Submitted:
12 June 2024
Posted:
13 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Tackling AMR in Aquaculture
4. Antimicrobials Use in Aquaculture
5. AMR Emergence, Transfer and Dissemination in Aquaculture
6. Clinical Breakpoints and Epidemiological Cut-Off Values
7. Detection Methods for AMR
8. Alternatives to Antimicrobial Treatment in Aquaculture
9. Risk Mitigation Strategies for AMR in Aquaculture
10. One Health to Tackle AMR in Aquaculture
11. Conclusions
List of Abbreviations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Iwu, C. D.; Korsten, L.; Okoh, A. The incidence of antibiotic resistance within and beyond the agricultural ecosystem: A concern for public health. Microbiology open 2020, 9(9), 1035. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antimicrobial resistance. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 6 March 2024).
- Zdolec, N.; Veskovic-Moracanin, S.; Filipovic, I.; Dobranic, V. Antimicrobial Resistance of Lactic Acid Bacteria in Fermented Meat Products. In Zdolec N. editor. Fermented Meat Products: Health Aspects. CRC Press. Taylor & Francis Group 2016, 316-339.
- Naghavi, M. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- OECD-European Commission Directorate-General for Health and Food Safety. A European One Health Action Plan against Antimicrobial Resistance (AMR). 2017. Available online: https://health.ec.europa.eu/system/files/2020-01/amr_2017_action-plan_0.pdf (accessed on 12 March 2024).
- OECD/WHO. Addressing the burden of infections and antimicrobial resistance associated with health care. 2022. Available online: https://www.oecd.org/health/Addressing-burden-of-infections-and-AMR-associated-with-health-care.pdf (accessed on 12 March 2024).
- Jasovský, D.; Littmann, J.; Zorzet, A.; Cars, O. Antimicrobial resistance-a threat to the world’s sustainable development. Ups. J. Med. Sci. 2016, 121(3), 159–164. [Google Scholar] [CrossRef]
- FAO. Report of the FAO expert working group meeting “Scoping exercise to increase the understanding of risks of antimicrobial resistance (AMR) in aquaculture. Palermo, Italy. Report No.: FIAA/R1268 (En). 2018. Available online: https://www.fao.org/3/ca7442en/CA7442EN.pdf (accessed on 15 February 2024).
- Alhaji, N. B.; Maikai B., V.; Kwaga, J. Antimicrobial use, residue and resistance dissemination in freshwater fish farms of north-central Nigeria: One health implications. Food Control. 2021, 130, 108–238. [Google Scholar] [CrossRef]
- World Bank. Drug-Resistant Infections: A Threat to Our Economic Future. Washington DC. 2017. Available online: https://documents1.worldbank.org/curated/en/323311493396993758/pdf/final-report.pdf (accessed on 18 February 2024).
- Deng, Y.; Xu, L.; Chen, H.; Liu, S.; Guo, Z.; Cheng, C.; Ma, H.; Feng, J. Prevalence, virulence genes, and antimicrobial resistance of Vibrio species isolated from diseased marine fish in South China. Scientific Reports. 2020, 10, 14329. [Google Scholar] [CrossRef]
- Woo, S. J.; Kim, M. S.; Jeong, M. G.; Do, M. Y.; Hwang, S. D.; Kim, W. J. Establishment of Epidemiological Cut-Off Values and the Distribution of Resistance Genes in Aeromonas hydrophila and Aeromonas veronii Isolated from Aquatic Animals. Antibiotics (Basel). 2022, 11(3), 343. [Google Scholar] [CrossRef]
- Oviedo-Bolaños, K.; Rodríguez-Rodríguez, J. A.; Sancho-Blanco, C.; Barquero-Chanto, J. E.; Pena-Navarro, N.; Escobedo-Bonilla, C. M.; Umana-Castro, R. Molecular identification of Streptococcus sp. and antibiotic resistance genes present in Tilapia farms (Oreochromis niloticus) from the Northern Pacific region, Costa Rica. Aquacult Int. 2021, 29, 2337–2355. [Google Scholar] [CrossRef]
- Algammal, A. M.; Mabrok, M.; Ezzat, M.; Alfifi, K. J.; Esawy, A. M.; Elmasry, N.; El-Tarabili, R. M. Prevalence, antimicrobial resistance (AMR) pattern, virulence determinant and AMR genes of emerging multi-drug resistant Edwardsiella tarda in Nile tilapia and African catfish. Aquaculture. 2022, 548, Part 1, 737643. [Google Scholar] [CrossRef]
- Schar, D.; Zhao, C.; Wang, Y.; Larsson, D. G. J. ; Gilbert, M; van Boeckel, T. P. Twenty-year trends in antimicrobial resistance from aquaculture and fisheries in Asia. Nat. Commun. 2021, 12(1), 5384. [CrossRef]
- Caputo, A.; Bondad-Reantaso, M. G.; Karunasagar, I.; Hao, B.; Gaunt, P.; Verner-Jeffreys, D.; Fridman, S.; Dorado-Garcia, A. Antimicrobial resistance in aquaculture: A global analysis of literature and national action plans. Rev Aquac. 2022, 15, 568–578. [Google Scholar] [CrossRef]
- Bobate, S.; Mahalle, S.; Dafale, N. A.; Bajaj, A. Emergence of environmental antibiotic resistance: Mechanism, monitoring and management. Environ. Adv. 2023, 13, 100409. [Google Scholar] [CrossRef]
- EU. Ban on antibiotics as growth promoters in animal feed enters into effect. Report No.:IP/05/1687. 2005. Available online: https://ec.europa.eu/commission/presscorner/detail/en/IP_05_1687 (accessed on 18 February 2024).
- EU. Regulation (EU) 2019/6 of the European Parliament and of the council of 11 December 2018 on veterinary medicinal products and repealing Directive 2001/82/EC. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R0006 (accessed on 8 March 2024).
- Kirchnelle, C. Swann Song. British antibiotic regulation in livestock production (1953-2006). Bull Hist Med. [CrossRef]
- Klein, E.; van Boeckel, T. P.; Martinez, E. M.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. PNAS. [CrossRef]
- Thornber, K.; Verner-Jeffreys, V.; Hincliffe, S.; Rahman, M. M.; Bass, D.; Tyler, C. R. Evaluating antimicrobial resistance in the global shrimp industry. Rev Aquac. 2020, 12, 966–986. [Google Scholar] [CrossRef] [PubMed]
- van Boeckel, T. P.; Brower, C.; Gilbert, M.; Grenfel, B. T.; Levin, S. A.; Robinson, T. P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. U.S.A. 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Singh, A.; Dar, M. A.; Kaur, R. J.; Charan, J.; Iskandar, K.; Haque, M.; Murti, K.; Ravichandiran, V.; Dhingra, S. Menace of antimicrobial resistance in LMICs: Current surveillance practices and control measures to tackle hostility. J. Infect. Public Health. 2022, 15(2), 172–181. [Google Scholar] [CrossRef] [PubMed]
- Brunton, L. A.; Desbois, A. P.; Garza, M.; Wieland, B.; Mohan, C. V.; Häsler, B.; Tam, C. C.; Le, P. N. T.; Phuong, N. T.; Van, P. T.; et al. Identifying hotspots for antibiotic resistance emergence and selection, and elucidating pathways to human exposure: application of a systems thinking approach to aquaculture systems. Sci. Total Environ. 2019, 687, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, P.; Rico, A.; Troell, M.; Klinger, D.; Buschmann, A.; Saksida, S.; Chadag, M.; Zhang, W. Unpacking factors influencing antimicrobial use in global aquaculture and their implication for management: a review from a systems perspective. Sustain. Sci. 2018, 13, 1105–1120. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Ramos, F. Antimicrobial resistance in aquaculture: Current knowledge and alternatives to tackle the problem. Int. J. Antimicrob. Agents. 2018, 52(2), 135–143. [Google Scholar] [CrossRef] [PubMed]
- Williams-Nguyen, J.; Sallach, J. B.; Bartelt-Hunt, S.; Boxall, A. B.; Durso, L. M.; McLain, J. E.; Singer, R. S.; Snow, D.; Zilles, J. L. Antibiotics and Antibiotic Resistance in Agroecosystems: State of the Science. J. Environ. Qual. 2016, 45, 394–406. [Google Scholar] [CrossRef]
- Government of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2006 Guelph. In: Public Health Agency of Canada. 2009. CIPARS_annualReport_e.indd (publications.gc.ca). (accessed on 18 January 2024).
- Kim, J. J.; Seong, H. J.; Johnson, T. A.; Cha, C. J.; Sul, W. J.; Chae, J. C. Persistence of antibiotic resistance from animal agricultural effluents to surface water revealed by genome-centric metagenomics. J. Hazard. Mater. 2023, 457. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yuan, Q.; Mathieu, J.; Stadler, L. B.; Senehi, N. L.; Sun, R.; Alvarez, P. J. J. Antibiotic resistance genes from livestock waste: occurrence, dissemination, and treatment. npj Clean Water. 2020, 3(4). 3. [CrossRef]
- Nastasijevic, I.; Proscia, F.; Jurica, K.; Veskovic-Moracanin, S. Tracking Antimicrobial Resistance Along the Meat Chain: One Health Context. Food Rev. Int. 2023. [CrossRef]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T. P.; van Boeckel, T. P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics (Basel). 2020, 9(12), 918. [Google Scholar] [CrossRef]
- Garza, M.; Mohan, C. V.; Brunton, L.; Wieland, B.; Hasler, B. Typology of interventions for antimicrobial use and antimicrobial resistance in aquaculture systems in low- and middle-income countries. Int. J. Antimicrob. Agents. 2022, 59. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Rheman, S.; Debnath, N.; Delamare-Deboutteville, J.; Akhtar, Z.; Ghosh, S.; Parveen, S.; Islam, K.; Islam, A.; Rashid, M.; Khan, Z. H.; Rahman, M.; Chadag, V. M.; Chowdhury, F. Antibiotics usage practices in aquaculture in Bangladesh and their associated factors. One Health. 2022, 15, 100445. [Google Scholar] [CrossRef] [PubMed]
- Cabello, F. C.; Godfrey, H. P.; Buschmann, A. H.; Dölz, H. J. Aquaculture as yet another environmental gateway to the development and globalisation of antimicrobial resistance, Lancet Infect. Dis. 2016, 16(127), 33. [Google Scholar] [CrossRef]
- Cabello, F. C.; Godfrey, H. P.; Tomova, A.; Ivanova, L.; Dölz, H.; Millanao, A.; Buschmann, A. H. Antimicrobial use in aquaculture re-examined: its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef] [PubMed]
- Miller, R. A.; Harbottle, H. Antimicrobial Drug Resistance in Fish Pathogens. Microbiol. Spectr. [CrossRef]
- Schar, D.; Klein, E. Y.; Laxminarayan, R.; Gilbert, M.; Van Boeckel, T. Global trends in antimicrobial use in aquaculture. Sci. Rep. 2020, 10, 21878. [Google Scholar] [CrossRef]
- Reed, T. A. N.; Krang, S.; Miliya, T.; Townell, N.; Letchford, J.; Bun, S.; Sar, B.; Osbjer, K.; Seng, S.; Chou, M.; et al. Antimicrobial resistance in Cambodia: a review. Int. J. of Infect. Dis. 2019, 85, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Choi, S.; Lee, M.; Shin, J.; Son, H.; Wang, J.; Kim, Y. M. Spatial and temporal effects of fish feed on antibiotic resistance in coastal aquaculture farms. Environ. Res. 2022, 212, Part A, 113177. [Google Scholar] [CrossRef]
- Osman, K. M.; da Silva Pires, A.; Franco, O. L.; Saad, A.; Hamed, M.; Naim, H.; Ali, A. H. M.; Elbehiry, A. Nile tilapia (Oreochromis niloticus) as an aquatic vector for Pseudomonas species of medical importance: Antibiotic Resistance Association with Biofilm Formation, Quorum Sensing and Virulence. Aquaculture, 2021, 532, 736068. [Google Scholar] [CrossRef]
- Girijan, S. K.; Paul, R.; Kumar V. J., R.; Pillai, D. Investigating the impact of hospital antibiotic usage on aquatic environment and aquaculture systems: A molecular study of quinolone resistance in Escherichia coli. Sci. Total Environ. 2020, 748, 141538. [Google Scholar] [CrossRef]
- Yang, J. -T. ; Xiao, D. -Y.; Zhang, L. -J.; Chen, H. -X.; Zheng, X. -R.; Xu, X. -L.; Jiang, H. -X. Antimicrobial resistome during the transition from an integrated to a monoculture aquaculture farm in southern China. Sci. Total Environ. 2023, 882, 163511. [Google Scholar] [CrossRef]
- Jones, D. C.; LaMartina, E. L.; Lewis, J. R.; Dahl, A. J.; Nadig, N.; Szabo, A.; Newton, R. J.; Skwor, T. A. One Health and Global Health View of Antimicrobial Susceptibility through the “Eye” of Aeromonas: Systematic Review and Meta-Analysis. Int. J. Antimicrob. Agents. 2023, 62(2), 106848. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Kaur, K.; Barathe, P.; Shriram, V.; Govarthanan, M.; Kumar, V. Antimicrobial resistance in urban river ecosystems. Microbiol. Res. 2022, 263, 127135. [Google Scholar] [CrossRef] [PubMed]
- Bell, A. G.; Thornber, K.; Chaput, D. L.; Hasan, N. A.; Alam, M. M.; Haque, M. M.; Cable, J.; Temperton, B.; Tyler, C. R. Metagenomic assessment of the diversity and ubiquity of antimicrobial resistance genes in Bangladeshi aquaculture ponds. Aquac. Rep. 2023, 29, 101462. [Google Scholar] [CrossRef]
- Kampouris, I.; Alygizakis, N.; Klümper, U.; Agrawal, S.; Lackner, S.; Cacace, D.; Kunze, S.; Thomaidis, N.; Slobdonik, J.; Berendonk, T. Elevated levels of antibiotic resistance in groundwater during treated wastewater irrigation associated with infiltration and accumulation of antibiotic residues. J. Hazard. Mater, 1: 423 Part B. [CrossRef]
- Romero, J.; Feijoo, C.; Navarrete, P. Antibiotics in Aquaculture – Use, Abuse and Alternatives. In: Carvalho E. editor. Health and Environment in Aquaculture, Intech. 2012. [CrossRef]
- Liu, X.; Steele, J.C.; Meng, X. Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: a review. Environ. Pollut. 2017, 223, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Lulijwa, R.; Rupia, E. J.; Alfaro, A. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: a review of the top 15 major producers. Rev Aquac. 2019, 12(2), 640–663. [Google Scholar] [CrossRef]
- Carrizo, J. C.; Griboff, J.; Bonansea, R. I.; Nimptsch, J.; Valdes, M. E.; Wunderlin, D. A.; Ame, M. V. Different antibiotic profiles in wild and farmed Chilean salmonids. Which is the maun source for antibiotic in fish? Sci. Total Environ. 2021, 800, 149–516. [Google Scholar] [CrossRef] [PubMed]
- WOAH. OIE List of Antimicrobials of Veterinary Importance. 2021. Available online: https://www.woah.org/app/uploads/2021/03/oie-list-antimicrobials.pdf (accessed on 10 March 2024.).
- WHO. Critically Important Antimicrobials for Human Medicine. 6th Revision 2018. Ranking of medically important antimicrobials for risk management of antimicrobial resistance due to non-human use. 2018. Available online: https://www.who.int/publications/i/item/9789241515528 (accessed on 11 December 2023).
- Zhang, Q. Z.; Li, X. M. Pharmacokinetics and residue elimination of 1108 oxytetracycline in grass carp, Ctenopharyngodon idellus. Aquaculture.
- Leal, J. F.; Santos, E. B. H.; Esteves, V. I. Oxytetracycline in intensive aquaculture: water quality during and after its administration, environmental fate, toxicity and bacterial resistance. Rev Aquac. 2019, 11, 1176–1194. [Google Scholar] [CrossRef]
- Navarrete, P.; Mardones, P.; Opazo, R.; Espejo, R.; Romero, J. Oxytetracycline treatment reduces bacterial diversity of intestinal microbiota of Atlantic salmon. J. Aquat. Anim. Health. 2008, 20(3), 177–83. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Samuelsen, O. B. Estimates, of the significance of out-washing of oxytetracycline from sediments under Atlantic salmon sea-cages. Aquaculture. [CrossRef]
- Almeida, S. A. A.; Heitor, A. M.; Montenegro, M. C. B. S. M.; Sales, M. G. F. Sulfadiazine-selective determination in aquaculture environment: Selective potentiometric transduction by neutral or charged ionophores. Talanta. 2011, 85(3), 1508–1516. [Google Scholar] [CrossRef]
- Pepi, M.; Focardi, S. Antibiotic-Resistant Bacteria in Aquaculture and Climate Change: A Challenge for Health in the Mediterranean Area. Int. J. Environ. Res. Public Health. 2021, 18(11), 5723. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Wang, B. -L.; Ma, P. -F.; Zhang, W. -X.; Nusrat, Z. S.; Ma, Z.; Zhang, Y.-Q.; Ying, L. Capabilities and mechanisms of microalgae on nutrients and florfenicol removing from marine aquaculture wastewater. J. Environ. Manage. 2022, 320, 115673. [Google Scholar] [CrossRef] [PubMed]
- Hektoen, H.; Berge, J. A.; Hormazabal, V.; Yndestad, M. Persistence of antibacterial agents in marine sediments. Aquaculture, 1995, 133, 175–184. [Google Scholar] [CrossRef]
- Stephen, J.; Mukherjee, S.; Lekshmi, M.; Kumar, S. H. Diseases and Antimicrobial Use in Aquaculture. In: Mothadaka, M. P.; Vaiyapuri, M.; Rao Badireddy, M.; Nagarajrao Ravishankar, C.; Bhatia, R.; Jena, J. (eds) Handbook on Antimicrobial Resistance. Springer, Singapore. 2023.
- Okocha, R. C.; Olatoye, I. O.; Adedeji, O. B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. [CrossRef]
- Varol, M.; Rasit, M. Environmental contaminants in fish species from a large dam reservoir and their potential risks to human health. Ecotoxicol. Environ. Saf. 2019, 169, 507–515. [Google Scholar] [CrossRef]
- Harwood, J.J. Molecular markers for identifying municipal, domestic and agricultural sources of organic matter in natural waters. Chemosphere. 2014, 95, 3–8. [Google Scholar] [CrossRef]
- Boxall, A. B. A.; Fogg, L. A.; Blackwell, P. A.; Kay, P.; Pemberton, E. J.; Croxford, A. Veterinary medicines in the environment. Rev. Environ. Contam. Toxicol. 2004, 180, 1–91. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T. P.; Bu, D. P.; Carrique-Mas, J.; Fèvre, E. M.; Gilbert, M.; Grace, D.; Hay, S. I.; Jiwakanon, J.; Kakkar, M.; Kariuki, S. Antibiotic resistance is the quintessential One Health issue. Antibiotic resistance is the quintessential One Health issue. Trans. R. Soc. Trop. Med. Hyg. 2016, 110(7), 377–380. [Google Scholar] [CrossRef] [PubMed]
- Yelin, I.; Kishony, R. Antibiotic Resistance. Cell. 1136. [Google Scholar] [CrossRef]
- Martinez, J. L. General principles of antibiotic resistance in bacteria. Drug Discov. Today Technol, 3: 11. [CrossRef]
- Arzanlou, M.; Chai, W. C.; Venter, H. Intrinsic, adaptive and acquired antimicrobial resistance in gram-negative bacteria. Essays Biochem. 2017, 61, 49–59. [Google Scholar] [CrossRef]
- Reygaert, W. C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4(3), 482–501. [Google Scholar] [CrossRef]
- Blair, J. M. A.; Webber, M. A.; Baylay, A. J.; Ogbolu, D. O.; Piddock, L. J. V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Cox, G.; Wright, G. D. Intrinsic antibiotic resistance: Mechanisms, origins, challenges and solutions. Int. J. Med. Microbiol. [CrossRef]
- Coculescu, B. I. Antimicrobial resistance induced by genetic changes. J Med Life. 2009, 2, 114–123. [Google Scholar]
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. [CrossRef]
- Berendonk, T.; Manaia, C.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M. N. Tackling antibiotic resistance: the environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Matthias, T.; Aminov, R. Potential effects of horizontal gene exchange in the human gut. Front. immunol. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- McInnes, R. S.; McCallum, G. E.; Lamberte, L. E.; van Schaik, W. Horizontal transfer of antibiotic resistance genes in the human gut microbiome. Curr. Opin. Microbiol. 2020, 53, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Lerminiaux, N. A.; Cameron, A. D. S. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019, 65(1), 34–44. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.; Kaur, P. Antibiotic resistance mechanisms in bacteria: relationships between resistance determinants of antibiotic producers, environmental bacteria, and clinical pathogens. Front. Microbiol. 2018, 9, 2928. [Google Scholar] [CrossRef]
- von Wintersdorff, C. J.; Penders, J.; van Niekerk, J. M.; Mills, N. D.; Majumder, S.; van Alphen, L. B.; Savelkoul, P. H.; Wolffs, P. F. Dissemination of Antimicrobial Resistance in Microbial Ecosystems through Horizontal Gene Transfer. Front. Microbiol. 2016, 19(7), 173. [Google Scholar] [CrossRef] [PubMed]
- Muziasari, W.; Pärnänen, K.; Johnson, T.; Lyra, C.; Karkman, A.; Stedtfeld, R.; Tamminen, M.; Tiedje, J.; Virta, M. Aquaculture changes the profile of antibiotic resistance and mobile genetic element associated genes in Baltic Sea sediments. FEMS Microbiol. Ecol. [CrossRef]
- Reverter, M.; Sarter, S.; Caruso, D.; Avarre, J. C.; Combe, M.; Pepey, E.; Pouyaud, L.; Vega-Heredía, S.; de Verdal, H.; Gozlan, R. Aquaculture at the crossroads of global warming and antimicrobial resistance. Nat. Commun. 2020, 11, 1870. [Google Scholar] [CrossRef] [PubMed]
- Bielen, A.; Šimatović, A.; Kosić-Vukšić, J.; Senta, I.; Ahel, M.; Babić, S.; Jurina, T.; Plaza, J. J. G.; Milaković, M.; Udiković-Kolić, N. Negative environmental impacts of antibiotic-contaminated effluents from pharmaceutical industries. Water Res. 2017, 126, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Hasan, J. , Rahman, H.; Ullah, R.; Mredul, M. H. Availability of aqua drugs and their uses in semi intensive culture farms at Patuakhali district in Bangladesh. H. Availability of aqua drugs and their uses in semi intensive culture farms at Patuakhali district in Bangladesh. Arch. agric. environ. sci. 2020, 5(3), 368–376. [Google Scholar] [CrossRef]
- Suyamud, B.; Chen, Y.; Quyen, D. T. T.; Dong, Z.; Zhao, C.; Hu, J. Antimicrobial resistance in aquaculture: Occurrence and strategies in Southeast Asia. Sci. Total Environ. 2024, 907, 167942. [Google Scholar] [CrossRef]
- Vilca, F. Z.; Galarza, N. C.; Tejedo, J. R.; Cuba, W. A. Z.; Quiróz, C. N. C.; Tornisielo, V. L. Occurrence of residues of veterinary antibiotics in water, sediment and trout tissue (Oncorhynchus mykiss) in the southern area of Lake Titicaca, Peru. J. Great Lakes Res. 2021, 47(4), 1219–1227. [Google Scholar] [CrossRef]
- Chen, J.; Li, W.; Zhang, J.; Qi, W.; Li, Y.; Chen, S.; Zhou, W. Prevalence of antibiotic resistance genes in drinking water and biofilms: The correlation with the microbial community and opportunistic pathogens. Chemosphere. 2020, 259, 127483. [Google Scholar] [CrossRef] [PubMed]
- Lécuyer, F.; Bourassa, J. S.; Gélinas, M.; Charron-Lamoureux, V.; Burrus, V.; Beauregard, P. Biofilm Formation Drives Transfer of the Conjugative Element ICEBs1 in Bacillus subtilis. Appl. Environ. Sci. [CrossRef]
- Abe, K.; Nomura, N.; Suzuki, S. Biofilms: hot spots of horizontal gene transfer (HGT) in aquatic environments, with a focus on a new HGT mechanism. FEMS Microbiol. Ecol. [CrossRef]
- Ge, Z.; Ma, Z.; Zou, J.; Zhang, Y.; Li, Y.; Zhang, L.; Zhang, J. Purification of aquaculture wastewater by macrophytes and biofilm systems: Efficient removal of trace antibiotics and enrichment of antibiotic resistance genes. Sci. Total Environ. 2023, 901, 165943. [Google Scholar] [CrossRef] [PubMed]
- Kampouris, I. D.; Klümper, U.; Kramer, L.; Sorum, H.; Wedekind, H.; Berendonk, T. U. Dissemination of antibiotic resistance in antibiotic-free recirculating aquaculture systems. J. Hazard. Mater. Adv. 2022, 8, 100201. [Google Scholar] [CrossRef]
- Casciaro, B.; Dutta, D.; Loffredo, M. R.; Marcheggiani, S.; McDermott, A. M.; Willcox, M. D.; Mangoni, M. L. Esculentin-1a derived peptides kill Pseudomonas aeruginosa biofilm on soft contact lenses and retain antibacterial activity upon immobilization to the lens surface. Pept. Sci. 2018, 110. [Google Scholar] [CrossRef] [PubMed]
- Crouzet, M.; Claverol, S.; Lomenech, A. M.; Le Senechal, C.; Costaglioli, P.; Barthe, C.; Garbay, B.; Bonneu, M.; Vilain, S. Pseudomonas aeruginosa cells attached to a surface display a typical proteome early as 20 min of incubation. PLoS ONE. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, W. F.; Silva, P. M. S.; Silva, R. C. S.; Silva, M. M.; Machado, G.; Coelho, L.; Correia, M. T. S. Staphylococcus aureus and Staphylococcus epidermidis infections on implants. J Hosp Infect. 2018, 98, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Cappiello, F.; Loffredo, M. R.; Del Plato, C.; Cammarone, S.; Casciaro, B.; Quaglio, D.; Mangoni, M. L.; Botta, B.; Ghirga, F. The Revaluation of Plant-Derived Terpenes to Fight Antibiotic-Resistant Infections. Antibiotics. [CrossRef]
- McGough, S. F.; MacFadden, D. R.; Hattab, M. W.; Mølbak, K.; Santillana, M. Rates of increase of antibiotic resistance and ambient temperature in Europe: Across-national analysis of 28 countries between 2000 and 2016. Euro Surveill. 2020, 25, 1900414. [Google Scholar] [CrossRef]
- Mancini, M. E.; Alessiani, A.; Donatiello, A.; Didonna, A.; D’Attoli, L.; Faleo, S.; Occhiochiuso, G.; Carella, F.; Di Taranto, P.; Pace, L.; et al. Systematic Survey of Vibrio spp. and Salmonella spp. in Bivalve Shellfish in Apulia Region (Italy): Prevalence and Antimicrobial Resistance. Microorganisms. 2023, 11, 450. [Google Scholar] [CrossRef]
- de Oliveira, T. F.; Queiroz, G. A.; Teixeira, J. P.; Figueiredo, H. C. P.; Leal, C. A. G. Recurrent Streptoccoccus agalactiae infection in Nile tilapia (Oreochromis niloticus) treated with florfenicol. Aquaculture 2018, 493, 51–60. [Google Scholar] [CrossRef]
- Kronvall, G. Normalized resistance interpretation as a tool for establishing epidemiological MIC susceptibility breakpoints. J. Clin. Microbiol. 2010, 48, 4445–4452. [Google Scholar] [CrossRef] [PubMed]
- Reis, F. Y. T.; Rocha, V. P.; Janampa-Sarmiento, P. C.; Costa, H. L.; Egger, R. C.; Passos, N. C.; de Assis, C. H. S.; Carneiro, S. P.; Santos, Á. F.; Silva, B. A. Edwardsiella tarda in Tambaqui (Colossoma macropomum): A Pathogenicity, Antimicrobial Susceptibility, and Genetic Analysis of Brazilian Isolates. Animals. [CrossRef]
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D. G. J. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol. Rev. [CrossRef]
- Jorgensen, J. H.; Turnidge, J. D. Susceptibility Test Methods: Dilution and Disk Diffusion Methods. In: Jorgensen, J. H.; Carroll, K. C.; Funke, G.; Pfaller, M. A.; Landry, M. L.; Richter, S. S.; Warnock, D. W.; Richter, S. S.; Patel, J. B. editors. Manual of Clinical Microbiology. 11th Edition. American Society of Microbiology. pp.1253-1273. 2015. [CrossRef]
- Yalew, S. T. Review on Antibiotic Resistance: Resistance Mechanisms, Methods of Detection and Its Controlling Strategies. Biomed J Sci Tech Res. [CrossRef]
- Aksentijević, K.; Ašanin, J.; Nišavić, J.; Marković, M.; Milanov, D.; Mišić, D. Antibiotic resistance in bacteria isolated from fish in Serbia. Veterinarski glasnik. 2017, 71(1), 24–34. [Google Scholar] [CrossRef]
- Michel, C.; Blanc, G. Minimal inhibitory concentration methodology in aquaculture: The temperature effect. Aquaculture. [CrossRef]
- Jiang, G. L.; Yu, S. Y.; Yu, W. Q.; Zhou, X.; Zhou, Z. M.; Li, H. Research progress in the detection techniques and methods of bacterial drug resistance and drug-resistant genes. J. Chin. Clin. Med. 2022, 15, 907–913. [Google Scholar] [CrossRef]
- Galhano, B. S. P.; Ferrari, R. G.; Panzenhagen, P.; de Jesus, A. C. S.; Conte-Junior, C. A. Antimicrobial Resistance Gene Detection Methods for Bacteria in Animal-Based Foods: A Brief Review of Highlights and Advantages. Microorganisms. 2021, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Piao, Y.; Hu, B.; Yang, C.; Zhang, X.; Zheng, Q.; Cao, J. Investigation of antibiotic resistance genotypic and phenotypic characteristics of marine aquaculture fish carried in the Dalian area of China. Front. Microbiol. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Benkova, M.; Soukup, O.; Marek, J. Antimicrobial susceptibility testing: Currently used methods and devices and the near future in clinical practice. J. Appl. Microbiol. 2020, 129(4), 806–822. [Google Scholar] [CrossRef] [PubMed]
- Gajic, I.; Kabic, J.; Kekic, D.; Jovicevic, M.; Milenkovic, M.; Mitic Culafic, D.; Trudic, A.; Ranin, L.; Opavski, N. Antimicrobial Susceptibility Testing: A Comprehensive Review of Currently Used Methods. Antibiotics (Basel). 2022, 23, 427. [Google Scholar] [CrossRef]
- Martinez, J. L. The role of natural environments in the evolution of resistance traits in pathogenic bacteria. Proc. Royal Soc. B-Biol. Sci. 2009, 276, 2521–2530. [Google Scholar] [CrossRef]
- Aedo, S.; Ivanova, L.; Tomova, A.; Cabello, F. C. Plasmid-related quinolone resistance determinants in epidemic Vibrio parahaemolyticus, uropathogenic Escherichia coli, and marine Bacteria from an aquaculture area in Chile. Microb. Ecol. 2014, 68, 324–328. [Google Scholar] [CrossRef]
- Rajan, V.; Sivaraman, G. K.; Vijayan, A.; Elangovan, R.; Prendiville, A.; Bachmann, T. T. Genotypes and phenotypes of methicillin-resistant staphylococci isolated from shrimp aquaculture farms. Environ. Microbiol. Rep. 2022, 14(3), 391–399. [Google Scholar] [CrossRef]
- OIE. Laboratory methodologies for bacterial antimicrobial susceptibility testing. In: OIE Terrestrial Manual. World Organisation for Animal Health. 2012. Available online: https://www.woah.org/fileadmin/Home/fr/Our_scientific_expertise/docs/pdf/GUIDE_2.1_ANTIMICROBIAL.pdf31:1-11. (accessed on 9 March 2024.).
- Uelze, L.; Grützke, J.; Borowiak, M.; Hammerl, J. A.; Juraschek, K.; Deneke, C.; Tausch, S. H.; Malorny, B. Typing methods based on whole genome sequencing data. One Health Outlook. [CrossRef]
- Devadas, S.; Zakaria, Z.; Shariff, M.; Bhassu, S.; Karim, M.; Natrah, I. Methodologies and standards for monitoring antimicrobial use and antimicrobial resistance in shrimp aquaculture. Aquaculture. 2024, 579, 740216. [Google Scholar] [CrossRef]
- FAO. Technical Meeting on the Impact of Whole Genome Sequencing on Food Safety Management within a One Health Approach. 2016. Available online: https://www.fao.org/3/i6582e/i6582e.pdf (accessed on 15 January 2024.).
- Manning, T. S.; Gibson, G. R. Prebiotics. Best Pract Res Clin Gastroenterol. 2004, 18(2), 287–298. [Google Scholar] [CrossRef] [PubMed]
- Bondad-Reantaso, M. G.; MacKinnon, B.; Karunasagar, I.; Fridman, S.; Alday-Sanz, V.; Brun, E.; Le Groumellec, M.; Li, A.; Surachetpong, W.; Karunasagar, I. Review of alternatives to antibiotic use in aquaculture. Review of alternatives to antibiotic use in aquaculture. Rev.Aquac. 2023, 15(4), 1421–1451. [Google Scholar] [CrossRef]
- Oviedo-Olvera, M. V.; Feregrino-Pérez, A. A.; Nieto-Ramírez, M. I.; Tovar-Ramírez, M. M.; Aguirre-Becerra, H.; García-Trejo, J. F. Forthcoming. Prebiotic emergent sources for aquaculture: Microalgae and insects. Aquac Fish. [CrossRef]
- Merrifield, D. L.; Dimitroglou, A.; Foey, A.; Davies, S. J.; Baker, R. T. M.; Bøgwald, J.; Castex, M.; Ringø, E. The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture, 2010, 302, 1–18. [Google Scholar] [CrossRef]
- Kumar, C. G.; Sripada, S.; Poornachandra, Y. Chapter 14 - Status and Future Prospects of Fructooligosaccharides as Nutraceuticals. In Grumezescu, M.; Holban A. M. editors. Handbook of Food Bioengineering, Role of Materials Science in Food Bioengineering. Academic Press, pp. 451-503. 2018. [CrossRef]
- Muzaffar, K.; Jan, R.; Bhat, N. A.; Gani, A.; Shagoo, M. A. Chapter 25 - Commercially Available Probiotics and Prebiotics Used in Human and Animal Nutrition. In: Dhanasekaran D, Sankaranarayanan A. editors. Advances in Probiotics. Academic Press, 2021. pp. 417-435. [CrossRef]
- Oktaviana, A.; Widanarni Yuhana, M. The Use of Synbiotics to Prevent IMNV and Vibrio harveyi Co-Infection in Litopenaeus vannamei. HAYATI J Biosci. 2014, 21(3), 127–134. [Google Scholar] [CrossRef]
- Banerjee, G.; Ray, A. K. The advancement of probiotics research and it application in fish farming industries. Res. Vet. Sci. 2017, 115, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Olmos, J.; Ochoa, L.; Paniagua-Michael, J.; Contreras, L. Functional feed assessment on Litopenaeus vannamei using 100% fish meal replacement by soybean meal, high levels of complex carbohydrates and Bacillus probiotic strains. Mar Drugs. 2011, 9(6), 1119–1132. [Google Scholar] [CrossRef] [PubMed]
- Antony, S.; Singh, I. S. B.; Jose, R. M.; Kumar, P. R. A.; Philip, R. Antimicrobial peptide gene expression in tiger shrimp, Penaeus monodon in response to gram-positive bacterial probionts and white spot virus challenge. Aquaculture. 2011, 316, 6–12. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, H. L.; Ma, R. L.; Lin, W. Y. Probiotic applications of two dominant gut Bacillus strains with antagonistic activity improved the growth performance and immune responses of grouper Epinephelus coioides. Fish Shellfish Immunol. 2010, 29(5), 803–809. [Google Scholar] [CrossRef]
- Tovar-Ramírez, D.; Mazurais, D.; Gatesoupe, J.; Patrick, Q. Dietary probiotic live yeast modulates antioxidant enzyme activities and gene expression of sea bass (Dicentrarchus labrax) larvae. Aquaculture. 2010, 300, 142–147 . [Google Scholar] [CrossRef]
- Abdeltawwab, M.; Abdelrahman, A.; Ismael, N. Evaluation of commercial live bakers’ yeast, Saccharomyces cerevisiae as a growth and immunity promoter for Fry Nile tilapia, Oreochromis niloticus (L.) challenged in situ with Aeromonas hydrophila. Aquaculture. 2008, 280, 185–189. [Google Scholar] [CrossRef]
- Vijayaram, S.; Ringø, E.; Zuorro, A.; van Doan, H.; Sun, Y. Forthcoming. Beneficial roles of nutrients as immunostimulants in aquaculture: A review. Aquac Fish. [CrossRef]
- Bragg, R. R.; Meyburgh, C. M.; Lee, J. Y.; Coetzee, M. Potential treatmentoptions in a post-antibiotic era. In: Adhikari, R.; Thapa, S. editors. Infectious diseases and nanomedicine. 3rd. Advances in experimental medicine and biology. 2018, 1052, pp. 51–61. [Google Scholar]
- Vijayaram, S.; Sun, Y. Z.; Zuorro, A.; Ghafarifarsani, H.; van Doan, H.; Hoseinifar, S. H. Bioactive immunostimulants as health-promoting feed additives in aquaculture: A review. Fish Shellfish Immunol. 2022, 130, 294–308. [Google Scholar] [CrossRef] [PubMed]
- El Basuini, M. F.; Shahin, S. A.; Teiba, I. I.; Zaki, M. A.; El-Hais, A. M.; Sewilam, H.; Almeer, R.; Abdelkhalek, N.; Dawood, M. A. O. The influence of dietary coenzyme Q10 and vitamin C on the growth rate, immunity, oxidative-related genes, and the resistance against Streptococcus agalactiae of Nile tilapia (Oreochromis niloticus). Aquaculture. 2021, 531. [Google Scholar] [CrossRef]
- Senthamarai, M. D.; Rajan, M. R.; Bharathi, P. V. Current risks of microbial infections in fish and their prevention methods: A review. Microb. Pathog. 2023, 185, 106400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, J.; Ma, Y.; Li, J.; Chen, X. The effective components of herbal medicines used for prevention and control of fish diseases. Fish Shellfish Immunol. 2022, 126, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Jang, I. S.; Ko, Y. H.; Kang, S. Y.; Lee, C. Y. Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chickens. Anim. Feed Sci. Technol. [CrossRef]
- Cross, D.; McDevitt, R. M.; Hillman, K.; Acamovic, T. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Br. Poult. Sci. 2007, 48, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, Jr. G.; Pês, T. S.; Saccol, E. M. H.; Sutili, F. J.; Rossi, Jr. W.; Murari, A. L.; Heinzmann, B. M.; Pavanato, M. A.; de Vargas, A. C.; de, L. Silva, L.; Baldisserotto, B. Potential uses of Ocimum gratissimum and Hesperozygis ringens essential oils in aquaculture. Ind. Crops Prod. 2017, 97, 484–491. [Google Scholar] [CrossRef]
- Lazzaro, B. P.; Zasloff, M.; Rolf, J. Antimicrobial peptides: application informed by evolution. Science. 2022, 368, 6490. [Google Scholar] [CrossRef]
- López-Sanmartín, M.; Rengel, R.; Lopez-Lopez, M.; Lebron, J. A.; Molina-Marquez, A.; de la Rosa, I.; Lopez-Cornejo, P.; Cuesta, A.; Vigara, J.; Leon, R. D-amino acid peptides as antimicrobial agents against vibrio-associated diseases in aquaculture. Aquaculture. 2023, 569, 739362. [Google Scholar] [CrossRef]
- Culot, A.; Grosset, N.; Gautier, M. Overcoming the challenges of phage therapy for industrial aquaculture: A review. Aquaculture. 2019, 513, 734423. [Google Scholar] [CrossRef]
- Fjalestad, K. T.; Gjedrem, T.; Gjerde, B. Genetic improvement of disease resistance in fish: an overview. Aquaculture. 1993, 111, 65–74. [Google Scholar] [CrossRef]
- Meuwissen, T. H. E.; Hayes, B. J.; Goddard, M. E. Prediction of Total Genetic Value Using Genome-Wide Dense Marker Maps. Genetics. 2001, 157(4), 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, R. L.; Evenhuis, J. P.; Cheng, H.; Fragomeni, B. O.; Gao, G.; Liu, S.; Long, R. L.; Shewbridge, K. L.; Silva, R. M. O.; Wiens, G. D. Genome-wide mapping of quantitative trait loci that can be used in marker-assisted selection for resistance to bacterial cold water disease in two commercial rainbow trout breeding populations. Aquaculture. 2022, 560, 738574. [Google Scholar] [CrossRef]
- Baquero, F.; Martinez, J. L.; Canton, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotech. 2008, 19(3), 260–265. [Google Scholar] [CrossRef] [PubMed]
- Pruden, A.; Larsson, D. G.; Amezquita, A.; Collignon, P.; Brandt, K. K.; Graham, D. W.; Lazorcha, J. M.; Zhu, Y. Management options for reducing the release of antibiotics and antibiotic resistance genes to the environment. Environ. Health Perspect. 2013, 121, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Moges, F.; Endris, M.; Belyhun, Y.; Worku, W. Isolation and characterization of multiple drug resistance bacterial pathogens from waste water in hospital and non-hospital environments, Northwest Ethiopia. BMC Res. Notes. 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Lall, S. P. Nutrition and health of fish. In: Cruz –Suárez, L. E.; Ricque-Marie, D.; Tapi- a-Salazar, M.; Olvera-Novoa, M. A.; Civera-Cerecedo, R. editors. Avances en Nutrición Acuícola V. Memorias del V Simposium Internacional de Nutrición Acuícola. Mérida, Yucatán, Mexico, p. 11. 2000.
- Nguyen, P. T. D.; Giovanni, A.; Maekawa, S.; Pham, T. H.; Wang, P. C.; Chen, S. C. An Integrated in silico and in vivo study of nucleic acid vaccine against Nocardia seriolae infection in orange-spotted grouper Epinephelus coioides. Fish Shellfish Immunol. 2023, 143, 109202. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, J.; Liu, G.; Du, H.; Liu, T.; Liu, T.; Li, P.; Yu, Q.; Wang, G.; Wang, E. A nanocarrier immersion vaccine encoding surface immunogenic protein confers cross-immunoprotection against Streptococcus agalactiae and Streptococcus iniae infection in tilapia. Fish Shellfish Immunol. 2024, 144, 109267. [Google Scholar] [CrossRef]
- Buttner, J. K.; Soderberg, R. W.; Terlizzi, D. E. An introduction to water chemistry in freshwater aquaculture. (Publication No. 170-1993). Dartmouth: University of Massachusetts, Northeastern Regional Aquaculture Center. 1993.
- Mramba, R. P.; Kahindi, E. J. Pond water quality and its relation to fish yield and disease occurrence in small-scale aquaculture in arid areas. Heliyon 9. 2023, e16753. [Google Scholar] [CrossRef]
- McNulty, K.; Soon, J. M.; Wallace, C. A.; Nastasijevic, I. Antimicrobial resistance monitoring and surveillance in the meat chain: A report from five countries in the European Union and European Economic Area. Trends Food Sci Technol. 2016, 58, 1–13. [Google Scholar] [CrossRef]
- WOAH. Aquatic Animal Health Code. Twenty-fourth Edition. 2022. Available online: https://rr-africa.woah.org/wp-content/uploads/2022/09/en_csaa-2022.pdf (accessed on 5 March 2024.).
- ESVAC. Sales of veterinary antimicrobial agents in 31 European countries in 2022. Trends from 2010 to 2022. Thirteenth ESVAC report. 2022. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2022-trends-2010-2022-thirteenth-esvac-report_en.pdf (accessed on 2 March 2024.).
- Kawsar, A.; Alam, T.; Pandit, D.; Rahman, M.; Mia, M.; Talukdar, A.; Sumon, T. A. Status of disease prevalence, drugs and antibiotics usage in pond-based aquaculture at Narsingdi district, Bangladesh: A major public health concern and strategic appraisal for mitigation. Heliyon 8. 2022, e09060. [Google Scholar] [CrossRef] [PubMed]
- FAO. 1989. In: Turner, G. E. editor. Codes of Practice and Manual of Procedures for Consideration of Introductions and Transfers of Marine and Freshwater Organisms. FAO Publications and Reports on Inland Fisheries and Aquaculture. Report No.: EIFAC/OP 23. 1989. Available online: https://www.fao.org/fishery/docs/CDrom/aquaculture/a0844t/docrep/009/AE989E/AE989E00.htm (accessed on 22 January 2024.).
- Murray, K. N.; Clark, T. S.; Kebus, M. J.; Kent, M. L. Specific Pathogen Free – A review of strategies in agriculture, aquaculture, and laboratory mammals and how they inform new recommendations for laboratory zebrafish. Res. Vet. Sci. 2022, 142, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Lee, E. Environmental health perspectives in the ancient world. Senior. Honors Thesis, . Chapel Hill: Department of Classics, University of North Carolina, 2019. [Google Scholar]
- Wei, L.; Su, Z.; Yue, Q.; Huang, X.; Wei, M.; Wang, J. Microplastics, heavy metals, antibiotics, and antibiotic resistance genes in recirculating aquaculture systems. TrAC Trends in Analytical Chemistry. 2024, 172, 117564. [Google Scholar] [CrossRef]
- FAO/NACA. Asia regional technical guidelines on health management for the responsible movement of live aquatic animals and the Beijing consensus and implementation strategy. Report No.: FAO Fisheries Technical Paper No. 402. 2000. Available online: https://www.fao.org/3/x8485e/x8485e.pdf (accessed on 4 March 2024.).
- FAO. Understanding and applying risk analysis in aquaculture. FAO Fisheries and Aquaculture technical paper 519. 2008. Available online: https://www.fao.org/3/i1136e/i1136e00.htm (accessed on 10 March 2024.).
- WHO. 68th World Health Assembly: WHA resolution 68.7. Geneva, Switzerland. Report No.: WHA68/2015/REC/1. 2015. Available online: https://apps.who.int/gb/ebwha/pdf_files/WHA68-REC1/A68_R1_REC1-en.pdf (accessed on 2 March 2024.).
- OIE. Combating Antimicrobial Resistance through a One Health Approach: Actions and OIE Strategy. OIE General Session. Report No.: 36. 2016. Available online: https://www.woah.org/fileadmin/Home/eng/Our_scientific_expertise/docs/pdf/AMR/A_RESO_AMR_2016.pdf (accessed on 10 January 2024.).
- FAO. CL 145/Report Food and Agriculture Organization 6. 6. 2015. Available online: https://www.fao.org/3/mo153e/mo153e.pdf (accessed on 15 March 2024.).
- FAO. The FAO Action Plan on Antimicrobial Resistance 2016-2020. 2016. Available online: https://www.fao.org/3/i5996e/i5996e.pdf (accessed on 10 March 2024.).
- UN. The Report of the Third High-Level Ministerial Conference on Antimicrobial Resistance. 2022. Available online: https://amrconference2022.om/index. (accessed on 11 March 2024.).
- WHO. Quadripartite launches a new platform to tackle antimicrobial resistance threat to human and animal health and ecosystems. 2022. Available online: https://www.who.int/news/item/18-11-2022-quadripartite-launches-a-new-platform-to-tackle-antimicrobial-resistance-threat-to-human-and-animal-health-and-ecosystems (accessed on 6 March 2024).
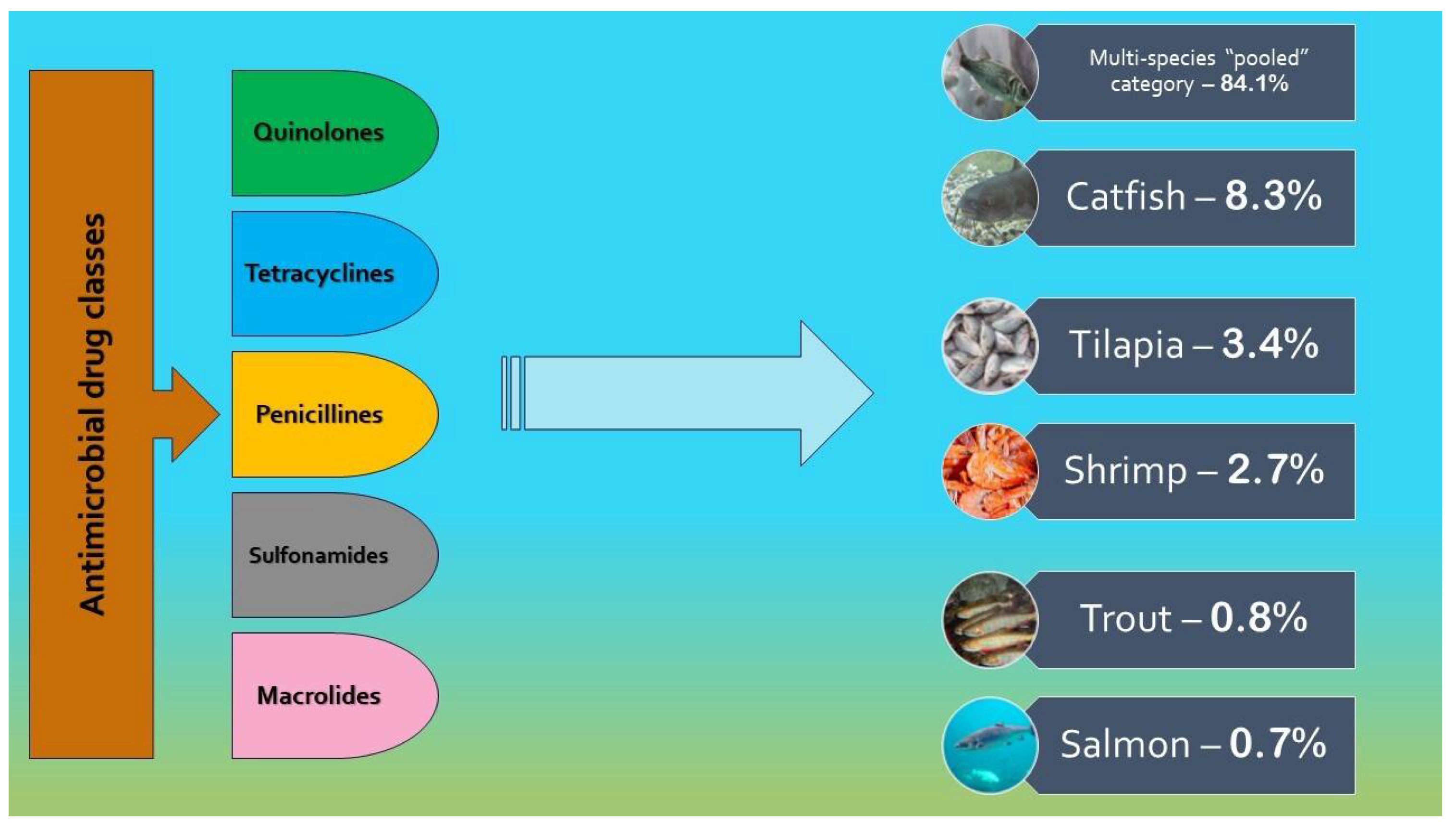
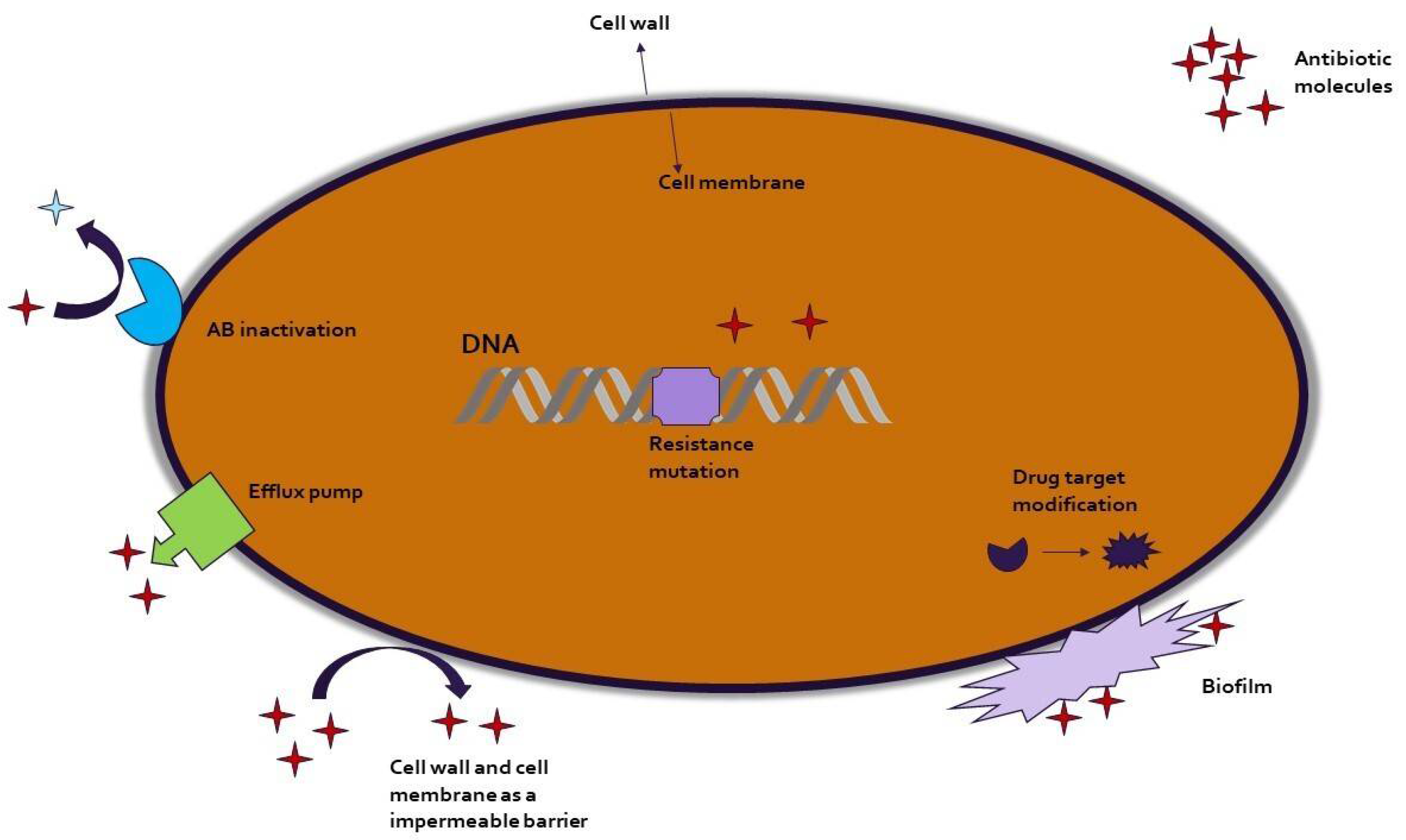
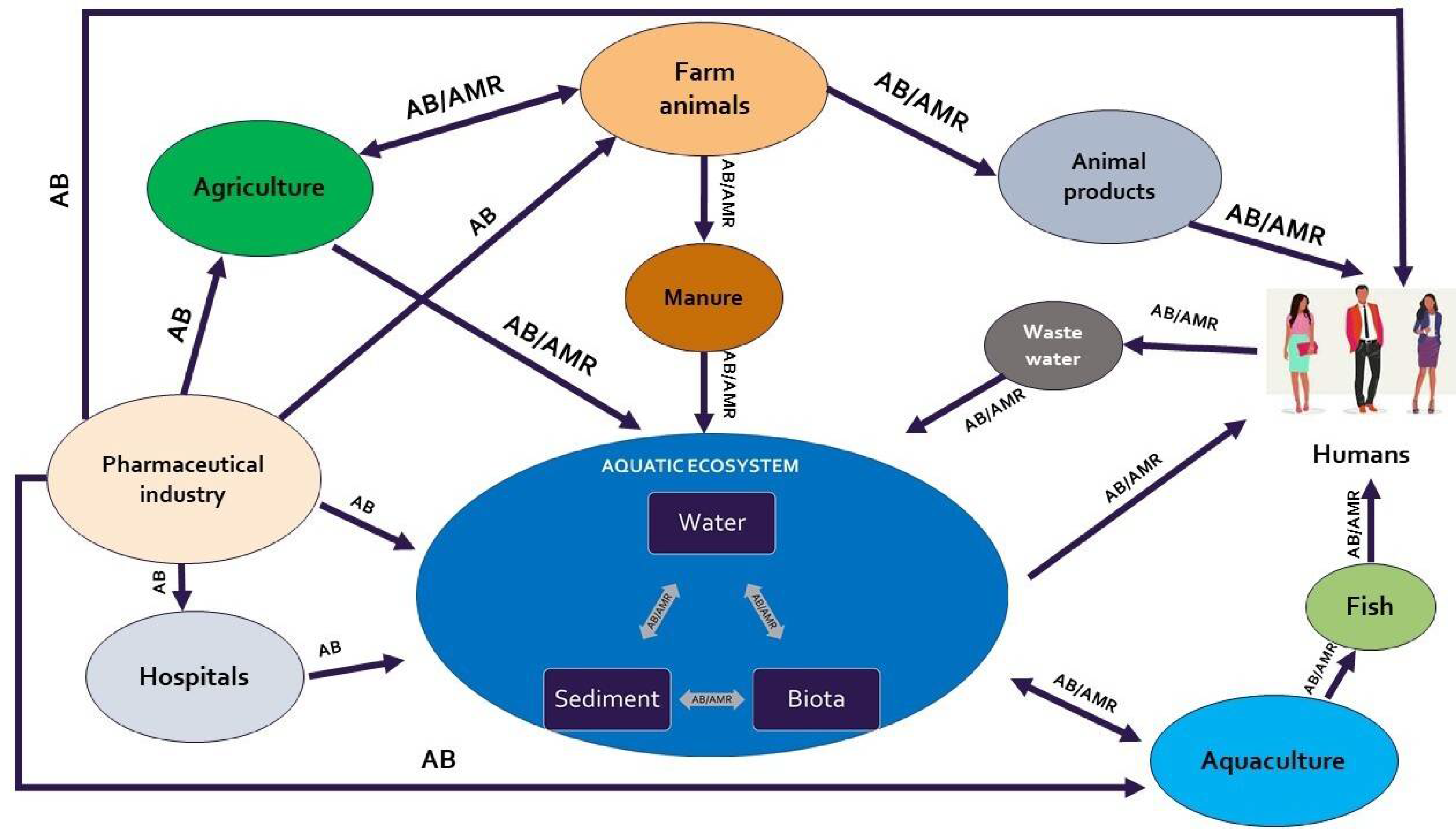
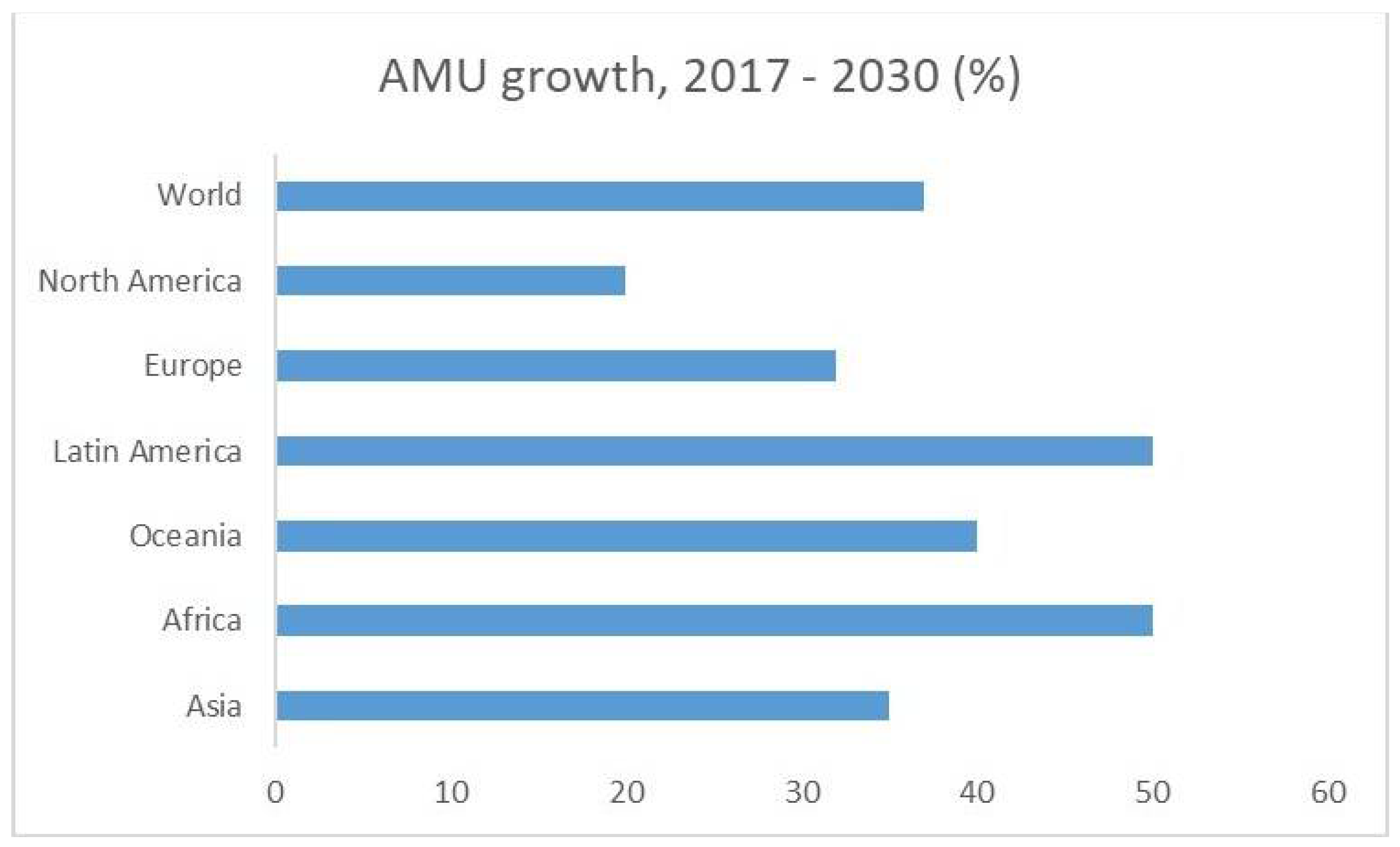

| Author(s) | Year | Study location | Microorganism(s) | Type of samples | Antimicrobial agent(s) | Phase in aquaculture production chain |
|---|---|---|---|---|---|---|
| Raza et al.[41] | 2022 | South Korea | / | Water samples | SulfonamidesVCIA,HIA, TetracyclinesVCIA,HIA, QuinolonesVCIA,CIA, Beta lactamsVCIA,CIA |
Pre-harvest |
| Osman et al.[42] | 2021 | Egypt | Pseudomonas spp. | Nile tilapia (Oreochromis niloticus) | Sulphamethoxazole/ TrimethoprimVCIA,HIA, AmikacinVCIA,CIA, ImipenemVCIA,CIA, TetracyclinesVCIA,HIA,, AmpicillinVCIA,CIA, Nalidixic acidVCIA,CIA, Chloramphenicol, GentamicinVCIA,CIA, CiprofloxacinVCIA,CIA, Aztreonam, Ampicillin/SulbactamVCIA,CIA, CefepimeVCIA,CIA, CeftriaxoneVCIA,CIA, CephalotinVCIA,CIA, CefotaximeVCIA,CIA, CeftazidimeVCIA,CIA |
Post-harvest |
| Algammal et al.[14] | 2022 | Egypt | Edwardsiella tarda | Nile tilapia (Oreochromis niloticus), African catfish (Clarias gariepinus) | AmoxicillinVCIA,CIA, AmpicillinVCIA,CIA, CefotaximeVCIA,CIA, ErythromycinVCIA,CIA, StreptomycinVCIA,CIA, GentamycinVCIA,CIA, EnrofloxacinVCIA,CIA, CiprofloxacinVCIA,CIA, Colistin-sulfate, TetracyclineVCIA,HIA, Trimethoprim-SulfamethoxazoleVCIA,HIA |
Pre-harvest |
| Brunton et al.[25] | 2019 | Vietnam | / | Striped catfish (Pangasianodon hypophthalmus), White-leg shrimp (Penaeus vannamei) |
N/A | Pre-harvest |
| Girijan et al.[43] | 2020 | India | Escherichia coli | Sediment, water, fish/shrimp/clams | CiprofloxacinVCIA,CIA, Levofloxacin, MoxifloxacinVCIA,CIA, OfloxacinVCIA,CIA, NorfloxacinVCIA,CIA, Nalidixic acidVCIA,CIA, GentamicinVCIA,CIA, AmikacinVCIA,CIA, CefotaximeVCIA,CIA, Cefotetan, CeftazidimeVCIA,CIA, ImipenemVCIA,CIA, ColistinVCIA,CIA |
Pre-harvest |
| Yang et al.[44] | 2023 | China |
Proteobacteria, Firmicutes, Enterococcus spp., Escherichia spp., Streptococcus spp., Klebsiella spp., Acinetobacter spp., Bacteroidetes, Cyanobacteria, Klebsiella pneumoniae, |
Farm worker feces, water, sediment, fish guts, duck manure | AminoglycosidesVCIA,CIA, PhenicolesVCIA,HIA, TetracyclinesVCIA,HIA, SulfonamidesVCIA,HIA | Pre-harvest |
| Garza et al.[34] | 2022 | Global/ review paper |
General | General | General | N/A |
| Jones et al.[45] | 2023 | 57 countries | Aeromonas spp. | Human, wastewater, drinking water, surface water, agriculture | AminoglycosidesVCIA,CIA, CarbapenemsVCIA,CIA, CephalosporinsVCIA,CIA, FluoroquinolonesVCIA,CIA, MacrolidesVCIA,CIA, Monobactams, PenicillinsVCIA,CIA, PhenicolsVCIA,HIA, Polypeptides, SulfonamidesVCIA,HIA, TetracyclinesVCIA,HIA |
N/A |
| Reddy et al.[46] | 2022 | Global/ review paper |
General | General | General | N/A |
| Bell et al.[47] | 2023 | Bangladesh | / | Water samples | AminoglycosidesVCIA,CIA, SulphonamidesVCIA,HIA, CarbapenemsVCIA,CIA, CephalosporinsVCIA,CIA, CephamycinsVCIA,CIA, PenamsVCIA,CIA, FluoroquinolonesVCIA,CIA, DiaminopyrimidinesVCIA,CIA, PhenicolsVCIA,HIA |
N/A |
| Chowdhury et al.[35] | 2022 | Bangladesh | / | Various fish species | OxytetracyclineVCIA,HIA, CiprofloxacinVCIA,CIA, AmoxicillinVCIA,CIA, LevofloxacinVCIA,CIA, ErythromycinVCIA,CIA, SulfadiazineVCIA,HIA, TrimethoprimVCIA,HIA |
N/A |
| Kampouris et al.[48] | 2022 | / | Flavobacterium, Pseudomonas, Lactococcus, Sphingomonas | Water samples, biofilm from plastic mechanical filters from ponds with African catfish (Clarias gariepinus) | SulfonamidesVCIA,HIA, Beta lactamsVCIA,CIA, QuinolonesVCIA,CIA, MacrolidesVCIA,CIA |
Pre-harvest |
| Alhaji [9] | 2021 | Nigeria | / | African catfish (Clarias gariepinus, Clarias lazera) | TetracyclinesVCIA,HIA, PenicillinVCIA,CIA, SulfonamidesVCIA,HIA, StreptomycinVCIA,CIA, NeomycinVCIA,CIA, AmpicillinVCIA,CIA, ColistinVCIA,CIA, ErythromycinVCIA,CIA, EnrofloxacinVCIA,CIA, ChloramphenicolVCIA,CIA |
Pre-harvest |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





