Submitted:
03 July 2024
Posted:
03 July 2024
You are already at the latest version
Abstract
Keywords:
MSC: 92-10; 92C05; 92C15; 92C40; 92C45; 80Axx; 82Cxx
1. Introduction
2. The Fundamental Molecules of Life are Dissipatively Structured UV-C Pigments
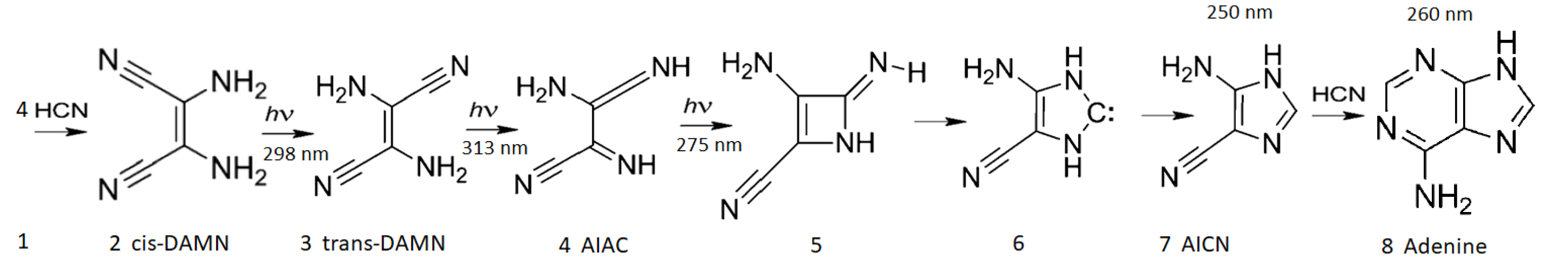
2.1. Precursors of the Fundamental Pigments of Life
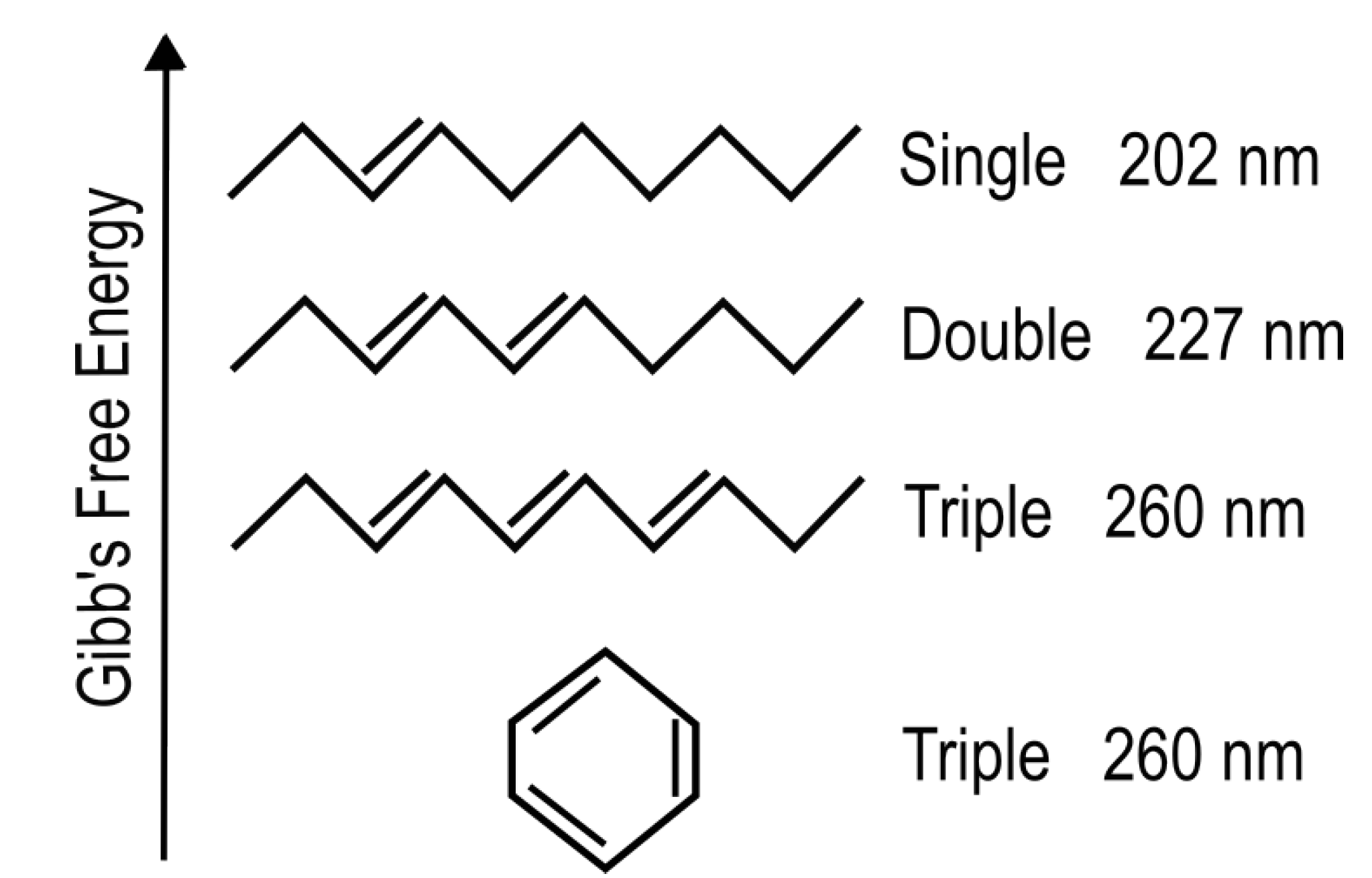
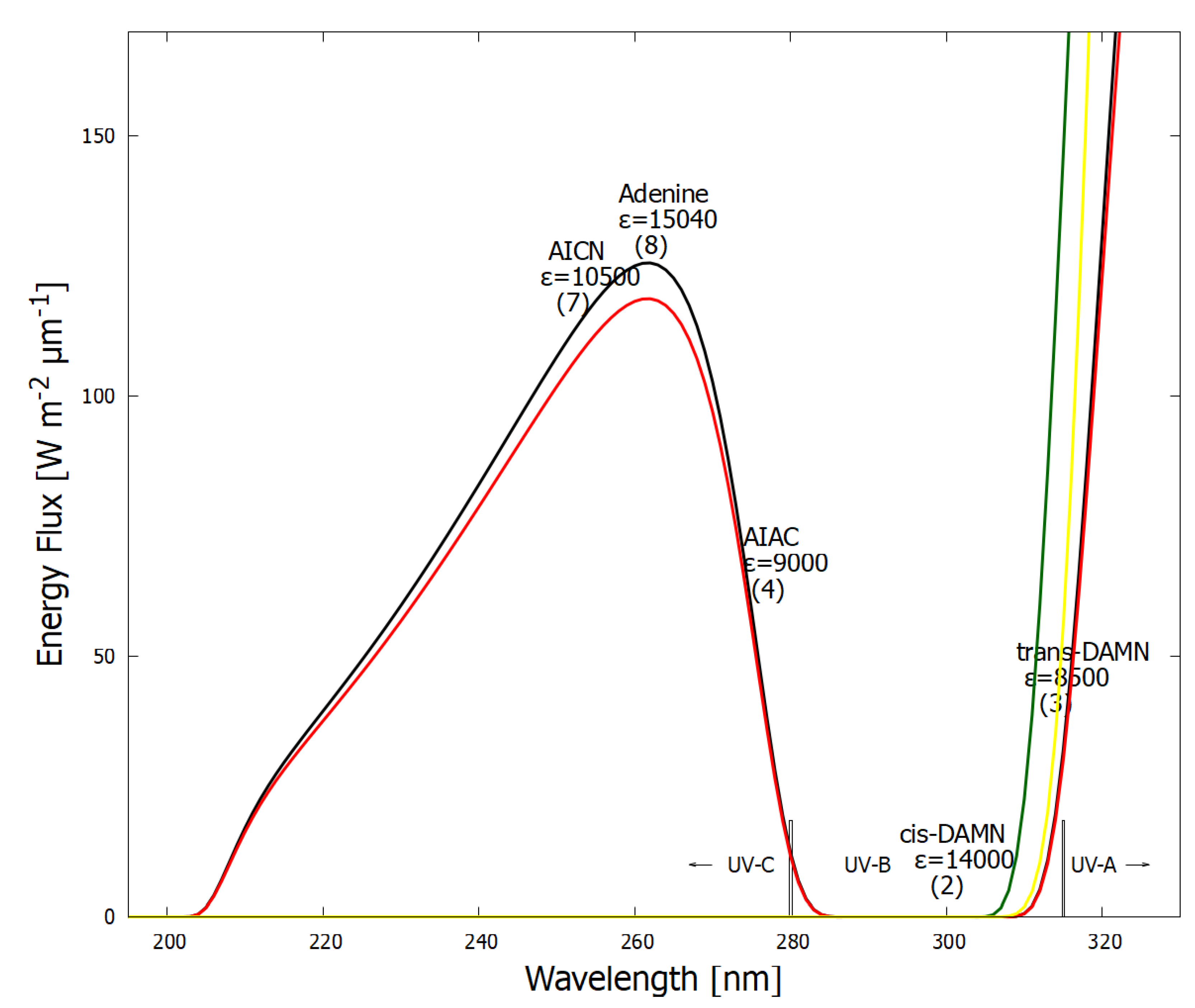
2.2. Strong UV-C Photon Absorption and Rapid Excited State Dissipation of Fundamental Molecules
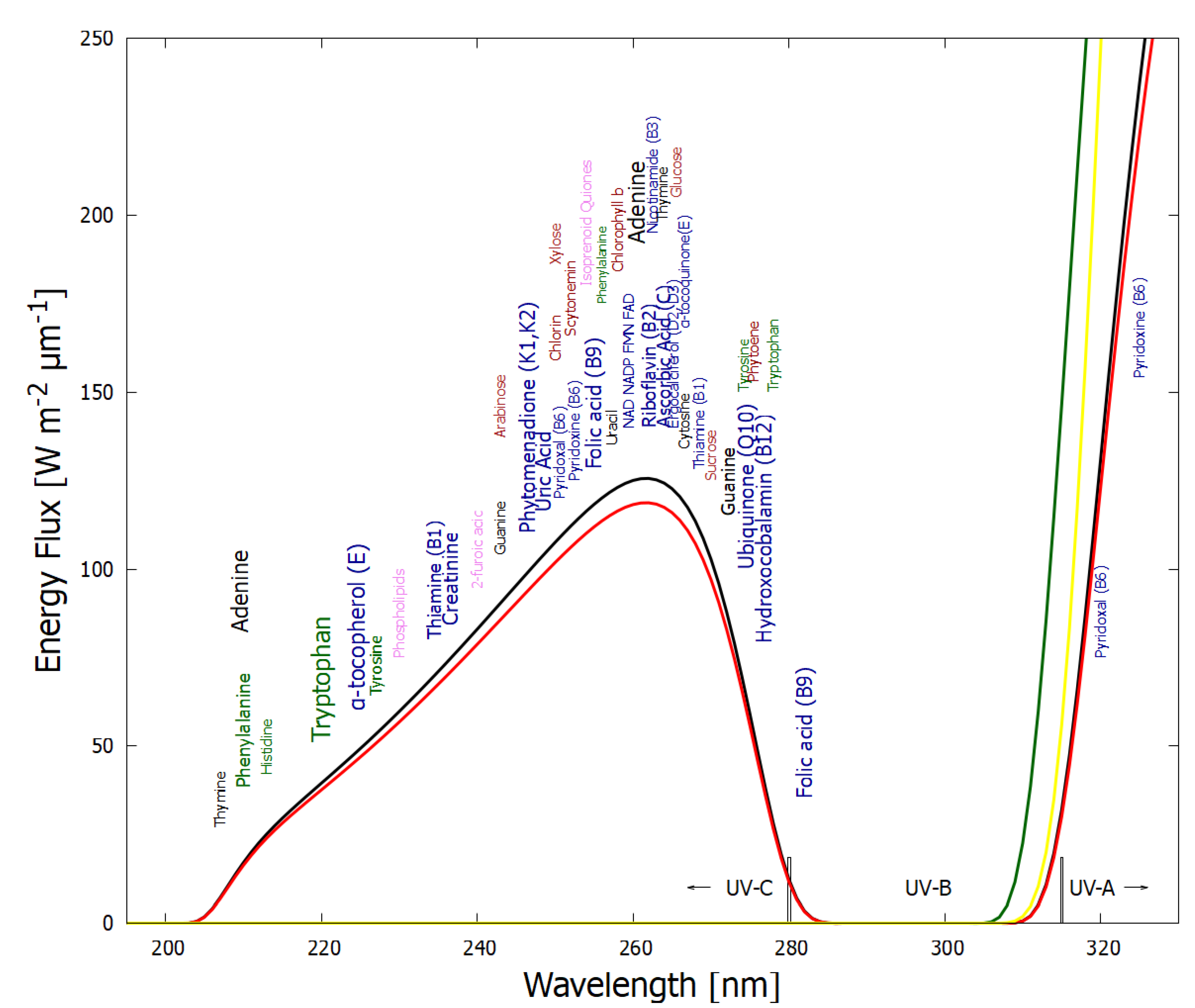
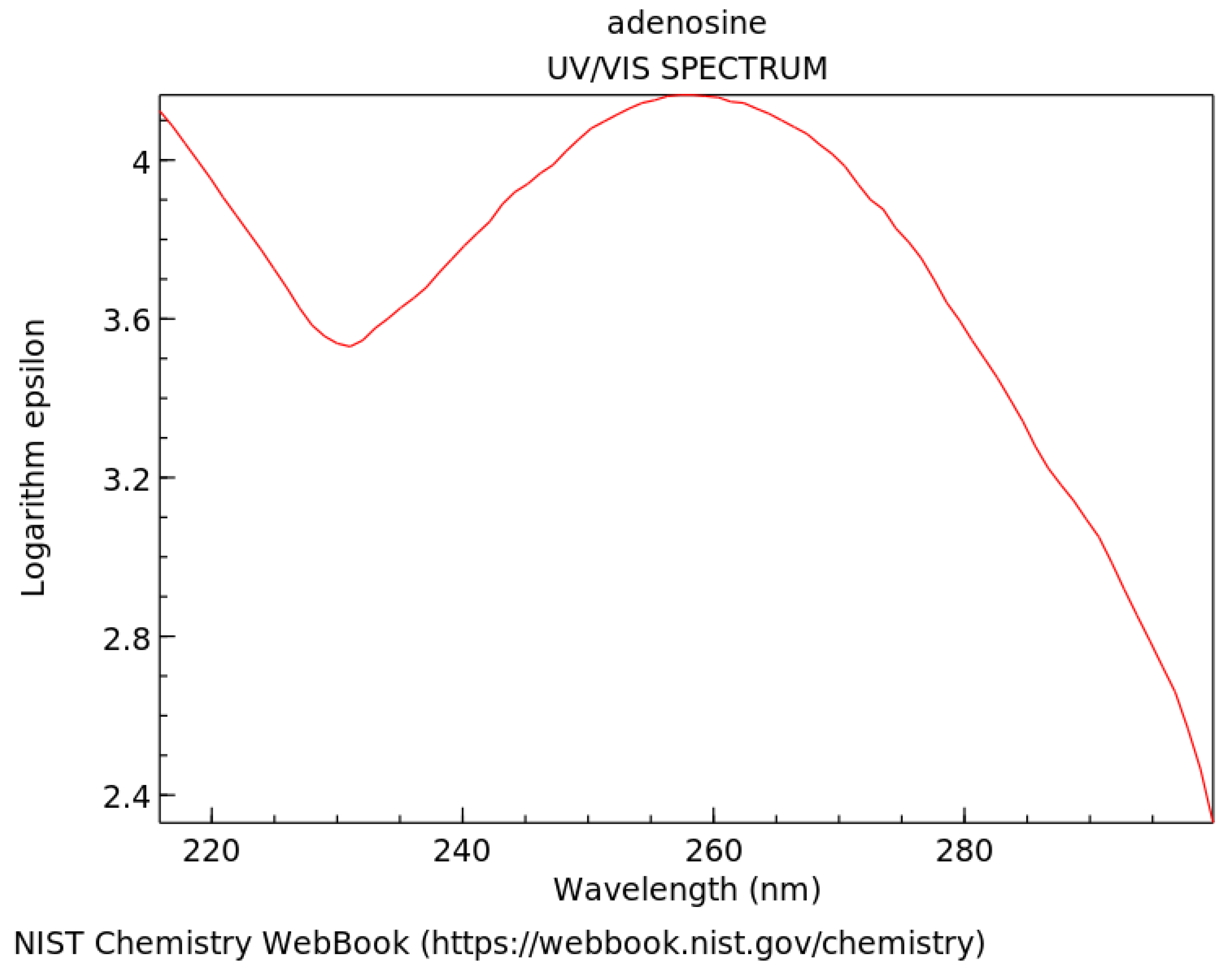
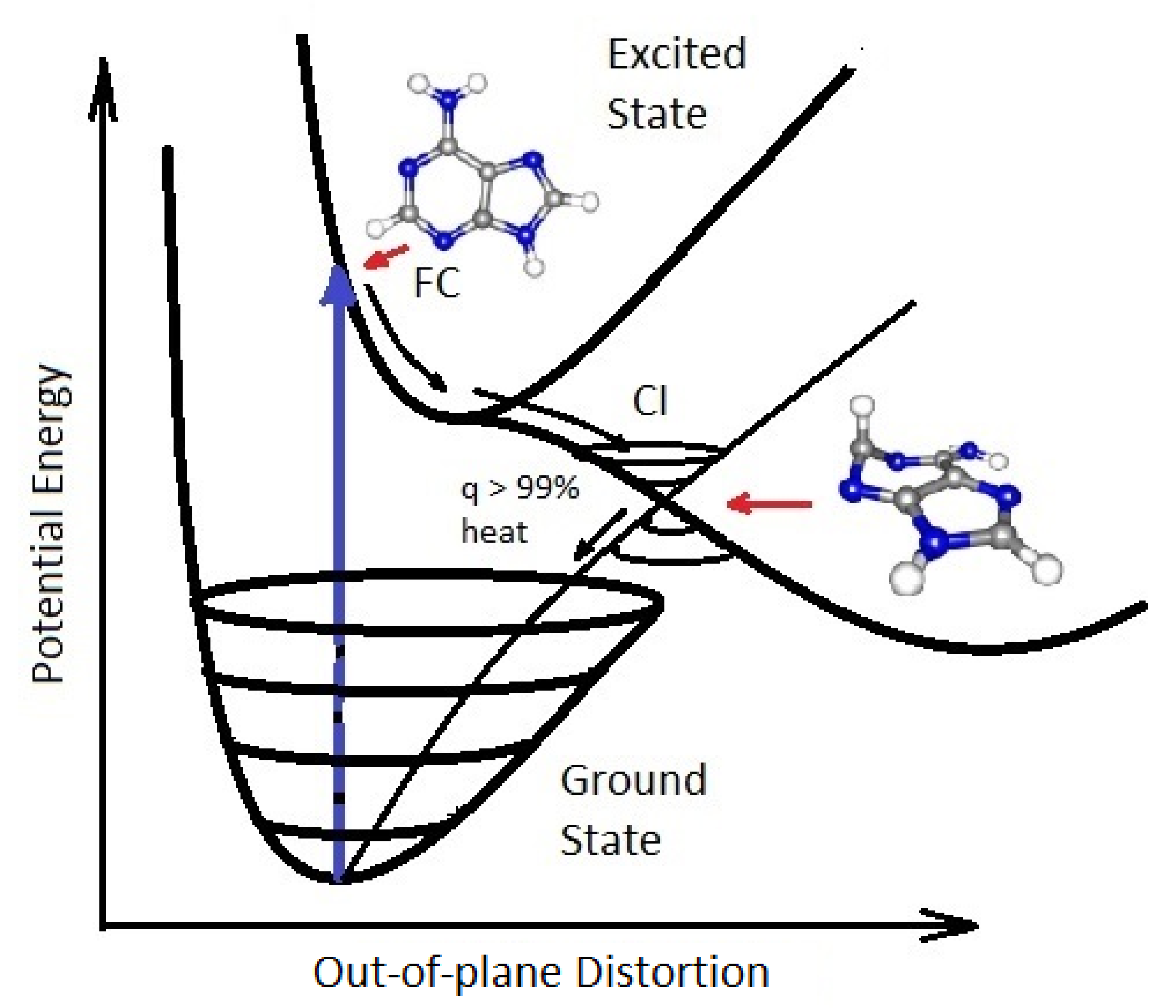
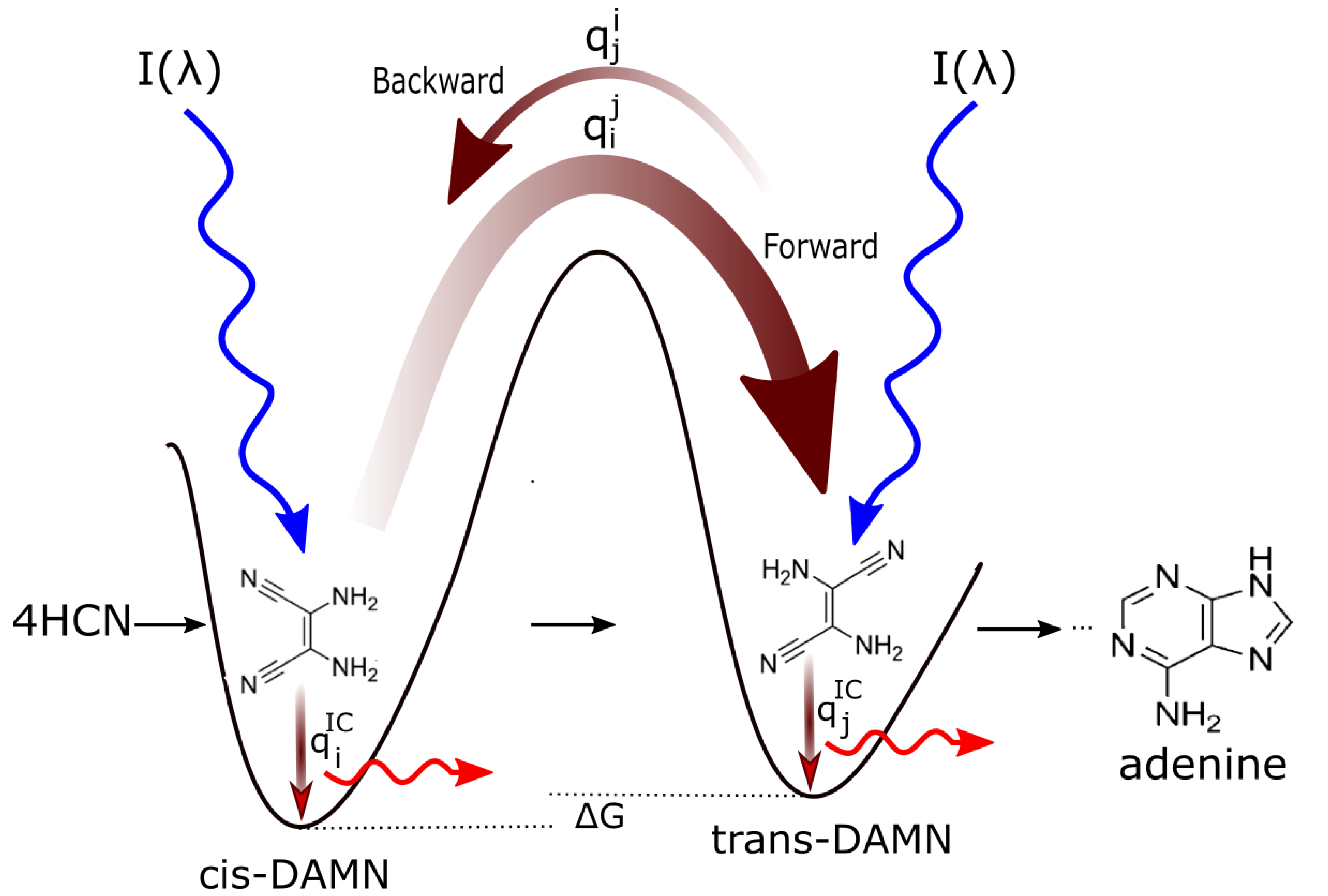
2.3. UV-C Photochemical Dissipative Structuring of the Fundamental Molecules
3. From Pigments to the Biosphere
3.1. Molecular Complexation through Natural Thermodynamic Selection on Dissipation
3.2. Organic Pigments in the Environment
3.3. Animals
3.3.1. Dispersal of Pigments; the Thermodynamic Role of Animals
3.3.2. The Survival Instinct
3.4. Coupling of Biotic and Abiotic Dissipative Processes in the Biosphere
3.5. Ecosystems
4. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AIAC | 2-amino-3-iminoacrylimidoyl cyanide |
| AICN | 4-aminoimidazole-5-carbonitrile |
| ATP | adenine triphosphate |
| CIT | Classical Irreversible Thermodynamics |
| DAMN | diaminomaleonitrile |
| DAFN | diaminofumaronitrile |
| HCN | hydrogen cyanide |
| NCCN | cyanogen |
| UV-A | light in the region 360-400 nm |
| UV-B | light in the region 285-360 nm (only the region 310-360 nm is relevant to dissipative structuring) |
| UV-C | light in the region 100-285 nm (only the soft UV-C region, 205-285, nm is relevant to dissipative structuring) |
| UVTAR | Ultraviolet and Temperature Assisted Replication |
References
- Prigogine, I. Introduction to Thermodynamics Of Irreversible Processes, third ed.; John Wiley & Sons: Hoboken, NJ, USA, 1967. [Google Scholar]
- Glansdorff, P.; Prigogine, I. Thermodynamic Theory of Structure, Stability and Fluctuations; Wiley - Interscience: Hoboken, NJ, USA, 1971. [Google Scholar]
- Prigogine, I.; Nicolis, G. Biological order, structure and instabilities. Quarterly Reviews of Biophysics 1971, 4, 107–144. [Google Scholar] [CrossRef] [PubMed]
- Prigogine, I.; Nicolis, G.; Babloyantz, A. Thermodynamics of evolution I. Physics Today 1972, 25, 23–28. [Google Scholar] [CrossRef]
- Prigogine, I.; Nicolis, G.; Babloyantz, A. Thermodynamics of evolution II. Physics Today 1972, 25, 38–44. [Google Scholar] [CrossRef]
- Michaelian, K. Thermodynamic stability of ecosystems. Journal of Theoretical Biology 2005, 237, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Lucia, U. Probability, ergodicity, irreversibility and dynamical systems. Proc. R. Soc. A. 2008, 464, 1089–1104. [Google Scholar] [CrossRef]
- Michaelian, K. Thermodynamic dissipation theory for the origin of life. Earth Syst. Dynam. 2011, 224, 37–51. [Google Scholar] [CrossRef]
- Lucia, U. Bio-engineering thermodynamics: an engineering science for thermodynamics of biosystems. International Journal of Thermodynamics 2015, 18, 254–265. [Google Scholar] [CrossRef]
- Michaelian, K. Microscopic Dissipative Structuring and Proliferation at the Origin of Life. Heliyon 2017, 3, e00424. [Google Scholar] [CrossRef]
- Fang, X.; Wang, J. Nonequilibrium Thermodynamics in Cell Biology: Extending Equilibrium Formalism to Cover Living Systems. Annual Review of Biophysics 2020, 49, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Michaelian, K. The Dissipative Photochemical Origin of Life: UVC Abiogenesis of Adenine. Entropy 2021, 23, https. [Google Scholar] [CrossRef]
- Michaelian, K. Non-Equilibrium Thermodynamic Foundations of the Origin of Life. Foundations 2022, 2, 308–337. [Google Scholar] [CrossRef]
- Michaelian, K. Thermodynamic origin of life. ArXiv 2009, arXiv:physics.gen-ph/0907.0042]. [Google Scholar]
- Sagan, C. Ultraviolet Selection Pressure on the Earliest Organisms. J. Theor. Biol. 1973, 39, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Michaelian, K. Thermodynamic Dissipation Theory of the Origina and Evolution of Life: Salient characteristics of RNA and DNA and other fundamental molecules suggest an origin of life driven by UV-C light; Self-published. Printed by CreateSpace. Mexico City. ISBN:9781541317482., 2016.
- Michaelian, K.; Simeonov, A. Fundamental molecules of life are pigments which arose and co-evolved as a response to the thermodynamic imperative of dissipating the prevailing solar spectrum. Biogeosciences 2015, 12, 4913–4937. [Google Scholar] [CrossRef]
- Solovchenko, A. PHOTOPROTECTION of PLANTS via OPTICAL SCREENING 2022.
- Gates, D.M. Biophysical Ecology; Springer-Verlag, 1980.
- Zvezdanovíc, J.; Markovíc. Bleaching of chlorophylls by UV irradiation in vitro: the effects on chlorophyll organization in acetone and n-hexane. J. Serb. Chem. Soc. 2008, 73, 271–282. [Google Scholar] [CrossRef]
- Blankenship, R.E.; Tiede, D.M.; Barber, J.; Brudvig, G.W.; Fleming, G.; Ghirardi, M.; Gunner, M.R.; Junge, W.; Kramer, D.M.; Melis, A.; Moore, T.A.; Moser, C.C.; Nocera, D.G.; Nozik, A.J.; Ort, D.R.; Parson, W.W.; Prince, R.C.; Sayre, R.T. Comparing Photosynthetic and Photovoltaic Efficiencies and Recognizing the Potential for Improvement. Science 2011, 332, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Michaelian, K.; Cano, R.E. A Photon Force and Flow for Dissipative Structuring: Application to Pigments, Plants and Ecosystems. Entropy 2022, 24, 76. [Google Scholar] [CrossRef]
- Wetherill, G. Late heavy bombardment of the moon and terrestrial planets. In Proc. 6th Lunar Sci. Conf., –21, Houston, TX; Pergamon, New York, 1975; p. 1539–1561. 17 March.
- Klein, F.; Grozeva, N.G.; Seewald, J.S. Abiotic methane synthesis and serpentinization in olivine-hosted fluid inclusions. Proceedings of the National Academy of Sciences 2019, 116, 17666–17672. [Google Scholar] [CrossRef] [PubMed]
- Dodonova, N.Y. Activation of Nitrogen by Vacuum Ultraviolet Radiation. Russ. J. Phys. Chem. 1966, 40, 523–524. [Google Scholar]
- Trainer, M.G.; Jimenez, J.L.; Yung, Y.L.; Toon, O.B.; Tolbert, M.A. Nitrogen Incorporation in CH4-N2 Photochemical Aerosol Produced by Far UV Irradiation. NASA archives, 2012. [Google Scholar]
- Ferris, J.P.; Orgel, L.E. An Unusual Photochemical Rearrangement in the Synthesis of Adenine from Hydrogen Cyanide. J. Am. Chem. Soc. 1966, 88, 1074–1074. [Google Scholar] [CrossRef]
- Ferris, J.P.; Chen, C.T. Chemical evolution. XXVI. Photochemistry of methane, nitrogen, and water mixtures as a model for the atmosphere of the primitive earth. Journal of the American Chemical Society 1975, 97, 2962–2967. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.; Joshi, P.; Edelson, E.; Lawless, J. HCN: a plausible source of purines, pyrimidines and amino acids on the primitive Earth. Journal of molecular evolution 1978, 11, 293–311. [Google Scholar] [CrossRef]
- Boulanger, E.; Anoop, A.; Nachtigallova, D.; Thiel, W.; Barbatti, M. Photochemical Steps in the Prebiotic Synthesis of Purine Precursors from HCN. Angew. Chem. Int. 2013, 52, 8000–8003. [Google Scholar] [CrossRef] [PubMed]
- Michaelian, K.; Rodriguez, O. Prebiotic fatty acid vesicles through photochemical dissipative structuring. Revista Cubana de Química 2019, 31, 354–370. [Google Scholar]
- Hernández, C.; Michaelian, K. Dissipative Photochemical Abiogenesis of the Purines. Entropy 2022, 24, 1027. [Google Scholar] [CrossRef] [PubMed]
- Michaelian, K. The Non-Equilibrium Thermodynamics of Natural Selection: From Molecules to the Biosphere. Entropy 2023, 25. [Google Scholar] [CrossRef]
- Petkowski, J.J.; Bains, W.; Seager, S. On the Potential of Silicon as a Building Block for Life. Life 2020, 10. [Google Scholar] [CrossRef]
- Domagal-Goldman, S.D.; Kasting, J.F.; Johnston, D.T.; Farquhar, J. Organic haze, glaciations and multiple sulfur isotopes in the Mid-Archean Era. Earth and Planetary Science Letters 2008, 269, 29–40. [Google Scholar] [CrossRef]
- Meller, R.; Moortgat, G.K. Temperature dependence of the absorption cross sections of formaldehyde between 223 and 323 K in the wavelength range 225–375 nm. Journal of Geophysical Research: Atmospheres 2000, 105, 7089–7101. [Google Scholar] [CrossRef]
- Shigemasa, Y.; Matsuda, Y.; Sakazawa, C.; Matsuura, T. Formose Reactions. II. The Photochemical Formose Reaction. Bulletin of the Chemical Society of Japan 1977, 50, 222–226. [Google Scholar] [CrossRef]
- Shapiro, R. Prebiotic ribose synthesis: A critical analysis. Origins Life Evol Biosphere 1988, 18, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Meixnerová, J.; Blum, J.D.; Johnson, M.W.; Stüeken, E.E.; Kipp, M.A.; Anbar, A.D.; Buick, R. Mercury abundance and isotopic composition indicate subaerial volcanism prior to the end-Archean “whiff” of oxygen. Proceedings of the National Academy of Sciences 2021, 118, e2107511118. [Google Scholar]
- Schuurman, M.S.; Stolow, A. Dynamics at Conical Intersections. Annu. Rev. Phys. Chem. 2018, 69, 427–450. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Perez, J.J.; de Vleeschouwer, F.; de Proft, F.; Mendive-Tapia, D.; Bearpark, M.J.; Robb, M.A. How the Conical Intersection Seam Controls Chemical Selectivity in the Photocycloaddition of Ethylene and Benzene. J. Org. Chem. 2013, 78, 1874–1886. [Google Scholar] [CrossRef] [PubMed]
- Polli, D.; Altoè, P.; Weingart, O.; Spillane, K.M.; Manzoni, C.; Brida, D.; Tomasello, G.; Orlandi, G.; Kukura, P.; Mathies, R.A.; Garavelli, M.; Cerullo, G. Conical intersection dynamics of the primary photoisomerization event in vision. Nature 2010, 467, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Michaelian, K.; Santillan, N. UVC photon-induced denaturing of DNA: A possible dissipative route to Archean enzyme-less replication. Heliyon 2019, 5, e01902. [Google Scholar] [CrossRef] [PubMed]
- Orr-Ewing, A. Reaction Dynamics –Relaxation Pathways. Lecture Notes; University of Bristol: Bristol, UK 2014; pp. 1–36. [Google Scholar]
- Roberts, G.M.; Marroux, H.J.B.; Grubb, M.P.; Ashfold, M.N.R.; Orr-Ewing, A.J. On the Participation of Photoinduced N–H Bond Fission in Aqueous Adenine at 266 and 220 nm: A Combined Ultrafast Transient Electronic and Vibrational Absorption Spectroscopy Study. The Journal of Physical Chemistry A 2014, 118, 11211–11225. [Google Scholar] [CrossRef] [PubMed]
- Kleinermanns, K.; Nachtigallová, D.; de Vries, M.S. Excited state dynamics of DNA bases. International Reviews in Physical Chemistry 2013, 32, 308–342. [Google Scholar] [CrossRef]
- Barbatti, M.; Aquino, A.; Szymczak, J.; Nachtigallová, D.; Hobza, P.; Lischka, H. Relaxation mechanisms of UV-photoexcited DNA and RNA nucleobases. Proc Natl Acad Sci U S A 2010, 107, 21453–21458. [Google Scholar] [CrossRef]
- Gnanadesikan, A.; Emanuel, K.; Vecchi, G.A.; Anderson, W.G.; Hallberg, R. How ocean color can steer Pacific tropical cyclones. Geophysical Research Letters 2010, 37, L18802. [Google Scholar] [CrossRef]
- Koch, T.; Rodehorst, R. Quantitative investigation of the photochemical conversion of diaminomaleonitrile to diaminofumaronitrile and 4-amino-5-cyanoimidazole. J. Am. Chem. Soc. 1974, 96, 6707–6710. [Google Scholar] [CrossRef]
- Sanchez, R.A.; Ferris, J.P.; Orgel, L.E. Studies in Prebiodc Synthesis II: Synthesis of Purine Precursors and Amino Acids from Aqueous Hydrogen Cyanide. J. Mol. Biol. 1967, 80, 223–253. [Google Scholar]
- Cavaluzzi, M.J.; Borer, P.N. Revised UV extinction coefficients for nucleoside-5’- monophosphates and unpaired DNA and RNA. Nucleic Acids Research 2004, 32, e13. [Google Scholar] [CrossRef] [PubMed]
- Mulkidjanian, A.Y.; Cherepanov, D.A.; Galperin, M.Y. Survival of the fittest before the beginning of life: selection of the first oligonucleotide-like polymers by UV light. BMC Evolutionary Biology 2003, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Goto, N.; Bazar, G.; Kovacs, Z.; Kunisada, M.; Morita, H.; Kizaki, S.; Sugiyama, H.; Tsenkova, R.; Nishigori, C. Detection of UV-induced cyclobutane pyrimidine dimers by near-infrared spectroscopy and aquaphotomics. Sci Rep 2015, 5, 11808. [Google Scholar] [CrossRef]
- Michaelian, K.; Hernández, C. Photochemical Microscopic Dissipative Structuring of the Pyrimidines. In process.
- Kopetzki, D.; Antonietti, M. Hydrothermal formose reaction. New J. Chem. 2011, 35, 1787–1794. [Google Scholar] [CrossRef]
- Meinert, C.; Myrgorodska, I.; de Marcellus, P.; Buhse, T.; Nahon, L.; Hoffmann, S.V.; Le Sergeant d’Hendecourt, L.; Meierhenrich, U.J. Ribose and related sugars from ultraviolet irradiation of interstellar ice analogs. Science 2016, 352, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Tuna, D.; Sobolewski, A.L.; Domcke, W. Conical-Intersection Topographies Suggest That Ribose Exhibits Enhanced UV Photostability. J. Phys. Chem. B 2016, 120, 10729–10735. [Google Scholar] [CrossRef]
- Nuevo, M.; Cooper, G.; Sandford, S. Deoxyribose and deoxysugar derivatives from photoprocessed astrophysical ice analogues and comparison to meteorites. Nat Commun, 2018; 5276. [Google Scholar]
- Michaelian, K.; Rodríguez, J.L. Photochemical Dissipative Structuring of Ribose. In process.
- Pinto, J.P.; Gladstone, G.R.; Yung, Y.L. Photochemical Production of Formaldehyde in Earth’s Primitive Atmosphere. Science 1980, 210, 183–185. [Google Scholar] [CrossRef]
- Ritson, D.; Sutherland, J. Prebiotic synthesis of simple sugars by photoredox systems chemistry. Nature Chem. 2012, 4, 895–899. [Google Scholar] [CrossRef]
- Todd, Z.R.; Fahrenbach, A.C.; Magnani, C.J.; Ranjan, S.; Björkbom, A.; Szostak, J.W.; Sasselov, D.D. Solvated-electron production using cyanocuprates is compatible with the UV-environment on a Hadean–Archaean Earth. Chem. Commun. 2018, 54, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Socha, R.; Weiss, A.; Sakharov, M. Autocatalysis in the formose reaction. React. Kinet. Catal. Lett. 1980, 14, 119–128. [Google Scholar] [CrossRef]
- Beeby, A.; Mohammed, D.b.H.; Sodeau, J.R. Photochemistry and photophysics of glycolaldehyde in solution. Journal of the American Chemical Society 1987, 109, 857–861. [Google Scholar] [CrossRef]
- Pigeot-Rémy, S.; Simonet, F.; Atlan, D.; Lazzaroni, J.; Guillard, C. Bactericidal efficiency and mode of action: A comparative study of photochemistry and photocatalysis. Water Research 2012, 46, 3208–3218. [Google Scholar] [CrossRef] [PubMed]
- Powner, M.; Gerland, B.; Sutherland, J. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 2009, 459, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.M.; Wang, H.g.; Zheng, X.; Phillips, D.L. Role of Ribose in the Initial Excited State Structural Dynamics of Thymidine in Water Solution: A Resonance Raman and Density Functional Theory Investigation. The Journal of Physical Chemistry B 2008, 112, 15828–15836. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, C.; Pluhařová, E.; Seidel, R.; Schroeder, W.; Faubel, M.; P. , S.; Winter, B.; Jungwirth, P.; Bradforth, S. Oxidation Half-Reaction of Aqueous Nucleosides and Nucleotides via Photoelectron Spectroscopy Augmented by ab Initio Calculations. J. Am. Chem. Soc. 2015, 137, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Ciciriello, F.; Di Mauro, E.; Costanzo, G. Origin of informational polymers: Differential stability of phosphoester bonds in ribomonomers and ribooligomers. J. Biol. Chem. 2006, 281, 5790–5796. [Google Scholar] [CrossRef] [PubMed]
- Mejía Morales, J.; Michaelian, K. Photon Dissipation as the Origin of Information Encoding in RNA and DNA. Entropy 2020, 22, https. [Google Scholar] [CrossRef]
- Ponnamperuma. ; Mariner, R.; Sagan, C. Formation of Adenosine by Ultraviolet Irradiation of a Solution of Adenine and Ribose. Nature 1963, 198, 1199–1200. [Google Scholar] [CrossRef]
- Szostak, J.W. The eightfold path to non-enzymatic RNA replication. J. Sys. Chem. 2012, 3, 2. [Google Scholar] [CrossRef]
- Schoffstall, A.M. Prebiotic phosphorylation of nucleosides in formamide. Origins Life Evol Biosphere 1976, 7, 399–412. [Google Scholar] [CrossRef]
- Pasek, M.A.; Harnmeijer, J.P.; Buick, R.; Gull, M.; Atlas, Z. Evidence for reactive reduced phosphorus species in the early Archean ocean. Proceedings of the National Academy of Sciences 2013, 110, 10089–10094. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Engelhart, A.E.; Zhang, W.; Adamala, K.; Szostak, J.W. Catalysis of Template-Directed Nonenzymatic RNA Copying by Iron(II). Journal of the American Chemical Society 2018, 140, 15016–15021. [Google Scholar] [CrossRef] [PubMed]
- Yarus, M. Amino Acids as RNA Ligands: A Direct-RNA-Template Theory for the Codes Origin. J. Mol. Evol. 1998; 109-17. [Google Scholar]
- Yarus, M.; Caporaso, J.G.; Knight, R. Origins The Genetic Code: The Escaped Triplet Theory. Annu. Rev. Biochem. 2005; 179-98. [Google Scholar]
- Rayner, D.M.; Szabo, A.G. Time resolved fluorescence of aqueous tryptophan. Can. J. Chem. 1974, 56, 743–745. [Google Scholar] [CrossRef]
- Arcaya, G.; Pantoja, M.E.; Pieber, M.; Romero, C.; Tohá, J.C. Molecular Interaction of L-Tryptophan with Bases, Ribonucleosides and DNA. Zeitschrift für Naturforschung B 1971, 26, 1026–1030. [Google Scholar] [CrossRef]
- Kleinwächter, V.; Koudelka, J. Thermal denaturation of deoxyribonucleic acid-acridine orange complexes. Biochimica et Biophysica Acta (BBA)-Specialized Section on Nucleic Acids and Related Subjects 1964, 91, 539–540. [Google Scholar] [CrossRef]
- Horowitz, E.D.; Engelhart, A.E.; Chen, M.C.; Quarles, K.A.; Smit, h.M.W.; Lynn, D.G.; Hud, N.V. Intercalation as a means to suppress cyclization and promote polymerization of base-pairing oligonucleotides in a prebiotic world. Proc. Natl. Acad. Sci. USA 2010, 107, 5288–5293. [Google Scholar] [CrossRef]
- Michaelian, K. Photochemical Dissipative Structuring of the Fundamental Molecules of Life. Proceedings, 5th International Electronic Conference on Entropy and Its Applications; Session: Biological Systems, 2019. [Google Scholar]
- Joyce, G.; Visser, G.; van Boeckel, C.; et al. . Chiral selection in poly(C)-directed synthesis of oligo(G). Nature 1984, 310, 602–604. [Google Scholar] [CrossRef]
- E. , O.L. Prebiotic Chemistry and the Origin of the RNA World. Critical Reviews in Biochemistry and Molecular Biology 2004, 39, 99–123. [Google Scholar] [CrossRef]
- Mejía, J.; Michaelian, K. Information Encoding in Nucleic Acids through a Dissipation-Replication Relation. ArXiv 2018, arXiv:physics.bio-ph/1804.05939], [arXiv:physics.bio–ph/180405939]. [Google Scholar] [CrossRef]
- Woese, C.R. The genetic code: the molecular basis for genetic expression; Harper & Row, 1967.
- Simeonov, A.; Michaelian, K. Properties of cyanobacterial UV-absorbing pigments suggest their evolution was driven by optimizing photon dissipation rather than photoprotection. ArXiv 2017, arXiv:physics.bio-ph/1702.03588], [arXiv:physics.bio–ph/170203588]. [Google Scholar]
- Stomp, M.; Huisman, J.; Stal, L.J.; Matthijs, H.C.P. Colorful niches of phototrophic microorganisms shaped by vibrations of the water molecule. The ISME Journal 2007, 1, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Saini, D.K.; Pabbi, S.; Shukla, P. Cyanobacterial pigments: Perspectives and biotechnological approaches. Food and Chemical Toxicology 2018, 120, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, DA and Vyverman, Wim and Verleyen, Elie and Sabbe, Koen and Leavitt, PR and Taton, A and Squier, AH and Keely, BJ. Environmental factors influencing the pigment composition of in situ benthic microbial communities in east Antarctic lakes. AQUATIC MICROBIAL ECOLOGY 2004, 37, 247–263. [Google Scholar] [CrossRef]
- Whitehead, K.; Hedges, J.I. Analysis of mycosporine-like amino acids in plankton by liquid chromatography electrospray ionization mass spectrometry. Marine Chemistry 2002, 80, 27–39. [Google Scholar] [CrossRef]
- Berkaloff, A.; Bourguet, J. and Favard, P.; Guinnebault, M., Biologia y Fisiologia Celula; Ediciones Omega, S. A, 1971.
- Niyogi, K.K. Safety valves for photosynthesis. Current Opinion in Plant Biology 2000, 3, 455–460. [Google Scholar] [CrossRef]
- Barry, R.; Chorley, R. Atmosphere, Weather, and Climate, 1st ed.; 2009.
- Wang, J.; Bastiaanssen, W.G.M.; Ma, Y.; Pelgrum, H. Aggregation of land surface parameters in the oasis–desert systems of north-west China. Hydrological Processes 1998, 12, 2133–2147. [Google Scholar] [CrossRef]
- Coakley, J. REFLECTANCE AND ALBEDO, SURFACE. In Encyclopedia of Atmospheric Sciences; Holton, J.R., Ed.; Academic Press: Oxford, 2003; pp. 1914–1923. [Google Scholar] [CrossRef]
- Varotsos, C.A.; Melnikova, I.N.; Cracknell, A.P.; Tzanis, C.; Vasilyev, A.V. New spectral functions of the near-ground albedo derived from aircraft diffraction spectrometer observations. Atmospheric Chemistry & Physics 2014, 14, 6953–6965. [Google Scholar] [CrossRef]
- Michaelian, K. Biological catalysis of the hydrological cycle: lifeś thermodynamic function. Hydrol. Earth Syst. Sci. 2012, 16, 2629–2645. [Google Scholar] [CrossRef]
- Cogley, J.G. The Albedo of Water as a Function of Latitude. Monthly Weather Review 1979, 107, 775–781. [Google Scholar] [CrossRef]
- Vernet, M.; Whitehead, K. Release of ultraviolet absorbing compounds by the red-tide dinoagellate Lingulodinium polyedra. Mar Biol. 1996, 127, 35–44. [Google Scholar] [CrossRef]
- Subramaniam, A.; Carpenter, E.J.; Karentz, D.; Falkowski, P.G. Bio-optical properties of the marine diazotrophic cyanobacteria Trichodesmium spp. I. Absorption and photosynthetic action spectra. Limnology and Oceanography 1999, 44, 608–617. [Google Scholar] [CrossRef]
- Steinberg, D.K.; Nelson, N.B.; Carlson, C.A.; Prusak, A.C. Production of chromophoric dissolved organic matter (CDOM) in the open ocean by zooplankton and the colonial cyanobacterium Trichodesmium spp. Marine Ecology Progress Series 2004, 267, 45–56. [Google Scholar] [CrossRef]
- Hannon, B. Total energy costs in ecosystems. Journal of Theoretical Biology 1979, 80, 271–293. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.; Phillips, R.; Milo, R. The biomass distribution on Earth. Proc Natl Acad Sci U S A 2018, 115, 6506–6511. [Google Scholar] [CrossRef] [PubMed]
- Flewelling, L.; Naar, J.; Abbott, J.; et al. . Red tides and marine mammal mortalities. Nature 2005, 435, 755–756. [Google Scholar] [CrossRef] [PubMed]
- Gaston, K. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Chichorro, F.; Juslén, A.; Cardoso, P. A review of the relation between species traits and extinction risk. Biological Conservation 2019, 237, 220–229. [Google Scholar] [CrossRef]
- Kleidon, A.; Fraedrich, K.; Heimann, M.A. Green Planet Versus a Desert World: Estimating the Maximum Effect of Vegetation on the Land Surface Climate. Climatic Change 2000, 44, 471–493. [Google Scholar] [CrossRef]
- Benton, J. Increase in Total Global Biomass over Time. Evolutionary Theory 1978, 4, 123–128. [Google Scholar]
- Buzhdygan, O.; Meyer, S.; Weisser, W.; Eisenhauer, N.; Ebeling, A.; Borrett, S.R.; Buchmann, N.; Cortois, R.; De Deyn, G.; de Kroon, H.; Gleixner, G.; Hertzog, L.R.; Hines, J.; Lange, M.; Mommer, L.; Ravenek, J.; Scherber, C.; Scherer-Lorenzen, M.; Scheu, S.; Schmid, B.; Steinauer, K.; Strecker, T.; Tietjen, B.; Vogel, A.; Weigelt, A.; Petermann, J.S. Biodiversity increases multitrophic energy use efficiency, flow and storage in grasslands. Nat Ecol Evol 2020, 4, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Ripple, W.J.; Beschta, R.L. Trophic cascades in Yellowstone: The first 15years after wolf reintroduction. Biological Conservation 2012, 145, 205–213. [Google Scholar] [CrossRef]
- Miller, S.L.; Orgel, L. , The origins of life on the earth; Prentice-Hall, Englewood Cliffs, N.J., 1974.
- Kang, H.; Lee, K.T.; Jung, B.; Ko, Y.J.; Kim, S.K. Intrinsic Lifetimes of the Excited State of DNA and RNA Bases. Journal of the American Chemical Society 2002, 124, 12958–12959. [Google Scholar] [CrossRef] [PubMed]
- Sagan, C.; Khare, B.N. Long-Wavelength Ultraviolet Photoproduction of Amino Acids on the Primitive Earth. Science 1971, 173, 417–420. [Google Scholar] [CrossRef]
- Ruiz-Bermejo, M.; Zorzano, M.P.; Osuna-Esteban, S. Simple Organics and Biomonomers Identified in HCN Polymers: An Overview. Life 2013, 3, 421–448. [Google Scholar] [CrossRef]
- Cohen, B.; Hare, P.M.; Kohler, B. Ultrafast Excited-State Dynamics of Adenine and Monomethylated Adenines in Solution: Implications for the Nonradiative Decay Mechanism. Journal of the American Chemical Society 2003, 125, 13594–13601. [Google Scholar] [CrossRef] [PubMed]
- Monson, R.; Jones, R.; Rosenstiel, T.; Schnitzler, J. Why only some plants emit isoprene. Plant Cell Environ. 2013, 36, 503–5016. [Google Scholar] [CrossRef]
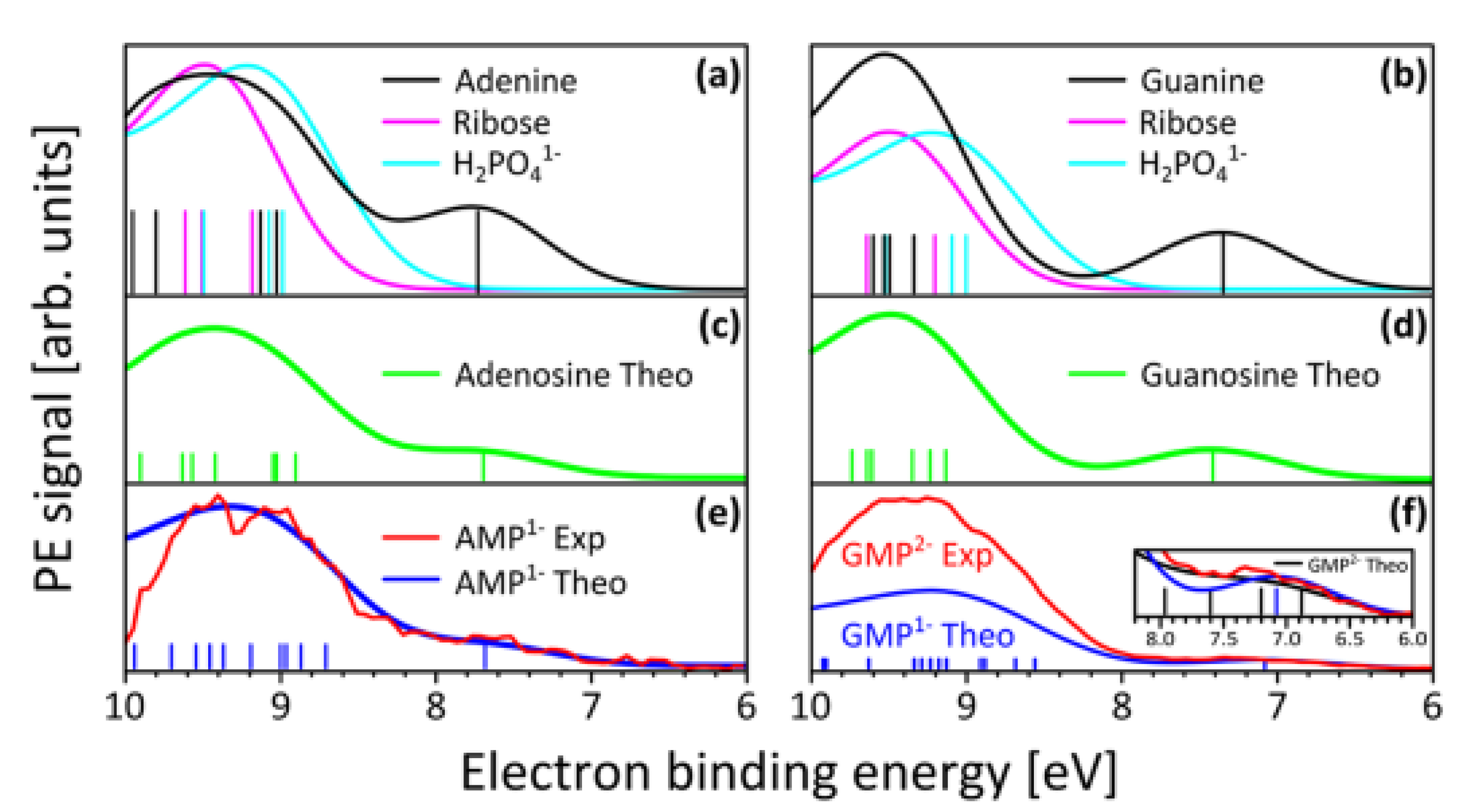
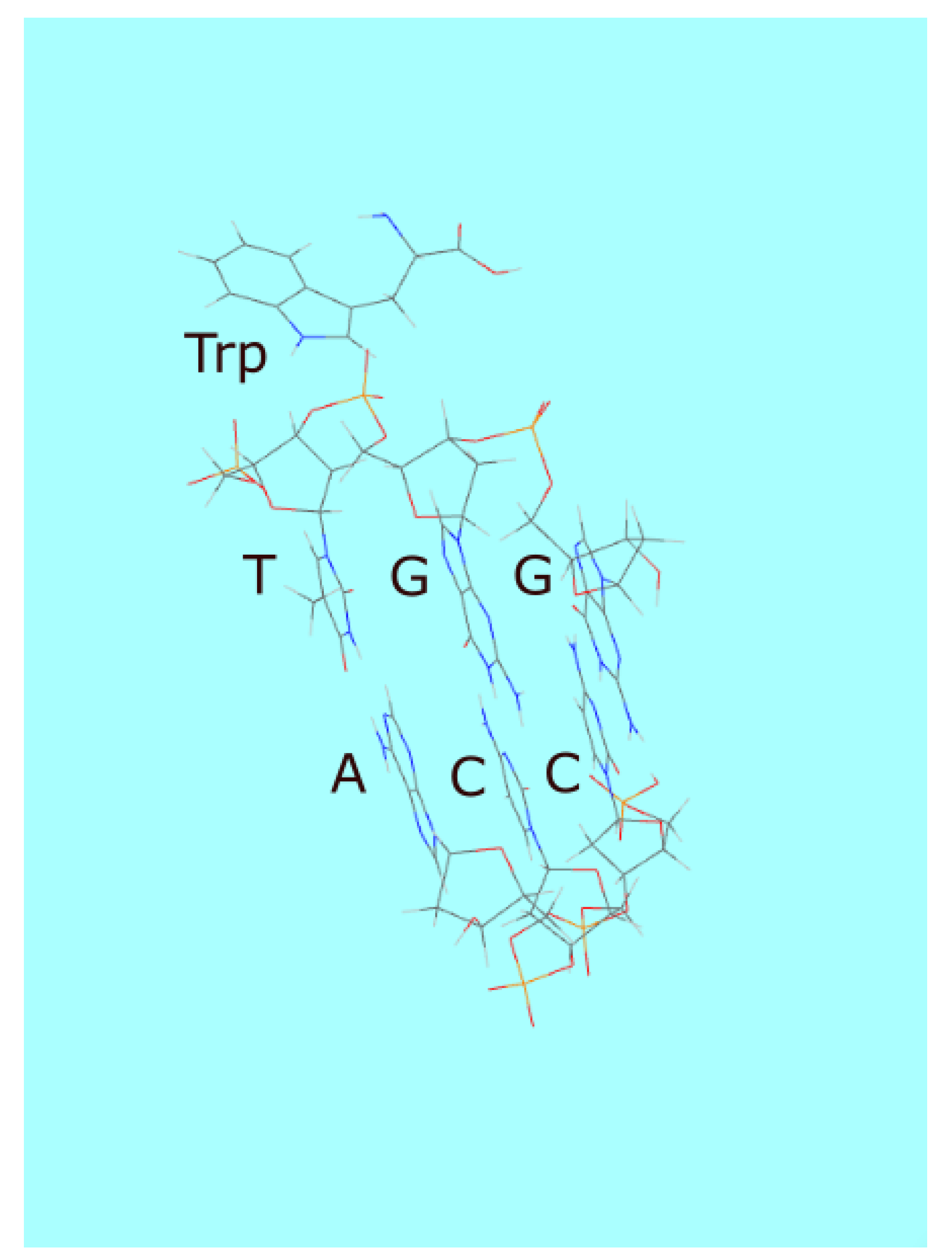
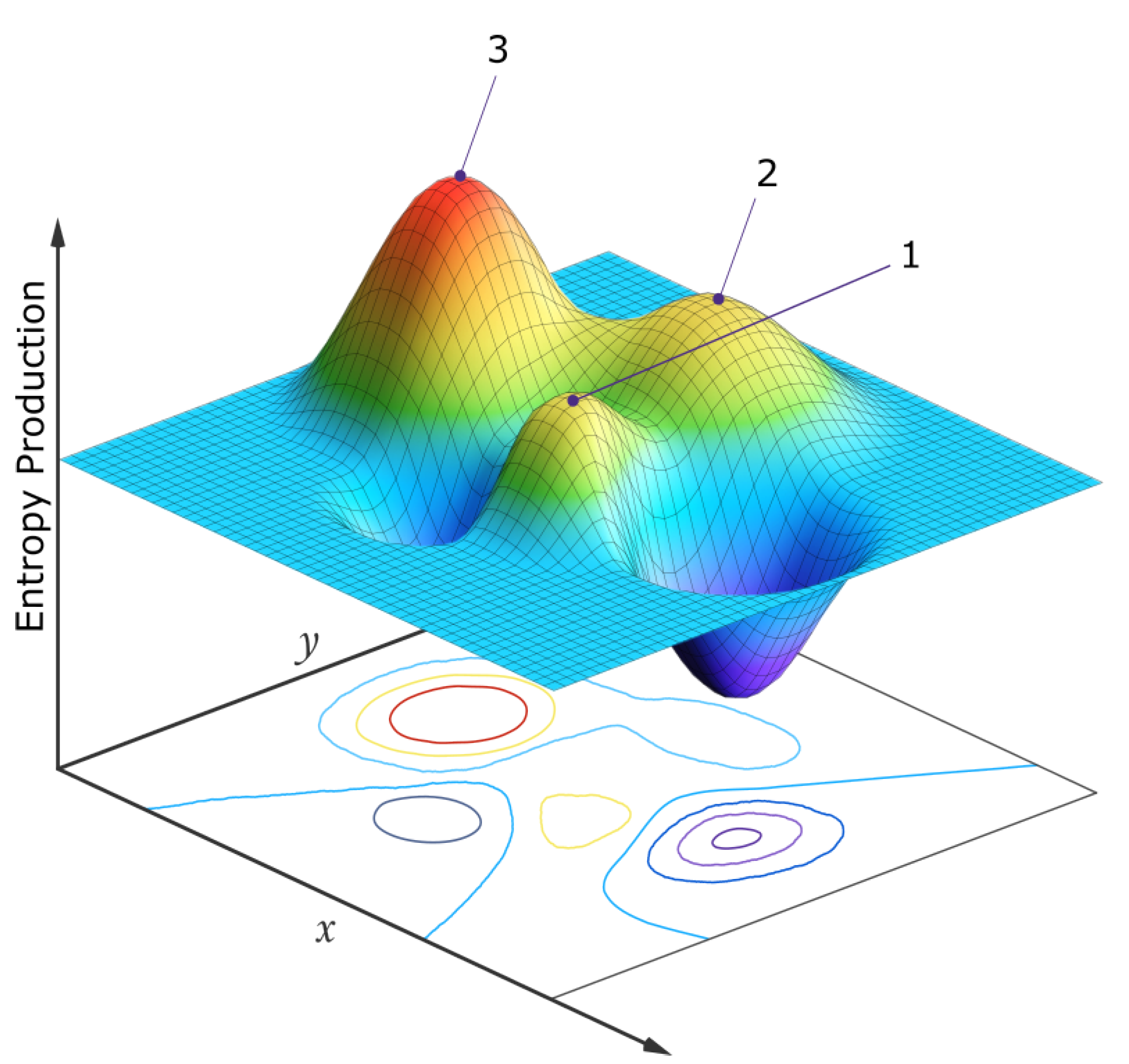
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




