1. Introduction
Increased fracture risk characterizes the skeletal disorder osteoporosis [
1]. The WHO criteria state that osteoporosis exists in cases with 2.5 or more standard deviations of lower bone mineral density (BMD) than the mean peak BMD for young healthy adults [
2]. Primary osteoporosis is induced by estrogen deficiency in postmenopausal women and is seen in men as well as in women in older age groups [
3]. Secondary osteoporosis is a result of a particular pathology or of treatment with some pharmacological agents [
4], for instance in rheumatic diseases and after bariatric surgery or in corticosteroid medication [
5,
6,
7]. Recent meta-analyses indicated a world-wide prevalence of osteoporosis of almost 20%, its prevalence, however, being characterized by significant geographic variations [
8,
9]. In Europe, osteoporotic fractures are considered one of the most significant pathological conditions responsible for reduced adjusted life years [
10]. And the burden of osteoporosis is anticipated to increase further in the nearest decades [
11].
Various exogenous factors including smoking, alcohol consumption, dietary habits and body weight will modify the risk of the disease [
12]. Dietary factors include intakes of calcium and of the vitamins D and K [
13]. The role of nutrient deficiencies in osteoporosis is also confirmed by observations on postoperative osteoporosis in subjects treated with bariatric surgery [6,7. It is well known that calcium (Ca) and phosphorus (P) represent the key minerals composing the inorganic bone matrix, viz. the hydroxyapatite (Ca
10(PO
4)
6(OH)
2) [
14]. The metabolism of these minerals is strictly regulated by parathyroid hormone (PTH) and 1,25-dihydroxyvitamin D (1,25(OH)
2D), which operate in collaboration with vitamin K [
6,
15]. The roles of Ca and vitamin D as key nutrients have been widely discussed in previous excellent reviews [
16]. Interestingly, although Ca is essential for bone health, systematic analysis shows that populations from developing countries with low Ca intake (below about 900 mg/day) are characterized by lower risk of osteoporotic bone fractures as compared to developed countries [
17]. Furthermore, it has been observed that the association between increased Ca intake and BMD appears clinically irrelevant provided a baseline intake of at least 900 mg/day [
18]. Apparently, the Ca
homeostasis rather than the dietary amount of Ca represent a key factor linking Ca to osteoporosis under these circumstances. By being regulators of the Ca homeostasis, the vitamins D and K are essential agents in the preventive treatment of bone loss [
19,
20]. However, the mechanisms of the multiple effects of these vitamins on bone physiology are still insufficiently characterized, and epidemiological studies sometimes reveal contradictory results.
The objective of the present study has been to review recent epidemiological and clinical data on the association of vitamin K deficiency and combined vitamin K and D deficiencies with osteoporosis and to discuss mechanisms underlying these associations.
The present narrative review is based on a search via Pubmed, Medline and Google Scholar, in addition to our own research. The keywords used in the search were Vitamin K, Vitamin D, AND Osteoporosis or Bone mineral density (BMD). The search was limited to papers in the English language published in the period 2000 - 2023.
2. Vitamin K, as Activator of Osteocalcin and the Bone Health
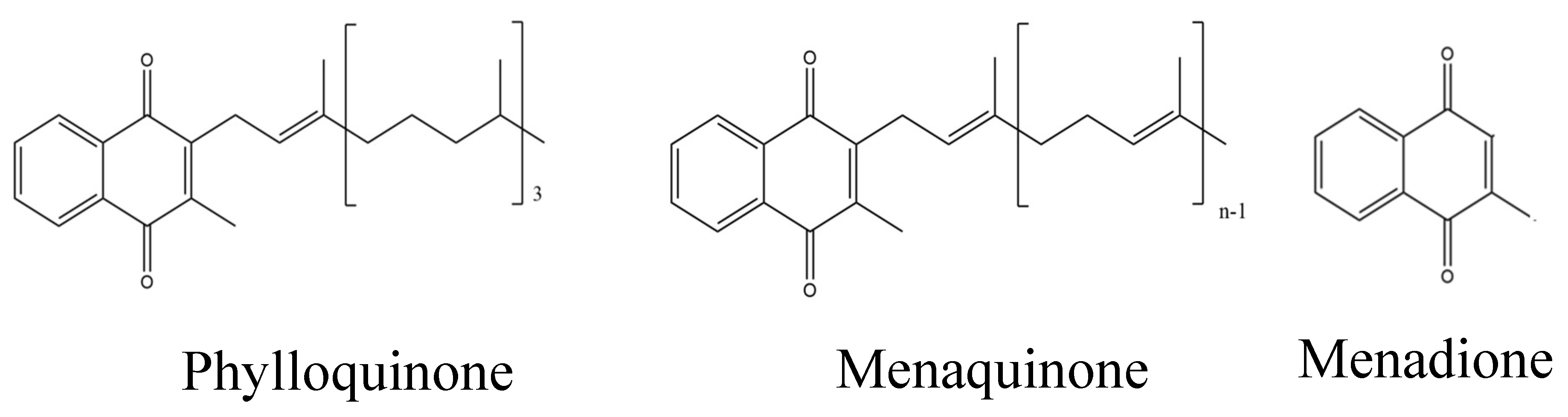
Vitamin K is a lipid-soluble vitamin that is found in various forms, viz. vitamins K1 (phylloquinone, PK) and K2 (menaquinone
s, MK). The derivative without the side chain is called menadione or K3 and is as such without vitamin K activity [
21]. It has been suggested that K1, the main form in liver is of particular importance for synthesis and carboxylation of several vitamin K dependent coagulation factors, while K2 appear to play a more important role in peripheral tissues with a beneficial effect on skeletal diseases [
22].
Especially vitamin K2 (
Figure 1) has been found to be involved in bone remodeling and bone health [
23,
24]. Most commonly, vitamin K2 exists in the form of menaquinone-4 to -10 (often denoted MK-4, to MK-10, respectively) [
25], where the numbers (n in
Figure 1) indicate the number of isoprenyl groups at C3 position. [
26].
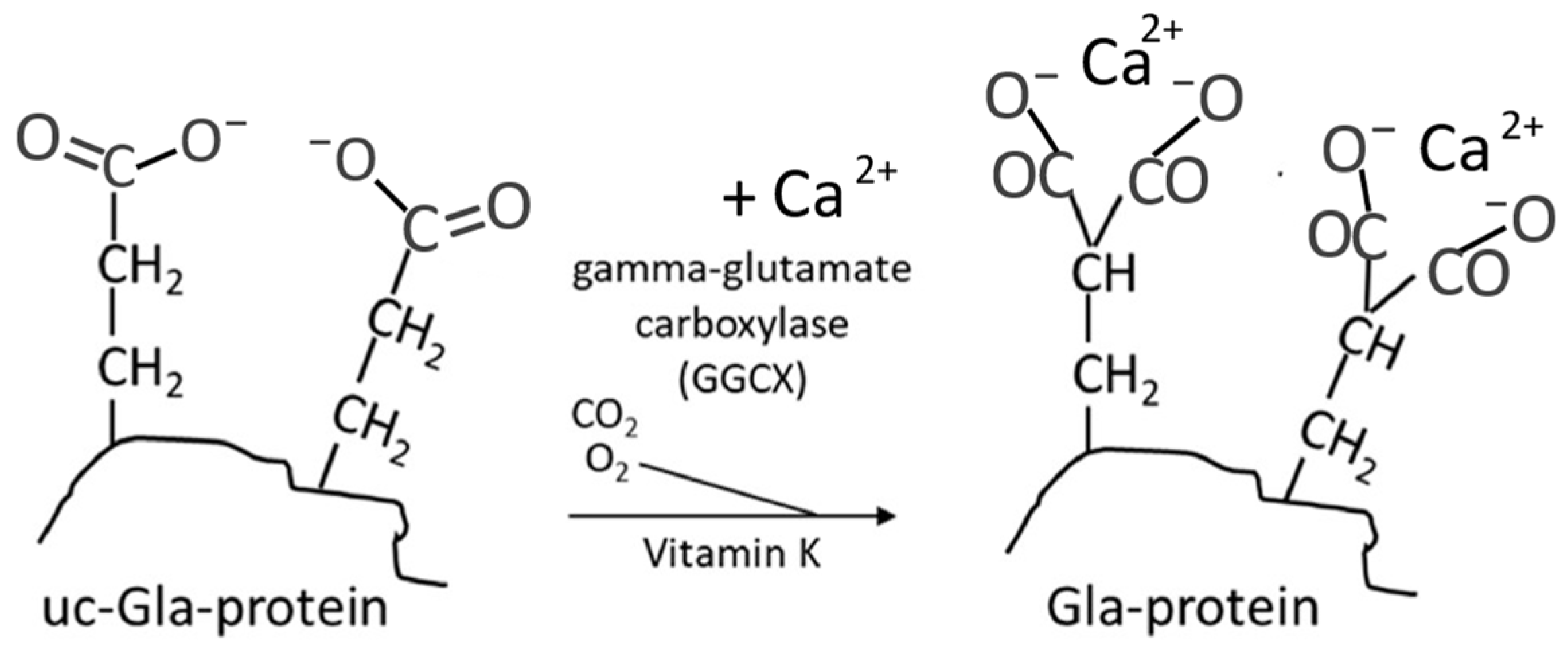
Vitamin K is a cofactor for an endoplasmic reticulum carboxylase that operates by activating some glutamic acid containing proteins (glu-proteins) into gamma-carboxyglutamic acid containing proteins (gla-proteins). The gla-amino acid is excreted in the urine when the protein is catabolized.
Among these proteins
osteocalcin is of particular importance for bone health. Osteocalcin is produced exclusively by the osteoblasts. The posttranslational activation involves an enzymatic carboxylation (
Figure 2) of three of its glutamic acid units thereby converting these units into calcium-chelating groups [
27]. One of the main functions of the activated osteocalcin appears to involve Ca import from blood and other tissues into bone for incorporation into hydroxyapatite, thereby influencing bone mineralization and making the skeleton less susceptible to fractures [
27]. A hypothesis states that osteocalcin can strengthen the bone through bridges between the bone components, as it can be bound both to hydroxyapatite and to the matrix protein osteopontin [
28].
The chemical state of osteocalcin has been used as a biomarker for vitamin K status. A low serum ratio of carboxylated (cOC) to undercarboxylated osteocalcin (ucOC) reflects an inadequate functional vitamin K, status, which appears to be associated with bone loss and increased risk of hip fractures [
29].
3. Observational and Interventional and Studies on Vitamin K and Bone
In general, bone density decreases with age, a process that is usually paralleled by increased vascular calcification [
26,
30]. A parallel phenomenon is a declining in vitamin K status that has been observed to result in a low ratio of carboxylated (cOC) to undercarboxylated osteocalcin (ucOC) [
31].
3.1. Observational Studies
Several studies have reported that low plasma vitamin K concentration is associated with increased risk of fractures in postmenopausal women. Beulens et al [
32] observed an inverse relationship between circulating vitamin K2 (MK-7) levels and the incidence of fractures in Japanese women. Associations between fractures and vitamin K1 levels have been observed in elderly Norwegians [
33] and in elderly Asians [
34] of both genders. A similar relation was seen in the Japanese general population as observed in a survey of unspecified vitamin K and the incidence of hip fractures [
35]. Other large observational studies of have confirmed these findings [
36]. However, some other researchers did not find an association of unspecified vitamin K intake and bone fracture risk, e.g. in Hong Kong [
37]. Nor was an association seen between K1 or K2 intake and vertebral fractures in the Hordaland Health Study of men and women in the age group 70 – 75 years. However, the intake of K1 in the lowest quartile was associated with an increased risk of hip fractures, while there was no association with the intake of K2 [
38]. In this Norwegian study the intake was assessed from food frequency questionnaires and the estimated intake of K2 was rather low (mean values below 14 µg/d).
3.2. Interventional Studies on Vitamin K and Osteoporosis
In a recent meta-analysis addressing the impact of vitamin K2 supplementation in postmenopausal women with and without osteoporosis 16 RCTs were included [
39]. In this meta-analysis studies that included vitamin K1 were excluded. Primary outcomes were change in BMD before and after treatment, fracture incidence, and change in ucOC and cOC. The studies comprised both studies supplementing with K2 alone or on top of vitamin D, calcium or a bisphosphonate. It was concluded that in 10 studies vitamin K2 supplementation improved lumbar spine BMD, however, in subgroup analysis only K2 given in combination with other remedies had a significant effect. K2 reduced fracture risk based on remaining five studies after removal of one study due to heterogeneity. K2 also decreased serum ucOC, while there was no change in cOC.
The effect of vitamin K2 (45 mg MK4/day for 12 months) in addition to risedronate, investigated in an RCT on 101 elderly women with postmenopausal osteoporosis, showed no significant difference between the risedronate group and the combined therapy group in terms of vertebral fracture incidence [
40]. However, Hirao et al [
41] in an RCT on postmenopausal women found a significant effect of K2 (45 mg/day for one year) on lumbar spine BMD, when given on top of alendronate. Møller et al. [
42] showed that a six-week period of supplementation with 180 μg/day of vitamin MK-7 in healthy subjects (20 - 66 years of age) was associated with an increase in serum cOC and a reduction in serum ucOC concentration.
A meta-analysis of 19 RCTs involving 6,759 study postmenopausal participants [
43], partly over-lapping with the meta-analysis of Ma et al. [
39], concluded that vitamin K2 played a significant role in the maintenance of vertebral BMD and in the prevention of fractures in their subgroup of postmenopausal women with osteoporosis. But in their non-osteoporotic subgroup vitamin K2 did not afford significant changes.
In 2022, Zhou et al. [
44] who reported the results of another meta-analysis of nine RCTs with 6,853 study participants, also partly over-lapping with the meta-analysis of Ma et al. [
39], concluded that vitamin K2 plays an important role for the maintenance of BMD. They also summarized observations showing that the vitamin decreases the levels of ucOC and increases cOC levels upon a long-term follow-up. The latter researchers concluded that vitamin K2 supplementation is safe and beneficial in the preventive treatment of osteoporosis for postmenopausal women.
Apparently, adequate intakes of vitamin K2 may improve bone quality in older age groups, which in turn reduces fracture risk, as has now been observed in several studies with population groups above the age of 50. Of note, variable results have been obtained depending on study design depending on length of study, whether vitamin K2 was given alone or on top of other treatments such as calcium, vitamin D or bisphosphonate and whether treatment groups had osteoporosis or not. Moreover, in several studies, vitamin K2, mostly MK4, was given in doses of 45 mg/day, which could be considered pharmacological doses as they are far above those necessary from a nutritional point of view. In several studies vitamin K2 resulted in a reduction in ucOC indicating a suboptimal or deficient vitamin K status. Although expression and activation of osteocalcin is apparently related to proper bone function; the precise
mechanisms of the vitamin K impacts are still under investigation [
45].
4. Mechanisms of Action of Vitamin K
In general, laboratory studies correspond to epidemiological studies in demonstrating the osteoprotective effect of vitamin K. In animal models of osteoporosis, vitamin K has demonstrated osteoprotective effects. Specifically, vitamin K supplementation was shown to be more efficient in improving bone characteristics in a model of immobilization osteoporosis as compared to combined Ca and vitamin D supplementation [
46]. A protective effect of MK-4 was also observed in a model of glucocorticoid induced bone loss [
47].
Important effects of vitamin K on Ca and skeletal homeostasis are known to be mediated through its role as a cofactor for the γ-glutamyl carboxylase enzyme that promotes conversion of glutamate (Glu) residues to gamma-carboxyglutamic (Gla) residues in the post-translational carboxylation of osteocalcin (OC) and matrix Gla protein (MGP) (
Figure 2), which may have a significant impact on osteogenesis [
48]. Activated MGP with five calcium and three phosphate chelating groups, in cooperation with osteocalcin with three dicarboxylic groups, appears to be essential for an adequate transfer of calcium from the circulation to the bones [
28]. In this respect, the action of these vitamin K dependent proteins (VKDPs) appears to have similarities with the action of the anti-osteoporotic bisphosphonates [
49]. However, some additional effects on bone health appear to be dependent on the forms of vitamin K species. For instance, the osteogenic effect of MK-4 is reported to be mediated partly by activation of the Wnt/β-catenin signaling pathway [
50], whereas MK-7 has been shown to increase osteoblast activation through a down-regulation of the Runx2 expression [
51], indicating that this homologue also may promote osteoblast maturation [
52]. It has been reported that vitamin K2 as MK-4 and MK-7 is more efficient than vitamin K1 in promoting osteoblast activity and inhibiting osteoclast activity [
53]. In an animal model of obesity, vitamin MK-4 appeared to promote osteogenesis by influencing the levels of activated OC and osteoprotegerin levels, while
reducing circulating RANKL levels [
53]. It is known that RANKL stimulates osteoclasts and bone resorption by being a receptor activator for the nuclear factor kappa-B ligand, whereas osteoprotegerin can counteract this effect. The inhibition of RANKL-induced osteoclastogenesis by MK-4 and MK-7 appears to be dose-dependent [
54]. Interestingly, the anti-osteoporotic drug denosumab (Prolia) acts by a similar mechanism [
55]. It has also been observed that MK-7 can reduce PTH-induced bone resorption [
56].
Another mechanism for the effects of vitamin K2 on bone appears to depend on a binding to the steroid and xenobiotic sensing nuclear receptor, SXR [
57,
58]. However, MK4 was the only vitamin K homologue that were bond to and thereby activated SXR [
59]. In osteoblasts, MK4 has been shown to activate the classical SXR target CYP3A4, in addition to its impacts on several genes and enzymes involved in bone formation, i.a. bone specific alkaline phosphatase (ALP), osteopontin (OPN), and osteoprotegerin [
57]. The SXR receptor appears to play an important role in bone maintenance as PXR (corresponding to SXR in humans) [
58] and knockout mice showed enhanced bone resorption and developed severe osteopenia despite adequate dietary vitamin K [
59].
Taken together, the existing clinical and laboratory data strongly indicate that appropriate vitamin K supplementation improves BMD and reduces fracture risk, especially in postmenopausal women.
5. Vitamin D and Vitamin K – Cooperators in Bone Protection?
Today vitamin D and vitamin K are considered key protective agents in the prevention of osteoporosis. The influence of vitamin D on bone functioning is not limited to the influence on Ca uptake and metabolism, but it also includes impacts on osteoblast and osteoclast functioning, these effects being mediated by the nuclear vitamin D receptor [
60,
61,
62]. Vitamin D exerts effects directly on osteoblasts by promoting osteoblast maturation and OC synthesis [
63,
64]. The multiple effects of vitamin D on bone are associated with high expression of the vitamin D receptor in several types of bone cells [
65].
Recent meta-analyses have confirmed previous indications that vitamin D
3 supplementation significantly increases BMD and reduces fracture risk in people above about 65 years when administered at a dose of at least 20 µg/day [
66,
67,
68]. Furthermore, supplementation with vitamin K2 on top of vitamin D3 supplementation appeared to improve BMD in postmenopausal women [
39].
A meta-analysis from 2020 of eight randomized clinical trials enrolling 971 study participants, concluded that vitamin K combined with vitamin D
3 significantly increased the total bone mineral density [
69]. Rønn et al. [
70] who conducted a placebo-controlled RCT using both MK-7 (375 μg/day) and vitamin D
3 plus calcium for three years in 142 postmenopausal women with osteopenia found that the combination increased carboxylation of osteocalcin, when compared with the placebo group which got only vitamin D
3 plus calcium. However, in this study the changes in bone turnover biomarkers were similar between the sub-groups with and without supplemented vitamin K.
In a recent RCT including 108 osteoporotic post-menopausal women with suboptimal vitamin K status Moore and co-workers [
71] investigated the effect of giving K1 (1 mg/day) or MK-4 (45 mg/day) in addition to bisphosphonate and calcium and vitamin D treatment (the latter combination referred to as placebo). In this study, there were no additional effects of vitamin K supplement on BMD or bone turnover markers, but a modest effect of K1 on hip geometry.
In another RCT 122 postmenopausal women were randomized into 4 groups [
72]. Three groups were fed farmed salmon containing either high levels of vitamin D and K1 or high vit D and low K1 or low vit D and high K1, together with calcium supplement. The fourth group were fed vitamin D and calcium. In all groups there were a positive effect on bone markers, but there were no significant differences between these groups.
A 2017 literature review on animal and human studies suggested that optimal concentrations of both vitamin D
3 and vitamin K are beneficial for bone health in postmenopausal women [
73]. The somewhat limited evidence (see also studies included in the meta-analyses of Ma et al. 2022 [
39] and Huang et al. 2015 [
43]) supports the hypothesis that combined vitamin D and vitamin K supplementation may be more effective than the supplementation with either vitamin alone for bone health.
6. Dietary Sources and Pharmacokinetics of the Vitamins K and D
The sources of vitamin K are different depending on the vitamers. Vitamin K1 is found mainly in dark green leafy vegetables, such as kale, spinach and broccoli where it is bound to the membranes of the chloroplasts [
74]. Menadione (K3) is often added as a supplement in animal feed and is converted to MK4 in their liver, which gives MK4 in animal food products. Fermented foods including fermented butter or cheese are sources of vitamin K2, especially the long-chain variants in the series MK5-10, depending on the starter culture in the cheese [
25]. Bioavailability of K1 depends on the food’s matrix. Thus, uptake of the vitamin from meals of pure spinach or broccoli is only 5-10%, but it is doubled with fat in the same meal [
25,
74]. It has been estimated that about 80% of the intake of vitamin K is as K1 worldwide [74,75}. Natto, a traditional Japanese soybean-based food, produced by fermentation, is a source of MK-7 [
75].
It is known that the vitamins K and D from food and supplements are incorporated into mixed micelles of lipids in the small intestines via the action of bile and pancreatic enzymes. The vitamins are absorbed together with the lipoid compounds in the small intestine [
26,
76].
As for vitamin K, the difference in structure between K1 and K2 has an effect that may be reflected in different absorption rates and bioavailability [
77]. Both the K1 and the K2 are absorbed via the enterocytes with the help of bile salts. From the blood circulation, the vitamin K is taken up by liver, where it is metabolized. Patients who have ileostomy or those who have underwent bariatric surgery are at risk of deficiency of the vitamin [
6]. But these patients can still absorb supplemental vitamin K2 given orally, provided a sufficient dose is given [
77]. After intake of vitamin K2, Møller et al. [
42] found a maximal blood serum concentration after 5 hours. However, the concentration did not return to the pre-administration level within the 72-hour observation period [
77] presumably due to the reaction of vitamin K in carboxylation of vitamin K dependent proteins and inefficient recycling of epoxidized vitamin K. A 2017 EFSA scientific panel concluded that dietary vitamin K2-MK-7 is more efficiently absorbed than synthetic free phylloquinone [
78]. However, it should be noted that dietary vitamin MK-7 does not contribute much to dietary vitamin K intake in most of the European countries. Thus, a significant difference for serum MK-7 was seen in women from Tokyo (5.3 ng/ml), Hiroshima (1.2 ng/ml) and Britain (0.37 ng/ml), with a corresponding inverse correlation with incidence of hip fractures, where the intake of natto was the only correlated food eaten, which gave MK-7 [
79].
It should be noted that the drug ezetimibe that is used for reducing cholesterol uptake from the gut also inhibits the vitamin K uptake [
80]. High-dosed statin therapy may also be a risk factor for osteoporosis [
81], since statin therapy leads to inhibition of the enzyme HMG CoA reductase that is necessary for the synthesis of the MK-4 vitamer, which is prevalent in peripheral tissues [
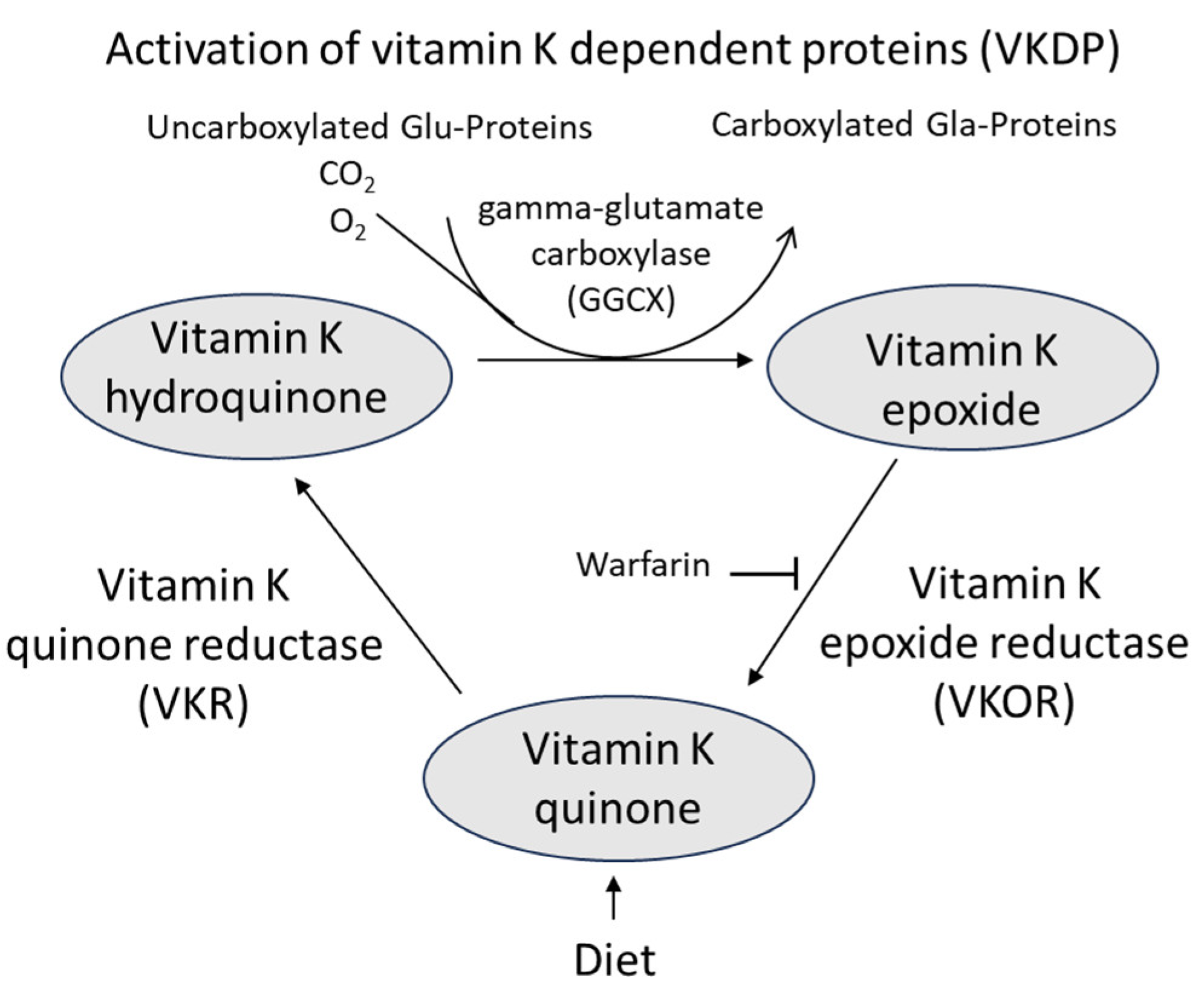
82]. It should also be noted that the anticoagulant drug warfarin that inhibits vitamin K epoxide reductase in the vitamin K cycle (
Figure 3) may lead to vitamin K deficiency, because of reduced recycling, also in bone tissue and thereby promote development of osteoporosis [
83]. More recently, direct working oral anticoagulants without this general impairing effect on all VKDP are about to replace warfarin today [
83].
A functional indicator for assessing the vitamin K status is the ratio of cOC-to-ucOC [
84]. It was reported that a supplemental intake of 250 μg/day of vitamin K1 activated osteocalcin appropriately, while a somewhat minor dose of vitamin K2-MK-7 (180 μg/day) resulted in a comparable effect [
84]. In that perspective doses of 45 mg/day of MK4 given in several studies [
39,
43] could be considered pharmacological. There is no conclusive information on the mechanism of uptake of vitamin K by the bones. However, it is known that the vitamin can be reactivated enzymatically by the vitamin K cycle [
85] and reused after its oxidation during carboxylation of osteocalcin and other proteins (
Figure 3).
During pregnancy, only small quantities of phylloquinone (PK) cross the placenta from mother to fetus. Blood concentrations of PK in the full-term newborn are about half of that of the mothers, the PK concentration in cord blood being as low as < 0.1 nmol/L [
75], explaining the importance of parenteral vitamin K supplementation to the newborn.
The 2017 EFSA scientific panel [
78] estimated that a mean of about 60% of injected PK is excreted in urine and faeces. No similar experiment has been done to assess the losses of metabolites after vitamin K2 ingestion.
As for vitamin D, an inactive precursor is cholecalciferol that must be converted to biologically active forms in the liver and kidneys. Following dietary intake or synthesis in the skin, the vitamin D precursors enter the circulation and are transported to the liver by the vitamin D-binding protein. In liver these precursors are converted to 25-hydroxyvitamin D, which make up the major circulating form of vitamin, the serum concentrations of this form being used as indicator of vitamin D
3 status [
86]. In the kidneys, the circulating compounds are hydroxylated to 1,25(OH)
2D
3 (1α,25-dihydroxyvitamin D / calcitriol). The production of in the kidneys is regulated by several factors, including serum calcium and parathyroid hormone (PTH). Most of the physiological effects of vitamin D in the body are related to the activity of calcitriol. Vitamin D acts to maintain Ca homeostasis in plasma and therefore, the action on vitamin D in bone is complicated as it may both stimulate mineralization, but in situations of low Ca it may promote osteoclasts and mobilize Ca from bone tissue [
87,
88]. Local activation of 25-(OH)D
3 to 1,25(OH)2D
3 may also take place in osteoblasts thereby exerting autocrine activity and maintenance of bone tissue [
87].
7. Conclusions and Perspectives
There exists an extensive body of evidence related to the positive effect of adequate vitamin K status and of vitamin K2 supplementation as regards the carboxylation of osteocalcin and the bone health. Less is known about the efficacy of vitamin K1 supplementation as most supplementation studies have been conducted with vitamin K2. Many studies have also used high and pharmacological doses of vitamin K2. Further research is necessary on the various mechanisms of actions of these vitamers for establishing vitamin K2 as a safe and cost-effective supplement for prevention of osteoporosis. Especially, there is a need for more research into questions related to a synergistic role of calcium and the vitamins D3 and K with respect to bone health. At present, it might be anticipated that the vitamins K and D3 would provide a preventive role, particularly in aged people. A combined regimen including vitamin K might also be of benefit in the prevention of osteoporosis following bariatric surgery as vitamin D3 and Ca do not seem to suffice. As for treatment of manifest osteoporosis drugs such as bisphosphonates or denosumab will still be essential as vitamin K2 appeared to have limited additional effect on BMD.
However, there remains research to establish data on optimized intakes and the ratio of vitamin K to vitamin D3 as well as data for optimal blood levels of the two protectors when supplemented in combination to individuals at risk. As for determination of the vitamin K status, the most reliable measure may be the ratio of total to undercarboxylated osteocalcin levels. It should be taken into consideration that the optimal vitamin K supplementation may vary from individual to individual.
Finally, there is a need for additional research into the long-term effects of combined supplementation with vitamin K and vitamin D3. Since the burden of osteoporosis is about to increase world-wide this research into preventive measures should be given high priority.
Author Contributions
Conceptualization, J.O.A., T.E.F. and J.A.; methodology, J.O.A., J.A., T.E.F. and M.A.; software, J.O.A., J.A., and M.A.; validation, J.O.A., and J.A.; formal analysis, J.O.A., J.A., and M.A.; investigation, J.O.A., J.A., and M.A.; resources, J.O.A.; data curation, J.O.A.; writing—original draft preparation, J.O.A.; writing—review and editing, J.O.A., M.A., T.E.F., and J.A.; visualization, J.A.; supervision, J.O.A., and J.A.; project administration, J.O.A..; funding acquisition, J.O.A.. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded from Innlandet Hospital Trust, Brumunddal, Norway and the Norwegian Institute of Public Health, Oslo, Norway.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used in this article are sourced from materials mentioned in the References section.
Acknowledgments
Innlandet Hospital Trust and the Norwegian Institute of Public Health are acknowledged for funding.
Conflicts of Interest
The authors declare that they have no confict of interest.
References
- Akkawi, I.; Zmerly, H. Osteoporosis: current concepts. Joints 2018, 6, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Lorentzon, M.; Cummings, S.R. Osteoporosis: the evolution of a diagnosis. Journal of internal medicine 2015, 277, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, G.; Boudin, E.; Van Hul, W. A look behind the scenes: the risk and pathogenesis of primary osteoporosis. Nature Reviews Rheumatology 2015, 11, 462–474. [Google Scholar] [CrossRef]
- Colangelo, L.; Biamonte, F.; Pepe, J.; Cipriani, C.; Minisola, S. Understanding and managing secondary osteoporosis. Expert Review of Endocrinology & Metabolism 2019, 14, 111–122. [Google Scholar]
- Ebeling, P.R.; Nguyen, H.H.; Aleksova, J.; Vincent, A.J.; Wong, P.; Milat, F. Secondary osteoporosis. Endocrine Reviews 2022, 43, 240–313. [Google Scholar]
- Aaseth, J.O.; Alexander, J. Postoperative Osteoporosis in Subjects with Morbid Obesity Undergoing Bariatric Surgery with Gastric Bypass or Sleeve Gastrectomy. Nutrients 2023, 15, 1302, https://www.mdpi.com/2072-6643/15/6/1302. [Google Scholar] [CrossRef] [PubMed]
- Blom-Høgestøl, I.K.; Hewitt, S.; Chahal-Kummen, M.; Brunborg, C.; Gulseth, H.L.; Kristinsson, J.A.; Eriksen, E.F.; Mala, T. (2019). Bone metabolism, bone mineral density and low-energy fractures 10 years after Roux-en-Y gastric bypass Bone 2019, 127, 436–445. [Google Scholar] [PubMed]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. 1. The global prevalence of osteoporosis in the world: a comprehensive systematic review and meta-analysis. Journal of orthopaedic surgery and research 2021, 16, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dennison, E.; Prieto-Alhambra, D. Osteoporosis epidemiology using international cohorts. Current Opinion in Rheumatology 2020, 32, 387–393. [Google Scholar] [CrossRef]
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The epidemiology of osteoporosis. British medical bulletin 2020, 133, 105–117. [Google Scholar] [CrossRef]
- Adami, G.; Fassio, A.; Gatti, D.; Viapiana, O.; Benini, C.; Danila, M.I.; Rossini, M. Osteoporosis in 10 years time: a glimpse into the future of osteoporosis. Therapeutic Advances in Musculoskeletal Disease 2022, 14, 1759720X221083541. [Google Scholar] [CrossRef] [PubMed]
- Pouresmaeili, F.; Kamalidehghan, B.; Kamarehei, M.; Goh, Y.M. A comprehensive overview on osteoporosis and its risk factors. Therapeutics and clinical risk management 2018, 2029–2049. Available online: https://www.doi/full/10.2147/TCRM.S138000. [CrossRef]
- Muñoz-Garach, A.; García-Fontana, B.; Muñoz-Torres, M. Nutrients and dietary patterns related to osteoporosis. Nutrients 2020, 12, 1986, https://www.mdpi.com/2072-6643/12/7/1986. [Google Scholar] [CrossRef] [PubMed]
- Black, J.D.; Tadros, B.J. Bone structure: from cortical to calcium. Orthopaedics and Trauma 2020, 34, 113–119. [Google Scholar] [CrossRef]
- Carmeliet, G.; Dermauw, V.; Bouillon, R. Vitamin D signaling in calcium and bone homeostasis: a delicate balance. Best Practice & Research Clinical Endocrinology & Metabolism 2015, 29, 621–631. [Google Scholar]
- Veldurthy, V.; Wei, R.; Oz, L.; Dhawan, P.; Jeon, Y.H.; Christakos, S. Vitamin D, calcium homeostasis and aging. Bone research 2016, 4, 1–7, https://www.nature.com/articles/boneres201641. [Google Scholar] [CrossRef]
- Tai, V.; Leung, W.; Grey, A.; Reid, I.R.; Bolland, M.J. Calcium intake and bone mineral density: systematic review and meta-analysis. Brit Med J 2015, 351. https://www.bmj.com/content/351/bmj.h4183.full.pdf+html. [Google Scholar] [CrossRef]
- Brincat, M.; Gambin, J.; Brincat, M.; Calleja-Agius, J. The role of vitamin D in osteoporosis. Maturitas 2015, 80, 329–332, https://www.sciencedirect.com/science/article/pii/S0378512214004083. [Google Scholar] [CrossRef]
- Kazemian, E.; Pourali, A.; Sedaghat, F.; Karimi, M.; Basirat, V.; Sajadi Hezaveh, Z.; Holick, M.F. Effect of supplemental vitamin D3 on bone mineral density: a systematic review and meta-analysis. Nutrition reviews 2023, 81, 511–530. [Google Scholar] [CrossRef]
- Fusaro, M.; Cianciolo, G.; Brandi, M.L.; Ferrari, S.; Nickolas, T.L.; Tripepi, G.; Plebani, M.; Cheung, A. Vitamin K and osteoporosis. Nutrients 2020, 12, 3625. [Google Scholar] [CrossRef]
- Bus, K.; Szterk, A. Relationship between structure and biological activity of various vitamin K forms. Foods 2021, 10, 3136. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Li, Y. Revisiting the interconnection between lipids and vitamin K metabolism; insights from recent research and potential therapeutic implications: a review. Nutrition & Metabolism 2024, 21, 6. [Google Scholar]
- Myneni, V.D.; Mezey, E. Regulation of bone remodeling by vitamin K2. Oral diseases 2017, 23, 1021–1028. [Google Scholar] [CrossRef]
- Stevenson, M.; Lloyd-Jones, M.; Papaioannou, D. Vitamin K to prevent fractures in older women: systematic review and economic evaluation. Health technology assessment (Winchester, England) 2009, 13, 1–134. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, C.; Joyce, R.; van’t Hoofd, C.; Knapen, M.H.J.; Xanthouela, S. Menaquinone content of cheese. Nutrients 2018, 10, 446. [Google Scholar] [CrossRef] [PubMed]
- Aaseth, J.O.; Alehagen, U.; Opstad, T.B.; Alexander, J. Vitamin K and calcium chelation in vascular health. Biomedicines 2023, 11, 3154. [Google Scholar] [CrossRef] [PubMed]
- Maresz, K. Proper calcium use: vitamin K2 as a promoter of bone and cardiovascular health. Integrative Medicine: A Clinician’s Journal 2015, 14, 34. [Google Scholar]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Levinger, I.; Scott, D.; Nicholson, G.C.; Stuart, A.L.; Duque, G.; McCorquodale, T.; Sanders, K.M. Undercarboxylated osteocalcin, muscle strength and indices of bone health in older women. Bone 2014, 64, 8–12. [Google Scholar] [CrossRef]
- Rodriguez, A.J.; Scott, D.; Ebeling, P.R. Exploring the links between common diseases of ageing—osteoporosis, sarcopenia and vascular calcification. Clinical Reviews in Bone and Mineral Metabolism 2019, 17, 1–23. [Google Scholar] [CrossRef]
- Rodriguez, M.; Fusaro, M.; Ciceri, P.; Gasperoni, L.; Cianciolo, G. The role of vitamin K in vascular calcification. Advances in chronic kidney disease 2019, 26, 437–444. [Google Scholar]
- Beulens, J.W.; Booth, S.L.; van den Heuvel, E.G.; Stoecklin, E.; Baka, A.; Vermeer, C. (2013). The role of menaquinones (vitamin K2) in human health. British Journal of Nutrition 2013, 110, 1357–1368. [Google Scholar] [CrossRef]
- Finnes, T.E; Lofthus, C.M.; Meyer, H.E.; Søgaard, A.J.; Tell, G.S.; Apalset, E.M. A combination of low serum concentrations of vitamins K1 and D is associated with increased risk of hip fractures in elderly Norwegians: a NOREPOS study. Osteoporosis international 2015, 27, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Tsugawa, N.; Kuwabara, A.; Kamao, M.; Tanaka, K.; Okano, T. High prevalence of hypovitaminosis D and K in patients with hip fracture. Asia Pac J Clin Nutr 2011, 20, 56–61. [Google Scholar] [PubMed]
- Yaegashi, Y.; Onoda, T.; Tanno, K.; Kuribayash, T.; Sakata, K.; Orimo, H. Association of hip fracture incidence and intake of calcium, magnesium, vitamin D, and vitamin K. Eur J Epidemiol 2008, 23, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, N.; Shiraki, M.; Suhara, Y.; Kamao, M.; Ozaki, R.; Tanaka, K. Low plasma phylloquinone concentration is associated with high incidence of vertebral fracture in Japanese women. J Bone Miner Metab 2008, 26, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.; Leung, J.; Woo, J. No association between dietary vitamin K intake and fracture risk in chinese community dwelling older men and women: a prospective study. Calcif Tissue Int 2012, 90, 396–403. [Google Scholar] [CrossRef]
- Apalset, E.M.; Gjesdal, C.G.; Eide, G.E.; Tell, G.S. Intake of vitamin K1 and K2 and risk of hip fractures: the Hordaland health study. Bone 2011, 49, 990–995. [Google Scholar] [CrossRef]
- Ma, M.L.; Ma, Z.J.; He, Y.L.; Sun, H.; Yang, B.; Ruan, B.J. : Wang, Y.X. Efficacy of vitamin K2 in the prevention and treatment of postmenopausal osteoporosis: A systematic review and meta-analysis of randomized controlled trials. Frontiers in Public Health 2022, 10, 2654. [Google Scholar] [CrossRef]
- Kasukawa, Y.; Miyakoshi, N.; Ebina, T.; Aizawa, T.; Hongo, M.; Nozaka, K. , et al. Effects of risedronate alone or combined with vitamin K2 on serum undercarboxylated osteocalcin and osteocalcin levels in postmenopausal osteoporosis. J Bone Miner Metab 2014, 32, 290–297. [Google Scholar] [CrossRef]
- Hirao, M. , Hashimoto, J., Ando, W., Ono, T., & Yoshikawa, H. (2008). Response of serum carboxylated and undercarboxylated osteocalcin to alendronate monotherapy and combined therapy with vitamin K 2 in postmenopausal women. Journal of bone and mineral metabolism 2008, 26, 260–264. [Google Scholar]
- Møller, M.; Gjelstad, I. M.F. : Baksaas, I.; Grande, T.; Aukrust, I.R.; Drevon, C.A.; Tone, G. Bioavailability and chemical/functional aspects of synthetic MK-7 vs. fermentation-derived MK-7 in randomised controlled trials. Int. J. Vitam. Nutr. Res 2016, 87, 1–15. [Google Scholar]
- Huang, Z-B.; Wan, S-L.; Lu, Y-J.; Ning L.; Liu, C.; Fan, S-W. Does vitamin K2 play a role in the prevention and treatment of osteoporosis for postmenopausal women: a meta-analysis of randomized controlled trials. Osteoporosis Int. 2015, 26, 1175–1186. [CrossRef] [PubMed]
- Zhou, M.; Han, S.; Zhang, W.; Wu, D. Efficacy and safety of vitamin K2 for postmenopausal women with osteoporosis at a long-term follow-up: meta-analysis and systematic review. Journal of Bone and Mineral Metabolism 2022, 40, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Halder, M.; Petsophonsakul, P.; Akbulut, A.C.; Pavlic, A.; Bohan, F.; Anderson, E.; Schurgers, L. Vitamin K: double bonds beyond coagulation insights into differences between vitamin K1 and K2 in health and disease. International journal of molecular sciences 2019, 20, 896. [Google Scholar] [CrossRef]
- El-Morsy, A.S.; Beshir, S.R.; Farrag, K.A.E.R.; Mohamed, M.S.; Hamam, G.G. Comparative study on the effect of vitamin K versus combined Ca and vitamin D administration on the prevention of experimentally-induced osteoporosis in adult male albino rats. Egyptian Journal of Histology 2011, 34, 5–14. [Google Scholar] [CrossRef]
- Sasaki, N.; Kusano, E.; Takahashi, H.; Ando, Y.; Yano, K.; Tsuda, E.; Asano, Y. Vitamin K2 inhibits glucocorticoid-induced bone loss partly by preventing the reduction of osteoprotegerin (OPG). Journal of bone and mineral metabolism 2005, 23, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Uitto, J.; Reutelingsperger, C.P. Vitamin K-dependent carboxylation of matrix Gla-protein: a crucial switch to control ectopic mineralization. Trends in molecular medicine 2013, 19, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Sahvieh, S. Effects of bisphosphonates on osteoporosis: Focus on zoledronate. Life Sciences 2021, 264, 118681. [Google Scholar] [CrossRef]
- Cui, Q.; Li, N.; Nie, F.; Yang, F.; Li, H.; Zhang, J. Vitamin K2 promotes the osteogenic differentiation of periodontal ligament stem cells via the Wnt/β-catenin signaling pathway. Archives of Oral Biology 2021, 124, 105057. [Google Scholar] [CrossRef]
- Lucero, C.M.; Vega, O.A.; Osorio, M.M.; Tapia, J.C; Antonelli, M.; Stein, G.S.; Galindo, M.A. The cancer-related transcription factor Runx2 modulates cell proliferation in human osteosarcoma cell lines. Journal of cellular physiology 2013, 228, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, A.C.; Wasilewski, G.B.; Rapp,N. ; Forin, F.; Singer, H.; Czogalla-Nitsche, K.J.; Schurgers, L.J. Menaquinone-7 supplementation improves osteogenesis in pluripotent stem cell derived mesenchymal stem cells. Frontiers in Cell and Developmental Biology 2021, 8, 618760. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Na, W.; Sohn, C. Vitamin K1 (phylloquinone) and K2 (menaquinone-4) supplementation improves bone formation in a high-fat diet-induced obese mice. Journal of clinical biochemistry and nutrition 2013, 53, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.J.; Kim, M.S.; Ahn, B.Y. The inhibitory effect of vitamin K on RANKL-induced osteoclast differentiation and bone resorption. Food & function 2015, 6, 3351–3358. [Google Scholar]
- Hanley, D.A.; Adachi, J.D.; Bell, A.; Brown, V. Denosumab: mechanism of action and clinical outcomes. International journal of clinical practice 2012, 66, 1139–1146. [Google Scholar] [CrossRef]
- Alonso, N.; Meinitzer, A.; Fritz-Petrin, E.; Enko, D.; Herrmann, M. Role of vitamin K in bone and muscle metabolism. Calcified Tissue International 2023, 112, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Tabb, M.M.; Sun, A.; Zhou, C.; Grun, F.; Errandi, J.; Romero, K.; Blumberg, B. Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. Journal of Biological Chemistry 2003, 278, 43919–43927. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Suhara, Y. New Aspects of Vitamin K Research with Synthetic Ligands: Transcriptional Activity via SXR and Neural Differentiation Activity. Int J Mol Sci. 2019, 20, 3006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Azuma, K.; Casey, S.C.; Ito, M.; Urano, T.; Horie, K.; Ouchi, Y.; Kirchner, S.; Blumberg, B.; Inoue, S. Pregnane X receptor knockout mice display osteopenia with reduced bone formation and enhanced bone resorption. J Endocrinol. 2010, 207, 257–63. [Google Scholar] [CrossRef]
- Li, A.; Cong, Q.; Xia, X.; Leong, W.F.; Yeh, J.; Miao, D.; Li, B. Pharmacologic calcitriol inhibits osteoclast lineage commitment via the BMP-Smad1 and IκB-NF-κB pathways. Journal of Bone and Mineral Research 2017, 32, 1406–1420. [Google Scholar] [CrossRef]
- Goltzman, D. Functions of vitamin D in bone. Histochemistry and cell biology 2018, 149, 305–312. [Google Scholar] [CrossRef]
- Kazemian, E.; Pourali, A.; Sedaghat, F.; Karimi, M.; Basirat, V.; Sajadi Hezaveh, Z.; Holick, M.F. Effect of supplemental vitamin D3 on bone mineral density: a systematic review and meta-analysis. Nutrition reviews 2023, 81, 511–530. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Ochiai-Shino, H.; Onodera, S.; Saito, A.; Shibahara, T.; Azuma, T. Promoting effect of 1, 25 (OH) 2 vitamin D3 in osteogenic differentiation from induced pluripotent stem cells to osteocyte-like cells. Open biology 2015, 5, 140201. [Google Scholar] [CrossRef] [PubMed]
- Borojević, A.; Jauković, A.; Kukolj, T.; Mojsilović, S.; Obradović, H.; Trivanović, D.; Bugarski, D. Vitamin D3 stimulates proliferation capacity, expression of pluripotency markers, and osteogenesis of human bone marrow mesenchymal stromal/stem cells, partly through sirt1 signaling. Biomolecules 2022, 12, 323. [Google Scholar] [CrossRef]
- Anderson, P.H. Vitamin D activity and metabolism in bone. Current Osteoporosis Reports 2017, 15, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.H.; Jang, H.N.; Kim, J.H.; Kim, S.W.; Shin, C.S. Effect of vitamin D supplementation on risk of fractures and falls according to dosage and interval: a meta-analysis. Endocrinology and Metabolism 2022, 37, 344–358. [Google Scholar] [CrossRef]
- Manoj, P.; Derwin, R.; George, S. What is the impact of daily oral supplementation of vitamin D3 (cholecalciferol) plus calcium on the incidence of hip fracture in older people? A systematic review and meta-analysis. International Journal of Older People Nursing 2023, 18, e12492. [Google Scholar] [CrossRef]
- Habibi Ghahfarrokhi, S.; Mohammadian-Hafshejani, A.; Sherwin, C.M.; Heidari-Soureshjani, S. Relationship between serum vitamin D and hip fracture in the elderly: a systematic review and meta-analysis. Journal of Bone and Mineral Metabolism 2022, 40, 541–553. [Google Scholar] [CrossRef]
- (Chakhtoura, M.; Bacha, D.S.; Gharios, C.; Ajjour, S.; Assaad, M.; Jabbour, Y.; El-Hajj Fuleihan, G. Vitamin D supplementation and fractures in adults: a systematic umbrella review of meta-analyses of controlled trials. The Journal of Clinical Endocrinology & Metabolism 2022, 107, 882–898. [Google Scholar]
- Kuang, X.; Liu, C.; Guo, X.; Li, K.; Deng, Q.; Li, D. The combination effect of vitamin K and vitamin D on human bone quality: A meta-analysis of randomized controlled trials. Food & function 2020, 11, 3280–3297. [Google Scholar]
- Rønn, S.H.; Harsløf, T.; Oei, L.; Pedersen, S.B.; Langdahl, B.L. (2021). The effect of vitamin MK-7 on bone mineral density and microarchitecture in postmenopausal women with osteopenia, a 3-year randomized, placebo-controlled clinical trial. Osteoporosis International 2021, 32, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.E.; Dulnoan, D.; Voong, K.; Ayis, S.; Mangelis, A.; Gorska, R.; Harrington, D.J.; Tang, J.C.Y.; Fraser, W.D.; Hampson, G. The additive effect of vitamin K supplementation and bisphosphonate on fracture risk in post-menopausal osteoporosis: a randomised placebo- controlled trial. Arch Osteoporos 2023, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Graff, I.E.; Øyen, J.; Kjellevold, M.; Frøyland, L.; Gjesdal, C.G.; Almås, B.; Rosenlund, G.; Lie, Ø. Reduced bone resorption by intake of dietary vitamin D and K from tailor-made Atlantic salmon: A randomized intervention trial. Oncotarget 2016, 7, 69200–69215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Ballegooijen, A.J.; Pilz, S.; Tomaschitz, A.; Grübler, M.R.; Verheyen, N. The synergistic interplay between vitamins D and K for bone and cardiovascular health: a narrative review. International journal of endocrinology 2017. [Google Scholar]
- Schurgers, L.J; Vermeer, C. Determination of Phylloquinone and Menaquinones in Food. Hemostasis 2000, 30, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Simes, D.C.; Viegas, C.S.; Araújo, N.; Marreiros, C. Vitamin K as a diet supplement with impact in human health: current evidence in age-related diseases. Nutrients 2020, 12, 138. [Google Scholar] [CrossRef]
- Silva, M.C.; Furlanetto, T.W. Intestinal absorption of vitamin D: a systematic review. Nutrition reviews 2018, 76, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D. Nutritional supplementation for vitamin B12 and vitamin K2 deficiency following ileostomy or colostomy formation. Gastrointestinal Nursing 2020, 18 (Suppl. S4), S12–S16. [Google Scholar] [CrossRef]
- <bold>Se referanse 42</bold>. Møller, M., Gjelstad, I. M. F., Baksaas, I., Grande, T., Aukrust, I. R., Drevon, C. A., ... & Tone, G. (2016). Bioavailability and chemical/functional aspects of synthetic MK-7 vs. fermentation-derived MK-7 in randomised controlled trials. Int. J. Vitam. Nutr. Res. 2016, 87, 1–15.
- EFSA. Dietary reference values for vitamin K. EFSA Journal 2017, 15, 4780. [Google Scholar]
- Kaneki, M.; Stephen, J.; Hosoi, T.; Fujiwara, S. Japanese fermented soybean food as the major determinant of the large geographic difference in circulating levels of vitamin K2: Possible implications for Hip-Fracture risk. Nutrition 2001, 17, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M.J.; Okano, T. Key Pathways and Regulators of Vitamin K Function and Intermediary Metabolism. Annu. Rev. Nutr. 2018, 38, 127–151. [Google Scholar] [CrossRef] [PubMed]
- Leutner, M.; Matzhold, C.; Bellach, L. Diagnosis of osteoporosis in statin-treated patients is dose-dependent. Ann Rheum Dis Dec 2019, 78, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Li, Y. (2024). Revisiting the interconnection between lipids and vitamin K metabolism; insights from recent research and potential therapeutic implications: a review. Nutrition & Metabolism 2024, 21, 6. [Google Scholar]
- Liu,Y. ; Xie, X.; Sun, Y.; Yu, T. Risk of osteoporosis in patients treated with direct oral anticoagulants vs. warfarin: an analysis of observational studies. Frontiers in Endocrinology 2023, 14, 1212570. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Inaba, N.; Yamashita, T. MK-7 and its effects on bone quality and strength. Nutrients 2020, 12, 965. [Google Scholar] [CrossRef]
- Ferland, G. The discovery of vitamin K and its clinical applications. Annals of Nutrition and Metabolism 2012, 61, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. A millenium perspective on Vitamin D. J Cell Biochem 2003, 88, 296–307. [Google Scholar] [CrossRef]
- Turner, A.G.; Hanrath, M.A.; Morris, H.A.; Atkins, G.J.; Anderson, P.H. The local production of 1,25(OH)2D3 promotes osteoblast and osteocyte maturation. J Steroid Biochem Mol Biol. 2014, 144 Pt A, A,114–118. [Google Scholar] [CrossRef]
- Udagawa, N.; Takahashi, E.; Jimi, K.; Matsuzaki, T.; Tsurukai, K.; Itoh, N.; Nakagawa, H.; Yasuda, M.; Goto, E.; Tsuda, K.; Higashio, M.T.; Gillespie, T.J.; Martin, T. Suda, Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor. Bone 1999, 25, 517–523. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).