1. Introduction
Coagulation is one of the most commonly used technologies for domestic sewage treatment at present [
1,
2,
3]. However, the conventional coagulation has many shortcomings. Firstly, the settling of flocs generated by coagulation is too slow and the settling time is too long, resulting in a large volume and occupied area of the sedimentation tank, which increases construction investment [
4,
5]. Secondly, research has shown that the efficiency of sewage coagulation treatment(SCT)is significantly related to the dosage of coagulants, that is, increasing the dosage of coagulants within a certain range can effectively improve the efficiency of SCT, but also increase the cost of water treatment [
6,
7].
In order to improve the above-mentioned shortcomings of conventional SCT, heavy media with fine particles such as magnetic powder and quartz sand are often used to improve its treatment effect [
8,
9,
10]. The principle of the improvement is that the heavy media is added to the conventional coagulation process, generating flocs with the heavy media particle as the core. Due to the characteristics of heavy and compact flocs, they can quickly settle at the bottom of the sedimentation tank. Therefore, this improvement method has the advantages of shortening the coagulation and sedimentation time, reducing the volume of the sedimentation tank, and its construction cost [
11]. Most sewage has the colloidal property of negative charge, and is often treated by the combined coagulation of polyaluminum chloride and cationic polyacrylamide [
12,
13,
14]. The coagulation effect is affected by many factors, such as the dosage of coagulant, the dosage and particle size of heavy medium, settling time, stirring time, sewage pH, etc, and their reasonable setting is critical to the coagulation effect [
15,
16,
17]. Only when each factor is set reasonably, can the flocculant play the best effect. Consequently, it is necessary to study the factors affecting the coagulation effect and their influencing rule.
Magnetic powder is currently the most commonly used heavy medium because it can’t only reduce sedimentation time but also improve water quality to a certain extent, however, its application is limited due to its high cost [
18]. Quartz sand has similar functions to magnetic powder in theory, but its price is significantly cheaper. If quartz sand can be used instead of magnetic powder, it can significantly reduce the processing cost of the coagulation method improved with heavy media. The method of using quartz sand as a heavy medium to enhance the SCT effect is termed the quartz sand-enhanced coagulation (QSEC). Moreover, centrifugal separation technology enables the separation and reuse of quartz sand from coagulation flocs, enhancing the application prospects of QSEC.
The response surface methodology (RSM) offers a good test in optimizing and verifying the scientific researches and industrial studies [
19]. It uses the multivariate quadratic regression equation to fit the relationship between index and influencing factors through regression equation analysis; this method aims to find the most excellent process parameters and has the ability to provide maximum information with minimum experience [
20]. The Box–Behnken design (BBD), a kind of RSM design, is the most frequently used design in pioneering studies because of its scientificity compared with other designs in RSM [
21].
At present, there is no report on the improvement of conventional SCT effect with quartz sand. In order to make up for the shortcomings of existing research, this study selected polyaluminum chloride(PAC) as the flocculant, cationic polyacrylamide(CPAM) as the coagulant aid, and quartz sand as the heavy medium for coagulation treatment of domestic sewage. Firstly, a preliminary study, namely a single factor experiment, was conducted to explore the influencing factors and their optimal range that affect the treatment effect. Then, response surface methodology (RSM) testing was conducted to accurately determine the optimal values of significant factors and obtain the best treatment effect.
2. Materials and Equipment
The sewage for coagulation treatment research was collected from the domestic sewage discharged from the student dormitories of Nanyang Normal University. The collected sewage must be analyzed and treated according to the designed treatment plan within the same day. The water quality of the raw sewage has a turbidity range of 180-352 NTU and a COD range of 450-700 mg·L-1. In order to maintain the comparability of the test results, the raw sewage is diluted to a chemical oxygen demand (COD) of 200 mg/L before being used for experimental treatment.
Cationic polyacrylamide (the aluminum oxide content is approximately 30%) and Cationic polyacrylamide (the molecular weight is approximately 1200000) were purchased from Gongyi Yiqing Water-Purifying Material Co., Ltd. (Gongyi, China). Quartz sand (the aluminum oxide content is approximately 90%) was purchased from Beijing Yinuo Green Technology Co., LTD, and had three particle sizes, namely 26-40, 70-110, and 200-300 mesh. Hydrochloric acid (HCl) and sodium hydroxide (NaOH), were of analytical grade. All aqueous and standard solutions were prepared with homemade deionized water.
Single Factor QSEC Test
3.1. Single Factor Test Design
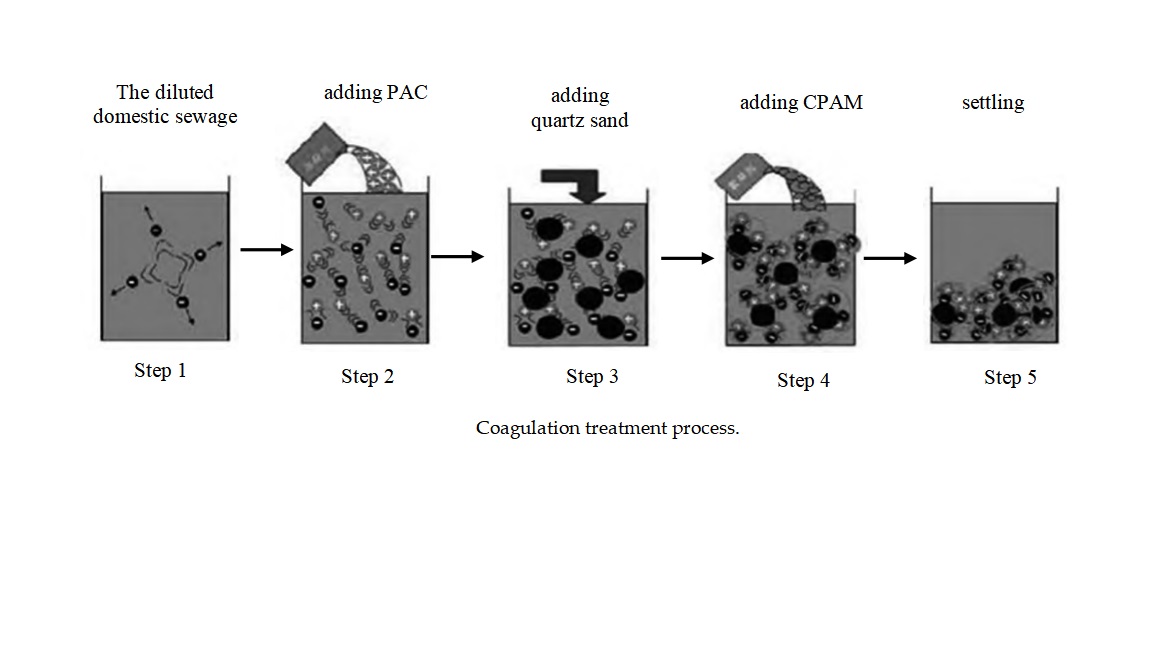
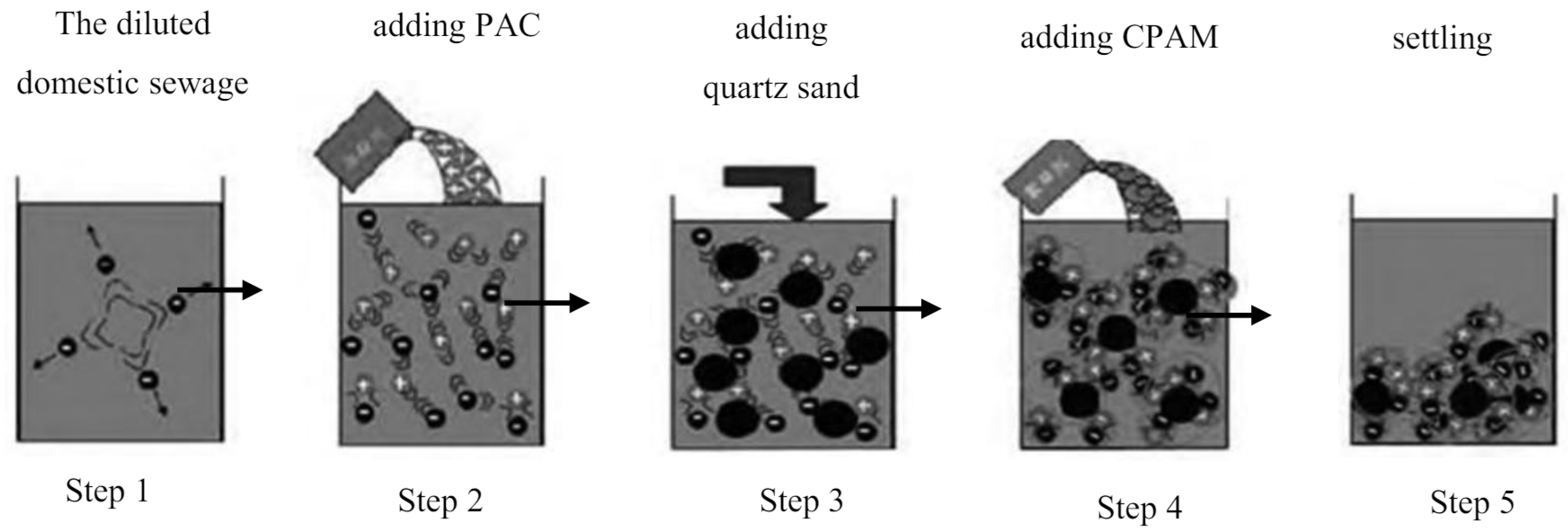
In order to study the influences of the particle and dose size of quartz sand, the coagulation dose, settling time, and sewage pH on the QSEC efficiency,the gradient coagulation tests of each factor were carried out to explore its optimal range, and provided data for the follow-up design of RSM test.The sewage QSEC treatment process is shown in
Figure 1, which includes the following 5 steps
Step 1: Put 1 liter of diluted sewage into a beaker;
Step 2: Add the prepared PAC solution to the sewage and stir with a ZR4-6 coagulation experiment blender (Shenzhen Zhongrunshui Industrial Technology Development Co., Ltd, China) at the stirring speed of 300 rpm for 2 minutes to induce coagulation reaction;
Step 3: During the stirring period, add quartz sand to the sewage;
Step 4: Add the prepared PAM solution to the sewage and continue stirring at the stirring speed of 180 rpm for 3 minutes to induce flocculation reaction;
Step 5: Settle the treated sewage to facilitate the settling of flocs.
During coagulation treatment process, change the values of influencing factors such as the coagulation dose, the dose and particle size of quartz sand, settling time, and sewage pH during the processing, and study the impact of these factors on the coagulation effect. The turbidity and the COD concentration of sewage were measured with the turbidity meter and the rapid digested spectrophotometry method, respectively, and the interface height changes of the sludge layer were observed and recorded.
To investigate the impact of quartz sand on coagulation, the conventional coagulation method was employed as a control. The process didn’t include the addition of quartz sand, and all other operations were identical to those of the quartz sand-enhanced coagulation (QSEC).
3.2. Results and Discussion of Single Factor Test
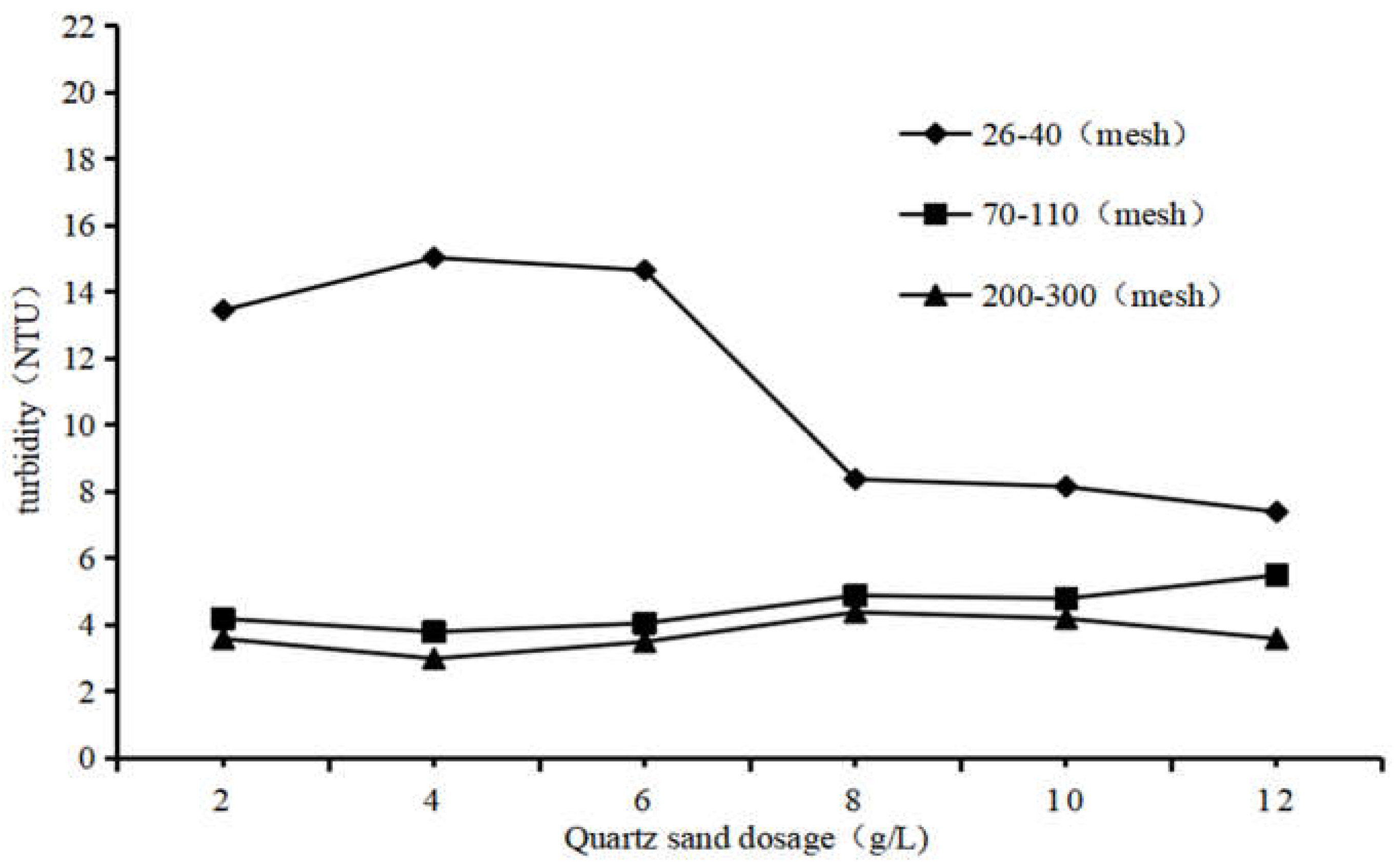
3.2.1. Impact of the Particle of Quartz Sand on QSEC
In order to study the impact of the particle of quartz sand on QSEC, the following experiments were performed, and the conditions of coagulation treatment were roughly as follows: the dosage of PAC and CPAM were 1.35 g·L
-1 and 1.8 mg·L
-1 respectively, the stirring time and settling time were 5 minutes and 30 minutes respectively, and the sewage pH were 7, three types of quartz sand with different particle sizes were added according to the predetermined gradient amounts. The turbidity and COD of the supernatant in each treatment experiment were detected and analyzed, and the results are shown in
Figure 2.
From
Figure 2, it could be seen that the impact of quartz sand with different particle sizes on sewage coagulation treatment is different. Quartz sand with a size of 200 to 300 mesh corresponds to the lowest sewage turbidity, followed by 70 to 110 mesh, and the worst is 24 to 40 mesh. This result proves that the fine particle size of quartz sand is not conducive to sewage coagulation treatment, which may be due to the small particle size of quartz sand, which makes the formed flocs difficult to settle [
22]. Therefore, all subsequent experimental studies utilized quartz sand with a particle size ranging from 200 to 300 mesh as the heavy medium to enhance the efficacy of conventional coagulation.
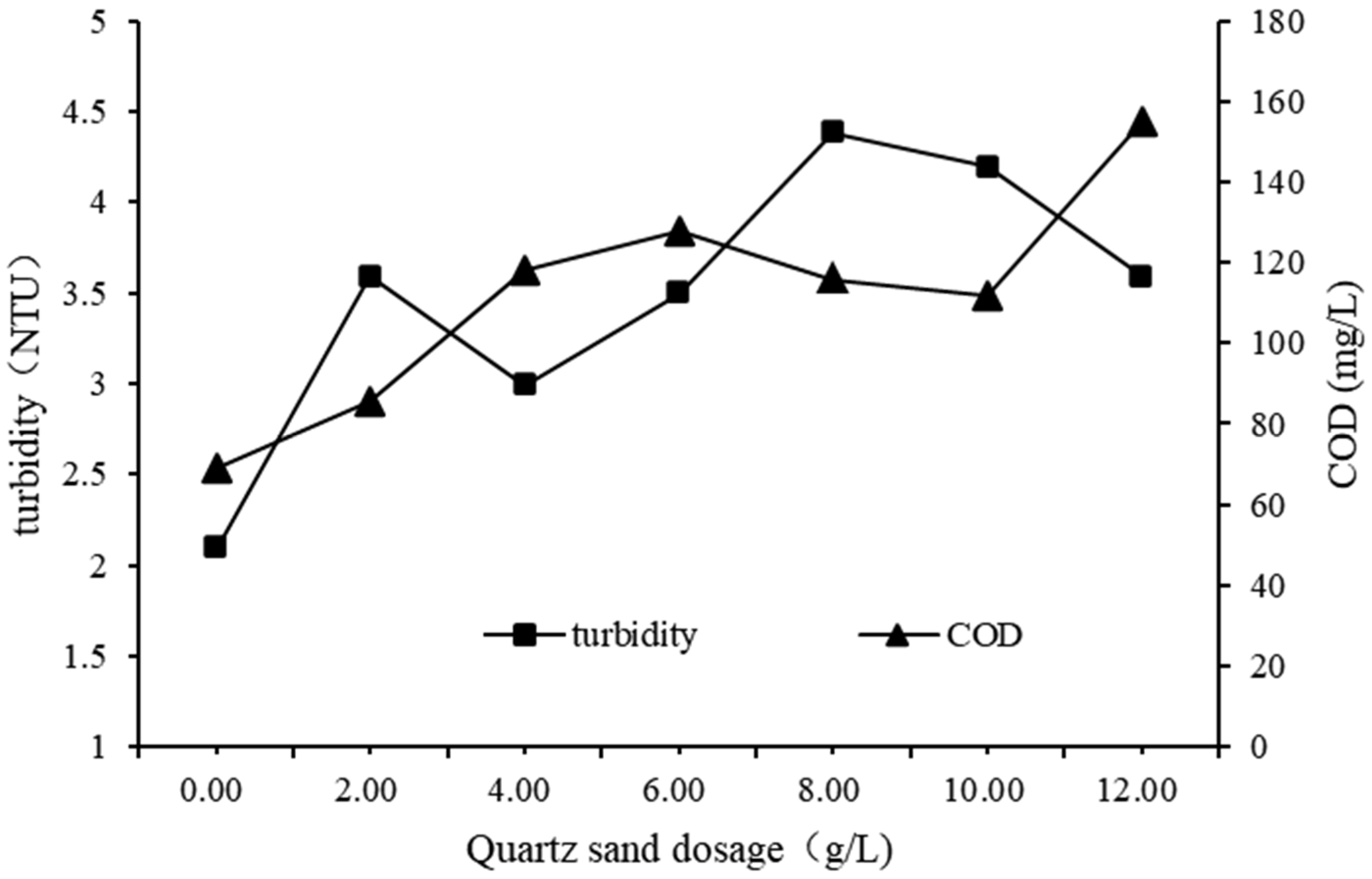
3.2.2. Impact of Dosage of Quartz Sand on QSEC
In order to study the impact of quartz sand dosage on QSEC, the following experiments were performed, and the conditions of coagulation treatment were roughly as follows: the dosages of PAC and CPAM were 1.35 g·L
-1 and 1.8 mg·L
-1 respectively, the stirring and settling time were 5 and 30 minutes respectively, the sewage pH were 7, and the dosages of quartz sand were added according to the predetermined gradient amounts. The control experimental group didn’t add quartz sand, and all other procedures were identical to those of the trial group. The turbidity and COD of the supernatant in each treatment experiment were detected and analyzed, and the results are shown in
Figure 3.
It could be seen from the
Figure 3 that the turbidity and COD all increased with the increase of quartz sand dosage, indicating that excessive quartz sand dosage is not conducive to coagulation. The reason may be that the settling rate of quartz sand is faster than that of flocs, which destroys the structure of flocs and produces some small and difficult to settle flocs, leading to a increase in the concentration of sewage quality [
23]. For cost saving considerations, the optimal dosage of quartz sand in subsequent experiments was determined to be 2g·L
-1.
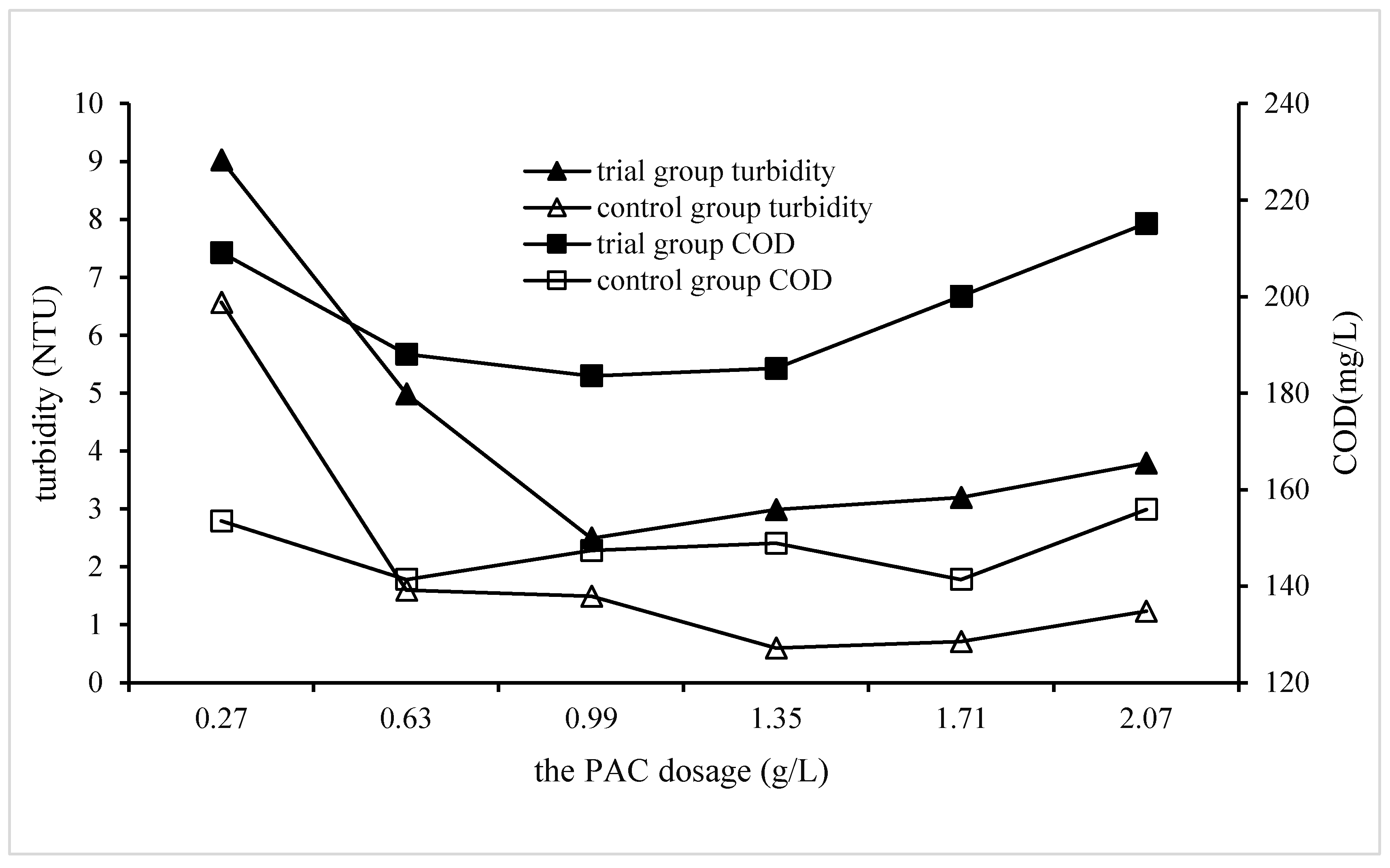
3.2.3. Impact of PAC dosage on QSEC
In order to study the impact of PAC dosage on QSEC, the following experiments were performed, and the conditions of coagulation treatment were roughly as follows: the dosage of CPAM and quartz sand were 1.8 mg·L
-1 and 2 g·L
-1 respectively, the stirring and settling time were 5 and 30 minutes respectively, the sewage pH were 7, and the PAC dosages were added according to the predetermined gradient amounts. The control experimental group didn’t add quartz sand, and all other procedures were identical to those of the trial group. The turbidity and COD of the supernatant in each treatment experiment were detected and analyzed, and the results are shown in
Figure 3.
It could be seen from
Figure 4 that the turbidity and COD of trial group had a similar trend, that was, with the increase of the dosage of PAC, the turbidity and COD of the sewage both first decreased to the minimum value, and then gradually increased. This phenomenon proved that excessive PAC dosage was not conducive to coagulation. When comparing the coagulation efficiencies of trial group with those of control group, it was found that the coagulation efficiency of control group were obviously better than those of trial group, and when the PAC dosages were 1.35 and 1.71 g·L
-1, the sewage turbidity and COD of control group reduced to the lowest values of 0.6 NTU and 141 mg·L
-1 respectively, while those of trial group were 2.5 NTU and 183.6 mg·L
-1 respectively, and its corresponding PAC dosage of was 0.99 g·L
-1. This result once again proves that the addition of quartz sand can’t improve the water quality of coagulation treatment. For cost saving considerations, the optimal dosages of PAC in subsequent experiments of QSEC was determined to be 0.99 g·L
-1.
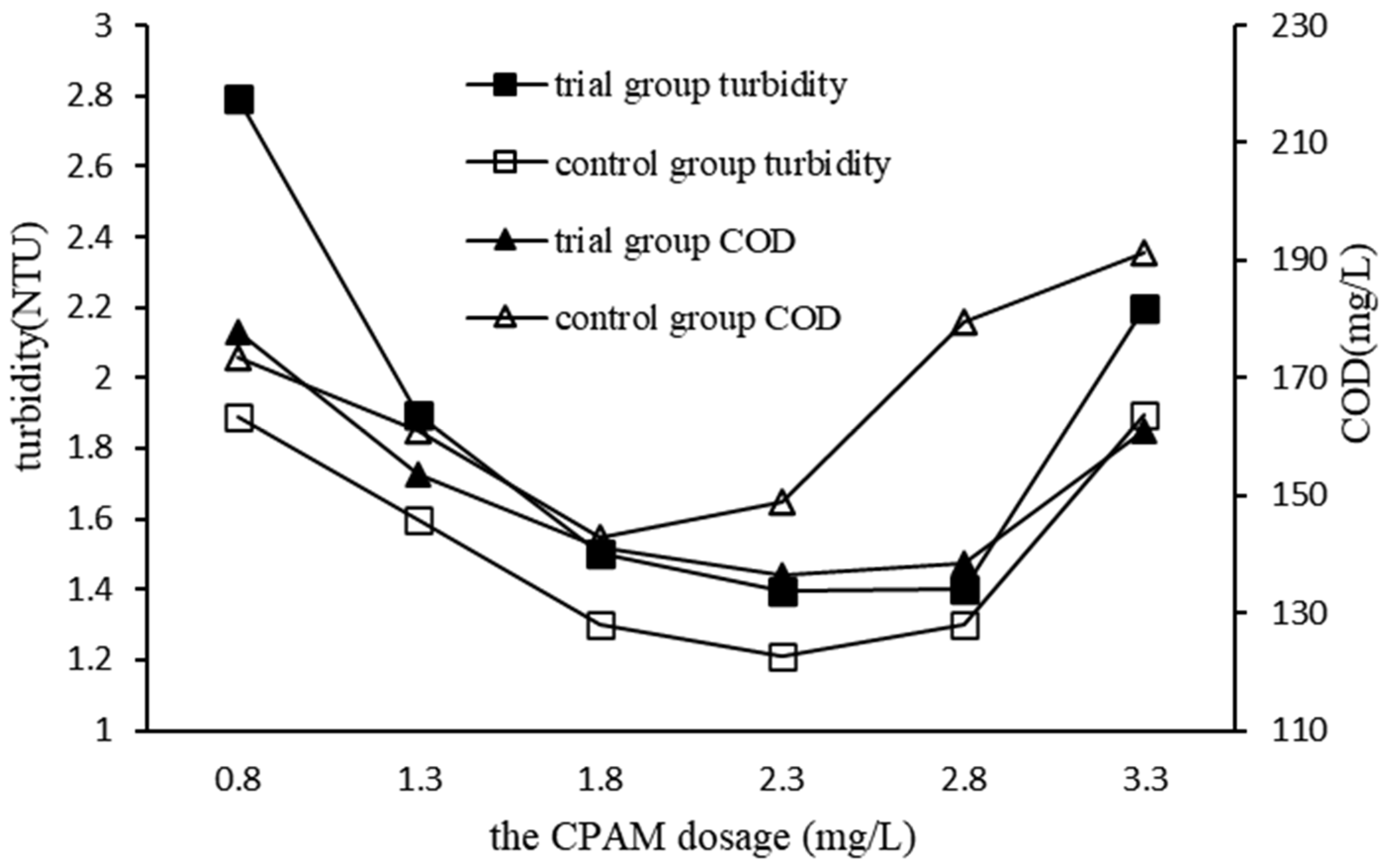
3.2.4. Impact of CPAM Dosage on QSEC
In order to study the impact of CPAM dosage on QSEC, the following experiments were performed, and the conditions of coagulation treatment were roughly as follows: the dosage of PAC and quartz sand were 0.99 and 2 g·L
-1 respectively, the stirring and settling time were 5 and 30 minutes respectively, the sewage pH was 7, and the CPAM were added according to the predetermined gradient amounts. and the dosages of CPAM were added according to the predetermined gradient amounts. The control experimental group didn’t add quartz sand, the dosage of PAC was 1.35 g·L
-1, and all other procedures were identical to those of the trial group. The turbidity and COD of the supernatant in each treatment experiment were detected and analyzed, and the results are shown in
Figure 5.
It could be seen from
Figure 5 that the turbidity and COD of trial group had a similar trend, that was, with the increase of the dosage of CPAM, the turbidity and COD of the sewage both first decreased to the minimum value, and then gradually increased. This phenomenon proved that excessive CPAM dosage was not conducive to coagulation. Comparing the coagulation efficiencies of trial group with those of control group, it was found that the coagulation efficiency of control group were obviously better than those of trial group, and when the CPAM dosage was 2.3 mg·L
-1, the turbidity and COD of trial groups reduced to the lowest values of 1.4 NTU and 142.9 mg·L
-1 respectively, while those of trial groups was 1.2 NTU and 142.9 mg·L
-1 respectively, and their corresponding CPAM dosages were 2.3 and 1.8 mg·L
-1 respectively. For cost saving considerations, the optimal dosage of CPAM in subsequent experiments of QSEC was determined to be 2.3 mg·L
-1.
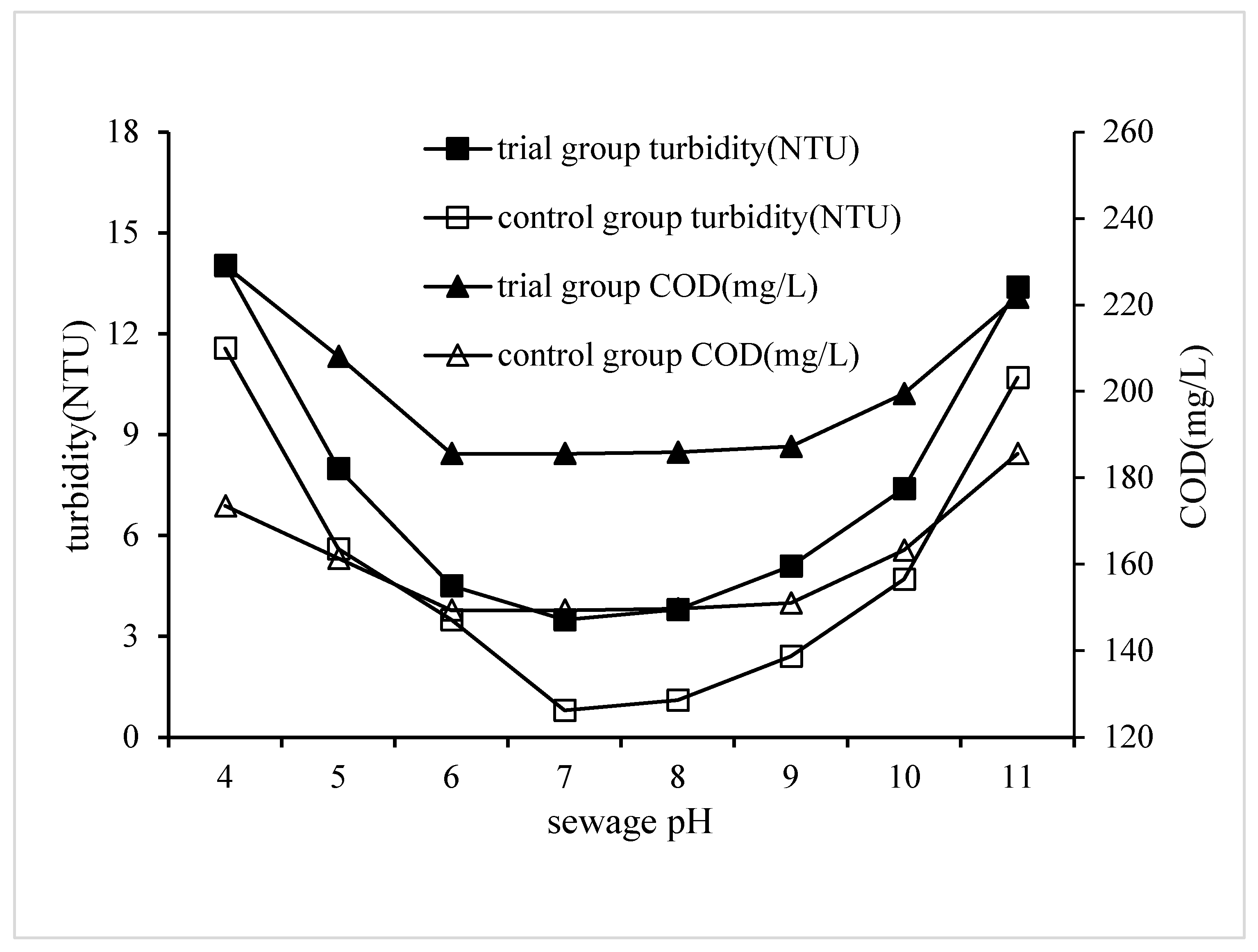
3.2.5. Impact of Sewage pH on QSEC
In order to study the impact of sewage pH on QSEC, the following experiments were performed, and the conditions of coagulation treatment were roughly as follows: the dosage of PAC, CPAM and quartz sand were 0.99 g·L
-1, 2.3 mg·L
-1 and 2 g·L
-1 respectively, the stirring and settling time were 5 and 30 minutes respectively, and the pHs of wastewater samples were adjusted according to the predetermined gradient value. The control groups didn’t add quartz sand, the dosage of PAC and CPAM were 1.35 g·L
-1 and 1.8 mg·L
-1 respectively, and all other procedures were identical to those of the trial group. The turbidity and COD of the supernatant in each treatment experiment were detected and analyzed, and the results are shown in
Figure 6.
It could be seen from the
Figure 6 that the COD changes in both the trial and control groups were as follows: the COD first decreases when the pH is less than 6, then remains stable between pH of 6 and 9, and then begins to rise when the pH exceeds 9. The minimum COD in all trial groups was 183.6 mg·L
-1, and the corresponding sewage pH was 6. The minimum COD of the trial and control groups was 185.9 and 149.7 mg·L
-1 respectively, and the corresponding sewage pHs were both 8. The test result indicated that the sewage pH had great influence on the coagulation efficiency, and the suitable pH range for coagulation was 6 to 9. The coagulation effect was very poor when the sewage pH was less than 5. The main reasons for this phenomenon were as follows: First, the negative charges carried by colloidal particles were neutralized by a large amount of hydrogen ions in sewage, colloidal particles became electrically neutral or even positively charged, and were difficult to coagulate and precipitate [
24]. In addition, CPAM can’t work with positive charge groups to play its advantages of charge neutralization, but further aggravate the electrostatic repulsion between colloidal particles. The decrease of sewage COD was mainly due to the adsorption bridging effect of CPAM [
25,
26].
The test result also proved that the coagulant performance deteriorated when the sewage pH exceeded 8. The main reason was that a large number of hydroxyl ions in wastewater neutralized the positive charge of CPAM and weakened its charge neutralization effect [
27,
28,
29].
The change in turbidity caused by changes in sewage pH is very similar to that of COD, indicating that the mechanism of pH's impact on turbidity is the same as that on COD. Under the same treatment conditions, the turbidity of the experimental group were higher than those of the control group. For example, the minimum turbidity values of the experimental group and the control group were 0.8 and 3.5 NTU, respectively.
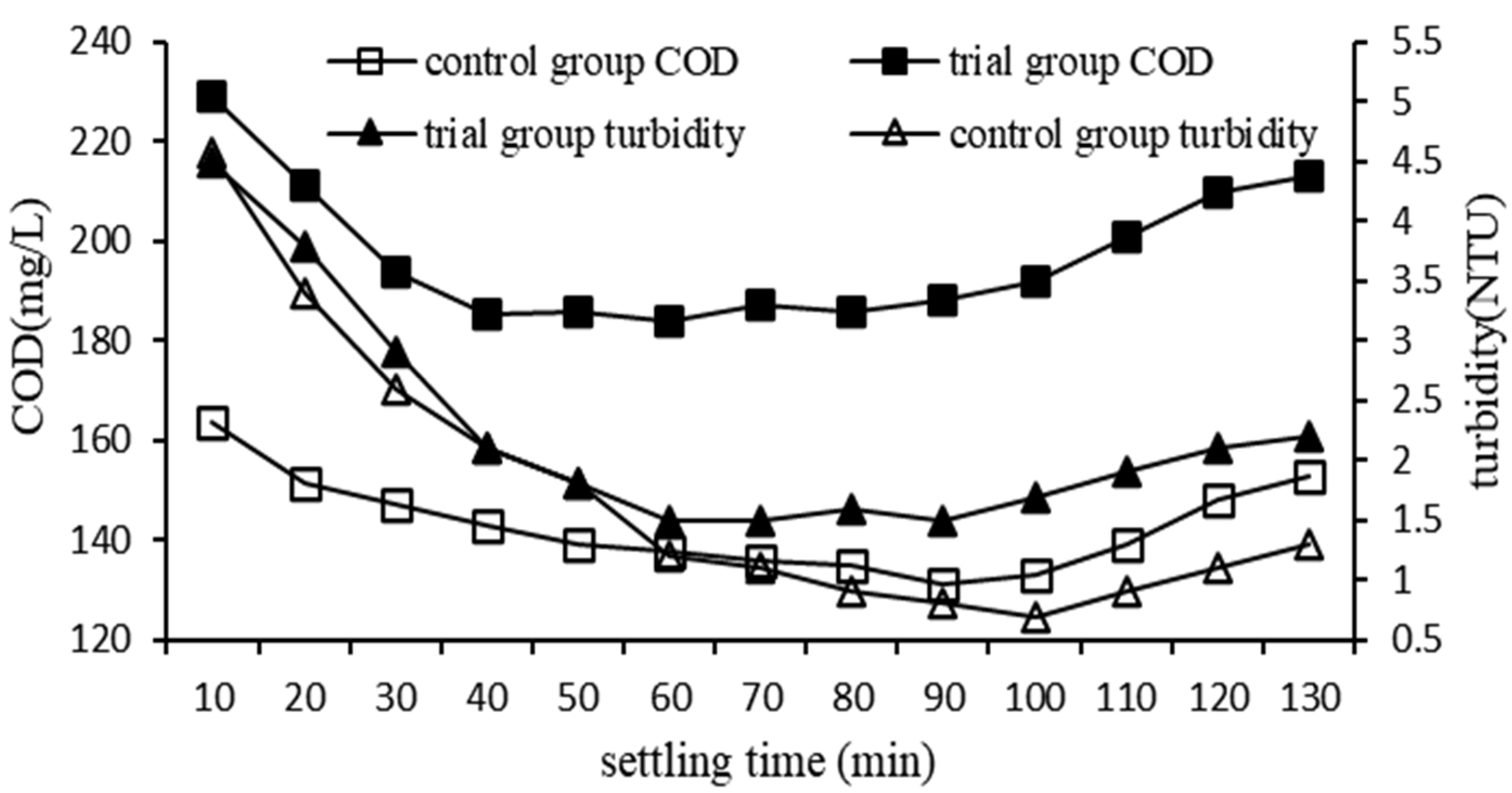
3.2.6. Impact of Settling Time on QSEC
In order to study the impact of sewage pH on QSEC, the following experiments were performed, and the conditions of coagulation treatment were roughly as follows: the dosage of PAC, CPAM and quartz sand were 0.99 g·L
-1, 2.3 mg·L
-1 and 2 g·L
-1 respectively, the stirring time were 5 minutes, the sewage pH were 7, and the settling times were set according to the predetermined gradient times. The control groups didn’t add quartz sand, the dosage of PAC and CPAM were 1.35 g·L
-1 and 1.8 mg·L
-1 respectively, and all other procedures were identical to those of the trial group. The turbidity and COD of the supernatant in each treatment experiment were detected and analyzed, and the results are shown in
Figure 7.
As can be seen from
Figure 7, the COD and turbidity of the trial and control group showed a trend of rapid decline at first, then stable, and slowly rising at last, indicating that too long sedimentation time was not conducive to coagulation, The reason was that the flocs contain a large amount of organic matter, which fermented and released gas, causing the flocs to float upwards. Throughout the entire precipitation process, the trial group consistently displayed higher COD and turbidity values compared to the control group. After approximately 70 minutes of settling, the trial group reached its minimum values for COD (184mg·L
-1) and turbidity (1.5 NTU), whereas it took 90 minutes for the control group to reach its respective minimum values (133mg·L
-1 for COD and 0.7 NTU for turbidity). This shows that the addition of quartz sand can’t improve the water quality of coagulation treatment, but it can accelerate the coagulation settlement process.
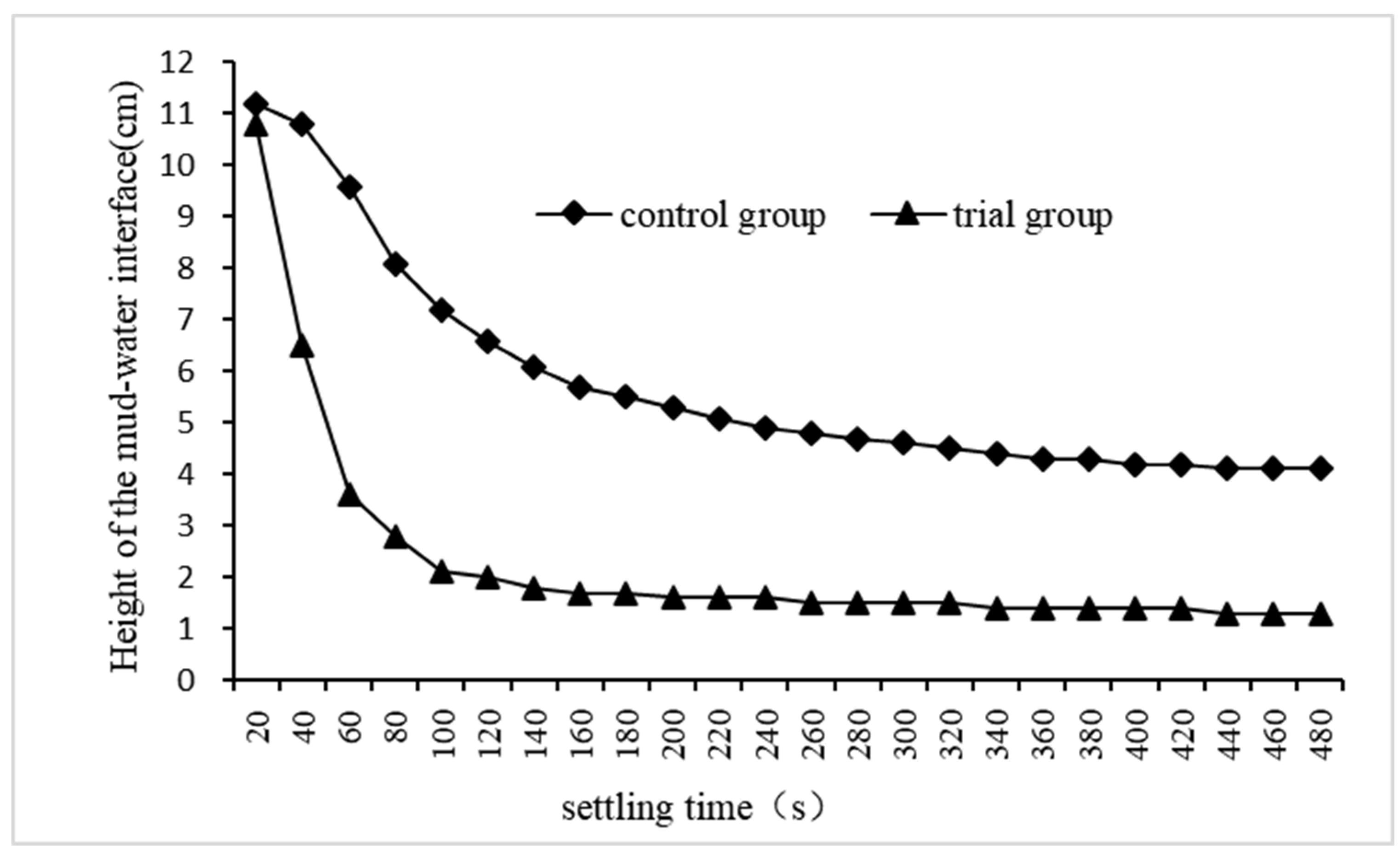
Select the experiment with the best treatment effect in the experimental group and that in the control group, record their dynamic changes in the height of the mud water interface, and the results were shown in
Figure 8, The height of the mud water interface first rapidly decreased, then slowed down until it stopped. The settling rate of floc in the trial group was significantly higher than that in the control group, and the height of the mud-water interface in the former remained stable after 140 seconds of precipitation, while that in the latter remained stable until 480 seconds. Throughout the sedimentation process, the mud-water interface height in the trial group consistently remained lower than that in the control group,and at 480 seconds of sedimentation, floc sedimentation volume accounted for 12.2% of total sewage volume in the test group, whereas it was 37.4% in the control group. These experimental findings demonstrate that adding quartz sand enhances floc settlement and reduces its volume. This is attributed to QSEC-formed flocs centered around quartz sand particles with a higher specific gravity, which not only accelerates their settlement but also compresses their volume.
4. RSM QSEC Test
4.1. RSM QSEC Test Design
Single factor test generally has the following two shortcomings: one is that the test ignores the influence of the interaction between variable factors on the test results; the other is that the span of the variable value is too large to accurately capture the optimal value of the variable [
30,
31]. To obtain the best variable level and test result, further optimization of test design, such as orthogonal design or RSM, should be carried out according to the single factor test results [
32]. RSM is an excellent method for optimizing and verifying scientific research and industrial studies. It uses the multivariate quadratic regression equation to fit the relationship between the index and influencing factors through regression equation analysis. This method aims to find the best process parameters and has the ability to provide maximum information with minimum experiments. Compared with orthogonal design, it is more intuitive and easier to reflect the optimal value of dependent variables [
33,
34] The Box–Behnken design (BBD), a kind of RSM design, is the most frequently used design in pioneering studies because it is more scientific compared with other designs in RSM.
The results of single-factor tests showed that the Quartz sand dosage, PAC dosage, CPAM dosage, sewage pH and settling time all had significant impacts on the coagulation, and the influences of settling time and quartz sand dosage on the coagulation effect were clear, and their optimums were obtained by the single factor test. However, the factors including CPAM dosage, sewage pH and PAC dosage had both positive and negative correlations with the coagulation efficiency of CPAM; thus, there were inflection points, and the variable values corresponding to inflection points were not necessarily the optimum but were close. Hence, on the basis of the single-factor test results, a BBD coagulation test was designed and conducted. The quartz sand with a particle size ranging from 200 to 300 mesh was selected as the heavy medium, and its dosage was 2 g·L
-1, the stirring and settling time were 5 and 30 minutes respectively. The sewage identical to that of the single-factor test was the treated object by coagulation, and reducing the sewage turbidity was the optimization goal, and the CPAM dosage, the sewage pH and PAC dosage were influencing factors. The BBD test was designed with Design-Expert 13 software, and each influence factor was set at three experimental levels, namely, high (+1), low (-1), and central point (basic level 0). The experimental levels and corresponding values of independent variables were determined and are listed in
Table 2. The settling times of all coagulation tests were the same, i.e., 30 minutes.
Table 1.
Experimental levels of independent test variables.
Table 1.
Experimental levels of independent test variables.
| Variable code |
Variables |
Variable levels and corresponding values |
| -1 |
0 |
1 |
| Z1
|
PAC dosage(g·L-1) |
0.63 |
0.99 |
1.35 |
| Z2
|
CPAM dosage(mg·L-1) |
1.8 |
2.3 |
2.8 |
| Z3
|
sewage pH |
6 |
7 |
8 |
4.2. Results and Discussion of RSM Test
4.2.1. Discussion of RSM Test Results
According to the scheme of the BBD test, a total of 17 groups of coagulation tests were carried out, including 12 factorial tests and 5 central tests for inspection errors. The variable values and the actual turbidity of each coagulation test are listed in
Table 3. The results showed that the turbidities of the 5 central tests were significantly lower than those of the others, which was consistent with the results of the single factor test and proved that the central point values of the influencing factors of the RSM tests were reasonable but not necessarily the optimal values, which could be obtained through further RSM analysis [
35].
Table 2.
The actual response values and predicted response values of BDD tests.
Table 2.
The actual response values and predicted response values of BDD tests.
| Run |
the CPAM dosage(mg·L-1) |
the sewage pH |
the stirring time (minutes) |
The response value of turbidity (NTU) |
| actual |
predicted |
| Eq. (2) |
Eq. (3) |
| 1 |
0.99 |
2.3 |
7.0 |
1.21 |
1.20 |
1.20 |
| 2 |
0.63 |
2.3 |
6.0 |
4.60 |
4.68 |
4.68 |
| 3 |
0.99 |
1.8 |
6.0 |
4.69 |
4.68 |
4.68 |
| 4 |
0.99 |
2.3 |
7.0 |
1.09 |
1.20 |
1.20 |
| 5 |
0.63 |
2.3 |
8.0 |
3.31 |
3.33 |
3.33 |
| 6 |
1.35 |
2.8 |
7.0 |
3.68 |
3.68 |
3.68 |
| 7 |
0.99 |
2.3 |
7.0 |
1.20 |
1.20 |
1.20 |
| 8 |
1.35 |
2.3 |
8.0 |
3.49 |
3.58 |
3.58 |
| 9 |
1.35 |
2.3 |
6.0 |
5.09 |
4.93 |
4.93 |
| 10 |
0.99 |
2.8 |
8.0 |
3.62 |
3.63 |
3.63 |
| 11 |
0.63 |
2.8 |
7.0 |
4.19 |
4.08 |
4.08 |
| 12 |
0.99 |
1.8 |
8.0 |
3.02 |
2.88 |
2.88 |
| 13 |
1.35 |
1.8 |
7.0 |
3.89 |
4.03 |
4.03 |
| 14 |
0.63 |
1.8 |
7.0 |
3.11 |
3.13 |
3.13 |
| 15 |
0.99 |
2.8 |
6.0 |
4.38 |
4.53 |
4.53 |
| 16 |
0.99 |
2.3 |
7.0 |
1.21 |
1.20 |
1.20 |
| 17 |
0.99 |
2.3 |
7.0 |
1.31 |
1.20 |
1.20 |
4.2.2. Model Fitting
The quadratic equation model (Y) in terms of linear, quadratic, and cross terms was constructed according to Eq. (1) as follows:
Eq. (1) reflects the relationship between variables and response.37,38 In this study, Y referred to the response to be modeled, i.e., sewage turbidity (NTU); Z1, Z2 and Z3 refer to the first-order terms of variables, i.e., the PAC dosage (g·L-1), the CPAM dosage (mg·L-1) and the sewage pH, respectively; Z12, Z22 and Z32 refer to their quadratic terms; and Z12, Z13 and Z23 refer to the corresponding terms of interaction effects between two variables, respectively. A0 was a constant term; A1, A2 and A3 refer to the primary linear coefficients of the PAC dosage (g·L-1), the CPAM dosage (mg·L-1) and the sewage pH, respectively; A11, A22 and A33 represent their secondary term coefficients, respectively; and A12, A13 and A23 represent the interaction term coefficients among variables, respectively.
According to the response results of the model, analysis of variance (ANOVA) was applied to analyze the feasibility of establishing the quadratic equation model between the variables and the responses [
36]. To check the statistical significance of the quadratic equation model and test variables, F tests and p values at the 95% confidence level were used. The modeling quality of the model was tested based on the coefficient of determination R
2 and adjusted R
2. Additionally, the interaction effects of the factors (Z
12, Z
13 and Z
23) on the response value were analyzed using three-dimensional plots and two-dimensional contour graphs [
37].
Using the data in
Table 3, regression simulation was conducted according to Eq. (1), the ternary quadratic polynomial regression model between response and variables was obtained, and the final equation in terms of actual factors is shown in Eq. (2) as follows:
The ANOVA for response surface quadratic model, i.e., Eq. (2), was conducted, the significance of the influence of each variable was tested, and the results are listed in
Table 4. Generally, “p values Prob > F” less than 0.0500 indicate that the model terms are significant [
38]. In this case, Z
1, Z
2, Z
3, Z
23, Z
23, Z
12, Z
22 and Z
32 were all significant model terms, and had significant impacts on sewage turbidity. The “p values Prob > F” of the model were less than 0.0500, which implied that the model was significant. The “Lack of Fit F value” of 5.67 implied that the lack of fit was not significant relative to the pure error and indicated that the equation was reliable [
39]. The “Pred R-Squared” of 0.9555 was in reasonable agreement with the “Adj R-Squared” of 0.9923, which indicated that Eq.(2) was well fitted and could be used to predict the turbidity of QSEC. The predicted turbidity with Eq.(2) values of all QSEC tests are listed in
Table 3.
Table 3.
ANOVAs for the response surface of Eq. (2) and Eq. (3).
Table 3.
ANOVAs for the response surface of Eq. (2) and Eq. (3).
| Source |
Sum of Squares |
Df |
Mean Squares |
F value |
p-value Prob > F |
Remark |
| Model |
Eq. (2) |
31.13 |
9 |
3.46 |
230.56 |
< 0.0001 |
significant |
| Eq. (3) |
31.1 |
8 |
3.89 |
243.95 |
< 0.0001 |
significant |
| Z1-the PAC dosage(g·L-1) |
Eq. (2) |
0.125 |
1 |
0.125 |
8.33 |
0.0234 |
|
| Eq. (3) |
0.125 |
1 |
0.125 |
7.84 |
0.0232 |
|
| Z2-the CPAM dosage(mg·L-1) |
Eq. (2) |
0.18 |
1 |
0.18 |
12 |
0.0105 |
|
| Eq. (3) |
0.18 |
1 |
0.18 |
11.29 |
0.0099 |
|
| Z3-the sewage pH |
Eq. (2) |
3.64 |
1 |
3.64 |
243 |
< 0.0001 |
|
| Eq. (3) |
3.64 |
1 |
3.64 |
228.71 |
< 0.0001 |
|
| Z12
|
Eq. (2) |
0.4225 |
1 |
0.4225 |
28.17 |
0.0011 |
|
| Eq. (3) |
0.4225 |
1 |
0.4225 |
26.51 |
0.0009 |
|
| Z13
|
Eq. (2) |
0.0225 |
1 |
0.0225 |
1.5 |
0.2603 |
|
| Eq. (3) |
----- |
----- |
----- |
----- |
----- |
|
| Z23
|
Eq. (2) |
0.2025 |
1 |
0.2025 |
13.5 |
0.0079 |
|
| Eq. (3) |
0.2025 |
1 |
0.2025 |
12.71 |
0.0074 |
|
| Z12
|
Eq. (2) |
7.82 |
1 |
7.82 |
521.1 |
< 0.0001 |
|
| Eq. (3) |
7.82 |
1 |
7.82 |
490.44 |
< 0.0001 |
|
| Z22
|
Eq. (2) |
5.69 |
1 |
5.69 |
379.34 |
< 0.0001 |
|
| Eq. (3) |
5.69 |
1 |
5.69 |
357.03 |
< 0.0001 |
|
| Z32
|
Eq. (2) |
10.28 |
1 |
10.28 |
685.31 |
< 0.0001 |
|
| Eq. (3) |
10.28 |
1 |
10.28 |
644.99 |
< 0.0001 |
|
| Residual |
Eq. (2) |
0.105 |
7 |
0.015 |
|
|
|
| Eq. (3) |
0.1275 |
8 |
0.0159 |
|
|
|
| Lack of Fit |
Eq. (2) |
0.085 |
3 |
0.0283 |
5.67 |
0.0635 |
not significant |
| Eq. (3) |
0.1075 |
4 |
0.0269 |
5.38 |
0.0661 |
not significant |
| Pure Error |
Eq. (2) |
0.02 |
4 |
0.005 |
|
|
|
| Eq. (3) |
0.02 |
4 |
0.005 |
|
|
|
| Cor Total |
Eq. (2) |
31.23 |
16 |
|
|
|
|
| Eq. (3) |
31.23 |
16 |
|
|
|
|
| R2Pre
|
Eq. (2) |
0.9555 |
|
|
|
|
|
| Eq. (3) |
0.9603 |
|
|
|
|
|
| R2adj
|
Eq. (2) |
0.9923 |
|
|
|
|
|
| Eq. (3) |
0.9918 |
|
|
|
|
|
The ANOVA result showed that Eq. (2) had a good fitting effect, but it also had a minor defect, that is, the “p-values Prob > F” of Z13 was greater than 0.0500, which implied the interaction between PAC dosage and sewage pH had insignificant impacts on the sewage turbidity. Therefore, the model, i.e. Eq. (2), could be further improved by removing the intercepts of insignificant terms from the coded model. After optimization, a better fitting model was obtained, and its final equation in terms of actual factors was shown in Eq. (3)
The ANOVA for Response Surface Quadratic Model, i.e. Eq. (3), was also conducted, and the significance of variable influence was tested. The result listed in
Table 4 showed that "Lack of Fit F-value" of model, i.e. Eq. (3), was approximately 5.38, which was less than that of Eq. (2), and implied the former was more reliable. Therefore, the Eq. (3) was chosen to predict the turbidity of QSEC, and the predicted turbidity values were listed in
Table 3.
4.2.3. Response Surface Analysis
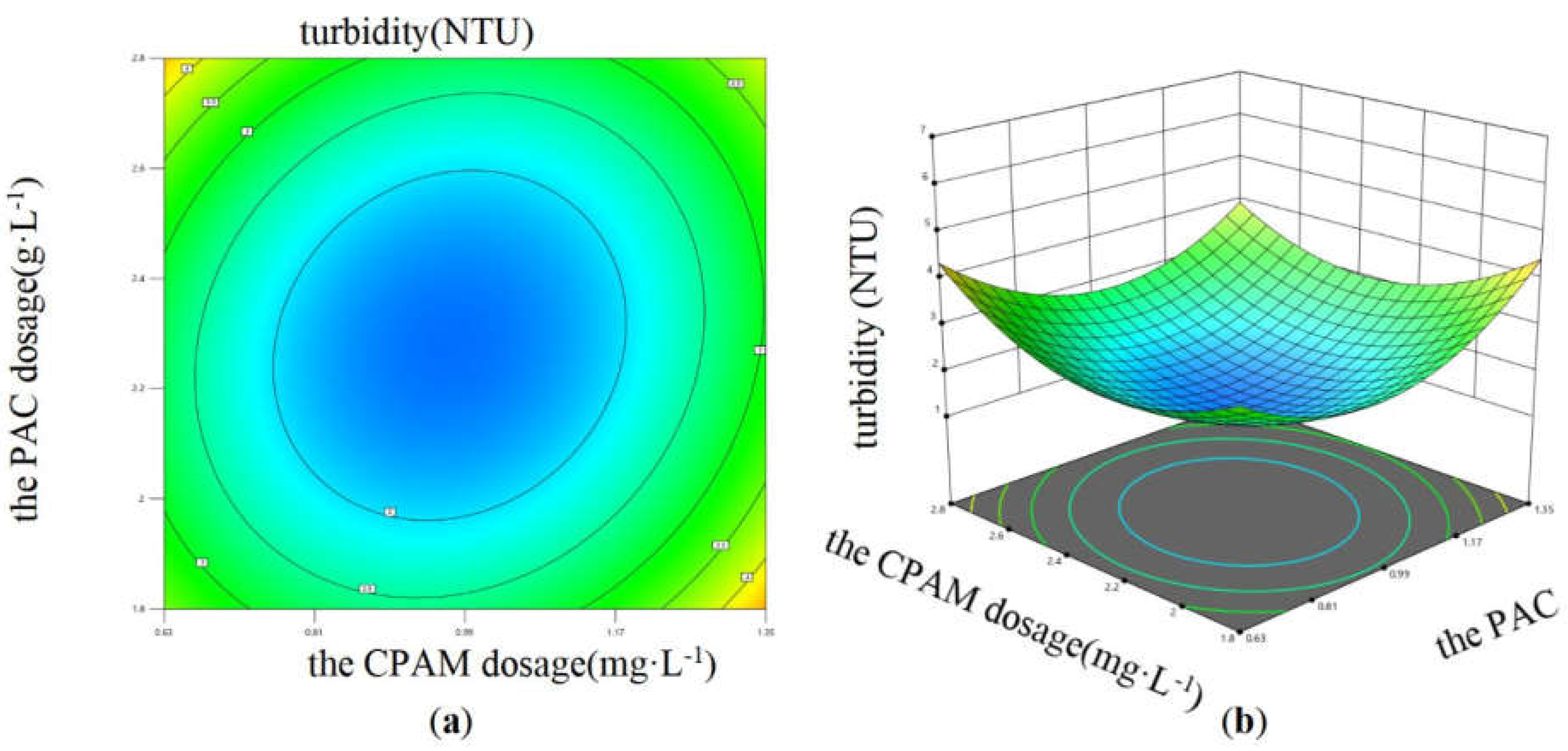
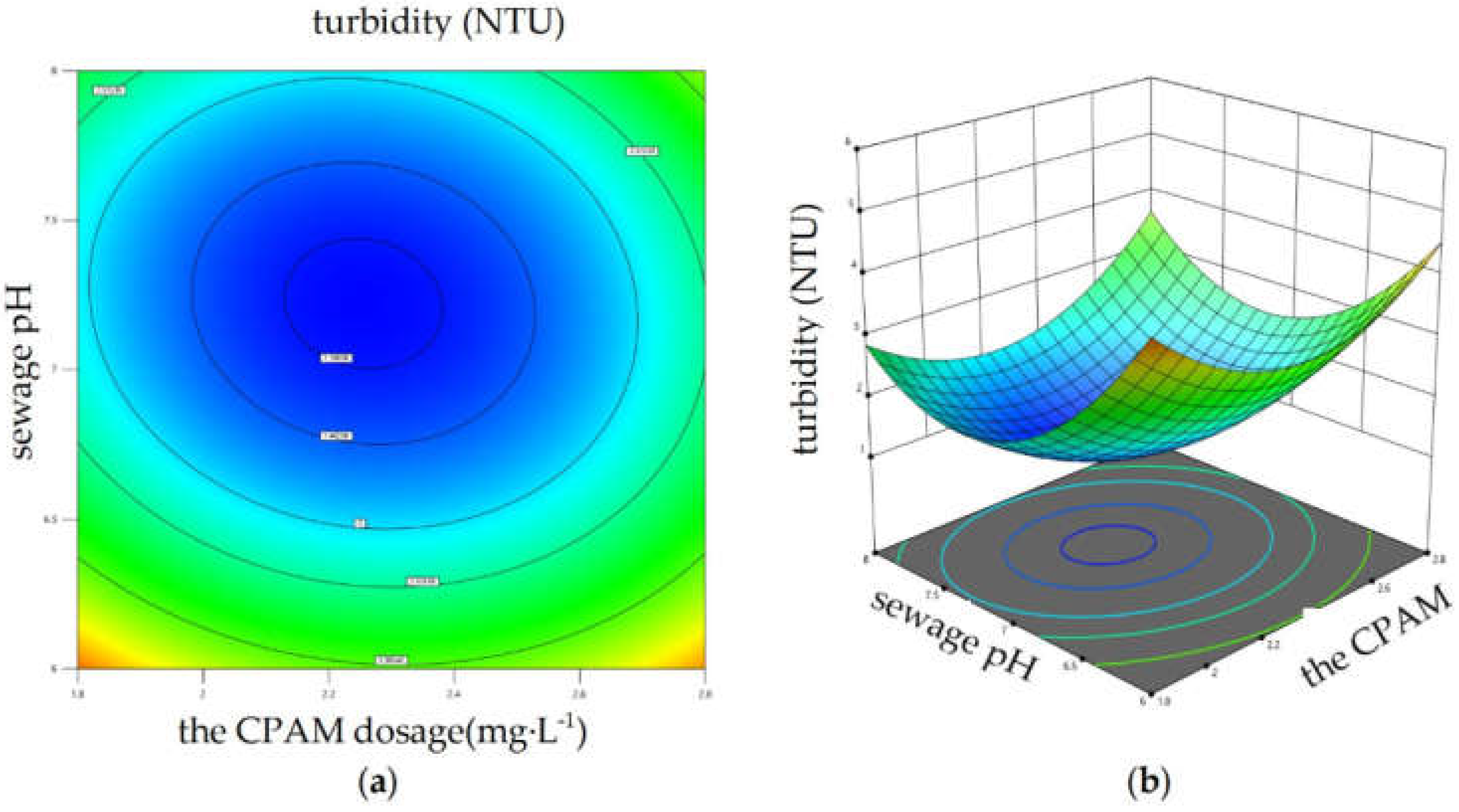
Eq. (3) was selected to perform ANOVA to determine the impacts of interactions between variables on the coagulation effect. The result showed that the interaction between PAC dosage and CPAM dosage, the interaction between sewage pH and CPAM dosage both had significant impact on the sewage turbidity. Design Expert 13 software was used to draw the response surface diagrams, which are shown in
Figure 9 and
Figure 10. The influence of each factor on the sewage turbidity could be judged by the steepness of the three-dimensional response surface. The steeper the slope of the response surface is, the more significant the influence of this factor on the test results [
40,
41]. The influence of the interaction between factors on the sewage turbidity could be judged by the shape of the two-dimensional contour graph. If the contour graph was oval, it indicated that the interaction effect of the corresponding factors had a significant influence on the sewage turbidity; however, when the contour tended to be circular, the influence was small [
42]. One of the factors was fixed, and the influences of the other two factors on the response value were investigated.
Figure 9 shows that with increasing PAC and CPAM dosage, the sewage turbidity first decreased and then increased under the condition of fixed sewage pH, and when the PAC and CPAM dosage were in the range of 0.81 to 1.17 g·L
-1 and 2 to 2.4 mg·L
-1, respectively, the sewage turbidity had a minimum value. Similarly, as shown in
Figure 10, when the PAC dosage was fixed, with increasing sewage pH and CPAM dosage, the sewage turbidity showed a trend of first increasing and then decreasing; when the sewage pH and CPAM dosage were in the range of 7 to 7.5 and 2 to 2.4 mg·L
-1, respectively, the sewage turbidity had a minimal value.
4.2.4. Coagulating Optimization and Model Validation
In Eq. (3), the first-order partial derivative was obtained and solved by being set to zero, and the optimal coagulating conditions were obtained as follows: the PAC dosage, the CPAM dosage and the sewage pH were 0.97 g·L
-1, 2.25 mg·L
-1, and 7.22 , respectively, and the predicted turbidity of the treated sewage was 1.11 NTU. To confirm the reliability of the prediction model, two runs of additional experiments were conducted under the coagulation conditions obtained from the model optimization. Other experimental conditions were that the quartz sand with a particle size ranging from 200 to 300 mesh was selected as the heavy medium, and its dosage was 2 g·L
-1, the stirring and settling time were 5 and 30 minutes respectively. The experimental results are listed in
Table 4 and show that the average of the measured turbidities was 1.12 NTU, which is very close to the predicted value of 1.11 NTU. The error between the measured turbidity and the predicted turbidity was only 3.6%, which indicated that the prediction model could be used to guide the quartz sand-enhanced coagulation.
Table 4.
Measured and predicted values of sewage turbidity.
Table 4.
Measured and predicted values of sewage turbidity.
| coagulation conditions |
sewage turbidity (NTU) |
| PAC dosage (g·L-1) |
CPAM dosage (mg·L-1) |
Sewage pH |
Quartz sand
dosage (g·L-1) |
Stirring time
(minutes) |
Settling time
(minutes) |
Average of measured value |
Predicted value |
| 0.97 |
2.25 |
7.22 |
2 |
5 |
30 |
1.15 |
1.11 |
5. Conclusions
To study the optimal coagulation conditions of QSEC, first, single-factor tests were conducted to preliminarily explore the optimal range of influencing factors, and then RSM tests were performed to accurately determine the optimums of influencing factors. The single-factor test results showed that the addition of quartz sand can’t improve the water quality of coagulation treatment, but it can accelerate the coagulation settlement process; the PAC dosage, CPAM dosage and sewage pH all had significant impacts on the effect of QSEC and existed optimums. A model that could guide QSEC was obtained by RSM tests. The results of model optimization showed that the optimal QSEC conditions for treating domestic sewage were as follows: the PAC dosage, the CPAM dosage, the sewage pH, quartz sand dosage, stirring time and settling time were 0.97 g·L-1, 2.25 mg·L-1, 7.22, 2 g·L-1, 5 minutes and 30 minutes, respectively, and the turbidity of treated sewage was reduced to 1.15 NTU.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, Zhengan Zhang and Yepu Li; Data curation, Yuying Li; Formal analysis, Zhengan Zhang; Funding acquisition, Zhengan Zhang and Zonghua Wang; Investigation, Yongzhi Liu, Dayang Wang, Lu Yan and B. Larry Li; Methodology, Zhengan Zhang and Yepu Li; Project administration, Jiayin Zhao; Resources, Yepu Li, Yongzhi Liu, Zonghua Wang, Dayang Wang, Lu Yan and B. Larry Li; Software, Zhengan Zhang, Yongzhi Liu and Zonghua Wang; Supervision, Jiayin Zhao; Validation, Zhengan Zhang, Yepu Li and Yuying Li; Visualization, Zhengan Zhang and Yuying Li; Writing – original draft, Zhengan Zhang; Writing – review & editing, Zhengan Zhang.
Funding
This research was funded by Henan Provincial Department of Science and Technology Research Project (grant numbers:242102320091, 242102321071, 242300420354, 242300420616), PhD Special Project of Nanyang Normal University (grant numbers:2024ZX020,2024ZX021), National Natural Science Foundation of China (52200052), Major Science and Technology Projects of Henan Province (grant number:221100320200), Key Research and Development Projects of Henan Province(grant number:221111520600), and Overseas expertise introduction center for discipline innovation of watershed ecological security in the water source area of the Middle Route of South-to-North Water Diversion Project (NO: D23015)
Institutional Review Board Statement
The study did not require ethical approval.
Informed Consent Statement
The study did not involve humans.
Data Availability
The data supporting this article have been included as part of the Supplementary Information.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, W.; Yue, Q.; Li, R.; Song, W.; Gao, B.; Shen, X. Investigating coagulation behavior of chitosan with different Al species dual-coagulants in dye wastewater treatment, J. Taiwan Inst Chem E. 2017, 78, 423–430. [Google Scholar] [CrossRef]
- Li, R.; Gao, B.; Guo, K.; Yue, Q.; Zheng, H.; Wang, Y. Effects of papermaking sludge-based polymer on coagulation behavior in the disperse and reactive dyes wastewater treatment, Bioresource Technol. 2017, 240, 59-67.
- Chen, N.; Liu, W.; Huang, J.; Qiu, X. Preparation of octopus-like lignin-grafted cationic polyacrylamide flocculant and its application for water flocculation. International journal of biological macromolecules. 2020, 146, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Chi, N.; Liu, J.; Lei, M.; Feng, L. Preparation of amphiphilic cationic polyacrylamide (CPAM) with cationic microblock structure to enhance printing and dyeing sludge dewatering and condition performance. Environmental Science and Pollution Research. 2022, 1–15. [Google Scholar] [CrossRef]
- Yang, K.; Chen, J.; Yao, C. Cationic polyacrylamide emulsion with ultra-high concentration as a flocculant for paper mill wastewater treatment. BioResources. 2020, 15, 3173–3189. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, X.; Dong, X.; Feng, Z.; Dong, Y. Characterisation of floc size, effective density and sedimentation under various flocculation mechanisms. Water Science and Technology. 2020, 82, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Lin, A.; Liu, X. Population balance modeling of cationic polyacrylamide (CPAM) induced flocculation process for lignin recovery from the pre-hydrolysis liquor of kraft pulping process. Separation and Purification Technology. 2019, 221, 152–158. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, Y.; Guo, J.; Li, F.; Fan, W.; Liao, Y.; et al. Characterization and Evaluation of Dewatering Properties of PADB, a Highly Efficient Cationic Flocculant. Industrial & Engineering Chemistry Research. 2014, 53, 2572–2582. [Google Scholar]
- Zhang, Z.; Zheng, H.; Huang, F.; Li, X.; He, S.; Zhao, C. Template Polymerization of a Novel Cationic Polyacrylamide: Sequence Distribution, Characterization, and Flocculation Performance. Industrial & Engineering Chemistry Research. 2016, 55, 9819–9828. [Google Scholar]
- Shang, H.Z.; Liu, J.P.; Zheng, Y.B.; Wang, L.G. Synthesis,characterization, and flocculation properties of poly(acrylamide-methacryloxyethyltrimethyl ammonium chloride-methacryloxypropyl-trimethoxy silane). J Appl Polym Sci 2009, 111, 1594–1599. [Google Scholar] [CrossRef]
- Zheng H, Sun Y, C Z, Guo J, Chun Z, Yi L, et al. UV-initiated polymerization of hydrophobically associating
cationic flocculants: Synthesis, characterization, and dewatering properties. Chem Eng J 2013, 234, 318−326.
- Daifa, M.; Shmoeli, E.; Domb, A.J. Enhanced flocculation activity of polyacrylamide-based flocculant for purification of industrial wastewater. Polymers for Advanced Technologies. 2019, 30, 2636–2646. [Google Scholar] [CrossRef]
- Fijałkowska, G.; Szewczuk-Karpisz, K.; Wiśniewska, M. Chromium (VI) and lead (II) accumulation at the montmorillonite/aqueous solution interface in the presence of polyacrylamide containing quaternary amine groups. Journal of Molecular Liquids. 2019, 293, 111514. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Chibowski, S.; Urban, T.; Terpiłowski, K. Investigations of chromium (III) oxide removal from the aqueous suspension using the mixed flocculant composed of anionic and cationic polyacrylamides. Journal of hazardous materials. 2019, 368, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Szewczuk-Karpisz, K.; Fijałkowska, G.; Wiśniewska, M.; Wójcik, G. Chromium (VI) reduction and accumulation on the kaolinite surface in the presence of cationic soil flocculant. Journal of Soils and Sediments. 2020, 20, 3688–3697. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, H.; Sun, Y.; Zhao, C.; Zhou, Y.; Tang, X.; et al. A combined process of chemical precipitation and flocculation for treating phosphating wastewater. Desalination and Water Treatment. 2016, 57, 25520–25531. [Google Scholar] [CrossRef]
- Harif, S.; Aboulhassan, M.A.; Bammou, L. Multi-response optimization for color removal from cardboard wastewater using polyaluminum chloride and cationic polyacrylamide. International Journal of Environmental Science and Technology. 2022, 1–12. [Google Scholar] [CrossRef]
- Khan, S.; Zheng, H.; Sun, Q.; Liu, Y.; Li, H.; Ding, W.; et al. Synthesis and characterization of a novel cationic polyacrylamide-based flocculants to remove Congo red efficiently in acid aqueous environment. Journal of Materials Science: Materials in Electronics. 2020, 31, 18832–18843. [Google Scholar] [CrossRef]
- Nourani, M.; Baghdadi, M.; Javan, M.; Bidhendi, G.N. Production of a biodegradable flocculant from cotton and evaluation of its performance in coagulation-flocculation of kaolin clay suspension: Optimization through response surface methodology (RSM). Journal of environmental chemical engineering. 2016, 4, 1996–2003. [Google Scholar] [CrossRef]
- Onukwuli, O.D.; Nnaji, P.C.; Menkiti, M.C.; Anadebe, V.C.; Oke, E.O.; Ude, C.N.; et al. Dual-purpose optimization of dye-polluted wastewater decontamination using bio-coagulants from multiple processing techniques via neural intelligence algorithm and response surface methodology. Journal of the Taiwan Institute of Chemical Engineers. 2021, 125, 372–386. [Google Scholar] [CrossRef]
- Rezania, N.; Hasani Zonoozi, M.; Saadatpour, M. Coagulation-flocculation of turbid water using graphene oxide: simulation through response surface methodology and process characterization. Environmental Science and Pollution Research. 2021, 28, 14812–14827. [Google Scholar] [CrossRef]
- Lapointe, M.; Farner, J.M.; Hernandez, L.M.; Tufenkji, N. Understanding and improving microplastic removal during water treatment: impact of coagulation and flocculation. Environmental science & technology. 2020, 54, 8719–8727. [Google Scholar]
- Liu, X.; Xu, Q.; Wang, D.; Wu, Y.; Yang, Q.; Liu, Y.; et al. Unveiling the mechanisms of how cationic polyacrylamide affects short-chain fatty acids accumulation during long-term anaerobic fermentation of waste activated sludge. Water research. 2019, 155, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cai, Q.; Feng, B.; Feng, S.; Tian, C.; Jiang, X.; et al. Improving the performance of shipboard rotary drum filters in the removal of cyanobacterial blooms by cationic polyacrylamide flocculation. Separation and Purification Technology. 2019, 215, 660–669. [Google Scholar] [CrossRef]
- Park JBK, Meerman, C. ; Craggs, R. Continuous low dosing of cationic polyacrylamide (PAM) to enhance algal harvest from a hectare-scale wastewater treatment high rate algal pond. New Zealand Journal of Botany. 2019, 57, 112–124. [Google Scholar] [CrossRef]
- Tajbakhsh, S.F.; Mohmmadipour, R.; Janani, H. One-pot production of a graft copolymer of cationic starch and cationic polyacrylamide applicable as flocculant for wastewater treatment. Journal of Macromolecular Science, Part, A. 2022, 59, 698–710. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, S.; Huang, F.; Li, X.; Shang, J.; Lai, J.; et al. Nitrogen and Phosphorus Removal by Activated Sludge Process: A Review. Mini-Reviews in Organic Chemistry. 2017, 14, 99–106. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Fijałkowska, G.; Szewczuk-Karpisz, K.; Urban, T.; Nosal-Wiercińska, A.; Wójcik, G. Comparison of adsorption affinity of anionic and cationic polyacrylamides for montmorillonite surface in the presence of chromium (VI) ions. Adsorption. 2019, 25, 41–50. [Google Scholar] [CrossRef]
- Zhou, S.; Bu, X.; Alheshibri, M.; Zhan, H.; Xie, G. Floc structure and dewatering performance of kaolin treated with cationic polyacrylamide degraded by hydrodynamic cavitation. Chemical Engineering Communications. 2022, 209, 798–807. [Google Scholar] [CrossRef]
- Agbovi, H.K.; Wilson, L.D. Flocculation optimization of orthophosphate with FeCl3 and alginate using the Box–Behnken response surface methodology. Industrial & Engineering Chemistry Research. 2017, 56, 3145–3155. [Google Scholar]
- Ahmad, T.; Ahmad, K.; Alam, M. Simultaneous modelling of coagulant recovery and reuse by response surface methodology. Journal of Environmental Management. 2021, 285, 112139. [Google Scholar] [CrossRef]
- Birjandi, N.; Younesi, H.; Bahramifar, N.; Ghafari, S.; Zinatizadeh, A.A.; Sethupathi, S. Optimization of coagulation-flocculation treatment on paper-recycling wastewater: application of response surface methodology. Journal of Environmental Science and Health, Part, A. 2013, 48, 1573–1582. [Google Scholar] [CrossRef]
- Wang, K.; Mao, Y.; Wang, C.; Ke, Q.; Zhao, M.; Wang, Q. Application of a combined response surface methodology (RSM)-artificial neural network (ANN) for multiple target optimization and prediction in a magnetic coagulation process for secondary effluent from municipal wastewater treatment plants. Environmental Science and Pollution Research. 2022, 29, 36075–36087. [Google Scholar] [CrossRef] [PubMed]
- Dbik, A.; El Messaoudi, N.; Bentahar, S.; El Khomri, M.; Lacherai, A.; Faska, N. Optimization of Methylene Blue Adsorption on Agricultural Solid Waste Using Box–Behnken Design (BBD) Combined with Response Surface Methodology (RSM) Modeling. Biointerface Research in Applied Chemistry. 2022, 12, 4567–4583. [Google Scholar]
- Ezemagu, I.G.; Ejimofor, M.I.; Menkiti, M.C.; Nwobi-Okoye, C.C. Modeling and optimization of turbidity removal from produced water using response surface methodology and artificial neural network. South African Journal of Chemical Engineering. 2021, 35, 78–88. [Google Scholar] [CrossRef]
- Gökçek, Ö.B.; Özdemir, S. Optimization of the coagulation–flocculation process for slaughterhouse wastewater using response surface methodology. CLEAN–Soil, Air, Water. 2020, 48, 2000033. [Google Scholar] [CrossRef]
- Heidari, M.; Vosoughi, M.; Sadeghi, H.; Dargahi, A.; Mokhtari, S.A. Degradation of diazinon from aqueous solutions by electro-Fenton process: effect of operating parameters, intermediate identification, degradation pathway, and optimization using response surface methodology (RSM). Separation Science and Technology. 2021, 56, 2287–2299. [Google Scholar] [CrossRef]
- Kim S-C. Application of response surface method as an experimental design to optimize coagulation–flocculation process for pre-treating paper wastewater. Journal of Industrial and Engineering Chemistry. 2016, 38, 93–102. [Google Scholar] [CrossRef]
- Luo, S.; Wu, X.; Jiang, H.; Yu, M.; Liu, Y.; Min, A.; et al. Edible fungi-assisted harvesting system for efficient microalgae bio-flocculation. Bioresource technology. 2019, 282, 325–330. [Google Scholar] [CrossRef]
- Ma, C.; Yu, H.; Gao, Y.; Xu, W.; Xu, T.; Wang, L.; et al. Operation parameters optimization of a hybrid dead-end/cross-flow forward osmosis system for microalgae dewatering by response surface methodology. Process Safety and Environmental Protection. 2020, 143, 14–24. [Google Scholar] [CrossRef]
- Singh, H.M.; Tyagi, V.V.; Ahmad, S.; Kothari, R. Optimization of flocculation efficiency of Chlorella pyrenoidosa with CaCl2 using the Box-Behnken design of response surface methodology: A cost effective statistical investigation. Biomass Conversion and Biorefinery. 2022, 1–13. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, L.; Lin, X.; Xu, Z.; Luo, W.; Luo, L. Response surface methodology to optimize self-flocculation harvesting of microalgae Desmodesmus sp. CHX1. Environmental Technology. 2021, 1–9. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).