1. Introduction
Grapes (
Vitis vinifera, L.) are one of the world's most popular and preferred fruit crops, since it is the world's fourth crop after citrus, apples, and bananas. Spain is the world's greatest grape producer, followed by France and Italy. In Egypt, grape is regarded as one of the most important fruit crops, coming as second after citrus. Minya governorate leads the Egypt's grape production, followed by Dakahlia and EL-Gharbia. Because of its high net return, the planted area has risen significantly over the last two decades and reached about 172.533 feddan that produced 1586342 tons [
1].
Chemical fertilizers that applied in excess have an adverse effect on ground water and cause eutrophication in aquatic environments. More focus has recently been placed on less expensive, safer, and more natural additives. Due to its nontoxicity, nutritional value, and ease of application, yeast has recently gained a lot of attention in the academic community. It is a type of soluble powder or paste that is created from fresh yeast that has a high level of biological activity, or brewer's yeast. Additionally, yeast extract has a wealth of beneficial components, including trace elements, amino acids, nucleotides, peptides, nitrogen, and low-molecular-weight organic materials. Furthermore, it has no harmful chemicals or chemically produced hormones [
2]. Both the yield and quality of needle mushrooms may be enhanced by yeast extract. Many vegetable crops showed considerable improvements in vegetative growth, yield, and quality when yeast was applied [
3,
7]. Vegetables also had higher elemental contents, including N, P, K, Fe, and Zn [
8].
Numerous elements, such as climate, irrigation, mineral nutrition, and vineyard maintenance, affect the grape output and quality. Also, managing nutrients effectively is essential to raise the output. Micronutrients particularly play a critical role in the growth and output of vines.
Manganese is a crucial microelement for the formation of chlorophyll, it has a clear effect on the metabolism of nitrogen, and the aqueous reactions involved in photosynthesis. It is crucial for the processes of reproduction, pollination, meristematic tissue cell division, vascular tissue repair, metabolism, and transfer of carbohydrates [
9].
Recently, nanotechnology has been applied to various fields, including agriculture. The term "nanomaterial" is defined as "a natural, incidental, or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50% or more of the particles in the number size distribution, one or more external dimensions is in the size range 1-100 nm" in the "Recommendation on the definition of a nanomaterial" adopted by the European Commission [
10], these materials' characteristics differ from those of micrometric or larger-sized materials because of their size. Also, have variations in electrical conductivity, chemical reactivity, and physical strength. Crop management may benefit greatly from the advancement of nanotechnology [
11].
The effects of nanostructured materials on plant germination and growth in recent years have been examined by many researchers. For instance, it is believed that certain plants produce more biomass and have a significantly higher germination rate because of the penetration of nanostructured materials into seeds [
12]. Foliar spray of almonds with Nano-chelate super plus (zinc, iron, and manganese) improved a variety of nut characteristics, including the yield, nut length, width, and fresh and dry weight. Further, the concentration of micro elements (Zn, Fe, Cu, and Mn) in the leaves increased significantly across all treatments [
13].
Many of biotic resources have been designed to leverage with them, such as algae, fungus, bacteria, and plant extracts, for the biogenesis production of metal/metal oxide nanoparticles. When compared to the methods that rely on yeast and bacteria for assistance, the use of plant extract is one of the most straightforward and straightforward eco-friendly methods for producing M/MO NPs in large quantities. Many kinds of naturally occurring bio-extracts from bacteria, yeast, and fungus have been used as effective sources for the manufacture of M/MO NPs (known as Nano green products). It has been demonstrated that the best stabilizing, capping, and reducing agent for the synthesis of regulated M/MO NPs is plant bio-extract. Techniques for biologic synthesis are highly helpful in controlling all properties, including shapes, sizes, structures, and other distinguishing qualities [
14].
2. Material and Methods
2.1. First: The Microbiological Experiment
2.1.1. Microbial Sample
This study used an Egyptian Saccharomyces cerevisiae sample that was received from the National Research Center's Microbial Biotechnology Laboratory. As a growth medium, peptone, glucose, and yeast extract were utilized (YEPD).
2.1.2. Enrichment of Yeast with Manganese Sulphate
Several incorporation procedures were employed in this work to enrich yeast with manganese, including the following: (1) In the initial process, manganese was added to the liquid medium just after the yeast was inoculated (growth phase). (2) After a 24-hour incubation period (the non-growth phase), it was inserted for integration in the second process [
15].
After sterilizing one liter of YEPD media, 200 ml of it was split among four conical flasks and aseptically inoculated. By dissolving 500 mg of powdered manganese sulphate in 100 ml of distilled water, a manganese sulphate solution was created. Each of the four conical flasks received varying quantities of manganese sulphate solution, with concentrations of 0.0195 g/l, 0.143 g/l, 0.2 g/l, and 0.4 g/l, sequentially. The flasks were then placed in an incubator/shaker and maintained at 30°C and 120 rpm. After a 24-hour period, the dry weight, absorbance, and total manganese concentration of the yeast samples were determined.
2.1.3. Biomass Concentration Determination
Spectronic Instruments, USA created a UV Spectrophotometer that was used to measure the biomass concentration at 620 nm. 30 milliliters of a 1:1 nitric acid solution (15 milliliters of concentrated nitric acid plus 15 milliliters of Millipore water) were used to dissolve 1.0 gram of manganese metal for use in an atomic absorption spectrophotometer. The manganese metal was entirely dissolved in this solution when it was heated to 60 °C for ten minutes. The solution was cooled and filled a 1000 ml volumetric flask with Millipore water.
2.1.4. Manganese Level in Yeast Cells Determination
Using an Atomic Absorption Spectrophotometer (AAS) (Shimadzu Scientific Instrument Inc., USA), the amount of manganese in yeast cells was measured. An adapted version of Demirci and Pometto's [
16] methodology was utilized to prepare samples for Atomic Absorption Spectroscopy (AAS). After centrifuging the yeast sample for 15 minutes at 10,000 rpm and 4°C. The supernatant was then removed. To remove all of the growth media that had adsorbed on the surface, the cells were washed three times with 0.9% saline water. A 300 ml Kjeldahl flask was filled with 0.1 g of dried yeast. After adding 5.0 ml of concentrated nitric acid to the dry material, it was heated to 160 °C until the violent reaction subsided. After adding 2.0 ml of concentrated sulfuric acid and continuing to boil the mixture, concentrated nitric acid was gradually added until the mixture was colorless. The heating procedure was continued until a dense sulfuric acid fume was produced. After cooling, the contents were filtered into a volumetric flask of 50 milliliters and the proper volume was adjusted by adding distilled water. Using a Flame Atomic Absorption Spectrophotometer, the sample's absorbance was determined at 213.9 nm.
2.2. Second: The Horticultural Experiment
Two seasons in consecutive years were used for this study on Flame Seedless grapes (Vitis vinifera L.). Grapevines were approximately ten years old, they were planted 1.5 by 3.5 meters apart under a flood irrigation system, trained using a bilateral horizontal cordon system, and had spur pruning (each with 2-3 eyes) at an individual grove, at Sammnod region, Gharbiya governorate, Egypt.
To ascertain the physical and chemical characteristics, soil samples were extracted from the soil at two depths: 0–30 and 30–60 cm below the soil's surface.
Results of the soil analysis (
Table 1) indicate that, the texture of the soil was loamy, EC ranged from 0.4 to 0.5 ds/m, pH was 8.4, and CaCO
3 ranged between 1.2- 2.0%. Accordingly, the soil in this location is loamy, none saline and none calcareous.
Three replicates were implemented in a randomized complete block design (RCBD) to set up the experiment. Treatments were applied as foliar spray twice in each season, the 1st was carried out before pollination and the 2nd was done three weeks after pollination as follows:
2.2.1. Treatments
T1: Manganese Sulphate at 2.0 g/l (as control).
T2: Manganese chelate 12 % w.w (amino and organic acids) at 2.0 g/l (as farm traditional application).
T3: Active fresh yeast enriched with manganese at 10 cm3/l.
T4: Active fresh yeast enriched with manganese at 20 cm3/l.
T5: Frozen yeast enriched with manganese at 10 cm3/l.
T6: Frozen yeast enriched with manganese at 20 cm3/l.
2.2.2. Measurements
Vegetative Measurements
Using a planimeter, the average leaf area (cm2) of each vine was determined in mid-July by measuring the fifth fully developed mature leaf from the stem tip. The diameter and average shoot length were calculated in centimeters. The number of leaves and shoots was counted.
Chemical Determinations
Leaf total chlorophyll:
By applying a Minolta chlorophyll meter (spad, 501), the total chlorophyll in leaves was measured in fresh leaf samples as spad units (spad = 100 mg chlorophyll/g fresh weight).
Leaf mineral content
In order to ascertain the mineral contents of the leaves, samples of twenty leaves, comprising the blade and petiole (the sixth leaf from the shoot tip) of each vine, were taken later in July. Following a 60–70°C oven drying period, leaves were cleaned with distilled water and allowed to reach a consistent weight. According to Jackson's approach [
17], 0.2 grams of each sample's ground material was digested using a mixture of perchloric acid and sulphoric acid 1:10 (v/v) after the dried samples were ground in a stainless steel knife mill. Using the Cottenie method [
18], the percentages of dry weight for nitrogen, phosphorus, and potassium were calculated. Using the atomic absorption apparatus, iron, zinc, and manganese were quantified in parts per million using the Cottenie method [
18].
Yield and Fruit Quality
Cluster numbers on each vine were totaled and weighed in order to estimate the total yield per vine (kg). The crop was picked at the ripening stage when TSS % reached 16% and color covered all bunch berries.
- 2.
Fruit properties
- 2.1
Physical properties
The weight of 100 berries (g), their juice weight (g), their average cluster dimension (length and width in cm), and their average cluster weight (g) were measured.
- 2.2
Chemical properties
Using a hand refractometer, the total soluble solids percentage (TSS %) in berry juice was calculated, and the total titratable acidity percentage was reported as tartaric acid /100 ml of juice [
19].
2.2.3. Statistical Analysis
Analysis of variance was performed on all the data in accordance with Snedecor and Cochran [
20]. Statistically, the least significant difference (LSD) test was used to analyze significant differences between means at p≤0.05 [
21]. The Co-stat program was used to do the statistical analysis.
3. Results
3.1. The Microbiological Experiment
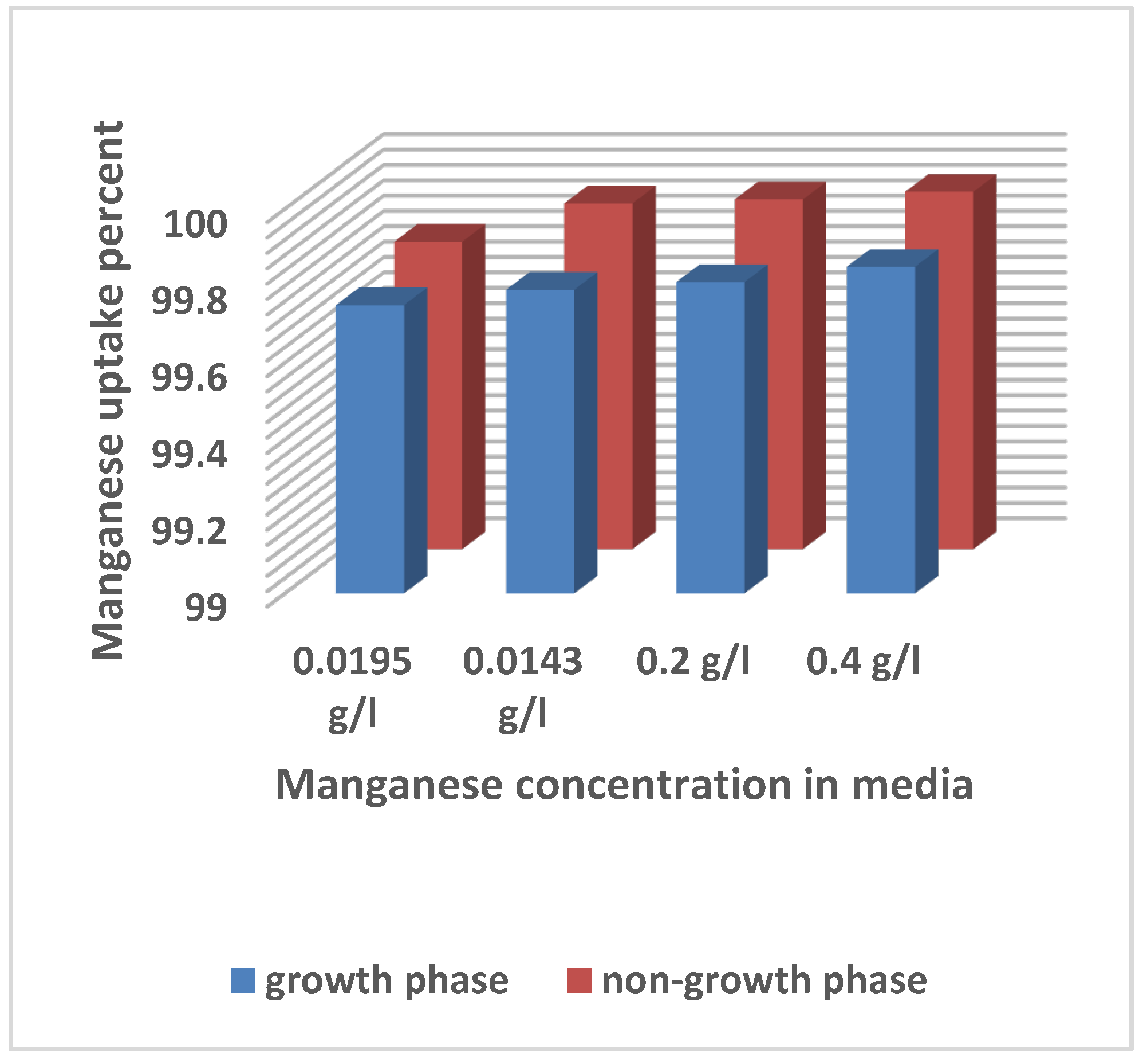
In this work, two distinct strategies for enriching yeast with manganese as a trace element were used. Manganese was supplied to the liquid medium immediately after the yeast was inoculated (growth phase) in the first approach, and after 24h of incubation (non-growth phase) in the second method for integration.
Figure 1 illustrates manganese content in yeast cells during growth and static phases with varying manganese sulphate concentrations in the culture medium. The results indicate that when the amount of manganese sulfate in the medium grew, so did the amount of manganese in yeast cells. Manganese accumulation in yeast cells was 99.93% higher at 0.4 g/l of manganese concentration throughout the media than it was at 0.0195 g/l of manganese concentration (
Figure 1).
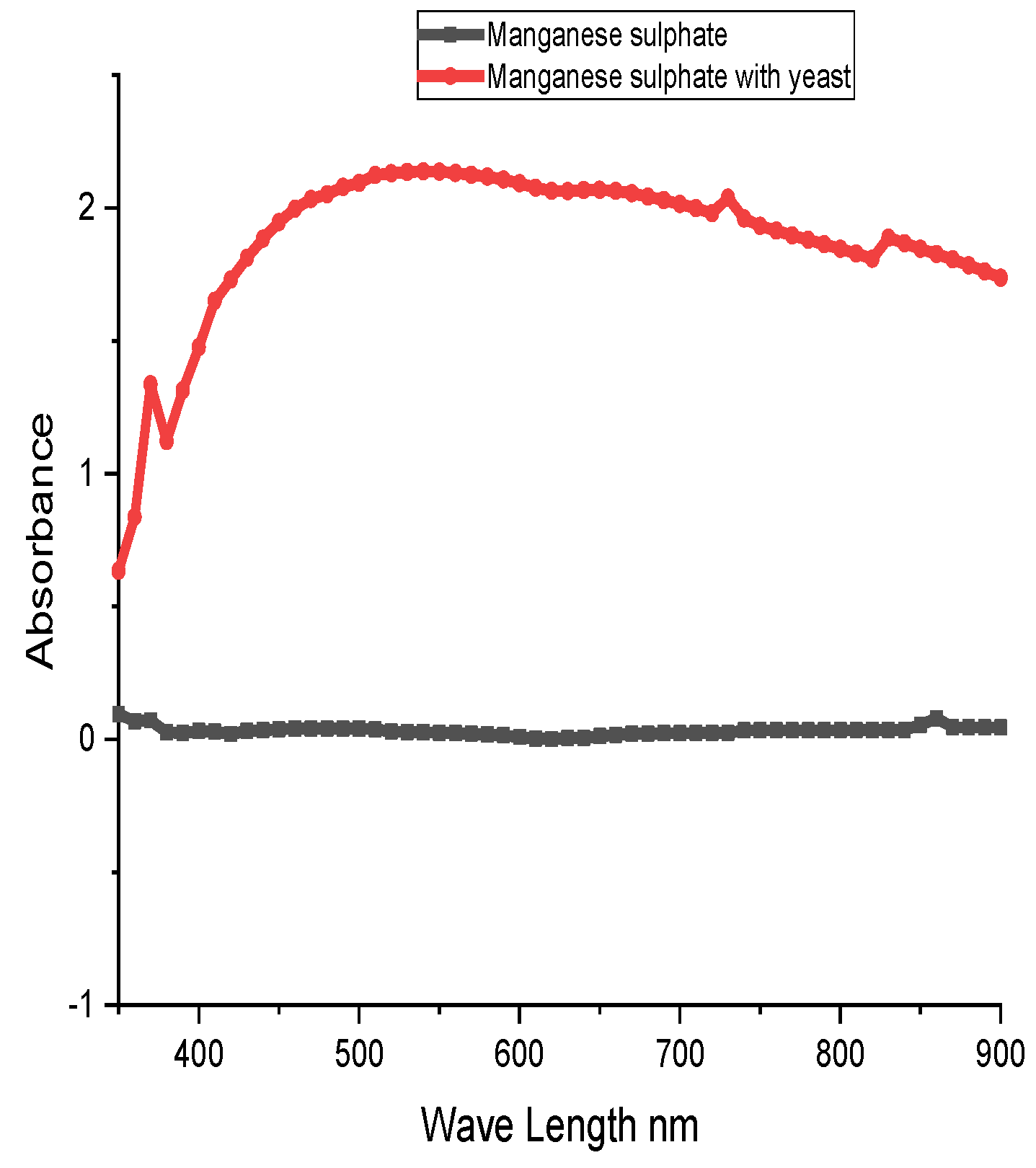
Effect of manganese on the yeast cell growth biomass was calculated using a UV spectrophotometer to measure manganese absorbance.
Figure 2 depicts the influence of manganese on yeast cell development. As demonstrated in the figure, the OD of manganese sulphate alone was not changed, however, the OD of yeast cell biomass was increased due to manganese concentration in the medium.
3.2. Horticultural Experiment
The results presented in
Table 2 indicate that the highest significant values of nitrogen (2.20 & 2.42%) in the leaves were reflected due to the frozen yeast enriched with Mn at 20 cm
3/l as foliar spray followed by 10 cm
3/l of the same treatment in both seasons, while, the lowest values of nitrogen (1.33 & 1.55%) were recorded from manganese sulphate treatment (control) as foliar spray in the two seasons. Regarding phosphorous percentage in the leaves, there were no significant differences among the treatments in the two experimental seasons. As for potassium percentage in the leaves, it is clear that spraying active fresh yeast at 20 cm
3/l had a positive effect and recorded the highest values (2.18 & 2.40%) in the two seasons followed without significance with the frozen yeast at 20 cm
3/l. The lowest values of potassium in the leaves (1.03 & 1.25%) were observed due to spraying manganese sulphate at 2.0 g/l. This was true in the two successive seasons.
The results tabulated in
Table 3 indicate that the frozen yeast extract enriched with manganese at the rate of 20 cm
3/l gave the highest values of Fe, Zn and Mn, since this treatment recorded the highest significant values of Fe (107.0 & 109.3 ppm), Zn (55.80 & 56.92 ppm) and Mn (49.53 & 50.65 ppm) in both seasons. Meanwhile, the lowest values of Fe (99.7& 101.0 ppm), Zn (29.50 & 30.62 ppm) and Mn (19.73 & 20.85 ppm) were obtained when the grapevines sprayed with manganese sulphate treatment in the two seasons.
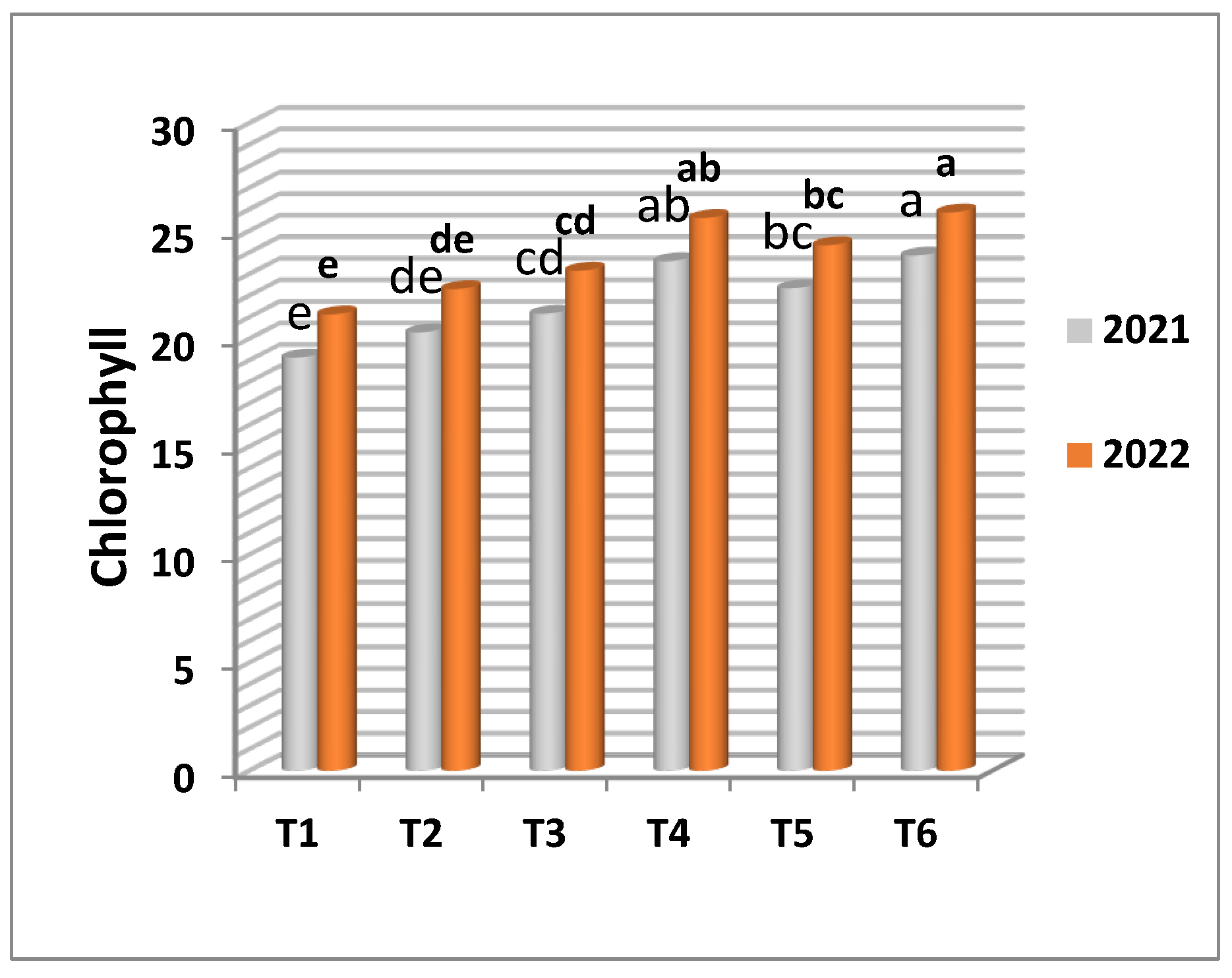
The results presented in
Table 4 and
Figure 3 and
Figure 4 show that spraying grapevines with the frozen yeast enriched with Mn at 20 cm
3/l was significantly responsible for motivating the growth parameters such as shoot length, number of leaves per shoot and leaf area compared with the other treatments. Increasing concentration of frozen yeast enriched with Mn in this study was associated with the promotion. The maximum values of shoot length, number of leaves per shoot, leaf area and chlorophyll content were observed on the vines received the frozen yeast enriched with manganese at 20 cm
3/l followed by 10 cm
3/l of the same form in both experimental seasons, while the vines treated with manganese sulphate and manganese chelate as foliar application recorded lower values in both seasons. Regarding shoot diameter, no significant difference was detected among the treatments.
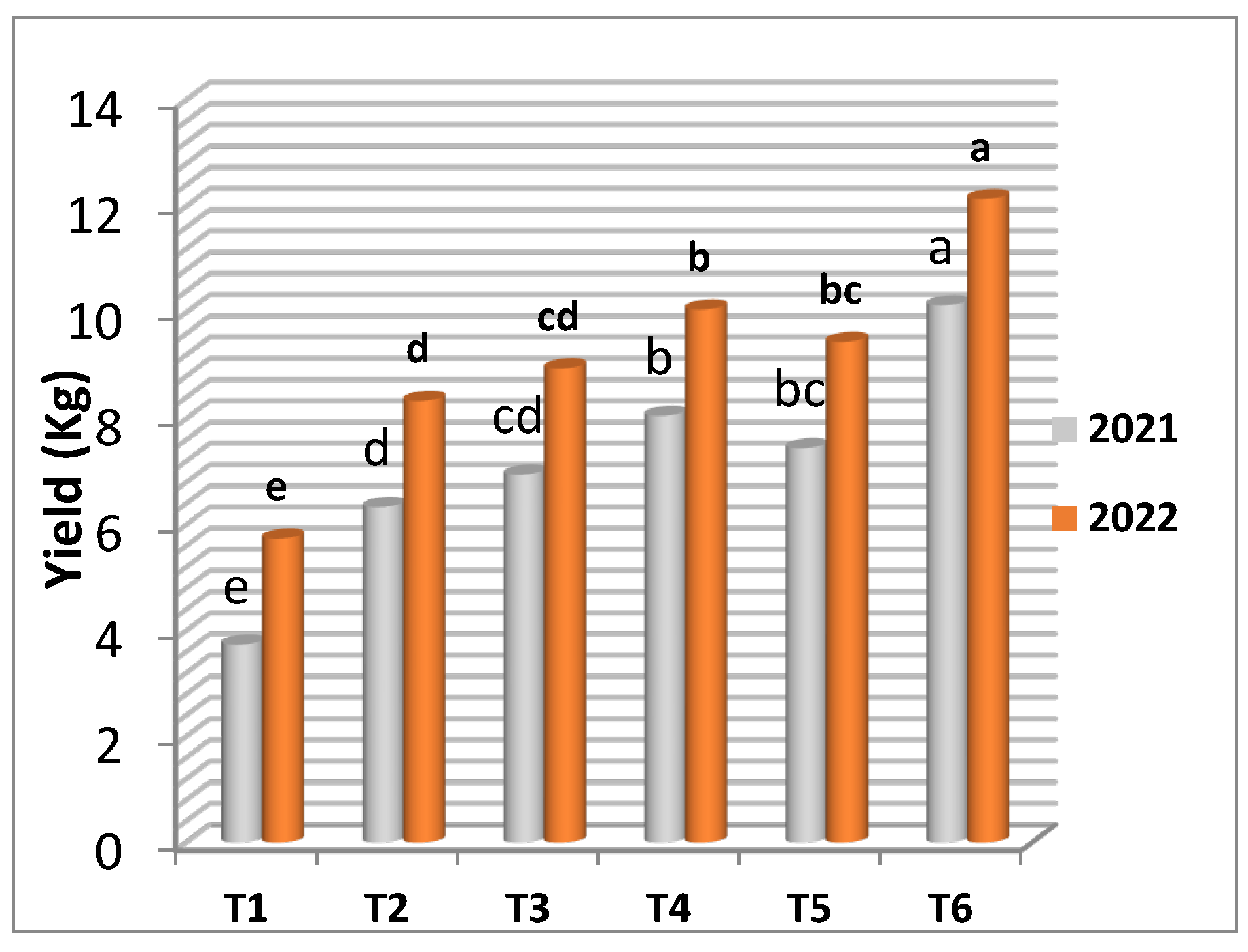
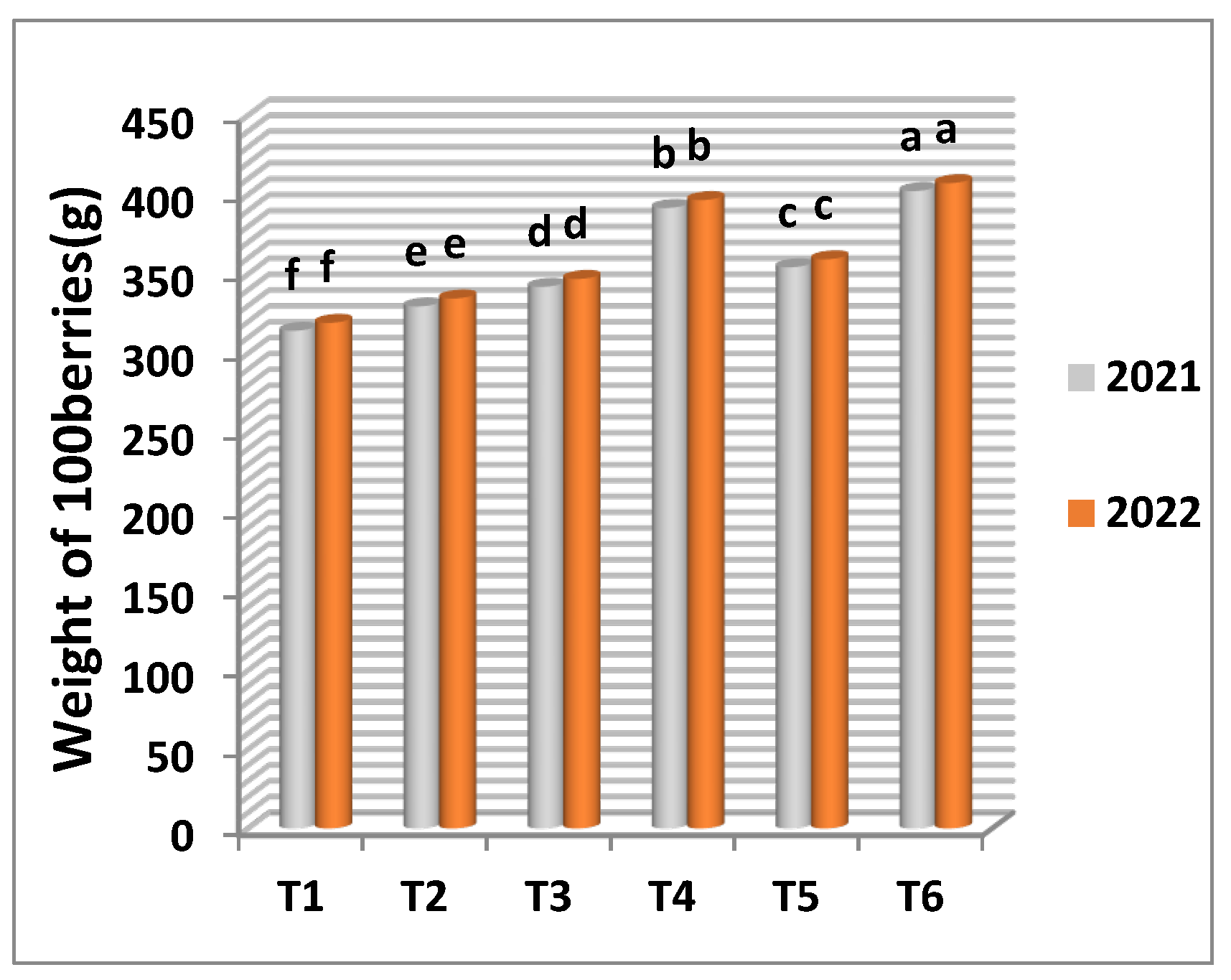
The results presented in
Table 5 and
Figure 5 and
Figure 6 illustrate that the highest significant values of cluster number per vine, cluster weight, weight of 100 berries and yield (kg) in the two seasons were obtained from the frozen yeast enriched with manganese at 20 cm
3/l as spraying application. Meanwhile, manganese sulphate (control) recorded the lowest number of clusters per vine, cluster weight, weight of 100 berries and yield per vine in the first and the second seasons.
The results in
Table 6 show that treating Flame Seedless grapevines with that yeast extract enriched with Mn significantly improved cluster length, cluster width and juice weight of 100 berries compared with the other treatments. Spraying frozen yeast extract enriched with manganese at 20 cm
3/l gave the highest values of cluster length, cluster width and juice weight of 100 berries in both experimental seasons. While spraying vines with manganese sulphate recorded the lowest values of cluster length, cluster width and juice weight of 100 berries.
As shown in
Table 7, spraying frozen yeast extract enriched with Mn at 20 cm
3/l scored the highest values of TSS. Regarding acidity, manganese sulphate treatment gave the highest values, while frozen yeast extract enriched with Mn at 20 cm
3/l scored the lowest values in the both seasons with significant difference among the treatments.
4. Discussion
Manganese (Mn) is essential for oxidation and reduction activities in the plant like electron transport during photosynthesis. In addition, manganese has a function in the synthesis of chlorophyll and is necessary for photosystem. Manganese is an activating element that activates more than 35 different enzymes [
9]. Using fertilizers containing manganese improves the rate at which carbohydrates like starch are synthesized and photosynthesized. A manganese shortage lowers the efficiency of photosynthesis, which lowers agricultural output and quality. This is due to the metabolic function of manganese in the activation of enzymes involved in the metabolism of carbohydrates and nitrate-reducing enzyme activity. Regarding the microbiological experiment, yeasts have been employed as a mean of delivering mineral supplements because of their high protein content and capacity to assimilate metals into their cells. It is well known that yeasts can gather metal ions from aqueous solutions through a variety of physicochemical processes. The concentration of manganese in yeast cells increased as the concentration of manganese sulphate in the medium increased because manganese incorporation in yeast cells was 99.93% higher at 0.4 g/l of manganese concentration in the medium than at 0.0195 g/l of manganese concentration in the medium. Prior research shown that 75 mg l
-1 MnSO
4 was incorporated in yeast cells [
22]. The two techniques for manganese enrichment of yeast did not differ significantly from one to another. Therefore, in order to reduce the possibility of medium contamination, the first approach (growth phase) was used. Consequently, the first strategy (growth phase) was selected in order to reduce the possibility of medium contamination. Our findings indicate that while manganese sulfate had no passive influence on yeast cell biomass, the quantity of manganese in the medium enhanced the OD of yeast cell biomass. Regarding this matter, reports have indicated that the ash of yeasts cultivated in diverse substrates often contains a wide range of substances. These findings showed that Cu
2+ and Mn
2+ ions induce cell death starting from several millimolar levels [
23].
Various amounts of copper, zinc, and manganese sulfate salts were applied to
Saccharomyces cerevsiae colonies because the cells' vitality decreased and they became inactive from a biological perspective at greater concentrations of microelements [
22]. The concentrations of the trace minerals and the method of addition had an impact on the biomass increase. The best outcome of the studies reported in this study was 14 g/l of yeast biomass with an enriched content of accumulated microelements, which included 33.9 mg of manganese, 1143.4 mg of zinc, and 1145.8 mg of copper.
Previous investigations using yeast revealed that certain metals, like copper, caused yeast cells to expand quickly over the course of 13–20 hours, during which time oxygen demand outpaced oxygen supply [
24]. Therefore, it is possible to draw the conclusion that the medium's composition, which aids in the yeast cells' constant oxygen supply and promotes the growth of the yeast as well as its capacity to incorporate the trace elements from the medium in high concentrations, is primarily responsible for the growth of the yeast in the presence of trace elements.
Concerns over the horticultural experiment's relationship between food safety and human health are growing. In this text, natural sources (compost, seaweed extracts, yeast, etc.) are crucial [
25,
28]. Farmers are growing more interested in employing natural sources as bio-control agents in agriculture to ensure food safety [
29,
30]. Comparatively speaking to the other treatments, the effects of spraying frozen yeast enhanced with manganese at a rate of 20 cm
3/l on shoot length and diameter, number of leaves / shoot, leaf area, chlorophyll content, cluster weight, yield, weight of 100 berries, TSS, and leaf mineral content are favorable. Many earlier research papers showed similar conclusions [
31,
35]. Applying 'Keitte' mango trees with 0.2 percent of yeast spray once had fully blossomed proved to be highly effective in boosting output and its constituents, as well as enhancing fruit quality [
31]. By applying algae extracts to sour orange trees twice at 1, 2 and 3% after fruit-set, fruit quality was improved. This was attributed to improvements in fruit length, width, fresh weight, and size as well as in fruit moisture, fruit juice, fruit peel moisture, total soluble solids, ascorbic acid content in juice, and peel thickness [
32]. Spraying grapevines with GA3 plus 0.4% active dry yeast obtain heavy and less compact cluster and hasten the ripening with fairly good Flame Seedless berries quality [
33]. The application of8% yeast and yeast enriched with zinc on grapes had improved fruit chemical characteristics i.e. soluble solid content and some physical characteristics (cluster width and weight of 100 berries) [
34,
35].
Crop development is positively impacted by yeast [
36,
37]. Yeast is a naturally occurring (harmless and nonpolluting) component that contains many of minerals, particularly N, P, and K, also proteins, vitamin B, and natural hormones like cytokinin and IAA. These factors may be connected to the effects of yeast extract. It has been discovered that axeins, hormones, vitamins, chelating agents, and yeast-produced enzymes stimulate cell growth and division, nutrition absorption, protein synthesis, and enhance net photosynthesis [
38,
39].
5. Conclusions
In the microbiological experiment, the first approach (growth phase) was chosen to lower the possibility of medium contamination, according to the results of several incorporation strategies employed to enrich yeast with manganese. As the quantity of manganese sulfate in the media increased, so did the concentration of manganese in yeast cells. Manganese incorporation in yeast cells was 99.93% higher at 0.4 g/l of manganese concentration in the media than it was at 0.0195 g/l of manganese concentration in the medium. Zinc content in the medium caused an increase in the optical density (OD) of yeast cell biomass, but manganese sulphate had no passive influence on it.
In relation to the horticultural experiment, it can be inferred that spraying frozen yeast enriched with manganese at 20 cm3/l is the most effective treatment followed by the same form at 10 cm3/l for improving shoot length, shoot diameter, number of leaves / shoot, leaf area, leaf chlorophyll content, cluster weight, yield per vine, weight of 100 berries, juice weight of 100 berries, TSS%, and leaf mineral content in the two studying seasons. In particular, when applied frozen yeast as foliar spray, it lowers the rate at which manganese fertilizer is utilized to achieve optimal production, hence mitigating the pollution that arises from the use of chemical fertilizers.
Author Contributions
Conceptualization, M.M.S.S. and S.R.H.; methodology, S.R.H. and R.S.A.-H.; data curation, M.M.S.S. and R.S.A.-H.; writing—original draft preparation, S.R.H., M.M.S.S. and R.S.A.-H.; writing—review and editing, A.M.A.-S. and M.H.F.; supervision, A.M.A.-S. and M.H.F.; funding acquisition, A.M.A.-S.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Researchers Supporting Project number (RSP2024R334), King Saud University, Riyadh, Saudi Arabia.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the required data are inserted in the manuscript..
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project number (RSP2024R334), King Saud University, Riyadh, Saudi Arabia. Also, the authors thank the National Research Centre, Cairo, Egypt for providing the equipment, laboratories, and materials used in this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. Statistical Database Food and Agriculture Organization of the United Nation, Rome, Italy. 2022, http://www.faostat.fao.org/site/339/default.
- Vieira, F.; Carvalho, J.; Pinto, E.; Cunha, S.; Almeida, A. A.; Ferreira I. Nutritive value, antioxidant activity and phenolic compounds profile of brewer’s spent yeast extract. J. Food Compos. Anal. 2016, 52, 44–51. [CrossRef]
- El-Tohamy, W.A.; El-Abagy, H.M.; El-Greadly, N.H.M. Studies on the effect of Putrescine, yeast and vitamin C on growth, yield and physiological responses of eggplant (Solanum melongena L.) under sandy soil conditions. Aust. J. Basic Appl. Sci. 2008; 2, 296–300. [Google Scholar] [CrossRef]
- Fawzy, Z.F. Increasing productivity of head lettuce by foliar spraying of some bio and organic compounds. Mesopotamia J. Agric. 2010, 38, 20–28. [Google Scholar] [CrossRef]
- Ahmed, A.A.; El-Baky, M.M.H.A.; Zaki, M.F.; Abd El-Aal, F.S. Effect of foliar application of active yeast extract and zinc on growth, yield and quality of potato plant (Solanum tuberosum L.). J. Appl. Sci. Res. 2011, 7, 2479–2488.
- Shehata, S.A.; Fawzy, Z.F.; El-Ramady, H.R. Response of cucumber plants to foliar application of chitosan and yeast under greenhouse conditions. Aust. J. Basic Appl. Sci. 2012, 6, 63–71. [Google Scholar]
- Shafek, M.R.; Asmaa, R.M.; Aisha, H.A.; Magda, M.H.; Singer, S.M. Effect of different levels of potassium applied with foliar spraying of yeast on growth, yield and root quality of turnip under sandy soil conditions. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 868–877. [Google Scholar]
- Dawood, M.G.; El-Lethy, S.R.; Mervat, S. Role of methanol and yeast in improving growth, yield, nutritive value and antioxidants of soybean. World Appl. Sci. J. 2013, 26, 6–14. [Google Scholar]
- Inani, N.; Nozulaidi, M.; Khairi, M.; Abdulkadir, A.B.; Jahan, M.S. Glutathione functions on physiological characters of corn plants to enhance Mn-induced corn production. Pertanika J. Tropical Agri. Sci. 2015, 38, 509–581. [Google Scholar]
- Josef, J.; Katarina, K. Application of nanotechnology in agriculture and food industry, its prospects and risks. Ecol. Chem. Eng. S. 2015, 22, 321–361. [Google Scholar]
- Nair, R.S.; Varghese, H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Li, Y.; Xu, Z.; Watanabe, F.; Biris, A.S. Carbon Nano tubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano 2009, 3, 3221–3227. [Google Scholar] [CrossRef] [PubMed]
- Kamiab, F.; Zamanibahramabadi, E. The Effect of foliar application of Nano-chelate super plus ZFM on fruit set and some quantitative and qualitative traits of almond commercial cultivars. J. Nuts 2016, 7, 9 – 20.
- Ajlouni, A.; Hamdan, E.H.; Alshalawi, R.A.E.; Shaik, M.R.; Khan, M.; Kuniyil, M.; Adil, S.F. Green synthesis of silver nanoparticles using aerial part extract of the anthemis pseudocotulaboiss plant and their biological activity. Molecules 2023, 28, 246. [Google Scholar] [CrossRef] [PubMed]
- Ponce de Leon, C.A.; Bayon, M.M.; Paquin, C.; Caruso, J.A. Selenium incorporation into Saccharomyces cerevisiae cells: a study of different incorporation methods. J. Appl. Microbiol. 2002, 92, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Demirci, A; Pometto, A.L. Production of organically bound selenium yeast by Continuous Fermentation. J. Agric. Food Chem. 1999, 47, 2491–2495. [CrossRef]
- Jackson, M.L. Jackson, M.L. Soil chemical analysis, New Jersey Prentice. Hall, Inc., Egleweed Cliffs, N.J. 1967, 331p.
- Cottenie, A.; Verloo, M.; Kiekens, L.; Velgle, G.; Amerlynuck, R. Chemical analysis of plant and soil. Laboratory of Analytical and Agroch., State Univ. of Belgium, Gent. 1982, pp. 43- 51.
- A.O.A.C. Association of Official Analytical Chemist 14th Ed. Benjamin Franklin Station, Washington, D.C., USA 1985, pp. 490-510.
- Snedecor, G.W.; Cochran, G.W. Statistical Methods 8th edition. Iowa state university press, Iowa, USA 1989.
- Duncan, D.B. Multiple F test. Biometrics 1955, 11:1-24.
- Barbulescu, I.D.; Rusu, N.; Rughinis, R.; Popa, O.; Stefaniu, A.; Casarca, A. Obtaining yeast biomass enriched with copper, zinc and manganese. Romanian Biotechnological Letters 2010, 15, 5008–5016. [Google Scholar]
- Liang, Q.; Zhou, B. Copper and manganese induce yeast apoptosis via different pathways. Molecular Biology of the Cell 2007, 18, 4741–4749. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.N.; He, X.X.; Zhang, L.B.; Cheng, Y.F.; Bai, X.M.; Wang, Z.Y.; He, X.P. Enhancement of copper uptake of yeast through systematic optimization of medium and the cultivation process of Saccharomyces cerevisiae. Appl. Bioch. & Biotech. 2022,. [CrossRef]
- Fornes, F.; Sanchez-Perales, M.; Guardiola, J. L. Effect of a seaweed extract on citrus fruit maturation. International Symposium on Quality of Fruit and Vegetable, Acta Hort. 1995, 379, 75-82.
- Kulik, M.M. The potential cyanobacteria (blue green algae) and algae for using in the biological control of plants. Pathogenic Bacteria and Fungi. European J. Plant Pathol. 1995, 101, 585–599. [Google Scholar] [CrossRef]
- Howgate, P. Review of the public health safety of products from aquaculture. Int. J. Food Sci. Technol. 1998, 33, 99–125. [Google Scholar] [CrossRef]
- Gott, J.; Morgenstern R.; Turnšek M. Aquaponics for the Anthropocene: Towards a ‘Sustainability First’ Agenda. In: Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M. (eds) Aquaponics Food Production Systems. Springer, Cham. 2019, . [CrossRef]
- https://doi.org/10.1007/978-3-030-15943- 6_16.
- Fleet, G.H. Yeasts in foods and beverages: Impact on product quality and safety. Current Opinion in Biotechnology 2007, 18, 170– 175. [CrossRef]
- Hassan-Hoda, M.I. Effect of algae extract on productivity of Balady orange trees. M. Sc. Thesis. Faculty of Agriculture, Minia University, Egypt 2008.
- Abd El-Motty, E.; Shahin, M.M.; El-Shiekh, M.; Abd El-Migeed, M. Effect of algae extract and yeast application on growth, nutritional status, yield and fruit quality of Keitte mango trees. Agric. Biol. J. N. Am. 2010, 1, 421– 429. [CrossRef]
- Al-Musawi, A.H.M. Effect of foliar application with algae extracts on fruit quality of sour orange (Citrus aurantium, L). J. Environ. Sci. Pollut. Res. 2018, 4, 250–252. [Google Scholar] [CrossRef]
- El-Halaby, E.H.S.; El-Salhy, A.M.; AlWasfy, M.M.; Ibrahim, R.A. Effect of GA3, urea and yeast spraying on fruiting of Flame Seedless grapevines under sandy soil conditions. Assiut J. Agric. Sci. 2015, 46, 95–106. [Google Scholar]
- Omar, A.E.; Ahmed, D.K.; Al-Obeed, M.A.A.; Alebidi, R.A. Influence of foliar applications of yeast extract, seaweed extract and different potassium sources fertilization on yield and fruit quality of ‘Flame Seedless’ grape. Acta Sci. Pol. Hortorum Cultus. 2020, 19, 143–150. [Google Scholar] [CrossRef]
- Shimaa, R. Hamed; Rasha, S. Abdel-Hak; Mohamed, M.S. S.; Abdel-Rahman, M.A. M. Impact of active fresh yeast enriched with zinc on yield and fruit quality of Flame Seedless grapes. Egypt. J. Chem. 2023, 66, 435 – 448.
- Sarhan, T.Z.; Ali, S.T.; Rasheed, S.M.S. Effect of bread yeast application and seaweed extract on cucumber (Cucumis sativus L.) plant growth, yield and fruit quality. Mesopotamia J. Agric. 2011, 39, 26-34.
- Alsaady, M.H.M.; Salim, H.A.; Abdulrazzaq, A.K.; Saleh, U.N.; Jassim, N.H.; Hamad, A.R.; Attia, J.A.; Darwish, J.J.; Hassan A.F. Response of cabbage plants to foliar application of yeast suspension and nitrogen fertilizer. Eco. Env. & Cons. 2020, 26, 832-836.
- Moor, T.C. Biochemistry and physiology of plant hormones. Pub. By Springer-Verlag New York, USA 1979.
- Idso, S.B.; Idso, K.E.; Garcia, R.L.; Kimball, B.A.; Hoober J.K. Effect of atomospheric CO2 enrichment and foliar methanol application on net photosynthesis of Sour orange trees (Citrus aurantium, Rutaceae) leaves. Amer. J. of Botany, 1995, 82, 26-30.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).