1. Introduction
Chilli peppers (
Capsicum annuum L.) are popular due to their unique taste, aroma, and color [
1]. In addition, the fruits of chilli peppers are rich in a variety of bioactive substances and vitamins, and have certain antioxidant properties [
2,
3], thus being considered the most valuable vegetables. From 2000 to 2010, more than 30% of global chilli pepper cultivation area took place in China, and by 2021, China took up 36.72% cultivation area of chilli pepper of the world [
4].
Capsaicin is the key substance that produces the pungent flavor of chilli peppers. Capsaicin has a variety of functions such as anti-inflammatory [
5], anticancer [
6], pain-relieving [
7], and antioxidant [
8]. Capsaicinoids are a class of alkaloids mainly composed of capsaicinoids, dihydrocapsaicinoids, descending dihydrocapsaicinoids, hypercapsaicinoids, and hyperdihydrocapsaicinoids. Capsaicinoids and dihydrocapsaicinoids account for 80% - 90% of the total amount of capsaicinoids [
9,
10]. The accumulation of capsaicinoids generally begins in the epidermal cells of the placenta, and different parts of the mature fruit such as the placenta, pulp, and seed contain capsaicinoids, with the highest content of capsaicinoids in the placenta [
11]. The metabolic pathway of capsaicin has been better defined, which is mainly formed by the condensation of vanillylamine with fatty acid fragments C9-C11. Phenylalanine is catalyzed by a series of enzymes to derive vanillylamine, and valine is catalyzed by a series of enzymes to derive the branched-chain fatty acids of 8-methyl-6-nonenyl-coenzyme A. The two are catalyzed by capsaicin synthase (CS) to form capsaicinoids [
12], which in turn produces a pungent taste [
13].
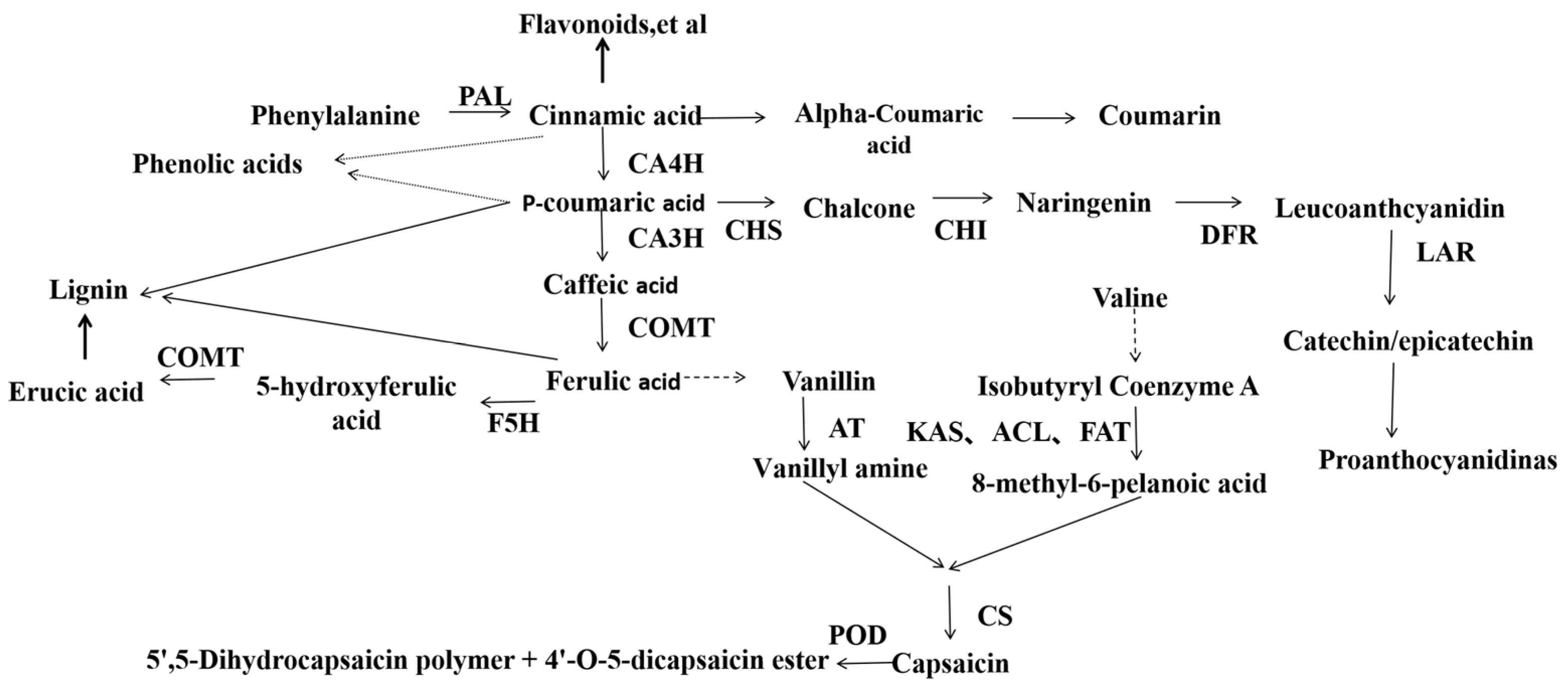
Figure 1.
Capsaicin biosynthetic pathway[
14]
.
Figure 1.
Capsaicin biosynthetic pathway[
14]
.
PAL: Phenylalanine ammonialyase;C4H: Cinnamic acid 4-hydroxylase;C3H:p-coumarate 3-hydroxylase; COMT: Caffeate/5-hydroxyferulate 3-O-methyltransferase;F5H: Ferulate 5-hydroxylase; CHS: Chalcone synthase; CHI: Chalcone isomerase;DFR: Fihydroflavonol 4-reductase; LAR: Leucoanthocyanidin reductase; POD:Peroxidase;AT: Acyltransferase;KAS: β-ketoacyl-ACP synthase;ACL: Acyl carrierprotein; FatA: Acyl-ACP thioesterase.
Phenolic substances in plants are precursors of flavonoids, lignans, tannins, and capsaicinoids. Flavonoids are mainly synthesized through the phenylpropane metabolic pathway, in which phenylalanine deaminase (PAL), cinnamic acid-4-hydroxylase (CA4H), and ligase of 4-coumarate CoA (4CL) are the key enzymes of this pathway, and these enzymes are also key enzymes for capsaicin synthesis. The biosynthetic processes of lignin and tannin are also initiated by phenylalanine. Thus, capsaicin and flavonoid, lignin, and tannin synthesis share common substrates, and to some extent there is comparative relationship of them. It was found that the higher the maturity of chilli fruits, the higher of the capsaicin content, at the same time, the accumulation of flavonoids declined[
15]. Estrada [
16] found that in mature chilli fruits, capsaicin content was somewhat negatively correlated with peroxidase (POD) and lignin content. The study showed an increase in capsaicin content within pepper fruits after fertilization, while the content of phenolic compounds and lignin decreased significantly[
17]. Currently, the capsaicin synthetic genes have been cloned. It was found that the expression of capsaicin synthetic genes ,such as
C4H, COMT, KAS, PAMT, and
AT3, in the placenta of chilli peppers were highly consistent with the capsaicin content[
18].
Nitrogen is the most critical nutrient in plant growth and is the main nutrient that determines crop yield. Excessive or inappropriate use of nitrogen fertilizer not only leads to fertilizer waste but also triggers environmental pollution [
19]. In general, excessive nitrogen causes spindling of the aboveground part, thus delaying maturity of fruits. When the nitrogen is in deficiency, the bottom leaves turn yellow, and the new leaves become smaller.
Chilli peppers are vegetables with long synchronized term of blooming and fruit setting, so pepper has high requirement of nitrogen fertilizer. Aminifard [
20] found that nitrogen application significantly increased the fruits number, yield. The ratio of nitrogen and potassium was found to significantly increase capsaicinoid content in fruits [
21]. Johnson [
22] et al. found that capsaicinoid content in fruits was maximum at a nitrogen fertilization level of 15 mmol.L-1, and that capsaicinoid content declined when nitrogen fertilization concentration was above or below this level. Similar results were obtained in the study of Soares [
23], whose research found that under nitrogen-deficient conditions, capsaicin and dihydrocapsaicin were minimum in the chilli pepper fruits. Tilen [
24] found that capsaicin content was strongly associated with nitrogen content.
Xinjiang is the most important base to produce dried chili peppers in China. Due to poor soil, large amount of nitrogen fertilizer most be applied to ensure plant growth..But the effect of nitrogen fertilizer on the spiciness of chilli peppers is not clear.
This study compared, the capsaicinoid content of the pepper fruit treated with different nitrogen fertilizer amounts. and analyzed the changes of capsaicinoid synthetic precursor substances, competitive substances, enzyme activities, and gene expressions to elucidate the influence of nitrogen fertilizer on pepper fruits spiciness. The study will provide a reference for fertilizer application of high spiciness peppers.
2. Materials and Methods
2.1. Overview of the Test Site
The experimental site was located in the Experimental Station of the College of Agriculture, Shihezi University. The annual sunshine duration was 2721-2818 h, the annual average temperature was 2-15°C, the ≥10°C effective cumulative temperature was 3570-3729°C, and the frost-free period was about 170 d. The soil pH was 7.45, the organic matter content was 11.8 g.kg-1, the alkaline dissolved nitrogen content was 43.7 g.kg-1, rapidly available potassium content was 155 mg.kg-1, rapidly available phosphorus content was 18.9 mg.kg-1.
2.2. Material
The 'Honglong 23' (spiciness 1000~2000SHU) and 'Hongxi' (15000~30000SHU) were hybrid generation of pigment peppers, which were provided by Xinjiang Tianjiao Hong'an Agricultural Science and Technology Limited Liability Company. Nitrogen fertilizer was urea (N≥46%) with potassium dihydrogen phosphate (K2O≥33.9%; P2O5≥51.5%), micronutrient fertilizer was formulated according to Hoagland Nutrient Solution, and the fertilizer was applied by drip irrigation under the mulch.
2.3. Fertilizer Treatment
The experiment was conducted in field, a one-row two-hole manner, the pepper seedlings were transplanted on May 8 with 100 cm row space and 30 cm plant space. After transplantation, the seedlings recovered for 10 days before fertilizer treatment. Five nitrogen fertilizer gradients were set up in the experiment: N1 (urea application 750 kg.hm
-2), N2 (urea 562.5 kg.hm
-2), N3 (urea 375 kg.hm
-2), N4 (urea 187.5 kg.hm
-2), and N0 (urea 0 kg.hm
-2). Fertilizers were applied every 10 days for total of eight times (
Table 1). The fertilizers were mixed with water for drip irrigation, 75% of the total amount of fertilizer was applied at the first five times and 25% of the fertilizer was applied at the last three times. Each row was 12 m long, and the plot area of each treatment was 1.0 m × 12 m = 12 m
2, with three plot replications.
2.4. Indicator Measurement
2.4.1. Determination of Fruit Morphological Indicators
Samples were taken at 20 d, 35 d and 50 d after flowering, each treatment had five plant replications, and the fruits were picked up from top, middle, and bottom of the pepper. The length and diameter of the fruits were measured with vernier calipers, and the fruit shape index was calculated as the fruit length divided by the diameter. The fruit weight and placentas weight were determined with an analytical balance, and then the ratio of the placentas weight to fruit was calculated.
2.4.2. Determination of Capsaicinoids the Precursors and Competitive Substances
Capsaicin contentwas measured with the high-performance liquid chromatographic described in Zhang Haiying [
25] et al. and Li Zhiwei [
26]. Total capsaicin content = (capsaicin content + dihydrocapsaicin content)/90%. Total phenols were determined by the Folin phenol method, flavonoids were determined by the determination of picking aluminum ion colorimetric method; tannins were determined by the Folin-Denis colorimetric method; lignin was determined by the acetylation method using a kit (provided by Suzhou Keming Biotechnology Co., Ltd.).
2.4.3. Determination of Capsaicin Related Enzyme Activity
PAL, POD, and PPO activities: 0.1 g of fruit pulp was ground with liquid nitrogen. The pulp was treated with the kit (provided by Suzhou Keming Biotechnology Co., Ltd.), and the enzyme activities were measured by Microplate reader. The light absorption values of PAL, POD, and PPO were measured at 290 nm, 470 nm, and 525 nm respectively.
2.4.4. qRT-PCR
RNA was extracted from pepper pulp with RNA kit (Xinjiang Kediyuan Biotechnology Co., Ltd.). cDNA was synthesized using HyperScript III RT SuperMix for qPCR with gDNA Remover (EnzyArtisan Biotech Co., Ltd.). cDNA was synthesized using a SYBR Green Real-Time PCR Master Mix kit (EnzyArtisan Biotech Co., Ltd.). Quantitative PCR (qRT-PCR) was performed using a SYBR Green Real-Time PCR Master Mix kit (EnzyArtisan Biotech Co., Ltd.). The relative expression level of genes was calculated using the (2-ΔΔCt) method.
Table 2.
qRT-PCR primer information of capsaicinoids related genes.
Table 2.
qRT-PCR primer information of capsaicinoids related genes.
| Gene Name |
Forward Primer |
Reverse Primer |
| Actin(Internal reference gene) |
CACCCTGTCCTGCTCACTG
|
AAGAATGGCATGCGGCAAAG
|
|
PAL
|
CACAGTTTCAACATTACCCTTAGC
|
AAATGGTGGCAGAGTTTAGGAA
|
|
AT3
|
TTCCCATATAGCCCACTTGC
|
CAGCTCCCATATCGTTACAGTC
|
|
C4H
|
CTTTGGGACGTTTGGTGCAG
|
TCTCCAGAGCCCCTTAACTGA
|
|
4CL
|
CTTCTTCTCAACCATCCCAACA
|
ACGAAATCCTTGACTTCATCCTC
|
|
COMT
|
TAGCACATAACCCAGGAGGC
|
CACAGCACACCTTACGGAATCT
|
|
HCT
|
GTGTGGTGGAGTCTGCTTAGGT
|
GGTCAGTTGGTCGCTTGTGATC
|
|
PAMT
|
ATTGCCGCTGTCCTTGTA
|
CAGTTCCCCTTATCTCCCC
|
2.5. Data Analysis
The data was conducted using Excel 2019, SPSS 26.0 software was used for variance analysis, and Origin 2021 was used for graphing.
3. Results
3.1. Effect of Nitrogen Fertilizer on the Morphological Characteristics of Dried Chilli Pepper Fruit
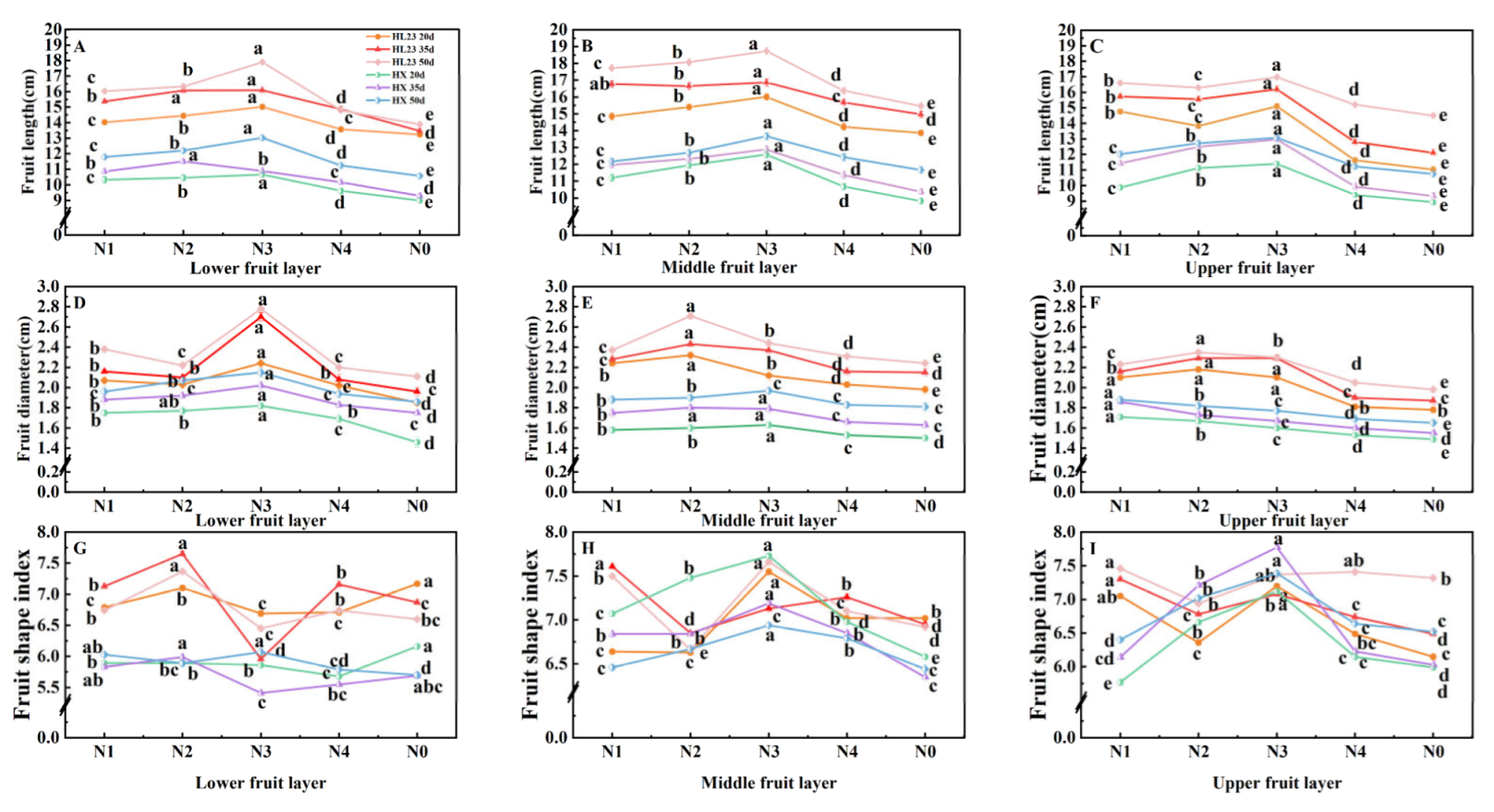
As shown in
Figure 2, the fruit length (
Figure 2ABC) and diameter (
Figure 2DEF) of 'Honglong 23' were larger than those of 'Hongxi'. The length and diameter of the fruits of both varieties reached a maximum values at 50 d after flowering. The length and diameter of the fruit in the middle layer were higher than those of the top and bottom layers; the fruit diameter in the middle layer of 'Honglong 23' was the best, and the diameter in the lower layer of 'Hongxi' was the best. The length and diameter of the fruits of two varieties treated with nitrogen fertilizer were higher than those of N0; except for the middle layer of 'Honglong 23', the length and diameter of the fruits treated with N3 were the highest.It can be infered that within a certain range of nitrogen anount, the length and diameter of pepper fruit increased with the increase of nitrogen application.
As shown in
Figure 2GHI, in the bottom layer, the highest and the lowest fruit shape index were found at N2 and N3 of 'Honglong 23'. The fruit shape index of the bottom layer of 'Hongxi' did not change much with the nitrogen amount. In the middle layer, the highest and the lowest fruit shape index were found at N3 and N2 of 'Honglong 23 and at N4 and N2 of 'Hongxi'.In the upper layer, the maximum and minimum fruit shape index were found at N1 of 'Honglong 23' and at N3, N1 of 'Hongxi' respectively. Overall, appropriate nitrogen reduction could improve the fruit shape index of 'Hongxi', and the fruit shape of 'Honglong 23' showed irregular changes to nitrogen ammounts.
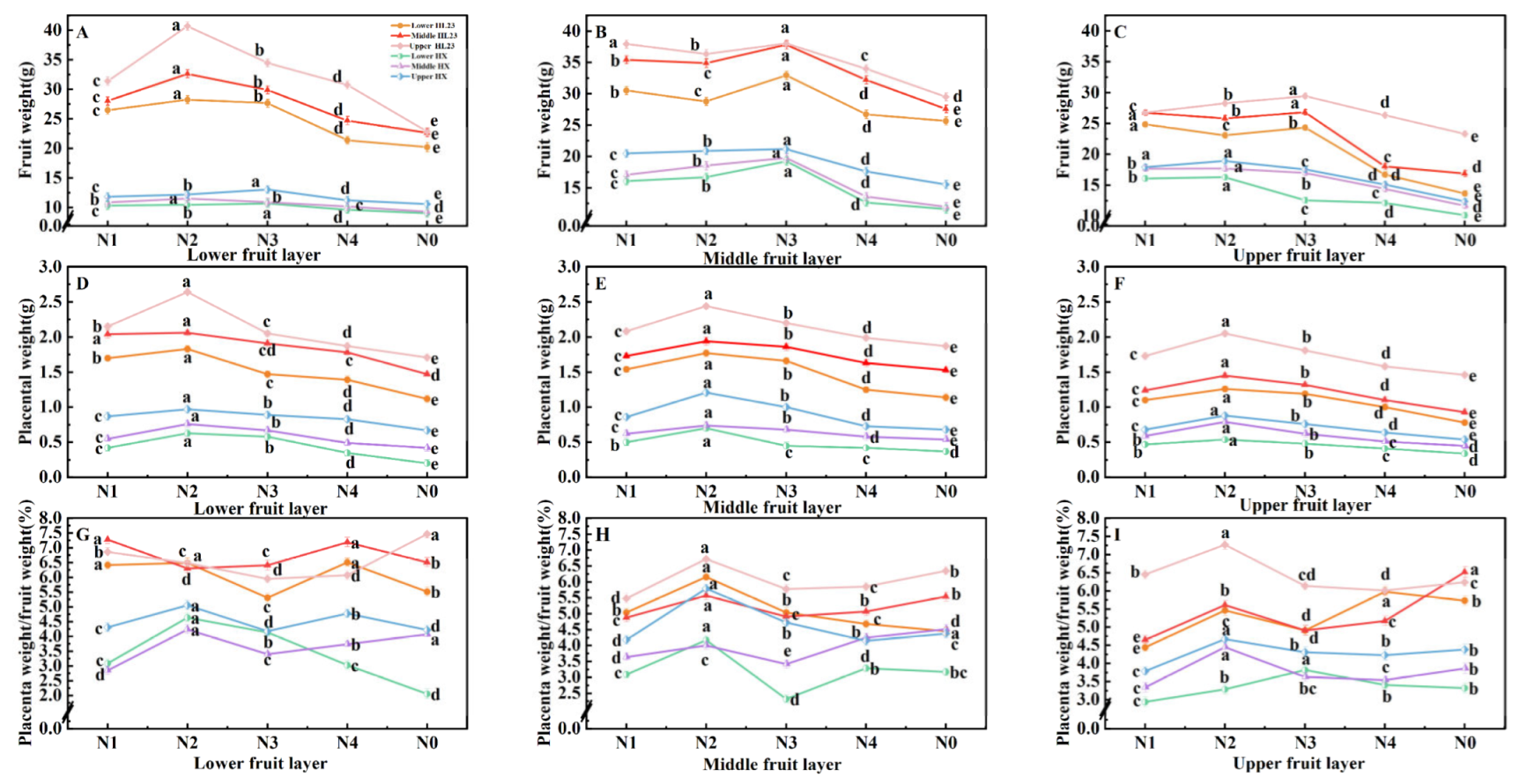
As shown in
Figure 3ABC, 'Honglong 23' exhibited a greater fruit weight compared to 'Hongxi'. Both varieties displayed higher fruit weight in the middle layer, The fruit weight of both peppers treated with nitrogen were greater than that of N0.Fruit weight of the bottom of 'Honglong 23' and the upper of 'Hongxi' were greater under the N2 treatment. The fruit weight of the middle and upper of 'Honglong 23' and the middle of 'Hongxi' were higher under the N3 treatment. Overall, the fruit weight of 'Honglong 23' showed greater sensitivity to nitrogen fertilizer, particularly there was minimal changes of the lower fruits of 'Hongxi' to nitrogen fertilizer concentrations.
As depicted in
Figure 3DEF, 'Honglong 23' had a heavier placenta weight than that of 'Hongxi'. The placenta weight of both varieties increased with fruit growth, and reached its maximum at 50 d after flowering. The middle layer fruit showed higher placenta weight in both varieties. the placenta weight of the treatments with nitrogen fertilizer were greater than those of N0 . Higher placenta weight was founde in the N2 treatment of different layers. Similar to the fruit weight, the placental weight of 'Honglong 23' exhibited greater sensitivity to nitrogen fertilizer ammount than 'Hongxi'.
As depicted in
Figure 3GHI, overall, the ratio of placenta weight to fruit weight of two varieties treated with nitrogen were greater than those of N0; the placenta/fruit of the bottom layer were higher under the N4 and N2 treatments, and lower under the N3 treatment of 'Honglong 23' and 'Hongxi' respectively. In the middle layer, the maxmium values of placenta/fruit were found at the N2, and minimum values were found at the N3. In the upper layer, values of placenta/fruit were higher under the N2 and lower under the N1 treatment.
3.2. Effect of Nitrogen Fertilizer on the Capsaicin Content of Dried Chilli Pepper Fruit
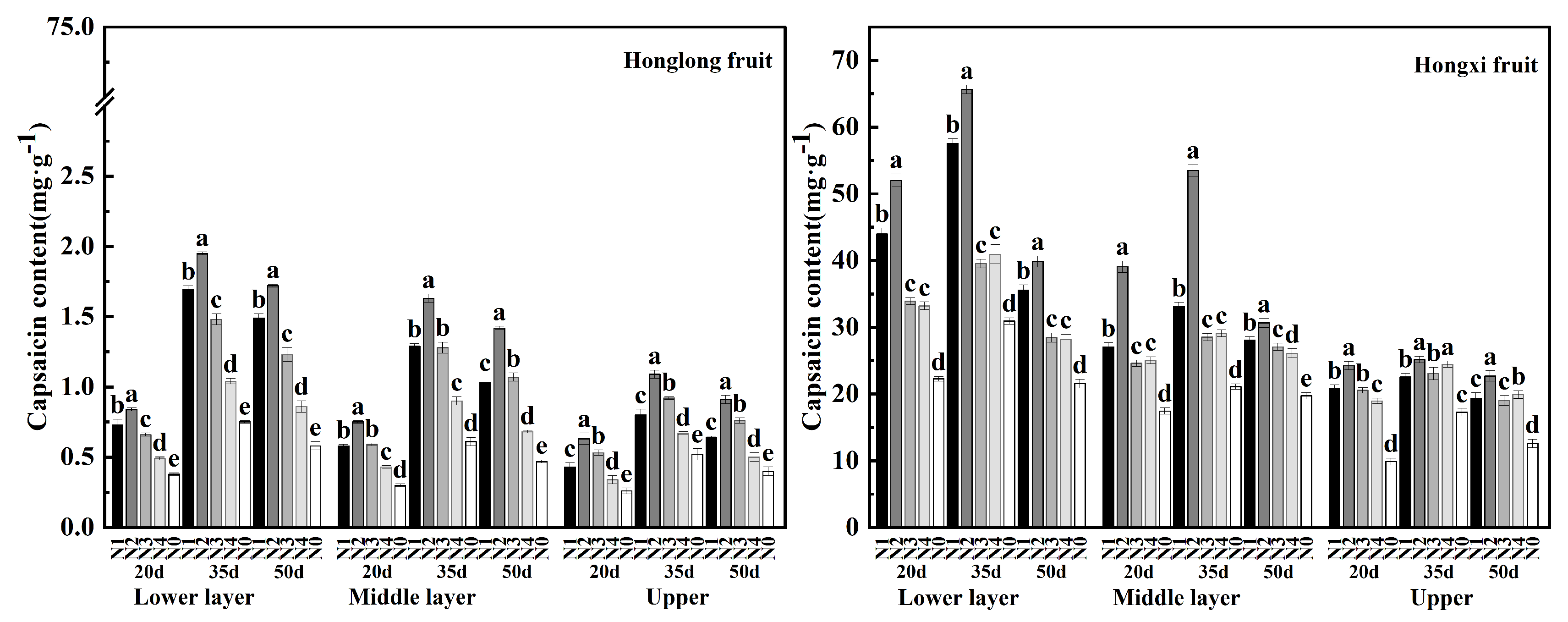
As shown in
Figure 4, the capsaicinoid content of 'Hongxi' fruits was higher than that of 'Honglong 23' , and the capsaicinoid contents of both varieties were ranked as: 35 d > 50 d > 20 d. the capsaicinoid contents of different layers of two varieties were following as: bottom > middle > upper, which indicated that capsaicinoid contents decreased with the fruiting layer increase. Compared with N0, nitrogen application increased capsaicinoid accumulation, and the capsaicinoid contents were higher at N2 in three fruit development stages, followed by the N1 treatment. Within a certain range of nitrogen application (N2, N3, N4, N0), capsaicin accumulation was positively related to the amount of nitrogen fertilizer.
3.3. Effect of Nitrogen Fertilizer on the Precursor Substances of Capsaicin
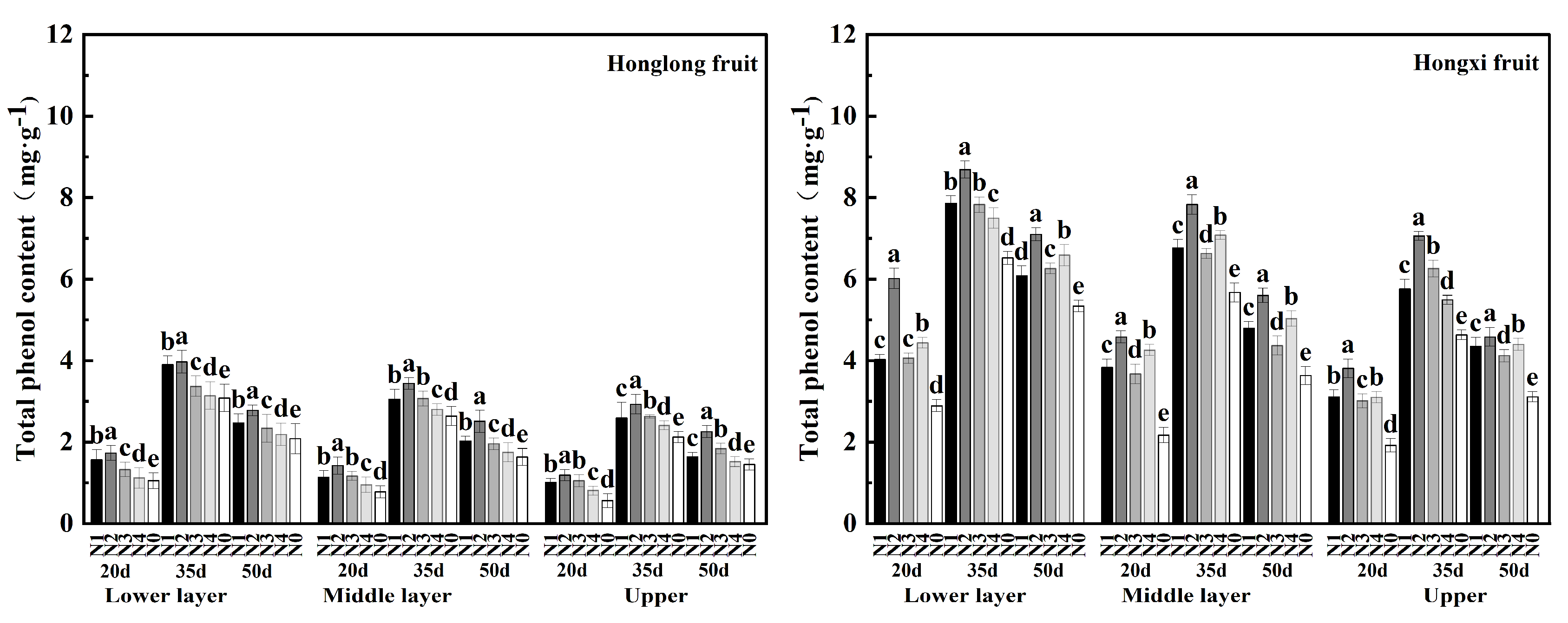
As shown in
Figure 5, the total phenolic content of 'Hongxi' was more than two times higher than that of 'Honglong 23' . The maximum total phenolic contents of both varieties occured at 35 d, followed by 50 d, which was consistent with the variation of capsaicin content. For different layers, total phenolic content showed the following pattern: bottom > middle > upper, which was consistent with the capsaicin contents of different fruit layers. Compared with N0, nitrogen application increased the accumulation of total phenols, and the highest total phenol content at three stages were found in the N2 treatment. The above results indicated that in a certain range, the accumulation of total phenols was positively related with the amount of nitrogen fertilizer.
3.4. Effect of Nitrogen Fertilizer on the Competitive Substances of Capsaicin
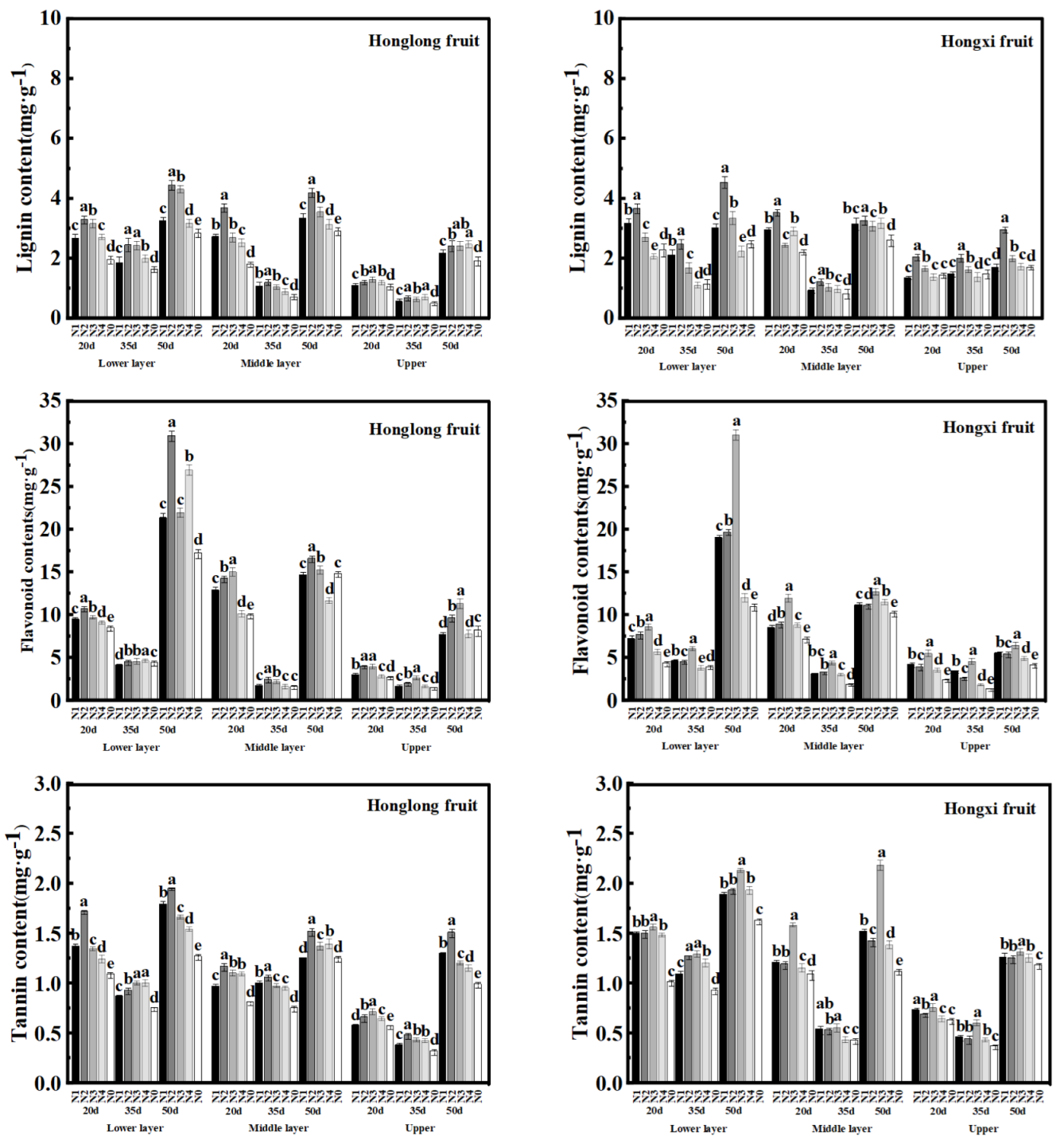
As shown in
Figure 6, the lignin and tannin contents of 'Hongxi' fruits were higher than those of 'Honglong 23' , while the flavonoid contents were lower than those of 'Honglong 23' during the same period. The lignin, flavonoid, and tannin contents of fruits of the two varieties were in the order of 50 d > 20 d > 35 d. During the same period, the contents of the three capsaicinoid competitors in fruits of the two varieties were lower > middle > upper, indicating a decrease in these substances with the upward shift of fruiting sites. Nitrogen application increased the lignin, flavonoid, and tannin contents in fruits compared with N0. The contents of the three competing substances of capsaicin in all layers of fruits at all three periods were highest under the N2 and N3 treatments. Similar to the changes in capsaicinoid content, the content of competitive substances of capsaicinoids in the fruit was positively related to the amount of nitrogen applied within a certain range.
3.5. Effect of Nitrogen Fertilizer on the Activity of Capsaicinoid-Related Enzymes in Dried Chilli Pepper Fruit
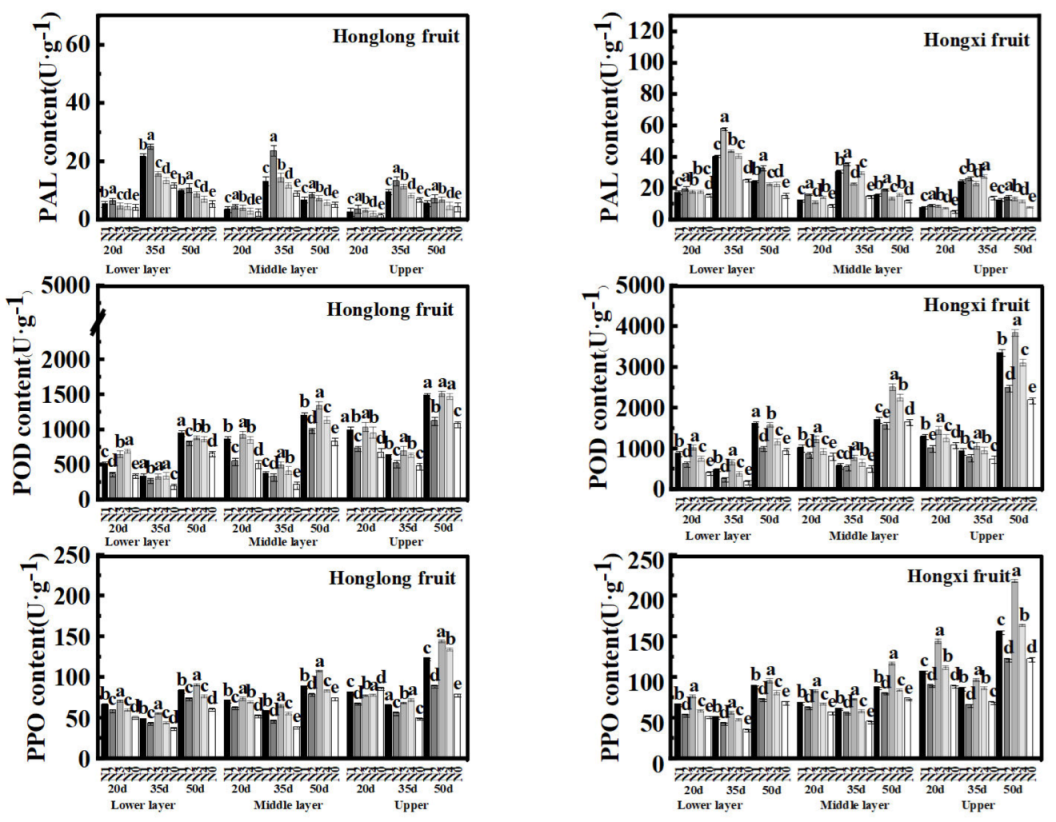
As shown in
Figure 7, the PAL, POD, and PPO enzyme activities of 'Hongxi' were higher than those of 'Honglong 23' . The PAL enzyme activities of both varieties showed the order as: 35 d > 50 d > 20 d; while POD and PPO enzyme activities showed the order as: 50 d > 20 d > 35 d. The PAL enzyme activities of different layers showed as: bottom > middle > upper, while POD and PPO enzyme activities showed as: upper > middle > bottom. Compared with N0, nitrogen application increased PAL, POD, and PPO enzyme activities, and N2 and N3 treatments exhibited higher enzyme activities of PAL, PPO, and POD of two varieties.
3.6. Effect of Nitrogen Fertilizer on the Expression of Capsaicin Synthetic Genes of the Dried Chilli Pepper Fruit
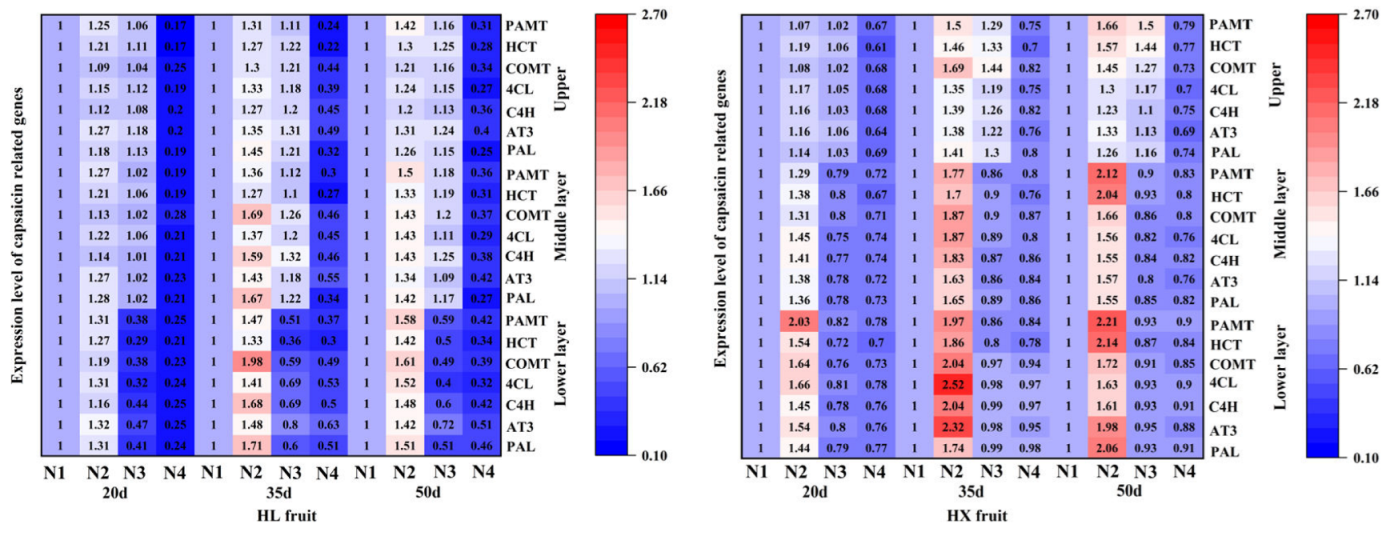
As shown in
Figure 8, the expression of capsaicinoid synthetic genes such as
PAL,
AT3,
C4H,
4CL,
COMT,
HCT, and
PAMT was higher of 'Hongxi' fruits than that of 'Honglong 23', and the expression of capsaicinoid synthetic genes of two varieties rankde as: 35 d > 50 d> 20 d. The expression level of seven capsaicinoid synthetic genes of different layers of two varieties showed the tendency as: bottom > middle > upper,which was consistent with the contents of capsaicin content and capsaicin relatede enzyme acitivity. Compared with N0, nitrogen application significantly up-regulated the expression of capsaicinoid synthetic genes, and N2 showed the highest expression of capsaicinoid synthetic genes of two varieties, for example, the expression of
AT3 genes of N2 of 'Honglong 23' and 'Hongxi' were1.48 and 2.32 times higher than those of the N1 at 35 days. That indicated, in a certain range, the expression of capsaicin synthetic genes was positively related with the amount of nitrogen fertilizer. Overall, 7 capsaicin synthetic genes of 'Hongxi' fruits were more sensitive to nitrogen fertilizer than those of 'Honglong 23'.
4. Discussion
4.1. Effect of Nitrogen Fertilizer on the Development of Pepper Fruits
Nitrogen is a key nutrient for plant growth and development. Nitrogen application can change the distribution and accumulation of nitrogen compounds in different organs of the plant. Studies of cotton have shown that reduction of nitrogen fertilizer properly increased the assimilates allocation in the fruit, and then increased the fruit production[
27]. It was reported that with the increase of nitrogen amount, the fruit weight of eggplant increased first and then decreased[
28]. Studies on cantaloupe melon surposed that nitrogen application favored the fruit weight, fruit length, fruit diameter, and fruit shape index [
29]. Studies on tomato showed that nitrogen application at 0.2 g/plant had a better promoting effect on fruit number, fruit dry weight soluble sugar, Vc and soluble solids content as compared to 0.05 g/plant [
30] . The above studies are in agreement with the performance of our study.
Studies on tomatoes indicated that compared to N0, the average fruit number were increased by 13.94%, 10.38%, and 10.68%, and yield were increased by 13.63%, 10.66%, and 8.42% in the N1 (150 kg.hm-2), N2 (300 kg.hm-2), and N3 (450 kg.hm-2) treatments, respectively. In this study, the fruit growth parameters treated with nitrogen were improved with varying degrees [
31]
. However, in this study, the growth index of chilli pepper fruits gradually decreased when fruit position increased from bottom to top. There were two reasons for this, one was the supplication of nitrogen fertilizer exceeded the requirement of the pepper, which broken the enzyme system and inhibited fruit growth [
32]
. Another reason was excessive nitrogen fertilizer caused fruit abscission
and reduced the fruit number [
33]
. Wu Yue [
34]
found that with the increase of fruit layers, the fruit weight of pepper gradually decreased, which was consistent with the results of this study. This may be related to the fact that fruits in higher layers were smaller and less competitive for photosynthetic products [
35]
.
4.2. Effect of Nitrogen Fertilizer on the Activity of Capsaicin Enzymes
Wang et al. reported that with the increase of urea amount, the peak time of capsaicinoids of pepper fruits was delayed , but the peak area was increased [
36]. Fa´tima Medina-Lara et al. [
37] reviewed that the application of nitrogen increased the capsaicinoid content of fruits compared to no nitrogen application. Han [
38] demonstrated that capsaicin and dihydrocapsaicin exhibited an increase first and then decrease with the amount of nitrogen amount. Wang Chunping [
39] found that 60% of nitrogen amount favored the formation of capsaicinoids and dihydrocapsaicinoids, which was consistent with the higher capsaicinoid content of the N2 treatment of this study. Studies mentioned above were consistent with the trend that capsaicin content was lower in N1 treatment and gradually decreased in the range of N2 to N4 in the present study. However, Huang [
40] found that there was linear correlation between,nitrogen amount and capsaicinoid content, and there was a significant interaction between nitrogen, phosphorus, and potassium. V. Pandhair [
2] found that capsaicinoid content of placenta was significantly higher than that of pericarp and seed of red pepper fruit , which was consistent with the results of the capsaicin content of 50 d was higher than that of 20 d in this study. The significantly higher contents of capsaicinoids and dihydrocapsaicinoids of placenta is due to the fact that capsaicinoid synthesis is controlled by a rate-limiting enzyme, this rate-limiting enzyme was first found on the vesicular membrane of the epidermal cells of the placenta, so the placenta is the starting site of capsaicin synthesis, and capsaicin of pulp was the secondary metabolites transported from the placenta[
41]. Bosland [
42] found that the fruits in the second layer of pepper were the hotest, and the spiciness decreased with the fruit position increase, which was in agreement with this study. Chen [
43] found that the spiciness of L-10 increased with the fruit site moveing- up, which differs from the conclusion of this study. This discrepancy may be related to the pepper varieties and the nitrogen fertilizer concentration. The phenomenon of decreased spiciness with upward movement of the fruit site may be closely associated with factors such as fruit number, early stages of fruit development, and relatively low competition for assimilates between stems and fruits. When the plant was in the period of intensive nutrition and reproductive growth, the competition for assimilates between stems, leaves, and fruits increased, which caused a gradual decrease of capsaicinoids of fruits [
44].
POD and PPO are associated with the decomposition of capsaicin [
45]. Chen [
46] reported that capsaicin content was positively correlated with PAL enzyme activity, negatively correlated with POD activity, and insignificantly correlated with PPO enzyme activity. Yang [
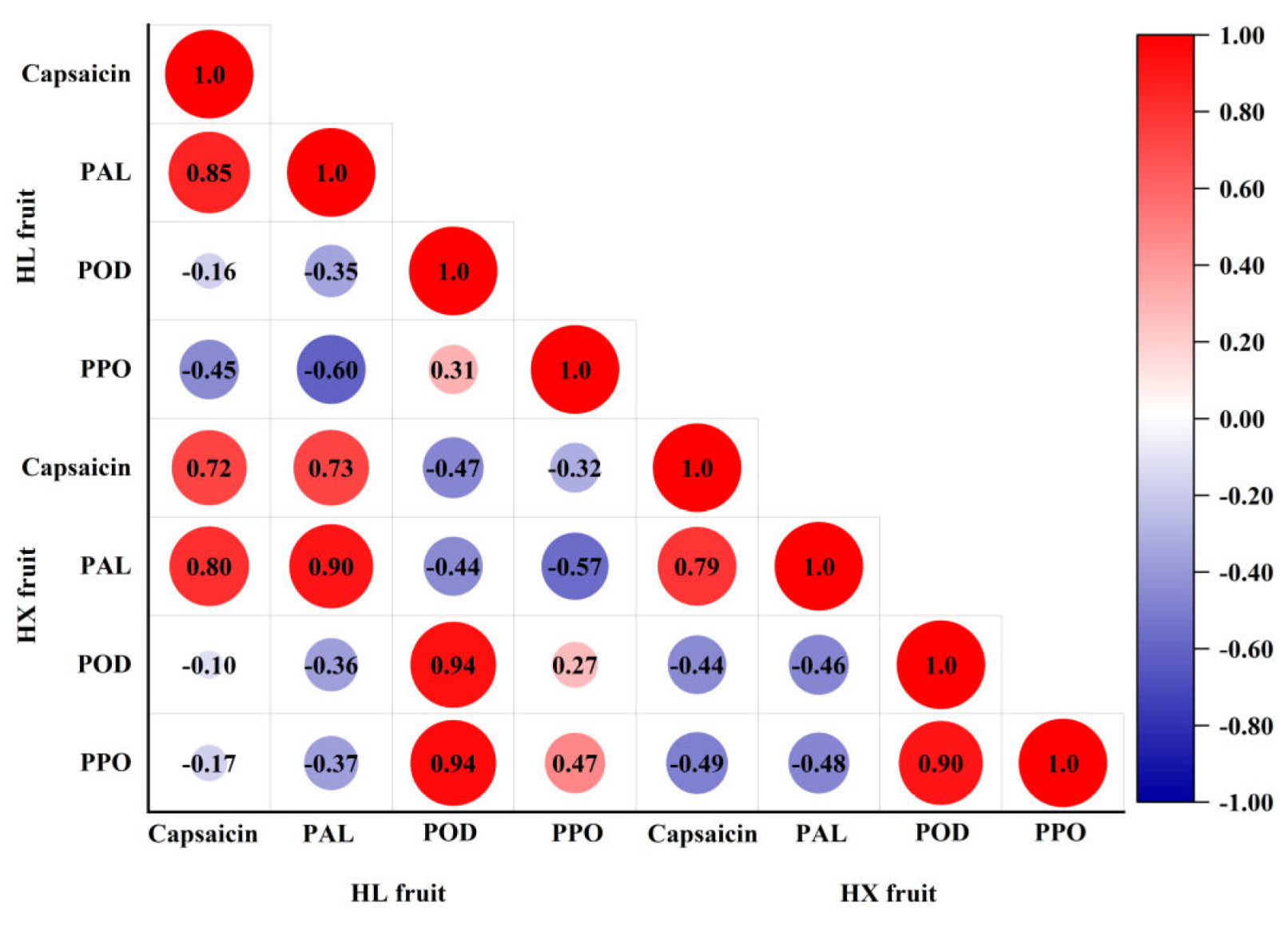
47] demonstrated that PAL enzyme activity was positively correlated with capsaicin content, while POD was negatively correlated with capsaicin. As illustrated in
Figure 9, capsaicin content was positively correlated with PAL enzyme activity and negatively correlated with POD and PPO enzyme activities in this study, which was consistent with previous researches. In this study, the capsaicin content of 'Honglong 23'fruits was significantly lower than that of 'Hongxi', possibly due to differences in capsaicin synthesis and degradation enzyme activities of 'Hongxi'. Furthermore, PAL enzyme activity was lower in 'Honglong 23' fruits and higher in 'Hongxi' fruits; PAL enzyme activity was lower and POD enzyme activity was higher in upper fruits compared to bottom fruits, resulting in higher capsaicinoid content in bottom fruits than that in upper fruits.
4.3. Effect of Nitrogen Fertilization on Capsaicin Precursors and Competitive Substances
Phenolic is the precursors of capsaicin. Hall and Yeoman[
48] indicated that there were different kinds of precursors of phenolic such as phenylalanine, p-coumaric acid, caffeic acid, and ferulic acid, these were also precursors of tannins, flavonoids, and lignans. Consequently, there is competition between the synthesis of capsaicinoids and tannins, flavonoids, lignans. Mira Elena Ionică[
49] demonstrated that the total phenolic and flavonoid contents increased with the development of chilli pepper fruits, which was different with this study, in whcih capsaicin content was higher at 35 d than that of 50 d, it might be due to different varieties [
49]. Chen [
50] discovered that high capsaicin content was found at 40 d after flowering, at the same time, contents of lignin and flavonoid were low; and when lignin and flavonoid content were high, capsaicin content would be reduced accordingly, there was an adverse variation of capsaicin content and its competitors, which consistent with the present study. In this study, more intermediates metabolite were allocated to the synthesis of flavonoids, lignin and tannin of fruit at 50 d after flowering, which resulted a decrease of capsaicin content. In this study, the total phenol content of 'Hongxi' was higher than that of'Honglong 23', it meant there was much more precursors to synthesis capsaicinoids of 'Hongxi', which contributedto the higher spiciness of 'Hongxi'. Additionally, with the fruit layer increaseing, the total phenol content decreased, and the precursor substance of capsaicin decreased, consequently,there was lower capsaicin content of upper layer fruits It has been demonstrated that the accumulation of nitrogen-free secondary metabolites such as terpenes and phenol increased when nitrogen fertilizer was in deficient, but when there was sufficient nitrogen fertilizer, the synthesis of nitrogen-containing secondary metabolites increased [
51].
4.4. Effect of Nitrogen Fertilizer on Capsaicin-Related Genes
A previous study found that the expressions of capsaicin-related genes such as
PAL, AT3, 4CL, C4H, COMT, PAMT, and
HCT were higher in the placenta than fruit pulp [
52]. In this study, the expression of capsaicin-related genes differed according to the fruit development. Li [
53] found that the expressions of
PAL, AT3, 4CL and
HCT increased first and then decreased with fruit development, which was consistent with our study, and the expressions of
COMT and
PAMT decreased continously. The expression of capsaicin-related genes varied in different varieties. Sarpras [
54] demonstrated that the expression of capsaicin synthetic genes such as
PAL, C4H, COMT, AMT, and
ACL differed in different spice pepper varieties, and the expression of these genes were higher of spice varieties, which was consistent with our finding that the 'Hongxi' had a higher expressions of capsaicin synthetic genes than that of the 'Honglong 23'. Compared with N1, the expression of capsaicin synthetic genes was elevated to different degrees after the reduction of nitrogen fertilization [
37,
55]. It was reported that nitrogen had a promoting effect on capsaicin accumulation.
5. Conclusions
Appropriate reduction of nitrogen has promoting effects on chilli peppers yield and quality, 25% to 50% reduction of nitrogen fertilizer leads to the maximum values of fruit weight, fruit diameter, fruit length, and placenta weight. Capsaicin accumulation exhibits a pattern of 35 d > 50 d > 20 d and bottom layer > middle layer > uppper layer of both varieties, competitive substances such as lignin, flavonoids, tannins, and the degradative enzymes POD and PPO increase with the increasing of fruit layers, while total phenol, PAL enzyme activity, and capsaicin synthetic gene expression decrease with the increase of fruit layers.With N2 treatment, there were peaks of the phenol content, PAL enzyme activity and expression of capsaicinoid synthetic genes,and vallyes of competitive substances. Considering yield and capsaicin content, the study infers that N2 (562.5 kg.hm-2) is an appropriate nitrogen fertilizer concentration for cultivating high spiciness peppers.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. Data Curation and Writing—Original Draft Preparation,Z.C.F;software ,S.L.F;Writing—Reviewing and Editing,J.X.H;Data Curation and software,Y.S.S,C.T;writing—review,L.M.L,Z.S.S;All authors have read and agreed to the published version of the manuscript.
Funding
National Natural Science Foundation of China (NSFC)(32060676,31860548).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Diana Penagos-Calvete.;Jonathan Guauque-Medina.;María Francisca Villegas-Torres.;Guillermo Montoya. Analysis of triacylglycerides, carotenoids and capsaicinoids as disposable molecules from Capsicum agroindustry[J]. Horticulture, Environment, and Biotechnology,2019,60(2). [CrossRef]
- Varindra Pandhair,S.;Sharma. Accumulation of Capsaicin in Seed, Pericarp and Placenta of Capsicum annuum L Fruit[J]. Journal of Plant Biochemistry and Biotechnology,2008,17(1). [CrossRef]
- R. Ananthan.;K. Subhash.;T. Longvah. Capsaicinoids, amino acid and fatty acid profiles in different fruit components of the world hottest Naga king chilli ( Capsicum chinense Jacq)[J]. Food Chemistry,2018,238. [CrossRef]
- Qiu Haifeng. China's annual production of chili peppers ranks first in the world[N]. China Food Safety News,2023-10-10(A01).https://x.cnki.net/xmlRead/xml.html?pageType=web&fileName=SPZL20231010A013&tableName=CCNDTOTAL&dbCode=CCND&topic=&fileSourceType=1&taskId=&from=&groupId=&appId=CRSP_BASIC_PSMC&act=&customReading=.
- Thongin Saowarose.;DenUdom Thittaya.;Uppakara Kwanchanok.;Sriwantana Thanaporn.;Sibmooh Nathawut.;Laolob Thanet.;Boonthip Chatchai.;Wichai Uthai.;Muta Kenjiro.;Ketsawatsomkron Pimonrat. Beneficial effects of capsaicin and dihydrocapsaicin on endothelial inflammation, nitric oxide production and antioxidant activity.[J]. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie,2022,154. [CrossRef]
- Min Hee Yang.;Sang Hoon Jung.;Gautam Sethi.;Kwang Seok Ahn. Pleiotropic Pharmacological Actions of Capsazepine, a Synthetic Analogue of Capsaicin, against Various Cancers and Inflammatory Diseases[J]. Molecules,2019,24(5). [CrossRef]
- Sendel Manon.;Dunst Andreas.;Forstenpointner Julia.;Hüllemann Philipp.;Baron Ralf. Capsaicin treatment in neuropathic pain: axon reflex vasodilatation after four weeks correlates with pain reduction[J].Pain,2022. [CrossRef]
- Rezazadeh Aida.;Hamishehkar Hamed.;Ehsani Ali.;Ghasempour Zahra.;Moghaddas Kia Ehsan. Applications of capsaicin in food industry: functionality, utilization and stabilization.[J]. Critical reviews in food science and nutrition,2021,63(19). [CrossRef]
- Stewart Charles.;Kang Byoung-Cheorl.;Liu Kede,Mazourek Michael.;Moore Shanna L.;Yoo Eun Young.;Kim Byung-Dong.;Paran Ilan.;Jahn Molly M. The Pun1 gene for pungency in pepper encodes a putative acyltransferase.[J]. The Plant journal : for cell and molecular biology,2005,42(5). [CrossRef]
- Kozukue Nobuyuki.;Han Jae-Sook.;Kozukue Etsuko.;Lee Sin-Jung.;Kim Joung-Ae.;Lee Kap-Rang.;Levin Carol E.;Friedman Mendel. Analysis of eight capsaicinoids in peppers and pepper-containing foods by high-performance liquid chromatography and liquid chromatography-mass spectrometry.[J]. Journal of agricultural and food chemistry,2005,53(23). [CrossRef]
- VázquezEspinosa Mercedes.;Fayos Oreto.;V. GonzálezdePeredo Ana.;EspadaBellido Estrella.;FerreiroGonzález Marta.;Palma Miguel.;GarcésClaver Ana,F. Barbero Gerardo. Changes in Capsiate Content in Four Chili Pepper Genotypes (Capsicum spp.) at Different Ripening Stages[J]. Agronomy,2020,10(9). [CrossRef]
- Md Aminul Islam.;Shyam Sundar Sharma.;Pratima Sinha.;Madan Singh Negi.;Bijoy Neog.;Shashi Bhushan Tripathi. Variability in capsaicinoid content in different landraces of Capsicum cultivated in north-eastern India[J]. Scientia Horticulturae,2015,183. [CrossRef]
- Mazourek Michael.;Pujar Anuradha.;Borovsky Yelena.;Paran Ilan.;Mueller Lukas.;Jahn Molly M. A dynamic interface for capsaicinoid systems biology.[J]. Plant physiology,2009,150(4). [CrossRef]
- GE Wenjia.; XIN Jianpan.; TIAN Runan. Phenylpropanoid pathway in plants and its role in response to heavy metal stress: a review[J]. Chinese Journal of Biotechnology, 2023, 39(2): 425-445. [CrossRef]
- Estrada Berta.;Bernal María A.;Díaz José,Pomar Federico.;Merino Fuencisla. Capsaicinoids in vegetative organs of Capsicum annuum L. in relation to fruiting.[J]. Journal of agricultural and food chemistry,2002,50(5). [CrossRef]
- Estrada B.;Bernal M A.;Díaz J,Pomar F.;Merino F. Fruit development in Capsicum annuum: changes in capsaicin, lignin, free phenolics, and peroxidase patterns.[J]. Journal of agricultural and food chemistry,2000,48(12). [CrossRef]
- Estrada B.;Bernal MA.;Pomar F.;Merino F. Identification and quantification of some capsaicinoids in P adron pepper (Capsicum annuum L. var. annuum) fruits[J]. Acta Alimentaria : An International Journal of Food Science,2001,30(4). [CrossRef]
- Arce-Rodríguez Magda L.;Ochoa-Alejo Neftalí. An R2R3-MYB Transcription Factor Regulates Capsaicinoid Biosynthesis.[J]. Plant physiology,2017,174(3). [CrossRef]
- ZHANG Ming-ming.;DONG Bao-di.;QIAO Yun-zhou.;SHI Chang-hai.;YANG Hong.;WANG Ya-kai.;LIU Meng-yu. Yield and water use responses of winter wheat to irrigation and nitrogen application in the North China Plain[J]. Journal of Agricultural Science:English version,2018,17(5). [CrossRef]
- M. H. Aminifard.;H. Aroiee.;H. Nemati.;M. Azizi.;M. Khayyat. EFFECT OF NITROGEN FERTILIZER ON VEGETATIVE AND REPRODUCTIVE GROWTH OF PEPPER PLANTS UNDER FIELD CONDITIONS[J]. Journal of Plant Nutrition,2012,35(2). [CrossRef]
- Yinghai Li.;Juncang Tian. Quality of Reclaimed Domestic Water Irrigated Peppers-NPK Couple Model Based on Optimized Combination Technique[J]. Mobile Information Systems,2022,2022. [CrossRef]
- Charles D. Johnson.;Dennis R. Decoteau. Nitrogen and Potassium Fertility Affects Jalapeño Pepper Plant Growth, Pod Yield, and Pungency[J]. HortScience,1996,31(7). [CrossRef]
- da Silva Mírian Peixoto Soares.;Mendonça Freitas Marta Simone.;Pereira José Armando Pires.;dos Santos Paulo Cesar.;de Carvalho Almy Junior Cordeiro.;Vieira Ivo José Curcino.;Rodrigues Rosana. Capsaicinoids and mineral composition of peppers produced under nutrient deficiencies[J]. Journal of Plant Nutrition,2021,44(6). [CrossRef]
- Zamljen Tilen.;Lojen Sonja.;Slatnar Ana.;Zupanc Vesna. Effect of deficit irrigation on nitrogen accumulation and capsaicinoid content in Capsicum plants using the isotope 15N[J]. Agricultural Water Management,2022,260. [CrossRef]
- Zhang Haiying. Effects of Salt Stress and Alkali salt Stress on the Growth and FruitQuality of the Industry Pepper[D].Shihezi University,2019.https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD202001&filename=1019662880.nh.
- Li Zhiwei.;Wang Shengli.Determination of Capsaicin and Dihydrocapsaicin in Capsicum by High Performance Liquid Chromatography[J].Chinese seasonings,2017,42(11):123-126.https://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjMxMjI2Eg56Z3R3cDIwMTcxMTAyNxoIc2xueTNubjc%3D.
- Liu Anda.;Ma Xiaolei.;Zhang Zhao.;Liu Jiahao.;Luo Dan.;Yang Lirong.;Lv Na.;Zhang Yanjun.;Yang Guozheng.;Dong Hezhong. Single dose fertilization at reduced nitrogen rate improves nitrogen utilization without yield reduction in late-planted cotton under a wheat–cotton cropping system[J]. Industrial Crops & Products,2022,176. [CrossRef]
- Mohammad HOSSEIN AMINIFARD.;Hossein AROIEE.;HAMIDE FATEMI,ATEFE AMERI.;SAJEDE KARIMPOUR. RESPONSES OF EGGPLANT (SOLANUM MELONGENA L.) TO DIFFERENT RATES OF NITROGEN UNDER FIELD CONDITIONS[J]. Journal of Central European Agriculture,2011,11(4).https://scholar.cnki.net/new/Detail/index/GARJ2011/SJDJA07955A96529B125E876F4DFD623CECC.
- Vendruscolo Eduardo Pradi.;Campos Luiz Fernandes Cardoso.;Seleguini Alexsander.;de Lima Sebastião Ferreira.;Bortolheiro Fernanda Pacheco de Almeida Prado.;Martins Murilo Battistuzzi.;Seron Cássio de Castro.;de Souza Maria Ingrid. Azospirillum brasilense and Nitrogen Fertilizer Affect the Development and Quality of Cantaloupe Melons[J]. Journal of Plant Growth Regulation,2023,42(9). [CrossRef]
- ZhangYanping. Effects of Water and Nitrogen Coupling on Matter Production and Nitrogen Absorption and Allocation of Tomato[D].Shanxi Agricultural University,2018..https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201901&filename=1019026365.nh.
- Li Huanhuan.;Song Jiawen.;Sun Jingsheng.;Wang Jinglei.;Qiang Xiaoman.;Liu Hao.;Zheng Ming.;LouYujun.Effects of Water and Nitrogen Applications on Yield Components and Nutritional Composition of Greenhouse Tomatoes in Different Trusses[J].Journal of Irrigation and Drainage,2023,42(06):1-9. [CrossRef]
- Krisztina Rios-Gonzalez.;László Erdei.;S.Herman Lips. The activity of antioxidant enzymes in maize and sunflower seedlings as affected by salinity and different nitrogen sources[J]. Plant Science,2002,162(6). [CrossRef]
- WANG Ji-dong.;CAO Yun.;CHANG Zhi-zhou.; ZHANG Yong-chun.; MA Hong-bo. Effects of combined application of biogas slurry with chemical fertilizers on fruit qualities of Prunus persica L. and soil nitrogen accumulation risk[J]. Journal of Plant Nutrition and Fertilizers, 2013, 19(2): 379-386. [CrossRef]
- Yue Wu. Effects of Nitrogen Supply on Pepper Quality and Mechanism[D].Southwest University,2020.https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD202101&filename=1020329028.nh.
- Ozlem Alan.;Benian Eser. Pepper Seed Yield and Quality in Relation to Fruit Position on the Mother Plant[J]. Pakistan Journal of Biological Sciences,2007,10(23).https://webofscience.clarivate.cn/wos/alldb/full-record/MEDLINE:19086580.
- Wang Pei. Study on the coupling and alternating effects between water and nitrogen(N)on yield and quality development of chili pepper(Capsicum annuum L. var.acuminatum Fingern)[D].Shihezi University,2015.https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201601&filename=1015994016.nh.
- Medina-LaraF.;Echevarria-Machado I.;Pacheco-Arjona R.;Ruiz-Lau N.;Guzman-Antonio A.;Martinez-Estevez M. Influence of nitrogen and potassium fertilization on fruiting and capsaicin content in Habanero pepper (Capsicum chinense Jacq.).[J]. HortScience,2008,43(5). [CrossRef]
- Shuang Han.;Xiaoqin Zhu.;Dongmei Liu.;Libo Wang.;Dongli Pei*. Optimisation of the amount of nitrogen enhances quality and yield of pepper[J]. Plant, Soil and Environment,2021,67(11). [CrossRef]
- Wang Chunping.;Zhang Shicai.;Huang Renzhong.;Tang Rongli.;Li Yifei.;Wu Hong.;Jiang Xiaoying.;Yang Xiaomiao.;Lei Kairong.;Huang Qizhong.;Liin Qing .Growth, Yield and Quality Characteristics of Processed Pepper under Different Nitrogen Levels[J].Molecular Plant Breeding,2020,18(04):1379-1384. [CrossRef]
- Huang Ke.;Liu Mingyue.;Cai Yanping.;Wen Qingfang.CORRELETION OF NPK APPLICATION WITH THE QUALITY OF HOT PEPPER[J].Southwest China Journal of Agricultural Sciences,2002(04):349-352+356.https:// doi.org/ 10.3969/j.issn.1673-9868.2002.04.020.
- Hideshi Fujiwake.;Tetsuya Suzuki.;Shinzaburo Oka.;Kazuo Iwai. Enzymatic Formation of Capsaicinoid from Vanillylamine and Iso-type Fatty Acids by Cell-free Extracts of Capsicum annuum var. annuum cv. Karayatsubusa[J]. Agricultural and Biological Chemistry,2014,44(12).https:// doi.org/ 10.1080/00021369.1980.10864424.
- Paul W. Bosland.;Yayeh Zewdie. Pungency of Chile (Capsicum annuum L.) Fruit is Affected by Node Position[J]. HortScience,2000,35(6). [CrossRef]
- Junqin Chen. Studies on capsaicin biosynthetic related substances and polyamines regulatory mechanisms for capsaicin in pepper(Capsicum annuum L.)[D].Shengyang Agricultural University,2015.https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CDFDLAST2016&filename=1016036637.nh.
- Di Yun.;Jiang Jianzhen.;Shi Zhengqiang.Current status of research on the metabolic physiology of capsaicinoids[J].China Vegetables,2000(03):50-52. [CrossRef]
- José Díaz.;Federico Pomar.;Angeles Bernal.;Fuencisla Merino. Peroxidases and the metabolism of capsaicin in Capsicum annuum L.[J]. Phytochemistry reviews: proceedings of the Phytochemical Society of Europe,2004,3(1-2). [CrossRef]
- Chen Junqin.;He Lili.;Zhang Kunpeng.;Liu Lei.Effects of PUT on Capsaicin,Endogenous Polyamines and Relevant Enzymes in Pepper Fruit[J].Journal of Shenyang Aricultural University,2015,46(05):521-525. [CrossRef]
- Yang Sun. Study on the changes of Capsaicinoids and Nutritional Quality and Related Enzymes in Pepper[D].Sichuan Agricultural University,2020. https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD202201&filename=1021681216.nh.
- Hall R D.;Yeoman M M. The influence of intracellular pools of phenylalanine derivatives upon the synthesis of capsaicin by immobilized cell cultures of the chilli pepper, Capsicum frutescens.[J]. Planta,1991,185(1). [CrossRef]
- Mira Elena Ionică.;Violeta Nour.;Ion Trandafir. Bioactive compounds and antioxidant activity of hot pepper fruits at different stages of growth and ripening[J]. Journal of Applied Botany and Food Quality,2017,90. [CrossRef]
- Chen Junqin.;He Lili.;Wang Shujie.Effects of Different Nitrogen Levels on Capsaicin Content and Relevant Substances to Capsaicin Metablic in Hot Pepper Fruit[J].Journal of Shenyang Aricultural University,2013,44(05):645-649. [CrossRef]
- Keski-Saari Sarita.;Julkunen-Tiitto Riitta. Resource allocation in different parts of juvenile mountain birch plants: effect of nitrogen supply on seedling phenolics and growth.[J]. Physiologia plantarum,2003,118(1). [CrossRef]
- Broderick.;C. E.Cooke.; P. H.. Fruit composition, tissues, and localization of antioxidants and capsaicinoids in Capsicum peppers by fluorescence microscopy.[J]. Acta Horticulturae,2009,2009(841).https://d.wanfangdata.com.cn/periodical/Ch9QZXJpb2RpY2FsRU5HTmV3UzIwMjQwNDI2MDEyNzAxEiBlMTcwOTJjZGJhYjRlZDQzM2NkN2E5MTAxYzcxNThmOBoIeW12amF2cm4%3D.
- Li Yanlei. Capsaicin Accumulation and Expression Analysis of Biosynthesis-related Genes during Pepper Fruit Development[D].Jilin University ,2013.https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201302&filename=1013196474.nh.
- Sarpras M.;Rashmi Gaur.;Vineet Sharma.;Sushil Satish Chhapekar.;Jharna Das.;Ajay Kumar.;Satish Kumar Yadava.;Mukesh Nitin.;Vijaya Brahma.;Suresh K Abraham.;Nirala Ramchiary. Comparative Analysis of Fruit Metabolites and Pungency Candidate Genes Expression between Bhut Jolokia and Other Capsicum Species.[J]. PLoS ONE,2017,11(12). [CrossRef]
- Monforte-González Miriam.;Guzmán-Antonio Adolfo.;Uuh-Chim Francisco.;Vázquez-Flota Felipe. Capsaicin accumulation is related to nitrate content in placentas of habanero peppers (Capsicum chinense Jacq.).[J]. Journal of the science of food and agriculture,2010,90(5). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).