1. Introduction
The discovery of antibiotics in the early 1930s was the miraculous solution that radically changed the management of various infections, whether community-acquired or nosocomial, and saved thousands of lives [
1]. Unfortunately, currently, the effectiveness and reliability of these antimicrobial agents are compromised due to the multitude of bacterial resistances that have begun to emerge for several decades, shortly after the beginning of antibiotic use. This evolution makes the choice of therapeutic protocol more complex and plunges the world into a critical post-antibiotic phase, with an increase in morbidities and mortalities [
2,
3,
4]. In 2021, the World Health Organization (WHO) classified antibiotic resistance as one of the top 10 threats to public health [
5]. The increasing numbers, with 2,868,700 infections due to resistant bacteria and 35,900 deaths due to antibiotic-resistant bacterial infections annually, confirm this observation [
5,
6,
7]. Hamad and al. estimate that if this problem is not addressed promptly, it could kill up to 10 million people annually by 2050 and incur expenses exceeding 100 trillion US dollars [
2]. Naturally, bacteria may have inherent resistance to certain antibiotics, but these resistances can be amplified by the acquisition of additional resistance genes often driven by human activities such as the inappropriate use of antibiotics [
7]. WHO advocates that only 50% of antibiotics are used correctly [
8]. It has been approved that the use, misuse, and overuse of antibiotics have led to direct and indirect correlations with the emergence of bacterial resistance through the phenomenon of selection pressure from resistant mutants [
9,
10].
Several studies have focused on the evolution of antibiotic consumption, and others on bacterial resistances prevailing in Tunisian hospitals, however the correlation between these two parameters has rarely been addressed in Tunisia, and it has never been study at our hospital or on a large scale in the Greater Tunis area, especially over an extended period.
In this context, the main objective of this study is to analyze the correlation between antibiotic consumption and the emergence of bacterial resistance at Charles Nicolle Hospital CNH in Tunis over 13 years period.
2. Results
2.1. Antibiotic Consumption
2.1.1. The "AWaRe" Classification
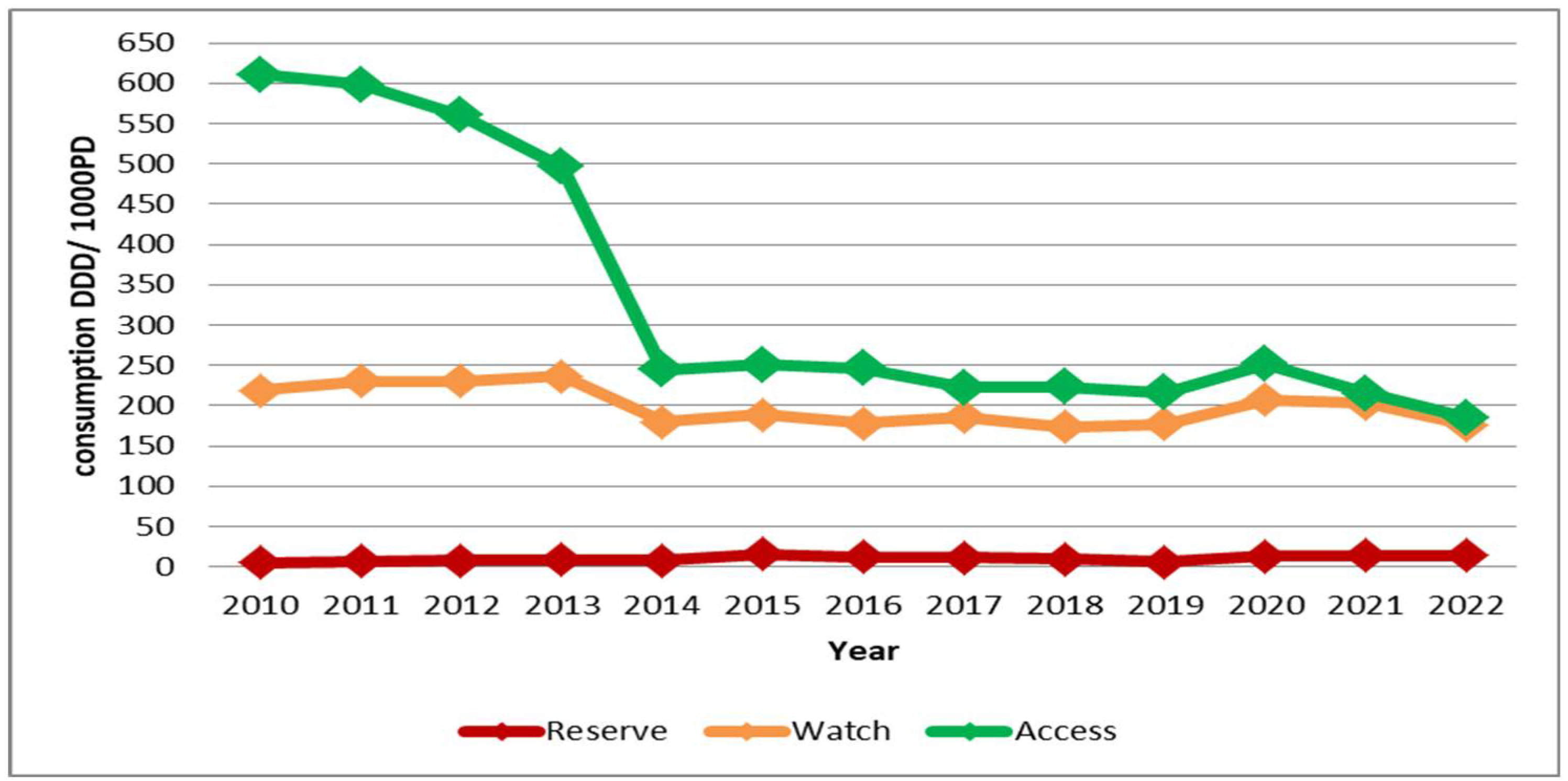
Antibiotic consumption in the "Access" group of the "AWaRe" classification was 610.84 DDD/1000 PD in 2010, but significantly decreased to 185.15 DDD/1000 PD by 2022.
For the "Watch" group, the variation in consumption between 2010 and 2022 was minimal, with at the lowest consumption recorder at 173.75 DDD/1000 PD.
The consumption of antibiotics in the "Reserve" group was fluctuated over the years. In 2010, it was only 5.3 DDD/1000 PD, gradually increasing to 16.25 DDD/1000 PD in 2015. A drop occurred in 2019 with 6.15 DDD/1000 PD, followed by a resurgence, reaching 14.17 DDD/1000 PD in 2022 (
Figure 1).
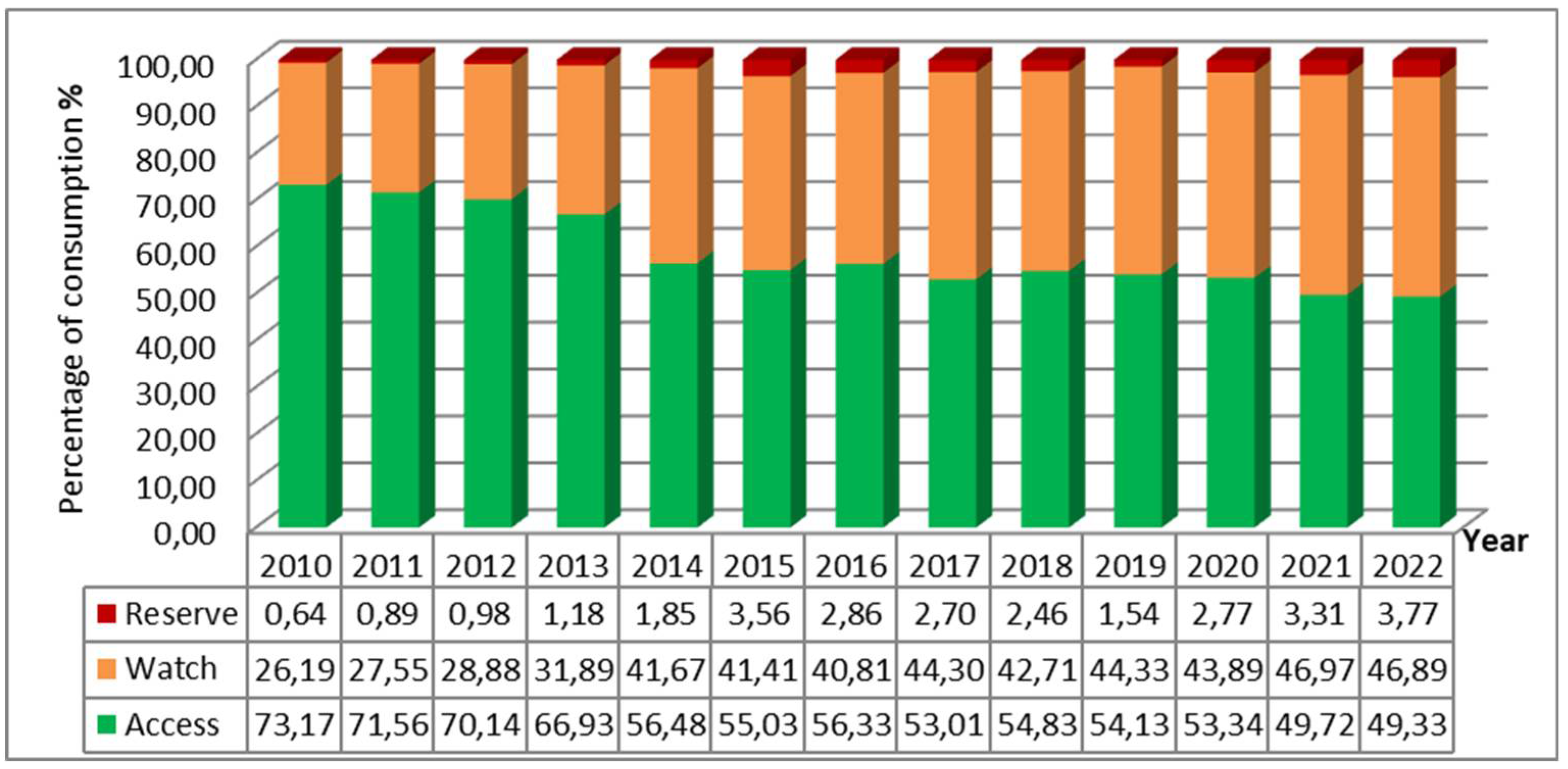
In 2010, the majority of antibiotic consumption was represented by the "Access" group with 73.17%, compared to only 26.19% for the "Watch" group and 0.64% for the "Reserve" group. Over the years, this distribution has changed significantly, with a considerable decrease in the share of the "Access" group and an increase in those of the "Watch" and "Reserve" groups. By 2022, consumption was almost evenly split between the two major groups, with 49.33% for "Access" and 46.89% for "Watch", and the consumption of the "Reserve" group also evolved, accounting for 3.77% (
Figure 2).
2.1.2. The Linear Regression of Antibiotic Consumption
The linear regression of overall consumption in DDD/1000PD is significant (P=0.000; β=-0.837), indicating a significant downward trend.
Table 1 shows the results of the linear regressions of the studied antibiotic consumptions.
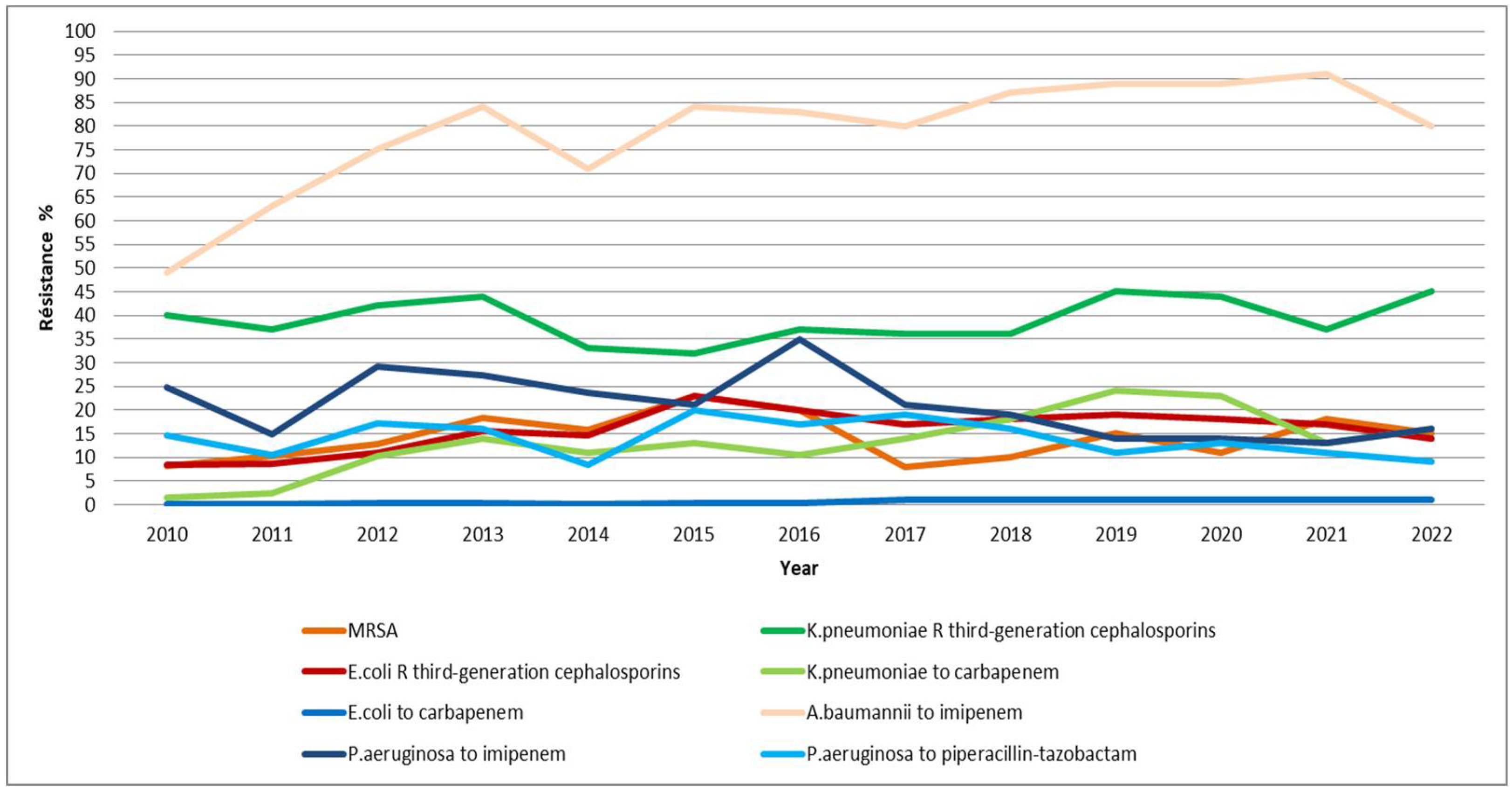
2.2. Bacterial Resistance (Figure 3)
2.2.1. Enterobacteriaceae Resistant to Third-Generation Cephalosporins
From 2010 to 2015, the resistance of E. coli strains to third-generation cephalosporins showed an upward trend, rising from 8.4% to 23%. However, from 2016 to 2022, this resistance gradually decreased reaching 14%.
The evolution of resistance in K. pneumoniae strains to third-generation cephalosporins has fluctuated over the years, with a minimum of 32% recorder in 2015 and a maximum of 45% observed in both 2019 and 2022.
2.2.2. Enterobacteriaceae Producing Extended-Spectrum Beta-Lactamases
The level of ESBL-producing E. coli steadily increased from 7.91% in 2011 to 20.68% in 2016, then began to decline, reaching its lowest value of 7.16% in 2020.
The percentage of ESBL-producing K. pneumoniae steadily decreased from 37% in 2012 to 12% in 2020. However, there was resurgence in 2021, with the percentage rising to 25.96%, and it further increased to 28.13% in 2022.
2.2.3. Enterobacteriaceae Resistant to Carbapenems
This study showed a low resistance (< 1) of E. coli strains to carbapenems .
The resistance of K. pneumoniae strains to carbapenems saw a sharp increase, rising from 1.42% in 2010 to 14.02% in 2013. A second peak occurred in 2019 at 24%, and finally settled at 16% in 2022
2.2.4. Pseudomonas Aeruginosa Resistant to Imipenem and Piperacillin-Tazobactam
The resistance of A. baumannii to imipenem has significantly evolved, increasing from 49% in 2010 to 80% in 2022, reaching a peak of 91% recorded in 2021.
2.2.6. Methicillin-Resistant Staphylococcus Aureus Strains
There is no overall increasing trend in methicillin resistance among S. aureus (MRSA). Minor variations, particularly noted from 2019 onwards, show a minimum resistance of 8% in 2017 and a peak resistance recorded in 2015 of 23%
2.2.4. The Strains of Pseudomonas Aeruginosa Resistant to Imipenem and Piperacillin-Tazobactam
Between 2010 and 2016, the resistance of P. aeruginosa to imipenem fluctuated, with a low of 14.78% in 2011 and a high of 35% in 2016. Thereafter, this resistance gradually decreased reaching 16% in 2022, with a minimum of 13% observed in 2021.
The resistance of P. aeruginosa to piperacillin-tazobactam PTZ has fluctuated over time. A minimum percentage was observed in 2014 at 8.48%, followed by another low in 2022 at 9%, while a peak of 20% was recorded in 2015.
2.2.5. The Strains of Acinetobacter Baumannii Resistant to Imipenem
The resistance of A. baumannii to imipenem has significantly evolved, increasing from 49% in 2010 to 80% in 2022, reaching a peak of 91% recorded in 2021.
2.2.6. Methicillin-Resistant Staphylococcus Aureus Strains
There is no overall increasing trend in methicillin resistance among S. aureus (MRSA). Minor variations, particularly noted from 2019 onwards, show a minimum resistance of 8% in 2017 and a peak resistance recorded in 2015 of 23%.
Figure 3.
Overlay of the evolution of resistance among the different bacterial strains studied. R: resistance; P. : Pseudomonas; K. : Klebsiella ; A. : Acinetobacter ; E. : Esherichia; MRSA: methicillin resistanceS. aureus
Figure 3.
Overlay of the evolution of resistance among the different bacterial strains studied. R: resistance; P. : Pseudomonas; K. : Klebsiella ; A. : Acinetobacter ; E. : Esherichia; MRSA: methicillin resistanceS. aureus
2.2.7. Linear Regressions of Bacterial Strain Resistance Densities
Table 2 presents the results of linear regressions of resistance densities of the studied bacterial strains to antibiotics. Four significant regressions were identified.
2.3. Correlations between Antibiotic Consumption and Bacterial Resistance Incidence Density
The correlation study between antibiotic consumption and the incidence density of bacterial resistance was conducted for each bacterial-antibiotic pair. Prior to this, the Shapiro-Wilk test was performed to differentiate between variables with normal distribution and those with non-normal distribution. Subsequently, the Spearman coefficient was used to assess correlation when both tested variables were non-normally distributed, while the Pearson coefficient was utilized when at least one of the variables followed a normal distribution. The direction of correlation is determined only if the regression of antibiotic consumption is significant.
Significant correlations found with Pseudomonas aeruginosa strains resistant to piperacillin-tazobactam are represented in
Table 8.
Significant correlations found with imipenem-resistant Acinetobacter baumannii strains are represented in
Table 9.
Significant correlations found with Methicillin-resistant Staphylococcus aureus (MRSA) strains are represented in
Table 10.
In total, 74 significant correlations were found. Among the most relevant are the correlations between carbapenem consumption and the increase in resistance density of E. coli strains to third-generation cephalosporins (C3G) and carbapenems, and of K. pneumoniae strains to carbapenems.
Seven of the 74 correlations had an undefined direction since the regression of the antibiotic consumption in question was not significant; otherefore, while a correlation exists, its trend cannot be defined. It is noteworthy that no significant correlation was found between antibiotic consumption and the increase in resistance density of ESBL-producing K. pneumoniae strains or imipenem-resistant P. aeruginosa strains.
3. Discussion
Four million nine hundred fifty thousand (4.95 million) deaths were estimated worldwide in 2019 due to antimicrobial resistance (AMR), with 1.27 million directly attributable to bacterial AMR [
11,
12].
A close correlation has been revealed between the irrational use of antibiotics and the occurrence of these uncontrollable resistances, particularly caused by hospital pathogens [
7,
13].
Given the absence of previous studies conducted in hospitals of Greater Tunis, particularly at our hospital, the largest in terms of number of beds in Tunisia, on this issue, we decided to reveal all correlations between antibiotic consumption and bacterial resistance.
The linear regression of overall antibiotic consumption was significant, showing a marked downward trend. Similarly, in India, consumption decreased by 3.6% between 2011 and 2019 [
14].
A slight deviation from this trend was observed during the COVID-19 period. Indeed, comparing the consumption in 2019 (399.19 DDD/1000 PD) to that in 2020 (471.92 DDD/1000 PD), we can see an 18.22% increase in consumption due to the pandemic. According to the review by Fukushige et al., studies conducted in hospitals in Lebanon, Italy, India, Spain, and the United Kingdom revealed an increase in antibiotic consumption in 2020 compared to 2019 [
15].
Overall, a considerable decrease in the consumption of antibiotics classified under "Access" group, according to the "AWaRe" classification, was noted. In 2010, this group accounted for the highest share share of antibiotic consumption at 73.17%, compared to only 49.33% in 2022. This percentage is well below the target of the WHO's 13th General Programme of Work, spanning from 2019 to 2023, which aimed for at least 60% of total antibiotic consumption be comprised of "Access" group drugs [
16,
17,
18].
This decrease in consumption is probably due to changes in medical prescriptions and increased consumption of molecules belonging to the other "AWaRe" groups. Our results align with those reported in the 2019 report by the French National Agency for Medicines and Health Products Safety (ANSM), where the "Access" group represented 72% of total antibiotic consumption in France [
19].
Antibiotics in the "Watch" group accounted for only 26.19% of consumption in 2010, but this percentage significantly increased in 2022 to 46.89%, nearly matching the consumption of the "Access" group. In a study conducted in the urology department of our hospital over the period from 2015 to 2019, it was revealed that the "Watch" group even surpassed the "Access" group with 60% of consumption compared to only 35% [
23]
. This percentage is alarming and absolutely discordant with WHO recommendations. This consumption must be reduced, notably by promoting therapeutic de-escalation and transitioning to an "Access" group medications whenever possible. Cephalosporins, particularly cefotaxime, are the most consumed molecules in this group. This is also the case in China, according to a study conducted in 153 hospitals [
20].
For the "Reserve" group, consumption has fluctuated over time. Antimicrobial use in this group is tightly controlled to preserve their effectiveness and reliability. A study conducted in the urology department of our hospital demonstrated that antimicrobial stewardship measures had significantly reducing the consumption of the "Reserve" group [
21].
Regarding the resistance profile, our study identified a significant upward trend in the incidence density of E. coli resistance to third-generation cephalosporins (3GC).These findings are very close to those published by the Antibiotic Resistance in Tunisia LART [
22].
In France, E. coli resistance to 3GC increased from 7% in 2010 to 8.3% in 2021[
23,
24] .
The results showed that the resistance density of K. pneumoniae to 3GC was highly variable, resulting in a non-significant linear regression of the distribution. These figures are consistent with those reported by LART [
22].
The average percentage of K. pneumoniae resistant to 3GC in European Union countries recorded in 2021 was 34.3%, a figure more or less comparable to that found in this study [
25].
An increase in the percentage of E. coli and K. pneumoniae ESBL was highlighted in this study. In Australia, the recorded percentages in 2018 were significantly lower than those in our study for K. pneumoniae (9.8%) but were comparable for E. coli (13.3%) [
26].
A significant upward trend in the resistance density of K. pneumoniae to carbapenems was recorded, with the percentage increasing from 1.42% in 2010 to 16% in 2022. Alarmingly high levels of carbapenem-resistant K. pneumoniae strains have been reported worldwide, with an upward trend [
27].
This trend is justified by the recently acquired ability of K. pneumoniae to develop resistance genes, particularly against carbapenems [
28].
The resistance of P. aeruginosa to imipenem and PTZ was marked by inconsistent trends, resulting in statistically non-significant evolution. For imipenem resistance, in a Tunisian burn intensive care unit, the average resistance was 74.9% between 2014 and 2019, higher than the result found in our study. This large difference in resistance rates is undoubtedly due to the increased involvement of P. aeruginosa in infections developed in burn patients [
29].
High levels of resistance of A. baumannii to imipenem have been demonstrated. These resistances increased from 49% in 2010 to 80% in 2022.The development of carbapenemases has made A. baumannii "an even more dangerous threat," as characterized by Ramirez et al. in their review[
30].
A study conducted in 9 hospitals in Greece revealed resistance rates ranging from 90.3% in 2010 to 94.5% in 2015 [
31].
The variations in MRSA were minimal during the study period with extreme percentages of 8% in 2017 and 23% in 2015. These results are close to those presented by LART with extreme figures of 15.2% in 2010 and 2022 and 23.4% in 2013 [
22].
A study conducted in Iran between 2010 and 2016 revealed an MRSA rate averaging 43%, almost double the result we found [
32].
This difference in resistance rates between our study and the one conducted in Iran can be justified by differences in healthcare systems, antibiotic consumption, antibiotic use protocols, and hygiene measures adopted in healthcare settings [
33].
Regarding the relationship between antibiotic consumption and the emergence of bacterial resistance, 74 correlations have been revealed.
Resistance of E. coli to 3GC has been associated with the consumption of several antibiotics, particularly 3GC such as cefotaxime. Chinese and Korean studies have confirmed these correlations [
34,
35].
However, unlike the result found in our study, in Italy, no link was found with the use of piperacillin-tazobactam [
36].
Furthermore, increased consumption of carbapenems, notably imipenem, has been correlated with increased resistance of E. coli to 3GC, as was the case in Korea [
37].
Conversely, a decrease in the consumption of fluoroquinolones, such as ciprofloxacin and ofloxacin, highlighting the importance of controlling the use of these antibiotics to reduce resistance rates, has been negatively associated with this correlation [
37].
Aminoglycosides have also shown a significant correlation with E. coli resistance to 3GC, as indicated in an Italian study [
38].
Likewise, a decrease in the consumption of cotrimoxazole has been associated with an increase in this resistance, according to the same Italian study [
38].
A significant negative correlation has been observed between the consumption of penicillins, particularly oxacillin and amoxicillin, and the occurrence of ESBL-producing E. coli. In China, Yang P et al. demonstrated this correlation [
39].
Similarly, significant correlations, positive with imipenem (and consequently with carbapenems) and negative with fluoroquinolones, notably ciprofloxacin, and with cotrimoxazole, have been revealed concerning the emergence of ESBL-producing E. coli. Similar correlations have been revealed in a Moroccan study (except for imipenem)[
40].
A significant increase in the consumption of carbapenems, particularly imipenem, has led to a notable correlation with the resistance density of E. coli to this class of antibiotics. Conversely, the decreased consumption of ertapenem has resulted in a significant negative correlation. A study conducted in a Chinese hospital between 2010 and 2016 highlighted the correlation between imipenem consumption and the emergence of this resistance [
41]. For ertapenem, similar findings were reported in a Serbian study [
13].
Additionally, a significant negative correlation has been observed between the consumption of fluoroquinolones and the resistance density of E. coli strains to carbapenems. This correlation was documented in a Thai study conducted between 2013 and 2016 [
42].
The increased consumption of 3GCs, particularly cefotaxime, has been correlated with the rise in resistance density of K. pneumoniae to 3GCs. Studies conducted in China between 2014 and 2016, as well as at the National Institute of Hygiene in Lomé between 2010 and 2017, have revealed these correlations [
43,
44].
The high consumption of imipenem in our hospital has been correlated with the emergence of carbapenem-resistant K. pneumoniae, as demonstrated by Pérez-Lazo et al. in a study conducted in a Peruvian hospital between 2015 and 2018 [
45].
Conversely, the reduced consumption of certain penicillins (oxacillin, amoxicillin, and piperacillin) and fluoroquinolones has shown significant negative correlations with resistance. This is supported by a Sicilian study for the entire beta-lactam family[
46] and a Thai study for fluoroquinolones [
42].
The increased consumption of PTZ, 3GC, and teicoplanin has been positively correlated with the resistance density of K. pneumoniae to carbapenems. For PTZ, the correlation was not significant in Peru [116], while for 3GCs, particularly ceftriaxone, and teicoplanin, these correlations were demonstrated in Thailand and China, respectively [
42,
47].
The consumption of the entire aminoglycoside family, particularly gentamicin, has shown correlations of an undefined nature with the resistance density of
P. aeruginosa to PTZ. This finding was also observed in Serbia within an intensive care unit between 2014 and 2018 [
48]. In our study, a significant positive correlation was recorded between the increased consumption of imipenem and the emergence of resistance in
A. baumannii to this molecule. Studies conducted in China, Jordan, and at Sahloul Hospital in Sousse have also noted the significance of this correlation [
33,
47,
49].
Indirect correlations with this resistance have been recorded: positive correlations with PTZ and 3GC, demonstrated respectively in Spain in 2019 [
50] and in Italy [
38], and negative correlations with amoxicillin, as revealed in China [
51], fluoroquinolones, as shown in a 15-year Chinese study and between 2015 and 2018 in Peru [
45,
52] , and rifampicin. For rifampicin, its synergistic action with colistin in treating infections caused by multidrug-resistant
A. baumannii, particularly those resistant to carbapenems, has produced favorable results [
53]. However, the increased consumption of rifampicin has begun to impact the emergence of resistance in
A. baumannii to carbapenems.
The consumption of oxacillin has shown a significant negative direct correlation with the occurrence of MRSA. Mascarello M. et al. highlighted this negative correlation [
36].
Regarding indirect correlations with MRSA, PTZ, 3GC, particularly ceftriaxone, imipenem, and aminoglycosides, have been implicated in significant positive correlations. These correlations have been demonstrated in Italian [
36] and Chinese studies [
43,
47,
54].
Other correlations revealed in our study were not statistically significant in the literature. For example, the correlation between the consumption of 3GCs and the emergence of carbapenem-resistant
E. coli [
20] and the correlation between vancomycin and ESBL-producing
E. coli [
55].
This pharmaco-epidemiological study underscores the critical importance of bolstering antibiotic stewardship programs to optimize antibiotic prescriptions and prevent the unfavorable progression of bacterial resistance, which could lead to a therapeutic dead end.
4. Materials and Methods
A descriptive retrospective study was conducted.
Systemic antibiotics administered orally or intravenously for curative or prophylactic purposes prescribed in all clinical departments of the hospital. These antibiotics are classified according to the WHO's "AWaRe" classification with their respective ATC (Anatomical Therapeutic Chemical Classification System) code [
56]
.
The "Access" group, which includes antibiotics recommended as first- and second-line treatments in protocols for common infections.The "Watch" group, which also includes first- and second-line antibiotics but for specific infections rather than common ones. Since the drugs in this group have a higher potential for developing resistance, they must be an integral part of antimicrobial stewardship programs ASP.The "Reserve" group, which includes last-resort antibiotics used when the patient's life is at risk due to an infection caused by multidrug-resistant bacteria [
11,
57,
58].
Non-redundant bacterial strains isolated from positive cultures obtained from various types of samples received from hospitalized patients at the hospital from January 1, 2010, to December 31, 2022, and analyzed in the microbiology laboratory was considered. The bacterial strains selected for study were chosen among the main bacteria posing a problem of antimicrobial resistance at our hospital and nationally: Methicillin-resistant Staphylococcus aureus (MRSA) strains, Klebsiella pneumoniae (K. pneumoniae) and Escherichia coli (E. coli) strains resistant to third-generation cephalosporins (3GC) with or without extended-spectrum beta-lactamase (ESBL) production, Carbapenem-resistant Klebsiella pneumoniae (K. pneumoniae) and Escherichia coli (E. coli) strains, Imipenem-resistant Acinetobacter baumannii (A. baumannii) strains, Imipenem and Piperacillin-tazobactam (PTZ) resistant Pseudomonas aeruginosa (P. aeruginosa) strains.
Information on the number of hospitalization days per year were obtained from the hospital administration. These administrative data detail the activity of all clinical departments in terms of annual hospitalization days.
Data on antibiotic consumption across all care departments were collected using STKMED® software utilized by the hospital pharmacy. These data were then converted into the number of Defined Daily Doses (DDDs) using EXCEL® software to calculate the international consumption indicator expressed in DDDs per 1000 hospitalization days as adopted by the WHO guidelines [
29,
59].
The collection of bacteriological data necessary for this study was carried out using the SIRSCAN® software from the microbiology laboratory at Charles Nicolle Hospital in Tunis.
Statistical analyses of data related to antibiotic consumption and bacterial resistance density were performed using SPSS® software version 23. It has been demonstrated that using the incidence density of resistance yields statistically more reliable results compared to using the percentage of bacterial strain resistance[
60].
The normality of the variables studied was assessed in SPSS® using the Shapiro-Wilk test [
61,
62]. A variable is considered to have a normal distribution if P > 0.05.
The pharmaco-epidemiological analysis translating the correlation, whether direct or indirect, between antibiotic consumption and the emergence of bacterial resistance was carried out using Pearson's R parametric test if the distribution of at least one of the two variables tested together is normal, or Spearman's rho non-parametric test if neither variable has a normal distribution [
63]. Correlations are significant if their p-value ≤ 0.05.
To evaluate trends, whether upward or downward, linear regressions were tested using SPSS® software. These values are significant and retained if their p-value ≤ 0.05.
There was no ethical approval for this study as no patients were involved.
5. Conclusions
The emergence of bacterial resistance is now one of the main challenges in the healthcare field of the 21st century. Addressing this alarming issue requires the implementation of strict measures through national and international action plans to rationalize the use of antibiotics, the primary factor promoting antimicrobial resistance.
The main objective of this work was to study the possible correlation between the consumption of various antibiotics and the increase in resistance density of the studied bacteria. This study revealed a significant decrease in overall antibiotic consumption between 2010 and 2022 by -55.04%, demonstrating the positive impact of actions taken by hospital pharmacists in collaboration with prescribers to rationalize antibiotic use. However, the "Watch" group accounted for almost half of the antibiotic consumption, which is quite high compared to WHO recommendations.
Regarding bacterial resistance to antibiotics, the results revealed certain significant upward trends, such as E. coli resistance to 3GCs and carbapenems, and K. pneumoniae resistance to carbapenems.
More than seventy correlations were recorded between antibiotic consumption and bacterial resistance density. Among the most relevant, we note the correlations between carbapenem consumption and the increased resistance density of E. coli to 3GCs and carbapenems, and of K. pneumoniae to carbapenems. This study revealed significant positive correlations between the increased consumption of PTZ and the emergence of several bacterial resistances, contrary to the findings in the literature.
Author Contributions
Conceptualization and methodology: Y.K., F.S., A.F., A.A, and I.B.B; investigation, analysis, and validation:Y.K., F.S., A.F.; interpretation: Y.K., F.S., A.F., A.A, and I.B.B; writing original draft: Y.K.F.S., A.F.; further drafts: Y.K., F.S., A.F., A.A, and I.B.B; administration: Y.K., F.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
There was no ethical approval for this study as no patients were involved
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cook MA, Wright GD. The past, present, and future of antibiotics. Sci Transl Med. 10 août 2022;14(657):eabo7793. [CrossRef]
- Hamad M, Al-Marzooq F, Orive G, Al-Tel TH. Superbugs but no drugs: steps in averting a post-antibiotic era. Drug Discovery Today. déc 2019;24(12):2225-8. [CrossRef]
- Bleuse A, Chabut C, Roy H, Lebel D, Ovetchkine P, Blackburn J, et al. Exploration de l’évolution de la consommation d’antibiotiques d’un centre hospitalier universitaire mère-enfant selon la classification AWaRe de l’Organisation mondiale de la santé. Annales Pharmaceutiques Françaises. 1 juill 2022;80(4):576-83. [CrossRef]
- Bleuse A, Roy H, Lebel D, Ovetchkine P, Blackburn J, Bussières JF. Exploration de l’association possible entre la consommation d’antibiotiques et l’émergence de résistance dans un centre hospitalier universitaire mère-enfant. Can J Hosp Pharm. 4 juill 2022;75(3):201-9. [CrossRef]
- Coello F, Rea M, Reinoza P. Antimicrobial Potential of Metallic Nanoparticles: Pathogens Staphylococcus aureus and Klebsiella pneumoniae. 10 févr 2024;6:944-56.
- Mitsoura E, Kopsidas I, Charalambous P, Papazisis G, Raikos N, Pana ZD. Community Antibiotic Consumption in Cyprus for the Period 2015 to 2022. Antibiotics (Basel). 4 janv 2024;13(1):52. [CrossRef]
- Abejew AA, Wubetu GY, Fenta TG. Relationship between Antibiotic Consumption and Resistance: A Systematic Review. Can J Infect Dis Med Microbiol. 5 mars 2024;2024:9958678. [CrossRef]
- Watkins RR. Antibiotic stewardship in the era of precision medicine. JAC Antimicrob Resist. juin 2022;4(3):dlac066. [CrossRef]
- Bellazreg F, Ben Lasfar N, Abid M, Rouis S, Hachfi W, Letaief A. Antibiotic stewardship team in a Tunisian university hospital:A four-yearexperience. Tunis Med. mai 2022;100(5):403-9.
- Klein EY, Van Boeckel TP, Martinez EM, Pant S, Gandra S, Levin SA, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci USA [Internet]. 10 avr 2018 [cité 20 avr 2024];115(15). [CrossRef]
- Hoxha I, Godman B, Malaj A, Meyer JC. 11-Year Trend in Antibiotic Consumption in a South-Eastern European Country; the Situation in Albania and the Implications for the Future. Antibiotics (Basel). 9 mai 2023;12(5):882. [CrossRef]
- Murray CJL, Ikuta KS, Sharara F, Swetschinski L, Aguilar GR, Gray A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet. 12 févr 2022;399(10325):629-55. [CrossRef]
- Medic D, Bozic Cvijan B, Bajcetic M. Impact of Antibiotic Consumption on Antimicrobial Resistance to Invasive Hospital Pathogens. Antibiotics (Basel). 28 janv 2023;12(2):259. [CrossRef]
- Fazaludeen Koya S, Ganesh S, Selvaraj S, Wirtz VJ, Galea S, Rockers PC. Antibiotic consumption in India: geographical variations and temporal changes between 2011 and 2019. JAC Antimicrob Resist. oct 2022;4(5):dlac112. [CrossRef]
- Fukushige M, Ngo NH, Lukmanto D, Fukuda S, Ohneda O. Effect of the COVID-19 pandemic on antibiotic consumption: A systematic review comparing 2019 and 2020 data. Front Public Health. 2022;10:946077. [CrossRef]
- Sharland M, Zanichelli V, Ombajo LA, Bazira J, Cappello B, Chitatanga R, et al. The WHO essential medicines list AWaRe book: from a list to a quality improvement system. Clin Microbiol Infect. déc 2022;28(12):1533-5. [CrossRef]
- Sulis G, Sayood S, Katukoori S, Bollam N, George I, Yaeger LH, et al. Exposure to World Health Organization’s AWaRe antibiotics and isolation of multidrug resistant bacteria: a systematic review and meta-analysis. Clinical Microbiology and Infection. 1 sept 2022;28(9):1193-202. [CrossRef]
- Klein EY, Milkowska-Shibata M, Tseng KK, Sharland M, Gandra S, Pulcini C, et al. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–15: an analysis of pharmaceutical sales data. The Lancet Infectious Diseases. 1 janv 2021;21(1):107-15. [CrossRef]
- Hider-Mlynarz K, Betansedi CO, Dhanani A, Pellanne I, Baril L, Vella P. Ce rapport a été rédigé par : 2000;
- Yang P, Chen Y, Jiang S, Shen P, Lu X, Xiao Y. Association between antibiotic consumption and the rate of carbapenem-resistant Gram-negative bacteria from China based on 153 tertiary hospitals data in 2014. Antimicrob Resist Infect Control. 2018;7:137. [CrossRef]
- Guettari M, Chakroun M, Sellami F, Ferjani A, Abassi A, Ben Slama M. Consommation d’antibiotiques d’un service d’urologie selon la classification AWaRe de L’OMS : étude sur 5 ans. Progrès en Urologie - FMC. 1 nov 2023;33(3, Supplement):S60. [CrossRef]
- Résistance bactérienne [Internet]. [cité 16 août 2023]. Disponible sur: https://www.infectiologie.org.tn/resistance.php.
- European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe : annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) 2010 [Internet]. LU: Publications Office; 2011 [cité 8 mai 2024]. [CrossRef]
- European Centre for Disease Prevention and Control., World Health Organization. Antimicrobial resistance surveillance in Europe 2023: 2021 data. [Internet]. LU: Publications Office; 2023 [cité 8 mai 2024]. [CrossRef]
- Veerapa-Mangroo L. An investigation into antibiotic consumption and use for a better understanding of antibiotic resistance on the island of Mauritius.
- Stewart AG, Price EP, Schabacker K, Birikmen M, Harris PNA, Choong K, et al. Molecular Epidemiology of Third-Generation-Cephalosporin-Resistant Enterobacteriaceae in Southeast Queensland, Australia. Antimicrob Agents Chemother. 65(6):e00130-21. [CrossRef]
- Messaoudi A, Mansour W, Jaidane N, Chaouch C, Boujaâfar N, Bouallègue O. Epidemiology of resistance and phenotypic characterization of carbapenem resistance mechanisms in Klebsiella pneumoniae isolates at Sahloul University Hospital-Sousse, Tunisia. Afr Health Sci. juin 2019;19(2):2008-20. [CrossRef]
- Yang X, Dong N, Chan EWC, Zhang R, Chen S. Carbapenem Resistance-Encoding and Virulence-Encoding Conjugative Plasmids in Klebsiella pneumoniae. Trends Microbiol. janv 2021;29(1):65-83. [CrossRef]
- Thabet L, Frigui S, Mellouli A, Gargouri M, Maamar B, Harzallal I, et al. Corrélation Entre La Consommation D’Antibiotiques Et Les Taux D’Antibiorésistance Chez P. Æruginosa Dans Un Service De Réanimation Des Brûlés Tunisien: Étude Sur 6 Ans (2014-2019). Ann Burns Fire Disasters. 30 sept 2021;34(3):245-51.
- Ramirez MS, Bonomo RA, Tolmasky ME. Carbapenemases: Transforming Acinetobacter baumannii into a Yet More Dangerous Menace. Biomolecules. 6 mai 2020;10(5):720. [CrossRef]
- Dafopoulou K, Tsakris A, Pournaras S. Changes in antimicrobial resistance of clinical isolates of Acinetobacter baumannii group isolated in Greece, 2010-2015. J Med Microbiol. avr 2018;67(4):496-8. [CrossRef]
- Dadashi M, Nasiri MJ, Fallah F, Owlia P, Hajikhani B, Emaneini M, et al. Methicillin-resistant Staphylococcus aureus (MRSA) in Iran: A systematic review and meta-analysis. Journal of Global Antimicrobial Resistance. 1 mars 2018;12:96-103. [CrossRef]
- Hayajneh WA, Al-Azzam S, Yusef D, Lattyak WJ, Lattyak EA, Gould I, et al. Identification of thresholds in relationships between specific antibiotic use and carbapenem-resistant Acinetobacter baumannii (CRAb) incidence rates in hospitalized patients in Jordan. J Antimicrob Chemother. 19 janv 2021;76(2):524-30. [CrossRef]
- Yang P, Chen Y, Jiang S, Shen P, Lu X, Xiao Y. Association between the rate of third generation cephalosporin-resistant Escherichia coli and Klebsiella pneumoniae and antibiotic consumption based on 143 Chinese tertiary hospitals data in 2014. Eur J Clin Microbiol Infect Dis. août 2020;39(8):1495-502. [CrossRef]
- Lee GW, Ryu S, Park J, Lee EJ, Lee KJ, Tae J, et al. Changes of antibiotic prescribing pattern and its resistance to E. Coli in South Korea: a 12-year retrospective observational study. Sci Rep. 11 mars 2021;11(1):5658. [CrossRef]
- Mascarello M, Simonetti O, Knezevich A, Carniel LI, Monticelli J, Busetti M, et al. Correlation between antibiotic consumption and resistance of bloodstream bacteria in a University Hospital in North Eastern Italy, 2008-2014. Infection. août 2017;45(4):459-67. [CrossRef]
- Kim B, Kim Y, Hwang H, Kim J, Kim SW, Bae IG, et al. Trends and correlation between antibiotic usage and resistance pattern among hospitalized patients at university hospitals in Korea, 2004 to 2012: A nationwide multicenter study. Medicine (Baltimore). déc 2018;97(51):e13719. [CrossRef]
- Barberi G, De Cola M, Dell’Utri D, Melardi S, Alagna B, Bramanti A, et al. Antimicrobial consumption and antimicrobial resistance: A snapshot of an Italian neuromuscular rehabilitation center. The new microbiologica. 3 avr 2017;40.
- Yang P, Chen Y, Jiang S, Shen P, Lu X, Xiao Y. Association between the rate of fluoroquinolones-resistant gram-negative bacteria and antibiotic consumption from China based on 145 tertiary hospitals data in 2014. BMC Infect Dis. 7 avr 2020;20(1):269. [CrossRef]
- El bouamri MC, Arsalane L, Kamouni Y, Yahyaoui H, Bennouar N, Berraha M, et al. Profil actuel de résistance aux antibiotiques des souches d’Escherichia coli uropathogènes et conséquences thérapeutiques. Progrès en Urologie. 1 déc 2014;24(16):1058-62. [CrossRef]
- Wang H, Wang H, Yu X, Zhou H, Li B, Chen G, et al. Impact of antimicrobial stewardship managed by clinical pharmacists on antibiotic use and drug resistance in a Chinese hospital, 2010-2016: a retrospective observational study. BMJ Open. 2 août 2019;9(8):e026072. [CrossRef]
- Prakobsrikul N, Malathum K, Santanirand P, Chumnumwat S, Piebpien P, Montakantikul P. Correlation between antimicrobial consumption and the prevalence of carbapenem-resistant Escherichia coli and carbapenem-resistant Klebsiella pneumoniae at a university hospital in Thailand. J Clin Pharm Ther. avr 2019;44(2):292-9. [CrossRef]
- Wushouer H, Zhang ZX, Wang JH, Ji P, Zhu QF, Aishan R, et al. Trends and relationship between antimicrobial resistance and antibiotic use in Xinjiang Uyghur Autonomous Region, China: Based on a 3 year surveillance data, 2014-2016. J Infect Public Health. 2018;11(3):339-46. [CrossRef]
- Salah FD, Sadji AY, Akolly K, Bidjada B, Awoussi KS, Abaya AM, et al. Augmentation de la résistance aux antibiotiques des Entérobactéries isolées à l’Institut National d’Hygiène de Lomé de 2010 à 2017. Journal of Interventional Epidemiology and Public Health [Internet]. 13 août 2021 [cité 28 avr 2024];4(3). Disponible sur: https://www.afenet-journal.net/content/series/4/3/3/full/#ref14.
- Pérez-Lazo G, Abarca-Salazar S, Lovón R, Rojas R, Ballena-López J, Morales-Moreno A, et al. Antibiotic Consumption and Its Relationship with Bacterial Resistance Profiles in ESKAPE Pathogens in a Peruvian Hospital. Antibiotics (Basel). 8 oct 2021;10(10):1221. [CrossRef]
- Barchitta M, Maugeri A, La Rosa MC, La Mastra C, Murolo G, Basile G, et al. Carbapenem Consumption and Rate of carbapenemresistant gram-negative bacteria: results from the Sicilian Surveillance System. Ann Ig. 2021;33(3):289-96. [CrossRef]
- Chen S, Li Z, Shi J, Zhou W, Zhang H, Chang H, et al. A Nonlinear Time-Series Analysis to Identify the Thresholds in Relationships Between Antimicrobial Consumption and Resistance in a Chinese Tertiary Hospital. Infect Dis Ther. 1 juin 2022;11(3):1019-32. [CrossRef]
- Popović R, Tomić Z, Tomas A, Anđelić N, Vicković S, Jovanović G, et al. Five-year surveillance and correlation of antibiotic consumption and resistance of Gram-negative bacteria at an intensive care unit in Serbia. J Chemother. oct 2020;32(6):294-303. [CrossRef]
- Nadia J, Wejdene M, Remy A. B, Meriam G, Cherifa C, Rachida G, et al. Temporal Variation in Antibiotic Resistance of Acinetobacter baumannii in a Teaching Hospital in Tunisia: Correlation with Antimicrobial Consumption. TOMICROJ. 30 avr 2019;13(1):106-11. [CrossRef]
- López-Lozano JM, Lawes T, Nebot C, Beyaert A, Bertrand X, Hocquet D, et al. A nonlinear time-series analysis approach to identify thresholds in associations between population antibiotic use and rates of resistance. Nat Microbiol. 8 avr 2019;4(7):1160-72. [CrossRef]
- Liang C, Zhang X, Zhou L, Meng G, Zhong L, Peng P. Trends and correlation between antibacterial consumption and carbapenem resistance in gram-negative bacteria in a tertiary hospital in China from 2012 to 2019. BMC Infect Dis. 17 mai 2021;21(1):444. [CrossRef]
- Guo W, Sun F, Liu F, Cao L, Yang J, Chen Y. Antimicrobial resistance surveillance and prediction of Gram-negative bacteria based on antimicrobial consumption in a hospital setting: A 15-year retrospective study. Medicine. sept 2019;98(37):e17157.
- Mohammadi M, Khayat H, Sayehmiri K, Soroush S, Sayehmiri F, Delfani S, et al. Synergistic Effect of Colistin and Rifampin Against Multidrug Resistant Acinetobacter baumannii: A Systematic Review and Meta-Analysis. TOMICROJ. 28 avr 2017;11(1):63-71. [CrossRef]
- Zhang D, Cui K, Wang T, Dong H, Feng W, Ma C, et al. Trends in and correlations between antibiotic consumption and resistance of Staphylococcus aureus at a tertiary hospital in China before and after introduction of an antimicrobial stewardship programme. Epidemiology & Infection. janv 2019;147:e48. [CrossRef]
- Tan SY, Khan RA, Khalid KE, Chong CW, Bakhtiar A. Correlation between antibiotic consumption and the occurrence of multidrug-resistant organisms in a Malaysian tertiary hospital: a 3-year observational study. Sci Rep. 24 févr 2022;12(1):3106. [CrossRef]
- Abdelsalam Elshenawy R, Umaru N, Aslanpour Z. WHO AWaRe classification for antibiotic stewardship: tackling antimicrobial resistance - a descriptive study from an English NHS Foundation Trust prior to and during the COVID-19 pandemic. Front Microbiol. 2023;14:1298858. [CrossRef]
- Sharland M, Gandra S, Huttner B, Moja L, Pulcini C, Zeng M, et al. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use-the new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect Dis. déc 2019;19(12):1278-80. [CrossRef]
- Sharland M, Pulcini C, Harbarth S, Zeng M, Gandra S, Mathur S, et al. Classifying antibiotics in the WHO Essential Medicines List for optimal use—be AWaRe. The Lancet Infectious Diseases. janv 2018;18(1):18-20. [CrossRef]
- Choutet P. Guide pour une méthode de calcul des consommations d’antibiotiques dans les établissements de santé et en ville.
- Wu HH, Liu HY, Lin YC, Hsueh PR, Lee YJ. Correlation between levofloxacin consumption and the incidence of nosocomial infections due to fluoroquinolone-resistant Escherichia coli. J Microbiol Immunol Infect. juin 2016;49(3):424-9. [CrossRef]
- Mishra P, Pandey CM, Singh U, Gupta A, Sahu C, Keshri A. Descriptive Statistics and Normality Tests for Statistical Data. Ann Card Anaesth. 2019;22(1):67-72. [CrossRef]
- Habibzadeh F. Data Distribution: Normal or Abnormal? J Korean Med Sci. 15 janv 2024;39(3):e35. [CrossRef]
- Rovetta A. Raiders of the Lost Correlation: A Guide on Using Pearson and Spearman Coefficients to Detect Hidden Correlations in Medical Sciences. Cureus. 12(11):e11794. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).