1. Introduction
Pollution from the oil industry has always caused short-, medium-, and long-term environmental impacts, such as the contamination of water, air, and soil, with adverse effects on both fauna and flora [
1,
2,
3,
4,
5], as well as economic impacts, mainly affecting fisheries, tourism, and coastal areas. All complications generated by the oil industry can be mitigated by a combination of rigorous regulations, advances in clean technologies, and a global commitment to the transition to renewable energy sources [
6,
7].
Oil in wastewater poses specific challenges in terms of removal and adequate treatment. Different types of oil may be present and the effectiveness of separation methods depends on the nature of the different forms of oil [
1]. Emulsions pose a particular challenge, as these water-in-oil or oil-in-water mixtures are stable and resistant to decomposition. Moreover, the presence of antifoaming and emulsifying agents makes the separation task more complex [
8,
9].
Medeiros
et al. (2022) [
1] specify different methods currently employed by the oil industry for the treatment of emulsified wastewater. Such methods are generally combined, depending on the type of emulsion (oil-in-water or water-in-oil), type of oil present, and percentage of oil in the mixture. The combination of methods is required due to the low efficiency of individual methods at removing all types of oily molecules, which range in size from 20 to 150 μm [
10].
Another important point is the care required when the water is to be reused in other processes, as the accumulation of oil can damage equipment, generating expenditures on maintenance and the loss of operating time. Oil content in reused water can also exert a negative impact heat exchange processes or lead to fines if disposal in waterbodies is not performed in compliance with the standards required by regulatory agencies [
8,
11].
To address this issue, companies and governments of various countries have allocated resources in search of more effective, faster, less expensive treatment processes that cause less secondary harm to the environment [
1]. Moreover, the optimisation of established processes can be achieved with the use of novel biotechnological materials that assist in the agglutination of oily molecules or can replace conventional membranes derived from petroleum in filtration processes [
12,
13,
14].
Among these novel biomaterials, bacterial cellulose (BC) is a biopolymer described as a revolutionary tool with vast applicability in various industrial sectors, such as wastewater treatment [
1,
3,
12,
15]. BC can be produced through the action of various microorganisms, such as the genera
Komagataeibacter and
Agrobacterium [
16,
17,
18]. Its unique characteristics, such as the reticular structure, high porosity, large number of fibres, hydrophilicity, and oleophobicity, make BC ideal for use as a filtration membrane in separation processes [
16,
19,
20,
21].
Therefore, the aim of the present study was to treat emulsified oily water using a filtration module previously described by Medeiros
et al. (2023) [
16] to determine its effectiveness as well as suggest possible improvements to the module through tests involving different pressures. As assessment of the membrane was also performed by testing its ability to filter oil, suspended solids, colour, and the microbiological load from real industrial wastewater.
4. Conclusions
The tests conducted in this work demonstrated that the filtration system created with the BC membrane was highly effective at retaining contaminants, resulting in significant reductions in colour, turbidity, and particulate matter in the initial effluent. The membrane proved to be extremely flexible as well as easy to manage and use, exhibiting the mechanical properties necessary for use as a filtering membrane.
The main objective of treatment, which is the complete removal of emulsified oil from wastewater, was achieved. Moreover, the membrane was capable of removing the entire microbiological load as a result of its nanometric structure. Due to the large quantity of oily and non-oily contaminants, the FTIR, XRD, and EDS characterisations of the membrane indicated a high rate of other components. The treatment system developed proved to be highly effective at filtration, surpassing the results of activated carbon and effluent treatment stations. To further improve the performance of the filtration system in the treatment of wastewater, further studies are needed to increase the flow volume of the equipment.
Author Contributions
Conceptualisation, L.A.S. and A.D.M.d.M.; validation, L.A.S., A.D.M.d.M., C.J.G.d.S.J., I.J.B.D., T.d.S.C., Y.d.F.C. and A.F.d.S.C.; writing—original draft preparation, A.D.M.d.M. and C.J.G.d.S.J.; writing—review and editing, A.D.M.d.M., C.J.G.d.S.J., I.J.B.D., T.d.S.C., Y.d.F.C. and A.F.d.S.C.; visualisation, A.D.M.d.M. and L.A.S.; supervision, L.A.S.; project administration, L.A.S.; funding acquisition, L.A.S. All authors have read and agreed to the version of the manuscript to be published.
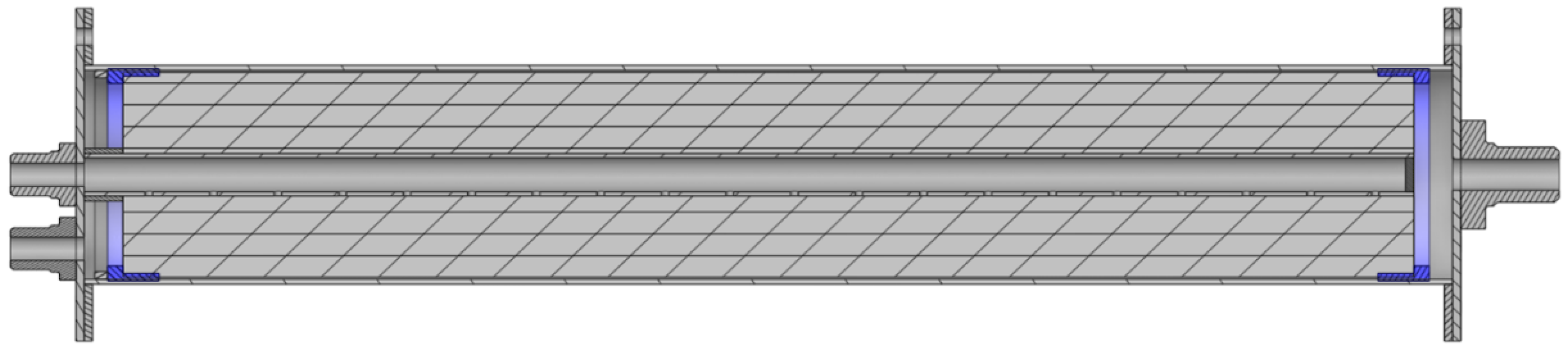
Figure 1.
Structural model of filtration module with cross-section showing external pressure support tubing and internal shaft in which bacterial cellulose membrane is packed.
Figure 1.
Structural model of filtration module with cross-section showing external pressure support tubing and internal shaft in which bacterial cellulose membrane is packed.
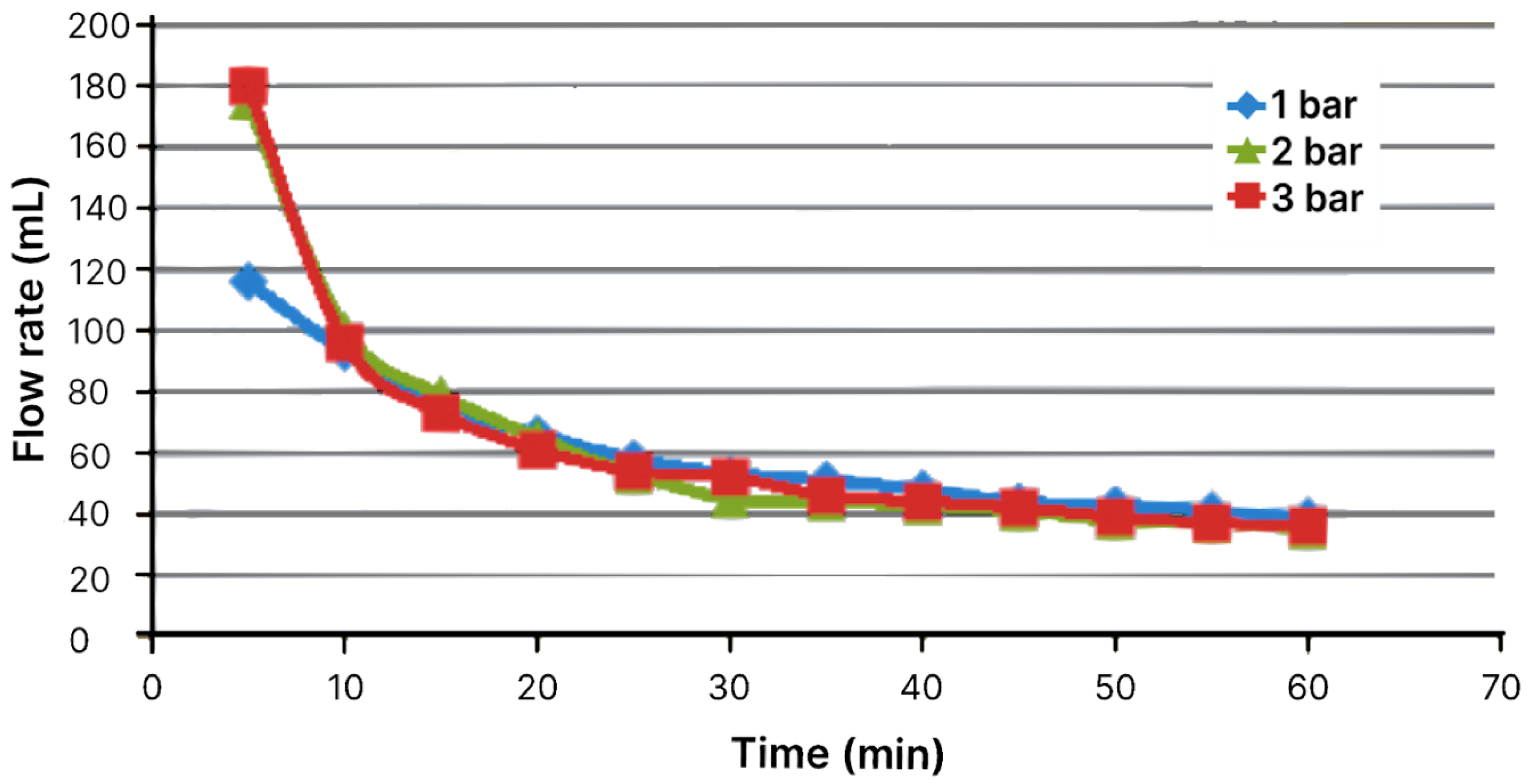
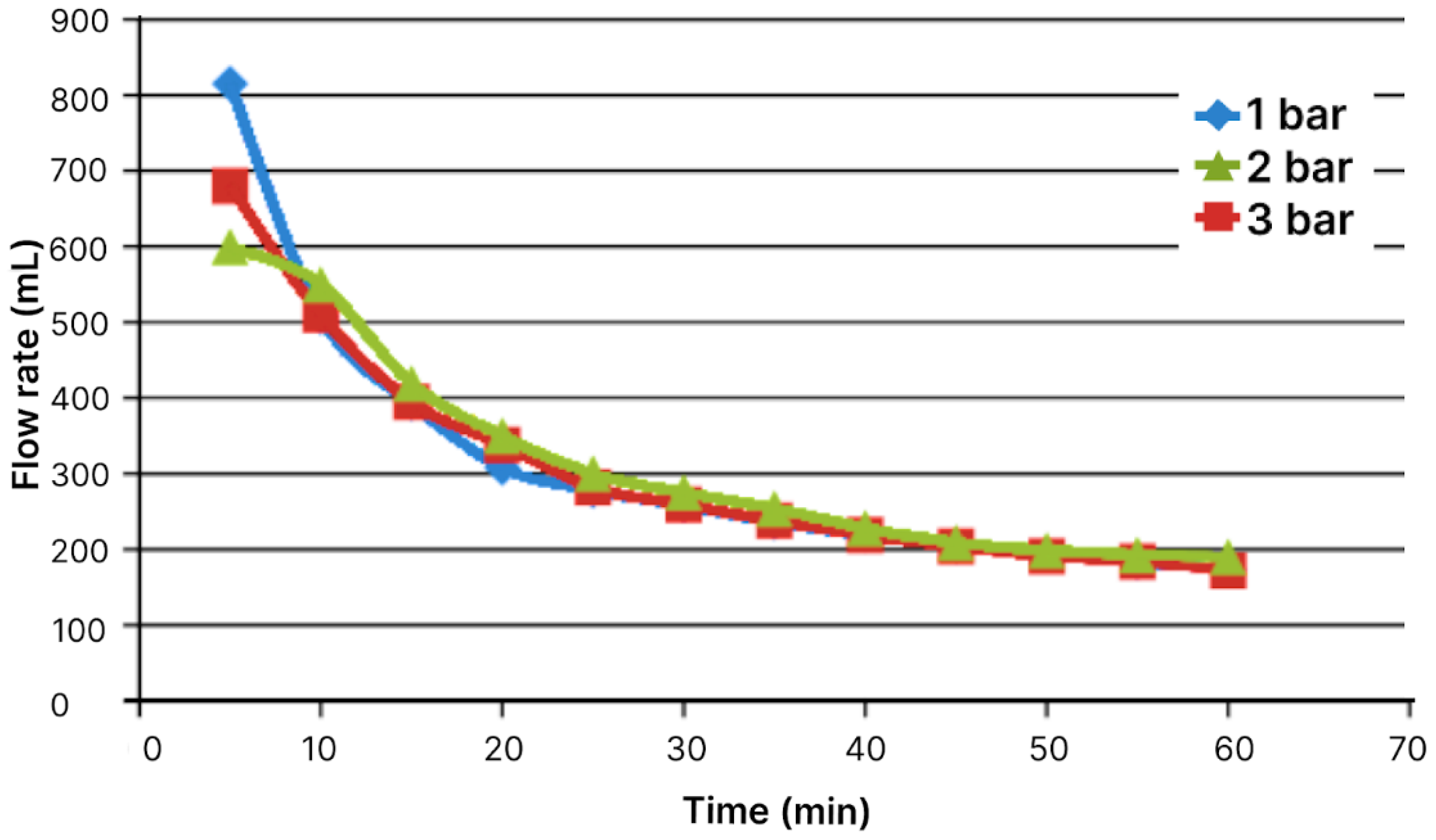
Figure 2.
Flow volume through module with area of 19.24 cm2 as function of time for pressures of 1, 2, and 3 bar.
Figure 2.
Flow volume through module with area of 19.24 cm2 as function of time for pressures of 1, 2, and 3 bar.
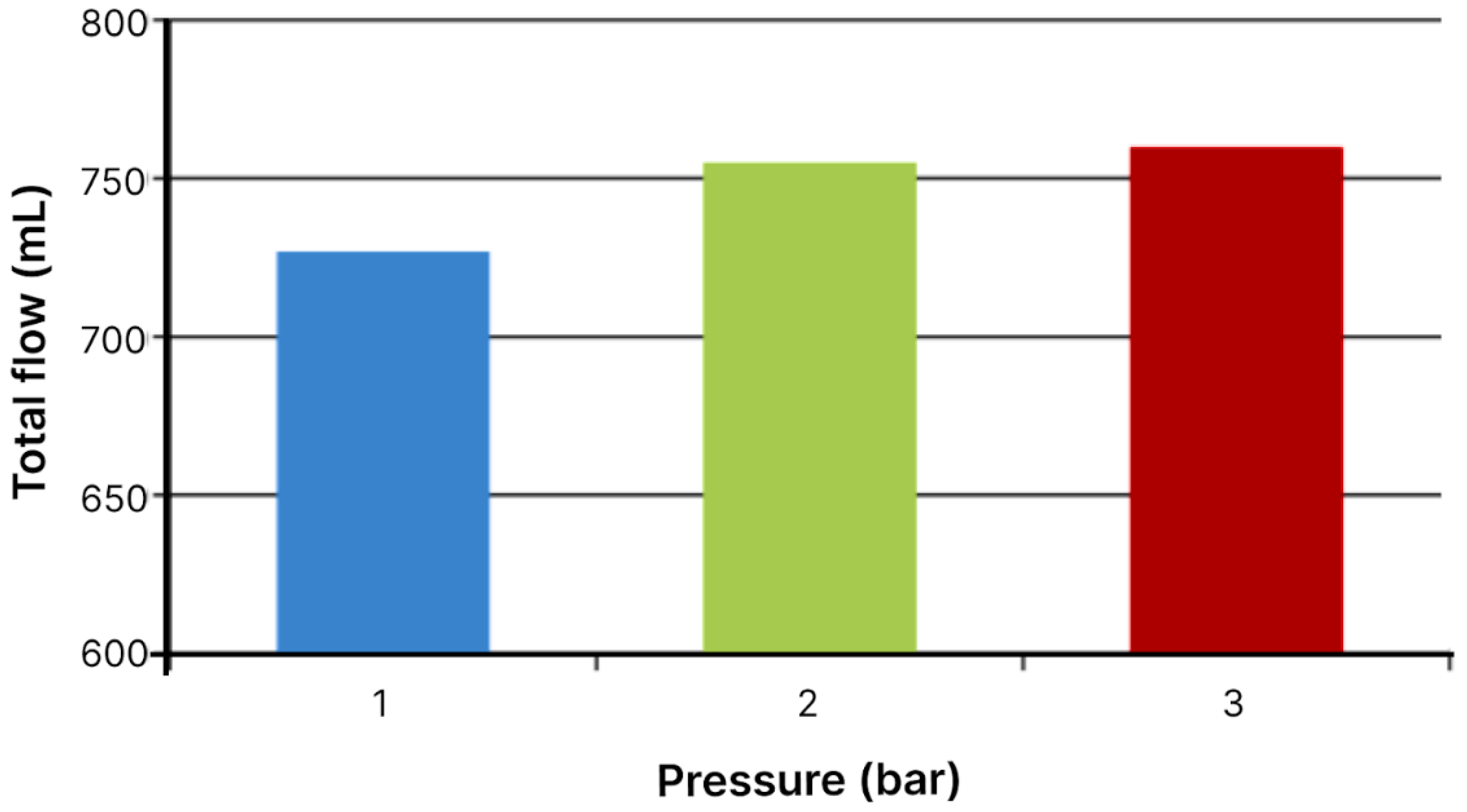
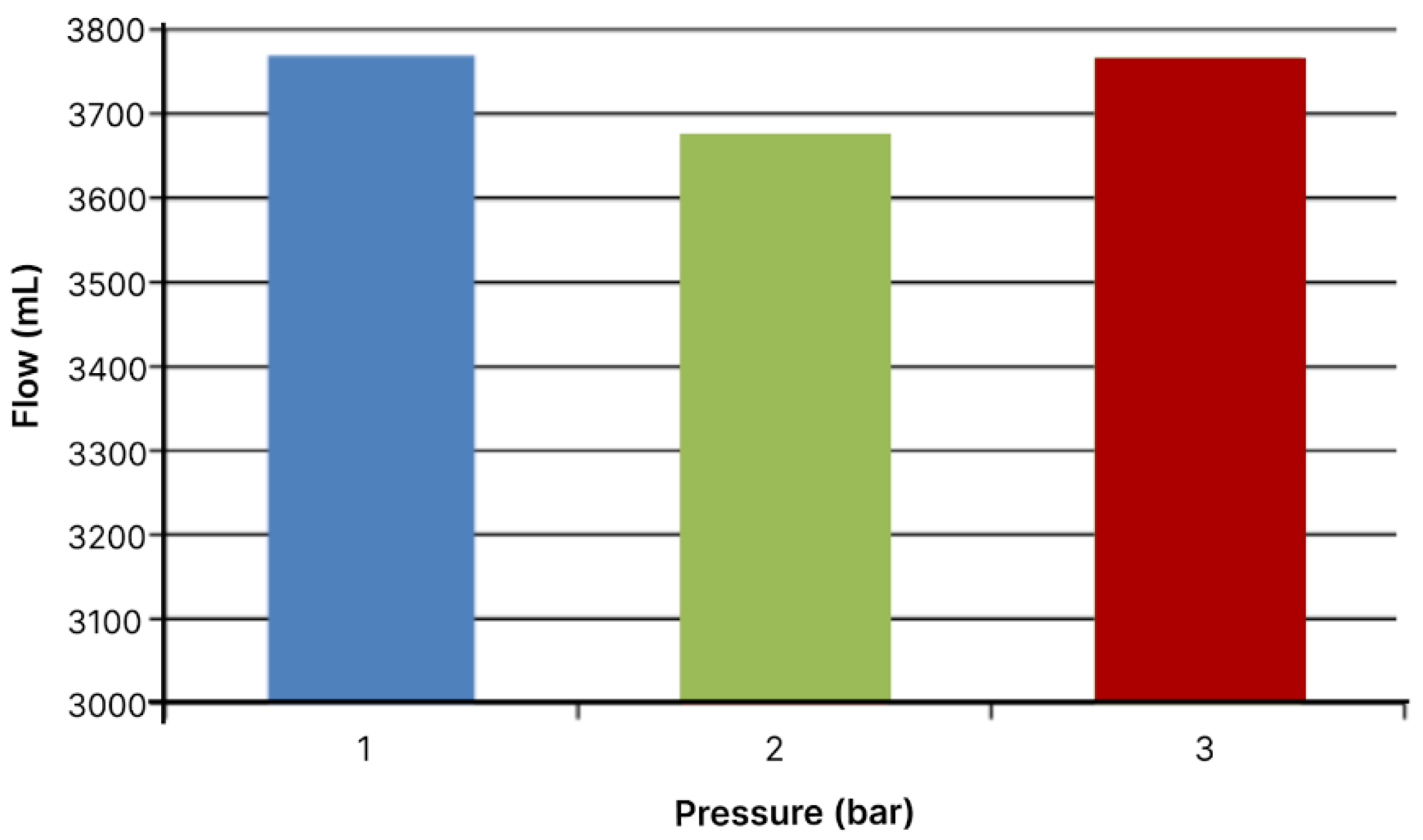
Figure 3.
Total flow volumes through module with area of 19.24 cm2 in 60 minutes.
Figure 3.
Total flow volumes through module with area of 19.24 cm2 in 60 minutes.
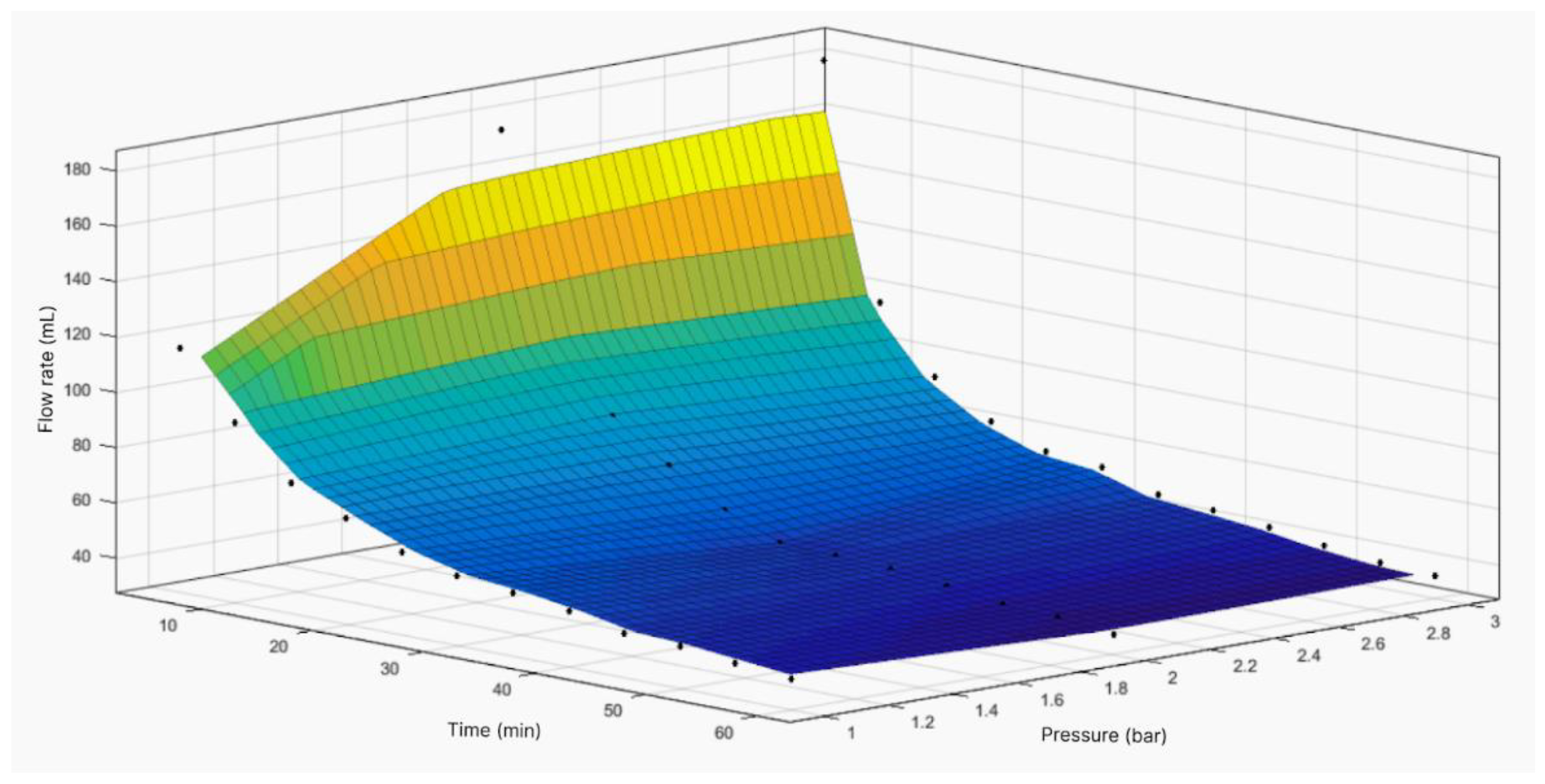
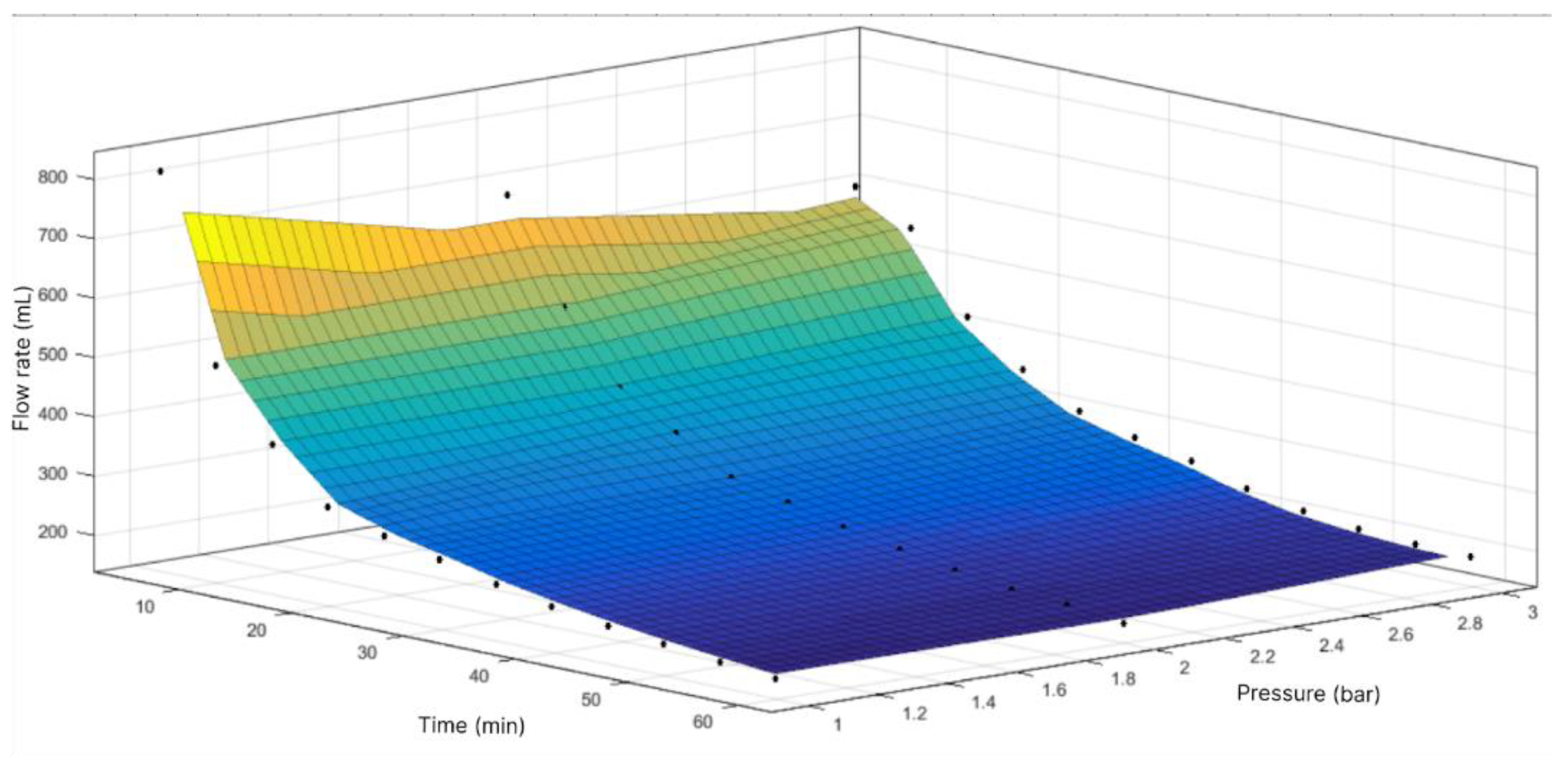
Figure 4.
Response surface of flow volume through filtration module with area of 19.24 cm2 as function of time and pressure.
Figure 4.
Response surface of flow volume through filtration module with area of 19.24 cm2 as function of time and pressure.
Figure 5.
Flow volume through module with area of 76.97 cm2 as function of time at pressures of 1, 2, and 3 bar.
Figure 5.
Flow volume through module with area of 76.97 cm2 as function of time at pressures of 1, 2, and 3 bar.
Figure 6.
Total flow volume through module with area of 76.97 cm2 in 60 minutes at pressure of 1, 2, and 3 bar.
Figure 6.
Total flow volume through module with area of 76.97 cm2 in 60 minutes at pressure of 1, 2, and 3 bar.
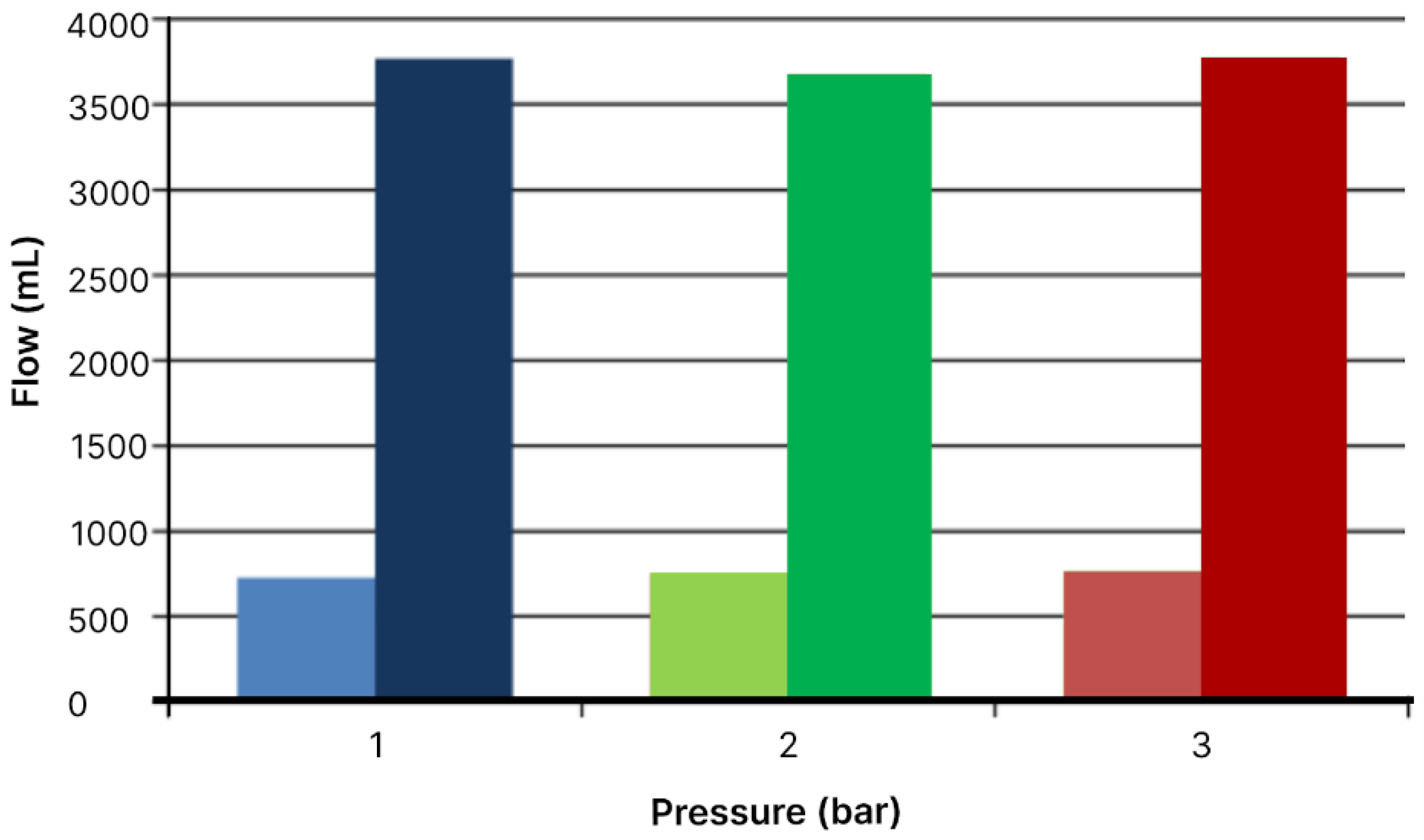
Figure 7.
Comparison of flow volumes at different pressures for the filtration module areas of 19.24 cm2 (left) and 76.97 cm2 (right).
Figure 7.
Comparison of flow volumes at different pressures for the filtration module areas of 19.24 cm2 (left) and 76.97 cm2 (right).
Figure 8.
Response surface of flow volume through module with area of 76.97 cm2 as function of time and pressure.
Figure 8.
Response surface of flow volume through module with area of 76.97 cm2 as function of time and pressure.
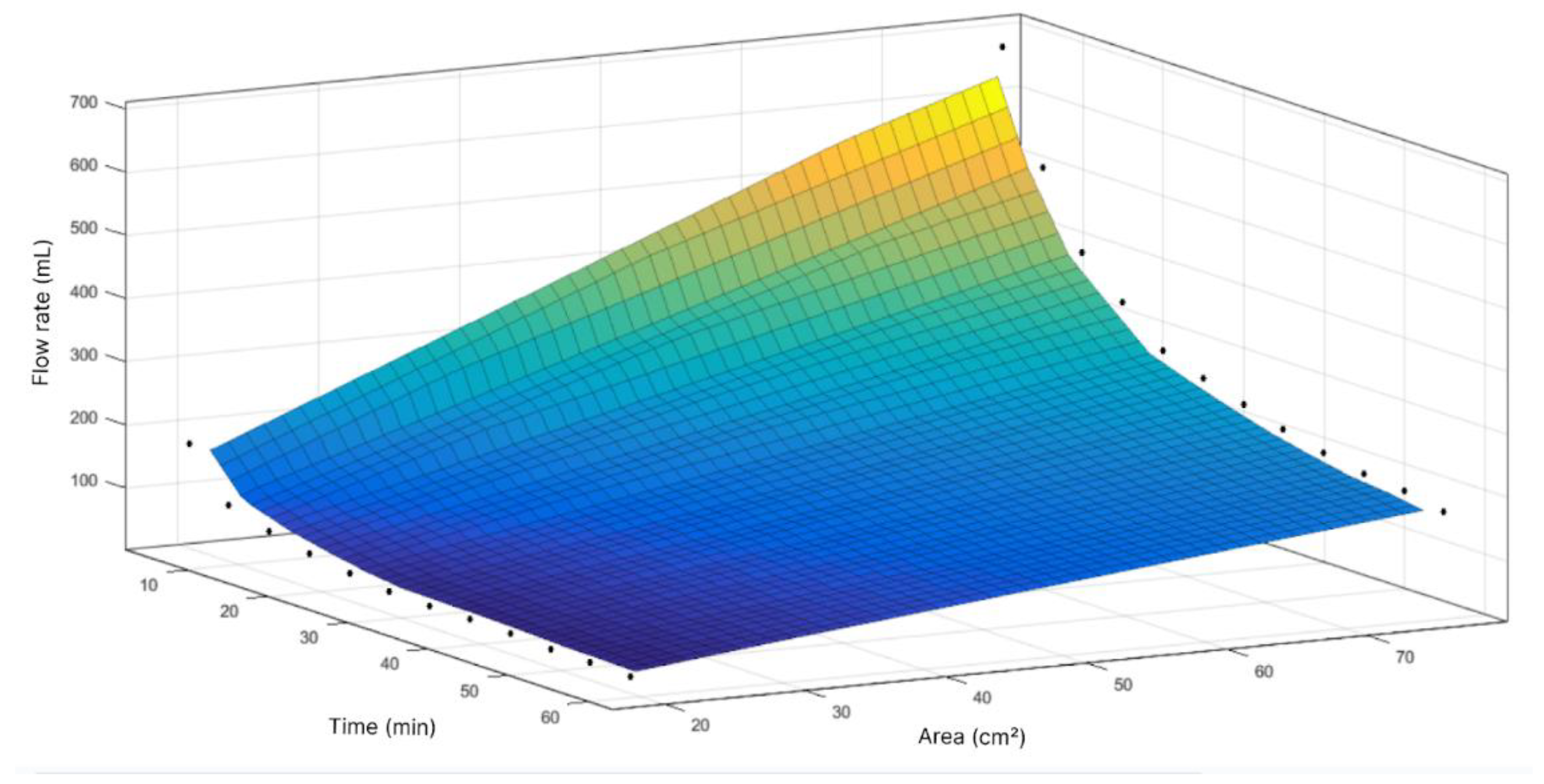
Figure 9.
Response surface of flow volume as function of time and area.
Figure 9.
Response surface of flow volume as function of time and area.
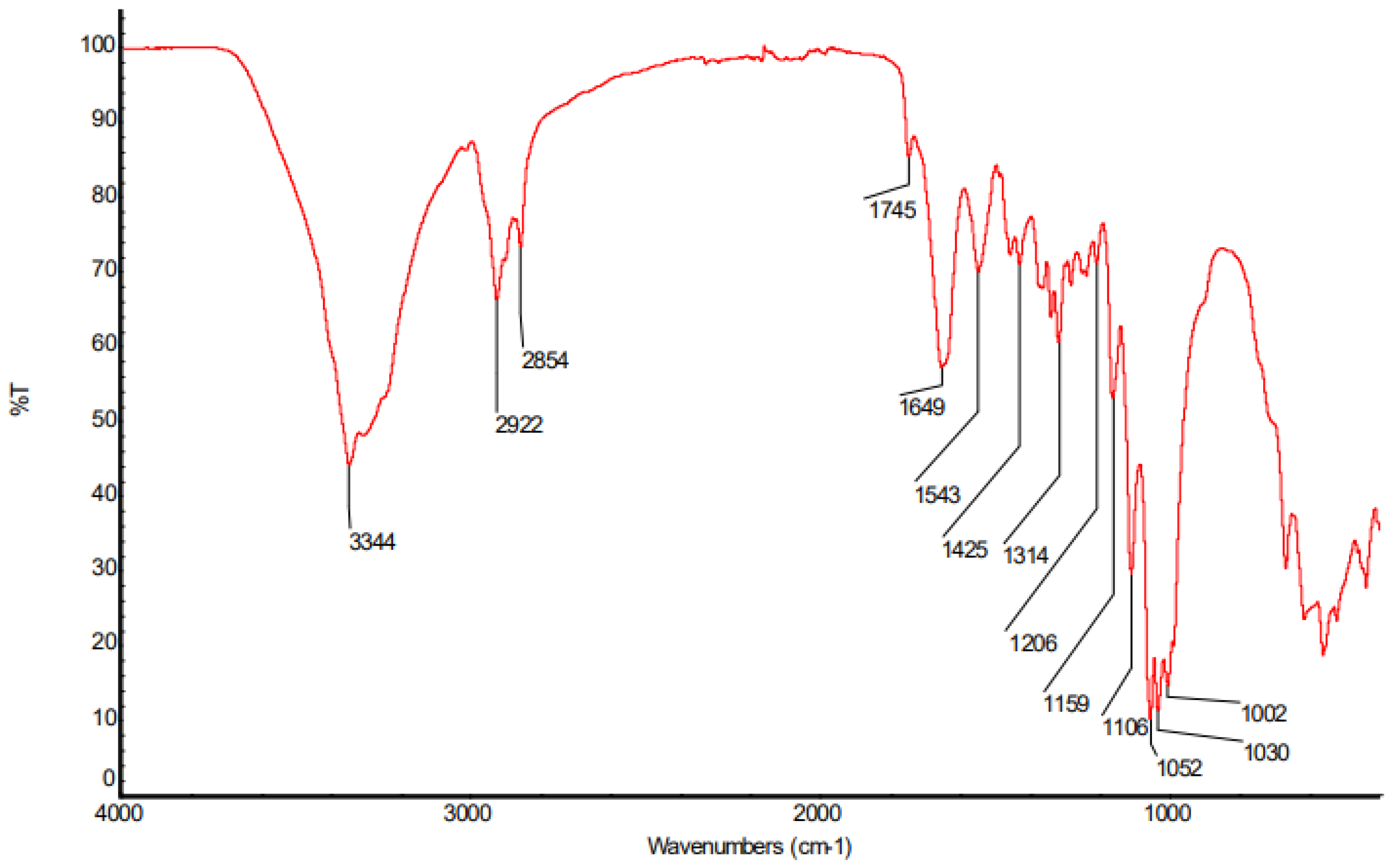
Figure 10.
Infrared spectrum of bacterial cellulose membrane after filtration.
Figure 10.
Infrared spectrum of bacterial cellulose membrane after filtration.
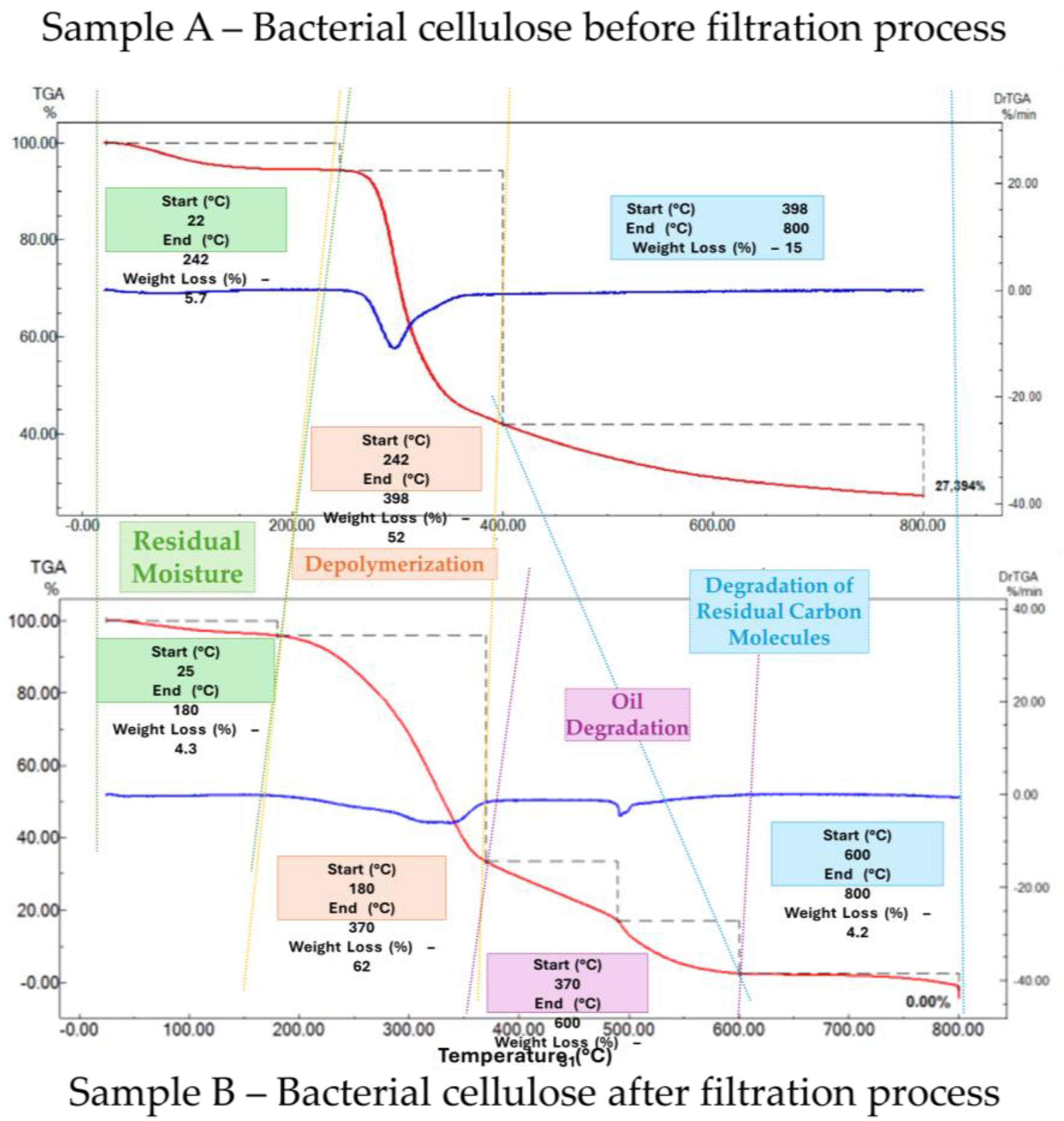
Figure 11.
Results of thermogravimetric analysis of BC samples before and after filtration of wastewater contaminated with oil.
Figure 11.
Results of thermogravimetric analysis of BC samples before and after filtration of wastewater contaminated with oil.
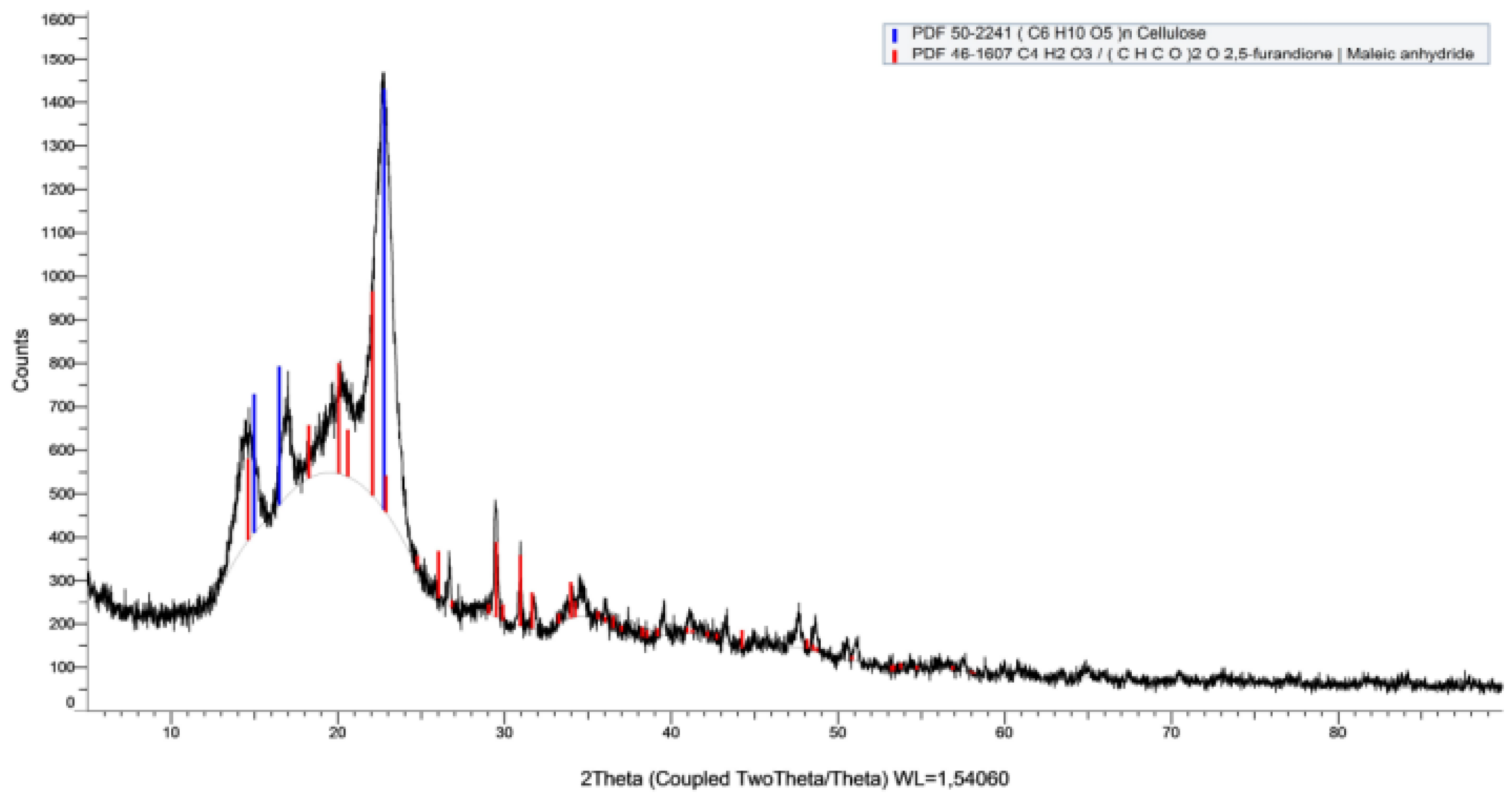
Figure 12.
Diffractogram of bacterial cellulose after filtration.
Figure 12.
Diffractogram of bacterial cellulose after filtration.
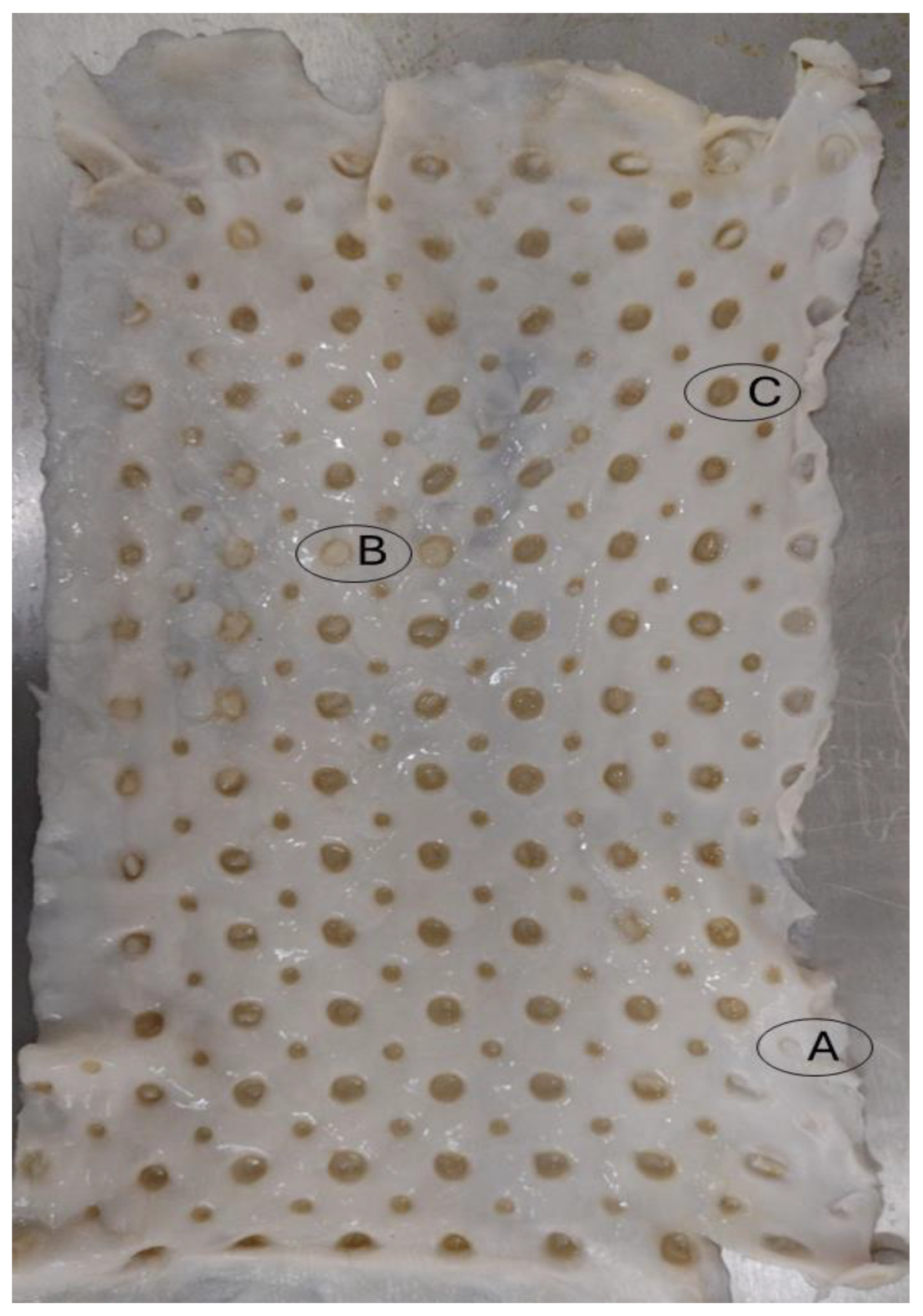
Figure 13.
Bacterial cellulose membrane used in filtration tests with nomenclature of “A”, “B”, to “C” to right of pores analysed.
Figure 13.
Bacterial cellulose membrane used in filtration tests with nomenclature of “A”, “B”, to “C” to right of pores analysed.
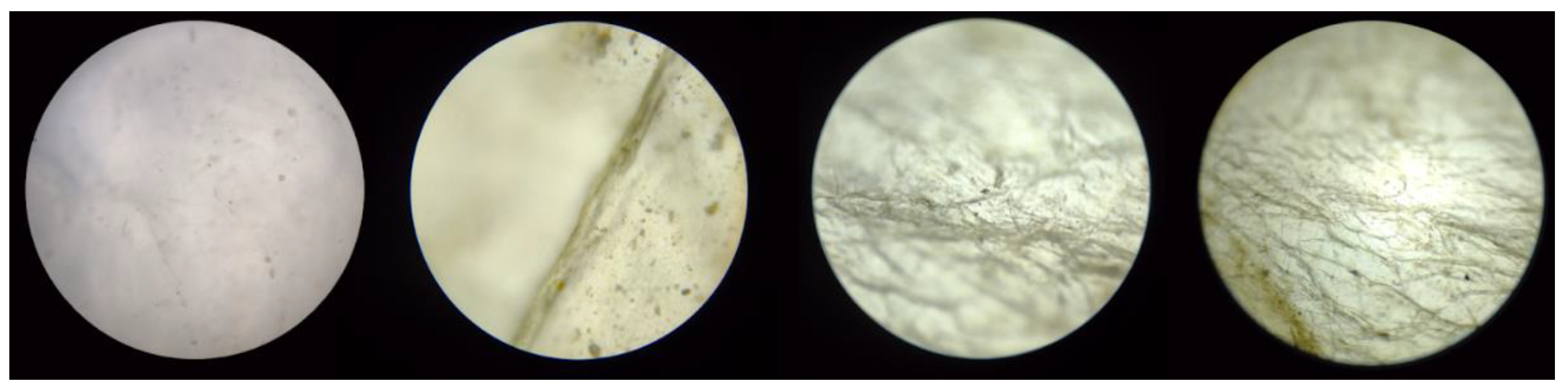
Figure 14.
Optical microscopy of pore A of BC membrane used for filtration (magnification left to right: 4x, 10x, 40x, and 100x).
Figure 14.
Optical microscopy of pore A of BC membrane used for filtration (magnification left to right: 4x, 10x, 40x, and 100x).
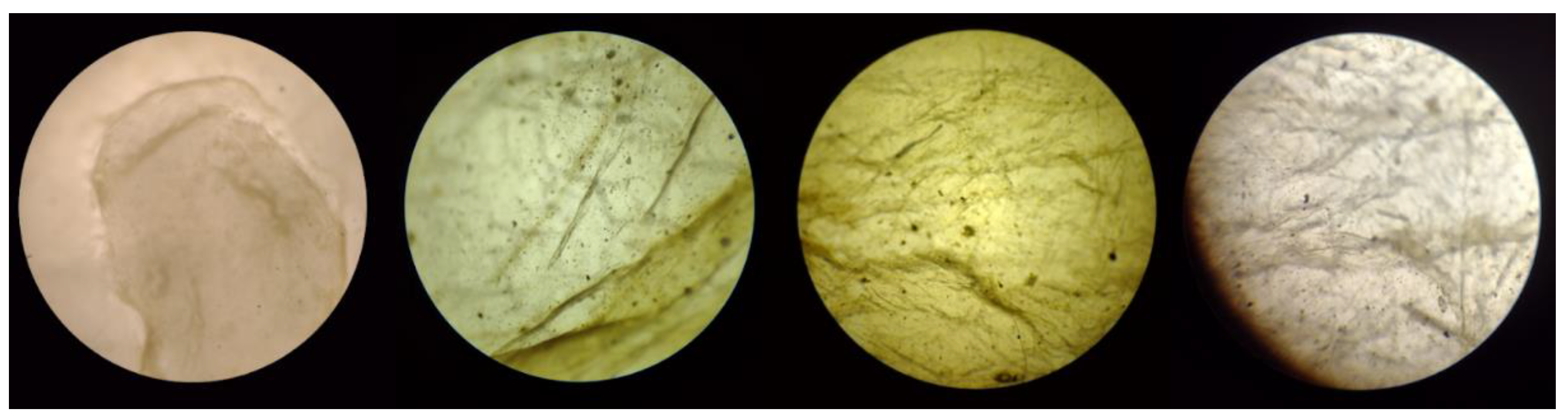
Figure 15.
Optical microscopy of pore B of BC membrane used for filtration (magnification left to right: 4x, 10x, 40x, and 100x).
Figure 15.
Optical microscopy of pore B of BC membrane used for filtration (magnification left to right: 4x, 10x, 40x, and 100x).
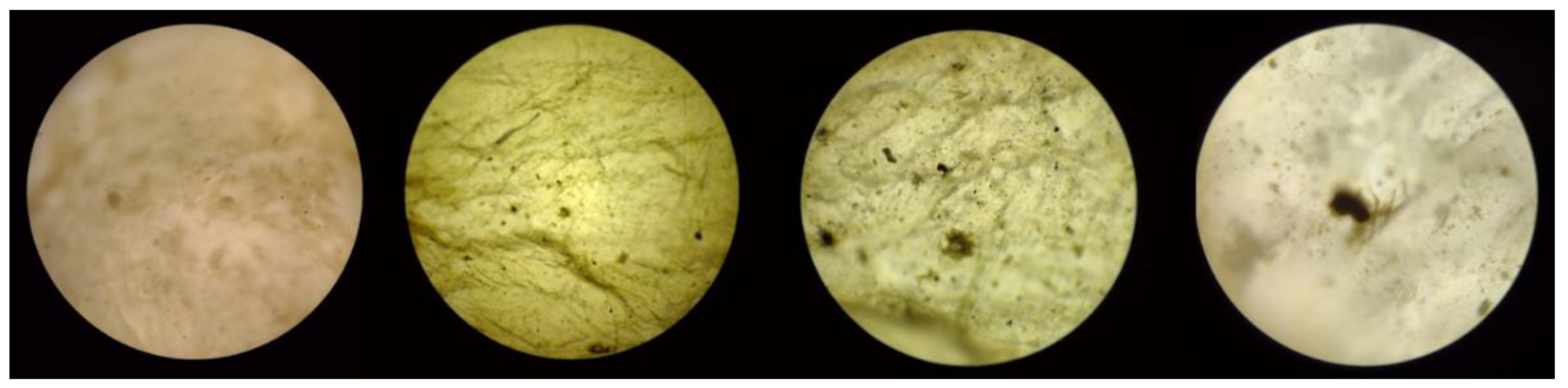
Figure 16.
Optical microscopy of pore C of BC membrane used for filtration (magnification left to right: 4x, 10x, 40x, and 100x).
Figure 16.
Optical microscopy of pore C of BC membrane used for filtration (magnification left to right: 4x, 10x, 40x, and 100x).
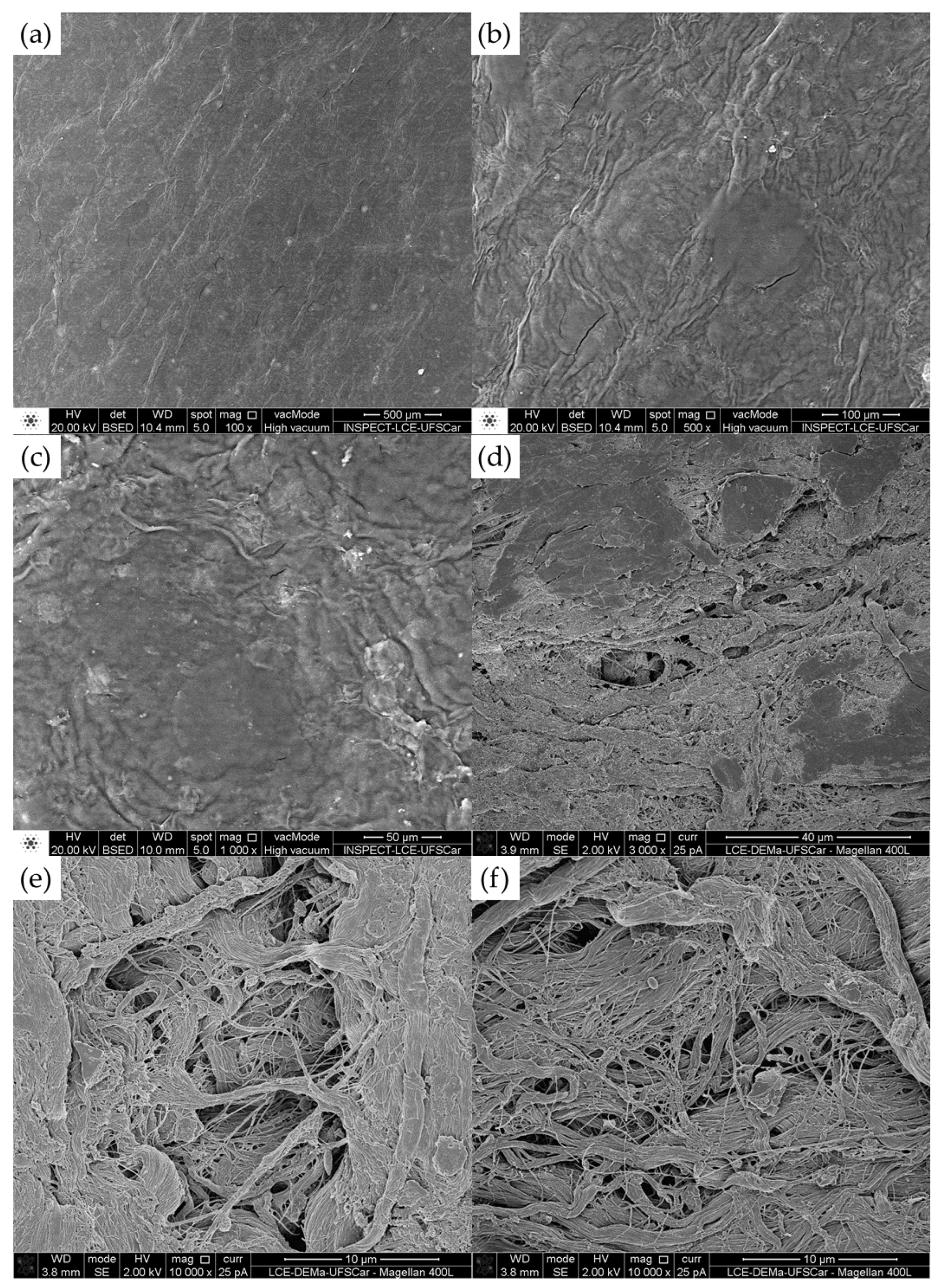
Figure 17.
Microphotographs of bacterial cellulose membrane before filtration process (magnification: 100, 500, 1000, 3000, and 10000 times).
Figure 17.
Microphotographs of bacterial cellulose membrane before filtration process (magnification: 100, 500, 1000, 3000, and 10000 times).
Figure 18.
Microphotographs of bacterial cellulose membrane after filtration process (magnification: 200, 1000, and 5000 times).
Figure 18.
Microphotographs of bacterial cellulose membrane after filtration process (magnification: 200, 1000, and 5000 times).
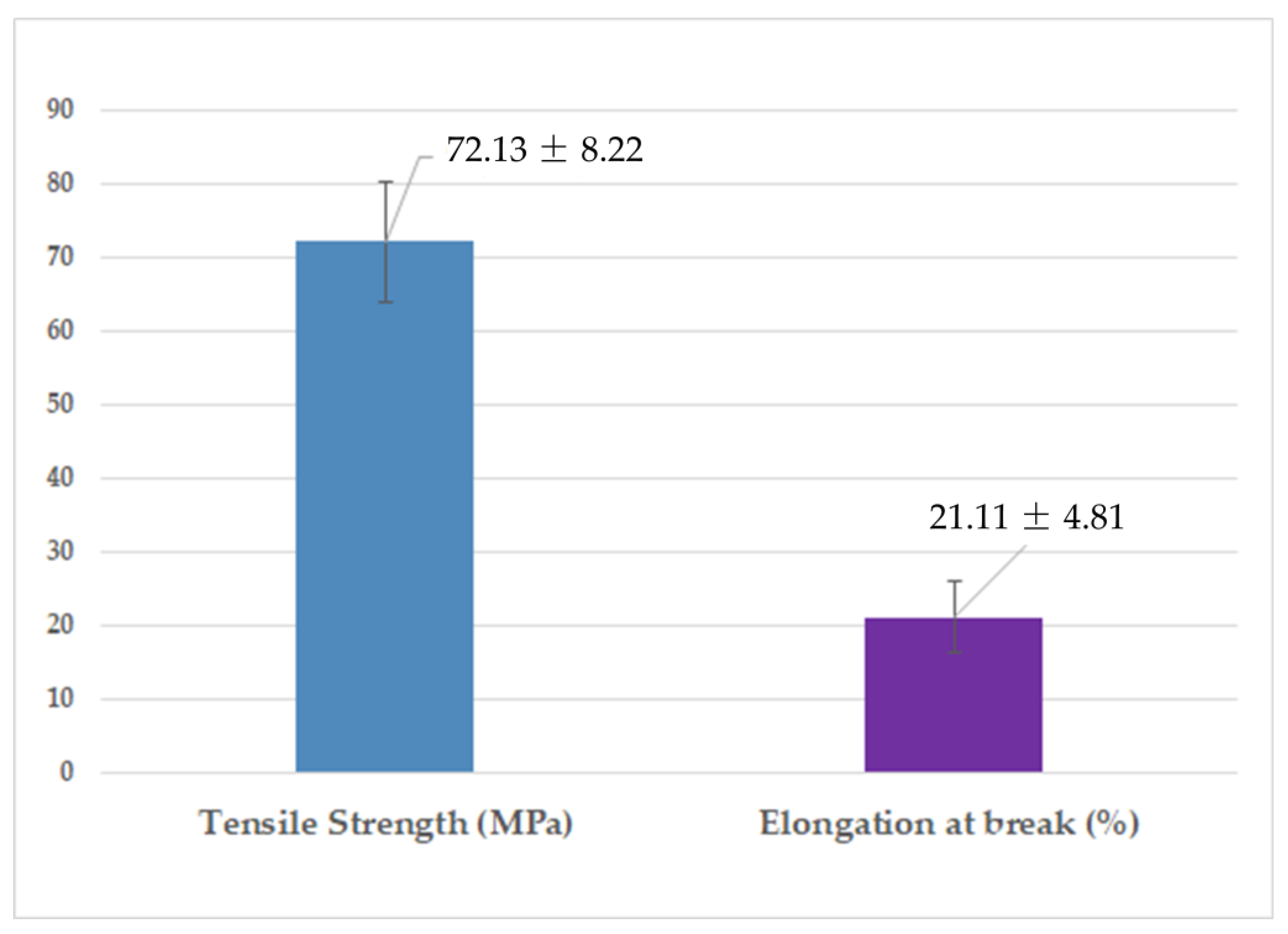
Figure 19.
Results of mechanical tests: Tensile strength (MPa) and elongation at break (%).
Figure 19.
Results of mechanical tests: Tensile strength (MPa) and elongation at break (%).
Figure 20.
Effluent (left to right) after treatment station, BC filtration system and activated charcoal filtering.
Figure 20.
Effluent (left to right) after treatment station, BC filtration system and activated charcoal filtering.
Figure 21.
Synthetic oily effluent before and after filtration with the BC membrane.
Figure 21.
Synthetic oily effluent before and after filtration with the BC membrane.
Table 1.
Yield and water retention capacity of bacterial cellulose membrane.
Table 1.
Yield and water retention capacity of bacterial cellulose membrane.
| Average weight (g/L) |
WRC (%) |
| Wet |
416.91 ± 16.32 |
97.58 ± 0.33 |
| Dry |
10.05 ± 1.46 |
Table 2.
Chemical groups in bacterial cellulose membrane.
Table 2.
Chemical groups in bacterial cellulose membrane.
| Wavenumber (cm-1) |
WRC (%) |
| 3344 |
Axial deformation of OH |
| 2922 |
Asymmetrical axial deformation of CH2 |
| 2854 |
Asymmetrical axial deformation of CH2 |
| 1745 |
Axial deformation of C=O |
| 1649 |
Angular deformation of C-O-H |
| 1543 |
Axial deformation of C=C |
| 1425 |
Angular deformation of CH2 |
| 1314 |
Angular deformation of CH |
| 1206 |
Angular deformation of CH |
| 1159 - 1002 |
Axial deformation of C-O |
Table 3.
Identification of crystalline phases of sample.
Table 3.
Identification of crystalline phases of sample.
| Sample |
Colour |
Reference code |
Chemical formula |
Name of compound |
| AFK239735 |
----- |
46-1607 |
C4H2O3/(CHCO)2O |
Maleic anhydride |
| ----- |
50-2241 |
(C6H10O5)n
|
Cellulose |
Table 4.
EDS results of bacterial cellulose membrane prior to filtration process.
Table 4.
EDS results of bacterial cellulose membrane prior to filtration process.
| lement |
Mass of Elements |
| A |
B |
C |
| C |
53.59 |
66.42 |
51.73 |
| O |
45.72 |
32.34 |
45.91 |
| P |
0.69 |
1.24 |
2.36 |
Table 5.
EDS results of bacterial cellulose membrane filtration process.
Table 5.
EDS results of bacterial cellulose membrane filtration process.
| Element |
Mass (%) |
| A |
B |
C |
D |
E |
F |
| C |
55.00 |
55.35 |
55.93 |
57.75 |
55.95 |
53.17 |
| O |
32.63 |
38.21 |
35.24 |
36.58 |
37.70 |
39.88 |
| Na |
5.02 |
2.99 |
3.77 |
2.28 |
2.78 |
3.13 |
| Mg |
0.87 |
0.30 |
0.88 |
0.43 |
0.30 |
- |
| Si |
0.97 |
0.33 |
0.56 |
- |
- |
- |
| Cl |
5.30 |
2.29 |
2.79 |
2.96 |
3.27 |
3.17 |
| P |
0.21 |
0.16 |
0.27 |
- |
- |
- |
| Ca |
- |
0.37 |
0.58 |
- |
- |
0.65 |
Table 6.
Physicochemical properties of effluent after treatment station, activated charcoal filtering and bacterial cellulose filtration system.
Table 6.
Physicochemical properties of effluent after treatment station, activated charcoal filtering and bacterial cellulose filtration system.
| Variable |
After effluent treatment station |
After activated charcoal filtering |
After BC filtration system |
| pH |
6.0 |
6.0 |
5.5 |
| Dissolved oxygen |
2.3 mg/L |
2.7 mg/L |
0.5 mg/L |
| Total oils and greases |
17.1 mg/L |
<10.0 mg/L |
<10.0 mg/L |
| Turbidity |
5.2 NTU |
26.3 NTU |
5.64 NTU |
| Apparent colour |
283.0 uH |
253.0 uH |
141.0 uH |
| Total hardness |
9200.0 mg/L |
10950.0 mg/L |
1387.0 mg/L |
| BOD5
|
214.0 mg/L |
384.0 mg/L |
<5.00 mg/L |
| COD |
750.0 mg/L |
607.0 mg/L |
<15.00 mg/L |
| Total alkalinity |
277.2 mg/L |
277.2 mg/L |
71.6 mg/L |
Table 7.
Results of microbiological tests of effluent after treatment station, activated charcoal filtering and bacterial cellulose filtration system.
Table 7.
Results of microbiological tests of effluent after treatment station, activated charcoal filtering and bacterial cellulose filtration system.
| Microorganisms |
After effluent treatment station |
After activated charcoal filtering |
After BC filtration system |
| Thermotolerant Coliforms |
28000 MPN/100 mL |
28000 MPN/100 mL |
<1.8 MPN/100 mL |
| Total coliforms |
28000 MPN/100 mL |
28000 MPN/100 mL |
<1.8 MPN/100 mL |
| Escherichia coli |
28000 MPN/100 mL |
28000 MPN/100 mL |
<1.8 MPN/100 mL |