The addition of L (L and M groups) and E (E and M groups) significantly (
p < 0.05) decreased the pH of silages. In addition, there was an interaction effect of W×E on pH (
p < 0.001). The without the addition of E (U and L groups) significantly increased pH compared to the addition of E (3.74, 3.72 vs. 3.64, 3.63,
p < 0.05). However, this effect was greater in W2 compared to W1 and W3. In contrast, the addition of L led to a decrease in pH (3.72, 3.63 vs. 3.74, 3.64), but L and W did not interact.
Water content significantly affected LA and PA content, with W2 having significantly higher LA content than W1 and W3 (20.3 g kg-1 DM vs. 16.9 g kg-1 DM, 14.6 g kg-1 DM, p < 0.05) and significantly lower PA content (0.00 g kg-1 DM vs. 0.03 g kg-1 DM, 7.34 g kg-1 DM, p < 0.05). The AA content of AC-silage was significantly lower (16.3 g kg-1 DM, vs. 17.8 g kg-1 DM) in group L compared to group U. The AA content of silage was significantly lower in group L compared to group U. The AA content of silage was significantly lower in group L compared to group U.
There was an interaction between W×E and W×L on BA content (p < 0.05). The BA content of the addition of E was significantly higher than that of BA without E (1.26 g kg-1 DM, 1.81 g kg-1 DM vs. 0.649 g kg-1 DM, 0.246 g kg-1 DM). With the addition of E, W3 had the lowest BA content. However, without the addition of E, W1 had the lowest BA content. The BA content with L addition was significantly lower than that without the addition of L (0.246 g kg-1 DM, 1.26 g kg-1 DM vs. 0.649 g kg-1 DM, 1.81 g kg-1 DM). Without the addition of L, BA content decreased with increasing water content. However, with the addition of L, the change in BA content with water content was not significant (p > 0.05).
There was an interaction between W × E, W × L, and L × E on NH3-N/TN (p < 0.05). NH3-N/TN decreased with decreasing water content (25.1 g kg-1 DM, 20.0 g kg-1 DM, and 19.3 g kg-1 DM, p < 0.05). However, without the addition of E, the mean NH3-N/TN was higher than that of the E-added group (22.9 g kg-1 DM vs. 20.1 g kg-1 DM, p < 0.05). Meanwhile, the NH3-N/TN with the addition of L was significantly lower than that without addition of L (20.6 g kg-1 DM vs. 22.4 g kg-1 DM, p < 0.05). With the addition of L, W1 has the lowest NH3-N/TN. And without the addition of L, W2 has the lowest NH3-N/TN. The addition of L significantly reduced NH3-N/TN, but with the addition of E, the mean NH3-N/TN was lower compared to without the addition of E (19.9 g kg-1 DM vs. 21.3 g kg-1 DM, p < 0.05).
3.3. Chemical Composition of AC-silage
Table 3 showed the chemical composition of AC-silage. The effect of water content and additives on the
in vitro digestibility of mixed with silage is shown in
Table 4. DM content increased with decreasing water content (287 g kg
-1, 330 g kg
-1, 389 g kg
-1,
p < 0.05). There was an interaction effect of L × E on DM content (
p = 0.010). The addition of L reduced the DM content of silage. However, the effect of E addition was greater compared to without the addition of E (329 g kg
-1 vs. 340 g kg
-1,
p < 0.05). There was an interaction effect of W × E on OM content (
p < 0.001). With the addition of E, OM content was significantly lower than without the addition of E (940 g kg
-1 DM, 941 g kg
-1 DM vs. 942 g kg
-1 DM, 942 g kg
-1 DM,
p < 0.05). However, this effect was minimized for W2 compared to W1 and W3.
There was an interaction between W × E and W × L on CP content (p < 0.05). With the addition of E, the CP content was higher than that of the group without E (121 g kg-1 DM vs. 118 g kg-1 DM, p < 0.05). However, this effect is smaller for W1 compared to W2 and W3. In the addition of L, CP content increased (p < 0.05) with increasing water content. However, it was unaffected (p > 0.05) in silage without the addition of L.
There was an interaction of W × L on NDF content (p = 0.024). The NDF content of the addition of L treatment was significantly lower than that without the addition of L (631 gkg-1 DM, 588 g kg-1 DM vs. 632 g kg-1 DM, 607 g kg-1 DM, p < 0.05). With the addition of L, W1 had the highest NDF content, whereas without the addition of L, W3 had the highest NDF content.
There was an interaction effect of W × E on ADF content (p = 0.033). ADF content decreased with decreasing water content (190 g kg-1 DM, 153 g kg-1 DM, 115 g kg-1 DM, p < 0.05). Without the addition of E, ADF content was significantly higher than with E addition (160 g kg-1 DM, 160 g kg-1 DM vs. 146 g kg-1 DM, 143 g kg-1 DM, p < 0.05). However, this effect was smaller for W1 compared to W2 and W3.
There was an interaction between W × E and W × L on GE (p < 0.05). The addition of E increased GE content under W2 (18.9 MJ kg-1 DM, 19.0 MJ kg-1 DM vs. 18.7 MJ kg-1 DM, 18.8 MJ kg-1 DM, p < 0.05), but there was no significant difference between W1 and W3 conditions with the addition of E. The effect of W × E on the GE content of silage with the addition of L was not significant (p < 0.05), and the effect of W × L on the GE content of silage with the addition of L was not significant (p < 0.05). In silage with L addition, GE decreased (p < 0.05) with increasing water content, but was unaffected (p > 0.05) in silage without L addition.
3.4. In Vitro Digestibility of AC-silage
There was an interaction effect of W×E on ivDMD, ivOMD, and ivCPD (p<0.05). ivDMD, ivOMD, and ivCPD were significantly lower in the without the addition of E than the addition of E (715 g kg-1 DM, 755 g kg-1 DM, 585 g kg-1 DM vs. 703 g kg-1 DM, 742 g kg-1 DM, 578 g kg-1 DM, p < 0.05). However, this effect was smaller in W1 compared to W2 and W3. There was an interaction effect of W×L on ivNDFD (p = 0.024). The addition of L increased ivNDFD content under W1 (595 g kg-1 DM, 565 g kg-1 DM vs. 594 g kg-1 DM, 560 g kg-1 DM, p < 0.05), but there was no significant difference between W2 and W3 conditions with the addition of E.
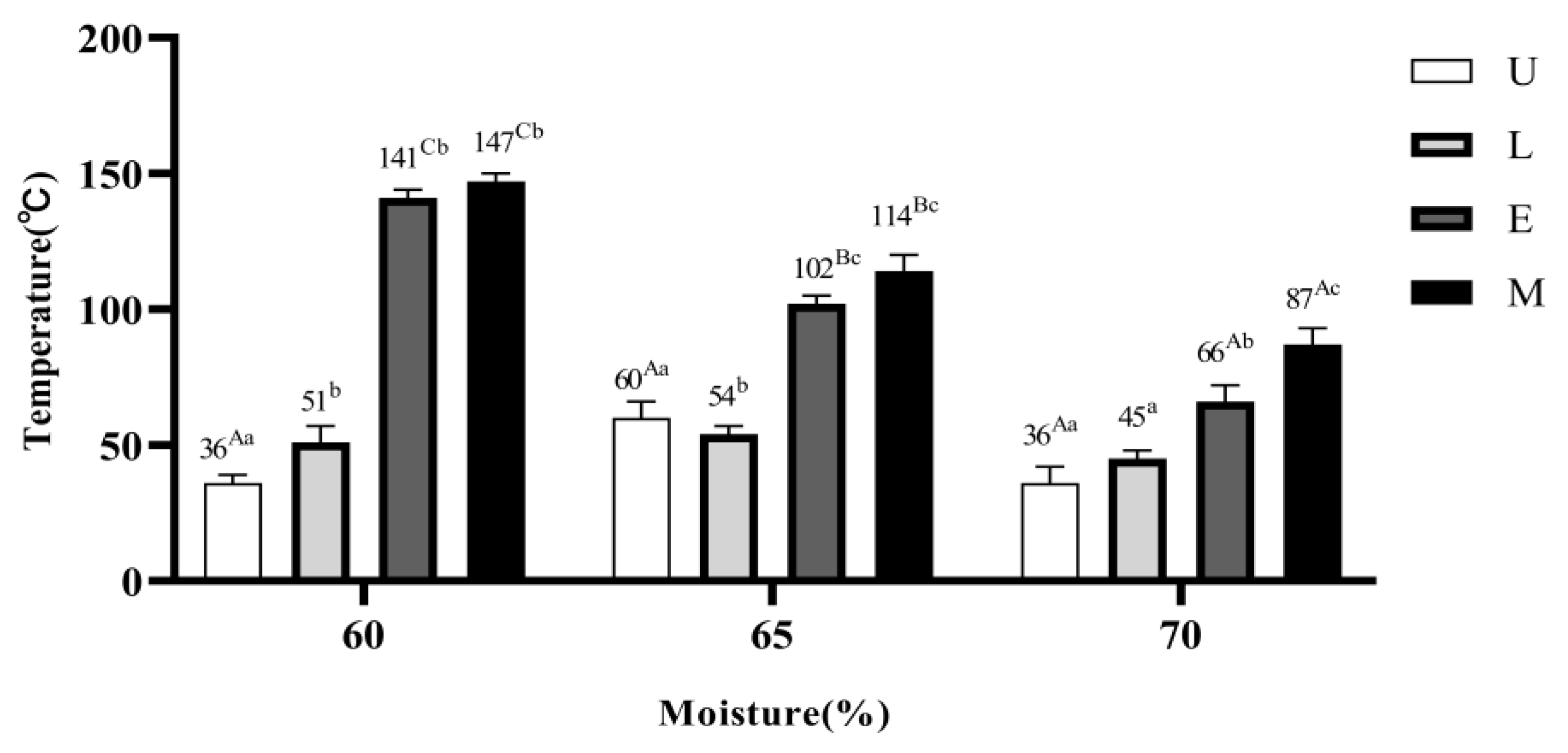
3.5. Aerobic Stability of AC-silage
The aerobic stability of AC-silage based on water content and additives is shown in
Figure 1. The aerobic stability decreased significantly (
p < 0.05) with increasing water content. The aerobic stability of the E and M treatments was significantly higher than that of the U treatment at all water contents.
4. Discussion
In our experimental hypotheses, we speculated that the addition of lactic acid bacteria and cellulase at W1content could induce earlier lactic acid fermentation in the mixed silage and improve fermentation quality,
in vitro digestibility, as well as aerobic stability. As shown in
Table 2, The M treatment had the best quality. According to
Table 4,
ivDMD and
ivOMD were the highest in M treatment at all water contents. As shown in
Figure 1, the M treatment had the highest aerobic stability, which is consistent with our previous speculation.
4.1. Effect of Moisture and Additives on the Fermentation Quality of AC-silage
Water content is the main factor affecting silage quality. When silage water content is too high, it can lead to a negative quality in silage [
20]. However, when the water content is too low, there will be more porous in the silage silos compared to silages with higher water content. Moreover, the low content of organic acids with antifungal activity (acetic acid) is not sufficient to inhibit the growth of yeasts, which can deteriorate quickly after opening [
21]. Muck et al. showed that fermentation quality was improved and nutrient losses were reduced at silage moisture of about 65% [
22]. Therefore, three moistures levels of 60%, 65%, and 70% were set in current study. The decrease in pH was more pronounced at lower water content conditions.This may be due to the higher DM content in low water content which provides more fermentation substrate. It enables lactic acid bacteria to produce large amounts of lactic acid while inhibiting the respiration of plant cells and reducing glycogen consumption and protein degradation. The experiment of Yahaya et al. found that high moisture silage with a high pH value was not as effective in fermentation as low moisture fermentation, which is consistent with the results of the present experiment [
23]. In the present study, W×E had an interaction effect, and the E addition treatment further reduced the pH of the silage. This is due to the addition of cellulase, which breaks down the plant cell wall during ensiling and provides soluble sugars to lactic acid bacterial. Increased sugar content during the early stages of ensiling, promoted lactic acid bacteria colonization. This leads to a rapid increase in lactic acid and a decrease in pH, which in turn inhibits the protein hydrolysing activity of harmful microorganisms and plant enzymes [24, 25].Generally, the low pH means high lactic acid concentration, and the typical concentration of lactic acid in silage ranging from 2% to 4% of the DM. Interestingly, although the pH in this experiment was below 4.0, the lactic acid content was not high. This may be due to the fact that enterobacteriaceae could convert nitrate to nitrite, which then was converted to NO and NO
3 in a 2:1 ratio under acidic conditions, resulting in a lower pH [21, 26]. The BA content of E-added or L-added treatments ranged from 0.00 to 1.78 g kg
-1 DM, which was low, indicating that lactic acid bacteria and cellulase preparations can reduce clostridial fermentation [
27].
The NH
3-N/TN ratio is an indicator of protein hydrolysis activity, amino acid deamination, and decarboxylation. This is mainly due to the fact that protein hydrolysing Clostridium perfringens ferments amino acids through valine and leucine deamination and redox reactions between alanine and glycine. This usually indicates the degradation of nutrients in mixed silage [
28]. The NH
3-N/TN ratiosof all silages in this experiment were within satisfactory limits (<10% TN), indicating that extensive protein hydrolysis did not occur [
29]. Li et al. found that the addition of cellulase to cassava leaf silage significantly reduced NH
3-N, supporting the results of the present treatment. In addition, it has been shown that the addition of lactic acid bacteria can reduce the microbial diversity of clover, annual ryegrass, and their mixed silage and improve silage quality [
30]. This may be due to the addition of exogenous lactic acid bacteria, shifting fermentation towards lactic acid with homofermentative lactic acid bacteria, or towards acetic acid with fermentative lactic acid bacteria. It also reduces the growth of clostridia and molds in silage, which reduces the degradation of proteins via the silage process and retains more nutrients in the silage, which is consistent with the results of this experimental [
31]. The combined action of lactic acid bacteria and cellulase improved fermentation quality, reduced plant cell wall fraction and protein loss, provided more digestible substrate for rumen microbial fermentation, and promoted rumen digestion. The combined treatment of lactic acid bacteria and cellulase may have beneficial synergistic effects on the fermentation quality of
AC-silage [
25].
4.2. Effect of Moisture and Additives on the Chemical Composition and In Vitro Digestibility of AC-silage
The DM, GE, and
in vitro digestibility of silage tended to decrease with increasing moisture. These results indicated that high moisture mixed silage had high loss of WSC and hemicellulose and low digestibility. This is in line with the results of Yahayaet al.'s study on orchard grass [
32]. At the same time, we found an interesting phenomenon:
Numbered lists can be added as follows:There was the highest DM in all treatment groups at W1, but the CP content was the lowest. This may be because the addition of corn meal to regulate the moisture. However, the protein content of corn meal was 32.6 g kg
-1 DM lower than the amaranth, thereby resulting in the lowest CP content in all treatment groups at W1. This is similar to the study of Mehrangiz et al., where it was found that the addition of molasses could lead to amaranth fermentation, increasing DM concentration [
33]. In the report of Mehrangiz et al., it was shown that soluble and degradable CP fractions of amaranth as well as effective CP degradability were not affected by wilting or any additives to silage [
33]. However, the addition of E treatment significantly increased the CP content in mixed silage compared to no E treatment. This may be because cellulase disrupts the plant cell wall and releases more plant proteins. The plant proteins continue to synthesize new bacterial proteins that are more easily digested and absorbed by the animals, which in turn promotes digestion and degradation and improves the
ivCPD. This was also indicated by the results of a previous study on the mixed silage of soybean residue and corn stover by Zhao et al. [
34]. W1 reduced the levels of ADF and ADL compared to W2 and W3. This is due to the increase of raw material leading to higher WSC content, and lower NDF and ADF levels in silage [
35]. It was demonstrated that high-moisture silage tends to have higher cellulose digestibility. Morrison reported a similar increase in cellulose digestibility due to the action of extracellular cellulase, which leads to the shortening of the cellulose chain length and makes it more susceptible to enzymatic attack [
36]. The addition of E treatment significantly reduced NDF and ADF content compared to no E treatment, which is similar to the findings of Lynchet al. on corn silage [
37]. This may reflect the fact that the added fibrocystic enzymes increased the hydrolysis of cell wall carbohydrates, decreased their fiber content, and increased the WSC content. This result is in agreement with the findings of Foster et al. that the addition of cellulase to warm-season legumes and Bahia grass silage increased WSC content [35, 38].
ivDMD and
ivOMD of mixed silage under M treatment were higher than the other groups, which may be due to the reduction of DM loss from silage with the addition of L and E treatments. As a result,
ivDMD and
ivOMD in the rumen were elevated. Low
ivNDFD was observed in mixed silage under M treatment. This result may have two reasons. One is related to the hydrolysis of hemicellulose due to silage fermentation. Hemi-cellulose is acid-unstable under strong acidic conditions, and silage fermentation leads to hydrolysis of the most readily available feed structural carbohydrate [
39]. Secondly, the addition of lactic acid bacteria enhanced NDF fermentation and increased hydrolysis. At the same time, cellulase treatment reduced the amount of available NDF degraded by rumen microorganisms in mixed silage [
40]. At W3,
in vitro digestibility was significantly increased in mixed silage under E and M treatments compared with U and L treatments. So we can infer that
in vitro digestibility and NDF and ADF contents were negatively correlated, and our conclusions were the same as those of Bao et al. [
35].
4.3. Effect of Moisture Content and Additives on the Aerobic Stability of AC-silage
Aerobic instability is the underlying cause of the loss of nutrients and DM, and mycotoxins produced from undesirable microorganisms also lead to health risks in people and animals. Therefore, aerobic stability is an important factor affecting the nutritional quality and subsequent feeding value of silage in ruminants [
29]. Aerobic microorganisms metabolize and consume nutrients, and a change in silage temperature is usually used as an important parameter to evaluate the aerobic stability of silage [
41].
In this experiment, the aerobic stability AC-silage decreased with increasing water content (Fig. 1). This may be due to the fact that a moist environment is more favorable for the growth of microorganisms such as yeasts, acetic acid bacteria and, acid-tolerant yeasts can survive in silage [
42]. Increased yeast growth rate in high moisture treatments was also demonstrated in a study of total mixed ration by Hao et al [
43].
The aerobic stability of L, E, and M treatments was improved at all water contents in this experiment. This is due to the fact that the inoculated lactic acid bacteria have anisolactic acid metabolic pathway that capable of producing acetic acid during fermentation after opening the silos. Thus, effectively controlling the yeast and filamentous fungi could improve aerobic stability [
44]. In addition, according to Kaewpila et al., the addition of cellulase can improve the aerobic stability of Napier Pakchong grass, which was consistent with our experimental results [
18]. The exposure time of all of the M treatment groups was longer than the other groups, which may be due to the synergistic effect of lactic acid bacteria and cellulase when used together. Many studies have shown that lactic acid bacteria or cellulase can have a positive effect on improving the aerobic stability of mixed silage by lowering pH and NH
3-N content, and reducing yeasts and clostridia [
25]. As a result, the M treatment group was more stable during aerobic exposure and reduced spoilage losses in silage fermentation.
5. Conclusions
In summary, silage water content, lactic acid bacteria and cellulase affect the fermentation quality, nutrient content, in vitro digestibility and aerobic stability of mixed amaranth and corn meal silage. In this study, the simultaneous addition of Lactobacillus and cellulase at 60% water content had the lowest pH, PA, AA, and NH3-N/TN and therefore the best fermentation quality. At the same time, mixed silage under the above conditions had the lowest content of ADF, the highest content of ivDMD, ivOMD, and ivCPD, and higher content of DM and OM, thus providing higher nutritional value and digestibility. However, further in suit experiments are needed in this experiment to evaluate the effect of seed amaranth and cornmeal silage mix on rumen growth performance.
Author Contributions
Conceptualization, X.L., B.Y., and F.L.; methodology, Y.J., J.D.,Q.Y., M.Y., and F.L..; software, X.L., T.Z., M.Y., J.D., and Y.J.; validation, Y.J., B.Y., P.W., J.D., M.Y., and T.Z.; formal analysis, X.L., P.W., and J.D.; investigation, F.L.; resources, B.Y., and F.L.; data analyze, Y.J., P.W., and M.Y; writing-original draft preparation,Y.J.,and P.W.; writing-review and editing, X.L., P.W., and T.Z; visualization, B.Y. and P.W.; supervision, B.Y. and P.W.; project administration, B.Y. and P.W.; funding acquisition, P.W., and Y.J. All authors have read and agreed to the published version of this manuscript.
Funding
This study was supported by the Jilin Province Agricultural Key Core Technology Demonstration and Promotion (Industrial Technology System) Project (JARS-2024-0703) and funds from the China Agriculture Research System (CARS-37).
Institutional Review Board Statement
This study strictly followed the Chinese Laboratory AnimalWelfare Ethical Review Guidelines and was approved by the Laboratory Animal Welfare EthicsCommittee of Jilin University (permit number: SY202009600).
Informed Consent Statement
Not applicable.
Data Availability Statement
The author is solely responsible for the completeness and accuracy of all data in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dong, Z.; Wang, S.; Zhao, J.; Li, J.; Shao, T. Effects of additives on the fermentation quality, in vitro digestibility and aerobic stability of mulberry (Morus alba L.) leaves silage. Asian Australas. J. Anim. Sci. 2020, 33, 1292–1300. [Google Scholar] [CrossRef]
- Cui, D.W. High-yield cultivation techniques of amaranthus hypochondriacus. Agricultural and Animal Husbandry Comprehensive Service Center, Happiness Town, Xiangfang District, Harbin City, Heilongjiang Province. [CrossRef]
- Nogoy, K.M.C.; Yu, J.; Song, Y.G.; Li, S.; Chung, J.-W.; Choi, S.H. Evaluation of the Nutrient Composition, In Vitro Fermentation Characteristics, and In Situ Degradability of Amaranthus caudatus, Amaranthus cruentus, and Amaranthus hypochondriacus in Cattle. Animals. 2021, 11. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, S.; Zhao, Y.; Yang, J.; Lv, L.; Zheng, Z.; Lu, H.; Ren, Y. Comparative Study of the Nutritional Value and Degradation Characteristics of Amaranth Hay in the Rumen of Goats at Different Growth Stages. Animals. 2023, 13. [Google Scholar] [CrossRef]
- Pond, W.G.; Lehmann, J.W. Nutritive value of a vegetable amaranth cultivar for growing lambs. J. Anim. Sci. 1989, 67, 3036–3039. [Google Scholar] [CrossRef]
- Calabro, S.; Oteri, M.; Vastolo, A.; Cutrignelli, M.I.; Todaro, M.; Chiofalo, B.; Gresta, F. Amaranthus grain as a new ingredient in diets for dairy cows: productive, qualitative, and in vitro fermentation traits. J. Sci. Food Agric. 2022, 102, 4121–4130. [Google Scholar] [CrossRef]
- Rezaei, J.; Rouzbehan, Y.; Fazaeli, H.; Zahedifar, M. Carcass characteristics, non-carcass components and blood parameters of fattening lambs fed on diets containing amaranth silage substituted for corn silage. Small Ruminant Res. 2013, 114, 225–232. [Google Scholar] [CrossRef]
- Shadi, H.; Rouzbehan, Y.; Rezaei, J.; Fazaeli, H. Yield, chemical composition, fermentation characteristics, in vitro ruminal variables, and degradability of ensiled amaranth (Amaranthus hypochondriacus) cultivars compared with corn (Zea mays) silage. Transl. Anim. Sci. 2020, 4. [Google Scholar] [CrossRef]
- Cajarville, C.; Britos, A.; Garciarena, D.; Luis Repetto, J. Temperate forages ensiled with molasses or fresh cheese whey: Effects on conservation quality, effluent losses and ruminal degradation. Anim. Feed Sci. Technol. 2012, 171, 14–19. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, X.; Elsabagh, M.; Lin, B.; Wang, H.-r. Effects of formic acid and corn flour supplementation of banana pseudostem silages on nutritional quality of silage, growth, digestion, rumen fermentation and cellulolytic bacterial community of Nubian black goats. J. Integr. Agric. 2021, 20, 2214–2226. [Google Scholar] [CrossRef]
- de Assis Pires, F.P.A.; Tomich, T.R.; Pereira, L.G.R.; Machado, F.S.; Campos, M.M.; de Oliveira, A.F.; Menezes, G.L.; de Menezes, R.A.; de Sousa, P.G.; Jayme, D.G.; et al. Effect of the Lactiplantibacillus plantarum and Lentilactobacillus buchneri on corn and sorghum silage quality and sheep energy partition under tropical conditions. Grass Forage Sci. 2023, 78, 224–235. [Google Scholar] [CrossRef]
- Owens, V.N.; Albrecht, K.A.; Muck, R.E. Protein degradation and ensiling characteristics of red clover and alfalfa wilted under varying levels of shade. Can. J. Plant Sci. 1999, 79, 209–222. [Google Scholar] [CrossRef]
- Robinson, D. Compensatory changes in the partitioning of dry matter in relation to nitrogen uptake and optimal variations in growth. Department of Soil Fertility, Macaulay Institute for Soil Research Aberdeen AB9 2QJ, UK. 1986, 58, 841–848. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC Int.: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Wylam, C.B. Analytical studies on the carbohydrates of grasses and clovers. IV.-further developments in the methods of estimation of mono-, di- and oligo-saccharides and fructosan. J. Sci. Food Agric. 1954, 5, 167–172. [Google Scholar] [CrossRef]
- Playne, M.J.; McDonald, P. The buffering constituents of herbage and of silage. J. Sci. Food Agric. 1966, 17, 264–268. [Google Scholar] [CrossRef]
- Kaewpila, C.; Khota, W.; Gunun, P.; Kesorn, P.; Cherdthong, A. Strategic addition of different additives to improve silage fermentation, aerobic stability and in vitro digestibility of napier grasses at late maturity stage. Agriculture-Basel. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- He, L.; Zhou, W.; Wang, Y.; Wang, C.; Chen, X.; Zhang, Q. Effect of applying lactic acid bacteria and cellulase on the fermentation quality, nutritive value, tannins profile and in vitro digestibility of Neolamarckia cadamba leaves silage. J. Anim. Physiol. Anim. Nutr. 2018, Vol.102, 1429–1436. [Google Scholar] [CrossRef]
- Coblentz, W.K.; Fritz, J.O.; Bolsen, K.K.; King, C.W.; Cochran, R.C. The effects of moisture concentration and type on quality characteristics of alfalfa hay baled under two density regimes in a model system. Anim. Feed Sci. Technol. 1998, 72, 53–69. [Google Scholar] [CrossRef]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Muck, R.E. Dry matter level effects on alfalfa silage quality. II. Fermentation products and starch hydrolysis. Trans. ASAE. 1990, 33, 373–381. [Google Scholar] [CrossRef]
- Yahaya, M.S.; Kawai, M.; Takahashi, J.; Matsuoka, S. The Effects of different moisture content and ensiling time on silo degradation of structural carbohydrate of orchardgrass. Asian Australas. J. Anim. Sci. 2002, 15, 213–217. [Google Scholar] [CrossRef]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Li, M.; Zhou, H.; Zi, X.; Cai, Y. Silage fermentation and ruminal degradation of stylo prepared with lactic acid bacteria and cellulase. Anim. Sci. J. 2017, 88, 1531–1537. [Google Scholar] [CrossRef]
- Pahlow, G.; Muck, R.E.; Driehuis, F.; Oude Elferink, S.J.W.H.; Spoelstra, S.F. Microbiology of ensiling. In Silage Science and Technology; 2015; pp. 31-93.
- Yang, W.; Yang, F.; Feng, C.; Zhao, S.; Zhang, X.; Wang, Y. Fermentation Properties and Bacterial Community Composition of Mixed Silage of Mulberry Leaves and Smooth Bromegrass with and without Lactobacillus plantarum Inoculation. Fermentation. 2023, 9, 279. [Google Scholar] [CrossRef]
- Zanine, A.d.M.; Bonelli, E.A.; Souza, A.L.d.; Ferreira, D.d.J.; Santos, E.M.; Ribeiro, M.D.; Geron, L.J.V.; Pinho, R.M.A. Effects of Streptococcus bovis Isolated from Bovine Rumen on the Fermentation Characteristics and Nutritive Value of Tanzania Grass Silage. The Scientific World JOURNAL. 2016, 8517698–8517696. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, S.; Zhao, J.; Li, J.; Shao, T. Effects of additives on the fermentation quality, in vitro digestibility and aerobic stability of mulberry (Morus alba L.) leaves silage. Asian Australas. J. Anim. Sci. 2020, 33, 1292–1300. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Gou, W.; Cheng, Q.; Bai, S.; Cai, Y. Silage fermentation and bacterial community of bur clover, annual ryegrass and their mixtures prepared with microbial inoculant and chemical additive. Anim. Feed Sci. Technol. 2019, 247, 285–293. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Weinberg, Z.G.; Ogunade, I.M.; Cervantes, A.A.P.; Arriola, K.G.; Jiang, Y.; Kim, D.; Li, X.; Gonçalves, M.C.M.; Vyas, D.; et al. Meta-analysis of effects of inoculation with homofermentative and facultative heterofermentative lactic acid bacteria on silage fermentation, aerobic stability, and the performance of dairy cows. J. Dairy Sci. 2017, 100, 4587–4603. [Google Scholar] [CrossRef]
- Yahaya, M.S.; Kawai, M.; Takahashi, J.; Matsuoka, S. The effect of different moisture contents at ensiling on silo degradation and digestibility of structural carbohydrates of orchardgrass. Anim. Feed Sci. Technol. 2002, 101, 127–133. [Google Scholar] [CrossRef]
- Abbasi, M.; Rouzbehan, Y.; Rezaei, J.; Jacobsen, S.E. The effect of lactic acid bacteria inoculation, molasses, or wilting on the fermentation quality and nutritive value of amaranth (Amaranthus hypochondriaus) silage. J. Anim. Sci. 2018, 96, 3983–3992. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, L.; Ma, G.; Jiang, X.; Yang, J.; Lv, J.; Zhang, Y. Cellulase interacts with lactic acid bacteria to affect fermentation quality, microbial community, and ruminal degradability in mixed silage of soybean residue and corn stover. Animals. 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Bao, J.; Wang, L.; Yu, Z. Effects of different moisture levels and additives on the ensiling characteristics and in vitro digestibility of stylosanthes silage. Animals. 2022, 12. [Google Scholar] [CrossRef]
- Morrison, I.M. Changes in the cell wall components of laboratory silages and the effect of various additives on these changes. J. Agric. Sci. 1979, 93, 581–586. [Google Scholar] [CrossRef]
- Lynch, J.P.; Baah, J.; Beauchemin, K.A. Conservation, fiber digestibility, and nutritive value of corn harvested at 2 cutting heights and ensiled with fibrolytic enzymes, either alone or with a ferulic acid esterase-producing inoculant. J. Dairy Sci. 2015, 98, 1214–1224. [Google Scholar] [CrossRef]
- Foster, J.L.; Carter, J.N.; Sollenberger, L.E.; Blount, A.R.; Myer, R.O.; Maddox, M.K.; Phatak, S.C.; Adesogan, A.T. Nutritive value, fermentation characteristics, and in situ disappearance kinetics of ensiled warm-season legumes and bahiagrass. J. Dairy Sci. 2011, 94, 2042–2050. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, Z.; Li, J.; Chen, L.; Bai, Y.; Jia, Y.; Shao, T. Effects of lactic acid bacteria and molasses on fermentation dynamics, structural and nonstructural carbohydrate composition and in vitro ruminal fermentation of rice straw silage. Asian Australas. J. Anim. Sci. 2019, 32, 783–791. [Google Scholar] [CrossRef]
- Chen, L.; Guo, G.; Yuan, X.; Zhang, J.; Li, J.; Shao, T. Effects of applying molasses, lactic acid bacteria and propionic acid on fermentation quality, aerobic stability and in vitrogas production of total mixed ration silage prepared with oat-common vetch intercrop on the Tibetan Plateau. J. Sci. Food Agric. 2016, 96, 1678–1685. [Google Scholar] [CrossRef]
- Ma, J.; Fan, X.; Ma, Z.; Huang, X.; Tang, M.; Yin, F.; Zhao, Z.; Gan, S. Silage additives improve fermentation quality, aerobic stability and rumen degradation in mixed silage composed of amaranth and corn straw. Front. Plant Sci. 2023, 14. [Google Scholar] [CrossRef]
- Cai, Y.; Du, Z.; Yamasaki, S.; Nguluve, D.; Tinga, B.; Macome, F.; Oya, T. Influence of microbial additive on microbial populations, ensiling characteristics, and spoilage loss of delayed sealing silage of Napier grass. Anim. Biosci. 2020, 33, 1103–1112. [Google Scholar] [CrossRef]
- Hao, W.; Wang, H.L.; Ning, T.T.; Yang, F.Y.; Xu, C.C. Aerobic stability and effects of yeasts during deterioration of non-fermented and fermented total mixed ration with different moisture levels. Asian Australas. J. Anim. Sci. 2015, 28, 816–826. [Google Scholar] [CrossRef]
- Wambacq, E.; Latré, J.P.; Haesaert, G. The effect of Lactobacillus buchneri inoculation on the aerobic stability and fermentation characteristics of alfalfa-ryegrass, red clover and maize silage. Agr. Food Sci. 2013, 22, 127–136. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).