1. Introduction
Mexico is considered one of the most diverse countries on the planet, harboring a large number of plant species, with an important number of native taxa, which are generally little studied (Hufnagel & Mics, 2021; Villaseñor, 2016; Villaseñor et al., 2023). Of the Amaryllidaceae family, there are species of the genera Hymenocallis, Sprekelia and Zephyranthes that are endemic to Mexico and have been identified with high potential for ornamental use (Leszczyñska-Borys et al., 2000; Tapia et al., 2012).
The latter genus includes plants that have been used in traditional oriental medicine for their antiviral properties (Nair & van Staden, 2023) and have recently been studied for their cytotoxic content in pharmaceutically important secondary metabolites such as those of the galanthamine group (Kohe-lová et al., 2021; Kulhánková et al., 2013; Luo et al., 2012; Wang et al., 2018; Zhan et al., 2016; Zhan, Liu, et al., 2017; Zhan, Zhou, et al., 2017). These compounds have been employed in palliative Alzheimer's therapy for several years now, with approval from the U.S. Food and Drug Administration (FDA) (Ates et al., 2021; Kohelová et al., 2021).
Of the 90 reported species of
Zephyranthes, 37 have been identified as endemic to Mexico (Tapia et al., 2012). However, there are only records of the presence of 11 them in the World Biodiversity Information Network (REMIB) (
http://www.conabio.mx/remib/doctos/remibnodosdb.html?#) throughout the national territory. Similarly, in Mexico, little research has been conducted on the study of these species. Existing works have focused on the generation of knowledge in taxonomic (Carnevali et al., 2010; Flagg et al., 2018), molecular (Tapia-Campos et al., 2016), chemical, pharmacological (Centeno-Betanzos et al., 2021), mineral nutrition and biostimulation (Rodríguez-Flores et al., 2023) fields. There is no record of studies that focus on aspects related to the ecology of these species.
Given the importance of Zephyranthes in Mexico in terms of biodiversity and uses, as well as the small number of research studies, it is crucial to promote the study of this genus. However, to initiate this process effectively, fundamental aspects of this group of plants must be understood, such as their distribution in the Mexican territory. This is relevant, since the lack of information on the spatial patterns of a species limits the ability to understand its ecology, conservation and potential applications (Franklin, 2013; Qazi et al., 2022).
Potential distribution modeling is a tool that has been used in ecology and biogeography for several years, in different taxonomic groups (Abdelaal et al., 2019; Jiménez et al., 2023; Li et al., 2023; Martínez-Méndez et al., 2016; Murillo-Pérez et al., 2022; Yun et al., 2022). This tool has been of great importance in the development of scientific strategies in the conservation of species or habitats (Austin, 2002; Beaumont et al., 2005; Espejo-Serna & López-Ferrari, 2018; López-Sandoval et al., 2015; Martínez-Méndez et al., 2016; Palma-Ordaz & Delgadillo Rodríguez, 2014; Rodríguez & López-Toledo, 2016; Soberón, 2010; Solís-Montero et al., 2022; Suárez-Mota et al., 2022; Villaseñor, 2018; Ye et al., 2021). From the model results, it is possible to define the geographic range that a species could occupy, if there were no significant restrictions in terms of biotic or abiotic factors (Elith et al., 2011).
One of the most widely used methodologies for modeling potential distribution is the maximum entropy method (MaxEnt), which calculates the probability of presence of a species, conditioned by the environment, and generates predictive models with high accuracy (Beaumont et al., 2005; Elith et al., 2011; Mota-Vargas et al., 2019). The application of the maximum entropy method in modeling potential distribution is of particular importance when dealing with endemic species, as MaxEnt has been shown to generate reliable results for entities with restricted distribution (Abdelaal et al., 2019; Murillo-Pérez et al., 2022; Qazi et al., 2022; Yun et al., 2022). The conservation of these species is crucial due to their uniqueness and the role they play in local biodiversity. This knowledge will not only enrich our understanding of Mexican biodiversity, but will also provide a basis for future studies within the Mexican territory.
The objective of this study is to determine the potential distribution of 11 species of Zephyranthes in Mexico, and to determine which environmental variables are most important in the prediction of the potential distribution area. It is assumed that each species has different factors that delimit its ecological niche and that these factors influence the geographic differentiation of the potential distribution. To clarify the above, the following questions will be answered: What are the bioclimatic variables that influence the distribution of each of the 11 species of the genus Zephyranthes? and What is the potential distribution of each of the species in the country?
2. Materials and Methods
2.1. Species Database
For the construction of the occurrence database, the World Biodiversity Information Network (REMIB) of Conabio (
http://www.conabio.gob.mx/remib/doctos/remibnodosdb.html? #), finding 169 occurrence records for 11 species of
Zephyranthes (
Table 1) in 6 herbaria: Instituto de Ecología (IE-XAL); Instituto Politécnico Nacional (ENCB); Universidad Autonoma Metropolitana (UAM-1); Herbarium of the University of Texas (LL, TEX); Herbarium of the Centro de Investigación Cientifica de Yucatán (CICY) and Herbarium of Geo. B. Hinton. All records and their geographic coordinates were subjected to two phases of cleaning. In the first phase, data of species whose taxonomic identity was unclear and repeated data were eliminated. Subsequently, atypical data were discarded for each species, i.e., data outside the natural distribution of the species.
2.2. Bioclimatic Variables
To generate the models, variables were obtained from the WORDCLIM database (
http://www.wordlclim.org).
Table 2 shows the 19 environmental variables used in the modeling, including 12 climatic variables, three edaphic variables, two topographic attributes and two vegetation cover variables.
2.3. Elimination of Spatial Autocorrelation
To reduce the spatial autocorrelation of the environmental predictors, the number of variables was reduced through a Principal Component Analysis (PCA), following the methodology proposed by Cruz-Cárdenas et al (2014). The PCA was performed with the FactoMineR and factoextra package in the RStudio environment for the R programming language (RStudio Team, 2021). For each component, the variables with the highest loading value were considered to generate the models (Jolliffe, 2002).
2.4. Distribution Model
The MaxEnt program was used to construct the models. The adjustment parameters used in the program were those that came by default in the software except for the Extrapolate and Do clamping options that were deactivated to avoid artificial extrapolations in the extreme values of the ecological variables (Elith et al., 2011; Phillips & Dudík, 2008). A regularization multiplier (1), maximum number of background points (10,000), convergence limit (0.00001) and maximum iterations (1000) parameter settings were used. A logistic type output was obtained using a minimum presence threshold; 75% of the randomly distributed records were considered for model training and 25% for validation.
The evaluation of the models was done with the results of the Area Under the Curve (AUC) analysis of the Receiver Operating Characteristic (ROC) and that, according to Peterson et al., (2011) values greater than 0.9 are accepted as very good models. Once the models were obtained and with the results of the Jackknife test given by MaxEnt, the variables with the highest contribution of each species model were selected and the model was repeated only with these variables.
Finally, the models in ASCII format were projected using Quantum GIS 3.26.1 (Quantum GIS Development Team, 2024) for the representation of a color gradient according to the suitability for the species studied.
For the interpretation of the potential distribution of each species, the 14 biogeographic provinces proposed by Morrone et al., (2017) were used. This zonation combines climatic, geological and biotic criteria that provide a better classification of the boundaries between ecoregions (Morrone et al., 2017).
3. Results
3.1. Layers of Bioclimatic Variables
For eight of the species, group PC1 explained more than 50% of the variance recorded in the data, so groups PC1 and PC2, which explained a large proportion of the accumulated variance (>80%), were selected. Regarding the species
Z. citrina,
Z. fosteri and
Z. verecunda, groups PC1, PC2 and PC3 were selected (
Table 3). The variables that were most determinant for the distribution of the species with the greatest contribution to the distribution models were BIO5, BIO8, BIO10, BIO12 (
Table 4).
3.2. Evaluation of the Models and Contribution of Bioclimatic Variables
The values of the area under the receiver-operated curve (ROC) obtained in the test models for the 11
Zephyranthes species were greater than 0.900, which allowed classifying the models as very good. Only one value in the models was found below this criterion (
Z. citrina), however, the value obtained (0.893) is considered a good model (Peterson et al., 2011) (
Table 5).
3.3. Known Distribution of the Genus Zephyranthes
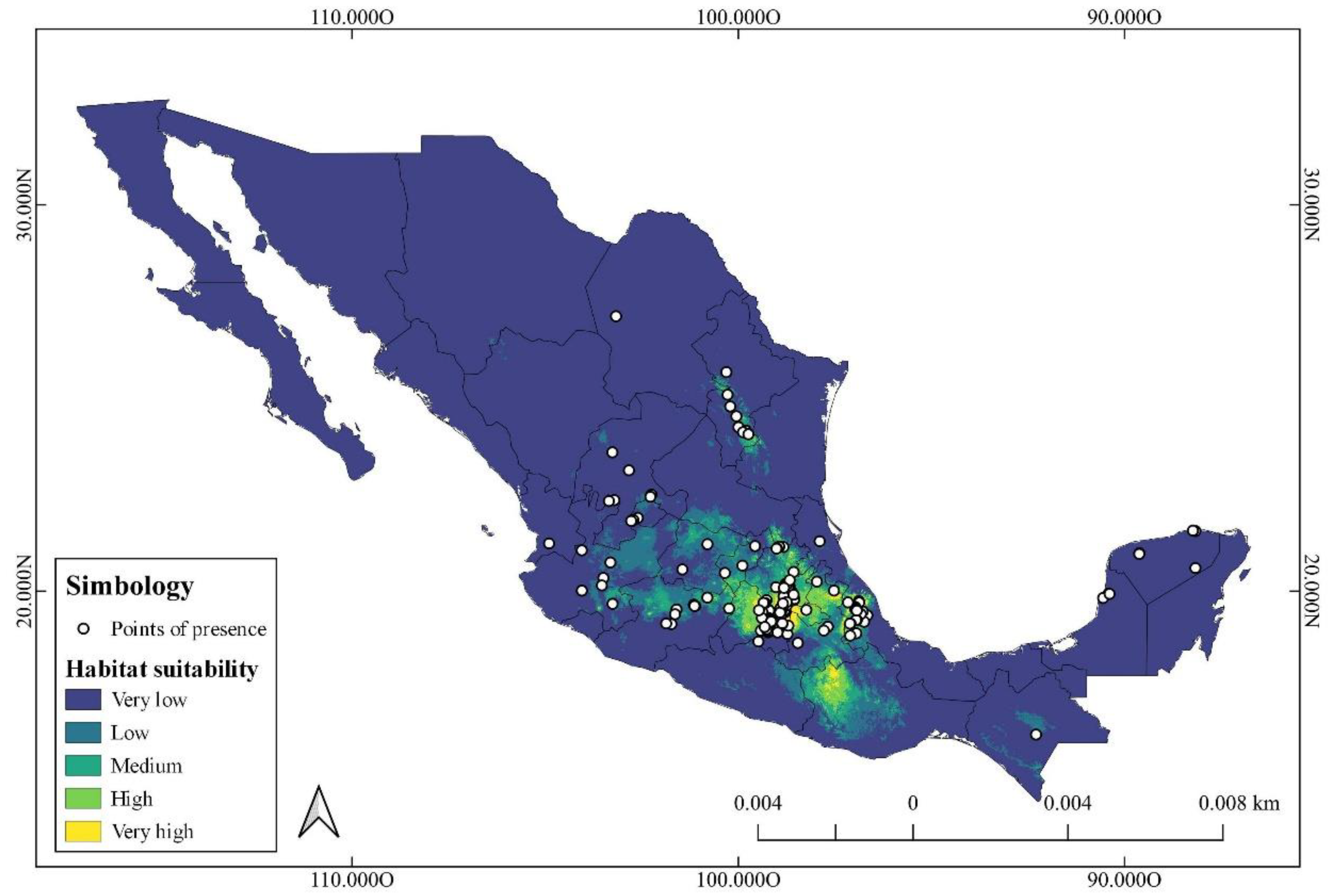
The actual distribution observed by the points of presence of 11 species of the genus
Zephyranthes, which can be seen in
Figure 1, indicates a wide distribution in the Mexican territory from the north of the country, where species such as
Z. lindleyana and
Z. morrisclintii are distributed in the northern zone of the Sierra Madre Oriental, to the Yucatan Peninsula where species such as
Z. citrina are distributed. However, the great majority of Zephyranthes species such as
Z. brevipes,
Z. carinata,
Z. concolor,
Z. clintiae,
Z. fosteri,
Z. longifolia,
Z. sessilis, and
Z. verecunda are concentrated in central Mexico.
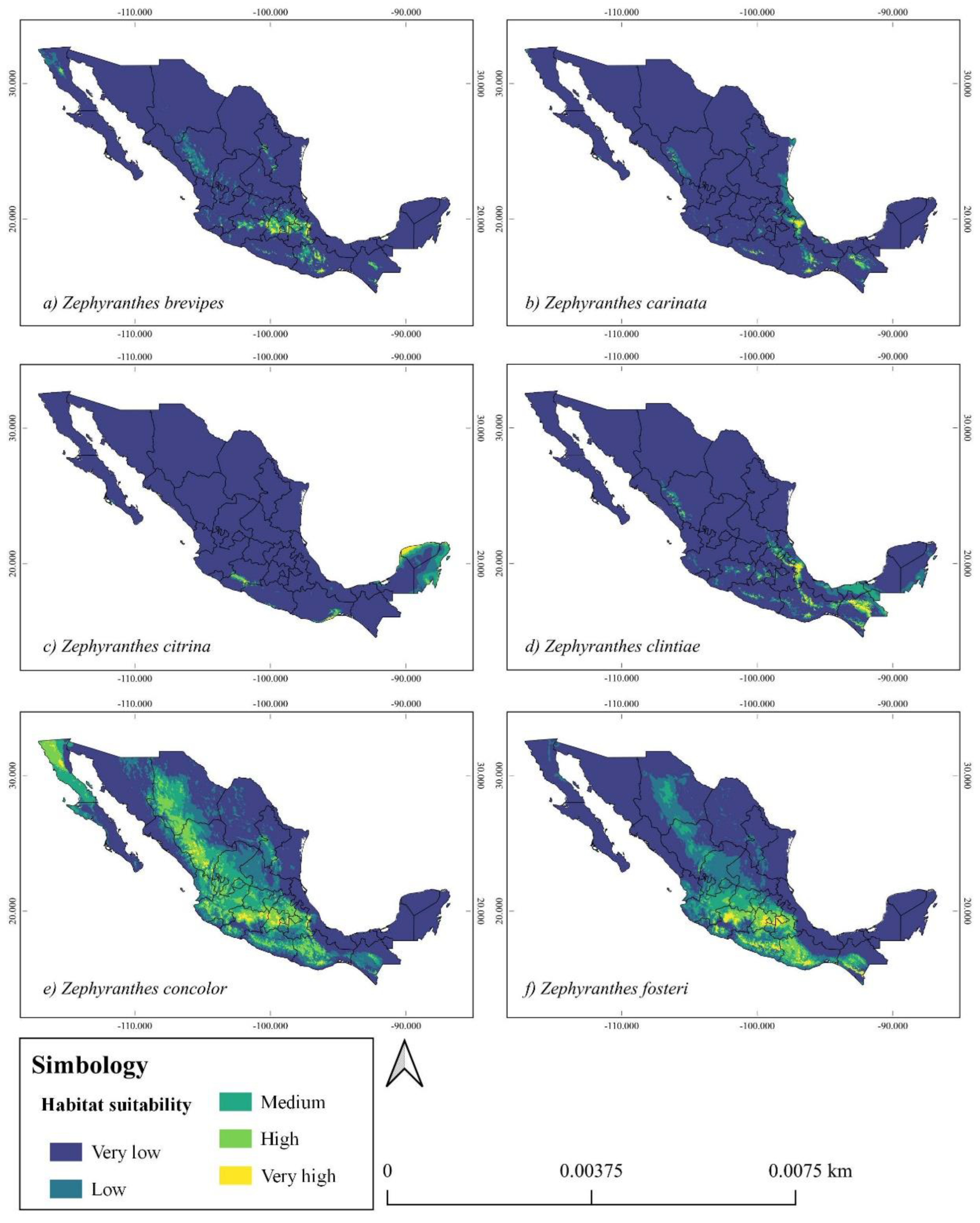
Zephyranthes brevipes. The highest probability for its presence is located in the province of the Transmexican Volcanic Belt in the states of Tlaxcala, State of Mexico, East-West of Puebla, South of the province of the Chihuahuan Desert in the states of Hidalgo (South), North of the State of Mexico and province of the Sierra Madre del Sur in the state of Oaxaca. A medium suitability in the Central Zone of the Chiapas Highlands, Southeast of the Chihuahuan Desert in the state of Durango, Michoacán in the East of the Western Sierra province and Baja California in the province of California. A low suitability in the state of Guerrero in the east of the Transmexican Volcanic Belt and east of the Sierra Madre del Sur in the state of Michoacán (
Figure 2a).
Zephyranthes carinata. This species has a high potential for geographic suitability in the central region of the state of Veracruz in the Balsas Basin province. It also has a medium potential distribution in the Highlands province of Chiapas and Oaxaca in the Sierra Madre del Sur province (
Figure 2b).
Zephyranthes citrina. The areas of greatest geographic distribution of the species are in the Yucatan Peninsula province in the states of Yucatan and Quintana Roo. In the province of the Balsas Basin in the central zone of the state of Michoacán it has a medium-low distribution (
Figure 2c).
Zephyranthes clintiae. This species has a high potential distribution in the southwestern part of the country in the province of Veracruz, the highlands of Chiapas and in Oaxaca in the province of the Sierra Madre del Sur. It also has a medium suitability in the Sierra Norte de Puebla in the Transmexican Volcanic Belt and in the eastern region of Quintana Roo in the Yucatan Peninsula (
Figure 2d).
Zephyranthes concolor. In the particular case of this species, it has a high potential distribution throughout the country, from the Pacific lowlands in the state of Oaxaca to the province of California. However, it presents a high habitat suitability in the province of the Transmexican Volcanic Belt in the states of Puebla, Mexico, Michoacan, in the Californian province in the central state of Baja California and in the Sierra Madre del Sur Occidental along the state of Durango. Medium habitat suitability in the provinces of the Sierra Norte Occidental, Chihuahuan Desert, Transmexican Volcanic Belt, Sierra Madre del Sur Cuenca de las Balsas, in the states of Aguascalientes, Chihuahua, Guerreo, Guanajuato, Hidalgo, Nayarit, Oaxaca, Puebla, San Luiz Potosí and Zacatecas (
Figure 2e).
Zephyranthes fosteri. This species has a potential distribution mainly in the central and southwestern part of the country. The high habitat suitability is present in the biogeographic provinces of the Transmexican Volcanic Belt, Balsas Basin, Sierra Madre del Sur and the highlands of Chiapas, in the states of Mexico, Michoacán, Tlaxcala, CDMX, Puebla, in the Sierra Madre del Sur in the state of Guerrero and Oaxaca and in the highlands of Chiapas. CDMX, Puebla, in the Sierra Madre del Sur in the states of Guerrero and Oaxaca and in the Chiapas Highlands. A medium potential distribution throughout the Sierra Madre Occidental and across the states of Chihuahua, Durango, Guanajuato, Jalisco and Zacatecas (
Figure 2f).
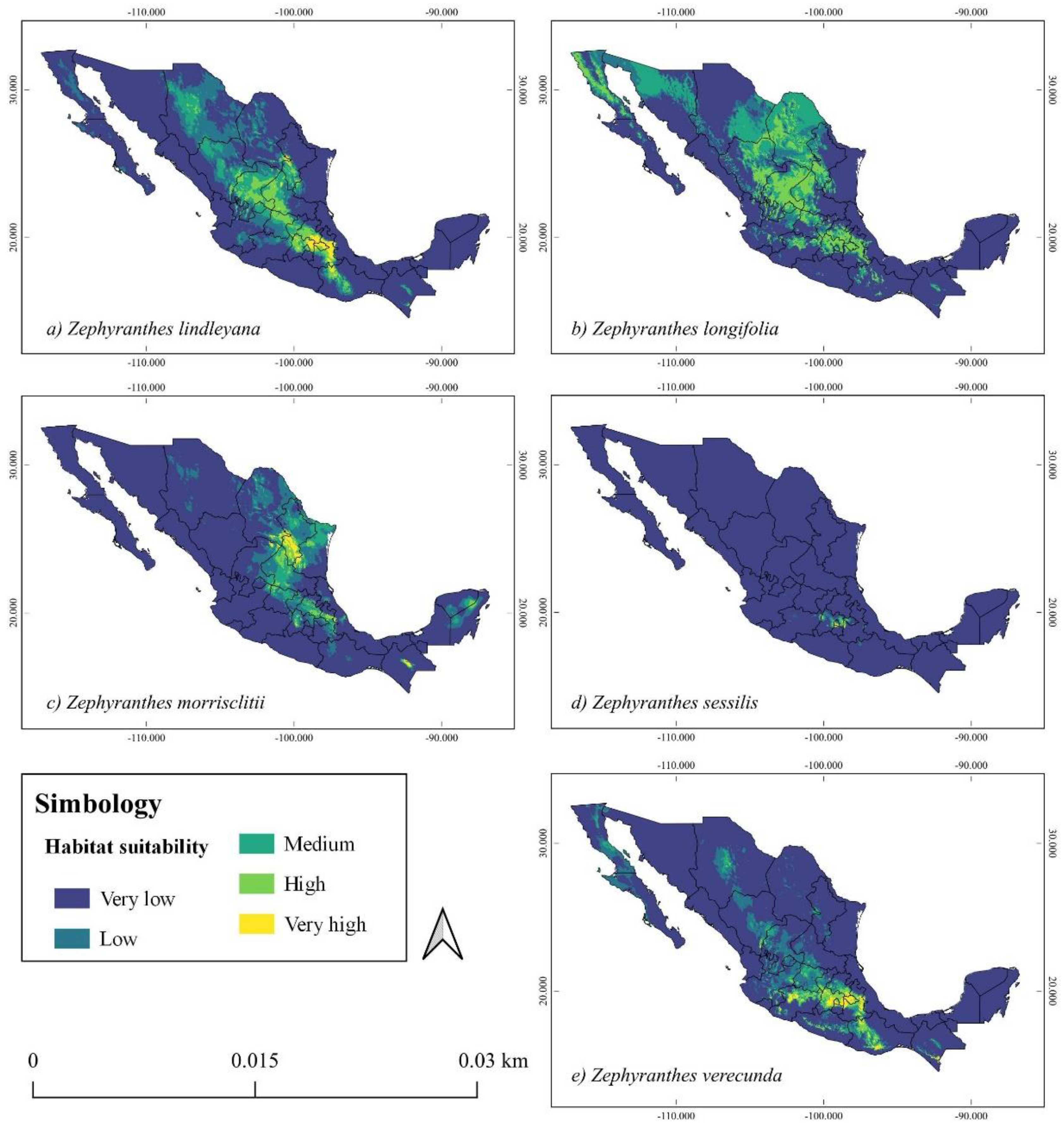
Zephyranthes lindleyana. The greatest potential distribution range for the species is in the Trans-Mexican Volcanic Belt in the states of Puebla, Tlaxcala, Hidalgo and Mexico. While the average potential distribution is along the Chihuahuan Desert province in the states of Chihuahua, Durango, Guanajuato, Querétaro, San Luis Potosí, Zacatecas and the Sierra Madre del Sur in the state of Oaxaca (
Figure 3a).
Zephyranthes longifolia. A high potential distribution was not found for this species, since the habitat suitability presents a medium potential distribution in the Chihuahuan Desert and Sierra Madre Oriental provinces, in the states of Aguascalientes, Coahuila, Chihuahua, Durango, San Luis Potosí, Zacatecas, Transmexican Volcanic Belt in the state of Mexico, Guanajuato, Puebla, Tlaxcala and in the Californian provinces in the states of Sonora, Baja California and Baja California Sur (
Figure 3b).
Zephyranthes morrisclintii. The high potential distribution of this species is mainly in the biogeographic provinces of the Chihuahuan Desert and Sierra Madre Oriental in the states of Nuevo León and Coahuila. A medium potential distribution in the provinces of Sonora, Sierra Madre Oriental, Transmexican Volcanic Belt in the states of Hidalgo, Mexico, Puebla, Queretaro, Sonora, San Luis Potosi and in the states of Quintana Roo and Yucatan in the province of the Yucatan Peninsula (
Figure 3c).
Zephyranthes sessilis. For this species, a more restricted potential distribution was found in the center of the Transmexican Volcanic Belt on the periphery of Mexico City in the states of Mexico, Morelos and Tlaxcala (
Figure 3d).
Zephyranthes verecunda. The high habitat suitability for this species is distributed in the states of Mexico, Tlaxcala, Puebla and Michoacan in the Transmexican Volcanic Belt and the Balsas Basin. The medium potential distribution was concentrated in the Balsas Basin, Sierra Madre del Sur, Sierra Madre Occidental and southern Chihuahuan Desert in the states of Oaxaca, Guerrero, Chihuahua, Durango, Guanajuato, Jalisco and Zacatecas (
Figure 3e).
Figure 3.
Potential distribution of five species of the genus Zephyranthes. a) Z. lindleyana, b) Z. longifolia, c) Z. morrisclintii, d) Z. sessilis, e) Z. verecunda.
Figure 3.
Potential distribution of five species of the genus Zephyranthes. a) Z. lindleyana, b) Z. longifolia, c) Z. morrisclintii, d) Z. sessilis, e) Z. verecunda.
4. Discussion
The genus Zephyranthes is distributed in both xerophytic and flooded environments (Knox, 2009), where temporal conditions of temperature and water availability are limiting factors. This is congruent with the environmental variables with greater weight in the generated models, since it was identified that the mean and maximum temperature of the warmest (BIO5, BIO10) and wettest (BIO8, BIO12) seasons, as well as the mean annual precipitation (BIO12) are determinants for the environmental suitability of Zephyranthes.
It has been reported that rainfall and temperature are fundamental for the reproduction of Zephyranthes, since flowering occurs after the first rains (Knox, 2009). The duration of flowers produced will be affected by both temperature and sun exposure (Tapia-Campos et al., 2012; Argueta-Guzmán et al., 2013). This implies that precipitation promotes the onset of the reproductive cycle, and temperature is related to the time available for pollination in Zephyranthes. This is reflected in the results obtained for the niche models of the species studied here.
There is other research on the ecological niche of Amaryllidaceae species. In these studies, it has been reported that temporal conditions of temperature and precipitation are the main abiotic factors that determine the suitability for Crinum malabaricum and Phaedranassa brevifolia (Oleas et al., 2014; Pulparambiland & Nediyaparambu, 2023). This coincides with what was reported in this research; future work on other taxa of Amaryllidaceae will allow us to determine if there is a pattern with respect to the variables with greater weight in the ecological niche, in the components of the family. Most of the species are distributed and have a greater probability of environmental suitability in the center and south of the country. This can be explained by the evolutionary origin of the genus; through phylogenetic analysis, it has been determined that Zephyranthes originated in South America and dispersed to Mexico through Central America (Meerow et al., 2000). This would explain the neotropical affinity of the Zephyranthes species studied. There are also species such as Z. longifolia and Z. morrisclintii that have dispersed to the north of the country. The most important variables for the niche models of these species are different from those relevant for species with neotropical affinity. This may reflect a niche differentiation between widely distributed species and those with more restricted geographic ranges. Morphological, phylogeographic and niche conservatism analyses will reveal whether these differences in the fundamental niche of Zephyranthes species in Mexico are informative at the interspecific level.
Temporal and maximum values of temperature and humidity, as well as annual precipitation, are the most important for the fundamental niche of Zephyranthes species distributed in the national territory. Both the known and potential distribution are congruent with the phylogenetic origin of the genus. However, there are species of the genus with a wide distribution, whose niche models revealed differences with respect to those restricted to central and southern Mexico. The models generated will allow the establishment of sampling strategies to identify new populations of Zephyranthes species, which will contribute to increase the number of available records of occurrence. This will allow making morphological, physiological, genetic and ecological comparisons between populations and species of Zephyranthes, which will integrate basic knowledge about this genus in Mexico.
5. Conclusions
In this study, the bioclimatic variables that influence the distribution of each of the 11 species of the genus Zephyranthes were obtained. The models generated with MaxEnt obtained an AUC value greater than 0.89, allowing prediction of the potential distribution of each of the species in Mexico. The bioclimatic variables with the greatest contribution as predictors to the distribution models were the average maximum temperature of the warmest period, average temperature of the rainiest four-month period, average temperature of the warmest four-month period and annual precipitation, which, according to the dimensionality reduction analysis (PCA), explained more than 80% of the accumulated variance.
Zephyranthes concolor, Z. fosteri, Z. lindleyana, Z. longifolia, Z. morrisclintii and Z. verecunda have a greater distribution in the north and center of the country. Meanwhile, Z. brevipes, Z. carinata, Z. citrina, Z. clintiae, and Z. sessilis have a variable distribution (from high to medium-low) in central and southern Mexico. The 169 records obtained and the analyses previously carried out for this work allowed us to know precisely the habitat in which the 11 species of the genus Zephyranthes studied develop, as well as the possible areas in which, with optimal conditions, they can be distributed.
The modeling of potential distribution has been one of the most important methods that have made it possible to know, study and conserve species, as well as areas of importance for the preservation of biological diversity. The studied species, endemic to Mexico, represent a little of the great diversity that exists in the country, however, little information is known. This work represents the importance of generating knowledge, in order to lay the foundations and provide a guideline for subsequent studies in which the potential of these and other taxa is made known.
Author Contributions
Z.E.R.F., designed the project, consulted and cleaned the presence data, interpreted the results, drafted the document. Y.L.F.P., designed the project, identified the study species, drafted the document. Y.M.R., consulted and cleaned the presence data, performed the statistical analysis to avoid collinearity of the environmental data, interpreted the results, drafted the document. J.A.R.V., performed statistical analysis to avoid collinearity of environmental data, interpreted the results, drafted the document.
Funding
Consejo Mexiquense de Ciencia y Tecnologia (COMECyT).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The main author is grateful to the Consejo Mexiquense de Ciencia y Tecnologia (COMECyT) for the grant awarded (folio ESYCA2023-1-4244) for a research stay under the program Investigadores e Investigadoras COMECyT EDOMÉX.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abdelaal, M., Fois, M., Fenu, G., & Bacchetta, G. (2019). Using MaxEnt modeling to predict the potential distribution of the endemic plant Rosa arabica Crép. in Egypt. Ecological Informatics, 50, 68–75. [CrossRef]
- Argueta-Guzmán Magda, Barrales-Alcalá Diego, Galicia-Pérez Aldanelly, Golubov Jordan & Mandujano María C. (2013). Sistema reproductivo y visitantes florales de Zephyranthes carinata Herb (Asparagales: Amaryllidaceae). Cact Suc Mex, 58(4):100-117.
- Ates, M. T., Yildirim, A. B., & Turker, A. U. (2021). Enhancement of alkaloid content (galanthamine and lycorine) and antioxidant activities (enzymatic and non-enzymatic) unders salt stress in summer snowflake (Leucojum aestivum L.). South African Journal of Botany, 140, 182–188. [CrossRef]
- Austin, M. P. (2002). Spatial prediction of species distribution: An interface between ecological theory and statistical modelling. Ecological Modelling, 157(2–3), 101–118. [CrossRef]
- Beaumont, L. J., Hughes, L., & Poulsen, M. (2005). Predicting species distributions: Use of climatic parameters in BIOCLIM and its impact on predictions of species’ current and future distributions. Ecological Modelling, 186(2), 251–270. [CrossRef]
- Carnevali, G., Duno, R., Tapia, J. L., & Ramírez, I. M. (2010). Reassessment of Zephyranthes (Amaryllidaceae) in the Yucatán Peninsula including a new species, Z. orellanae. 137(1), 39-48. [CrossRef]
- Centeno-Betanzos, L. Y., Reyes-Chilpa, R., Pigni, N. B., Jankowski, C. K., Torras-Claveria, L., & Bastida, J. (2021). Plants of the ‘Libellus de Medicinalibus Indorum Herbis’ from Mexico, 1552. Zephyranthes fosteri (Amaryllidaceae) Alkaloids. Chemistry & Biodiversity, 18(3), e2000834. [CrossRef]
- Cruz-Cárdenas, G., López-Mata, L., Villaseñor, J. L., & Ortiz, E. (2014). Potential species distribution modeling and the use of principal component analysis as predictor variables. Revista Mexicana de Biodiversidad, 85(1), 189–199. [CrossRef]
- Elith, J., Phillips, S. J., Hastie, T., Dudík, M., Chee, Y. E., & Yates, C. J. (2011). A statistical explanation of MaxEnt for ecologists. Diversity and Distributions, 17(1), 43–57. [CrossRef]
- Espejo-Serna, A., & López-Ferrari, A. R. (2018). La familia Bromeliaceae en México. Botanical Sciences, 96(3), 533–554. [CrossRef]
- Flagg, R. O., Smith, G. L., & García-Mendoza, A. J. (2018). Zephyranthes pseudoconcolor (Amaryllidaceae: Amaryllidoideae), a New Species from Mexico, and Clarification of Z. concolor. Novon: A Journal for Botanical Nomenclature, 26(3), 290–297. [CrossRef]
- Franklin, J. (2013). Species distribution models in conservation biogeography: Developments and challenges. Diversity and Distributions, 19(10), 1217–1223. [CrossRef]
- Hufnagel, L., & Mics, F. (2021). Introductory Chapter: Biodiversity of Mexico. En L. Hufnagel (Ed.), Natural History and Ecology of Mexico and Central America (Vol. 1, pp. 3–15). IntechOpen. [CrossRef]
- Jiménez, A. A., Marceleño Flores, S. M. L., González, O. N., & Vilchez, F. F. (2023). Potential Coffee Distribution in a Central-Western Region of Mexico. Ecologies, 4(2), 269–287. [CrossRef]
- Jolliffe, I. T. (2002). Choosing a Subset of Principal Components or Variables. En Principal Component Analysis (2a ed., Vol. 1, pp. 111–149). Springer New York, NY. [CrossRef]
- Knox, G W. (2009). Rainly, Zephyranthes and Habranthus spp.: Low maintenance flowering bulbs for Florida gardens. Environmental Horticulture Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences. University of Florida:Florida, USA. 12 pp.
- Kohelová, E., Maříková, J., Korábečný, J., Hulcová, D., Kučera, T., Jun, D., Chlebek, J., Jenčo, J., Šafratová, M., Hrabinová, M., Ritomská, A., Malaník, M., Peřinová, R., Breiterová, K., Kuneš, J., Nováková, L., Opletal, L., & Cahlíková, L. (2021). Alkaloids of Zephyranthes citrina (Amaryllidaceae) and their implication to Alzheimer’s disease: Isolation, structural elucidation and biological activity. Bioorganic Chemistry, 107. [CrossRef]
- Kulhánková, A., Cahlíková, L., Novák, Z., Macáková, K., Kuneš, J., & Opletal, L. (2013). Alkaloids from Zephyranthes robusta Baker and Their Acetylcholinesterase-and Butyrylcholinesterase-Inhibitory Activity. Chemistry & Biodiversity, 10(2013), 1120–1127. [CrossRef]
- Leszczyñska-Borys, H., Borys, M. W., & Espejo Serna, A. (2000). MEXICAN GEOPHYTES - BIODIVERSITY, CONSERVATION AND HORTICULTURAL APLICATION. Acta Horticulturae, 523(523), 205–210. [CrossRef]
- Li, L., Wu, H., Gao, Y., & Zhang, S. (2023). Predicting Ecologically Suitable Areas of Cotton Cultivation Using the MaxEnt Model in Xinjiang, China. Ecologies, 4(4), 654–670. [CrossRef]
- López-Sandoval, J. A., López-Mata, L., Cruz-Cárdenas, G., Vibrans, H., Vargas, O., & Martínez, M. (2015). Modelado de los factores ambientales que determinan la distribución de especies sinantrópicas de Physalis. Botanical Sciences, 93(4), 755–764. [CrossRef]
- Luo, Z., Wang, F., Zhang, J., Li, X., Zhang, M., Hao, X., Xue, Y., Li, Y., Horgen, F. D., Yao, G., & Zhang, Y. (2012). Cytotoxic alkaloids from the whole plants of zephyranthes candida. Journal of Natural Products, 75(12), 2113–2120. [CrossRef]
- Martínez-Méndez, N., Aguirre-Planter, E., Eguiarte, L. E., & Jaramillo-Correa, J. P. (2016). Modelado de nicho ecológico de las especies del género Abies (pinaceae) en México: Algunas implicaciones taxonómicas y para la conservación. Botanical Sciences, 94(1), 362–371. [CrossRef]
- Meerow, A. W., Guy, C. L., Li, Q.-B., & Yang, S.-L. (2000). Phylogeny of the American Amaryllidaceae Based on nrDNA ITS Sequences. Systematic Botany, 25(4), 708. [CrossRef]
- Morrone, J. J., Escalante, T., & Rodríguez-Tapia, G. (2017). Mexican biogeographic provinces: Map and shapefiles. En Zootaxa (Vol. 4277, Número 2, pp. 277–279). Magnolia Press. [CrossRef]
- Mota-Vargas, C., Luévano-Encarnación, A., Ortega-Andrade, H. M., & Prieto-Torres, D. A. (2019). Una breve introducción a los modelos de nicho ecológico. En C. Moreno (Ed.), La biodiversidad en un mundo cambiante: Fundamentos teóricos y metodológicos para su estudio (1a ed., Vol. 1, pp. 39–63). Universidad Autónoma del Estado de Hidalgo. https://www.researchgate.net/publication/339181920.
- Murillo-Pérez, G., Rodríguez, A., Sánchez-Carbajal, D., Ruiz-Sanchez, E., Carrillo-Reyes, P., & Munguía-Lino, G. (2022). Spatial distribution of species richness and endemism of Solanum(Solanaceae) in Mexico. Phytotaxa, 558(2), 147–177. [CrossRef]
- Nair, J. J., & van Staden, J. (2023). Antiviral alkaloid principles of the plant family Amaryllidaceae. Phytomedicine, 108, 154480. [CrossRef]
- Nora H. Oleas 1,2,3*, Alan W. Meerow 4, Kenneth J. Feeley 1,3, Jennifer Gebelein 5 and Javier FranciscoOrtega. (2014). Using species distribution models as a tool to discover new populations of Phaedranassa brevifolia Meerow, 1987 (Liliopsida: Amaryllidaceae) in Northern Ecuador. [CrossRef]
- Palma-Ordaz, S., & Delgadillo-Rodríguez, Y. J. (2014). Distribución pontencial de ocho especies exóticas de carácter invasor en el estado de Baja California, México. Botanical Sciences, 92(4), 587–597.
- Peterson, A. T., Pearson, R. G., Anderson, R. P., Martínez-Meyer, E., Nakamura, M., & Araújo, M. B. (2011). Ecological Niches and Geographic Distributions. Princeton University Press. [CrossRef]
- Phillips, S. J., & Dudík, M. (2008). Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography, 31(2), 161–175. [CrossRef]
- Pulparambil, H., & Pradeep, N. S. (2023). Ecological niche modelling in identifying ábitats for effective species conservation: A study on Endemic aquatic plant Crinum malabaricum. Journal for Nature Conservation, 76, 126517. [CrossRef]
- Qazi, A. W., Saqib, Z., & Zaman-ul-Haq, M. (2022). Trends in species distribution modelling in context of rare and endemic plants: a systematic review. Ecological Processes, 11(1), 1–11. [CrossRef]
- R Core Team, R.A. Lang, Environ. Stat. Comput, R Found. Stat. Comput., Vienna, Austria, 2018. URL. https://www.r-project.org/.
- Rodríguez, P., & López-Toledo, L. (2016). Modeling the distribution of Mexican plants: Knowledge and challenges in the face of biodiversity loss. En Botanical Sciences (Vol. 94, Número 1). Sociedad Botanica de Mexico, A.C. [CrossRef]
- Rodríguez-Flores, Z. E., Fernández-Pavía, Y. L., García-Cué, J., Cruz-Torres, E. D. la, & Jaen-Contreras, D. (2023). Evaluation of the application of fertilizers and biostimulants in Zephyranthes lindleyana Herb (Amarylidaceae) under greenhouse conditions. Agro Productividad. [CrossRef]
- Soberón, J. M. (2010). Niche and area of distribution modeling: A population ecology perspective. Ecography, 33(1), 159–167. [CrossRef]
- Solís-Montero, L., Vega-Polanco, M., Vázquez-Sánchez, M., & Suárez-Mota, M. E. (2022). Ecological niche modeling of interactions in a buzz-pollinated invasive weed. Global Ecology and Conservation, 39. [CrossRef]
- Suárez-Mota, M. E., Ramírez, J. M. H., Bautista, L. L., Díaz, M. M. M., Santiago-García, W., & Ruiz-Aquino, F. (2022). Potential distribution of riparian trees in the Bajo Río Grijalva sub basin. Botanical Sciences, 100(3), 534–549. [CrossRef]
- Tapia, E. C., Rodriguez, J. M. D., De Los Milagros Revuelta, M. A., Van, J. M. T., & Gónzalez, R. B. (2012). Mexican geophytes II. The genera Hymenocallis, Sprekelia and Zephyranthes. Floriculture and Ornamental Biotechnology, 6(1), 129–139.
- Tapia-Campos, E., Rodriguez-Dominguez, J. M., Quiñones-Aguilar, E. E., Dupre, P., & Barba-Gonzalez, R. (2016). Molecular cytogenetic characterization of wild mexican geophytes. Acta Horticulturae, 1000, 499–504. [CrossRef]
- Tapia-Campos, E., Rodríguez-Domínguez, JM., Revuelta-Arreola, M.M., Van-Tuyl, J., Barba-González, R. 2012. Mexican Geophytes II. The Genera Hymenocallis, Sprekelia and Zephyranthes. Floriculture and Ornamental Biotech 6(1): 129-138.
- Villaseñor, J. L. (2016). Checklist of the native vascular plants of Mexico. Revista Mexicana de Biodiversidad, 87(3), 559–902. [CrossRef]
- Villaseñor, J. L. (2018). Diversidad y distribucion de la familia Asteraceae en Mexico. Botanical Sciences, 96(2), 332–358. [CrossRef]
- Villaseñor, J. L., Ortiz, E., & Hernández-Flores, M. M. (2023). THE VASCULAR PLANT SPECIES ENDEMIC OR NEARLY ENDEMIC TO PUEBLA, MEXICO. Botanical Sciences, 101(4), 1207–1221. [CrossRef]
- Wang, H. Y., Qu, S. M., Wang, Y., & Wang, H. T. (2018). Cytotoxic and anti-inflammatory active plicamine alkaloids from Zephyranthes grandiflora. Fitoterapia, 130(10), 163–168. [CrossRef]
- Ye, P., Zhang, G., Zhao, X., Chen, H., Si, Q., & Wu, J. (2021). Potential geographical distribution and environmental explanations of rare and endangered plant species through combined modeling: A case study of Northwest Yunnan, China. Ecology and Evolution, 11(19), 13052–13067. [CrossRef]
- Yun, H.-G., Lee, J.-W., An, J.-B., Yu, S.-B., Bak, G.-P., Shin, H.-T., Park, W.-G., & Kim, S.-J. (2022). Prediction of Potential Habitat and Damage Amount of Rare·Endemic Plants (Sophora Koreensis Nakai) Using NBR and MaxEnt Model Analysis - For the Forest Fire Area of Bibongsan (Mt.) in Yanggu -. Korean Journal of Plant Resources 2022 35:2, 35(2), 169–182. [CrossRef]
- Zhan, G., Liu, J., Zhou, J., Sun, B., Aisa, H. A., & Yao, G. (2017). Amaryllidaceae alkaloids with new framework types from Zephyranthes candida as potent acetylcholinesterase inhibitors. European Journal of Medicinal Chemistry, 127, 771–780. [CrossRef]
- Zhan, G., Zhou, J., Liu, J., Huang, J., Zhang, H., Liu, R., & Yao, G. (2017). Acetylcholinesterase Inhibitory Alkaloids from the Whole Plants of Zephyranthes carinata. Journal of Natural Products, 80(9), 2462–2471. [CrossRef]
- Zhan, G., Zhou, J., Liu, R., Liu, T., Guo, G., Wang, J., Xiang, M., Xue, Y., Luo, Z., Zhang, Y., & Yao, G. (2016). Galanthamine, Plicamine, and Secoplicamine Alkaloids from Zephyranthes candida and Their Anti-acetylcholinesterase and Anti-inflammatory Activities. Journal of Natural Products, 79(4), 760–766. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).