Submitted:
09 June 2024
Posted:
13 June 2024
You are already at the latest version
Abstract
Keywords:
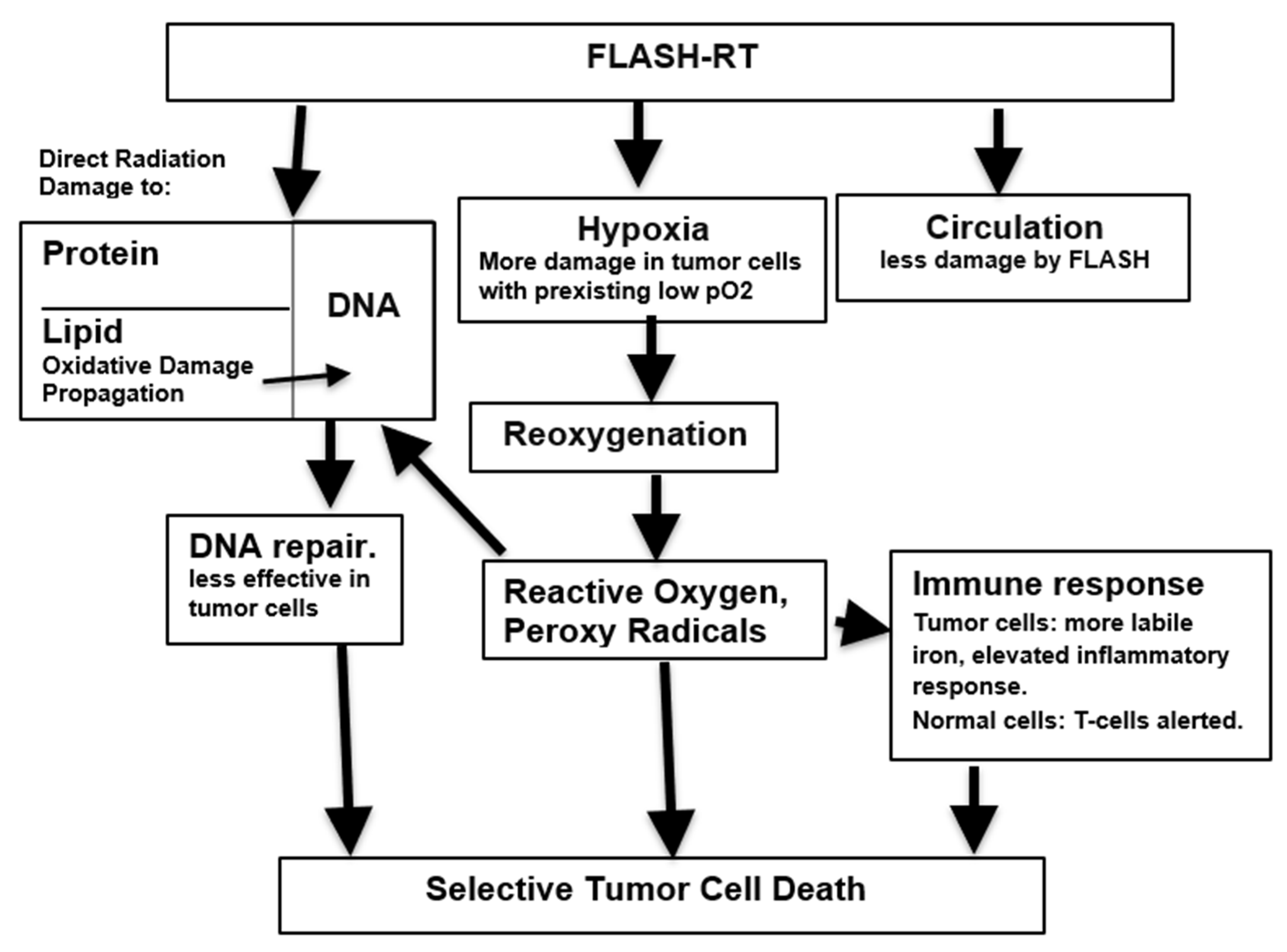
1. Introduction
2. Accounting for the Differences between FLASH-RT and CONV-RT
2.1. Why Is FLASH-RT Less Lethal to Normal Cells Than the Same Dose of CONV-RT?
2.1.1. Anoxia
2.1.2. The Role of Reactive Oxygen Species
2.1.3. Damage to DNA
2.1.4. Inflammation
2.2. Why Are Tumor Cells More Sensitive Than Normal Cells to FLASH-RT?
2.2.1. Tumor Metabolism is Unlike That of Normal Tissue
2.2.2. Differing Production and Disposition of Reactive Oxygen Species
2.2.3. Reduction of Bystander Effects by FLASH
2.2.4. Immune Responses
3. Outcome
Funding
Conflicts of Interest
References
- Begg, A.C.; Stewart, F.A.; Vens, C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011, 11, 239–253. [Google Scholar] [CrossRef]
- Dewey, D.L.; Boag, J.W. Modification of the oxygen effect when bacteria are given large pulses of radiation. Nature. 1959, 183, 1450–1451. [Google Scholar] [CrossRef]
- Vozenin M,C,; De Fornel, P. ; Petersson, K.; Favaudon, V.; Jaccard, M.; Germond, J.F.; Petit, B.; Burki, M.; Ferrand, G.; Patin, D.; Bouchaab,H.; Ozsahin, M.; Bochud, F.; Bailat, C.; Devauchelle, P.; Bourhis, J. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin Cancer Res. 2019, 25, 35–42.
- Lin, B.; Gao, F.; Yang, Y.; Wu, D.; Zhang, Y.; Feng, G.; Dai, T.; Du, X. FLASH Radiotherapy: History and Future. Front Oncol. 2021, 11, 644400. [Google Scholar] [CrossRef]
- Jin, J.Y.; Gu, A.; Wang, W.; Oleinick, N.L.; Machtay, M.; Spring Kong, F.M. Ultra-high dose rate effect on circulating immune cells: A potential mechanism for FLASH effect? Radiother Oncol. 2020, 149, 55–62. [Google Scholar] [CrossRef]
- Favaudon, V.; Labarbe, R.; Limoli, C.L. Model studies of the role of oxygen in the FLASH effect. Med Phys. 2022, 49, 2068–2081. [Google Scholar] [CrossRef]
- Hubenak, J.R.; Zhang, Q.; Branch, C.D.; Kronowitz, S.J. Mechanisms of injury to normal tissue after radiotherapy: a review. Plast Reconstr Surg. 2014, 133, 49e–56e. [Google Scholar] [CrossRef]
- Bourhis, J.; Sozzi, W.J.; Jorge, P.G.; Gaide, O.; Bailat, C.; Duclos, F.; Patin, D.; Ozsahin, M.; Bochud, F.; Germond, J.F.; Moeckli, R.; Vozenin, M.C. Treatment of a first patient with FLASH-radiotherapy. Radiother Oncol, 2019; 139, 18–22. [Google Scholar]
- Konradsson, E.; Szecsenyi, R.E.; Adrian, G.; Coskun, M. ; Børresen B.; Arendt, M.L.; Erhart, K.; Bäck,S.Å.; Petersson, K.; Ceberg, C. Evaluation of intensity-modulated electron FLASH radiotherapy in a clinical setting using veterinary cases. Med. Phys. 2023, 50, 6569–6579. [Google Scholar] [CrossRef]
- Børresen, B.; Arendt, M.L.; Konradsson, E.; Bastholm Jensen, K.; Bäck, S.Å.; Munck, A.f.; Rosenschöld, P.; Ceberg, C.; Petersson, K. Evaluation of single-fraction high dose FLASH radiotherapy in a cohort of canine oral cancer patients. Front Oncol. 2023, 13, 256760. [Google Scholar] [CrossRef]
- Swarts, S.G.; Flood, A.B.; Swartz, H.M. Implications of “flash” radiotherapy for biodosimetry. Radiat Prot Dosimetry. 2023, 199, 1450–1459. [Google Scholar] [CrossRef]
- Bourhis, J.; Montay-Gruel, P.; Gonçalves Jorge, P.; Bailat, C.; Petit, B.; Ollivier, J.; Jeanneret-Sozzi, W.; Ozsahin, M.; Bochud, F.; Moeckli, R.; Germond, J.F.; Vozenin, M.C. Clinical translation of FLASH radiotherapy: Why and how? Radiother Oncol. 2019, 139, 11–17. [Google Scholar] [CrossRef]
- Hendry, J.H.; Moore, J.V.; Hodgson, B.W.; Keene, J.P. The constant low oxygen concentration in all the target cells for mouse tail radionecrosis. Radiat Res. 1982, 92, 172–181. [Google Scholar] [CrossRef]
- Hall, E.J.; Brenner, D.J. The dose-rate effect revisited: radiobiological considerations of importance in radiotherapy. Int J Radiat Oncol Biol Phys. 1991, 21, 1403–1414. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Acharya, M.M.; Petersson, K.; Alikhani, L.; Yakkala, C.; Allen, B.D.; Ollivier, J.; Petit, B.; Jorge, P.G.; Syage, A.R.; Nguyen, T.A.; Baddour, A.A.D.; Lu, C.; Singh, P.; Moeckli, R.; Bochud, F.; Germond, J.F.; Froidevaux, P.; Bailat, C.; Bourhis, J.; Vozenin, M.C.; Limoli, C.L. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc Natl Acad Sci U S A. 2019, 116, 10943–10951. [Google Scholar] [CrossRef]
- Friedl, A.A.; Prise, K.M.; Butterworth, K.T.; Montay-Gruel, P.; Favaudon, V. Radiobiology of the FLASH effect. Med Phys. 2022, 49, 1993–2013. [Google Scholar] [CrossRef]
- Limoli, C.L.; Kramár, E.A.; Almeida, A.; Petit, B.; Grilj, V.; Baulch, J.E.; Ballesteros-Zebadua, P.; Loo, B.W. Jr.; Wood, M.A.; Vozenin, M.C. The sparing effect of FLASH-RT on synaptic plasticity is maintained in mice with standard fractionation. Radiother Oncol. 2023, 186, 109767. [Google Scholar] [CrossRef]
- Tavakkoli, A.D.; Clark, M.A.; Kheirollah, A.; Sloop, A.M.; Soderholm, H.E.; Daniel, N.J.; Petusseau, A.F.; Huang, Y.H.; Thomas, C.R. Jr.; Jarvis, L.A.; Zhang, R.; Pogue, B.W.; Gladstone, D.J.; Hoopes, P.J. Anesthetic Oxygen Use and Sex Are Critical Factors in the FLASH Sparing Effect. Adv Radiat Oncol. 2024, 9, 101492. [Google Scholar] [CrossRef]
- Adrian, G.; Konradsson, E.; Lempart, M.; Bäck, S.; Ceberg, C.; Petersson, K. The FLASH effect depends on oxygen concentration. Br J Radiol. 2020, 93, 20190702. [Google Scholar] [CrossRef]
- Moon, E.J.; Petersson, K.; Olcina, M.M. The importance of hypoxia in radiotherapy for the immune response, metastatic potential and FLASH-RT. Int J Radiat Biol. 2022, 98, 439–451. [Google Scholar] [CrossRef]
- El Khatib, M.; Van Slyke, A.L.; Velalopoulou, A.; Kim, M.M.; Shoniyozov, K.; Allu, S.R.; Diffenderfer, E.E.; Busch, T.M.; Wiersma, R.D.; Koch, C.J.; Vinogradov, S.A. Ultrafast Tracking of Oxygen Dynamics During Proton FLASH. Int J Radiat Oncol Biol Phys. 2022, 113, 624–634. [Google Scholar] [CrossRef]
- Labarbe, R.; Hotoiu, L.; Barbier, J.; Favaudon, V. A physicochemical model of reaction kinetics supports peroxyl radical recombination as the main determinant of the FLASH effect. Radiother Oncol. 2020, 153, 303–310. [Google Scholar] [CrossRef]
- Favaudon V, Labarbe R, Limoli CL. Model studies of the role of oxygen in the FLASH effect. Med Phys. 2022 Mar;49(3):2068-2081. Epub 2021 Aug 18. PMCID: PMC8854455. [CrossRef] [PubMed]
- Ramos-Méndez, J.; Domínguez-Kondo, N.; Schuemann, J.; McNamara, A.; Moreno-Barbosa, E.; Faddegon, B. LET-Dependent Intertrack Yields in Proton Irradiation at Ultra-High Dose Rates Relevant for FLASH Therapy. Radiat Res. 2020, 194, 351–362. [Google Scholar] [CrossRef]
- Perstin, A.; Poirier, Y.; Sawant, A.; Tambasco, M. Quantifying the DNA-damaging Effects of FLASH Irradiation With Plasmid DNA. Int J Radiat Oncol Biol Phys. 2022, 113, 437–447. [Google Scholar] [CrossRef]
- Alizadeh, E.; Sanz, A.G.; García, G.; Sanche, L. Radiation Damage to DNA: The Indirect Effect of Low Energy Electrons. J Phys Chem Lett. 2013, 4, 820–825. [Google Scholar] [CrossRef]
- Garty, G.; Obaid, R.; Deoli, N.; Royba, E.; Tan, Y.; Harken, A.D.; Brenner, D.J. Ultra-high dose rate FLASH irradiator at the radiological research accelerator facility. Sci Rep. 2022, 12, 22149. [Google Scholar] [CrossRef]
- Schüler, E.; Acharya, M.; Montay-Gruel, P.; Loo, B.W. Jr.; Vozenin, M.C.; Maxim, P.G. Ultra-high dose rate electron beams and the FLASH effect: From preclinical evidence to a new radiotherapy paradigm. Med Phys. 2022, 49, 2082–2095. [Google Scholar] [CrossRef]
- Small, K.L.; Henthorn, N.T.; Angal-Kalinin, D. Chadwick, A. L.; Santina, E.; Aitkenhead, A.; Kirkby, K.J.; Smith, R.J.; Surman, M.; Jones, J.; Farabolini, W.; Corsini, R.; Gamba, D.; Gilardi. A.; Merchant, M.J.; Jones, R.M. Evaluating very high energy electron RBE from nanodosimetric pBR322 plasmid DNA damage. Sci Rep. 2021, 11, 3341. [Google Scholar]
- Ohsawa, D.; Hiroyama, Y.; Kobayashi, A.; Kusumoto, T.; Kitamura, H.; Hojo, S.; Kodaira, S.; Konishi, T. DNA strand break induction of aqueous plasmid DNA exposed to 30 MeV protons at ultra-high dose rate. J Radiat Res. 2022, 63, 255–260. [Google Scholar] [CrossRef]
- Kumar, K.; Kumar, S.; Datta, K.; Fornace, A.J. Jr.; Suman, S. High-LET-Radiation-Induced Persistent DNA Damage Response Signaling and Gastrointestinal Cancer Development. Curr Oncol. 2023, 30, 5497–5514. [Google Scholar] [CrossRef]
- Iturri, L.; Bertho, A.; Lamirault, C.; Juchaux, M.; Gilbert, C.; Espenon, J.; Sebrie, C.; Jourdain, L.; Pouzoulet, F.; Verrelle, P.; De Marzi, L.; Prezado, Y. Proton FLASH Radiation Therapy and Immune Infiltration: Evaluation in an Orthotopic Glioma Rat Model. Int J Radiat Oncol Biol Phys. 2023, 116, 655–665. [Google Scholar] [CrossRef]
- Bondy, S.C. Mitochondrial Dysfunction as the Major Basis of Brain Aging. Biomolecules. 2024, 14, 402. [Google Scholar] [CrossRef]
- Zhou, X.; Li, N.; Wang, Y.; Wang, Y.; Zhang, X.; Zhang, H. Effects of X-irradiation on mitochondrial DNA damage and its supercoiling formation change. Mitochondrion. 2011, 11, 886–892. [Google Scholar] [CrossRef]
- Guo, Z.; Buonanno, M.; Harken, A.; Zhou, G.; Hei, T.K. Mitochondrial Damage Response and Fate of Normal Cells Exposed to FLASH Irradiation with Protons. Radiat Res. 2022, 197, 569–582. [Google Scholar] [CrossRef]
- Trappetti, V.; Fazzari, J.; Fernandez-Palomo, C.; Smyth, L.; Potez, M.; Shintani, N.; de Breuyn Dietler, B.; Martin, O.A.; Djonov, V. Targeted Accumulation of Macrophages Induced by Microbeam Irradiation in a Tissue-Dependent Manner. Biomedicines. 2022, 10, 735. [Google Scholar] [CrossRef]
- Buonanno, M.; Grilj, V.; Brenner, D.J. Biological effects in normal cells exposed to FLASH dose rate protons. Radiother Oncol. 2019, 139, 51–55. [Google Scholar] [CrossRef]
- Elbakrawy, E.; Kaur Bains, S.; Bright, S.; Al-Abedi, R.; Mayah, A.; Goodwin, E.; Kadhim, M. Radiation-Induced Senescence Bystander Effect: The Role of Exosomes. Biology (Basel). 2020, 9, 191. [Google Scholar] [CrossRef]
- Fouillade, C.; Curras-Alonso, S.; Giuranno, L.; Quelennec, E.; Heinrich, S.; Bonnet-Boissinot, S.; Beddok, A.; Leboucher, S.; Karakurt, H.U.; Bohec, M.; Baulande, S.; Vooijs, M.; Verrelle, P.; Dutreix, M.; Londoño-Vallejo, A.; Favaudon, V. FLASH Irradiation Spares Lung Progenitor Cells and Limits the Incidence of Radio-induced Senescence. Clin Cancer Res. 2020, 26, 1497–1506. [Google Scholar] [CrossRef]
- Giaccia, A.J. Hypoxic Stress Proteins: Survival of the Fittest. Semin Radiat Oncol. 1996, 6, 46–58. [Google Scholar] [CrossRef]
- Adekola, K.; Rosen, S.T.; Shanmugam, M. Glucose transporters in cancer metabolism. Curr Opin Oncol. 2012, 24, 650–654. [Google Scholar] [CrossRef]
- Ganapathy-Kanniappan, S.; Geschwind, J.F. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013, 12, 152. [Google Scholar] [CrossRef]
- Aboelella, N.S.; Brandle, C.; Kim, T.; Ding, Z.C.; Zhou, G. Oxidative Stress in the Tumor Microenvironment and Its Relevance to Cancer Immunotherapy. Cancers (Basel). 2021, 13, 986. [Google Scholar] [CrossRef]
- Alanazi, A.; Jay-Gerin, J.P.; Blázquez-Castro, A. Is singlet oxygen involved in FLASH-RT? J Appl Clin Med Phys. 2023, 24, e13974. [Google Scholar] [CrossRef]
- Leavitt, R.J.; Almeida, A.; Grilj, V.; Montay-Gruel, P.; Godfroid, C.; Petit, B.; Bailat, C.; Limoli, C.L.; Vozenin, M.C. Acute Hypoxia Does Not Alter Tumor Sensitivity to FLASH Radiation Therapy. Int J Radiat Oncol Biol Phys. 2024, S0360-3016, 00320–00321. [Google Scholar] [CrossRef]
- Abolfath, R.; Grosshans, D.; Mohan, R. Oxygen depletion in FLASH ultra-high-dose-rate radiotherapy: A molecular dynamics simulation. Med Phys. 2020, 47, 6551–6561. [Google Scholar] [CrossRef]
- Ma, J.; Gao, H.; Shen, X.; Bai, X.; Tang, M. A FLASH model of radiolytic oxygen depletion and reactive oxygen species for differential tumor and normal-tissue response. medRxiv. 2023, 2023.10.20.23297337. [Google Scholar]
- Spitz, D.R.; Buettner, G.R.; Petronek, M.S.; St-Aubin, J.J.; Flynn, R.T.; Waldron, T.J.; Limoli, C.L. An integrated physico-chemical approach for explaining the differential impact of FLASH versus conventional dose rate irradiation on cancer and normal tissue responses. Radiother Oncol. 2019, 139, 23–27. [Google Scholar] [CrossRef]
- Prasad, K.N.; Sinha, P.K.; Ramanujam, M.; Sakamoto, A. Sodium ascorbate potentiates the growth inhibitory effect of certain agents on neuroblastoma cells in culture. Proc Natl Acad Sci U S A. 1979, 76, 829–832. [Google Scholar] [CrossRef]
- Prasad, K.N.; Bondy, S.C. FLASH Radiation vs. Conventional Radiation: Basis of Varying Effects and Potential Improvement of Their Differential Effects on Normal and Malignant Cells. J. Adv. Med. Sci. 2024. (in press) [Google Scholar]
- Wallis, C.J.; Mahar, A.L.; Choo, R.; Herschorn, S.; Kodama, R.T.; Shah, P.S.; Danjoux, C.; Narod, S.A.; Nam, R.K. Second malignancies after radiotherapy for prostate cancer: systematic review and meta-analysis. BMJ. 2016, 352, i851. [Google Scholar] [CrossRef]
- Casey, D.L.; Vogelius, I.R.; Brodin, N.P.; Roberts, K.B.; Avanzo, M.; Moni, J.; Owens, C.; Ronckers, C.M.; Constine, L.S.; Bentzen, S.M.; Olch, A. Risk of Subsequent Neoplasms in Childhood Cancer Survivors After Radiation Therapy: A PENTEC Comprehensive Review. Int J Radiat Oncol Biol Phys. 2024, 119, 640–654. [Google Scholar] [CrossRef]
- Marozik, P.; Mothersill, C.; Seymour, C.B.; Mosse, I.; Melnov, S. Bystander effects induced by serum from survivors of the Chernobyl accident. Exp Hematol. 2007, 35, 55–63. [Google Scholar] [CrossRef]
- Tang, H.; Cai, L.; He, X.; Niu, Z.; Huang, H.; Hu, W.; Bian, H.; Huang, H. Radiation-induced bystander effect and its clinical implications. Front Oncol. 2023, 13, 1124412. [Google Scholar] [CrossRef]
- Rudigkeit, S.; Schmid, T.E.; Dombrowsky, A.C.; Stolz, J.; Bartzsch, S.; Chen, C.B.; Matejka, N.; Sammer, M.; Bergmaier, A.; Dollinger, G.; Reindl, J. Proton-FLASH: effects of ultra-high dose rate irradiation on an in-vivo mouse ear model. Sci Rep. 2024, 14, 1418. [Google Scholar] [CrossRef]
- Kim, Y.E.; Gwak, S.H.; Hong, B.J.; Oh, J.M.; Choi, H.S.; Kim, M.S.; Oh, D.; Lartey, F.M.; Rafat, M.; Schüler, E.; Kim, H.S.; von Eyben, R.; Weissman, I.L.; Koch, C.J.; Maxim, P.G.; Loo, B.W. Jr.; Ahn, G.O. Effects of Ultra-high dose rate FLASH Irradiation on the Tumor Microenvironment in Lewis Lung Carcinoma: Role of Myosin Light Chain. Int J Radiat Oncol Biol Phys. 2021, 109, 1440–1453. [Google Scholar] [CrossRef]
- Foroumadi, R.; Rashedi, S.; Asgarian, S.; Mardani, M.; Keykhaei, M.; Farrokhpour, H.; Javanshir, S.; Sarallah, R.; Rezaei, N. Circular RNA MYLK as a prognostic biomarker in patients with cancers: A systematic review and meta-analysis. Cancer Rep (Hoboken). 2022, 5, e1653. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, J.; Xu, Y.; Ye, Y.; Zhong, G.; Chen, T.; Qiu, L. PRPF19 facilitates colorectal cancer liver metastasis through activation of the Src-YAP1 pathway via K63-linked ubiquitination of MYL9. Cell Death Dis. 2023, 14, 258. [Google Scholar] [CrossRef]
- Bogaerts, E.; Macaeva, E.; Isebaert, S.; Haustermans, K. Potential Molecular Mechanisms behind the Ultra-High Dose Rate “FLASH” Effect. Int J Mol Sci. 2022, 23, 12109. [Google Scholar] [CrossRef]
- Tang, R.; Yin, J.; Liu, Y.; Xue, J. FLASH radiotherapy: A new milestone in the field of cancer radiotherapy. Cancer Lett. 2024, 587, 216651. [Google Scholar] [CrossRef]
- Park, H.; Chung, H.T.; Kim, J.W.; Dho, Y.S.; Lee, E.J. A 3-month survival model after Gamma Knife surgery in patients with brain metastasis from lung cancer with Karnofsky performance status ≤ 70. Sci Rep. 2023, 13, 13159. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, R.; Chang, C.W.; Charyyev, S.; Zhou, J.; Bradley, J.D.; Liu, T.; Yang, X. A potential revolution in cancer treatment: A topical review of FLASH radiotherapy. J Appl Clin Med Phys. 2022, 23, e13790. [Google Scholar] [CrossRef]
- Venkatesulu, B.P.; Sharma, A.; Pollard-Larkin, J.M.; Sadagopan, R.; Symons, J.; Neri, S.; Singh, P.K.; Tailor, R.; Lin, S.H.; Krishnan, S. Ultra high dose rate (35 Gy/sec) radiation does not spare the normal tissue in cardiac and splenic models of lymphopenia and gastrointestinal syndrome. Sci Rep. 2019, 9, 17180. [Google Scholar] [CrossRef]
- Zhang, Q.; Gerweck, L.E.; Cascio, E.; Gu, L.; Yang, Q.; Dong, X.; Huang, P.; Bertolet, A.; Nesteruk, K.P.; Sung, W.; McNamara, A.L.; Schuemann, J. Absence of Tissue-Sparing Effects in Partial Proton FLASH Irradiation in Murine Intestine. Cancers (Basel). 2023, 15, 2269. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Smirnova, O.A. Effects of Flash Radiotherapy on Blood Lymphocytes in Humans and Small Laboratory Animals. Radiat Res. 2023, 199, 240–251. [Google Scholar] [CrossRef]
- Galts, A.; Hammi, A. FLASH radiotherapy sparing effect on the circulating lymphocytes in pencil beam scanning proton therapy: impact of hypofractionation and dose rate. Phys Med Biol. 2024, 69. [Google Scholar] [CrossRef]
- Wilson, J.D.; Hammond, E.M.; Higgins, G.S.; Petersson, K. Ultra-High Dose Rate (FLASH) Radiotherapy: Silver Bullet or Fool’s Gold? Front Oncol. 2020, 9, 1563. [Google Scholar] [CrossRef]
- Melia, E.; Parsons, J.L. DNA damage and repair dependencies of ionising radiation modalities. Biosci Rep. 2023, 43, BSR20222586. [Google Scholar] [CrossRef]
- Böhlen, T.T.; Germond, J.F.; Desorgher, L.; Veres, I.; Bratel, A.; Landström, E.; Engwall, E.; Herrera, F.G.; Ozsahin, E.M.; Bourhis, J.; Bochud, F.; Moeckli, R. Very high-energy electron therapy as light-particle alternative to transmission proton FLASH therapy - An evaluation of dosimetric performances. Radiother Oncol. 2024, 194, 110177. [Google Scholar] [CrossRef]
- Moeckli, R.; Gonçalves Jorge, P.; Grilj, V.; Oesterle, R.; Cherbuin, N.; Bourhis, J.; Vozenin, M.C.; Germond, J.F.; Bochud, F.; Bailat, C. Commissioning of an ultra-high dose rate pulsed electron beam medical LINAC for FLASH RT preclinical animal experiments and future clinical human protocols. Med Phys. 2021, 48, 3134–3142. [Google Scholar] [CrossRef]
- Liljedahl, E.; Konradsson, E.; Linderfalk, K.; Gustafsson, E.; Petersson, K.; Ceberg, C.; Redebrandt, H.N. Comparable survival in rats with intracranial glioblastoma irradiated with single-fraction conventional radiotherapy or FLASH radiotherapy. Front Oncol. 2024, 13, 1309174. [Google Scholar] [CrossRef]
- Allen, B.D.; Alaghband, Y.; Kramár, E.A.; Ru, N.; Petit, B.; Grilj, V.; Petronek, M.S.; Pulliam, C.F.; Kim, R.Y.; Doan, N.L.; Baulch, J.E.; Wood, M.A.; Bailat, C.; Spitz, D.R.; Vozenin, M.C.; Limoli, C.L. Elucidating the neurological mechanism of the FLASH effect in juvenile mice exposed to hypofractionated radiotherapy. Neuro Oncol. 2023, 25, 927–939. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




