Submitted:
09 June 2024
Posted:
12 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. Extracting Efficiency of the Various Components
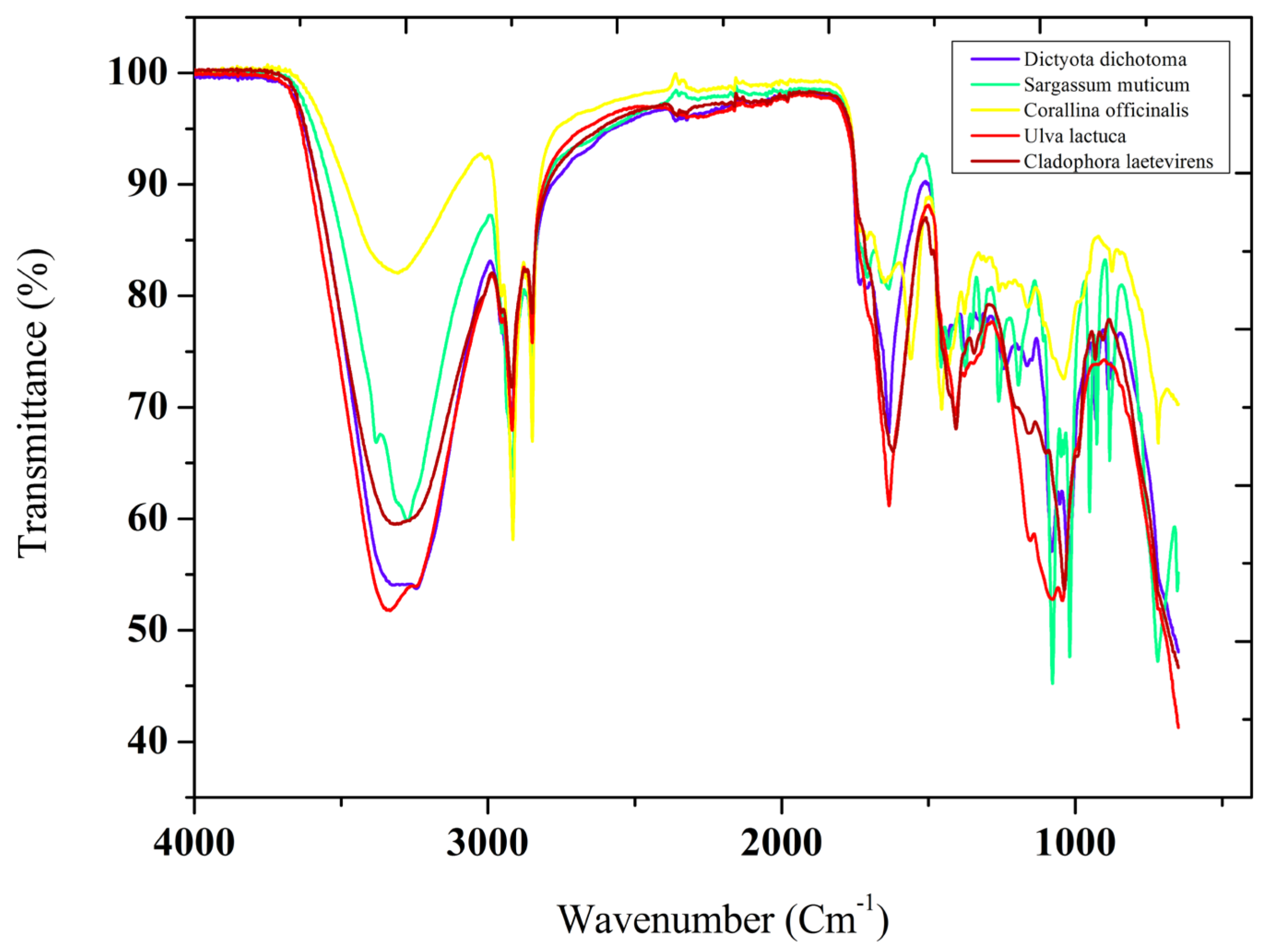
2.2. Seaweed Extracts Analysed by FT-IR
2.3. Seaweed’s Elementary Analysis Using X-Ray
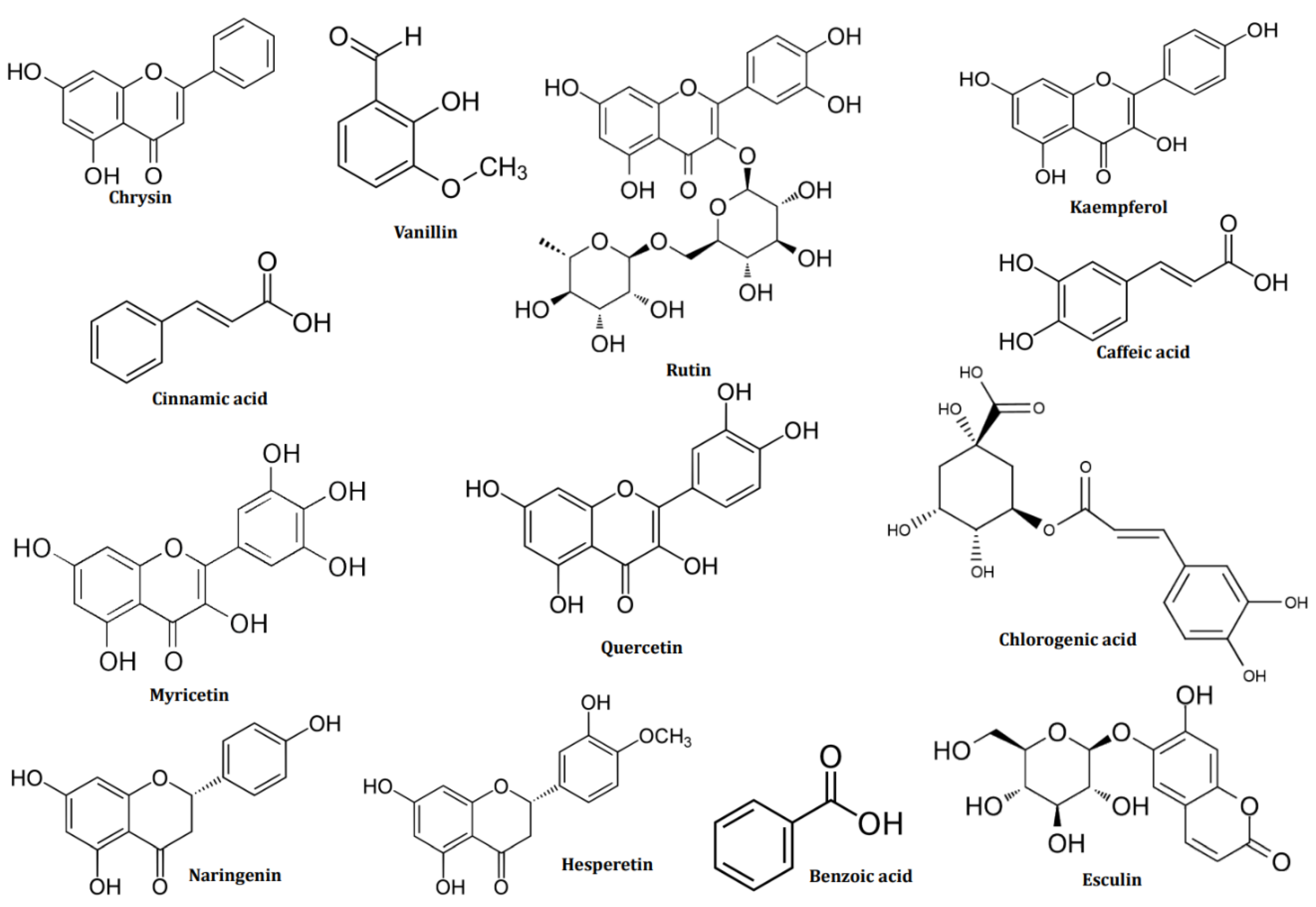
2.4. Seaweed-Based Analysis by UPLC-ESI-MS/MS
2.5. Assessment of the Nutritional Compounds
2.5.1. Lipid Content
2.5.2. Protein Content
2.5.3. Total Sugar Content
2.6. Assessment of the Total Phenolic and Total Flavonoid Compounds
2.6.1. Total Phenolic Content
2.6.2. Total Flavonoid Content
2.7. Antioxidant Activity
2.7.1. ABTS Radical-Scavenging Activity
2.7.2. DPPH Radical-Scavenging Activity
2.7.3. Reducing-Power Activity
2.7.4. O-Phenanthroline Chelating Activity
2.7.5. Silver Nanoparticles
2.8. Correlation between Antioxidant Assays
2.9. Antibacterial Activity
| Inhibition diameter (mm) | |||||
|---|---|---|---|---|---|
| Seaweed | Bacteria | 1000 µg.ml-1 | 500 µg.ml-1 | 250 µg.ml-1 | 125 µg.ml-1 |
| Dictyota dichotoma | E. coli | 26.00a±1.14 | 19.50a±0.71 | 15.50a±0.71 | 13.50a±0.71 |
| S. aureus | 14.75b±0.35 | 13.50b±0.71 | 12.50b±0.71 | 12.5b±0.71 | |
| Mean | 20.4 | 16.50 | 14.0 | 12.5 | |
| LSDp0.05 | 1.1 | 0.90 | 0.7 | 0.7 | |
| Sargassum muticum | E. coli | 26.50a±0.71 | 19.25a±1.06 | 17.25a±1.77 | 15.25a±0.35 |
| S. aureus | 14.25b±0.35 | 13,00b±1.41 | 11.25b±1.06 | 9.75b±1.06 | |
| Mean | 20.4 | 16.1 | 14.3 | 12.5 | |
| LSDp0.05 | 1.1 | 0.9 | 0.8 | 0.7 | |
| Corallina officinalis | E. coli | 22.50a±0.71 | 18.50a±0.71 | 16.25a±1.06 | 11.50a±0.71 |
| S. aureus | 11.50b±0.71 | 10.75b±0.35 | 9.75b±1.06 | 9.75b±1.06 | |
| Mean | 17.0 | 14.6 | 13.0 | 10.6 | |
| LSDp0.05 | 0.9 | ns*** | 0.7 | 0.6 | |
| Ulva lactuca | E. coli | 16.00a±1.41 | 12.50a±0.71 | 10.50a±0.71 | 9.50a±0.71 |
| S. aureus | 13.50b±0.71 | 11.50b±0.71 | 10.50a±0.71 | 9,00a±1.41 | |
| Mean | 14.8 | 12.0 | 10.5 | 9.3 | |
| LSDp0.05 | 0.8 | 0.7 | ns | ns | |
| Cladophora laetevirens | E. coli | 15.75a±1.06 | 13,00a±1.41 | 11.50a±0.71 | 8.50a±0.71 |
| S. aureus | 12.50b±0.71 | 11.25b±0.35 | 10.25b±0.35 | 7.50b±0.71 | |
| Mean | 14.1 | 12.1 | 10.9 | 8.0 | |
| LSDp0.05 | 0.8 | 0.7 | 0.6 | 0.5 | |
3. Materials and Methods
3.1. Sampled Seaweed
| Seaweed | Color | Size (cm) | Thalles morphology |
|---|---|---|---|
|
Dictyota spiralis (Kützing) Grunow |
Dark brown and olive green | 10 and 20 | Thallus in the form of flattened, branched stems, with branches pointing in different directions. The fronds are divided into lobes or segments, giving it a feathery appearance. |
| Sargassum muticum (Yendo) Fensholt | Golden brown and slightly yellow | 45 and 60 | Branched stems (stolons) Leaves are toothed and lanceolate. |
| Corallina officinalis Linnaeus | Light red, slightly brown with dark pink areas | 10 and 25 | Thallus is composed of branched, calcified structures resembling small branches forming dense tufts |
| Ulva lactuca Linnaeus | Bright green | 3 and 5 | Its thallus consists of thin, small, flat, smooth and relatively translucent, ribbon-shaped green leaves. |
| Cladophora laetevirens (Dillwyn) Kützing | Slightly dark green | 20 and 40 | Its thallus consists of cylindrical branched filaments that form dense tufts. |
3.2. Extraction of Bioactive Compounds
3.3. Polysaccharides Extraction
3.3.1. Extraction of Alginate
3.3.2. Extraction of Agar
3.3.3. Extraction of Carrageenan
3.3.4. Extraction of Ulvan
3.4. Elemental Composition of Raw Seaweed
3.5. Determination of Polysaccharides, Carbohydrates, Total Phenolic and Total Flavonoid Contents
3.5.1. Yield Content
3.5.2. Lipid Content
- P0 : Sample test point;
- PI : Weight of the empty flask;
- PF : Weight of the flask containing the fatty extract.
3.5.3. Soluble Protein Content
3.5.4. Sugar Content
3.5.5. Total Phenolic Content
3.5.6. Total Flavonoid Content
3.6. Antioxidant Activity
3.6.1. DPPH Radical-Scavenging Activity
3.6.2. ABTS Radical-Scavenging Activity
3.6.3. Reducing-Power Activity
3.6.4. O-Phenanthroline Chelating Activity
3.6.5. Silver Nanoparticles
3.7. Antibacterial Activity
3.8. Statistical Calculations
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pérez-Lloréns, J.L.; Mouritsen, O.G.; Rhatigan, P.; Cornish, M.L.; Critchley, A.T. Seaweeds in Mythology, Folklore, Poetry, and Life. J. Appl. Phycol. 2020, 32, 3157–3182. [Google Scholar] [CrossRef]
- Lomartire, S.; Gonçalves, A.M.M. An Overview of Potential Seaweed-Derived Bioactive Compounds for Pharmaceutical Applications. Mar. Drugs 2022, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Janarthanan, M.; Senthil Kumar, M. The Properties of Bioactive Substances Obtained from Seaweeds and Their Applications in Textile Industries. J. Ind. Text. 2018, 48, 361–401. [Google Scholar] [CrossRef]
- Mendes, G.d.S.; Soares, A.R.; Martins, F.O.; Albuquerque, M.C.M.d.; Costa, S.S.; Yoneshigue-Valentin, Y.; Gestinari, L.M.d.S.; Santos, N.; Romanos, M.T.V. Antiviral Activity of the Green Marine Alga Ulva Fasciata on the Replication of Human Metapneumovirus. Rev. Inst. Med. Trop. São Paulo 2010, 52, 03–10. [Google Scholar] [CrossRef] [PubMed]
- Mac Monagail, M.; Cornish, L.; Morrison, L.; Araújo, R.; Critchley, A.T. Sustainable Harvesting of Wild Seaweed Resources. Eur. J. Phycol. 2017, 52, 371–390. [Google Scholar] [CrossRef]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef] [PubMed]
- 7 Mutalipassi, M.; Esposito, R.; Ruocco, N.; Viel, T.; Costantini, M.; Zupo, V. Bioactive Compounds of Nutraceutical Value from Fishery and Aquaculture Discards. Foods 2021, 10, 1495. [Google Scholar] [CrossRef]
- Braña, A.F.; Fiedler, H.-P.; Nava, H.; González, V.; Sarmiento-Vizcaíno, A.; Molina, A.; Acuña, J.L.; García, L.A.; Blanco, G. Two Streptomyces Species Producing Antibiotic, Antitumor, and Anti-Inflammatory Compounds Are Widespread Among Intertidal Macroalgae and Deep-Sea Coral Reef Invertebrates from the Central Cantabrian Sea. Microb. Ecol. 2015, 69, 512–524. [Google Scholar] [CrossRef]
- Morales, J.L.; Cantillo-Ciau, Z.O.; Sánchez-Molina, I.; Mena-Rejón, G.J. Screening of Antibacterial and Antifungal Activities of Six Marine Macroalgae from Coasts of Yucatán Peninsula. Pharm. Biol. 2006, 44, 632–635. [Google Scholar] [CrossRef]
- Safafar, H.; Van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef]
- Raja, K.; Kadirvel, V.; Subramaniyan, T. Seaweeds, an Aquatic Plant-Based Protein for Sustainable Nutrition - A Review. Future Foods 2022, 5, 100142. [Google Scholar] [CrossRef]
- Schmid, M.; Kraft, L.G.K.; van der Loos, L.M.; Kraft, G.T.; Virtue, P.; Nichols, P.D.; Hurd, C.L. Southern Australian Seaweeds: A Promising Resource for Omega-3 Fatty Acids. Food Chem. 2018, 265, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Tanna, B.; Mishra, A. Nutraceutical Potential of Seaweed Polysaccharides: Structure, Bioactivity, Safety, and Toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.; Inácio, A.; Coutinho, T.; Ministro, M.; Cotas, J.; Pereira, L.; Bahcevandziev, K. Seaweed Potential in the Animal Feed: A Review. J. Mar. Sci. Eng. 2020, 8, 559. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Ferdous, U.T.; Balia Yusof, Z.N. Insight into Potential Anticancer Activity of Algal Flavonoids: Current Status and Challenges. Molecules 2021, 26, 6844. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Belhaouari, B.; Bezzina, Z. Study of the Macroalgae and Application of Ecological Evaluation Index (EEI-c) in the Coastal Waters of Algeria |. International Journal of Aquatic Biology 2019. [Google Scholar]
- Benattouche, Z.; Raho, G.B.; Sahnouni, F.; Hariri, A.; Bouhadi, G.; Benchohra, M. ANTIOXIDANT ACTIVITIES OF SULFATED POLYSACCHARIDE OBTAINED FROM RED ALGAE CORALLINA OFFICINALIS. Int. J. Pharmacogn. 2017, 4. [Google Scholar]
- Saim, S.; Sahnouni, F.; Bouhadi, D.; Kharbouche, S. The Antimicrobial Activity of Two Marine Red Algae Collected from Algerian West Coast. Trends Pharm. Sci. 2021, 7, 233–242. [Google Scholar] [CrossRef]
- Benfares, R.; Kord, A.; Boudjema, K.; Bouarab, M.; Benrabah, S.; Boudjemaa, K.; Švarc-Gajić, J. Chemical Characterization of Essential Oils and Antioxidant Activity of Dictyota Dichotoma and Dictyopteris Membranacea. Acta Period. Technol. 2019, No. 50, 33–42. [Google Scholar] [CrossRef]
- Benali, M.; Djebri, I.; Bellouis, D.; Sellam, L.-N.; Rebzani-Zahaf, C. First Record of Drifting Sargassum Muticum (Yendo) Fensholt Thalli on the Algerian Coasts of Cherchell and Sidi Fredj. BioInvasions Rec. 2019, 8, 575–581. [Google Scholar] [CrossRef]
- Nabti, E.; Leila, B.; Nassira, T. Effect of the Marine Algae Cystoseira Mediterranea on Growth of Hordeum Vulgare (l.) and It Chlorophyll Content. Trends Hortic. 2018, 1. [Google Scholar] [CrossRef]
- Kozak-Balkan, A.; Alcitepe, I.; Uysal, S.A.; Kuruoglu, B.N.; Baykan, S.; Tezcanli-Kaymaz, B.; Tuney, I. Seaweed Extracts as a Promising Natural Source Exert a Therapeutic Approach via Inducing Cytotoxicity and Apoptosis in Chronic Myeloid Leukemia Cell Model. 2023. [Google Scholar]
- Dixit, D.; Suthar, P.; Trivedi, M.H.; Reddy, C.R.K.; Gadhavi, D. Evaluation of Tropical Edible Seaweeds Across the Untapped Frontier of Gok for Boosting the Expanse’s Fiscal Valorization. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2023, 93, 577–588. [Google Scholar] [CrossRef]
- Houchi, S.; Mahdadi, R.; Khenchouche, A.; Song, J.; Zhang, W.; Pang, X.; Zhang, L.; Sandalli, C.; Du, G. Investigation of Common Chemical Components and Inhibitory Effect on GES-Type β-Lactamase (GES22) in Methanolic Extracts of Algerian Seaweeds. Microb. Pathog. 2019, 126, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Farvin, K.H.S.; Surendraraj, A.; Al-Ghunaim, A.; Al-Yamani, F. Chemical Profile and Antioxidant Activities of 26 Selected Species of Seaweeds from Kuwait Coast. J. Appl. Phycol. 2019, 31, 2653–2668. [Google Scholar] [CrossRef]
- Aziz, S.; Jafarah, N.; Sabri, S.; Wahab, A.; Balia Yusof, Z.N. Antifungal Activities against Oil Palm Pathogen Ganoderma Boninense from Seaweed Sources. Asia Pac. J. Mol. Biol. Biotechnol. 2019, 75–83. [Google Scholar] [CrossRef]
- Sanger, G.; Rarung, L.K.; Kaseger, B.E.; Assa, J.R.; Agustin, T. Phenolic Content and Antioxidant Activities of Five Seaweeds from North Sulawesi, Indonesia. 2019, 12. [Google Scholar]
- El-Sheekh, M.M.; Bases, E.; El Shafay, S.M.; El-shenody, R. Influence of Seasonal Variations on Extract Yield and Antioxidant Activities of Some Seaweed Species. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2023. [Google Scholar] [CrossRef]
- Petchsomrit, A.; Chanthathamrongsiri, N.; Jiangseubchatveera, N.; Manmuan, S.; Leelakanok, N.; Plianwong, S.; Siranonthana, N.; Sirirak, T. Extraction, Antioxidant Activity, and Hydrogel Formulation of Marine Cladophora Glomerata. Algal Res. 2023, 71, 103011. [Google Scholar] [CrossRef]
- Dangar, K.; Varsani, V.; Vyas, S. Characterization of Sodium Alginate Extracted from Brown Seaweeds Growing on Veraval Coast, Gujarat. Plant Sci. Today 2021, 8, 45–48. [Google Scholar] [CrossRef]
- Osman, N.; Suli̇Man, T.; Osman, K. Characterization of Native Alginates of Common Alginophytes from the Red Sea Coast of Sudan. Int. J. Second. Metab. 2020, 7, 266–274. [Google Scholar] [CrossRef]
- Balboa, E.M.; Rivas, S.; Moure, A.; Domínguez, H.; Parajó, J.C. Simultaneous Extraction and Depolymerization of Fucoidan from Sargassum Muticum in Aqueous Media. Mar. Drugs 2013, 11, 4612–4627. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.; Amer, M.S. Characterization and Biological Properties of Sulfated Polysaccharides of Corallina Officinalis and Pterocladia Capillacea. Acta Bot. Bras. 2021, 34, 623–632. [Google Scholar] [CrossRef]
- Chidambaram, P.; Jeyprakash, A.; Chinnathambi, P. Characterisation of Carrageenan Extracted from Fresh and Defatted Red Algae along the Pamban Coast, Tamilnadu, India. Vegetos 2019, 32, 281–287. [Google Scholar] [CrossRef]
- Scholz, D.B.; Örlygsson, D.G.; Moss, D.C.; Karsten, D.U. Biorefinery of Red Algae for Multiple High-Value Products. 2022. [Google Scholar]
- Madany, M.A.; Abdel-Kareem, M.S.; Al-Oufy, A.K.; Haroun, M.; Sheweita, S.A. The Biopolymer Ulvan from Ulva Fasciata: Extraction towards Nanofibers Fabrication. Int. J. Biol. Macromol. 2021, 177, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.-H. R.; Chen, G.-W.; Pan, C.-L.; Lin, H.-T. V. Production of Ulvan Oligosaccharides with Antioxidant and Angiotensin-Converting Enzyme-Inhibitory Activities by Microbial Enzymatic Hydrolysis. Fermentation 2021, 7, 160. [Google Scholar] [CrossRef]
- Qin, L.; Yang, Y.; Mao, W. Anticoagulant Property of a Sulfated Polysaccharide with Unique Structural Characteristics from the Green Alga Chaetomorpha Aerea. Mar. Drugs 2023, 21, 88. [Google Scholar] [CrossRef]
- Dı́ez, I.; Santolaria, A.; Gorostiaga, J.M. The Relationship of Environmental Factors to the Structure and Distribution of Subtidal Seaweed Vegetation of the Western Basque Coast (N Spain). Estuar. Coast. Shelf Sci. 2003, 56, 1041–1054. [Google Scholar] [CrossRef]
- Breuer, F.; Janz, P.; Farrelly, E.; Ebke, K.-P. Environmental and Structural Factors Influencing Algal Communities in Small Streams and Ditches in Central Germany. J. Freshw. Ecol. 2017, 32, 65–83. [Google Scholar] [CrossRef]
- Sumayya, S.S.; Lubaina, A.S.; Murugan, K. Phytochemical, HPLC and FTIR Analysis of Methanolic Extract from Gracilaria Dura (C Agardh) J Agardh. J. Drug Deliv. Ther. 2020, 10, 114–118. [Google Scholar] [CrossRef]
- John Peter Paul, J.P.P. Phytochemical Analysis of Sargassum Linearifolium (Turner) C.Ag. (Brown Seaweed) Using UV-VIS, FTIR and HPLC. 2017, 14–17. [Google Scholar]
- Aravinth, A.; Dhanasundaram, S.; Perumal, P.; Vengateshwaran, T.D.; Thavamurugan, S.; Rajaram, R. Biological Activities of the Brown Seaweed Dictyota Ciliolata with Special Reference to the Human Diseases Transmitting Aedes Aegypti’s Larvae. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Imran, M.; Iqbal, A.; Badshah, S.L.; Sher, A.A.; Ullah, H.; Ayaz, M.; Mosa, O.F.; Mostafa, N.M.; Daglia, M. Chemical and Nutritional Profiling of the Seaweed Dictyota Dichotoma and Evaluation of Its Antioxidant, Antimicrobial and Hypoglycemic Potentials. Mar. Drugs 2023, 21, 273. [Google Scholar] [CrossRef] [PubMed]
- Haque, K.; Chy, S.; Akter, S.; Nath, K. Collection, Identification and Biochemical Analyses of Different Sea Weeds from Saint Martin’s Island. Bangladesh J. Agric. Res. 2010, 34. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Ashraf, M.Y.; Saifullah; Ahmad, M. Improving Agricultural Water Use Efficiency by Nutrient Management in Crop Plants. Acta Agric. Scand. Sect. B — Soil Plant Sci. 2011, 61, 291–304. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Efficiency of Hydrothermal Pretreatment on the Anaerobic Digestion of Pelagic Sargassum for Biogas and Fertiliser Recovery. Fuel 2020, 279, 118527. [Google Scholar] [CrossRef]
- Yavaş, İ.; Ünay, A. The Role of Silicon under Biotic and Abiotic Stress Conditions. Türkiye Tarımsal Araştırmalar Derg. 2017, 4, 204–209. [Google Scholar] [CrossRef]
- Marsham, S.; Scott, G.W.; Tobin, M.L. Comparison of Nutritive Chemistry of a Range of Temperate Seaweeds. Food Chem. 2007, 100, 1331–1336. [Google Scholar] [CrossRef]
- Parsa, M.; Jalilzadeh, H.; Pazoki, M.; Ghasemzadeh, R.; Abduli, M. Hydrothermal Liquefaction of Gracilaria Gracilis and Cladophora Glomerata Macro-Algae for Biocrude Production. Bioresour. Technol. 2018, 250, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Tabarsa, M.; Rezaei, M.; Ramezanpour, Z.; Waaland, J.R. Chemical Compositions of the Marine Algae Gracilaria Salicornia (Rhodophyta) and Ulva Lactuca (Chlorophyta) as a Potential Food Source. J. Sci. Food Agric. 2012, 92, 2500–2506. [Google Scholar] [CrossRef] [PubMed]
- Hawas, U.W.; Hussein, S.; El-Kassem, L.T.A.; Taie, H.A.A.; El-Sherbiny, M.M. Biochemical Assessment of Some Red Sea Brown Algae with Potential of Antioxidant and Antimicrobial Agents. preprint; In Review. 2023. [Google Scholar] [CrossRef]
- Kumar, Y.; Singhal, S.; Tarafdar, A.; Pharande, A.; Ganesan, M.; Badgujar, P.C. Ultrasound Assisted Extraction of Selected Edible Macroalgae: Effect on Antioxidant Activity and Quantitative Assessment of Polyphenols by Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS). Algal Res. 2020, 52, 102114. [Google Scholar] [CrossRef]
- Grina, F.; Ullah, Z.; Kaplaner, E.; Moujahid, A.; Eddoha, R.; Nasser, B.; Terzioğlu, P.; Yilmaz, M.A.; Ertaş, A.; Öztürk, M.; Essamadi, A. In Vitro Enzyme Inhibitory Properties, Antioxidant Activities, and Phytochemical Fingerprints of Five Moroccan Seaweeds. South Afr. J. Bot. 2020, 128, 152–160. [Google Scholar] [CrossRef]
- Yarnpakdee, S.; Senphan, T.; Wangtueai, S.; Jaisan, C.; Nalinanon, S. Characteristic and Antioxidant Activity of Cladophora Glomerata Ethanolic Extract as Affected by Prior Chlorophyll Removal and Drying Methods. J. Food Process. Preserv. 2022, 46. [Google Scholar] [CrossRef]
- El-Bilawy, E.H.; Al-Mansori, A.-N. A.; Alotibi, F.O.; Al-Askar, A.A.; Arishi, A.A.; Teiba, I.I.; Sabry, A.E.-N.; Elsharkawy, M.M.; Heflish, A.A.; Behiry, S.I.; Abdelkhalek, A. Antiviral and Antifungal of Ulva Fasciata Extract: HPLC Analysis of Polyphenolic Compounds. Sustainability 2022, 14, 12799. [Google Scholar] [CrossRef]
- Korzeniowska, K.; Łęska, B.; Wieczorek, P.P. Isolation and Determination of Phenolic Compounds from Freshwater Cladophora Glomerata. Algal Res. 2020, 48, 101912. [Google Scholar] [CrossRef]
- Tester, P.A.; Litaker, R.W.; Berdalet, E. Climate Change and Harmful Benthic Microalgae. Harmful Algae 2020, 91, 101655. [Google Scholar] [CrossRef]
- Theuerkauf, S.J.; Barrett, L.T.; Alleway, H.K.; Costa-Pierce, B.A.; St. Gelais, A.; Jones, R.C. Habitat Value of Bivalve Shellfish and Seaweed Aquaculture for Fish and Invertebrates: Pathways, Synthesis and next Steps. Rev. Aquac. 2022, 14, 54–72. [Google Scholar] [CrossRef]
- Gür, İ.; Polat, S. Seasonal Changes in Proximate and Bioactive Compounds of Brown and Red Seaweeds from İskenderun Bay, the North-Eastern Mediterranean Sea. Çanakkale Onsekiz Mart Univ. J. Mar. Sci. Fish. 2023, 6, 33–43. [Google Scholar] [CrossRef]
- Almutairi, A.W.; El-Sayed, A.E.-K. B.; Reda, M.M. Combined Effect of Salinity and pH on Lipid Content and Fatty Acid Composition of Tisochrysis Lutea. Saudi J. Biol. Sci. 2020, 27, 3553–3558. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.M.U.; Che Radziah, C.; Ibrahim, S.; Latiff, F.; Othman, M.F.; Abdullah, M.A. Effects of Photoperiod, Salinity and pH on Cell Growth and Lipid Content of Pavlova Lutheri. Ann. Microbiol. 2014, 64, 157–164. [Google Scholar] [CrossRef]
- Savage, A.M.; Trapido-Rosenthal, H.; Douglas, A.E. On the Functional Significance of Molecular Variation in Symbiodinium, the Symbiotic Algae of Cnidaria: Photosynthetic Response to Irradiance. Mar. Ecol. Prog. Ser. 2002, 244, 27–37. [Google Scholar] [CrossRef]
- Sharma, S.; Neves, L.; Funderud, J.; Mydland, L.T.; Øverland, M.; Horn, S.J. Seasonal and Depth Variations in the Chemical Composition of Cultivated Saccharina Latissima. Algal Res. 2018, 32, 107–112. [Google Scholar] [CrossRef]
- Bartwal, A.; Mall, R.; Lohani, P.; Guru, S.K.; Arora, S. Role of Secondary Metabolites and Brassinosteroids in Plant Defense Against Environmental Stresses. J. Plant Growth Regul. 2013, 32, 216–232. [Google Scholar] [CrossRef]
- Ratnasonia, S.G.; Fachri, B.A.; Palupi, B. Extraction of Antioxidant Compounds from Sargassum Sp. Using Water and Ultrasound Assisted Extraction Method as A Derivation of Green Chemistry Principles. J. Biobased Chem. 2022, 2, 29–40. [Google Scholar] [CrossRef]
- Hsaine, L. Radical Scavenging Activity and Phenolic Contents of Brown Seaweeds Harvested from the Coast of Sidi Bouzid. 2019. [Google Scholar]
- Aleksandrova, A.; Nesterkina, M.; Gvozdii, S.; Kravchenko, I. Phytochemical Analysis and Anti-Inflammatory Activity of Cladophora Aegagropila Extract. J. Herbmed Pharmacol. 2019, 9, 81–85. [Google Scholar] [CrossRef]
- Mridha, A.; Paul, S. Cladophora Glomerata, a Newly Emerging Green Alga, Acting as a Repository of Potent Antioxidant. Res. J. Biotechnol. 2021, 16, 155–161. [Google Scholar]
- El-Shaibany, A.; AL-Habori, M.; Al-Maqtari, T.; Al-Mahbashi, H. The Yemeni Brown Algae Dictyota Dichotoma Exhibit High In Vitro Anticancer Activity Independent of Its Antioxidant Capability. BioMed Res. Int. 2020, 2020, e2425693. [Google Scholar] [CrossRef]
- Chouh, A.; Nouadri, T.; Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Phlorotannins of the Brown Algae Sargassum Vulgare from the Mediterranean Sea Coast. Antioxidants 2022, 11, 1055. [Google Scholar] [CrossRef]
- Velankanni, S.V. Extraction Of Bioactive Compounds From Seaweed Dictyota Dichotoma (Hudson) J.V. Lamouroux And Assessment Of Its Antioxidant Activity. J. Pharm. Negat. Results 2022, 3528–3539. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Martyasari, N.W.R.; Abidin, A.S.; Ilhami, B.T.K.; Padmi, H.; Widyastuti, S.; Sunarwidhi, A.L.; Sunarpi, H. Antioxidant Activity of Brown Macroalgae Sargassum Ethanol Extract from Lombok Coast, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 712, 012038. [Google Scholar] [CrossRef]
- Li, Y.; Sun, S.; Pu, X.; Yang, Y.; Zhu, F.; Zhang, S.; Xu, N. Evaluation of Antimicrobial Activities of Seaweed Resources from Zhejiang Coast, China. Sustainability 2018, 10, 2158. [Google Scholar] [CrossRef]
- Baleta, F.N.; Bolaños, J.M.; Ruma, O.C.; Baleta, A.N.; Cairel, J.D. Phytochemicals Screening and Antimicrobial Properties of Sargassum Oligocystum and Sargassum Crassifolium Extracts. 2016. [Google Scholar]
- Zouaoui, B.; Ghalem, B.R. The Phenolic Contents and Antimicrobial Activities of Some Marine Algae from the Mediterranean Sea (Algeria). Russ. J. Mar. Biol. 2017, 43, 491–495. [Google Scholar] [CrossRef]
- Saadatmand, S.; Khavarinejad, R.; Nejadsattari, T.; Soltani, S. Antioxidant and Antibacterial Activities of Cladophora Glomerata (L.) Kütz. in Caspian Sea Coast, Iran. Afr. J. Biotechnol. 2011, 10, 7684–7689. [Google Scholar]
- Garreta, A.G.; Gallardo, T.; Ribera, M.A.; Cormaci, M.; Furnari, G.; Giaccone, G.; Boudouresque, C.F. Checklist of Mediterranean Seaweeds. III. Rhodophyceae Rabenh. 1. Ceramiales Oltm. 2001, 44, 425–460. [Google Scholar] [CrossRef]
- Okudan, E.; Dural, B.; Demir, V.; Erduğan, H.; Aysel, V. Biodiversity of Marine Benthic Macroflora (Seaweeds/Macroalgae and Seagrasses) of the Mediterranean Sea; 2016; pp 107–135.
- Shabaka, S.H. Checklist of Seaweeds and Seagrasses of Egypt (Mediterranean Sea): A Review. Egypt. J. Aquat. Res. 2018, 44, 203–212. [Google Scholar] [CrossRef]
- Torres, M.R.; Sousa, A.P.A.; Silva Filho, E.A.T.; Melo, D.F.; Feitosa, J.P.A.; De Paula, R.C.M.; Lima, M.G.S. Extraction and Physicochemical Characterization of Sargassum Vulgare Alginate from Brazil. Carbohydr. Res. 2007, 342, 2067–2074. [Google Scholar] [CrossRef]
- Marinho-Soriano, E. Agar Polysaccharides from Gracilaria Species (Rhodophyta, Gracilariaceae). J. Biotechnol. 2001, 89, 81–84. [Google Scholar] [CrossRef]
- González-López, N.; Moure, A.; Domínguez, H. Hydrothermal Fractionation of Sargassum Muticum Biomass. J. Appl. Phycol. 2012, 24, 1569–1578. [Google Scholar] [CrossRef]
- Robic, A. Etude de La Variabilité Chimique, Physico-Chimique et Rhéologique Des Ulvanes, Polysaccharides Des Parois Cellulaires d’algues Marines Vertes de La Famille Des Ulves (Ulvales, Chlorophyta). These de doctorat, Nantes, 2008. https://www.theses.fr/2008NANT2104 (accessed 2023-09-08).
- Pliego-Cortés, H.; Hardouin, K.; Bedoux, G.; Marty, C.; Cérantola, S.; Freile-Pelegrín, Y.; Robledo, D.; Bourgougnon, N. Sulfated Polysaccharides from Seaweed Strandings as Renewable Source for Potential Antivirals against Herpes Simplex Virus 1. Mar. Drugs 2022, 20, 116. [Google Scholar] [CrossRef]
- Barford, R.A. Interactions of Proteins with Bonded-Phase Ion Exchangers. J. Chromatogr. A 1979, 185, 393–402. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Topçu, G.; Ay, M.; Bilici, A.; Sarıkürkcü, C.; Öztürk, M.; Ulubelen, A. A New Flavone from Antioxidant Extracts of Pistacia Terebinthus. Food Chem. 2007, 103, 816–822. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, (9–10). [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on Products of Browning Reaction. Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Szydlowskaczerniak, A.; Dianoczki, C.; Recseg, K.; Karlovits, G.; Szlyk, E. Determination of Antioxidant Capacities of Vegetable Oils by Ferric-Ion Spectrophotometric Methods. Talanta 2008, 76, 899–905. [Google Scholar] [CrossRef]
- Özyürek, M.; Güngör, N.; Baki, S.; Güçlü, K.; Apak, R. Development of a Silver Nanoparticle-Based Method for the Antioxidant Capacity Measurement of Polyphenols. Anal. Chem. 2012, 84, 8052–8059. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT®9.2 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Bordens, K.S.; Abbott, B.B. Research Design and Methods: A Process Approach, 5th ed.; Research design and methods: A process approach, 5th ed; McGraw-Hill: New York, NY, US, 2002; pp. xv, 490. [Google Scholar]
| Seaweed | Yields (%) | ||
|---|---|---|---|
| Polysaccharide extracts | Bioactive extracts | ||
| Dictyota dichotoma | Alginate | 14.15b*±0.19** | 27.07a±1.19 |
| Sargassum muticum | Alginate | 17.40a±0.95 | 5.29e±0.44 |
| Corallina officinalis | Agar agar | 8.85c±0.29 | 6.11d±0.12 |
| Carrageenan | 8.19d±0.18 | ||
| Ulva lactuca | Ulvan | 2.47d±0.10 | 9.55c±0.12 |
| Cladophora laetevirens | Ulvan | 2.78e±0.07 | 12.07b±0.44 |
| Mean | 8.97 | 12.02 | |
| LSDp0.05 | 0.46 | 0.61 | |
| Element | Dictyota dichotoma (%) | Sargassum muticum (%) | Corallina officinalis (%) | Ulva lactuca (%) | Cladophora laetevirens (%) | Mean | LSDp0.05 |
|---|---|---|---|---|---|---|---|
| L.E | 79.86a*±0.000 | 76.26a±0.000 | 52.64e±0.000 | 74.76b±0.000 | 70.00d±0.000 | 70.70 | 3.68 |
| Aluminum (Al) | 1.20d±0.035 | 2.08b±0.045 | 2.39a±0.056 | 1.71c±0.038 | 0.945e±0.032 | 1.67 | 0.09 |
| Phosphorus (P) | 0.184b±0.005 | 0.155c±0.006 | Nd | 0.230a±0.006 | 0.086d±0.005 | 0.13 | 0.01 |
| Potassium (K) | 6.58b±0.012 | 7.23a±0.013 | 0.455e±0.004 | 2.31d±0.007 | 3.32c±0.010 | 3.979 | 0.207 |
| Iron (Fe) | 0.175d±0.004 | 0.226c±0.005 | 0.379b±0.009 | 2.05a±0.016 | 0.223c±0.005 | 0.611 | 0.032 |
| Zinc (Zn) | 0.027a±0.001 | 0.006c±0.000 | Nd | 0.005c±0.000 | 0.018b±0.001 | 0.013 | 0.002 |
| Rubidium (Rb) | 0.006a±0.000 | 0.012a±0.000 | 0.005a±0.000 | 0.009a±0.000 | 0.004a±0.000 | 0.007 | ns*** |
| Magnesium (Mg) | 3.96c±0.340 | 2.20d±0.386 | 4.31b±0.433 | 1.61e±0.361 | 6.26a±0.269 | 3.67 | 0.19 |
| Silicon (Si) | 0.959d±0.017 | 4.10b±0.031 | 3.06c±0.031 | 6.57a±0.036 | 3.21c±0.028 | 3.58 | 0.19 |
| Sulfur (S) | 4.93c±0.013 | 2.82d±0.011 | 1.03e ±0.008 | 6.62b±0.016 | 13.65a±0.022 | 5.81 | 0.30 |
| Calcium (Ca) | 2.02d±0.006 | 4.69b±0.010 | 36.48a±0.035 | 3.67c±0.008 | 2.21d±0.007 | 9.81 | 0.51 |
| Copper (Cu) | 0.006a±0.000 | 0.001a±0.000 | Nd | 0.003a±0.000 | 0.018a±0.001 | 0.006 | ns |
| Arsenic (As) | 0.004a±0.000 | 0.013a±0.000 | 0.002a±0.000 | 0.004a±0.000 | 0.003a±0.000 | 0.005 | ns |
| Strontium (Sr) | 0.090c±0.000 | 0.160b±0.001 | 0.179a±0.001 | Nd | 0.007d±0.000 | 0.087 | 0.005 |
| Titanium (Ti) | Nd | 0.031d±0.008 | 0.050c±0.013 | 0.338a±0018 | 0.022b±0.007 | 0.088 | 0.005 |
| Manganese (Mn) | Nd | 0.015a±0.002 | 0.015a±0.003 | 0.026a±0.003 | 0.009a±0.001 | 0.015 | ns |
| Seaweed species | Lipids | Soluble Proteins | Total sugar |
|---|---|---|---|
| Dictyota dichotoma | 3.07a*±0.13** | 4.51bc±0.005 | 57.87a±0.04 |
| Sargassum muticum | 0.34c±0.06 | 4.32c±0.003 | 46.43b±0.12 |
| Coralina officinalis | 0.16d±0.01 | 4.71b±0.004 | 34.93d±0.11 |
| Ulva lactuca | 0.16d±0.02 | 4.49bc±0.002 | 24.53e±0.04 |
| Cladophora laetevirens | 0.50b±0.03 | 5.15a±0.002 | 40.67c±0.09 |
| Mean | 0.85 | 4.64 | 40.89 |
| LSDp0.05 | 0.04 | 0.23 | 2.17 |
| Extracts | Total phenolic compounds content | Flavonoid’s content | |
|---|---|---|---|
| Dictyota dichotoma | MeOH | 105.96±2.37 c | 41.32±0.52 c |
| EtOAc | 189.33±3.11a | 98.45±1.12 a | |
| n-BuOH | 116.07±1.49 b | 48.04±0.89 b | |
| Sargassum muticum | MeOH | 130.53±2.06 c | 115±0.75 c |
| EtOAc | 235.67±1.13 a | 175.01±0.87 a | |
| n-BuOH | 197.3±2.70 b | 149±1.67 b | |
| Corallina officinalis | MeOH | 102.28±4.78 c | 55.83±0.21 c |
| EtOAc | 211.04±2.35 a | 75.41±1.87 a | |
| n-BuOH | 158.37±1.68 b | 57.07±1.12 b | |
| Ulva lactuca | MeOH | 110.53±3.45 c | 70.35±0.67 c |
| EtOAc | 158.89±2.79 a | 112.05±1.89 a | |
| n-BuOH | 117.03±0.75 b | 99±0.31 b | |
| Cladophora laetevirens | MeOH | 133.33±2.90 c | 201.18±0.73 c |
| EtOAc | 215±2.33 a | 331.05±3.11 a | |
| n-BuOH | 187.67±1.15 b | 286.29±2.89 b | |
| Mean LSDp0.05 |
157.87 | 127.62 | |
| 2.34 | 1.24 | ||
| Extracts | DPPH assay IC50 (µg.mL-1) | ABTS assay IC50 (µg.mL-1) | Reducing Power assay A0.5 (µg.mL-1) | Phenanthroline assay A0.5 (µg.mL-1) | SNP assay A0.5 (µg.mL-1) | |
|---|---|---|---|---|---|---|
| Dictyota dichotoma | MeOH | 369.48±1.28b | 162.89±2.46 a | >200 | 190.28±3.94 a | 112.20±1.21 a |
| EtOAc | 288.56±2.98 c | 129.28±1.78 c | >200 | 85.71±1.32 c | 109.87±1.89 b | |
| n-BuOH | 327.89±3.02 a | 142.56±2.14 b | >200 | 112.07±2.11 b | 107.31±2.11 c | |
| Sargassum muticum | MeOH | 356.64±2.20 a | 594.06±1.15 a | >200 | 170.28±0.96 a | 62.50±2.50 a |
| EtOAc | 276.23±1.98 c | 389.11±2.13 c | >200 | 72.07±1.89 c | 45.79±1.21 c | |
| n-BuOH | 347.74±3.29 b | 499.07±2.89 b | >200 | 98.27±1.11 b | 58.34±1.78 b | |
| Corallina officinalis | MeOH | 579.26±1.39 a | 176.62±2.10 a | >200 | 107.28±0.96 a | 36.46±3.11 a |
| EtOAc | 477.05±2.94 b | 127.05±1.89 c | 197.16±2.43 a | 83.45±0.88 c | 11.58±0.79 c | |
| n-BuOH | >800 | 169.03±0.79 b | >200 | 99.07±1.06 b | 27.87±1.11 b | |
| Ulva lactuca | MeOH | 675.74±3.66 a | 150.28±1.15 a | >200 | 26.61±1.93 a | 118.24±2.93 a |
| EtOAc | 588.16±3.22 b | 102.74±1.89 c | 190.78±3.84 a | 5.67±0.82 c | 98.32±1.87 c | |
| n-BuOH | >800 | 136.01±2.11 b | >200 | 19.97±0.97 b | 110.89±2.87 b | |
| Cladophora laetevirens | MeOH | 118.01±1.12 b | 107.55±1.62 a | >200 | 28.98±1.10 a | 63.71±2.84 a |
| EtOAc | 89.11±0.98 c | 78.65±0.96 c | 189.28±2.94 a | 10.06±0.88 c | 24.57±1.03 c | |
| n-BuOH | 102.55±1.29 a | 91.07±1.12 b | >200 | 23.50±1.14 b | 43.79±1.22 b | |
| BHA* | 6.14±0.41 e | 1.81±0.10 e | 8.41±0.67 b | 0,93±0,07 e | NT | |
| BHT* | 12.99±0.41 f | 1.29±0.30 f | > 200 | 2,24±0,17 f | NT | |
| α-Tocopherol* | 13.02±5,17 g | NT | 34.93±2.38 c | NT | NT | |
| Ascorbic acid* | NT | NT | 6.77±1.15 d | NT | 7.14±0.05 d | |
| Trolox* | NT | NT | NT | NT | 34.17±1.23 e | |
| Mean | 413.01 | 203.65 | 198.46 | 75.46 | 68.62 | |
| LSDp0.05 | 34.16 | 22.97 | 0.55 | 8.29 | 5.44 | |
| Specification | DPPH | ABTS | Reducing power | Phenanthroline | SNP | TFC | TPC |
|---|---|---|---|---|---|---|---|
| Mean | 413.01 | 203.65 | 198.46 | 75.46 | 68.62 | 127.62 | 157.87 |
| Standard error | 34.16 | 22.97 | 0.55 | 8.29 | 5.44 | 12.71 | 6.56 |
| Median | 356.23 | 142.22 | 200.00 | 83.01 | 60.87 | 99.21 | 156.69 |
| Standard deviation | 229.16 | 154.07 | 3.67 | 55.58 | 36.50 | 85.29 | 43.99 |
| Kurtosis | -0.95 | 1.33 | 4.51 | -0.53 | -1.54 | 0.55 | -1.37 |
| Skewness | 0.29 | 1.65 | -2.37 | 0.55 | 0.04 | 1.23 | 0.27 |
| Range | 711.87 | 517.52 | 13.66 | 189.37 | 110.38 | 293.30 | 139.23 |
| Minimum | 88.13 | 77.69 | 186.34 | 4.85 | 10.79 | 40.80 | 97.50 |
| Maximum | 800.00 | 595.21 | 200.00 | 194.22 | 121.17 | 334.10 | 236.73 |
| Coefficient of variation V (%) | 55.49 | 75.65 | 1.85 | 73.66 | 53.20 | 66.83 | 27.86 |
| Specification | DPPH | ABTS | Reducing power | Phenanthroline | SNP |
|---|---|---|---|---|---|
| DPPH | 1,00 | ||||
| ABTS | 0,18 | 1,00 | |||
| Reducing power | 0,36 | 0,30 | 1,00 | ||
| Phenanthroline | 0,23 | 0,60 | 0,32 | 1,00 | |
| SNP | 0,39 | 0,07 | 0,38 | 0,21 | 1,00 |
| Specifications | DPPH | ABTS | Reducing power | Phenanthroline | SNP | TFC | TPC |
|---|---|---|---|---|---|---|---|
| DPPH | 1.00 | ||||||
| ABTS | -0.05 | 1.00 | |||||
| Reducing power | 0.14 | 0.31 | 1.00 | ||||
| Phenanthroline | 0.02 | 0.52 | 0.45 | 1.00 | |||
| SNP | 0.22 | -0.12 | 0.19 | 0.04 | 1.00 | ||
| TFC | -0.70 | -0.08 | -0.41 | -0.54 | -0.41 | 1.00 | |
| TPC | -0.43 | 0.13 | -0.32 | -0.28 | -0.55 | 0.57 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





