1. Introduction
Australian birds have a unique role in global ornithology as the major early centre of passerines’ radiation and diversification of parrots (Garnett et al., 2015). Around 45% of bird species can only be found in Australia (Narayan, 2017). However, as climatic conditions continue to change drastically and rapidly around the world, the distribution, physiology, and behaviour of many native bird species across Australia have been seriously affected by extreme climate events such as high temperature (Crino et al., 2017; Gardner et al., 2017). The vulnerability of native species are modified by climate change and human activities in a variety of ways, including habitat degradation, the disruption of existing competitive interactions, fire regime changes (Reside et al., 2015), lack of available water sources during heatwave (McKechnie et al., 2012) and the timing of migration (Chambers, 2008). Mainland Australia and islands have already lost 29 native bird taxa over the last 200 years (Szabo et al., 2012). Previous study suggests that unless continuous efforts are made to enhance management, around 10 species of Australian birds like orange-bellied parrot and Western ground parrot will become extinct in the next two decades (Geyle et al., 2018). Therefore, the efficiency and quality of wildlife rescue and rehabilitation has been a globally noteworthy issue. Each year, a great number of animals are rescued and rehabilitated in Australia. From 2013 to 2018, 469,553 rescues were reported in New South Wales, 688 species of bird were involved, and the Spring to Summer period witnessed most admissions due to increasing movement, breeding and rearing behaviour (Kwok et al., 2021). Wildlife rehabilitation centres (WRC) are also good information sources which enable researchers to analyse the causes of admission and resultant outcomes, understand potential threats to local species, and quantify both natural and anthropogenic elements that may result in survival threat of wildlife (Taylor-Brown et al., 2019).
Previous study has indicated that many species such as Rainbow Lorikeets (Trichoglossus moluccanus), Australian Magpie (Gymnorhina tibicen), Pigeon and Cockatoo are the common species admitted, and the common reasons for admittance include impact injury, vehicle-related injuries, animal attack, abnormal behaviour, heat stress, etc. (Janssen et al., 2020). Except from these common stressors, diseases such as Beak and Feather disease (BFD), allostatic load can all lead to chronic stress (Taylor-Brown et al., 2019), and ultimately cause high mortality in wild bird rehabilitation. Previous study suggests that in New South Wales, only around 37.1% of rescued animals could recover and be subsequently released after rehabilitation (Kwok et al., 2021). Among admissions in a WRC in Queensland from 2006 to 2017, the mortality rate reached as high as 55.7% in avian species (Taylor-Brown et al., 2019). Another study held in Catalonia, Spain showed the similar result as only 47.2% of birds could be successfully released back into the wild, other admissions resulted in mortality, euthanasia or permanent captivity (Molina-López et al., 2013). The high mortality suggests that multiple bird species are exposed to various short- and long-term stressors in the wild and in the captive environments they are brought into for rehabilitation purposes. So far, however, there has been little discussion about the role of stress levels in wildlife management and rehabilitation.
Similar with mammals, in avian species the hypothalamo-pituitary-adrenal (HPA) axis plays an important role in the process of responding to a physical or anticipated challenge (Smulders et al, 2021). Corticotropin releasing hormone (CRH) released by hypothalamus stimulates the release of adrenocorticotropin hormone (ACTH) in pituitary, which results in the secretion of corticosterone (CORT) (the predominant glucocorticoid in avian species) in adrenal gland (Dickens and Bentley, 2014). The release of CORT triggers a series of physiological changes such as the mobilization of stored energy, gluconeogenesis, enhanced substrate delivery to muscle, and the stimulation of immune function (Sapolsky et al., 2000). In previous studies, blood sample were most widely used for quantifying CORT, providing an invasive “snapshot” of an individual's CORT concentration. Recent years, minimally invasive or non-invasive techniques such as measuring CORT in bird feathers have been well developed (Lattin et al.,2011). During the period of feather growth, CORT is deposited in feathers, making feathers CORT an ideal historical record of the amplitude and duration of CORT release (Harris et al., 2016; Jenni-Eiermann et al., 2015) and a good tool which can be used to determine the long-term health of an individual (Johns et al., 2018). Moreover, the stress caused by collecting a fully grown feather from an animal would not affect the CORT levels in the feather (Legagneux et al., 2013), and unlike plasma samples, no specialized storage requirement should be met for feathers samples, storing at room temperature with a bag or envelope can keep the stability of CORT concentration in feathers for about 14 years, making it possible for researcher to use dead, absent birds or even museum specimens to understand avian stress response and physiology (Beattie and Romero, 2023).
The primary aim of this study was to use non-invasive hormone monitoring technique to qualifying glucocorticoid (primary CORT in birds) levels in 30 samples collected from14 species of birds under rehabilitation, assist in optimising rehabilitation and improving conservation strategies. We also did a retrospective analysis using secondary data collected from the same population to analyse the trends and reasons for admissions, assist future researchers, wildlife managers and carers with making informed decisions around conservative management in both captivity and the wild.
2. Materials and Methods
2.1. Approval
Non-invasive sample collection was performed in accordance with relevant guidelines and regulations. Formal approval was granted by The University of Queensland Ethics (ACEC) Committee (approval number: AE000396).
2.2. Study Site, Animals and Sample Size
The research study was conducted in collaboration with the Adelaide Koala and Wildlife Centre (AKWC) in 282 Anzac Highway, Plympton, South Australia (GPS coordinates: 34° 57' 49.2048'' S, 138° 33' 20.3292'' E). A total of 14 bird species were admitted at the AKWC, and the patient admission data was provided by AKWC from 2022-2023. 30 individuals of 14 species were sampled and analysed in the lab for this study. The total number of feather samples from all individuals accounted for N = 30, one sample was taken from each individual.
2.3. Feather Sample Collection
Feather samples were collected from 30 euthanised birds admitted to AKWC during the period of study. Samples were cut as close to the skin from the shoulder area of each bird, then were labelled and stored at room temperature in brown paper bags before being posted to the University of Queensland.
2.4. Feather Sample Processing and Hormone Extraction
The weighting and measurement of feather pieces follow the methods used by Aharon- Rotman (2017). The scale of each feather sample was measured and recorded with a ruler. A portion of each sample was taken to fill a weigh tray and 3mL of Isopropanol was added into each tray. Then samples were dried in a desiccator overnight to remove any oils or dirt. Once completely dried, the samples were cut up into fine pieces and clumped together. The cut-up pieces were taken out of weight tray using forceps and then placed on a zeroed scale. A 0.06 grams (g) +/− 0.002g sample was weighed out into a labelled microtube and then stored at room temperature.
For hormone extraction, 1mL of 100% methanol was added to each microtube. Then samples were left overnight in the fridge (4℃). Once removed from the fridge, microtubes were put in a centrifuge for 5 minutes, 15,000 rpm. Then a 0.5 mL (500 microlitres) solution was extracted from each centrifuged sample using a pipette and was then placed into a new labelled microtube. Samples were then left in the fume hood for 48 hours in order to evaporate all liquids or alcohol.
2.5. Hormone Analysis
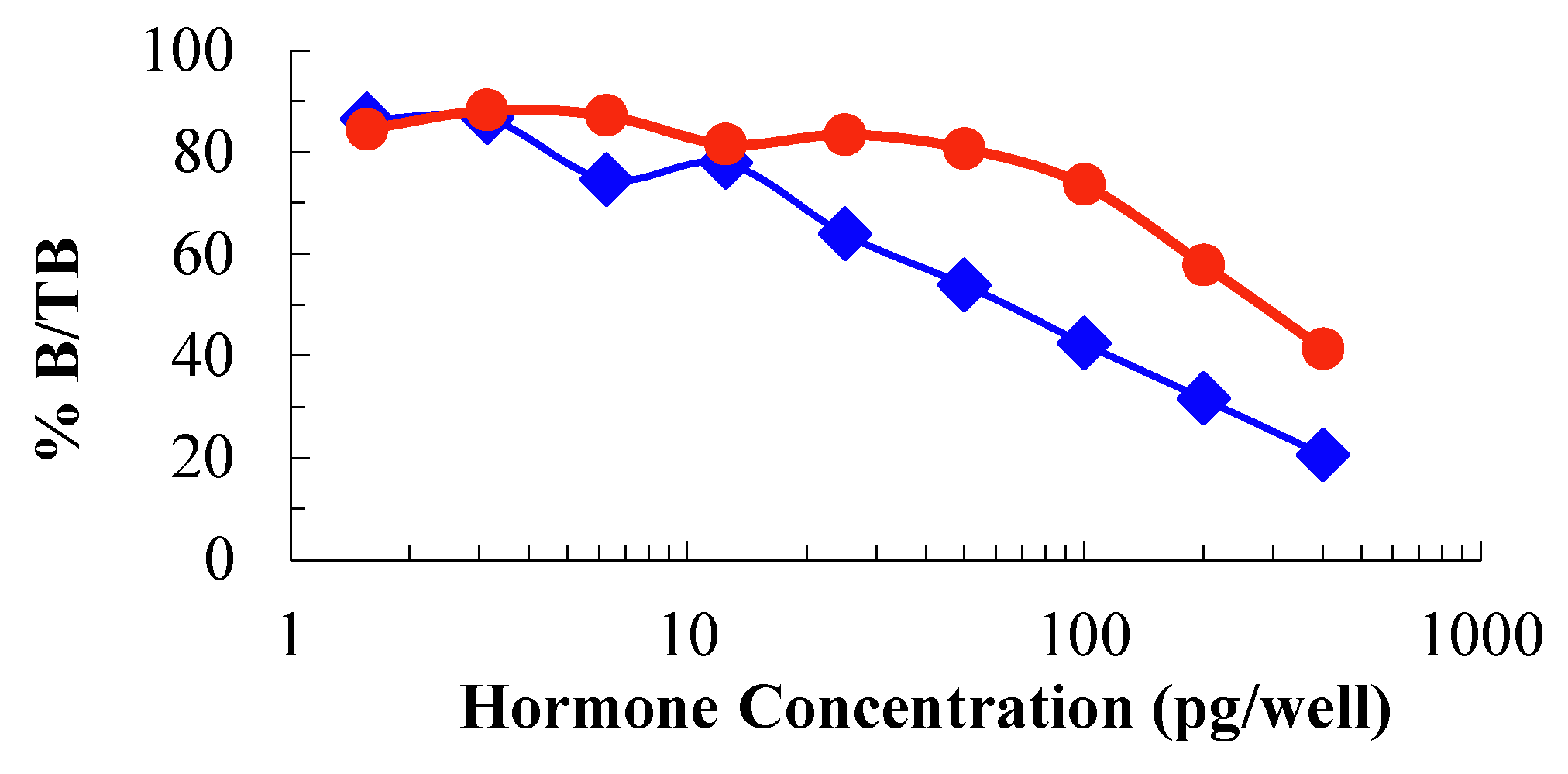
Corticosterone concentrations in feather samples were determined using a polyclonal anti-corticosterone antiserum (CJM06, UC Davis California) that was diluted 1:45,000, corticosterone-horseradish peroxidase (HRP) that was diluted 1:120,000, corticosterone standards (1.56- 400 pg well-1) and control stocks made using a pool of feather samples with high corticosterone levels (150 pg well-1). The pool was compared with standard results to produce a parallelism and validate laboratory results collected from the assay, following the methods used by previous study (Lattin et al, 2011). 50µL of standard, control and sample per well was pipetted, 50µL of diluted corticosterone HRP was then added into all wells that contain standard, control or sample. Plates were incubated at room temperature for 2 hours. Then 50µL TMB substrate (1µL 30% H202, 75µL 1% TMB and 7.425mL substrate buffer) was added to all wells. Plate was then incubated at room temperature for 15min before the assay was stopped with 50µL Stop Solution (26.4mL 0.5 M H2SO4 added to 973.6mL Milli-Q water) to all wells. Plate was read at 450nm. Optimal reading for 0 wells was >0.7 to <1 Optical density (OD).
2.6. Statistical Analysis
Statistical analysis was performed in SPSS. Descriptive statistics was used to summarize data collected from samples which measured CORT. A Nonparametric One-sample Chi-square Test was carried to determine the significance of CORT levels of each sample in the laboratory sample population. Then the sample population was divided into two groups, acute group and chronic group, according to the diagnoses. Previous study defined acute stress as “a short-lived negative situation that allows a quick and quite complete recovery of the physiological balance”, and chronic stress as “a long-lasting condition from which the subject cannot fully recover” (Trevisi & Bertoni, 2009). Stressors include trauma and fracture were classified as acute stressors due to the short courses, and stressors include BFD, pneumonia, neurological disorder, metabolic bone disorder, malnutrition and emaciation were classified as chronic stressors. A Mann-Whitney Test was then carried to compare differences between two independent groups.
2.7. Retrospective Study on the Rescues of Native Bird Species to a Clinical Setting
Secondary data was obtained from the Adelaide Koala and Wildlife Centre (AKWC) in the form of patient records. Patient records included diagnosis, species ID, admission date and outcome date from the 2022-2023 period. Data assessed was categorized and used to assess the variation between species admitted, the seasonal differences and diagnoses presented. For seasonal admission analysis, admissions were separated based on the Australian seasonal year with Late Summer (January-February), Autumn (March-May), Winter (June-August), Spring (September-November) and Early Summer (December) leading into the next year (Australian Government, 2023) in order to observe spikes in admissions. For diagnosis analysis, data was separated based on the causes of admission. The common diagnoses were analysed to provide insights into how diagnoses relate to environmental and population health.
3. Results
3.1. Measured Corticosterone of Sample Population in Laboratory Analysis
A total of 30 samples collected from AKWC were analysed in laboratory work. The corticosterone levels for each of the 30 birds under rehabilitation were determined. Assay validation indicated that results from the assay were accurate since the parallelism run parallel to the standards (
Figure 1). The Nonparametric One-sample Chi-square Test suggested that CORT levels of different samples occurred with equal probabilities (X
2 =0.000, df=29, P=1.000).
3.2. Seasonal Admission Analysis
Secondary data collected from AKWC was analysed from the 2022-2023 period. It should be noticed that in 2023, data was only collected from the summer to autumn period due the time of this study. Seasonal data from the 2022 period suggests that the Rainbow Lorikeet had the highest admission rate over the whole year in comparison to other species in this study (See
Table 1). For the whole year, there were 192 (192/544, 35.29%) Rainbow Lorikeet admissions with most (67/192, 34.90%) occurring in Summer (January- February). The Magpie had the second highest admission rates with 124 (124/544, 22.79%) for the whole year with most (43/124, 34.68%) occurring in Summer (December) which follow similar trends to the 2023 period. For the 2023 period, the number of admissions overall decreased compared to 2022. The number of Rainbow Lorikeet admissions (51/174, 29.31%) also decreased from the previous year while the number of Galah (31/174, 17.82%) admissions increased.
3.3. Diagnoses Analysis
For the 2022 period, musculoskeletal injury was the most prevalent diagnosis across all species with Magpies having the highest rates (91/124, 73.39%) across all diagnoses and Rainbow Lorikeets the second highest (70/192, 36.46%) (See
Table 2). It should be notice that among all species, lorikeet species showed high rate of Beak and Feather Disease (BFD). For Rainbow Lorikeets, Beak and Feather (66/192, 34.38%) was the second highest diagnosis. Similarly, the second most common diagnosis after musculoskeletal injury for Musk Lorikeets was also Beak and Feather (15/56, 26.79%).
The 2023 period followed similar trends in musculoskeletal injury being the most common diagnosis (89/168, 52.98%) (See
Table 3). Rainbow lorikeets were the most commonly admitted for this diagnosis (26/89, 46.43%).
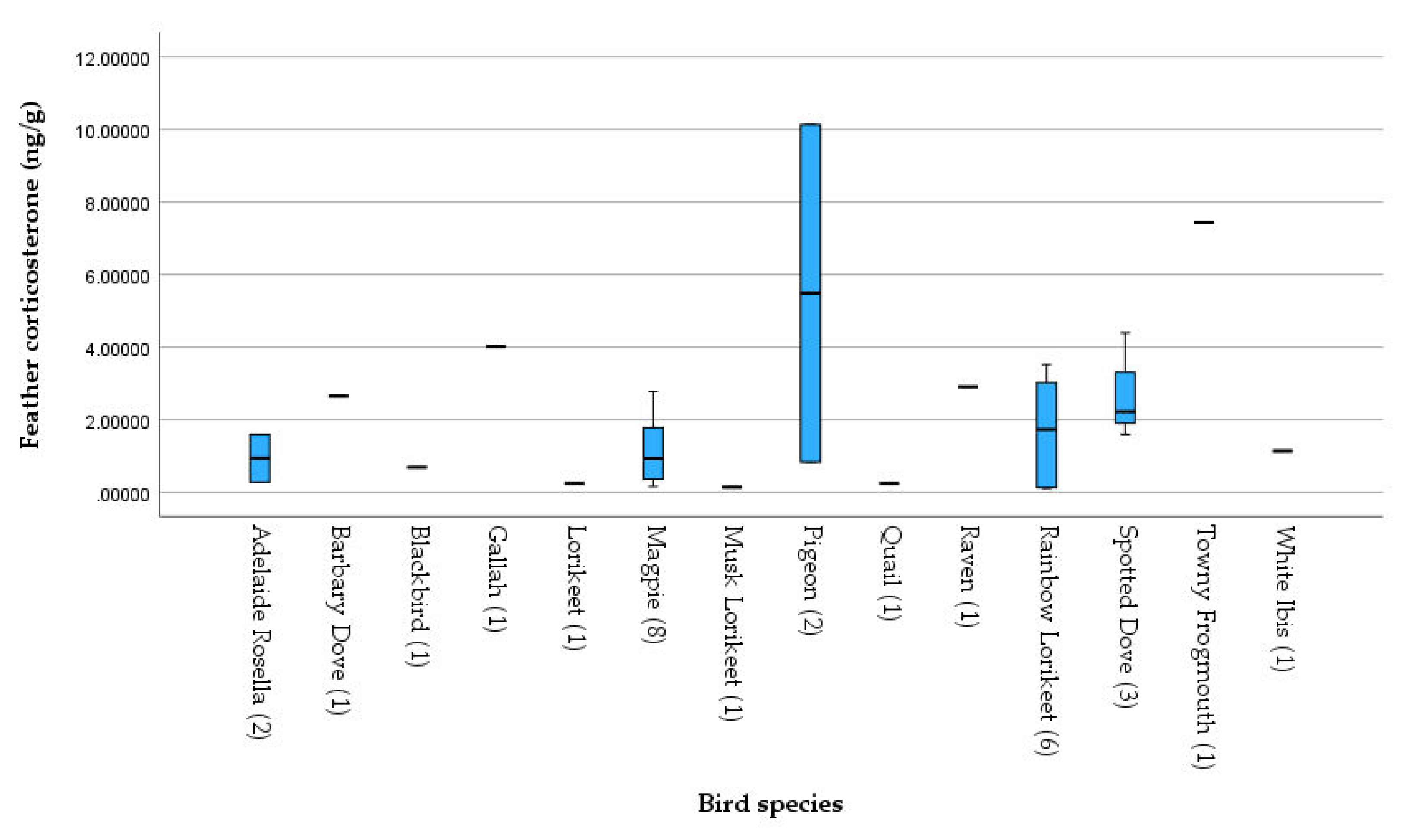
3.4. Comparing Corticosterone Levels between different Species
Data assessed in laboratory hormone analysis was categorized by species, and CORT levels were compared between species (
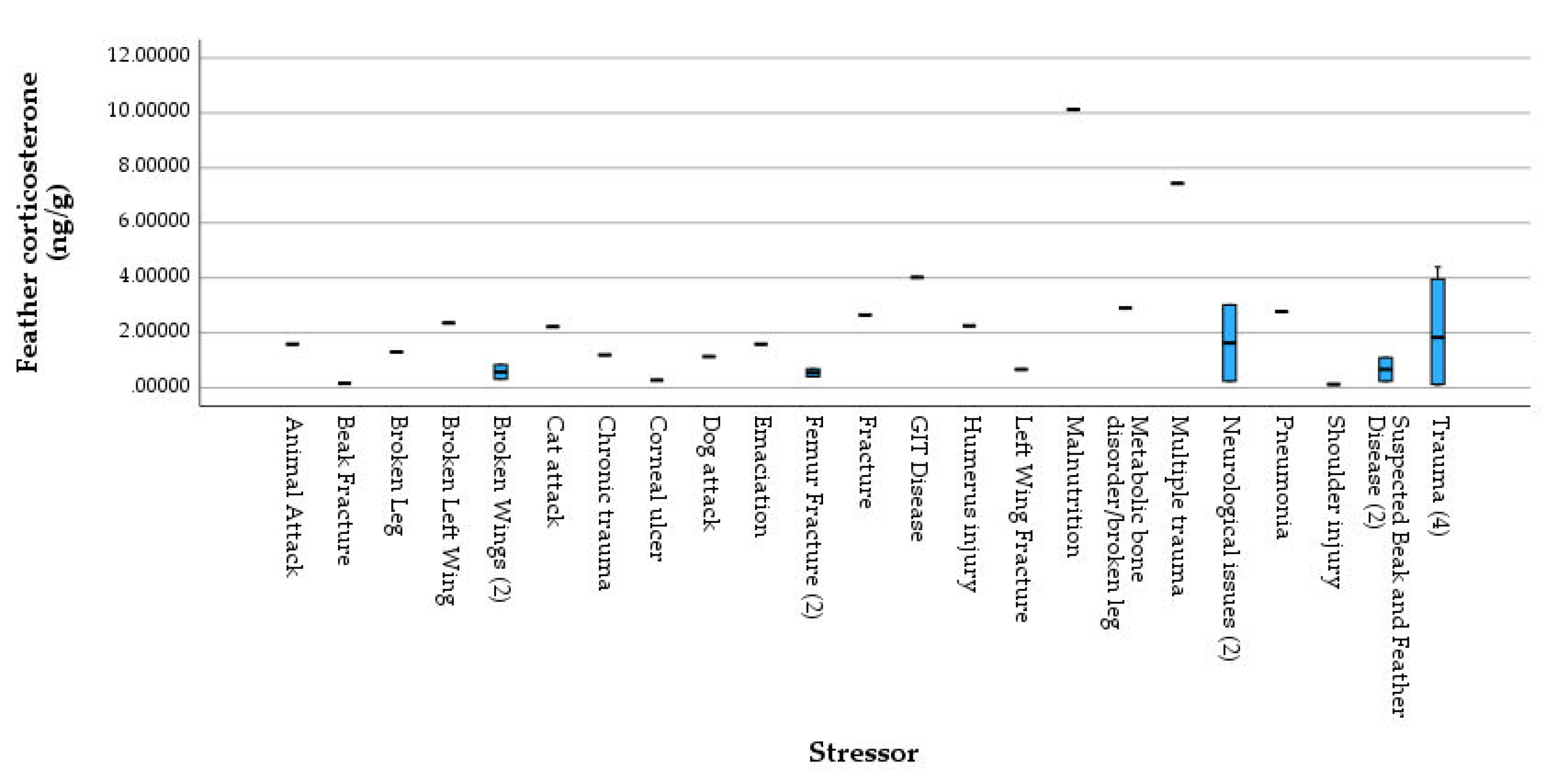
Figure 2) and stressor categories (
Figure 3).
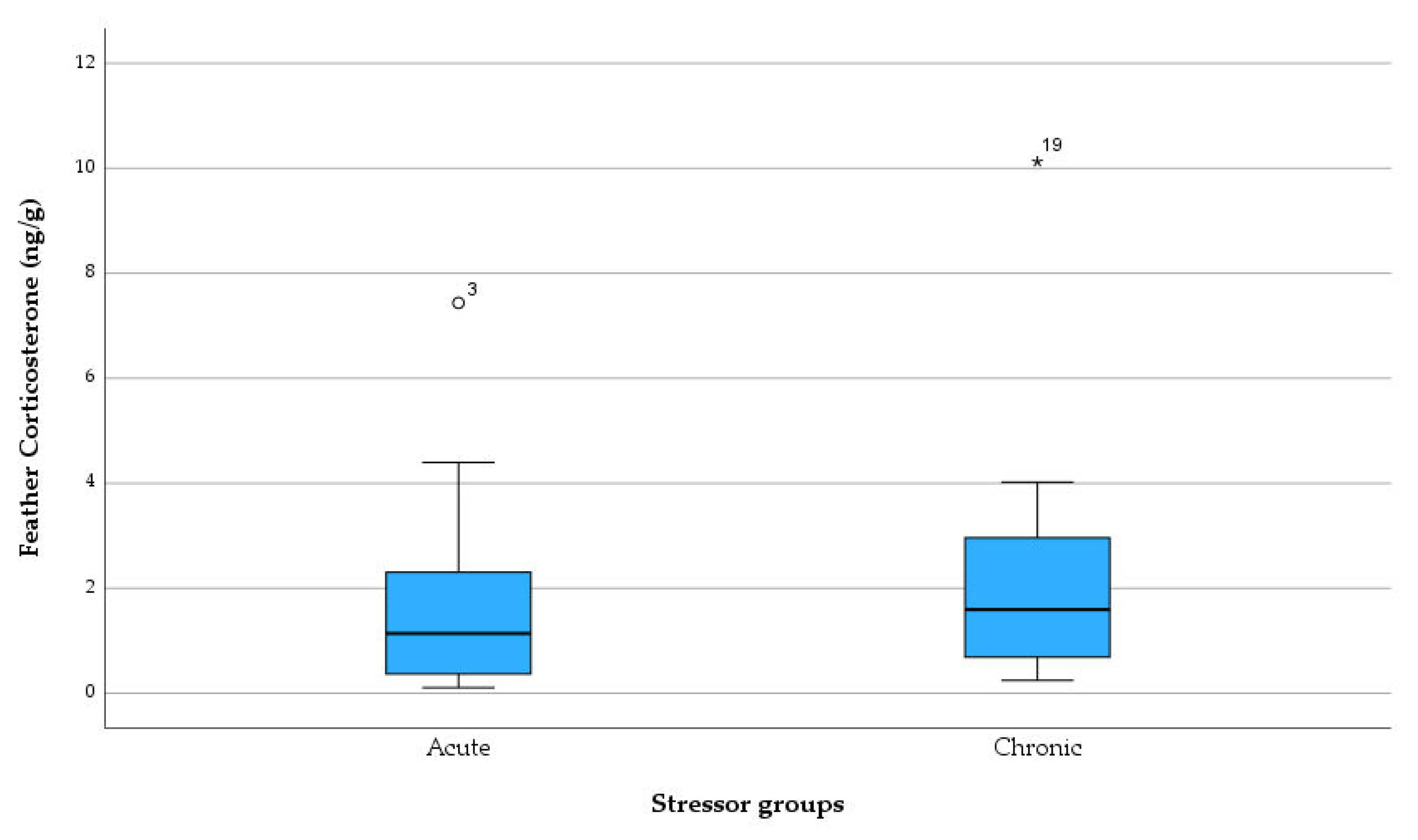
3.5. Comparing Corticosterone Levels between different Types Of Diagnoses
Data assessed in laboratory hormone analysis was categorized by reasons of admissions, and CORT levels were compared between 2 different types of diagnoses (
Figure 4). The distributions in the two groups were not differ significantly from each other (Mann–Whitney U = 82.500, Z= -0.947, P= 0.344 two-tailed). The highest CORT level was observed in a Pigeon diagnosed as malnutrition, followed by the second highest CORT level in a Tawny Frogmouth diagnosed as barbed wire inflicted wing trauma and infection.
4. Discussion
In this pilot study we used available data-set from a veterinary clinic to determine the species and stressors/diagnosis for native Australian birds. We also attempted to quantify the absolute levels of the stress hormone corticosterone using non-invasive feather CORT assay. Retrospective study in this study indicated that Magpie and Rainbow Lorikeet were the most commonly admitted species. Summer was the season when the highest admission rate was witnessed. Musculoskeletal injury and BFD were prevalent diagnoses across all species and contributed to the high euthanasia rate.
A parallelism was conducted successfully in this study to validate the feather corticosterone enzyme-immunoassay. Previous studies have used this test to validate EIA results which have also consisted of the comparison of two curves which show the diluted concentrations of corticosterone standard against the serial diluted curve obtained from the pooled samples (Barbosa-Moyano et al., 2023). Future research should also consider conducting biological validation using a pharmacological challenge such as ACTH challenge (Nilsson et al., 2008). However, this can be highly challenging for validating feather CORT because of the varying rates of deposition of corticosterone in feather due to the age and moulting stages. The nearest best means of biologically validating the feather CORT in these species could be through the comparison of absolute levels of feather CORT between acute and chronic stress states. However, our study was limited by low sample size and poor background information on the individual species (e.g. age, gender, etc.). Still however, we have provided the first report of feather CORT in these Australian native bird species. Future research could use feather as a biological sample to index the stress levels of individual species living in specific areas such as urban landscape or disturbed forests.
In our sampled dataset, the Pigeon showed the most variation in measured corticosterone as well as the highest visually noted CORT reading overall. Corticosterone was also relatively high in the Spotted Dove due to diagnoses presented (acute stressors as trauma and animal attack or chronic stressors as infection in all admissions). Rainbow Lorikeets were another commonly admitted species with diagnoses ranging from trauma, injury (broken wings or legs) or suspected BFD which is related with chronic stress and usually results in high rates of mortality. BFD is a disease caused by the beak and feather disease virus (BFDV), a DNA virus that predominantly infects wild Psittaciformes population, resulting in immune-suppression, feather loss, weight loss, anaemia and other tissue damages (Ortiz-Catedral et al., 2022). Our results suggest that lorikeet species showed high rate of BFD (34.38% of Rainbow Lorikeets and 26.79% of Musk Lorikeets were diagnosed as BFD), making BFD is a non-negligible stressor and health risk for the wild bird populations. This finding corroborates previous study which indicated that BFDV could be tested positive in different Australian native Psittaciformes species such as Galahs, Rosella and Rainbow Lorikeet (Martens et al., 2020). It should also be noticed that in our study, apart from parrots, BFDV was also tested positive in other species such as Pigeon and Magpies. Researchers in a previous study detected BFDV DNA in 20.0 % of non-psittacine birds, suggesting that different from previous cognition, BFDV is actually common in non-psittacine avian hosts (Amery-Gale et al., 2017). In order to avoid quick spread of BFDV, it is very important for wildlife carers and managers to effectively use disinfectants such as Virkon S, and apply a Quarantine Period before introducing birds of unknown health status to a health population (Australian Government, 2006).
The retrospective data in this study showed similar trends in many aspects of both species admissions and presenting diagnoses. It was found that both the Magpie and Rainbow Lorikeet were the most commonly admitted species in both primary and secondary data. These results are also supported in other previous research conducted in 2013, 2014, 2016 and 2017 where Magpies and Rainbow Lorikeets are shown to have the highest stressor counts (Janssen et al. 2020). However, it is also important to note that changes in admissions and diagnoses will vary year to year based on climate, weather events and other changes in the environment and populations. Therefore, future study should still pay attention to the regional species admissions and diagnoses trends to provide more reliable conservation suggestions.
Throughout the period of feather growth, birds produce corticosterone in response to stressors, environmental pressures or to cope with energy demands which maintains homeostasis. CORT is incorporated into feathers in a non-specific manner during feather growth (Bortolotti et al., 2009; Mikkelsen et al., 2022). Thus, feather CORT is a good tool which enables the long-term measure of the HPA axis activity and the assessment of stress in an individual over months in a cumulative manner (Frongia et al., 2020), integrating hormonal effects caused by the time course and magnitude of the stress response, the number of stressors experienced (Hõrak et al., 2013). In this study, individual diagnosed as malnutrition showed the highest CORT level among all of the 30 samples. Previous researches suggested that restricted diet could result in higher levels of feather CORT in captive seabird, and feather CORT can be used as a sensitive tool of detecting nutritional stress (Kitaysky et al., 2010; Will et al, 2014). The second highest CORT level was observed in a Tawny Frogmouth with multiple traumas and infection. Our previous study in AKWC suggests that trauma and fracture were the most common secondary stressors in avian patients (Janssen et al., 2020). While the effects of multiple traumas and bone fractures are less understood in wild avian species, in captive hens, bone fractures have been proved to be related with a depressive- like state of chronic stress (Armstrong et al., 2020). These findings can be examined in further research which may investigate other geographical regions or admissions at other rehabilitation facilities to see trends in other populations. They may also be particularly relevant for studies regarding threatened or endangered species and their management in the future.
This study was somewhat limited in terms of obtaining the full history of stress in an individual as the only corticosterone present would be for the period of feather growth before admission. It is difficult to determine the timing of feather molting since it varies within and between species (Contina et al., 2023) and can be regulated by various environmental signals like the annual changes in food abundance, precipitation, and photoperiod (González-Gómez et al., 2013; Ndlovu et al., 2017), making it difficult to complete inter-individual comparisons and represent the majority of the annual cycle (Romero & Fairhurst, 2016). And as the corticosterone stored in feathers is reflective of all produced in the period of feather growth it is difficult to identify the exact moment an individual is exposed to a stressor. However, CORT levels measured in this study do provide an overall absolute amount of stress an animal has experienced throughout the time of feather growth. This directly relates to any diagnoses which may be present and how much stress this may have caused an animal. Collecting blood sample to assess the individual stress responses to specific stressors is still a standard procedure (Huber et al., 2021) that researchers should consider when studying a defined stressor or stress levels in nestlings (Beaugeard et al., 2019).
5. Conclusions
The primary aim of this study was to measure glucocorticoids in non-invasive sampling of various bird species undergoing rehabilitation and analyse the trends of admissions during the 2022-2023 period in a wildlife hospital. Retrospective study in this study indicated that Magpie and Rainbow Lorikeet were the most commonly admitted species in both primary and secondary data. Summer was the season when the highest admission rate was witnessed. Musculoskeletal injury and Beak and Feather Disease were prevalent diagnoses across all species and contributed significantly to the high euthanasia rate. We also measured corticosterone concentrations in 30 feather samples and analysed the different stress levels caused by various diagnoses (stressors) in different avian species. In this study, no statistically significant difference was found between stress levels induced by acute stressors or chronic stressors. However, levels were variable between the diagnosis, with malnutrition showing the highest levels of feather CORT.
This is the first study to use feather CORT EIA to assess stress levels in rescued Australian native avian species. Additionally, it lays the groundwork for future research, enabling more comprehensive analyses of wild populations and a diverse array of species.
Author Contributions
Conceptualization, E.N. and E.D.; methodology, E.N. E.D. and H.P.; formal analysis, E.N. E.D. and H.P.; investigation, E.N. E.D. and J.Z; resources, E.N.; data curation, E.N.; writing—original draft preparation, E.N., J.Z.; writing—review and editing, E.N. and J.Z; supervision, E.N.; project administration, E.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of The University of Queensland (approval number: AE000045).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Acknowledgments
We thank the Adelaide Koala and Wildlife Centre for collaboration. We also thank two anonymous reviewers for their valuable comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- AUSTRALIAN GOVERNMENT, 2023. Climate Glossary. [Online]. Available: http://www.bom.gov.au/climate/glossary/seasons.shtml#:~:text=Spring%20%2D%20the%20three%20transition%20months,months%20June%2C%20July%20and%20August.
- AUSTRALIAN GOVERNMENT, 2006. Hygiene Protocols for the Prevention and Control of Diseases (Particularly Beak and Feather Disease) in Australian Birds. [Online]. Available: https://www.dcceew.gov.au/environment/invasive-species/publications/hygiene-protocols-preventionand-control-diseases-particularly-beak-and-feather-disease#daff-page-main.
- AHARON-ROTMAN Y., BUCHANAN K., KLAASSEN M., BUTTEMER W. 2017. An experimental examination of interindividual variation in feather corticosterone content in the house sparrow, Passer domesticus in southeast Australia. General and Comparative Endocrinology, 244, 93-100.
- AMERY-GALE, J., MARENDA, M. S., OWENS, J., EDEN, P. A., BROWNING, G. F., & DEVLIN, J. M. 2017. A high prevalence of beak and feather disease virus in non-psittacine Australian birds. Journal of Medical Microbiology, 66, 1005–1013.
- ARMSTRONG, E. A., RUFENER, C., TOSCANO, M. J., EASTHAM, J. E., GUY, J. H., SANDILANDS, V., BOSWELL, T., & SMULDERS, T. V. 2020. Keel bone fractures induce a depressive-like state in laying hens. Scientific Reports, 10, 3007–3007.
- BARBOSA-MOYANO H., SOBRAL G., DE OLIVEIRA C.A. 2023. Glucocorticoid metabolites in an ex situ nocturnal bird, the tropical screech owl Megascops choliba: effects of sex, activity period and inter-individual variation. Conservation Physiology, 11. coad016–coad016.
- BEATTIE, U. K., & ROMERO, L. M. 2023. Long term stability of corticosterone in feathers. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology, 283, 111472–111472.
- BEAUGEARD, E., BRISCHOUX, F., HENRY, P., PARENTEAU, C., TROUVÉ, C., & ANGELIER, F. 2019. Does urbanization cause stress in wild birds during development? Insights from feather corticosterone levels in juvenile house sparrows (Passer domesticus). Ecology and Evolution, 9, 640–652.
- BORTOLOTTI, G. R., MARCHANT, T., BLAS, J., & CABEZAS, S. 2009. Tracking stress: Localisation, deposition and stability of corticosterone in feathers. Journal of Experimental Biology, 212, 1477–1482.
- CHAMBERS, L. E. 2008. Trends in Timing of Migration of South-Western Australian Birds and Their Relationship to Climate. Emu, 108, 1–14.
- CONTINA, A., BOSSU, C. M., ALLEN, D., WUNDER, M. B., & RUEGG, K. C. 2023. Genetic and ecological drivers of molt in a migratory bird. Scientific Reports, 13, 814–814.
- CRINO, O. L., BUCHANAN, K. L., TROMPF, L., MAINWARING, M. C., & GRIFFITH, S. C. 2017. Stress reactivity, condition, and foraging behavior in zebra finches: effects on boldness, exploration, and sociality. General and Comparative Endocrinology, 244, 101–107.
- DICKENS, M. J., & BENTLEY, G. E. 2014. Stress, captivity, and reproduction in a wild bird species. Hormones and Behavior, 66, 685–693.
- FRONGIA G., PERIC T., LEONI G., SATTA V., BERLINGUER F., MUZZEDDU M., PRANDI A., NAITANA S., COMIN A. 2020. Assessment of Cortisol and DHEA Concentrations in Griffon Vulture (Gyps fulvus) Feather to Evaluate its Allostatic Load. Annals of Animal Science, 20, 85-96.
- GARDNER, J. L., ROWLEY, E., DE REBEIRA, P., DE REBEIRA, A., & BROUWER, L. 2017. Effects of extreme weather on two sympatric Australian passerine bird species. Philosophical Transactions of the Royal Society B: Biological Sciences, 372, 20160148–20160148.
- GARNETT, S. T., DUURSMA, D. E., EHMKE, G., GUAY, P. J., STEWART, A., SZABO, J. K., WESTON, M. A., BENNETT, S., CROWLEY, G. M., DRYNAN, D., DUTSON, G., FITZHERBERT, K., & FRANKLIN, D. C. 2015. Biological, ecological, conservation and legal information for all species and subspecies of Australian bird. Scientific Data, 2, 150061–150061.
- GEYLE, H. M., WOINARSKI, J. C. Z., BAKER, G. B., DICKMAN, C. R., DUTSON, G., FISHER, D. O., FORD, H., HOLDSWORTH, M., JONES, M. E., KUTT, A., LEGGE, S., LEIPER, I., LOYN, R., MURPHY, B. P., MENKHORST, P., RESIDE, A. E., RITCHIE, E. G., ROBERTS, F. E., TINGLEY, R., & GARNETT, S. T. 2018. Quantifying extinction risk and forecasting the number of impending Australian bird and mammal extinctions. Pacific Conservation Biology, 24, 157–167.
- GONZÁLEZ-GÓMEZ, P. L., MERRILL, L., ELLIS, V. A., VENEGAS, C., PANTOJA, J. I., VASQUEZ, R. A., & WINGFIELD, J. C. 2013. Breaking down seasonality: Androgen modulation and stress response in a highly stable environment. General and Comparative Endocrinology, 191, 1–12.
- HARRIS, C. M., MADLIGER, C. L., & LOVE, O. P. 2016. Temporal overlap and repeatability of feather corticosterone levels: Practical considerations for use as a biomarker. Conservation Physiology, 4, cow051.
- HÕRAK, P., MÄNNISTE, M., MEITERN, R., SILD, E., SAKS, L., & SEPP, T. 2013. Dexamethasone inhibits corticosterone deposition in feathers of greenfinches. General and Comparative Endocrinology, 191, 210–214.
- HUBER, N., MAHR, K., TÓTH, Z., SZARKA, E. Z., ÇINAR, Y. U., SALMÓN, P., & LENDVAI, ÁDÁM Z. 2021. The stressed bird in the hand: Influence of sampling design on the physiological stress response in a free-living songbird. Physiology & Behavior, 238, 113488–113488.
- JANSSEN, K., MARSLAND, C., BARRETO, M. O., CHARALAMBOUS, R., & NARAYAN, E. 2020. Identifying the stressors impacting rescued avian wildlife. Animals (Basel), 10, 1–9.
- JENNI-EIERMANN, S., HELFENSTEIN, F., VALLAT, A., GLAUSER, G., JENNI, L., & FISHER, D. 2015. Corticosterone: effects on feather quality and deposition into feathers. Methods in Ecology and Evolution, 6, 237–246.
- JOHNS, D., MARCHANT, T., FAIRHURST, G., SPEAKMAN, J., & CLARK, R. 2018. Biomarker of burden: Feather corticosterone reflects energetic expenditure and allostatic overload in captive waterfowl. Functional ecology, 32, 345-357.
- KITAYSKY, A. S., PIATT, J. F., HATCH, S. A.,. KITAISKAIA, E.V., BENOWITZ-FREDERICKS, Z. M., SHULTZ, M. T., & WINGFIELD, J. C. 2009. Food availability and population processes: severity of nutritional stress during reproduction predicts survival of long-lived seabirds. Functional Ecology, 24, 625–637.
- KWOK, A. B. C., HAERING, R., TRAVERS, S. K., & STATHIS, P. 2021. Trends in wildlife rehabilitation rescues and animal fate across a six-year period in New South Wales, Australia. PloS One, 16, e0257209–e0257209.
- LATTIN, C. R., REED, J. M., DESROCHERS, D. W., & ROMERO, L. M. 2011. Elevated corticosterone in feathers correlates with corticosterone-induced decreased feather quality: a validation study. Journal of Avian Biology, 42, 247–252.
- LEGAGNEUX, P., HARMS, N. J., GAUTHIER, G., CHASTEL, O., GILCHRIST, H. G., BORTOLOTTI, G., BÊTY, J., & SOOS, C. 2013. Does feather corticosterone reflect individual quality or external stress in arctic-nesting migratory birds? PloS One, 8, e82644–e82644.
- MARTENS, J. M., STOKES, H. S., BERG, M. L., WALDER, K., RAIDAL, S. R., MAGRATH, M. J. L., & BENNETT, A. T. D. 2020. Beak and feather disease virus (BFDV) prevalence, load and excretion in seven species of wild caught common Australian parrots. PloS One, 15, e0235406–e0235406.
- MCKECHNIE, A. E., HOCKEY, P. A. R., & WOLF, B. O. 2012. Feeling the heat: Australian landbirds and climate change. Emu, 112, i–vii.
- MIKKELSEN, A. J., LESMEISTER, D. B., O’REILLY, K. M., & DUGGER, K. M. 2022. Feather corticosterone reveals developmental challenges in a long-term study of juvenile northern spotted owls. Functional Ecology, 36, 51–63.
- MOLINA-LÓPEZ, R. A., CASAL, J., & DARWICH, L. 2013. Final Disposition and Quality Auditing of the Rehabilitation Process in Wild Raptors Admitted to a Wildlife Rehabilitation Centre in Catalonia, Spain, during a Twelve Year Period (1995-2007). PloS One, 8, e60242–e60242.
- NARAYAN, E. 2017. Evaluation of physiological stress in Australian wildlife: Embracing pioneering and current knowledge as a guide to future research directions. General and Comparative Endocrinology, 244, 30-39.
- NILSSON, P. B., HOLLMÉN, T. E., ATKINSON, S., MASHBURN, K. L., TUOMI, P. A., ESLER, D., MULCAHY, D. M., & RIZZOLO, D. J. 2008. Effects of ACTH, capture, and short term confinement on glucocorticoid concentrations in harlequin ducks (Histrionicus histrionicus). Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology, 149, 275–283.
- NDLOVU, M., HOCKEY, P. A. R., & CUMMING, G. S. 2017. Geographic variation in factors that influence timing of moult and breeding in waterfowl. Zoology (Jena), 122, 100–106.
- ORTIZ-CATEDRAL, L., WALLACE, C. J., HEINSOHN, R., KREBS, E. A., LANGMORE, N. E., VUKELIC, D., BUCHER, E. H., VARSANI, A., & MASELLO, J. F. 2022. A PCR-Based Retrospective Study for Beak and Feather Disease Virus (BFDV) in Five Wild Populations of Parrots from Australia, Argentina and New Zealand. Diversity (Basel), 14, 148.
- RESIDE, A. E., VANDERWAL, J., GARNETT, S. T., & KUTT, A. S. 2015. Vulnerability of Australian tropical savanna birds to climate change. Austral Ecology, 41, 106–116.
- ROMERO, L. M., & FAIRHURST, G. D. 2016. Measuring corticosterone in feathers: Strengths, limitations, and suggestions for the future. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology, 202, 112–122.
- SAPOLSKY, R. M., ROMERO, L. M., & MUNCK, A. U. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews, 21, 55–89.
- SMULDERS, T. V. 2021. Telencephalic regulation of the HPA axis in birds. Neurobiology of Stress, 15, 100351–100351.
- SZABO, J. K., KHWAJA, N., GARNETT, S. T., & BUTCHART, S. H. M. 2012. Global Patterns and Drivers of Avian Extinctions at the Species and Subspecies Level. PloS One, 7, e47080–e47080.
- TAYLOR-BROWN, A., BOOTH, R., GILLETT, A., MEALY, E., OGBOURNE, S. M., POLKINGHORNE, A., & CONROY, G. C. 2019. The impact of human activities on Australian wildlife. PloS One, 14, e0206958–e0206958.
- TREVISI, E., & BERTONI, G. 2009. Some physiological and biochemical methods for acute and chronic stress evaluationin dairy cows. Italian Journal of Animal Science, 8, 265–286.
- WILL, A. P., SUZUKI, Y., ELLIOTT, K. H., HATCH, S. A., WATANUKI, Y., & KITAYSKY, A. S. 2014. Feather corticosterone reveals developmental stress in seabirds. Journal of Experimental Biology, 217, 2371–2376.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).