1. Introduction
Locally advanced or metastatic prostate cancer (PCa) can be treated with surgery, radiotherapy, chemotherapy and hormonal therapy, depending on the stage of the tumor, the age, previous treatment and comorbidity. The seminal work of Huggins and Hodges in 1941 demonstrated that androgens are integral to the development of PCa and that decreasing the activity of androgens through castration with surgical orchidectomy or estrogenic injections inhibits PCa [
1]. Treatments that suppress and inactivate the androgen testosterone (T) remain the cornerstone of PCa treatment, although surgical orchidectomy and estrogens have been replaced with hormonal anti-androgen treatment by androgen deprivation therapy (ADT) and anti-androgenic drugs such as the androgen receptor signaling inhibitors (ARSI’s) [
2].
Gonadotrophin-releasing hormone (GnRH) analogues are established ADT options that are continued lifelong, except when used with radiotherapy for locally advanced PCa. Before the GnRH analogues were available, estrogens had been an effective treatment option for 30 years, able to reduce T levels to inhibit the growth of androgen-sensitive PCa [
3,
4]. Although the efficacy of estrogen and ADT was comparable, estrogens had more major adverse cardiovascular events (MACE) and were replaced by GnRH analogues for safety reasons [
4]. However, this treatment paradigm change from estrogen to GnRH analogues has created a consequential problem of estrogen deficiency. By suppressing T, its metabolite estradiol (E2) is also decreased by about 80% [
5], causing serious estrogen deficiency side-effects affecting quality-of-life (QoL) including arthralgia, hot flushes, weight gain, muscle atrophy (sarcopenia), bone loss and fractures, the metabolic syndrome, an increased MACE risk, fatigue, sleeping problems, loss of energy, apathy, mood changes and depression, and cognition and memory problems (
Table S1) [
6,
7].
Estrogen treatment using diethylstilbestrol (DES), ethinylestradiol or oral E2 has been associated with an increased risk of MACE [
4,
7,
8]. However, new safer estrogen therapies including transdermal E2 (tE2) [
9], and the oral fetal estrogen estetrol (E4) [
5,
6,
7,
10] have shown promising anti-tumor effects, either combined with ADT (E4) or without ADT (tE2), and a reconsideration of the use of these estrogens is warranted, especially as these compounds may prevent/treat estrogen deficiency signs and symptoms.
Estetrol is a natural human estrogen, produced by the fetal liver during pregnancy only. Its physiological function is unknown, and it has no active or toxic metabolites [
11]. Oral administration of E4 has a low impact on coagulation and hemostatic liver factors, at doses up to 20 mg for hormone replacement therapy (HRT) [
12], or at a dose of 15 mg in combination with the progestin drospirenone for contraception [
13]. An E4 dose-finding study in healthy older males to estimate the optimal dose of E4 for the treatment of advanced PCa resulted in the selection of a dose of 40 mg E4 for further development [
14]. The phase II PCombi study (Co-treatment of PCa with ADT and oral E4) was conducted to assess the efficacy and safety of 40 mg E4 in patients with advanced PCa who started ADT treatment with an LHRH agonist (LHRHa) [
5,
6]. We have previously reported that E4 cotreatment was well tolerated, and associated with improvements in estrogen-deficiency symptoms compared to placebo, while also demonstrating more potent suppression of endocrine parameters consistent with enhanced anti-tumor effects [
5,
6]. In the present paper, we further evaluate the anti-tumor effects of oral E4 co-treatment on the tumor stimulators total and free T, prostate-specific antigen (PSA), follicle-stimulating hormone (FSH) and insulin-like growth factor-1 (IGF-1). In particular, special emphasis has been put on FSH and IGF-1 since the important role of these tumor stimulators is generally underestimated.
2. Patients and Methods
2.1. Patients
Male patients with a body mass index of 18.0–35.0 kg/m
2, recently diagnosed with locally advanced or metastatic PCa and qualifying for treatment with an LHRHa as ADT were eligible. In addition, patients had to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 [
15] and a life expectancy of at least 2 years. The supplementary information provides a full list of the inclusion and exclusion criteria.
2.1. Study Design and Treatments
A randomized, double-blind, placebo-controlled, phase II, proof-of concept study in patients with advanced PCa (PCombi) was conducted at four sites in the Netherlands (ClinicalTrials.gov NCT03361969; EudraCT 2017-003708-34) [
5]. Eligible patients were randomized at baseline at a 2:1 ratio to receive once-daily oral co-treatment of ADT with 40 mg E4 or matching placebo for a treatment duration of 24 weeks (wks). Blinded study medication was packed per subject number according to a computer-generated randomization list that was only known to an independent biostatistician.
The study was approved by an independent ethics committee (Evaluation of Ethics in Biomedical Research, Assen, The Netherlands) and was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Council for Harmonization. All patients provided written informed consent.
2.3. Endpoints and Assessments
Prior papers have reported on the favorable effects of oral E4 on hot flushes, arthralgia, endocrine and bone parameters, and QoL in patients with PCa [
5,
6]. The present paper is focusing in more detail on the analysis of the effects on endocrinological tumor stimulators. For these analyses, blood was withdrawn before treatment (baseline), and at 2, 4, 6, 8, 12, 18 and 24 wks for total T, free T and PSA, and at baseline, 12 and 24 wks for FSH and IGF-1. Total T levels were determined by LC-MS/MS at Ardena Bioanalysis, Assen, The Netherlands. Free T was assessed using an enzyme immunoassay at IBL, Hamburg, Germany. PSA, FSH and IGF-1 levels were assessed by electrochemiluminescence immunoassay at BARC Central Laboratory, Ghent, Belgium.
2.4. Statistical Analysis
The per-protocol (PP) population was considered the primary population for the analysis of all the secondary endpoints involving endocrine parameters. Patient demographic data were compared using a chi-square exact test or t test. Differences between treatment groups over time for total T, free T, and PSA were analyzed using a repeated-measure mixed model on log-transformed values (Wilcoxon rank-sum test). All endocrine parameters at the 24-wk visit were compared using Kruskal-Wallis testing. Individual data, recorded at wks 2, 6, 12 and 24 were used to visualize changes versus baseline in every patient.
3. Results
In total, 63 patients were randomized and 62 received study medication (41 ADT+E4 and 21 ADT+placebo). The PP population consisted of 57 patients: 37 on E4 and 20 on placebo (
Figure S1 CONSORT diagram). Five patients discontinued treatment early, three due to adverse events (
Figure S1). Baseline patient characteristics did not differ between the treatment groups, except for BMI and ECOG performance status (
Table S2) [
5].
Circulating levels of total T to castrate levels of less than 0.6 nmol/l (20 ng/dl) were achieved in almost all patients in both treatment groups from wk 4 onwards (
Figure S2A,
Tables S3 and S4). Compared to placebo, patients using E4 showed significantly earlier suppression of total T during the first 2–6 wks of treatment (
p<0.05;
Figure S2A), and were similar thereafter. Apart from one case of increased total T at 2 wks, suggesting a flare, individual maximal total T suppression was achieved earlier with E4 (
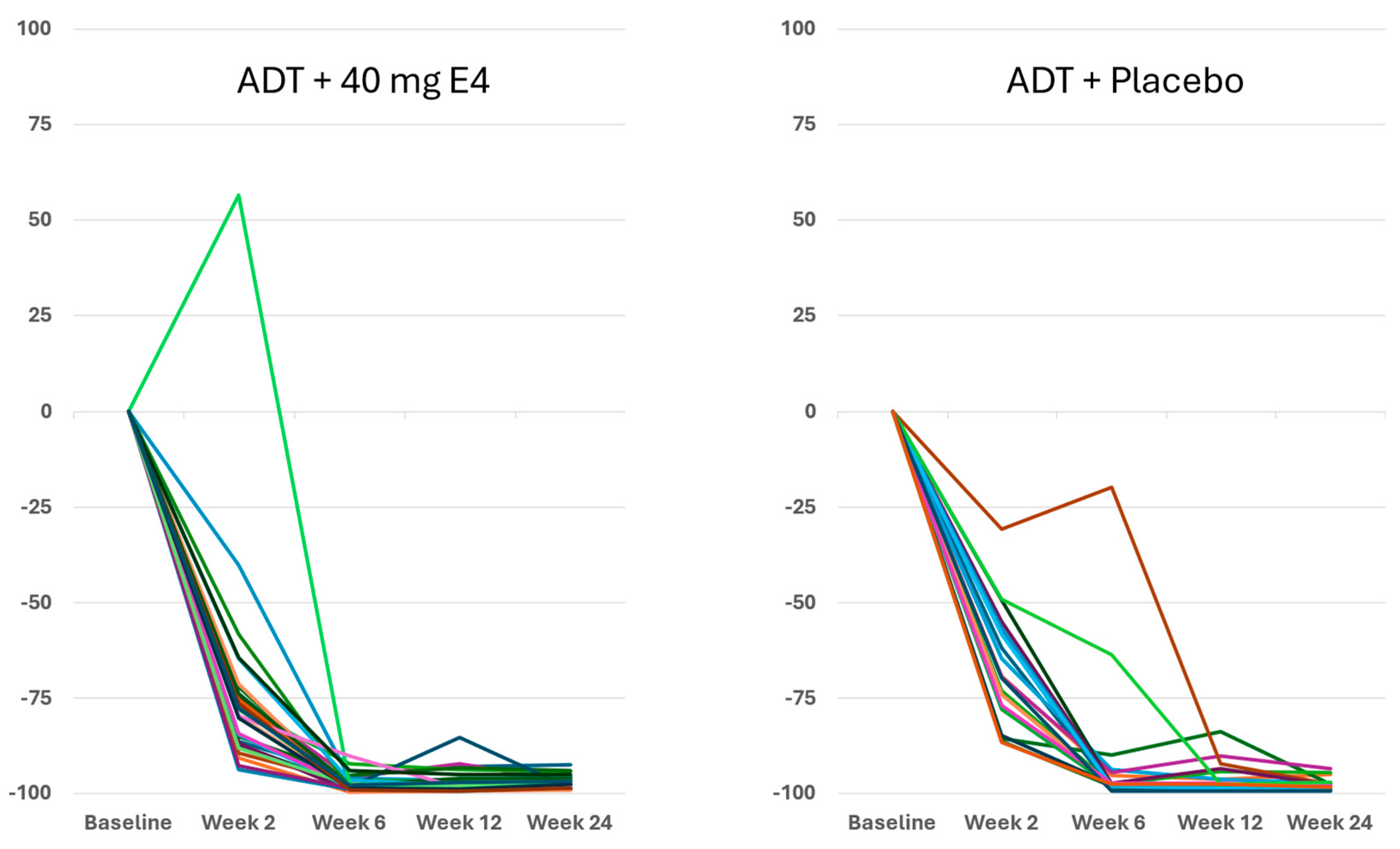
Figure 1 and
Figure S2A,
Table S4).
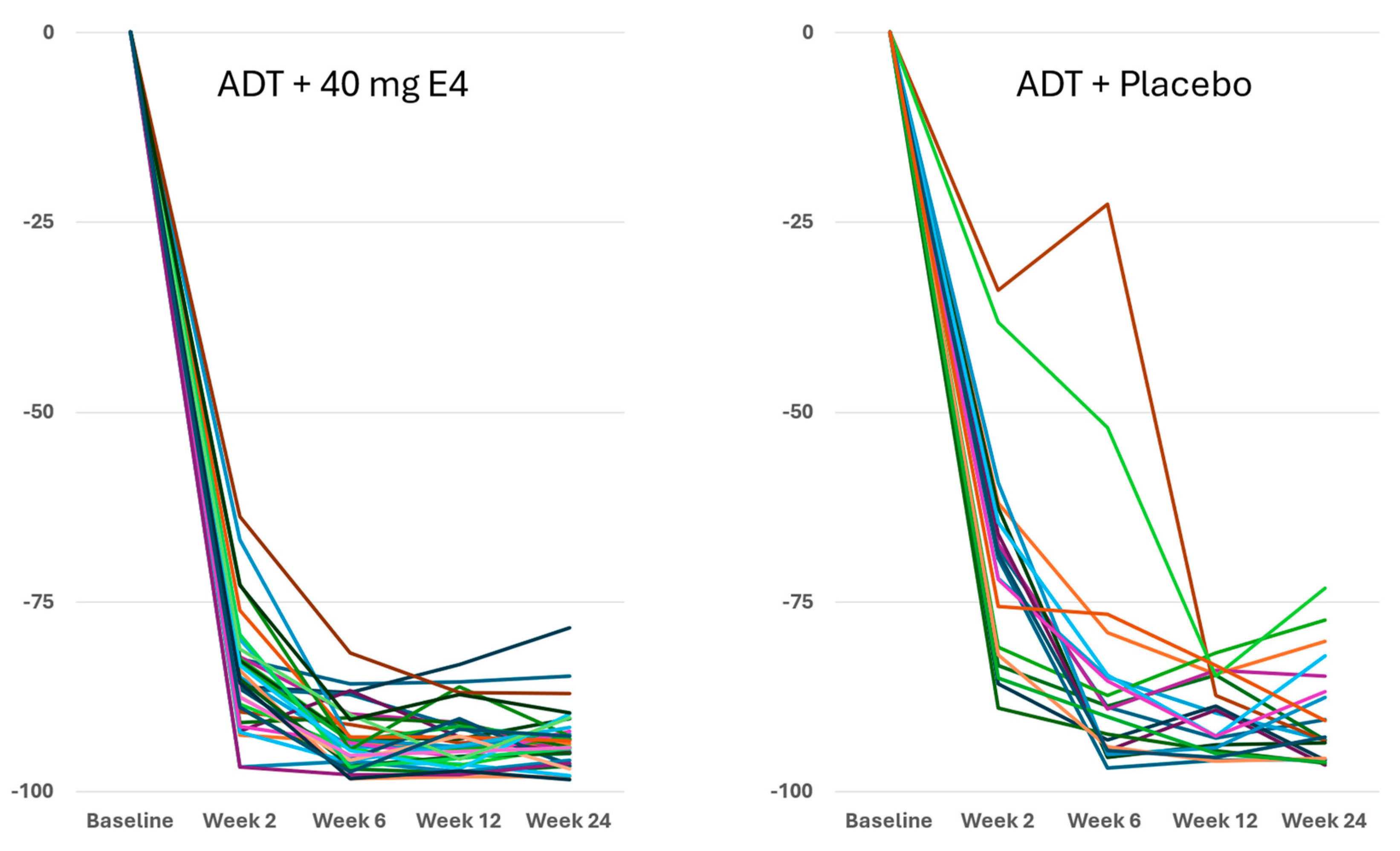
Measured free T levels were significantly lower with E4 at all time points during the study (all
p<0.05;
Figure S2B,
Table 1,
Table S3 and S5). At the end of the study at 24 wks, the mean level of free T had decreased from 42.0 pmol/l at baseline to 2.4 pmol/l (-93.2%) with E4, and significantly less with placebo, from 37.7 pmol/l to 3.2 pmol/l (-89.7%) (
p<0.05;
Table 1 and
Table S5). Across all the individuals, free T levels decreased faster and better with E4, with adequate suppression from wk 2 onwards (
Figure 2).
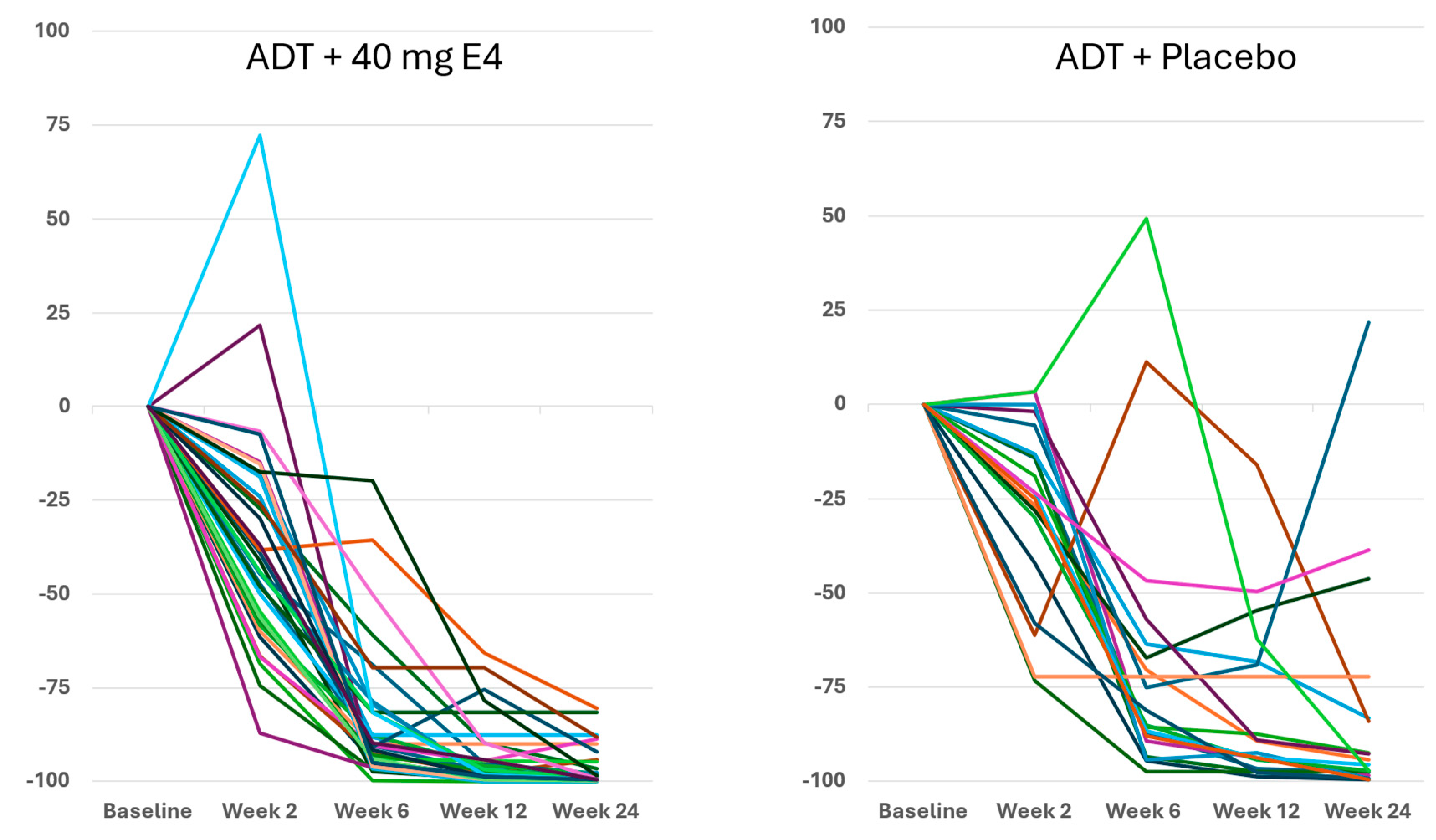
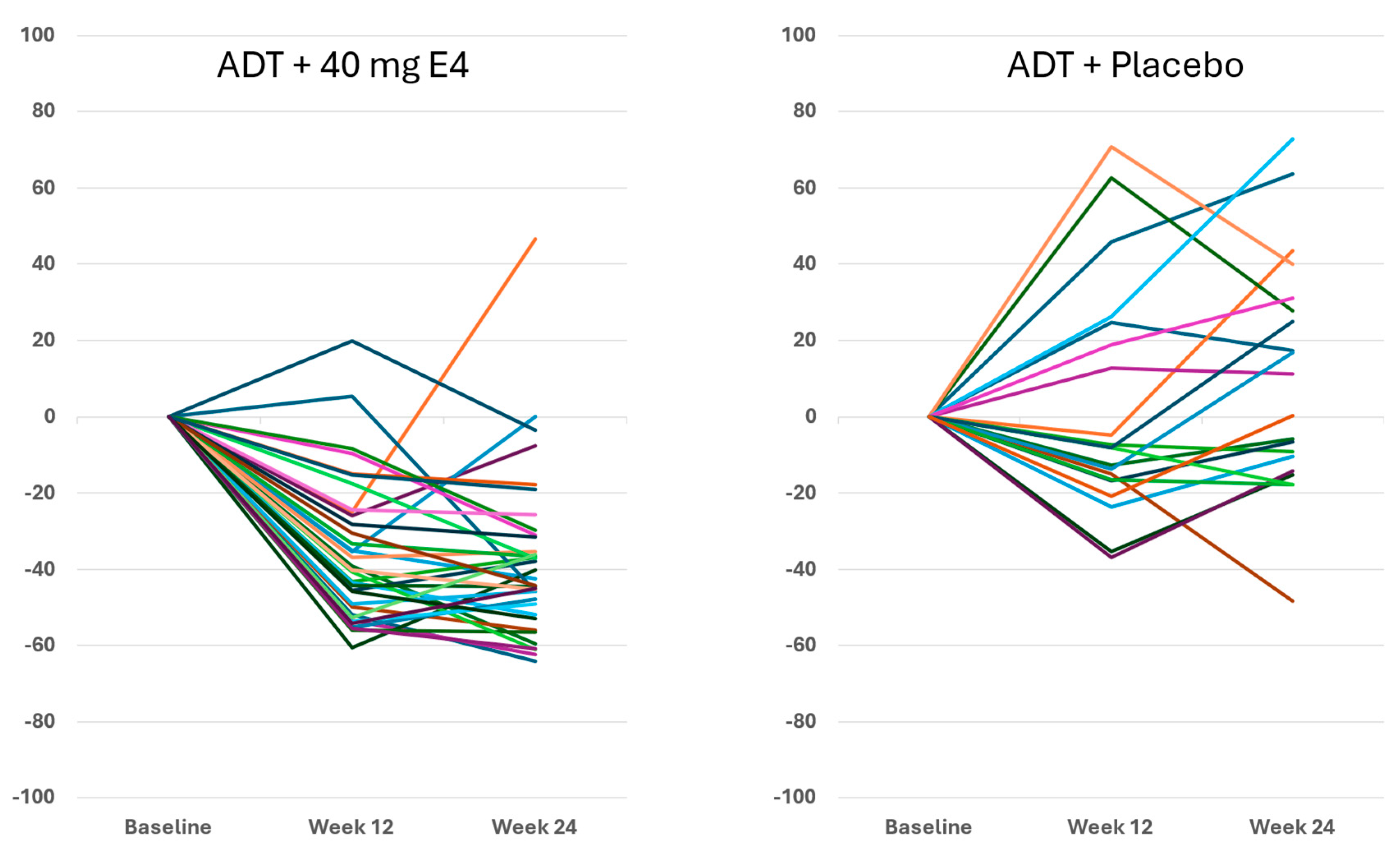
The decrease of PSA with E4 occurred earlier than with placebo in the majority of patients, with mean levels of 13.4 ng/ml with E4 (-27.2%) and 27.4 ng/ml with placebo (-18.2%) after 2 wks (
Figure S2C,
Tables S3 and S6). At 24 wks, the mean level of PSA had decreased from 18.4 ng/ml at baseline to 0.6 ng/ml with E4 (-96.4%), and significantly less with placebo, from 33.5 ng/ml to 4.0 ng/ml (-83.1%) (
p<0.005;
Tables S3 and S6). The differences between treatment groups were significant at all time points (
p<0.05;
Figure S2C).
Figure 3 shows the enhanced and consistent PSA suppression from 6-12 wks onwards in all individuals.
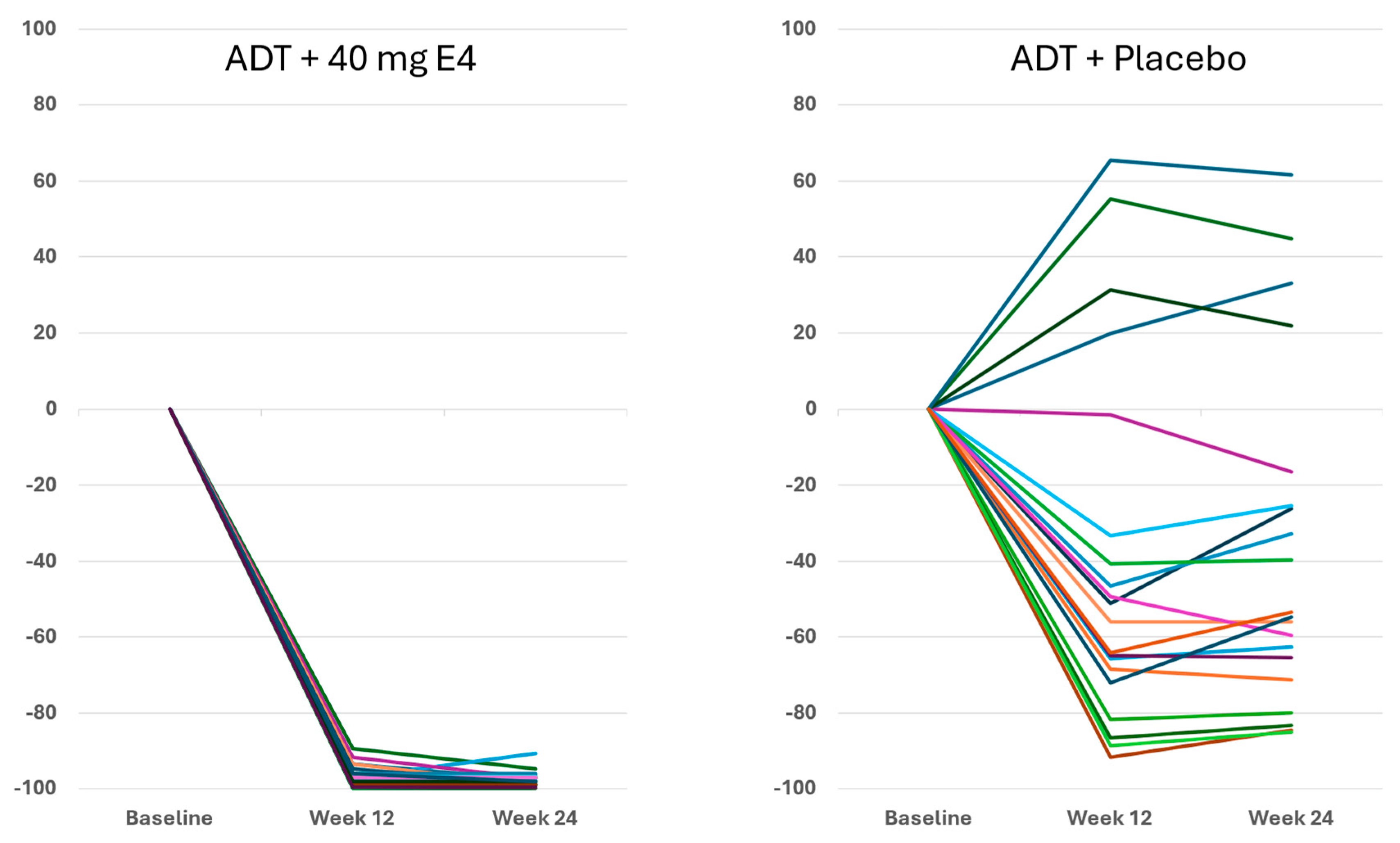
For FSH,
Figure 4 shows that with E4 FSH was almost completely suppressed in all patients. In the placebo group, FSH decreased in 16 of 20 patients and to a much lower extent than with E4 (
p<0.001) (
Figure 4,
Table 1). The mean level of FSH decreased from 11.3 IU/l at baseline to 0.2 IU/l both at 12 wks and at 24 wks with E4 (-97.8%), and from 12.7 IU/l to 5.5 IU/l at 24 wks with placebo (-36.7%) (
p<0.001;
Figure 4 and
Figure S3;
Table 1 and Table S7).
For IGF-1, the suppressive effect of E4 in all except one patient at 24 wks is shown in
Figure 5. With placebo, IGF-1 levels showed a more variable effect with both increases and decreases. With E4, the mean level of IGF-1 decreased from 174.0 ng/ml at baseline to 110.2 ng/ml (-35.8%) and to 100.4 ng/ml (-41.4%) at 12 wks and 24 wks, respectively (Figures 5 and S3, Tables 1 and S8). In contrast, the mean levels of IGF-1 increased slightly in the placebo group, from 153.1 ng/ml at baseline to 161.3 ng/ml (+2.1%) and 171.2 ng/ml (+10.2%), respectively (
Figure S3 and
Table S3) The differences between treatment groups were significant (
p<0.0001).
4. Discussion
Publications reporting clinical trials generally do not include individual effects. The size of the study reported in this paper, with 37 PCa patients treated by ADT+E4 and 20 patients treated by ADT+placebo, allows us to show all individual assessments of the four endocrine tumor stimulators, i.e testosterone (T), PSA, FSH and IGF-1, during the 24 wks treatment period. Figures 1, 2 and S2 show that total T and free T both respond faster with E4 during the first 2-6 wks of treatment, and free T is better suppressed at all time points thereafter. From wk 6 onwards, PSA nadirs are more profound and sustained with E4 (Figures 3 and S2C). FSH and IGF-1, both measured at 12 and 24 wks show a striking difference with almost complete suppression of FSH in all patients (
Figure 4) and a decrease in IGF-1 in all but one patient (
Figure 5). In the placebo group, 6 of 20 patients even showed an increase of FSH and 10 of 20 patients showed an increase of IGF-1. This confirms that all E4 treated patients demonstrated a favorable effect of E4 on endocrine tumor stimulators.
The principle aim of ADT as treatment for advanced PCa is to reduce the circulating levels of testosterone to castrate levels of total T of less than 0.6 nmol/l (20 ng/dl) [
16,
17], which was achieved in all patients in both treatment groups from wk 4 through wk 24 in this study. GnRH analogues used for ADT act by suppression of (LHRH agonists) or by competition with (GnRH antagonist) the pituitary luteinizing hormone (LH).
In the present PCombi study in both treatment groups LHRHa’s were used for ADT and in both groups the suppression of LH is 97.6% [
5], and therefore the addition of E4 does not suppress LH further. Therefore, it is not surprising that the effect on total T is comparable in both groups, except for the earlier suppression with E4, which can be explained by the negative feedback of estrogens on the hypothalamus. The earlier suppression of free T can be explained by the same feedback mechanism. The significant further inhibition of free T, considered a better predictor than total T for castration resistance [
18], can be explained by the increase of sex hormone-binding globulin (SHBG) by estrogens as also shown for E4 (
Table S9), and the binding and thereby inactivation of testosterone by SHBG.
The rather strong and significant effect of E4 on PSA is a new observation. PSA is kallikrein-3, a protease enzyme, that stimulates the growth of PCa [
19] and the rise in PSA means higher production of this tumor stimulator, which explains that a suppression of the tumor stimulator PSA by ADT is associated with clinical benefits [
20] and also explains why PSA is a marker for PCa tumor progression.
The mitogenic effect of FSH plays an important stimulating role in the development and progression of PCa [
21,
22]. The name of the hormone FSH is derived from its physiological mitogenic effect in women on ovarian follicular granulosa cells [
23]. In the male, FSH is the prime inducer of proliferation of spermatogonia [
24]. FSH levels are suppressed by ADT alone [
21] and by ADT combined with an ARSI [
25], or by tE2 only [
26]. The almost complete suppression of FSH (97.8%) in all patients co-treated with E4 is therefore a significant anti-tumor effect just like the significantly enhanced suppression of the mitogen IGF-1. High levels of serum IGF-1 and activated IGF-1 receptor (IGF-1R) in the prostate are found in PCa [
27]. Not only enhanced serum IGF-1 but also the activation of IGF-1R and its downstream signaling components has been increasingly recognized to play a vital role in driving the development of PCa [
27]. Treatment modalities targeting IGF-1 are potential strategies for cancer therapy [
27], and the observed effects of E4 in the PCombi study suggest a favorable effect on IGF-1.
New endocrine treatments for advanced PCa are generally based on a novel mode of action to inhibit or antagonize testosterone, all at the expense of the concomitant loss of estrogens. This results in a loss of QoL by serious subjective side effects including hot flushes and arthralgia, and objective symptoms such as bone loss and fractures, arterial vascular disease and unfavorable brain effects. In the past, estrogens have been used very effectively for PCa treatment, but have been replaced by LHRHa’s because of an increased risk of MACE. So far, the risk of MACE when using tE2 or E4 is reassuring and seems to be lower than the risk of the older estrogens used in the past [
5,
8,
9,
10,
28].
5. Limitations
The current findings are robust for an enhanced suppression of total T, free T, PSA, FSH and IGF-1 during E4 cotreatment with ADT. However, larger and longer-duration studies are needed to confirm that these secondary tumor suppression endpoints are predictive and representative for a better progression-free survival and overall survival in men with advanced prostate cancer.
6. Conclusion
Estetrol co-treatment with ADT suppresses the tumor stimulator FSH almost completely and augments the suppression of total and free T, PSA and IGF-1, suggesting enhanced anti-cancer treatment efficacy.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Study concept and design: Coelingh Bennink, Debruyne, Reisman. Acquisition, analysis, and interpretation of data: Coelingh Bennink, Roos, van Moorselaar, van Melick, Somford, Roeleveld, de Haan, Reisman, Schultz, Krijgh, Debruyne. Drafting of the manuscript: Coelingh Bennink. Critical revision of the manuscript for important intellectual content: Coelingh Bennink, Roos, van Moorselaar, van Melick, Somford, Roeleveld, de Haan, Reisman, Schultz, Krijgh, Debruyne. Statistical analysis: Coelingh Bennink. Obtaining funding: Coelingh Bennink. Administrative, technical or material support: Krijgh, Debruyne. Supervision: Coelingh Bennink, Debruyne.
Financial Disclosures
Dr Coelingh Bennink is president and a shareholder of Pantarhei Oncology, an affiliate of Pantarhei Bioscience BV, and Dr Krijgh is chief medical officer of Pantarhei Oncology. Dr van Moorselaar received grants and fees from Astellas, Ipsen, Astra Zeneca, Bayer and Janssen. Dr Somford is member of advisory boards of Astellas and Janssen and research grant from Astellas. The other authors declare no conflict of interest.
Funding/Support and role of the sponsor
All funding for the study was provided by the sponsor, Pantarhei Oncology. Pantarhei Oncology, in collaboration with academic authors, had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication. The sponsor did not have the right to veto publication. The decision regarding to which journal the paper was to be submitted was collaboratively made between the lead authors and the sponsor.
Acknowledgements
Statistical analysis was performed by Gonnie van Osta at Author! et al, Hilversum, The Netherlands. Astrid van den Heuvel (Pantarhei Bioscience), Jan Egberts and Amanda Prowse (at Terminal 4 Communications, Hilversum, The Netherlands) are acknowledged for their contribution to the preparation of the manuscript. The authors are also grateful to Professor Marc Garnick (Harvard Medical School, Boston, MA, USA), Professor Bertrand Tombal (Cliniques Universitaires Saint Luc, UCLouvain, Brussels, Belgium) and Professor Neal Shore (Carolina Urologic Research Center, Myrtle Beach, USA) for their support and valuable suggestions.
Data Availability Statement
Individual data regarding the tumor stimulators investigated are presented in the Appendix. Other data are available from the corresponding author upon request.
Clinical Trial
PCombi) was conducted at four sites in the Netherlands; ClinicalTrials.gov NCT03361969; EudraCT 2017-003708-34. The study was approved by an independent ethics committee (Evaluation of Ethics in Biomedical Research, Assen, The Netherlands) and was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Council for Harmonization. All patients provided written informed consent.
Material from other Sources
References
- Huggins C, Hodges CV. Studies on Prostatic Cancer. I. The Effect of Castration, of Estrogen and of Androgen Injection on Serum Phosphatases in Metastatic Carcinoma of the Prostate. Cancer Res. 1941;1:293-7.
- Gillessen S, Bossi A, Davis ID, de Bono J, Fizazi K, James ND, et al. Management of patients with advanced prostate cancer-metastatic and/or castration-resistant prostate cancer: Report of the Advanced Prostate Cancer Consensus Conference (APCCC) 2022. Eur J Cancer. 2023;185:178-215. [CrossRef]
- Presti JC, Jr. Estrogen therapy for prostate carcinoma. JAMA. 1996;275:1153. [CrossRef]
- Turo R, Smolski M, Esler R, Kujawa ML, Bromage SJ, Oakley N, et al. Diethylstilboestrol for the treatment of prostate cancer: past, present and future. Scand J Urol. 2014;48:4-14. [CrossRef]
- Coelingh Bennink HJT, Van Moorselaar JA, Crawford ED, Roos EPM, Somford DM, Roeleveld TA, et al. Estetrol Cotreatment of Androgen Deprivation Therapy in Infiltrating or Metastatic, Castration-Sensitive Prostate Cancer: A Randomized, Double-blind, Phase II Trial (PCombi). Eur Urol Open Sci. 2021;28:52-61. [CrossRef]
- Zimmerman Y, Frydenberg M, van Poppel H, van Moorselaar RJA, Roos EPM, Somford DM, et al. Estetrol Prevents Hot Flushes and Improves Quality of Life in Patients with Advanced Prostate Cancer Treated with Androgen Deprivation Therapy: The PCombi Study. Eur Urol Open Sci. 2022;45:59-67. [CrossRef]
- Coelingh Bennink HJT, Krijgh J, Egberts JF, Slootweg M, van Melick HH, Roos EP, et al. Maintaining bone health by estrogen therapy in patients with advanced prostate cancer: a narrative review. Endocr Connect. 2022;11:e220182. [CrossRef]
- Reis LO, Zani EL, Garcia-Perdomo HA. Estrogen therapy in patients with prostate cancer: a contemporary systematic review. Int Urol Nephrol. 2018;50:993-1003. [CrossRef]
- Langley RE, Gilbert DC, Duong T, Clarke NW, Nankivell M, Rosen SD, et al. Transdermal oestradiol for androgen suppression in prostate cancer: long-term cardiovascular outcomes from the randomised Prostate Adenocarcinoma Transcutaneous Hormone (PATCH) trial programme. Lancet. 2021;397:581-91. [CrossRef]
- Coelingh Bennink HJT, Prowse A, Egberts JFM, Debruyne FMJ, Huhtaniemi IT, Tombal B. The loss of estradiol by androgen deprivation in prostate cancer patients shows the importance of estrogens in males. J Endocr Soc. 2024:bvae107. [CrossRef]
- Gerard C, Arnal JF, Jost M, Douxfils J, Lenfant F, Fontaine C, et al. Profile of estetrol, a promising native estrogen for oral contraception and the relief of climacteric symptoms of menopause. Expert Rev Clin Pharmacol. 2022;15:121-37. [CrossRef]
- Douxfils J, Gaspard U, Taziaux M, Jost M, Bouvy C, Lobo RA, et al. Impact of estetrol (E4) on hemostasis, metabolism and bone turnover in postmenopausal women. Climacteric. 2023;26:55-63. [CrossRef]
- Douxfils J, Klipping C, Duijkers I, Kinet V, Mawet M, Maillard C, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402. [CrossRef]
- Coelingh Bennink HJT, Zimmerman Y, Verhoeven C, Dutman AE, Mensinga T, Kluft C, et al. A Dose-Escalating Study With the Fetal Estrogen Estetrol in Healthy Men. J Clin Endocrinol Metab. 2018;103:3239-49. [CrossRef]
- Azam F, Latif MF, Farooq A, Tirmazy SH, AlShahrani S, Bashir S, et al. Performance Status Assessment by Using ECOG (Eastern Cooperative Oncology Group) Score for Cancer Patients by Oncology Healthcare Professionals. Case Rep Oncol. 2019;12:728-36. [CrossRef]
- Oefelein MG, Feng A, Scolieri MJ, Ricchiutti D, Resnick MI. Reassessment of the definition of castrate levels of testosterone: implications for clinical decision making. Urology. 2000;56:1021-4. [CrossRef]
- Crawford ED, Heidenreich A, Lawrentschuk N, Tombal B, Pompeo ACL, Mendoza-Valdes A, et al. Androgen-targeted therapy in men with prostate cancer: evolving practice and future considerations. Prostate Cancer Prostatic Dis. 2019;22:24-38. [CrossRef]
- Regis L, Planas J, Carles J, Maldonado X, Comas I, Ferrer R, et al. Free Testosterone During Androgen Deprivation Therapy Predicts Castration-Resistant Progression Better Than Total Testosterone. Prostate. 2017;77:114-20. [CrossRef]
- Moradi A, Srinivasan S, Clements J, Batra J. Beyond the biomarker role: prostate-specific antigen (PSA) in the prostate cancer microenvironment. Cancer Metastasis Rev. 2019;38:333-46. [CrossRef]
- Saad F, Small EJ, Feng FY, Graff JN, Olmos D, Hadaschik BA, et al. Deep Prostate-specific Antigen Response following Addition of Apalutamide to Ongoing Androgen Deprivation Therapy and Long-term Clinical Benefit in SPARTAN. Eur Urol. 2022;81:184-92. [CrossRef]
- Crawford ED, Rove KO, Schally AV, Rick FG, Block NL, Beveridge TJR, et al. The Role of the FSH System in the Development and Progression of Prostate Cancer. Am J Hematol/Oncol. 2014;10:5-13.
- Crawford ED, Schally AV. The role of FSH and LH in prostate cancer and cardiometabolic comorbidities. Can J Urol. 2020;27:10167-73.
- Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. 1997;18:739-73. [CrossRef]
- Simoni M, Gromoll J, Hoppner W, Kamischke A, Krafft T, Stahle D, et al. Mutational analysis of the follicle-stimulating hormone (FSH) receptor in normal and infertile men: identification and characterization of two discrete FSH receptor isoforms. J Clin Endocrinol Metab. 1999;84:751-5. [CrossRef]
- Sawazaki H, Araki D, Kitamura Y, Yagi K. Metabolic changes with degarelix vs. leuprolide plus bicalutamide in patients with prostate cancer: a randomized clinical study. World J Urol. 2020;38:1465-71. [CrossRef]
- Russell N, Hoermann R, Cheung AS, Ching M, Zajac JD, Handelsman DJ, et al. Short-term effects of transdermal estradiol in men undergoing androgen deprivation therapy for prostate cancer: a randomized placebo-controlled trial. Eur J Endocrinol. 2018;178:565-76. [CrossRef]
- Liu G, Zhu M, Zhang M, Pan F. Emerging Role of IGF-1 in Prostate Cancer: A Promising Biomarker and Therapeutic Target. Cancers (Basel). 2023;15:1287. [CrossRef]
- Gilbert DC, Nankivell M, Rush H, Clarke NW, Mangar S, Al-Hasso A, et al. A Repurposing Programme Evaluating Transdermal Oestradiol Patches for the Treatment of Prostate Cancer Within the PATCH and STAMPEDE Trials: Current Results and Adapting Trial Design. Clin Oncol (R Coll Radiol). 2024;36:e11-e9. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).