Submitted:
12 June 2024
Posted:
12 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. X-ray Analysis
3.2. Scanning Electron Microscope
3.3. Electrophysical Research
- a)
- Calculation of the width of the forbidden zone (∆E) in the range 293-313 K:
Т, К lg R 293 5,22 313 5,01 - b)
- Calculation of the width of the forbidden zone (∆E) in the range 333-393 K:
Т, К lg R 333 5,31 393 3,91 - c)
- Calculation of the width of the forbidden zone (∆E) in the range 453-483 K:
Т, К lg R 453 5,94 483 5,71
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hossain, M.D., Jamil, A. T. M. K., Hossain, M. S., Ahmed, S. J., Das, H. N., Rashid, R., Khan, M. N. I. Investigation on structure, thermodynamic and multifunctional properties of Ni–Zn–Co ferrite for Gd3+ substitution. RSC Adv., 2022. 12, 4656. [CrossRef]
- Gonçalves, J. M., Silva, M. N., Naik, K. K., Martins, P. R., Rocha, D. P., Nossol, E., Rout, C. S. Multifunctional spinel MnCo2O4 based materials for energy storage and conversion: a review on emerging trends, recent developments and future perspectives. J. Mater. Chem.,2021, 9, 3095. [CrossRef]
- Yao, C., Ismail, M., Hao, A., Thatikonda, S. K., Huang, W., Qin, N., Bao, D. Annealing atmosphere effect on the resistive switching and magnetic properties of spinel Co3O4 thin films prepared by a sol–gel technique. RSC Adv., 2019, 9, 12615. [CrossRef]
- Chandel, M., Moitra, D., Makkar, P., Sinha, H., Hora, H. S., Ghosh, N. N. Synthesis of multifunctional CuFe2O4–reduced graphene oxide nanocomposite: an efficient magnetically separable catalyst as well as high performance supercapacitor and first-principles calculations of its electronic structures. RSC Adv., 2018, 8, 27725. [CrossRef]
- Li, Z., Wang, Z. L., Wang, Z. In situ tuning of crystallization pathways by electron beam irradiation and heating in amorphous bismuth ferrite films. RSC Adv., 2018, 8, 23522. [CrossRef]
- Nilar, L., Fauzi, M.A.N., Sreekantan, S., Othman, R. Physical and electromagnetic properties of nanosized Gd substituted Mg–Mn ferrites by solution combustion method. PhysicaB. 2015 461, 134, . [CrossRef]
- XiuBo, X., Wang, B., Wang, Y., Ni, C., Sun, X., Du, W. Spinel structured MFe2O4 (M = Fe, Co, Ni, Mn, Zn) and their composites for microwave absorption: A review. Chem. Engg. Journ. 2022, 428 , 131160, . [CrossRef]
- Ahmad, I., Farid, M.T. Characterization of cobalt based spinel ferrites with small substitution of gadolinium. World Appl. Sci. J. 2012. 19, 464. [CrossRef]
- Fu, Y.P., Lin, C.H., Liu, C.W. Preparation and magnetic properties of Ni0.25Cu0.25Zn0.5 ferrite from microwave-induced combustion. J. Magn. Magn. Mater. 2004. 283, 59. [CrossRef]
- Ravinder, D., Balachander, L., Venudhar, Y. C. Electrical conductivity in manganese-substituted lithium ferrites. Mater. Lett. 2001. 49, 267. [CrossRef]
- Trivedi, U. N., Jani, K. H., Modi, K. B., Joshi, H. H. Study of cation distribution in lithium doped nickel ferrite. J. Mater. Sci. Lett. 2000. 19, 1271.
- Abo El Ata, A. M., Attia, S. M., El Kony, D., Al-Hammadi, A. H. Spectral, initial magnetic permeability and transport studies of Li0.5−0.5xCoxFe2.5−0.5xO4 spinel ferrite. J. Magn. Magn. Mater. 2005. 295, 28. [CrossRef]
- Ravinder, D., Reddy, P. V. B. Thermoelectric power studies of polycrystalline magnesium substituted lithium ferrites. J. Magn. Magn. Mater. 2003. 263, 127. [CrossRef]
- Laishram, R., Prakash, C. Magnetic properties of Cr3+ substituted Li–Sb ferrites. J. Magn. Magn. Mater. 2006. 305, 35. [CrossRef]
- Sattar, A.A., El-Sayed, H.M., Agami, W.R., Ghani, A.A. Magnetic properties and electrical resistivity of Zr4+ substituted Li-Zn ferrite. Am. J. Appl. Sci. 2007. 4 (2) 89. 16.
- Surzhikov, P., Pritulov, A. M., Ivanov, Yu F., Shabardin, R. S., Usmanov, R. U. Electron-Microscopic Study of Morphology and Phase Composition of Lithium-Titanium Ferrites. Russ. Phys. J. 2001. 44 (4) 420.
- Mataev, M.М., Madiyarova, A.M., Patrin , G.S., Abduraimova, М.R., Nurbekova, M.A., Tursunova, Zh.I. Synthesis and physico – chemical characteristics of complex ferrite CrNaFe2O5. Chem. J. Kaz., 2024, 1(85), 109-118. [CrossRef]
- Mataev, M.M., Mustafin, E. S., Kasenov, R. Z., Pudov, A. M., Kaikenov, D. A., Bogzhanova, Zh. K. X ray Diffraction Study of the YbMIIFe5O12 (MII = Mg, Ca, Sr) Ferrites. Inorganic Materials, 2014, Vol. 50, No. 6, pp. 622–624. [CrossRef]
- Mataev, M. M, Abdraimova, M.R., Saxena, S.X., Nuketaeva, D.Zh., Zheksembieva, B.T. Syntesis and X-Ray analysis of complex ferrites. Key Engineering Materials, -2017,-Vol.744? pp 393-398.
- Okazaki, K. Technology of ceramic dielectrics. − M.: Energiya, 1976. – 256 p.
- Kasenov, B.K.; Kasenova, S.B.; Sagintaeva, Z.I.; Baisanov, S.; Lu, N.Y.; Nukhuly, A.; Kuanyshbekov, E.E. Heat Capacity and Thermodynamic Functions of Titanium-Manganites of Lanthanum, Lithium and Sodium of LaLi2TiMnO6 and LaNa2TiMnO6. Molecules 2023, 28, 5194. [CrossRef]
- Fesenko, E.G. The perovskite family and ferroelectricity. M.: Atomizdat, 1972.
- Venevtsev, Yu.N., Politova, E.D., Ivanov, S.A. Ferroelectric and antisegnetoelectrics of the barium titanate family. Moscow: Chemistry, 1985.
- Lines, M., Glass, A. Ferroelectrics and related materials. Moscow: Mir, 1981.
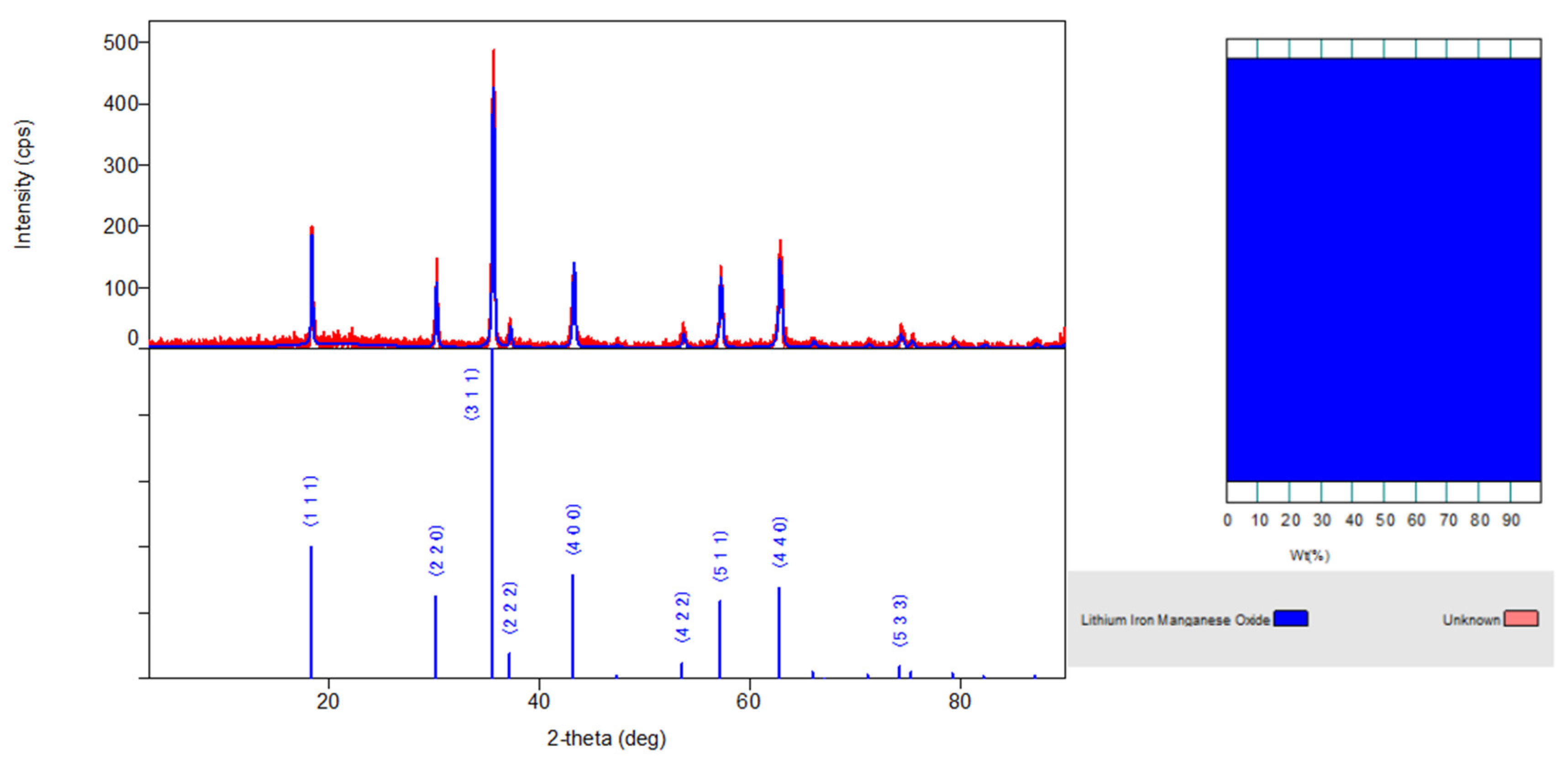
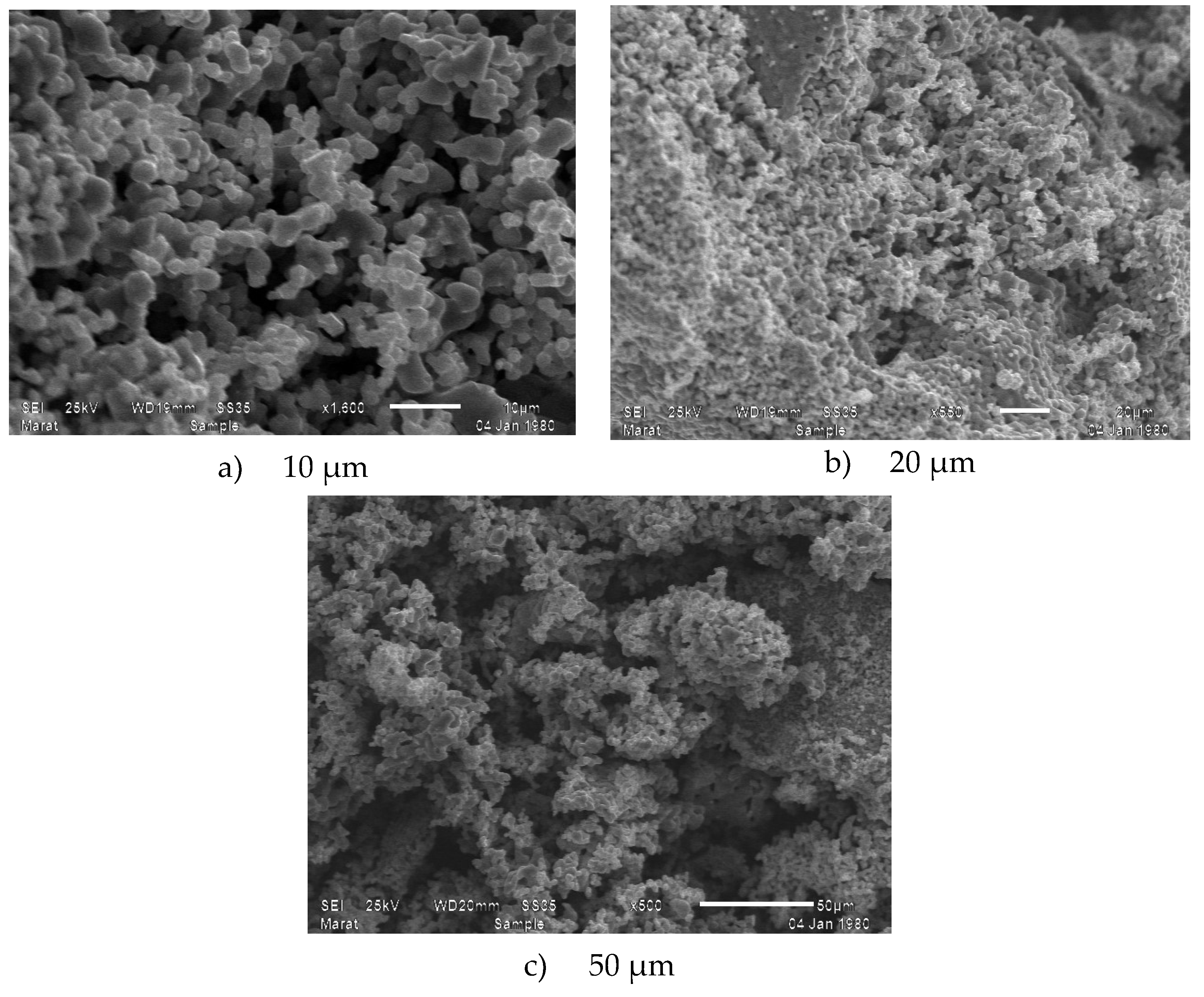

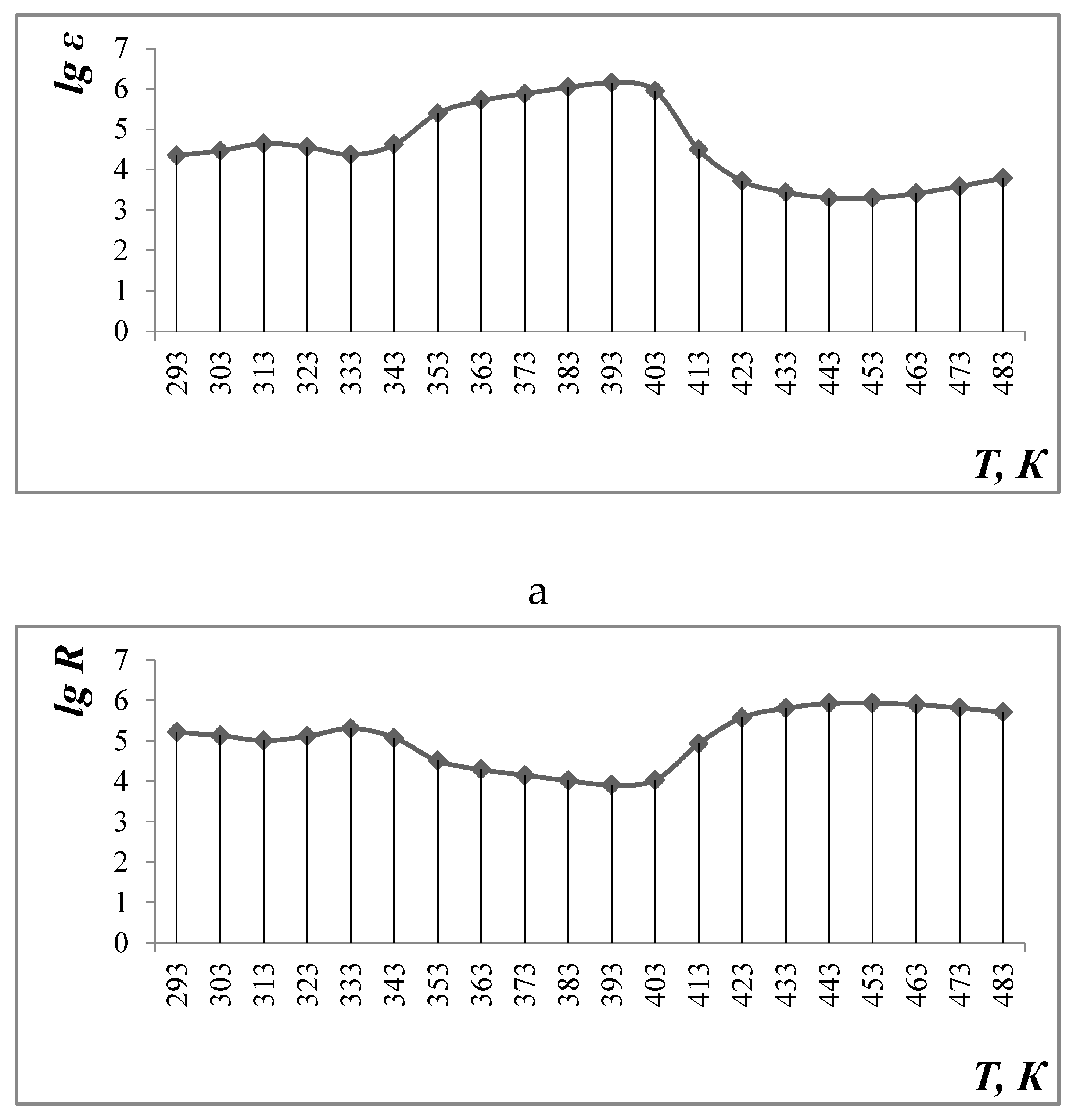
| Sample | Li0,5MnFe1.5O4 |
|---|---|
| Space group | , cubic side centered |
| Z Parameter cell (Å) a = b = c = V(A^3) α β γ According to Scherrer’s formula, the average size of crystallites is X-ray density (g/cm3) Pycnometric density (g/cm3)* |
8 8.3677(9) 8.3677(9) 8.3677(9) 585.90(11) 90 90 90 40.6 μm 4.678 4.675 |
| Т, К | C, nF | R, Oм | ε | lgε | lgR |
|---|---|---|---|---|---|
| Measurement frequency at 1 kHz | |||||
| 293 303 313 323 333 343 353 363 373 383 393 403 413 423 433 443 453 463 473 483 |
0,27278 0,27426 0,27715 0,28125 0,28772 0,29313 0,29916 0,30751 0,31202 0,31702 0,32255 0,32967 0,3423 0,35119 0,36668 0,38018 0,39802 0,4169 0,43147 0,45456 |
13400 13270 12910 12560 11890 11210 10290 9383 8831 9061 8814 7881 7098 6902 6153 6317 6010 5584 5149 4656 |
1296 1303 1316 1336 1367 1392 1421 1461 1482 1506 1532 1566 1626 1668 1742 1806 1891 1980 2050 2159 |
3,11 3,11 3,12 3,13 3,14 3,14 3,15 3,16 3,17 3,18 3,19 3,19 3,21 3,22 3,24 3,26 3,28 3,30 3,31 3,33 |
4,13 4,12 4,11 4,10 4,08 4,05 4,01 3,97 3,95 3,96 3,95 3,90 3,85 3,84 3,79 3,80 3,78 3,75 3,71 3,67 |
| Measurement frequency at 5 kHz | |||||
| 293 303 313 323 333 343 353 363 373 383 393 403 413 423 433 443 453 463 473 483 |
0,25678 0,2683 0,2775 0,28638 0,29667 0,30226 0,30787 0,31283 0,31843 0,32148 0,32578 0,32976 0,33303 0,33948 0,35613 0,3713 0,3925 0,41682 0,44245 |
29630 21650 13080 5236 4301 4733 3296 2966 2805 2529 2669 3172 4434 6377 9644 11520 10430 8021 5978 3799 |
1220 1274 1318 1360 1389 1409 1436 1462 1486 1513 1527 1547 1566 1582 1613 1692 1764 1864 1980 2102 |
3,09 3,11 3,12 3,13 3,14 3,15 3,16 3,17 3,17 3,18 3,18 3,19 3,19 3,20 3,21 3,23 3,25 3,27 3,30 3,32 |
4,47 4,34 4,12 3,72 3,63 3,68 3,52 3,47 3,45 3,40 3,43 3,50 3,65 3,80 3,98 4,06 4,02 3,90 3,78 3,58 |
| Measurement frequency at 10 kHz | |||||
| 293 303 313 323 333 343 353 363 373 383 393 403 413 423 433 443 453 463 473 483 |
0,11814 0,18494 0,22927 0,25954 0,27501 0,28531 0,29302 0,29988 0,30652 0,31215 0,31667 0,32294 0,32779 0,33406 0,34256 0,35658 0,378 0,39475 0,41687 0,44203 |
152300 70790 32200 11870 4842 3312 2689 2257 1946 1689 1737 3130 5945 8231 8805 8052 5967 4604 3343 2353 |
561 878 1089 1233 1306 1355 1392 1424 1456 1483 1504 1534 1557 1587 1627 1694 1796 1875 1980 2100 |
2,75 2,94 3,04 3,09 3,12 3,13 3,14 3,15 3,16 3,17 3,18 3,19 3,19 3,20 3,21 3,23 3,25 3,27 3,30 3,32 |
5,18 4,85 4,51 4,07 3,69 3,52 3,43 3,35 3,29 3,23 3,24 3,50 3,77 3,92 3,94 3,91 3,78 3,66 3,52 3,37 |
| Т, К | C, nF | R, Oм | ε | lgε | lgR |
|---|---|---|---|---|---|
| Measurement frequency at 1 kHz | |||||
| 293 303 313 323 333 343 353 363 373 383 393 403 413 423 433 443 453 463 473 483 |
7,7977 10,302 15,68 12,489 8,1684 14,516 86,933 176,65 265,14 378,56 495,27 312,87 11,039 1,8105 0,95809 0,68585 0,70009 0,89737 1,3483 2,1329 |
165700 135800 101700 131900 203600 121200 32510 19300 14110 10520 8184 10720 85500 379400 649900 843600 874900 796100 666100 511600 |
22448 29658 45140 35954 23515 41789 250266 508547 763295 1089813 1425802 900702 31779 5212 2758 1974 2015 2583 3882 6140 |
4,35 4,47 4,65 4,56 4,37 4,62 5,40 5,71 5,88 6,04 6,15 5,95 4,50 3,72 3,44 3,30 3,30 3,41 3,59 3,79 |
5,22 5,13 5,01 5,12 5,31 5,08 4,51 4,29 4,15 4,02 3,91 4,03 4,93 5,58 5,81 5,93 5,94 5,90 5,82 5,71 |
| Measurement frequency at 5 kHz | |||||
| 293 303 313 323 333 343 353 363 373 383 393 403 413 423 433 443 453 463 473 483 |
1,3926 1,8886 2,728 1,517 0,9064 2,5719 15,47 31,031 45,909 64,608 82,944 40,501 1,7403 0,28075 0,13233 0,09429 0,09084 0,10483 0,13985 0,19866 |
140100 115400 89690 128100 184900 93070 29090 17900 13060 9799 7702 11420 79660 293500 455500 533500 555900 536400 477500 401900 |
4009 5437 7853 4367 2609 7404 44536 89333 132165 185996 238782 116596 5010 808 381 271 262 302 403 572 |
3,60 3,74 3,90 3,64 3,42 3,87 4,65 4,95 5,12 5,27 5,38 5,07 3,70 2,91 2,58 2,43 2,42 2,48 2,60 2,76 |
5,15 5,06 4,95 5,11 5,27 4,97 4,46 4,25 4,12 3,99 3,89 4,06 4,90 5,47 5,66 5,73 5,74 5,73 5,68 5,60 |
| Measurement frequency at 10 kHz | |||||
| 293 303 313 323 333 343 353 363 373 383 393 403 413 423 433 443 453 463 473 483 |
0,62512 0,85581 1,188 0,50394 0,3238 1,2082 7,2162 14,584 21,676 30,192 39,355 14,483 0,64942 0,12128 0,06145 0,04865 0,04694 0,05137 0,06369 0,08415 |
126300 103700 83510 127500 169200 76460 26640 16680 12440 9304 7426 12940 83960 243700 329700 351900 362500 360400 339200 306100 |
1800 2464 3420 1451 932 3478 20774 41985 62402 86918 113297 41694 1870 349 177 140 135 148 183 242 |
3,26 3,39 3,53 3,16 2,97 3,54 4,32 4,62 4,80 4,94 5,05 4,62 3,27 2,54 2,25 2,15 2,13 2,17 2,26 2,38 |
5,10 5,02 4,92 5,11 5,23 4,88 4,43 4,22 4,09 3,97 3,87 4,11 4,92 5,39 5,52 5,55 5,56 5,56 5,53 5,49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




