Submitted:
10 June 2024
Posted:
12 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Clinical Information
2.3. Construction of Tissue Microarray
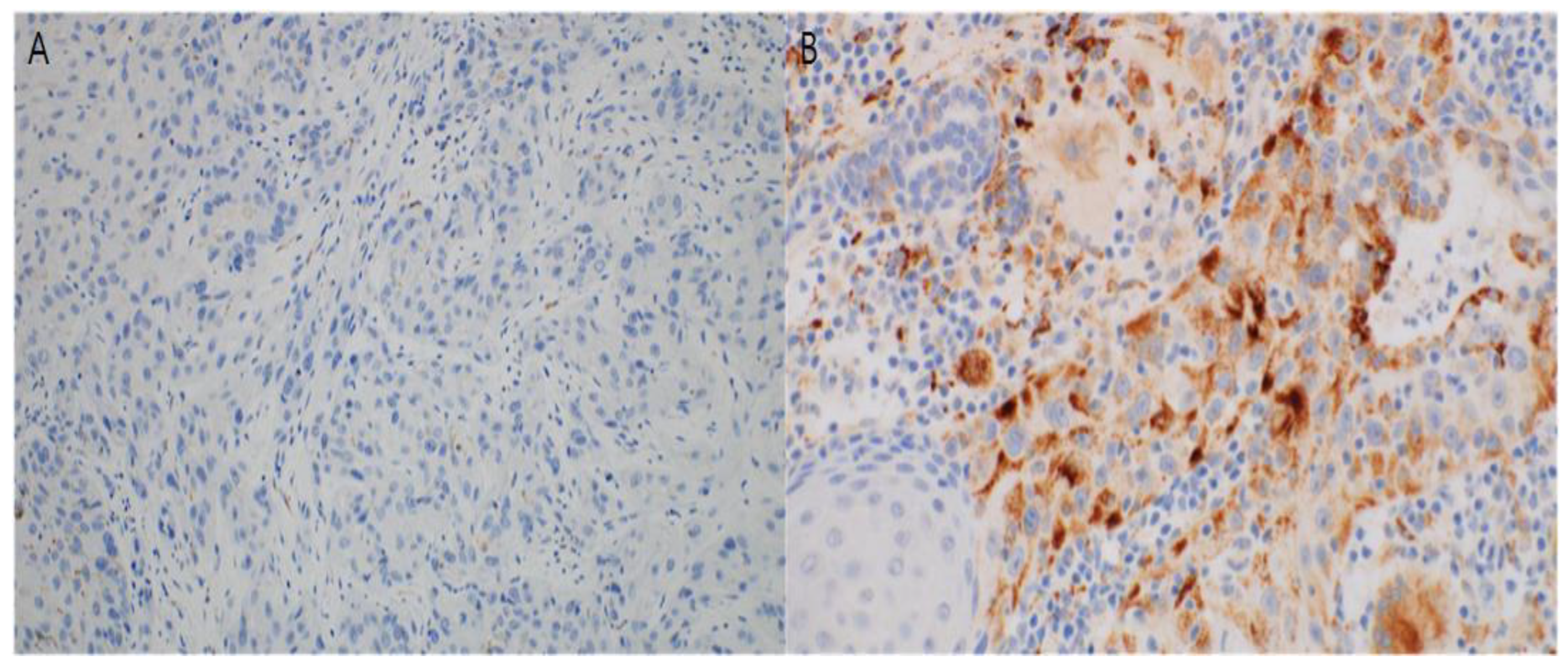
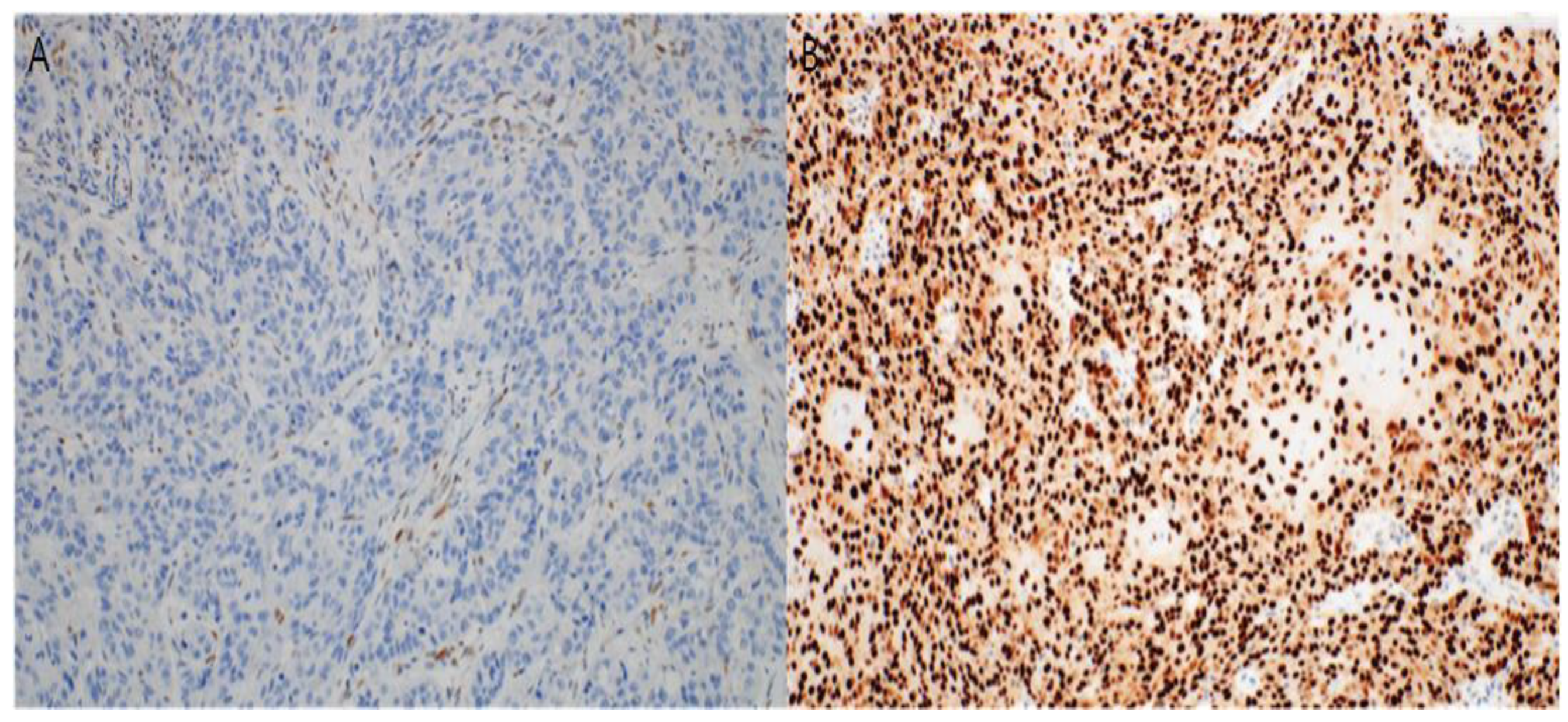
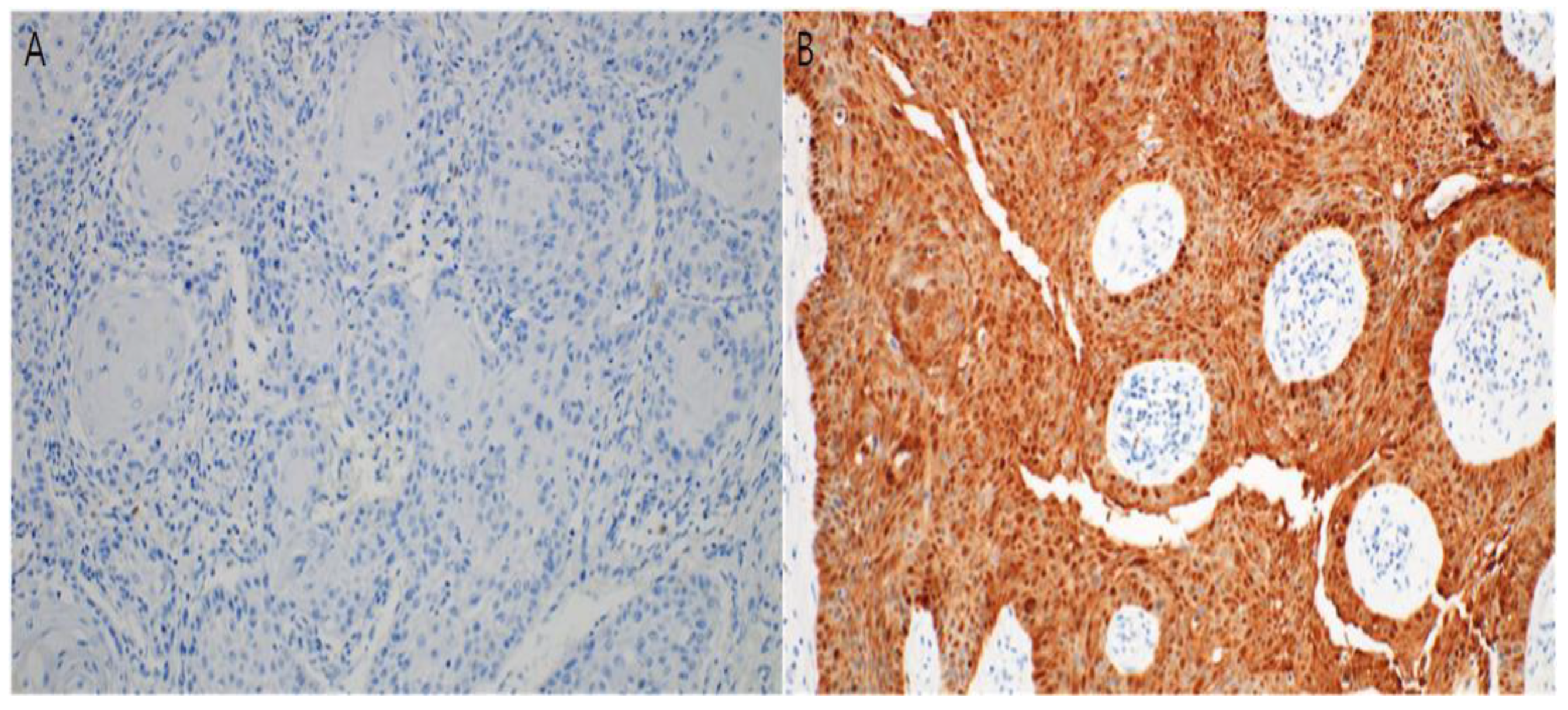
2.4. Immunohistochemistry and Interpretation
2.5. Statistical Analysis
3. Results
3.1. Patients Demographics and Tumor Characteristics
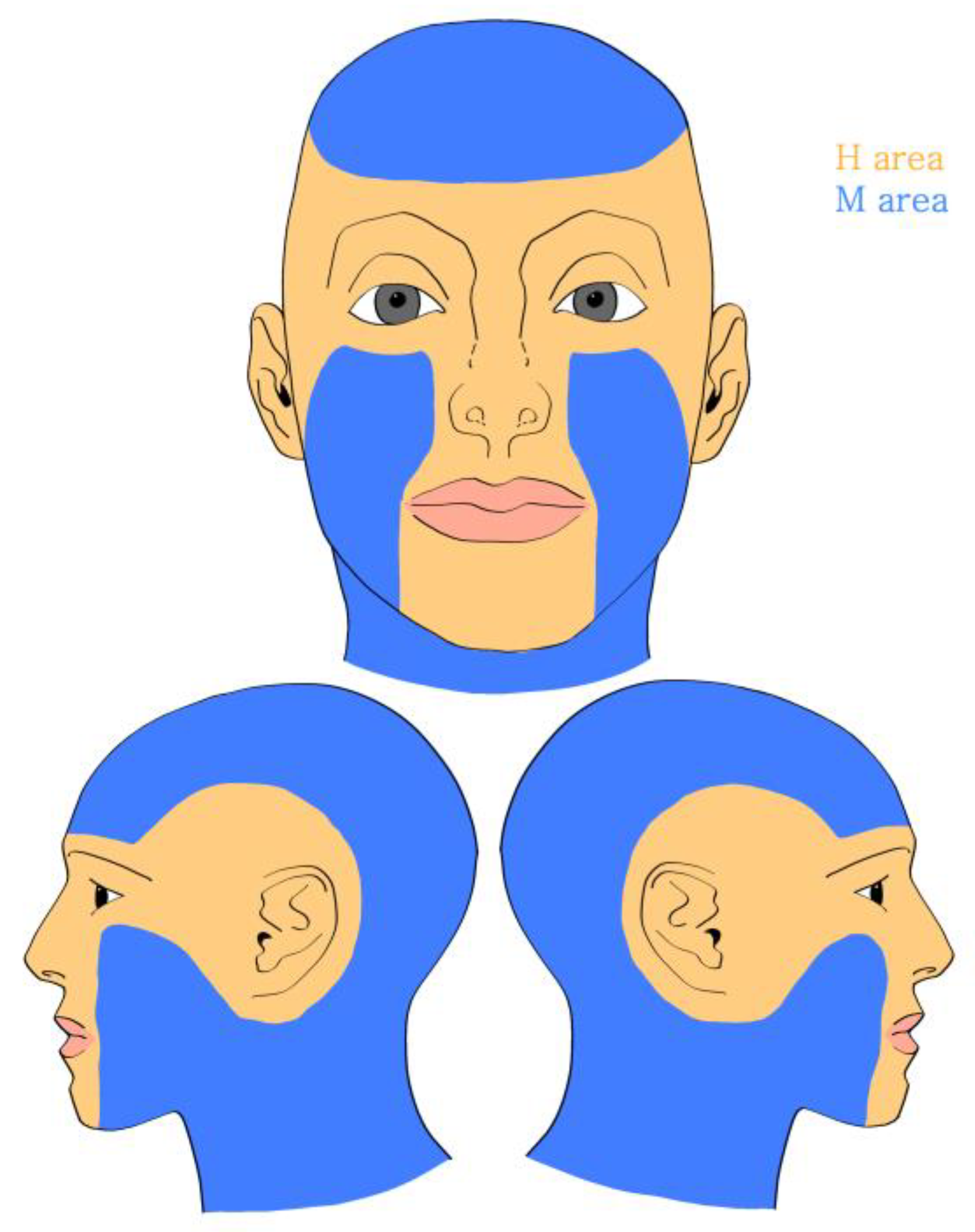
3.2. Location of Tumors
3.3. Immunohistochemical Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aldabagh, B.; Angeles, J.G.C.; Cardones, A.R.; Arron, S.T. Cutaneous squamous cell carcinoma and human papillomavirus: is there an association? Dermatol Surg 2013, 39, 1–23. [Google Scholar] [CrossRef]
- Harwood, C.A.; McGregor, J.M.; Proby, C.M.; Breuer, J. Human papillomavirus and the development of non-melanoma skin cancer. J Clin Pathol 1999, 52, 249–253. [Google Scholar] [CrossRef]
- Wookey, V.B.; Appiah, A.K.; Kallam, A.; Ernani, V.; Smith, L.M.; Ganti, A.K. HPV status and survival in non-oropharyngeal squamous cell carcinoma of the head and neck. Anticancer Res 2019, 39, 1907–1914. [Google Scholar] [CrossRef]
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol 2018, 78, 237–247. [Google Scholar] [CrossRef]
- Jiromaru, R.; Yamamoto, H.; Yasumatsu, R.; Hongo, T.; Nozaki, Y.; Nakano, T.; Hashimoto, K.; Nakagawa, T.; Oda, Y. p16 overexpression and Rb loss correlate with high-risk HPV infection in oropharyngeal squamous cell carcinoma. Histopathology 2021, 79, 358–369. [Google Scholar] [CrossRef]
- Ciążyńska, M.; Kamińska-Winciorek, G.; Lange, D.; Lewandowski, B.; Reich, A.; Sławińska, M.; Pabianek, M.; Szczepaniak, K.; Hankiewicz, A.; Ułańska, M.; et al. The incidence and clinical analysis of non-melanoma skin cancer. Sci Rep 2021, 11, 4337. [Google Scholar] [CrossRef]
- Zur Hausen, H. Papillomaviruses in the causation of human cancers – a brief historical account. Virology 2009, 384, 260–265. [Google Scholar] [CrossRef]
- Syrjänen, K.; Syrjänen, S.; Lamberg, M.; Pyrhönen, S.; Nuutinen, J. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg 1983, 12, 418–424. [Google Scholar] [CrossRef]
- Gordis, T.M.; Cagle, J.L.; Nguyen, S.A.; Newman, J.G. Human papillomavirus-associated oropharyngeal squamous cell carcinoma: A systematic review and meta-analysis of clinical trial demographics. Cancers (Basel) 2022, 14, 4061. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Clifford, G.M.; Boyle, P.; Franceschi, S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 2005, 14, 467–475. [Google Scholar] [CrossRef]
- Lewis Jr., J. S.; Thorstad, W.L.; Chernock, R.D.; Haughey, B.H.; Yip, J.H.; Zhang, Q.; El-Mofty, S.K. p16 positive oropharyngeal squamous cell carcinoma:an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol 2010, 34, 1088–1096. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- D’Souza, G.; Kreimer, A.R.; Viscidi, R.; Pawlita, M.; Fakhry, C.; Koch, W.M.; Westra, W.H.; Gillison, M.L. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 2007, 356, 1944–1956. [Google Scholar] [CrossRef]
- Fakhry, C.; Westra, W.H.; Li, S.; Cmelak, A.; Ridge, J.A.; Pinto, H.; Forastiere, A.; Gillison, M.L. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 2008, 100, 261–269. [Google Scholar] [CrossRef]
- Westra, W.H. The changing face of head and neck cancer in the 21st century: the impact of HPV on the epidemiology and pathology of oral cancer. Head Neck Pathol 2009, 3, 78–81. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Lassen, P.; Eriksen, J.G.; Hamilton-Dutoit, S.; Tramm, T.; Alsner, J.; Overgaard, J.; Danish Head and Neck Cancer Group (DAHANCA). HPV-associated p16-expression and response to hypoxic modification of radiotherapy in head and neck cancer. Radiother Oncol 2010, 94, 30–35. [Google Scholar] [CrossRef]
- D’Souza, G.; Dempsey, A. The role of HPV in head and neck cancer and review of the HPV vaccine. Prev Med 2011, 53, Suppl 1(Suppl 1):S5-S11, S5–S11. [CrossRef]
- O’Rorke, M.A.; Ellison, M.V.; Murray, L.J.; Moran, M.; James, J.; Anderson, L.A. Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis. Oral Oncol 2012, 48, 1191–1201. [Google Scholar] [CrossRef]
- Dayyani, F.; Etzel, C.J.; Liu, M.; Ho, C.H.; Lippman, S.M.; Tsao, A.S. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol 2010, 2, 15. [Google Scholar] [CrossRef]
- Gillison, M.L.; Broutian, T.; Pickard, R.K.L.; Tong, Z.Y.; Xiao, W.; Kahle, L.; Graubard, B.I.; Chaturvedi, A.K. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA 2012, 307, 693–703. [Google Scholar] [CrossRef]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol 2015, 33, 3235–3242. [Google Scholar] [CrossRef]
- Hasche, D.; Stephan, S.; Braspenning-Wesch, I.; Mikulec, J.; Niebler, M.; Gröne, H.J.; Flechtenmacher, C.; Akgül, B.; Rösl, F.; Vinzón, S.E. The interplay of UV and cutaneous papillomavirus infection in skin cancer development. PLOS Pathog 2017, 13, e1006723. [Google Scholar] [CrossRef]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 2000, 92, 709–720. [Google Scholar] [CrossRef]
- Mehanna, H.; Beech, T.; Nicholson, T.; El-Hariry, I.; McConkey, C.; Paleri, V.; Roberts, S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer—systematic review and meta-analysis of trends by time and region. Head Neck 2013, 35, 747–755. [Google Scholar] [CrossRef]
- Awan, M.S.; Irfan, B.; Zahid, I.; Mirza, Y.; Ali, S.A. Comparison of polymerase chain reaction and immunohistochemistry assays for analysing human papillomavirus infection in oral squamous cell carcinoma. J Clin Diagn Res 2017, 11, XC10–XC13. [Google Scholar] [CrossRef]
- Soussi, T.; Wiman, K.G. Shaping genetic alterations in human cancer: the p53 mutation paradigm. Cancer Cell 2007, 12, 303–312. [Google Scholar] [CrossRef]
- Tandon, S.; Tudur-Smith, C.; Riley, R.D.; Boyd, M.T.; Jones, T.M. A systematic review of p53 as a prognostic factor of survival in squamous cell carcinoma of the four main anatomical subsites of the head and neck. Cancer Epidemiol Biomarkers Prev 2010, 19, 574–587. [Google Scholar] [CrossRef]
- Dong, W.; Arpin, C.; Accardi, R.; Gissmann, L.; Sylla, B.S.; Marvel, J.; Tommasino, M. Loss of p53 or p73 in human papillomavirus type 38 E6 and E7 transgenic mice partially restores the UV-activated cell cycle checkpoints. Oncogene 2008, 27, 2923–2928. [Google Scholar] [CrossRef]
- Münger, K.; Baldwin, A.; Edwards, K.M.; Hayakawa, H.; Nguyen, C.L.; Owens, M.; Grace, M.; Huh, K. Mechanisms of human papillomavirus-induced oncogenesis. J Virol 2004, 78, 11451–11460. [Google Scholar] [CrossRef]
- Thomas, M.; Pim, D.; Banks, L. The role of the E6-p53 interaction in the molecular pathogenesis of HPV. Oncogene 1999, 18, 7690–7700. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, M.; Yu, D.S.; Lee, Y.B. Identification of genetic mutations of cutaneous squamous cell carcinoma using whole exome sequencing in non-Caucasian population. J Dermatol Sci 2022, 106, 70–77. [Google Scholar] [CrossRef]
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol 2012, 166, 1069–1080. [Google Scholar] [CrossRef]
- Satgunaseelan, L.; Chia, N.; Suh, H.; Virk, S.; Ashford, B.; Lum, T.; Ranson, M.; Clark, J.; Gupta, R. p16 expression in cutaneous squamous cell carcinoma of the head and neck is not associated with integration of high risk HPV DNA or prognosis. Pathology 2017, 49, 494–498. [Google Scholar] [CrossRef]
- Schmults, C.D.; Blitzblau, R.; Aasi, S.Z.; Alam, M.; Andersen, J.S.; Baumann, B.C.; Bordeaux, J.; Chen, P.L.; Chin, R.; Contreras, C.M.; et al. [NCCN guidelines]® Insights: Squamous Cell Skin Cancer. J Natl Compr Canc Netw, version 1.2022, 2021; Vol. 19; pp. 1382–1394.
- Wentzensen, N.; Schwartz, L.; Zuna, R.E.; Smith, K.; Mathews, C.; Gold, M.A.; Allen, R.A.; Zhang, R.; Dunn, S.T.; Walker, J.L.; et al. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin Cancer Res 2012, 18, 4154–4162. [Google Scholar] [CrossRef]
| N (%) | N=62 (%) |
|---|---|
| Sex | |
| Male | 20 (32.26) |
| Female | 42 (67.74) |
| Age group (years) | |
| ≤50 | 2 (3.22) |
| 51-65 | 2 (3.22) |
| >65 | 58 (93.55) |
| Location | |
| Area H | 19 (30.65) |
| Area M | 43 (69.35) |
| Size of tumor (cm2) |
2.73 ± 4.60 |
| Invasion depth (mm) |
4.06 ± 3.62 |
| Lymphovascular invasion |
|
| (+) | 3 |
| (-) | 59 |
| Perineural invasion |
|
| (+) | 3 |
| (-) | 59 |
| DM | |
| (+) | 9 |
| (-) | 53 |
| HTN | |
| (+) | 27 |
| (-) | 35 |
| N (%) | N=62 (%) |
|---|---|
| Area H | 19 (30.65) |
| Eyelid | 5 (8.06) |
| Nose | 3 (4.84) |
| Lip | 5 (8.06) |
| Chin | 2 (3.23) |
| Ear | 4 (6.46) |
| Area M | 43 (69.35) |
| Forehead | 11 (17.74) |
| Scalp | 3 (4.84) |
| Cheek | 29 (46.77) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





