Submitted:
11 June 2024
Posted:
11 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
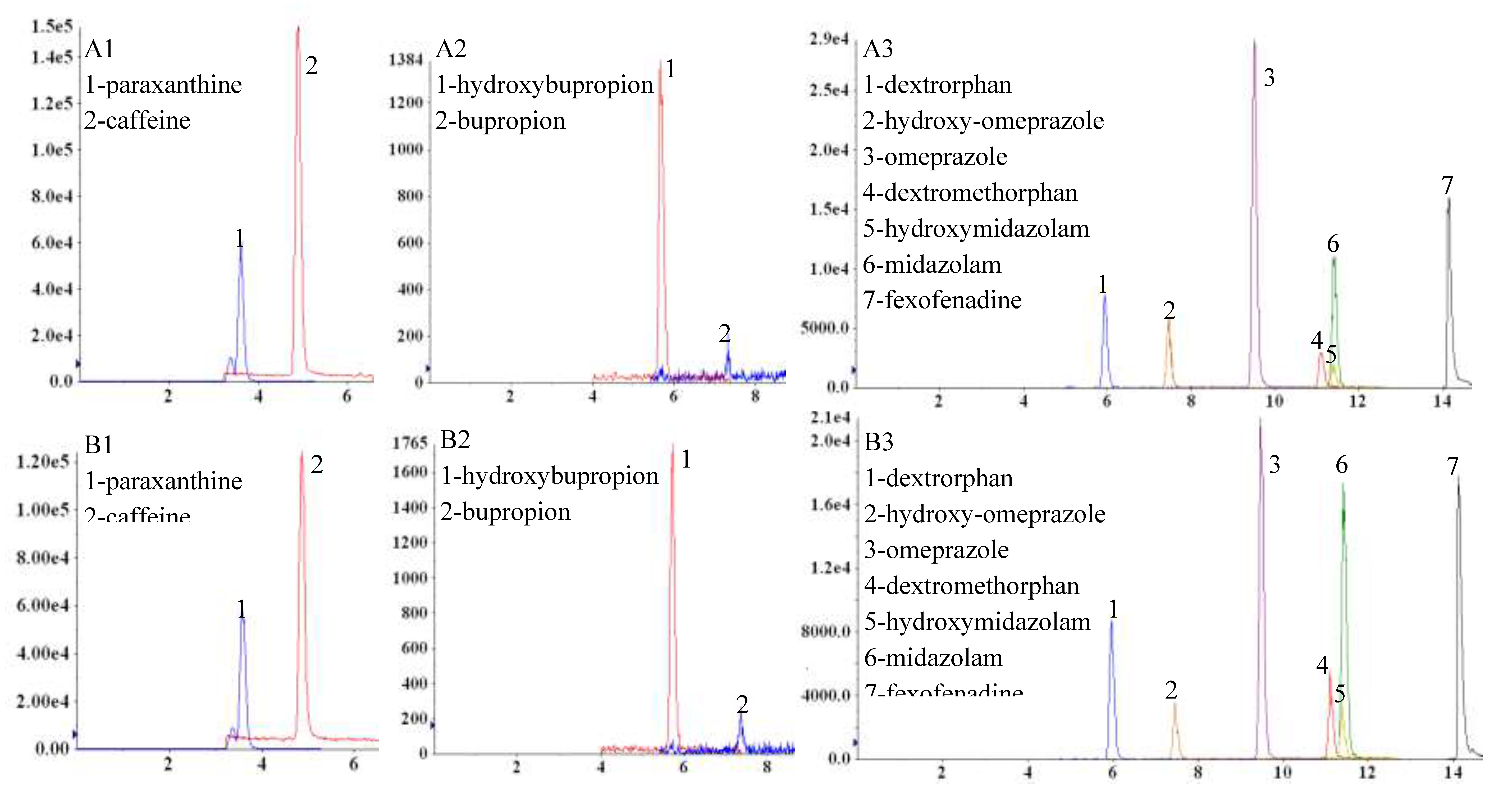
2.1. Analytical Methods
2.1.1. Chemicals and Reagents
2.1.2. Preparation of Stock and Working Standard Solutions
2.1.3. Instrumentation
2.1.4. Mass Spectrometric Conditions
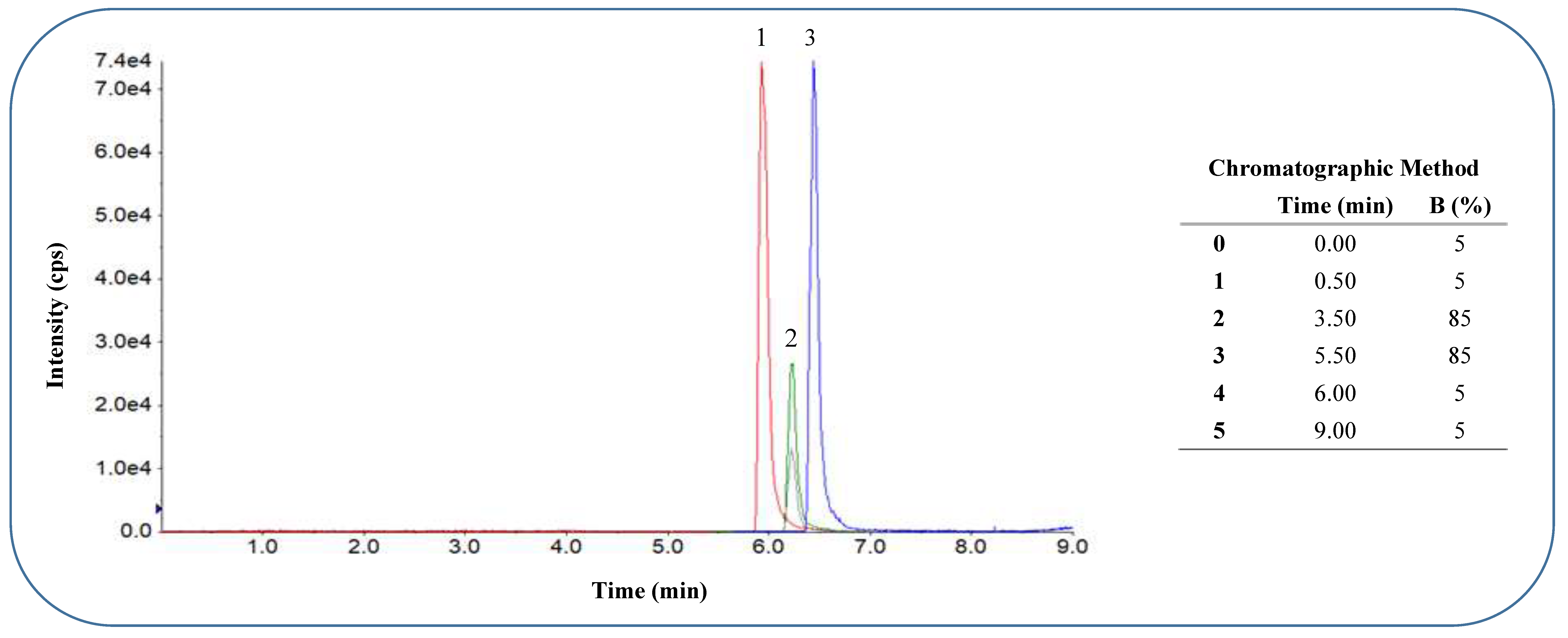
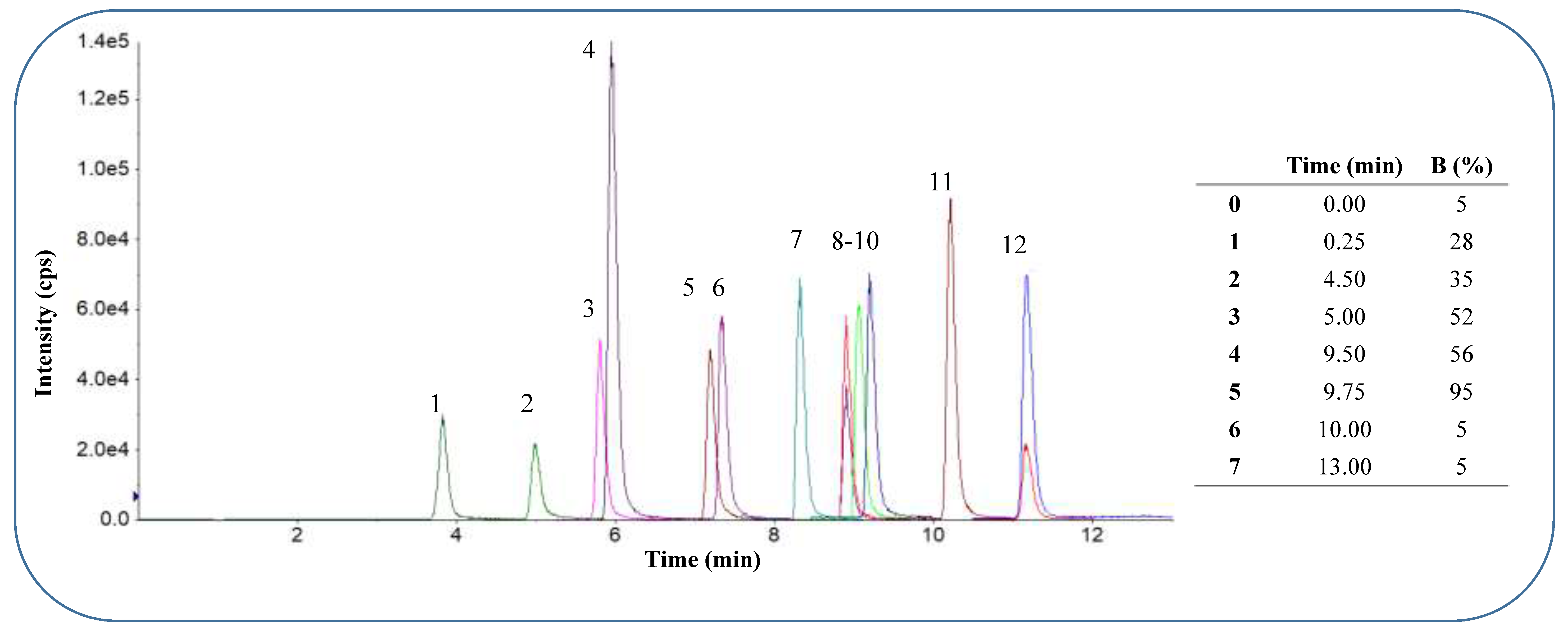
2.1.5. Chromatographic Conditions
2.1.6. Human Plasma and Whole Blood Collection and Storage
2.1.7. Preparation of Calibration Standards for Validation in Plasma and Dried Blood Spots
2.1.8. Extraction from Plasma and DBS Mitra Tips
2.1.9. LCMSMS Method Validation
2.2. Pilot Pharmacokinetic Study in a Healthy Volunteer
2.3. Statistical Analysis of Agreement between DBS Collected with the Mitra™ Device and Plasma Sampling
3. Results and Discussion
3.1. LCMSMS Method Validation
3.1.1. Evaluation of Matrix Effects
3.1.2. Evaluation of Recovery from Plasma and Dried Blood Spots
3.1.3. Linearity, LOD, LLOQ
3.1.4. Carry-Over
3.1.4. Intra- and Inter-Day Precision and Accuracy
3.1.5. Analyte Stability
3.2. Pilot Pharmacokinetic Study in a Healthy Volunteer
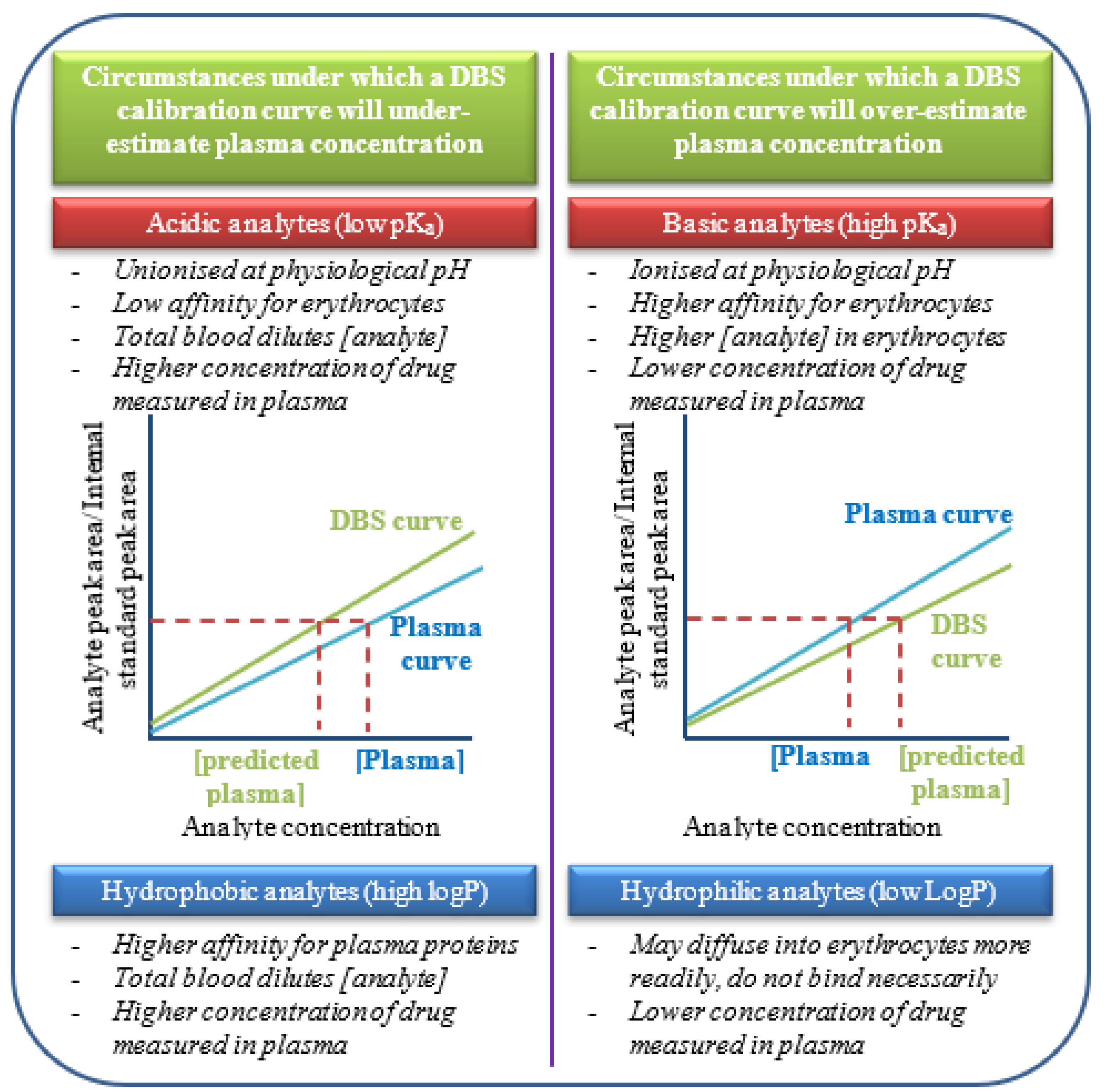
3.3. Assessment of Inter-Method Agreement between Dried Blood Spots and Plasma Sampling
Supplementary Materials
Funding
Conflicts of Interest
References
- Johansson, I. and M. Ingelman-Sundberg, Genetic polymorphism and toxicology—with emphasis on cytochrome p450. Toxicological sciences, 2010. 120(1): p. 1-13. [CrossRef]
- Patel, T.K. and P.B. Patel, Mortality among patients due to adverse drug reactions that lead to hospitalization: a meta-analysis. European journal of clinical pharmacology, 2018. 74(6): p. 819-832. [CrossRef]
- Samer, C.F., et al., Applications of CYP450 testing in the clinical setting. Molecular diagnosis & therapy, 2013. 17(3): p. 165-184.
- Giacomini, K.M., et al., Membrane transporters in drug development. Nature reviews Drug discovery, 2010. 9(3): p. 215-236. [CrossRef]
- Rajman, I., et al., African genetic diversity: implications for cytochrome P450-mediated drug metabolism and drug development. EBioMedicine, 2017. 17: p. 67-74.
- Boer, T., et al., Application of dried blood spot sampling combined with LC-MS/MS for genotyping and phenotyping of CYP450 enzymes in healthy volunteers. Biomedical Chromatography, 2011. 25(10): p. 1112-1123.
- Bosilkovska, M., et al., Geneva cocktail for Cytochrome P450 and P-Glycoprotein activity assessment using dried blood spots. Clinical Pharmacology & Therapeutics, 2014. 96(3): p. 349-359.
- Donzelli, M., et al., The Basel cocktail for simultaneous phenotyping of human cytochrome P450 isoforms in plasma, saliva and dried blood spots. Clinical pharmacokinetics, 2014. 53(3): p. 271-282.
- Denniff, P. and N. Spooner, Volumetric absorptive microsampling: a dried sample collection technique for quantitative bioanalysis. Analytical chemistry, 2014. 86(16): p. 8489-8495.
- Evans, C., et al., Implementing dried blood spot sampling for clinical pharmacokinetic determinations: considerations from the IQ Consortium Microsampling Working Group. 2015, Springer.
- Ye, Z. and H. Gao, Evaluation of sample extraction methods for minimizing hematocrit effect on whole blood analysis with volumetric absorptive microsampling. Bioanalysis, 2017. 9(4): p. 349-357.
- Guideline, I.H.T. Validation of analytical procedures: text and methodology Q2 (R1). in International conference on harmonization, Geneva, Switzerland. 2005.
- Matuszewski, B., Standard line slopes as a measure of a relative matrix effect in quantitative HPLC–MS bioanalysis. Journal of Chromatography B, 2006. 830(2): p. 293-300. [CrossRef]
- De Kesel, P.M. E. Lambert, and C.P. Stove, Does volumetric absorptive microsampling eliminate the hematocrit bias for caffeine and paraxanthine in dried blood samples? A comparative study. Analytica chimica acta, 2015. 881: p. 65-73.
- Bosilkovska, M., et al., Simultaneous LC-MS/MS quantification of P-glycoprotein and cytochrome P450 probe substrates and their metabolites in DBS and plasma. Bioanalysis, 2014. 6(2): p. 151-164.
- Kok, M.G.and M. Fillet, Volumetric absorptive microsampling: current advances and applications. Journal of pharmaceutical and biomedical analysis, 2017. [CrossRef]
- O’byrne, P.M., et al., The aqueous stability of bupropion. Journal of pharmaceutical and biomedical analysis, 2010. 53(3): p. 376-381.
- Rowland, M. and G.T. Emmons, Use of dried blood spots in drug development: pharmacokinetic considerations. The AAPS journal, 2010. 12(3): p. 290-293.
- Emmons, G. and M. Rowland, Pharmacokinetic considerations as to when to use dried blood spot sampling. Bioanalysis, 2010. 2(11): p. 1791-6.
| Negative ionisation mode [M – H] - | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyte | CYP probe | Q1 (m/z) | Q3 (m/z) | DP (V) | CE (V) | CXP (V) | |||||
| Flurbiprofen | CYP2C9 | 243.20 | 199.30 | -12 | -14 | -12 | |||||
| Hydroxyflurbiprofen | 259.20 | 215.20 | -40 | -11 | -4 | ||||||
| Internal Standard | |||||||||||
| Probenecid | 284.30 | 240.30 | -50 | -24 | -10 | ||||||
| Positive ionisation mode [M+H] + | |||||||||||
| Fexofenadine | P-gp | 502.7 | 466.6; 484.7 | 100 | 38 | 12 | |||||
| Caffeine | CYP1A2 | 195.3 | 138.2 | 20 | 25 | 6 | |||||
| Paraxanthine | 181.1 | 124.2 | 70 | 27 | 5 | ||||||
| Bupropion | CYP2B6 | 240.4 | 131.3 | 20 | 50 | 11 | |||||
| Hydroxybupropion | 256.4 | 103.1 | 50 | 52 | 3 | ||||||
| Omeprazole | CYP2C19 | 346.3 | 198.1 | 25 | 30 | 10 | |||||
| Hydroxyomeprazole | 362.1 | 214.4 | 50 | 15 | 10 | ||||||
| Dextromethorphan | CYP2D6 | 272.4 | 147.4; 171.5 | 90 | 50 | 10 | |||||
| Dextrorphan | 258.4 | 157.2 | 80 | 45 | 4 | ||||||
| Midazolam | CYP3A4 | 326.3 | 291.4 | 80 | 35 | 14 | |||||
| Hydroxymidazolam | 342.2 | 324.1 | 89 | 29 | 18 | ||||||
| Internal Standard | |||||||||||
| Imipramine | 281.5 | 86.1 | 50 | 50 | 10 | ||||||
| Analyte | Human plasma | Dried Blood Spots (Mitra™) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Linearity (r2) | LOD (ng.mL-1) | LLOQ (ng.mL-1) |
Recovery (%) (CV%) | Linearity (r2) | LOD (ng.mL-1) |
LLOQ (ng.mL-1) |
Recovery (%) (CV%) | |||
| FEX | 0.9997 | 0.86 | 2.86 | 106.99 | (6.76) | 0.9994 | 1.02 | 3.41 | 100.16 | (13.88) |
| CAF | 0.9958 | 9.53 | 31.78 | 97.35 | (11.46) | 0.9971 | 8.65 | 28.85 | 118.23 | (26.38) |
| PAR | 0.9973 | 1.76 | 5.86 | 102.23 | (11.44) | 0.9929 | 1.45 | 4.84 | 116.30 | (30.11) |
| BUP | 0.9990 | 0.62 | 2.07 | 98.86 | (7.20) | 0.9983 | 0.94 | 3.13 | 32.01 | (0.15) |
| OH-BUP | 0.9997 | 0.68 | 2.26 | 108.18 | (2.74) | 0.9995 | 0.70 | 2.34 | 107.64 | (15.41) |
| FLB | 0.9936 | 10.42 | 34.72 | 99.43 | (4.21) | 0.9981 | 3.69 | 12.30 | 107.98 | (15.09) |
| OH-FLB | 0.9939 | 1.75 | 5.82 | 100.52 | (5.12) | 0.9983 | 2.30 | 7.65 | 93.25 | (8.77) |
| OPZ | 0.9968 | 0.22 | 0.72 | 113.73 | (12.46) | 0.9986 | 0.49 | 1.63 | 89.88 | (10.42) |
| OH-FLB | 0.9985 | 0.50 | 1.67 | 108.24 | (8.11) | 0.9979 | 0.82 | 2.73 | 94.29 | (4.28) |
| DEX | 0.9958 | 0.67 | 2.23 | 110.60 | (10.74) | 0.9997 | 0.74 | 2.46 | 94.23 | (17.90) |
| DTP | 0.9999 | 0.29 | 0.96 | 109.28 | (14.16) | 0.9996 | 0.34 | 1.14 | 97.34 | (18.68) |
| MDZ | 0.9976 | 0.27 | 0.90 | 112.68 | (13.21) | 0.9987 | 0.18 | 0.61 | 107.46 | (18.60) |
| OH-MDZ | 0.9978 | 0.71 | 2.38 | 102.87 | (11.37) | 0.9988 | 0.47 | 1.58 | 106.93 | (13.27) |
| Analyte | Spiked conc. (ng.mL-1) | Inter-day plasma (n=9) | Spiked conc. (ng.mL-1) | Inter-day DBS (n=9) (Mitra™) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Found (mean ± SD in ng.mL-1) | Accuracy (%) | Precision (%) | Found (mean ± SD in ng.mL-1) | Accuracy (%) | Precision (%) | |||||||
| FEX | 75.00 | 73.08 | ± | 2.24 | 97.45 | 3.07 | 75.00 | 70.45 | ± | 3.95 | 93.93 | 5.61 |
| 150.00 | 153.58 | ± | 5.88 | 102.39 | 3.83 | 150.00 | 149.20 | ± | 2.63 | 99.47 | 1.76 | |
| 750.00 | 785.30 | ± | 5.76 | 104.71 | 0.73 | 750.00 | 739.89 | ± | 96.38 | 98.65 | 13.03 | |
| 1500.00 | 1522.38 | ± | 17.35 | 101.49 | 1.14 | 1500.00 | 1556.97 | ± | 25.56 | 103.80 | 1.64 | |
| 4500.00 | 4446.33 | ± | 25.58 | 98.81 | 0.58 | 4500.00 | 4404.97 | ± | 50.71 | 97.89 | 1.15 | |
| CAF | 150.00 | 150.67 | ± | 11.70 | 100.45 | 7.77 | 150.00 | 142.91 | ± | 7.73 | 95.27 | 5.41 |
| 300.00 | 299.66 | ± | 8.11 | 99.89 | 2.71 | 300.00 | 295.94 | ± | 12.07 | 98.65 | 4.08 | |
| 1500.00 | 1548.55 | ± | 44.42 | 103.24 | 2.87 | 1500.00 | 1451.72 | ± | 156.33 | 96.78 | 10.77 | |
| 3000.00 | 2980.31 | ± | 112.20 | 99.34 | 3.76 | 3000.00 | 3012.33 | ± | 53.93 | 100.41 | 1.79 | |
| 9000.00 | 8884.12 | ± | 137.15 | 98.71 | 1.54 | 9000.00 | 8931.49 | ± | 65.62 | 99.24 | 0.73 | |
| PAR | 75.00 | 75.36 | ± | 5.12 | 100.48 | 6.79 | 75.00 | 73.93 | ± | 3.42 | 98.58 | 4.63 |
| 150.00 | 144.48 | ± | 5.57 | 96.32 | 3.86 | 150.00 | 147.19 | ± | 6.86 | 98.12 | 4.66 | |
| 750.00 | 784.73 | ± | 33.99 | 104.63 | 4.33 | 750.00 | 774.79 | ± | 47.41 | 103.31 | 6.12 | |
| 1500.00 | 1517.20 | ± | 32.91 | 101.15 | 2.17 | 1500.00 | 1535.04 | ± | 87.08 | 102.34 | 5.67 | |
| 4500.00 | 4436.14 | ± | 69.15 | 98.58 | 1.56 | 4500.00 | 4428.43 | ± | 136.32 | 98.41 | 3.08 | |
| BUP | 75.00 | 79.49 | ± | 1.72 | 105.99 | 2.17 | 75.00 | 79.11 | ± | 3.64 | 105.48 | 4.60 |
| 150.00 | 137.87 | ± | 5.26 | 91.91 | 3.82 | 750.00 | 148.92 | ± | 2.57 | 99.28 | 1.72 | |
| 750.00 | 793.77 | ± | 36.09 | 105.84 | 4.55 | 1500.00 | 746.10 | ± | 18.83 | 99.48 | 2.52 | |
| 1500.00 | 1542.74 | ± | 34.11 | 102.85 | 2.21 | 3000.00 | 1468.00 | ± | 8.00 | 97.87 | 0.54 | |
| 4500.00 | 4405.34 | ± | 93.61 | 97.90 | 2.12 | 4500.00 | 4515.67 | ± | 154.22 | 100.35 | 3.42 | |
| Analyte | Spiked conc. (ng.mL-1) | Inter-day plasma (n=9) | Spiked conc. (ng.mL-1) | Inter-day DBS (n=9) | ||||||||
| Found (mean ± SD in ng.mL-1) | Accuracy (%) | Precision (%) | Found (mean ± SD in ng.mL-1) | Accuracy (%) | Precision (%) | |||||||
| OH-BUP | 30.00 | 30.29 | ± | 3.66 | 100.96 | 12.07 | 75.00 | 72.59 | ± | 1.70 | 96.79 | 2.34 |
| 150.00 | 142.18 | ± | 7.68 | 94.79 | 5.40 | 150.00 | 145.60 | ± | 7.25 | 97.07 | 4.98 | |
| 750.00 | 766.23 | ± | 21.70 | 102.16 | 2.83 | 750.00 | 761.55 | ± | 33.97 | 101.54 | 4.46 | |
| 1500.00 | 1511.25 | ± | 43.93 | 100.75 | 2.91 | 1500.00 | 1514.68 | ± | 9.77 | 100.98 | 0.65 | |
| 4500.00 | 4459.10 | ± | 110.87 | 99.09 | 2.49 | 4500.00 | 4404.56 | ± | 109.85 | 97.88 | 2.49 | |
| FLB | 150.00 | 142.43 | ± | 6.32 | 94.95 | 4.44 | 150.00 | 148.60 | ± | 6.60 | 99.06 | 4.44 |
| 300.00 | 303.85 | ± | 8.42 | 101.28 | 2.77 | 300.00 | 307.07 | ± | 16.19 | 102.36 | 5.27 | |
| 1500.00 | 1544.51 | ± | 17.87 | 102.97 | 1.16 | 1500.00 | 1461.66 | ± | 90.79 | 97.44 | 6.21 | |
| 3000.00 | 3057.69 | ± | 151.34 | 101.92 | 4.95 | 3000.00 | 3044.17 | ± | 158.49 | 101.47 | 5.21 | |
| 9000.00 | 8936.24 | ± | 128.58 | 99.29 | 1.44 | 9000.00 | 8942.83 | ± | 529.40 | 99.36 | 5.92 | |
| OH-FLB | 75.00 | 71.56 | ± | 2.18 | 95.41 | 3.05 | 75.00 | 75.65 | ± | 2.79 | 100.87 | 3.68 |
| 150.00 | 149.78 | ± | 3.18 | 99.85 | 2.12 | 150.00 | 150.20 | ± | 6.35 | 100.13 | 4.23 | |
| 750.00 | 783.89 | ± | 20.70 | 104.52 | 2.64 | 750.00 | 734.04 | ± | 23.47 | 97.87 | 3.20 | |
| 1500.00 | 1520.17 | ± | 54.33 | 101.34 | 3.57 | 1500.00 | 1512.70 | ± | 57.85 | 100.85 | 3.82 | |
| 4500.00 | 4458.25 | ± | 47.52 | 99.07 | 1.07 | 4500.00 | 4474.10 | ± | 91.90 | 99.42 | 2.05 | |
| OPZ | 30.00 | 27.72 | ± | 1.29 | 92.39 | 4.67 | 75.00 | 71.33 | ± | 4.33 | 95.11 | 6.07 |
| 150.00 | 155.65 | ± | 10.34 | 103.77 | 6.65 | 150.00 | 151.96 | ± | 5.89 | 101.31 | 3.87 | |
| 750.00 | 785.52 | ± | 6.70 | 104.74 | 0.85 | 750.00 | 768.41 | ± | 31.38 | 102.46 | 4.08 | |
| 1500.00 | 1564.87 | ± | 26.32 | 104.32 | 1.68 | 1500.00 | 1522.43 | ± | 12.65 | 101.50 | 0.83 | |
| 4500.00 | 4423.55 | ± | 22.13 | 98.30 | 0.50 | 3000.00 | 4449.65 | ± | 23.97 | 98.88 | 0.54 | |
| OH-OPZ | 75.00 | 74.59 | ± | 2.88 | 99.46 | 3.86 | 30.00 | 70.46 | ± | 1.99 | 93.94 | 2.82 |
| 150.00 | 161.45 | ± | 14.09 | 107.63 | 8.73 | 75.00 | 149.75 | ± | 0.74 | 99.83 | 0.50 | |
| 750.00 | 784.63 | ± | 57.01 | 104.62 | 7.27 | 750.00 | 785.00 | ± | 71.40 | 104.67 | 9.10 | |
| 1500.00 | 1505.75 | ± | 13.97 | 100.38 | 0.93 | 1500.00 | 1503.30 | ± | 24.74 | 100.22 | 1.65 | |
| 4500.00 | 4098.65 | ± | 353.24 | 91.08 | 8.62 | 3000.00 | 4463.32 | ± | 18.64 | 99.18 | 0.42 | |
| Analyte | Spiked conc. (ng.mL-1) | Inter-day plasma (n=9) | Spiked conc. (ng.mL-1) | Inter-day DBS (n=9) | ||||||||
| Found (mean ± SD in ng.mL-1) | Accuracy (%) | Precision (%) | Found (mean ± SD in ng.mL-1) | Accuracy (%) | Precision (%) | |||||||
| DEX | 75.00 | 72.71 | ± | 4.60 | 96.94 | 6.32 | 75.00 | 73.64 | ± | 1.55 | 98.19 | 2.10 |
| 150.00 | 147.26 | ± | 6.04 | 98.17 | 4.10 | 150.00 | 151.34 | ± | 2.26 | 100.89 | 1.50 | |
| 750.00 | 791.79 | ± | 44.43 | 105.57 | 5.61 | 750.00 | 743.62 | ± | 19.24 | 99.15 | 2.59 | |
| 1500.00 | 1554.21 | ± | 73.08 | 103.61 | 4.70 | 1500.00 | 1506.87 | ± | 21.87 | 100.46 | 1.45 | |
| 4500.00 | 4361.56 | ± | 181.78 | 96.92 | 4.17 | 4500.00 | 4477.94 | ± | 20.06 | 99.51 | 0.45 | |
| DTP | 75.00 | 71.69 | ± | 1.66 | 95.59 | 2.31 | 75.00 | 73.45 | ± | 2.82 | 97.94 | 3.84 |
| 150.00 | 151.68 | ± | 3.26 | 101.12 | 2.15 | 150.00 | 151.35 | ± | 2.75 | 100.90 | 1.82 | |
| 750.00 | 787.06 | ± | 31.41 | 104.94 | 3.99 | 750.00 | 740.02 | ± | 17.83 | 98.67 | 2.41 | |
| 1500.00 | 1548.95 | ± | 77.14 | 103.26 | 4.98 | 1500.00 | 1484.57 | ± | 14.08 | 98.97 | 0.95 | |
| 4500.00 | 4403.00 | ± | 118.14 | 97.84 | 2.68 | 4500.00 | 4508.65 | ± | 11.75 | 100.19 | 0.26 | |
| MDZ | 30.00 | 29.42 | ± | 2.01 | 98.07 | 6.82 | 30.00 | 27.77 | ± | 0.86 | 92.55 | 3.09 |
| 60.00 | 59.57 | ± | 3.30 | 99.28 | 5.54 | 60.00 | 60.37 | ± | 1.91 | 100.62 | 3.17 | |
| 300.00 | 306.27 | ± | 5.23 | 102.09 | 1.71 | 300.00 | 307.86 | ± | 21.64 | 102.62 | 7.03 | |
| 600.00 | 599.51 | ± | 2.43 | 99.92 | 0.41 | 600.00 | 615.30 | ± | 13.62 | 102.55 | 2.21 | |
| 1800.00 | 1795.05 | ± | 7.09 | 99.72 | 0.40 | 1800.00 | 1785.79 | ± | 19.80 | 99.21 | 1.11 | |
| OH-MDZ | 30.00 | 28.87 | ± | 0.31 | 96.25 | 1.09 | 30.00 | 28.59 | ± | 2.67 | 95.31 | 9.33 |
| 60.00 | 59.40 | ± | 0.60 | 99.00 | 1.01 | 60.00 | 60.01 | ± | 1.49 | 100.02 | 2.49 | |
| 300.00 | 310.92 | ± | 1.91 | 103.64 | 0.61 | 300.00 | 302.20 | ± | 14.90 | 100.73 | 4.93 | |
| 600.00 | 609.72 | ± | 13.92 | 101.62 | 2.28 | 600.00 | 609.52 | ± | 21.61 | 101.59 | 3.55 | |
| 1800.00 | 1782.55 | ± | 12.59 | 99.03 | 0.71 | 1800.00 | 1778.79 | ± | 34.44 | 98.82 | 1.94 | |
| Human plasma | Dried Blood Spots ( Mitra™ ) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spiked conc. (ng.mL-1) | Found (mean ± SD in ng.mL-1) | Accuracy (%) | Precision (%) | Spiked conc. (ng.mL-1) | Found (mean ± SD in ng.mL-1) | Accuracy (%) | Precision (%) | |||||
| Fexofenadine | 150.00 | 142.17 | ± | 15.74 | 94.53 | 11.07 | 150.00 | 136.97 | ± | 1.86 | 91.31 | 1.36 |
| 600.00 | 587.21 | ± | 86.94 | 97.87 | 14.81 | 450.00 | 410.73 | ± | 29.24 | 91.27 | 7.12 | |
| 3000.00 | 2953.25 | ± | 349.62 | 98.44 | 11.84 | 3000.00 | 2529.27 | ± | 95.76 | 84.31 | 3.79 | |
| Caffeine | 150.00 | 105.25 | ± | 17.76 | 70.16 | 16.87 | 150.00 | 131.88 | ± | 2.28 | 87.92 | 1.73 |
| 600.00 | 566.56 | ± | 57.78 | 94.43 | 10.20 | 600.00 | 515.05 | ± | 9.32 | 85.84 | 1.81 | |
| 3000.00 | 2770.61 | ± | 378.45 | 92.35 | 13.66 | 3000.00 | 2642.36 | ± | 53.60 | 88.08 | 2.03 | |
| Paraxanthine | 75.00 | 58.02 | ± | 7.03 | 77.36 | 12.12 | 75.00 | 63.54 | ± | 4.04 | 84.73 | 6.36 |
| 300.00 | 285.58 | ± | 36.22 | 95.19 | 12.68 | 300.00 | 280.23 | ± | 17.40 | 93.41 | 6.21 | |
| 1500.00 | 1439.21 | ± | 184.95 | 95.95 | 12.85 | 1500.00 | 1389.55 | ± | 72.03 | 92.64 | 5.18 | |
| Bupropion | 150.00 | 117.58 | ± | 26.11 | 78.39 | 22.20 | 150.00 | 138.67 | ± | 10.00 | 92.44 | 7.21 |
| 750.00 | 671.12 | ± | 51.89 | 89.48 | 7.73 | 450.00 | 462.01 | ± | 36.69 | 102.67 | 7.94 | |
| 3000.00 | 3270.21 | ± | 217.32 | 109.01 | 6.65 | 3000.00 | 2822.43 | ± | 220.29 | 94.08 | 7.81 | |
| Hydroxybupropion | 75.00 | 66.98 | ± | 3.96 | 89.31 | 5.91 | 75.00 | 71.09 | ± | 2.51 | 94.78 | 3.54 |
| 300.00 | 320.10 | ± | 8.88 | 106.70 | 2.77 | 300.00 | 278.04 | ± | 21.46 | 92.68 | 7.72 | |
| 1500.00 | 1635.18 | ± | 12.05 | 109.01 | 0.74 | 1500.00 | 1576.19 | ± | 31.09 | 105.08 | 1.97 | |
| Flurbiprofen | 150.00 | 169.06 | ± | 18.58 | 112.71 | 10.99 | 150.00 | 193.96 | ± | 2.54 | 129.30 | 1.31 |
| 600.00 | 711.69 | ± | 54.04 | 118.61 | 7.59 | 600.00 | 650.30 | ± | 82.69 | 108.38 | 12.72 | |
| 3000.00 | 3497.46 | ± | 115.23 | 116.58 | 3.29 | 3000.00 | 3283.55 | ± | 493.34 | 109.45 | 15.02 | |
| Hydroxyflurbiprofen | 75.00 | 65.43 | ± | 0.90 | 87.24 | 1.38 | 150.00 | 122.48 | ± | 4.42 | 81.65 | 3.61 |
| 300.00 | 284.31 | ± | 16.94 | 94.77 | 5.96 | 300.00 | 299.39 | ± | 14.40 | 99.80 | 4.81 | |
| 1500.00 | 1511.98 | ± | 88.64 | 100.80 | 5.86 | 1500.00 | 1481.57 | ± | 6.22 | 98.77 | 0.42 | |
| Analyte | Human plasma | Dried Blood Spots (Mitra™ ) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spiked conc. (ng.mL-1) | Found (mean ± SD in ng.mL-1) | Accuracy (%) | Precision (%) | Spiked conc. (ng.mL-1) | Found (mean ± SD in ng.mL-1) | Accuracy (%) | Precision (%) | ||||||
| Omeprazole | 150.00 | 160.38 | ± | 19.83 | 106.92 | 12.37 | 75.00 | 54.21 | ± | 2.61 | 72.29 | 4.81 | |
| 300.00 | 251.81 | ± | 26.33 | 83.94 | 10.46 | 300.00 | 281.02 | ± | 5.71 | 93.67 | 2.03 | ||
| 1500.00 | 1399.75 | ± | 237.21 | 93.32 | 16.95 | 1500.00 | 1338.81 | ± | 55.19 | 89.25 | 4.12 | ||
| Hydroxy-omeprazole | 75.00 | 68.39 | ± | 1.65 | 91.19 | 2.41 | 75.00 | 71.15 | ± | 3.37 | 94.87 | 4.74 | |
| 300.00 | 313.34 | ± | 26.25 | 104.45 | 8.38 | 300.00 | 294.24 | ± | 12.17 | 98.08 | 4.13 | ||
| 1500.00 | 1457.62 | ± | 76.06 | 97.17 | 5.22 | 1500.00 | 1474.07 | ± | 39.84 | 98.27 | 2.70 | ||
| Dextromethorphan | 75.00 | 77.41 | ± | 6.88 | 103.21 | 8.89 | 75.00 | 75.36 | ± | 4.96 | 100.48 | 6.58 | |
| 300.00 | 333.85 | ± | 16.94 | 111.28 | 5.07 | 300.00 | 274.74 | ± | 10.84 | 91.58 | 3.95 | ||
| 1500.00 | 1629.79 | ± | 97.39 | 108.65 | 5.98 | 1500.00 | 1543.52 | ± | 50.39 | 102.90 | 3.26 | ||
| Dextrorphan | 150.00 | 150.04 | ± | 4.32 | 100.03 | 2.88 | 150.00 | 151.49 | ± | 5.92 | 100.99 | 3.91 | |
| 750.00 | 790.96 | ± | 48.57 | 105.46 | 6.14 | 750.00 | 659.63 | ± | 17.17 | 87.95 | 2.60 | ||
| 3000.00 | 3127.85 | ± | 104.21 | 104.26 | 3.33 | 3000.00 | 3131.03 | ± | 141.39 | 104.37 | 4.52 | ||
| Midazolam | 30.00 | 26.14 | ± | 3.14 | 87.14 | 12.01 | 30.00 | 24.78 | ± | 1.97 | 82.62 | 7.93 | |
| 120.00 | 137.37 | ± | 16.05 | 114.48 | 11.69 | 120.00 | 115.10 | ± | 2.26 | 95.91 | 1.96 | ||
| 600.00 | 650.37 | ± | 55.62 | 108.40 | 8.55 | 600.00 | 559.28 | ± | 36.62 | 93.21 | 6.55 | ||
| Hydroxy-midazolam | 30.00 | 34.19 | ± | 5.72 | 113.97 | 16.73 | 60.00 | 49.65 | ± | 1.37 | 82.74 | 2.75 | |
| 180.00 | 205.49 | ± | 12.36 | 114.16 | 6.01 | 180.00 | 164.88 | ± | 8.48 | 91.60 | 5.15 | ||
| 1200.00 | 1090.39 | ± | 129.74 | 90.87 | 11.90 | 1200.00 | 979.89 | ± | 31.29 | 81.66 | 3.19 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





