Submitted:
07 June 2024
Posted:
11 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
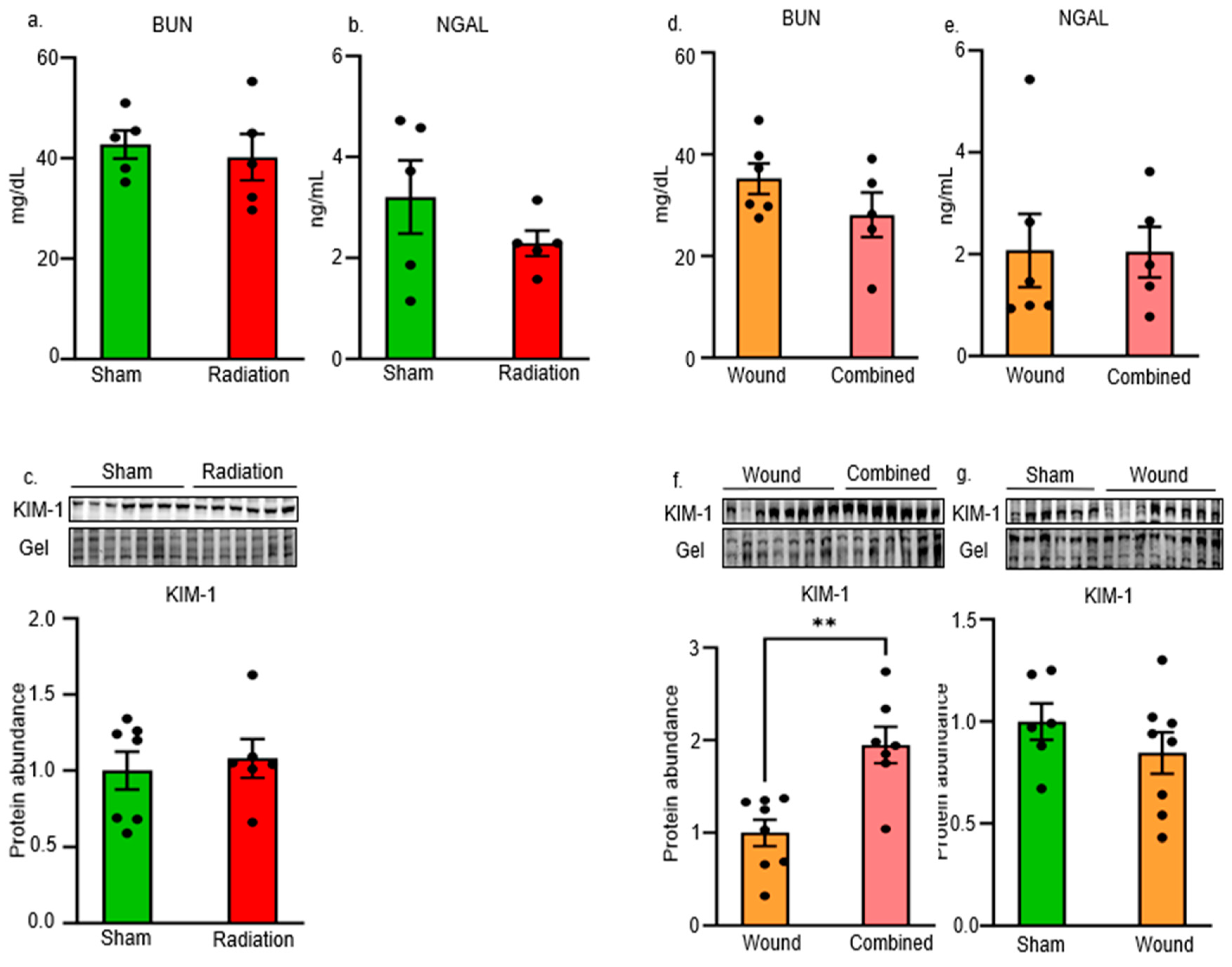
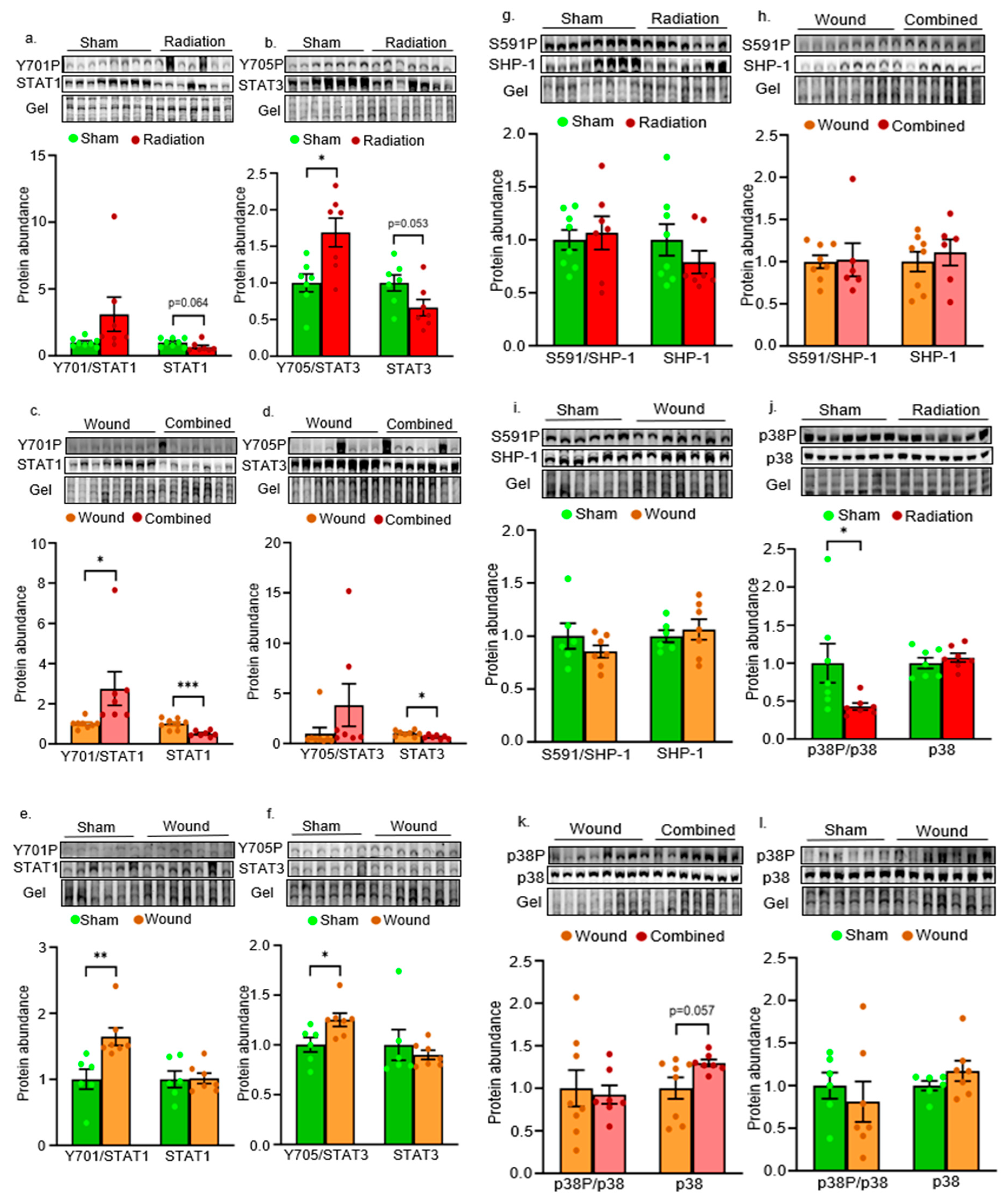
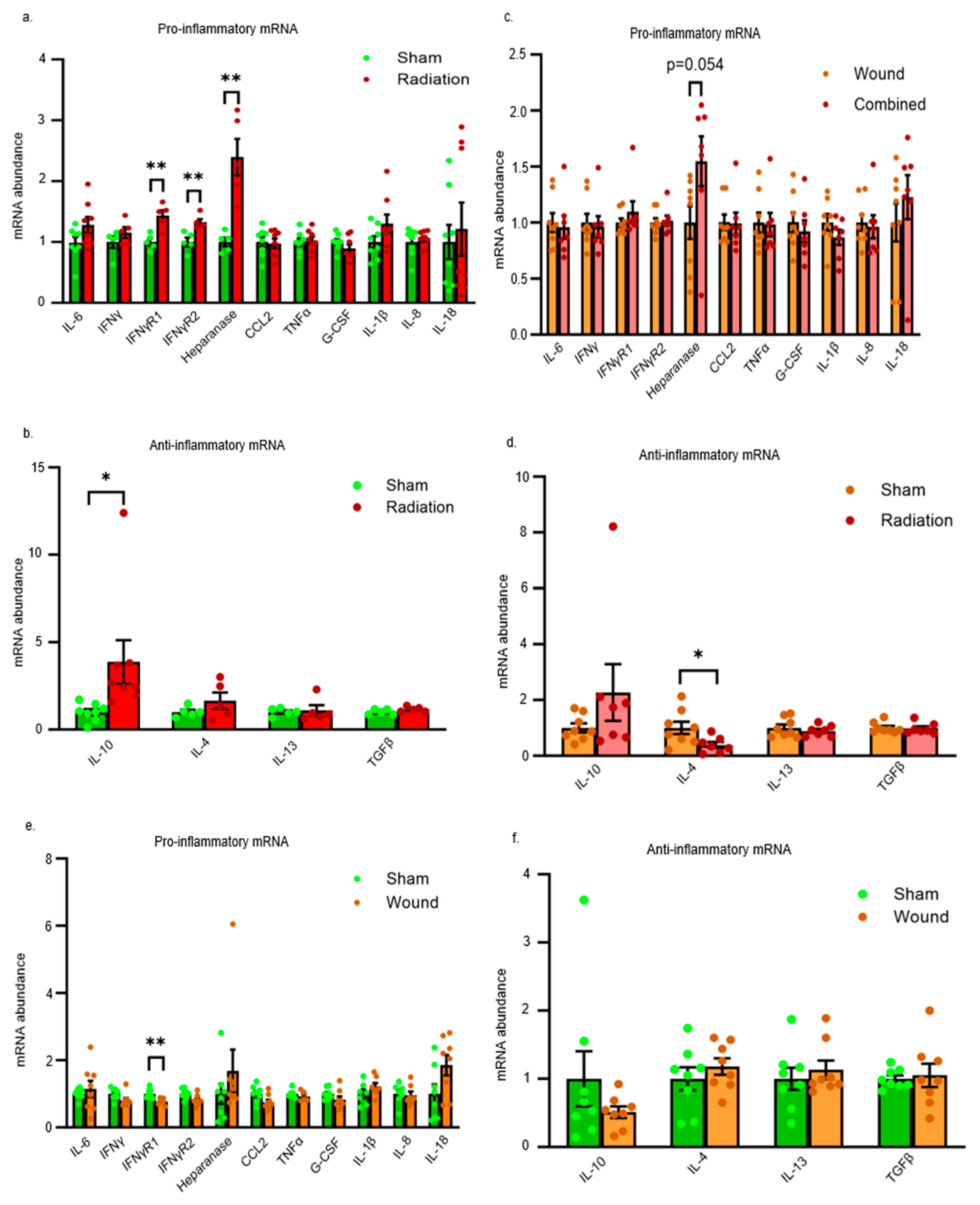
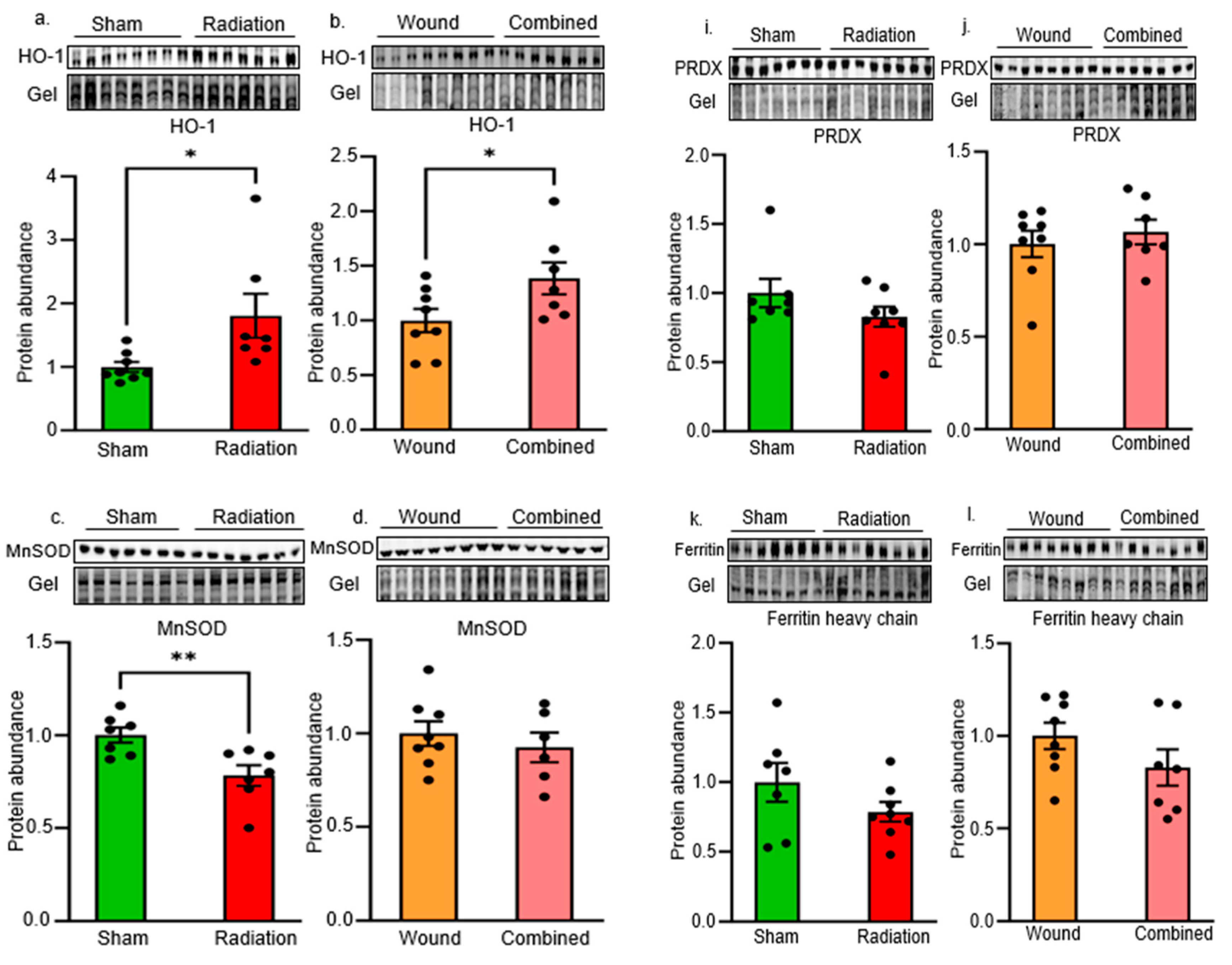
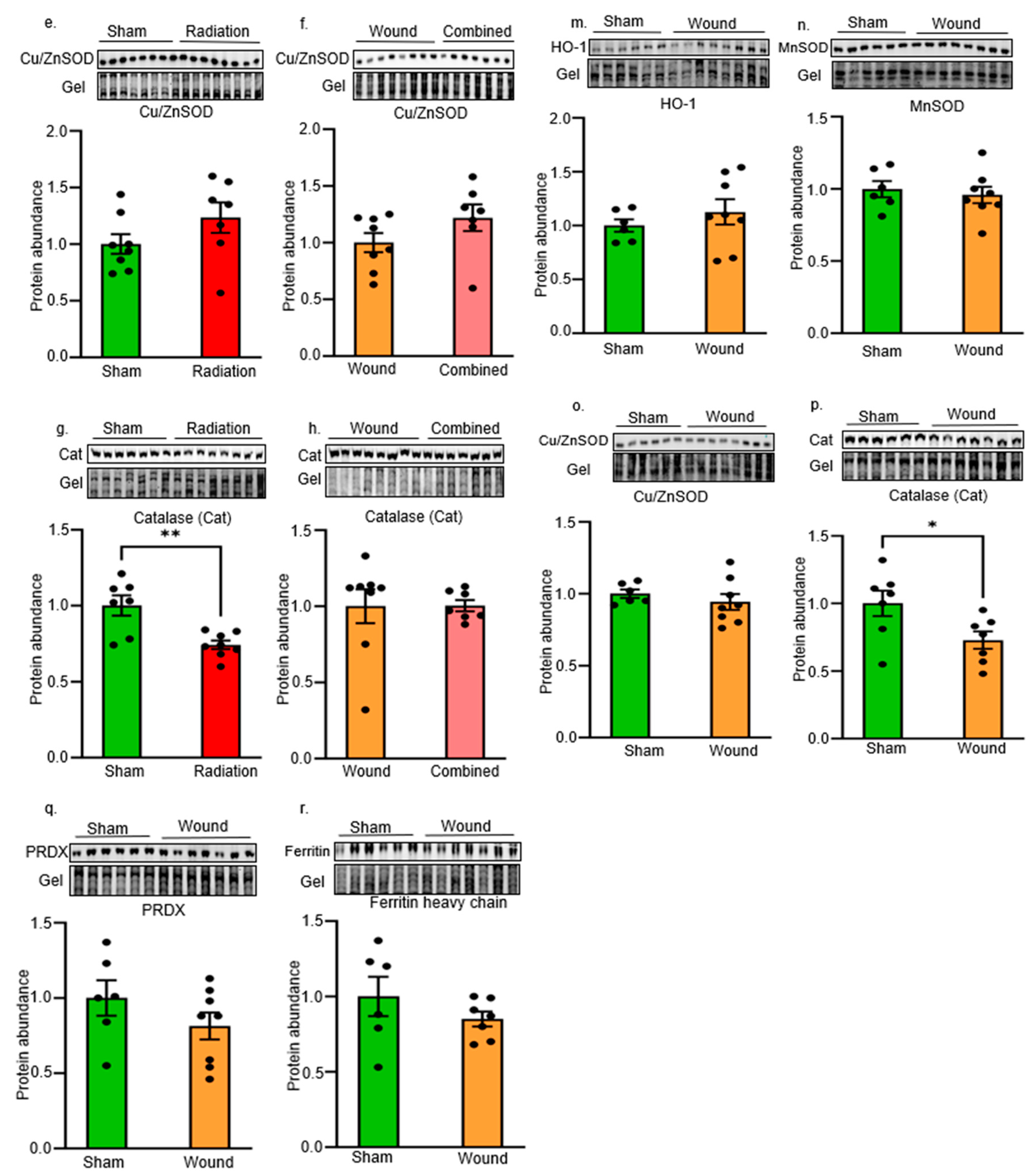
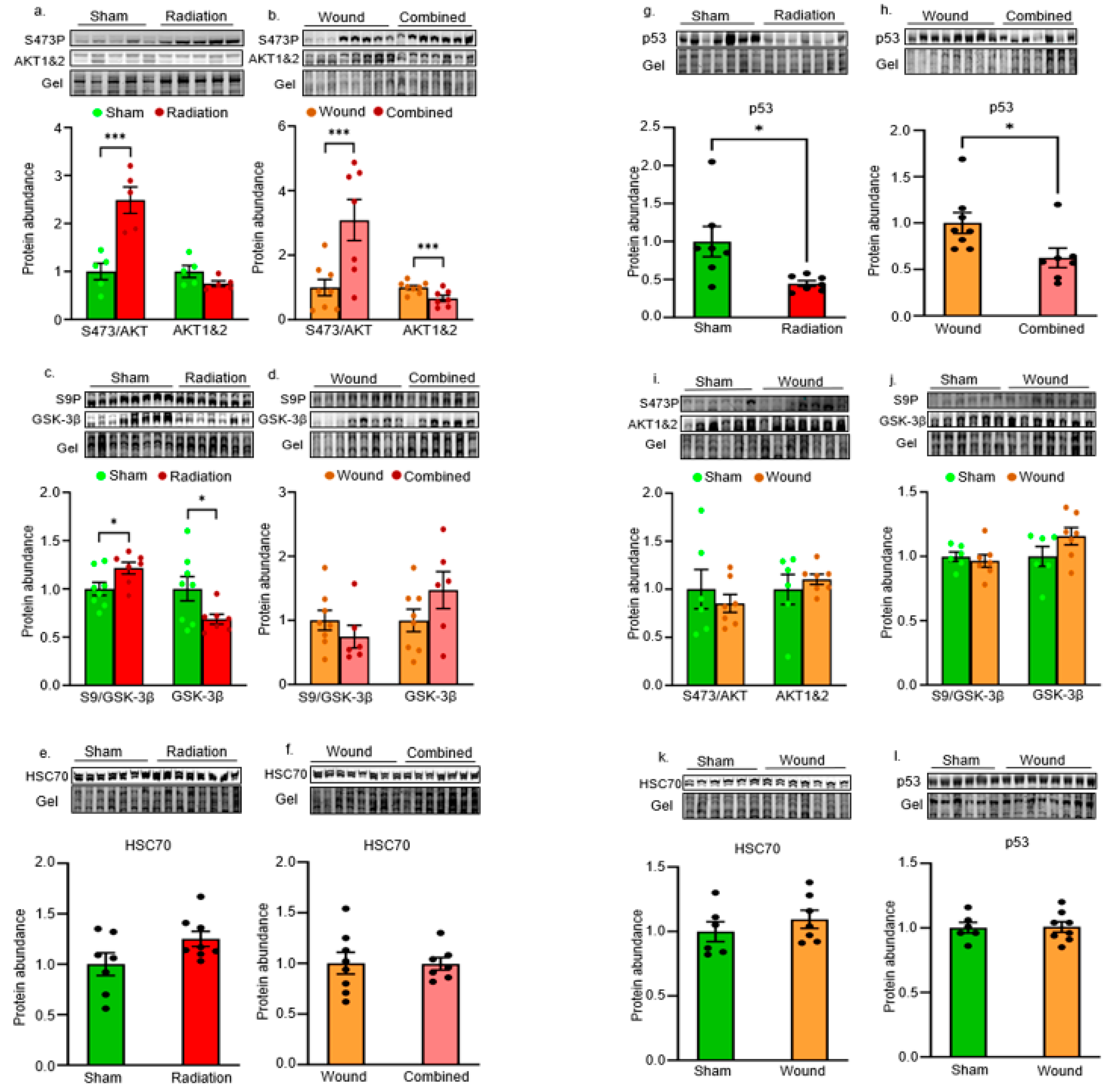
2. Results
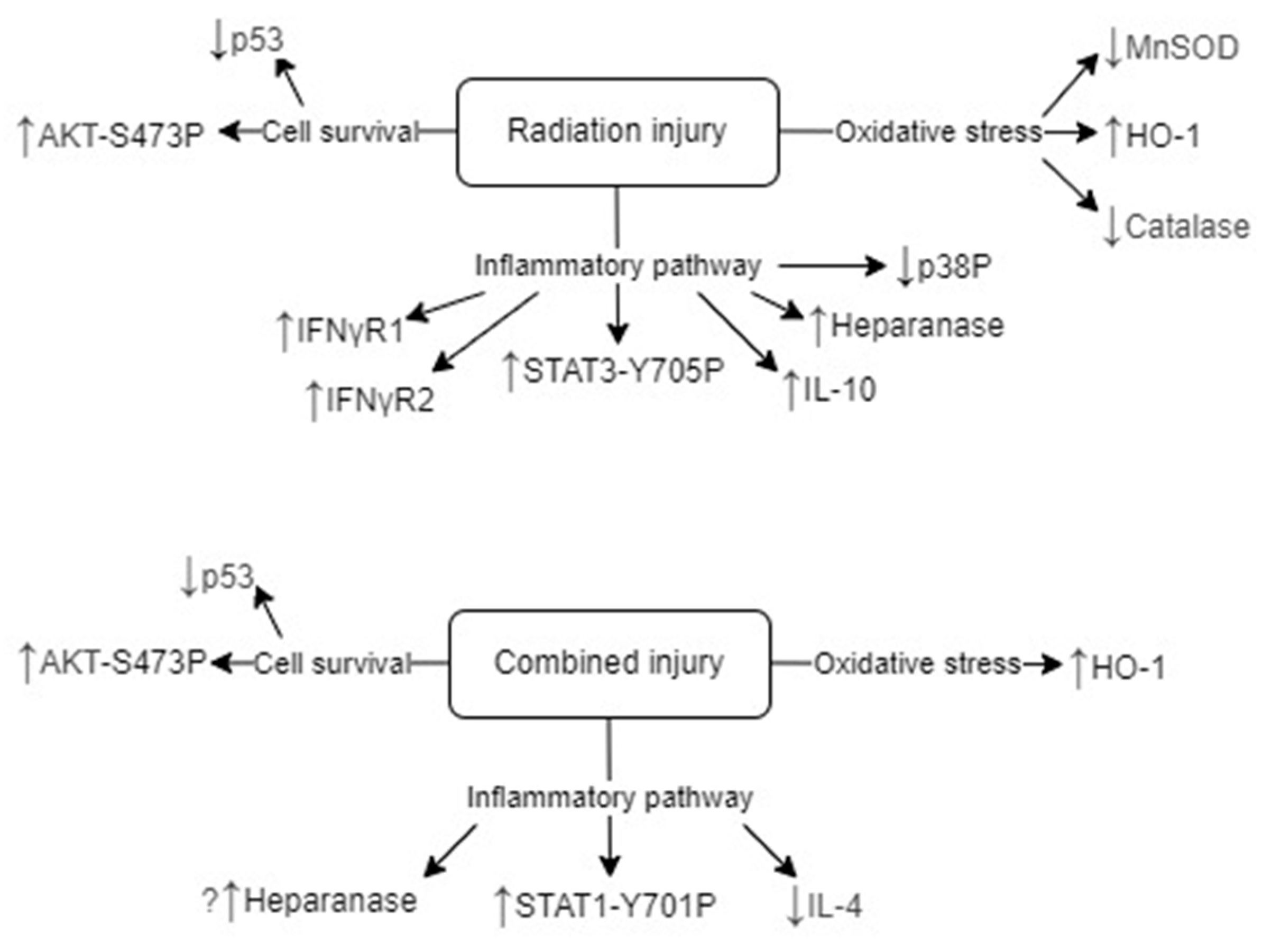
3. Discussion
Inflammatory Pathways
Oxidative Stress and Antioxidant Defense
Cell Survival Pathways
Limitations of the Study
Summary
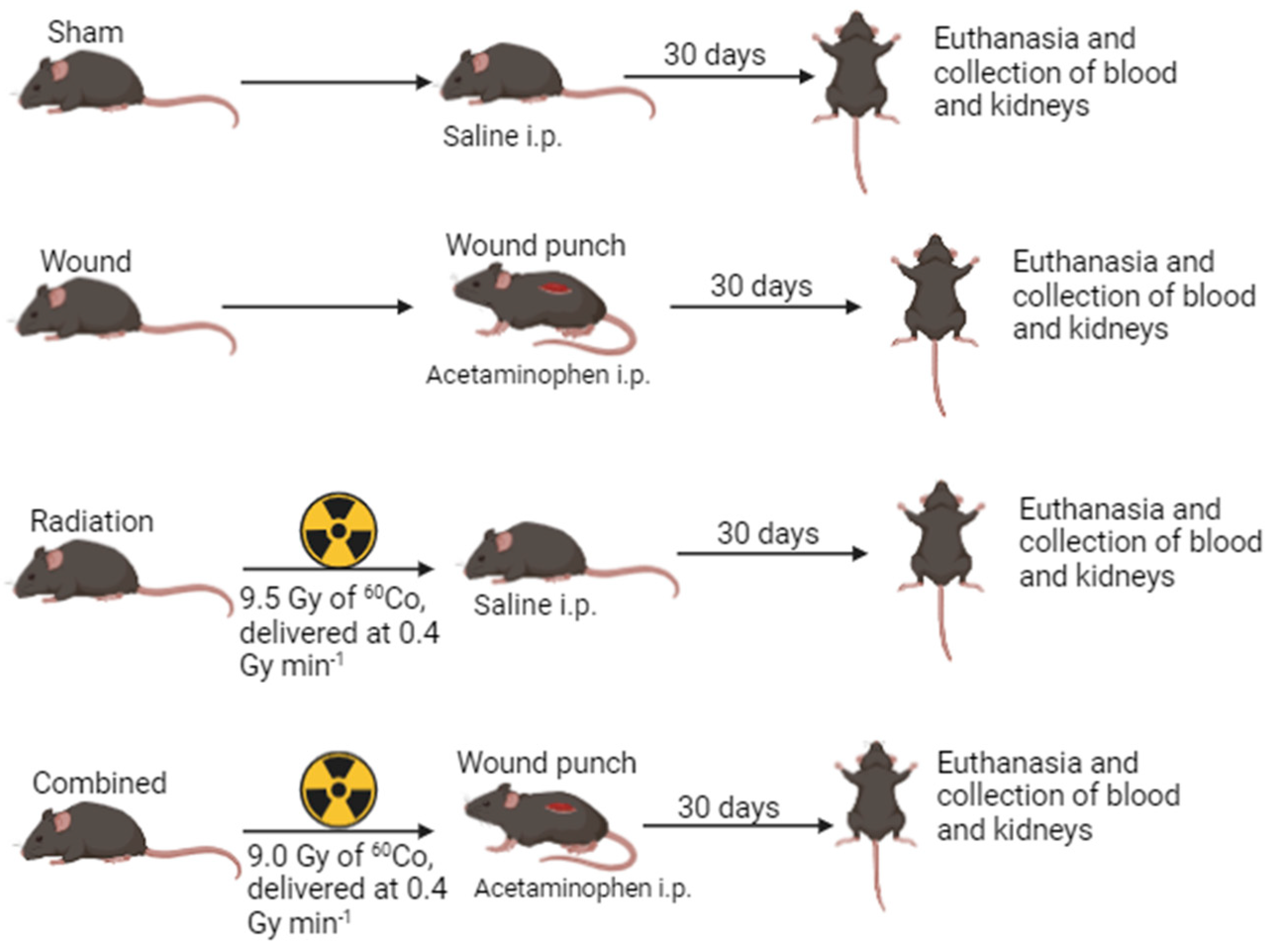
4. Methods and Materials
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, E.P.; Hankey, K.G.; Farese, A.M.; Parker, G.A.; Jones, J.W.; Kane, M.A.; Bennett, A.; MacVittie, T.J. Radiation Nephropathy in a Nonhuman Primate Model of Partial-body Irradiation with Minimal Bone Marrow Sparing-Part 1: Acute and Chronic Kidney Injury and the Influence of Neupogen. Health Phys 2019, 116, 401–408. [Google Scholar] [CrossRef]
- Parker, G.A.; Cohen, E.P.; Li, N.; Takayama, K.; Farese, A.M.; MacVittie, T.J. Radiation nephropathy in a nonhuman primate model of partial-body irradiation with minimal bone marrow sparing—Part 2: histopathology, mediators, and mechanisms. Health physics 2019, 116, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Farese, A.M.; Booth, C.; Tudor, G.L.; Cui, W.; Cohen, E.P.; Parker, G.A.; Hankey, K.G.; MacVittie, T.J. The Natural History of Acute Radiation-induced H-ARS and Concomitant Multi-organ Injury in the Non-human Primate: The MCART Experience. Health Phys 2021, 121, 282–303. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.; Hegi-Johnson, F. Radiotherapy toxicity. Nature Reviews Disease Primers 2019, 5, 13. [Google Scholar] [CrossRef]
- Ahmad, A.; Shi, J.; Ansari, S.; Afaghani, J.; Molina, J.; Pollack, A.; Merscher, S.; Zeidan, Y.H.; Fornoni, A.; Marples, B. Noninvasive assessment of radiation-induced renal injury in mice. Int J Radiat Biol 2021, 97, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Ozasa, K.; Akiba, S.; Niwa, O.; Kodama, K.; Takamura, N.; Zaharieva, E.K.; Kimura, Y.; Wakeford, R. Long-term effects of radiation exposure on health. Lancet 2015, 386, 469–478. [Google Scholar] [CrossRef]
- Cohen, E.P.; Fish, B.L.; Moulder, J.E. Late-onset effects of radiation and chronic kidney disease. Lancet 2015, 386, 1737–1738. [Google Scholar] [CrossRef]
- Klaus, R.; Niyazi, M.; Lange-Sperandio, B. Radiation-induced kidney toxicity: molecular and cellular pathogenesis. Radiat Oncol 2021, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Blakely, W.F. Combined radiation injury and its impacts on radiation countermeasures and biodosimetry. Int J Radiat Biol 2023, 99, 1055–1065. [Google Scholar] [CrossRef]
- Mostaghimi, S.; Mehrvar, S.; Foomani, F.H.; Narayanan, J.; Fish, B.; Camara, A.K.S.; Medhora, M.; Ranji, M. Vascular regression in the kidney: changes in 3D vessel structure with time post-irradiation. Biomed Opt Express 2022, 13, 4338–4352. [Google Scholar] [CrossRef]
- Yamaga, S.; Aziz, M.; Murao, A.; Brenner, M.; Wang, P. DAMPs and radiation injury. Front Immunol 2024, 15, 1353990. [Google Scholar] [CrossRef] [PubMed]
- Molinar-Inglis, O.; DiCarlo, A.L.; Lapinskas, P.J.; Rios, C.I.; Satyamitra, M.M.; Silverman, T.A.; Winters, T.A.; Cassatt, D.R. Radiation-induced multi-organ injury. Int J Radiat Biol 2024, 100, 486–504. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X. Reducing Oxygen Demand to Alleviate Acute Kidney Injury. Front Biosci (Landmark Ed) 2023, 28, 62. [Google Scholar] [CrossRef] [PubMed]
- Dawson, L.A.; Kavanagh, B.D.; Paulino, A.C.; Das, S.K.; Miften, M.; Li, X.A.; Pan, C.; Ten Haken, R.K.; Schultheiss, T.E. Radiation-associated kidney injury. Int J Radiat Oncol Biol Phys 2010, 76, (3 Suppl), S108–15. [Google Scholar] [CrossRef]
- Trécul, A.; Morceau, F.; Dicato, M.; Diederich, M. Dietary compounds as potent inhibitors of the signal transducers and activators of transcription (STAT) 3 regulatory network. Genes Nutr 2012, 7, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.-C.; Teng, H.-W.; Shiau, C.-W.; Tai, W.-T.; Hung, M.-H.; Yang, S.-H.; Jiang, J.-K.; Chen, K.-F. Pharmacological targeting SHP-1-STAT3 signaling is a promising therapeutic approach for the treatment of colorectal cancer. Neoplasia 2015, 17, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Yan-Sanders, Y.; Lyn-Cook, B.D.; Wang, T.; Tamae, D.; Ogi, J.; Khaletskiy, A.; Li, Z.; Weydert, C.; Longmate, J.A.; Huang, T.T.; Spitz, D.R.; Oberley, L.W.; Li, J.J. Manganese superoxide dismutase-mediated gene expression in radiation-induced adaptive responses. Mol.Cell Biol. 2003, 23, 2362–2378. [Google Scholar] [CrossRef]
- Wei, J.; Wang, B.; Wang, H.; Meng, L.; Zhao, Q.; Li, X.; Xin, Y.; Jiang, X. Radiation-Induced Normal Tissue Damage: Oxidative Stress and Epigenetic Mechanisms. Oxid Med Cell Longev 2019, 2019, 3010342. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Miao, B.; Cao, H.; Tian, X.; Shen, L.; Yang, Z.; Yuan, F.; Ding, Y. Monkfish (Lophius litulon) Peptides Ameliorate High-Fat-Diet-Induced Nephrotoxicity by Reducing Oxidative Stress and Inflammation via Regulation of Intestinal Flora. Molecules 2023, 28, 245. [Google Scholar] [CrossRef] [PubMed]
- Nath, M.; Agarwal, A. New insights into the role of heme oxygenase-1 in acute kidney injury. Kidney Res Clin Pract 2020, 39, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhao, R.; Pu, Q.; Jiang, S.; Yu, F.; Yang, Z.; Han, T. Investigation of nephrotoxicity on mice exposed to polystyrene nanoplastics and the potential amelioration effects of DHA-enriched phosphatidylserine. Sci Total Environ 2023, 892, 164808. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, K.; Han, Z.; Chi, M.; Sai, X.; Zhu, P.; Ding, Z.; Song, L.; Liu, C. Immunomodulatory Effects of Heme Oxygenase-1 in Kidney Disease. Front Med (Lausanne) 2021, 8, 708453. [Google Scholar] [CrossRef] [PubMed]
- Gambini, J.; Stromsnes, K. Oxidative Stress and Inflammation: From Mechanisms to Therapeutic Approaches. Biomedicines 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Covington, S.M.; Bauler, L.D.; Toledo-Pereyra, L.H. Akt: A Therapeutic Target in Hepatic Ischemia-Reperfusion Injury. J Invest Surg 2017, 30, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, R.; Yan, Y.; He, B.; Miao, C.; Fang, Y.; Wan, H.; Zhou, G. Exosomes-Mediated Signaling Pathway: A New Direction for Treatment of Organ Ischemia-Reperfusion Injury. Biomedicines 2024, 12. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Smith, J.T.; Anderson, M.N.; Umali, M.V.; Ho, C.; Zhai, M.; Lin, B.; Jiang, S. A novel therapy, using Ghrelin with pegylated G-CSF, inhibits brain hemorrhage from ionizing radiation or combined radiation injury. Pharm Pharmacol Int J 2019, 7, 133–145. [Google Scholar]
- Abraham, A.G.; O'Neill, E. PI3K/Akt-mediated regulation of p53 in cancer. Biochem Soc Trans 2014, 42, 798–803. [Google Scholar] [CrossRef]
- Liu, A.; Zhu, Y.; Chen, W.; Merlino, G.; Yu, Y. PTEN Dual Lipid- and Protein-Phosphatase Function in Tumor Progression. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, X.; Wang, L.; Lin, B.; Zhai, M.; Hull, L.; Zizzo, A.; Cui, W.; Kiang, J.G. Skin Wound following Irradiation Aggravates Radiation-Induced Brain Injury in a Mouse Model. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Deng, L.; Wang, S.; Leng, X.; Yao, P.; Li, C.; Zheng, Y. Combining network pharmacology and in vitro and in vivo experiments to study the mechanism of Keluoxin in the treatment of radiation nephropathy†. J Radiat Res 2023, 64, 769–782. [Google Scholar] [CrossRef]
- Kiang, J.G.; Smith, J.T.; Anderson, M.N.; Elliott, T.B.; Gupta, P.; Balakathiresan, N.S.; Maheshwari, R.K.; Knollmann-Ritschel, B. Hemorrhage enhances cytokine, complement component 3, and caspase-3, and regulates microRNAs associated with intestinal damage after whole-body gamma-irradiation in combined injury. PLoS One 2017, 12, e0184393. [Google Scholar] [CrossRef]
- Abassi, Z.; Goligorsky, M.S. Heparanase in Acute Kidney Injury. Adv Exp Med Biol 2020, 1221, 685–702. [Google Scholar] [PubMed]
- Li, J.C.; Wang, L.J.; Feng, F.; Chen, T.T.; Shi, W.G.; Liu, L.P. Role of heparanase in sepsis-related acute kidney injury (Review). Exp Ther Med 2023, 26, 379. [Google Scholar] [CrossRef]
- Masola, V.; Zaza, G.; Onisto, M.; Lupo, A.; Gambaro, G. Impact of heparanase on renal fibrosis. Journal of Translational Medicine 2015, 13, 181. [Google Scholar] [CrossRef] [PubMed]
- Danikowski, K.M.; Jayaraman, S.; Prabhakar, B.S. Regulatory T cells in multiple sclerosis and myasthenia gravis. J Neuroinflammation 2017, 14, 117. [Google Scholar] [CrossRef]
- Kushwah, R.; Hu, J. Role of dendritic cells in the induction of regulatory T cells. Cell Biosci 2011, 1, 20. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Anderton, S.M. Cytokines in the induction and resolution of experimental autoimmune encephalomyelitis. Cytokine 2005, 32, 81–84. [Google Scholar] [CrossRef]
- Multhoff, G.; Radons, J. Radiation, inflammation, and immune responses in cancer. Front Oncol 2012, 2, 58. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Liongue, C.; Sobah, M.L.; Ward, A.C. Signal Transducer and Activator of Transcription Proteins at the Nexus of Immunodeficiency, Autoimmunity and Cancer. Biomedicines 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, C.; Leung, S.; Salvekar, A.; Mano, H.; Schindler, U. Repression of IL-4-induced gene expression by IFN-gamma requires Stat1 activation. J Immunol 1999, 162, 4053–4061. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.L.; Newcomb, D.C.; Parekh, V.V.; Van Kaer, L.; Collins, R.D.; Zhou, W.; Goleniewska, K.; Chi, M.H.; Mitchell, D.; Boyce, J.A.; Durbin, J.E.; Sturkie, C.; Peebles, R.S. Jr. , STAT1 negatively regulates lung basophil IL-4 expression induced by respiratory syncytial virus infection. J Immunol 2009, 183, 2016–2026. [Google Scholar] [CrossRef]
- Tolomeo, M.; Cavalli, A.; Cascio, A. STAT1 and Its Crucial Role in the Control of Viral Infections. International Journal of Molecular Sciences 2022, 23, 4095. [Google Scholar] [CrossRef]
- Menon, P.R.; Staab, J.; Gregus, A.; Wirths, O.; Meyer, T. An inhibitory effect on the nuclear accumulation of phospho-STAT1 by its unphosphorylated form. Cell Communication and Signaling 2022, 20, 42. [Google Scholar] [CrossRef]
- Schieven, G.L. The biology of p38 kinase: a central role in inflammation. Curr Top Med Chem 2005, 5, 921–928. [Google Scholar] [CrossRef]
- Sisakht, M.; Darabian, M.; Mahmoodzadeh, A.; Bazi, A.; Shafiee, S.M.; Mokarram, P.; Khoshdel, Z. The role of radiation induced oxidative stress as a regulator of radio-adaptive responses. Int J Radiat Biol 2020, 96, 561–576. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, H.; Guo, W.; Yu, L. Potential role of ghrelin in the regulation of inflammation. Faseb j 2022, 36, e22508. [Google Scholar] [CrossRef]
- Kiang, J.G.; Cannon, G.; Olson, M.G.; Smith, J.T.; Anderson, M.N.; Zhai, M.; Umali, M.V.; Ho, K.; Ho, C.; Cui, W.; Xiao, M. Female Mice are More Resistant to the Mixed-Field (67% Neutron + 33% Gamma) Radiation-Induced Injury in Bone Marrow and Small Intestine than Male Mice due to Sustained Increases in G-CSF and the Bcl-2/Bax Ratio and Lower miR-34a and MAPK Activation. Radiat Res 2022, 198, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Zhou, D. Inhibition of p38 MAPK attenuates ionizing radiation-induced hematopoietic cell senescence and residual bone marrow injury. Radiat Res 2011, 176, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Kato, T.; Isogai, T.; Gu, Y.; Yamashita, T. The Potential Effects of Taurine in Mitigation of Radiation Nephropathy. Adv Exp Med Biol 2019, 1155, 497–505. [Google Scholar] [PubMed]
- Geng, R.; Fang, J.; Kang, S.G.; Huang, K.; Tong, T. Chronic exposure to UVB induces nephritis and gut microbiota dysbiosis in mice based on the integration of renal transcriptome profiles and 16S rRNA sequencing data. Environ Pollut 2023, 333, 122035. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Wilkinson, F.L.; Sandhu, M.A.; Lightfoot, A.P. The Interplay of Oxidative Stress and Inflammation: Mechanistic Insights and Therapeutic Potential of Antioxidants. Oxidative Medicine and Cellular Longevity 2021, 2021, 9851914. [Google Scholar] [CrossRef]
- Gupta, R.; Liu, L.; Zhang, X.; Fan, X.; Krishnamurthy, P.; Verma, S.; Tongers, J.; Misener, S.; Ashcherkin, N.; Sun, H.; Tian, J.; Kishore, R. IL-10 provides cardioprotection in diabetic myocardial infarction via upregulation of Heme clearance pathways. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Smith, J.T.; Cannon, G.; Anderson, M.N.; Ho, C.; Zhai, M.; Cui, W.; Xiao, M. Ghrelin, a novel therapy, corrects cytokine and NF-κB-AKT-MAPK network and mitigates intestinal injury induced by combined radiation and skin-wound trauma. Cell Biosci 2020, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Wade, M.; Li, Y.C.; Wahl, G.M. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer 2013, 13, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Thomasova, D.; Anders, H.J. Cell cycle control in the kidney. Nephrol Dial Transplant 2015, 30, 1622–1630. [Google Scholar] [CrossRef]
- Packialakshmi, B.; Stewart, I.J.; Burmeister, D.M.; Feng, Y.; McDaniel, D.P.; Chung, K.K.; Zhou, X. Tourniquet-induced lower limb ischemia/reperfusion reduces mitochondrial function by decreasing mitochondrial biogenesis in acute kidney injury in mice. Physiol Rep 2022, 10, e15181. [Google Scholar] [CrossRef]
- Rahmatullah, M.; Boyde, T.R.C. Improvements in the determination of urea using diacetyl monoxime; methods with and without deproteinisation. Clinica Chimica Acta 1980, 107, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Packialakshmi, B.; Xiao, Y.; Nurmukhambetova, S.; Lees, J.R. Progression of experimental autoimmune encephalomyelitis is associated with up-regulation of major sodium transporters in the mouse kidney cortex under a normal salt diet. Cell Immunol 2017, 317, 18–25. [Google Scholar] [CrossRef] [PubMed]
| Gene names | Forward | Reverse |
|---|---|---|
| IL-6 | AGTGGCTAAGGACCAAGACC | ACCACAGTGAGGAATGTCCA |
| IFNγ | CTGCTGATGGGAGGAGATGT | CACATTCGAGTGCTGTCTGG |
| IFNγR1 | CGAAGCAGCAGAACAGGAAG | CGTCTTTGTGTCGGAGTTGG |
| IFNγR2 | GTTCCCTCGTCATACACTTCTC | CCTGTTCCTGTTGGGTTTCT |
| Heparanase | GCCTCGAGGGAAGACAGTTA | GCGATGCGTCCATTCAAGTA |
| CCL2 | AACTGCATCTGCCCTAAGGT | CTGTCACACTGGTCACTCCT |
| TNFα | AGCCCATATACCTGGGAGGA | TGGATGAACACCCATTCCCT |
| G-CSF | CAGCCTTCACTTCTGCCTTC | CTGGAAAGGGCTTTCTGCTC |
| IL-18 | CTTCTGCAACCTCCAGCATC | GTGAAGTCGGCCAAAGTTGT |
| IL-1β | ACTCATTGTGGCTGTGGAGA | AGCCTGTAGTGCAGTTGTCT |
| IL-8 | GCACCCAAACCGAAGTCATA | TCTGAACCAAGGGAGCTTCA |
| IL-10 | AGAGAAGCATGGCCCAGAAA | ACACCTTGGTCTTGGAGCTT |
| IL-4 | AACGAGGTCACAGGAGAAGG | CTGCAGCTCCATGAGAACAC |
| IL-13 | GGATTCCCTGACCAACATCTC | AGGGATGGTCTCTCCTCATT |
| TGFβ | CAGACATTCGGGAAGCAGTG | TTCCACATGTTGCTCCACAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





