1. Introduction
Thyroid surgery represents a common operation with approximately 93,000 thyroidectomies being performed in the United States each year, while parathyroidectomy is comparatively rare, as the incidence of primary hyperparathyroidism is approximately 25 cases per 100 000.1
Postoperative pain related to thyroid or parathyroid surgery can be a major challenge for patient care. Pain control after thyroid surgery improves patients’ quality of life and allows a quick return to normal daily activities. Adequate postoperative pain control is customarily managed by a broad spectrum of analgesics including nonsteroidal anti-inflammatory drugs (NSAIDs) or opioid analgesics which constitute the top analgesic choices. 2,3 Yet, both regimens are frequently linked to adverse effects.
Local wound infiltration has long been used in general surgery to reduce postoperative pain intensity and minimize the need for analgesics. Evidence indicates that thyroidectomy wound infiltration constitutes an easy-to-implement technique operated by the surgeon himself, while it elongates the time frame until rescue analgesia.4 Currently, magnesium sulfate is widely applied in the perioperative setting for its multifactorial properties and has been shown to decrease the anesthesia and analgesia requirements effectively. 5-8 Relevant reports indicate that the implementation of wound infiltration with magnesium sulfate and ropivacaine mixture seems to reduce postoperative tramadol requirements after radical prostatectomy. 5-8
To the authors’ knowledge, no studies investigating the use of magnesium sulfate as an adjunct to local anesthetics for wound infiltration thyroidectomy/parathyroidectomy exist in the current literature. Thus the present study aims to evaluate the effectiveness of wound infiltration with ropivacaine plus magnesium sulfate mixture in the management of postoperative pain in patients undergoing thyroid or parathyroid surgery with the safety of this practice constituting the secondary study end-point.
2. Materials and Methods
2.1. General Information
After institutional approval was obtained from the Ethics Committee adult patients of both sexes and American Society of Anesthesiologists (ASA) classification I-II, scheduled for thyroidectomy/parathyreoidectomy were enrolled in the study. The study was conducted in accordance with the Helsinki Declaration guidelines, while written informed consent was obtained from all patients before participation. The trial was registered in the ClinicalTrials.gov database with the unique reference ID NCT05294393. The exclusion criteria included the indication for selective lateral neck dissection, previous cervical operation, long-term analgesic drug use, known or suspected allergy to local anesthetics, and a major complication of surgery or anesthesia (major bleeding, allergy to anesthetic products).
2.2. Randomization and Masking
Patients were allocated to three groups using computer randomization by a person irrelevant to the study investigators. An anesthesia nurse being unaware of the study protocol prepared an unlabeled syringe of 15 ml solution including normal saline which served as a placebo (group P), ropivacaine 100 mg (group R), or a mixture of 100 mg ropivacaine plus 10mg/kg magnesium sulfate (group RMg).
2.3. Anesthesia and Surgical Procedure
A standardized protocol was applied to all patients. Surgeries were performed by the same anesthesiologic and surgical team. After ensuring venous access with an 18- 20 G IV cannula in the operating room, standard hemodynamic monitoring was employed for all patients. After preoxygenation with 100% oxygen for 3 minutes, anesthesia induction was executed with intravenous 2-2.5 mg.kg-1 propofol and 0.2mg.kg-1. Spiral endotracheal tubes via the orotracheal way with an inner diameter of 7.5 and 8 mm for females and males, respectively, were employed. Maintenance of anesthesia was ensured with inhaled desflurane while a target-control infusion (TCI) of remifentanil was applied for intraoperative analgesia. An increase of 20% in heart rate or mean blood pressure necessitated a change in TCI. Total remifentanil consumption was measured at the end of the operation.
Patients were placed in the supine position on the operating table, with the neck hyperextended. Through a 4–7 cm skin incision (depending on the size of the thyroid), sub-platysmal flaps were created, and strap muscles were separated in the midline and reflected laterally. The middle, superior, and inferior thyroid vessels were then divided. The same steps were repeated for the removal of the contralateral lobe. After complete hemostasis and before wound closure the surgeon blinded to the applied medications soaked a pad with the solution over the anterior face of the trachea, then infiltrated using a 21-gauge needle, the strap muscles and subcutaneous tissues in both flaps as well as the surgical edges. Laryngeal nerves were not anesthetized so that laryngeal function could be maintained after surgery. Finally, the wound was closed using interrupted 3-0 and 4-0 polyglactin sutures to approximate the strap muscles and the platysmal layer respectively. The skin was closed with 4-0 interrupted polypropylene sutured. Suction drains were not used. All patients were extubated after ensuring enough muscle strength and the ability to follow all commands. Τime from incision to wound closure and time since anesthesia interruption to extubation were also noted for each patient.
2.4. Assessment of Postoperative Pain
All patients were familiarized with a 10 cm Visual Analogue Scale (VAS) preoperatively for pain intensity evaluation. All study participants were followed for 24 hours concerning the evaluation of pain and adverse effects. The VAS score was recorded at 30 minutes as well as 1, 2, 4, 6, 12, and 24 hours after operation completion both at rest and at movement. In cases of moderate pain (VAS score of 4–6), 1000 mg paracetamol was administered as a rescue analgesic drug, while in cases of severe pain (VAS score >6), 40 mg parecoxib or 200 mg tramadol was administered targeting a VAS < 4. Total analgesic requirements up to 24 hours postoperatively were calculated in morphine equivalents. Blood samples for determination of cortisol, TNF-α, and IL-6 levels were collected 30 minutes prior to infiltration, as well as 6 and 24 hours postoperatively. After centrifugation sera were stored in deep freeze. Patient satisfaction was also evaluated with a 5-point scale upon hospital discharge.
2.5. Outcomes
The primary outcome was the analgesic efficacy as assessed by VAS scores at the predefined time points, time to first analgesic request, and total analgesic consumption up to 24 hours after study completion, while the magnitude of the inflammatory response, occurrence of adverse effects, and patient satisfaction constituted secondary study endpoints.
2.6. Statistical Analysis
Based on the findings of a previous relevant clinical study in patients undergoing lumbar spine,8 a total of 58 patients (29 per group) were estimated to detect a 24-hour reduction in the consumption of supplemental analgesics (in morphine equivalents) by 20% (standard deviation 8) in the RMg versus R group (α=0.05 and power 80%). Taking into account a dropout of the order of 15%, a total of 68 patients were included in this study.
Data normality was assessed by the Kolmogorov-Smirnoff test. Non-parametric tests were performed for continuous variables comparisons such as VAS scores at rest and movement and total analgesic requirement. So, the Friedman test was applied for detecting differences between the 7-time points regardless of patient groups, while pairwise comparisons between the time points regardless of patient groups were achieved by the Durbin-Conover test. Comparisons by group for each time point were performed by the Kruskal–Wallis test, while the Dwass-Steel-Critchlow-Fligner test was used for pair comparison. Cross-tabulations were applied for qualitative variables comparisons.
Data of inflammatory markers were normalized by inverse normal transformation through their fractional rank so the use of a parametric test was feasible. A mixed ANOVA analysis was performed. The sphericity testing criterion with the Mauchly test was not met (p < .05) and a Greenhouse-Geisser correction was applied. The equality of variances test for equality of covariance in the three patient groups indicated equality of variances at all time points.
Regarding overall patient satisfaction, the Kruskal–Wallis test was used for comparisons between patients by group. Pairwise comparisons were applied using the Dwass-Steel-Critchlow-Fligner test. The SPSS package (version 29.0) was used for statistical analysis, while a P value < 0.05 was considered statistically significant.
3. Results
3.1. Clinical and Demographic Characteristics of Study Patients
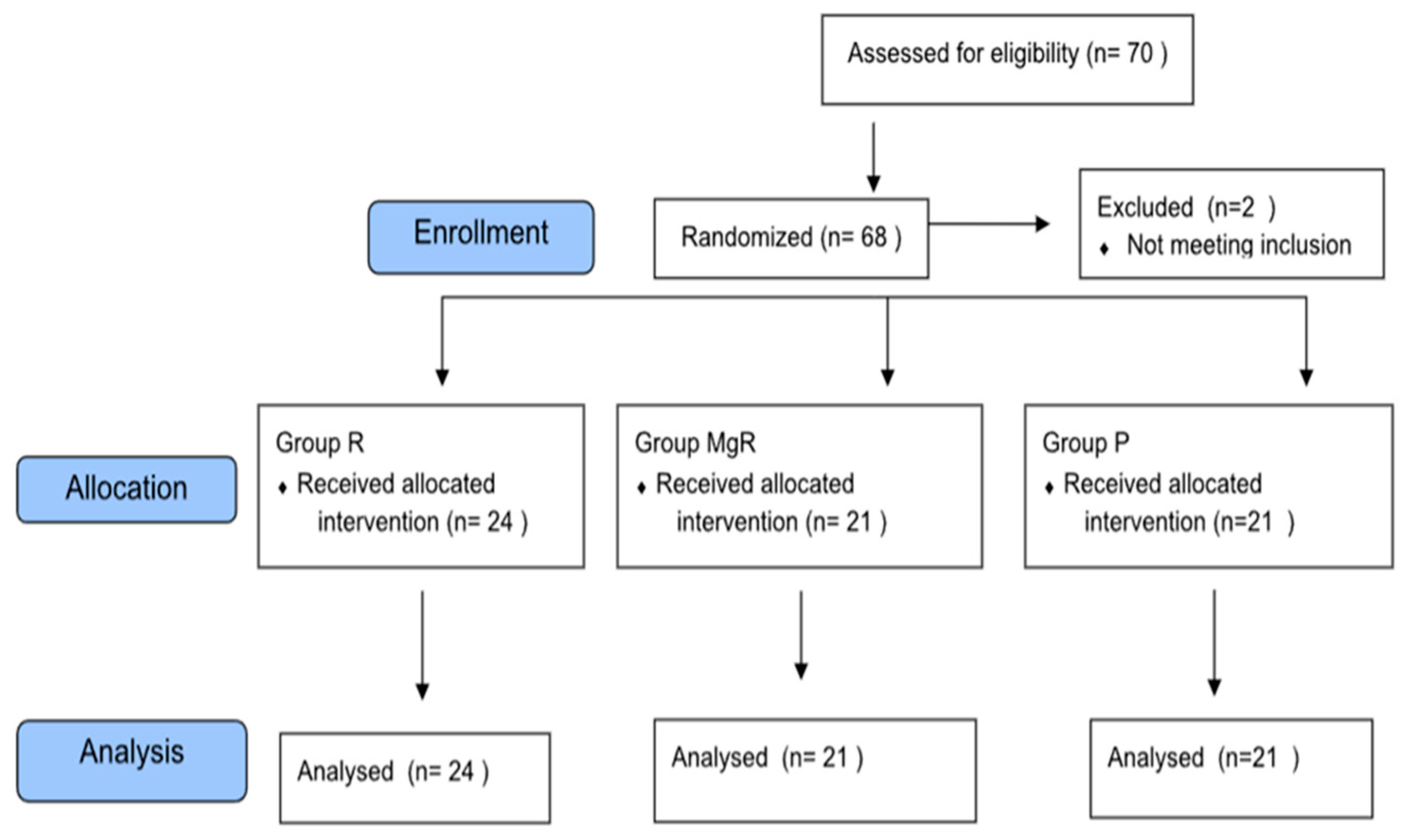
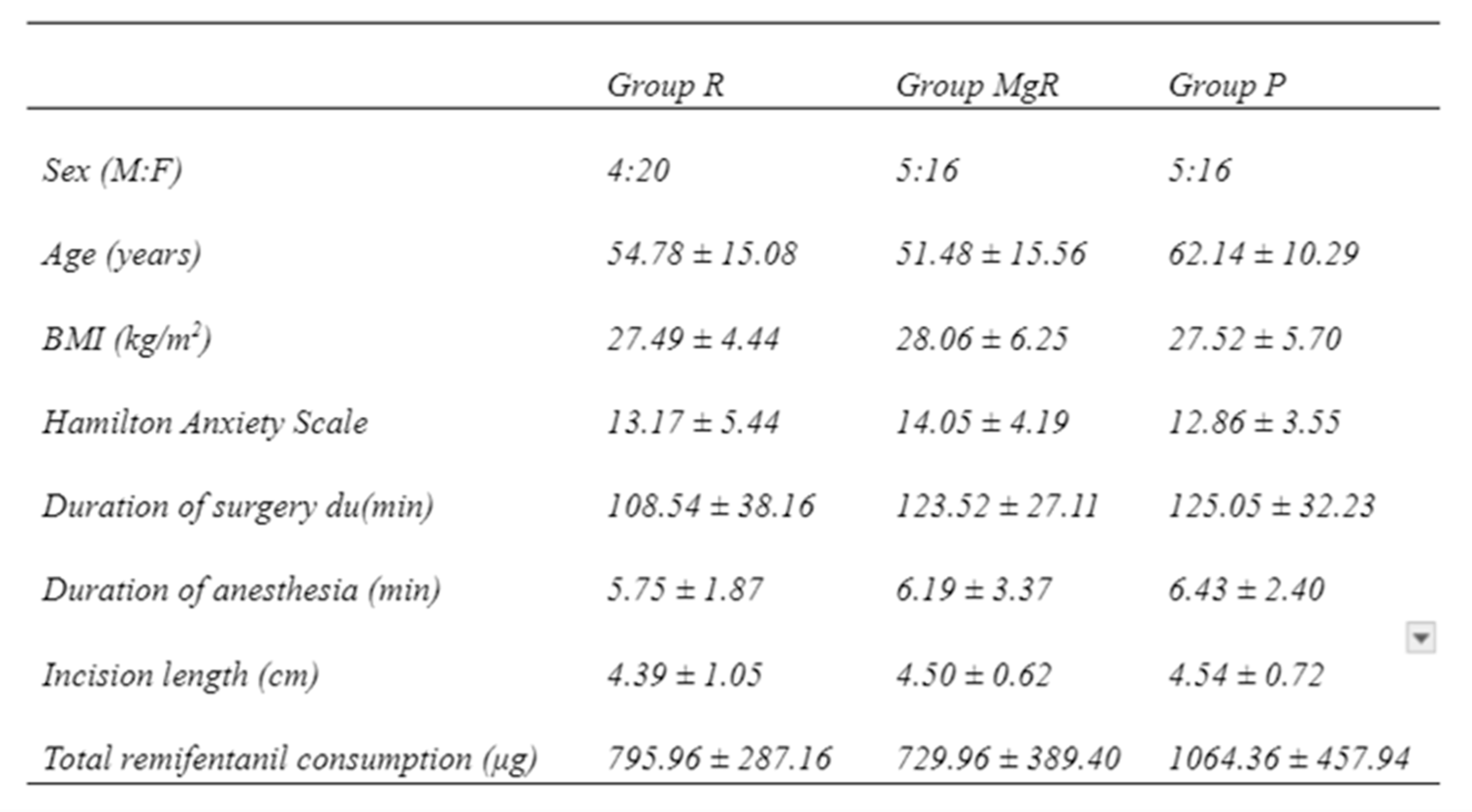
From the total of 68 patients initially stratified in this study, two were excluded due to re-operation for severe bleeding control, leaving 66 patients for final analysis (
Figure 1). Demographic and intraoperative data of the study groups are presented in
Table 1. There was no statistically significant difference between the groups. As far as the type of surgery is concerned 41 thyroidectomies were performed for multinodular disease, 7 for toxic goiter, 6 for carcinoma, 5 for concurrent hyperparathyroidism and goiter, and 7 for hyperparathyroidism.
3.2. Pain Evaluation
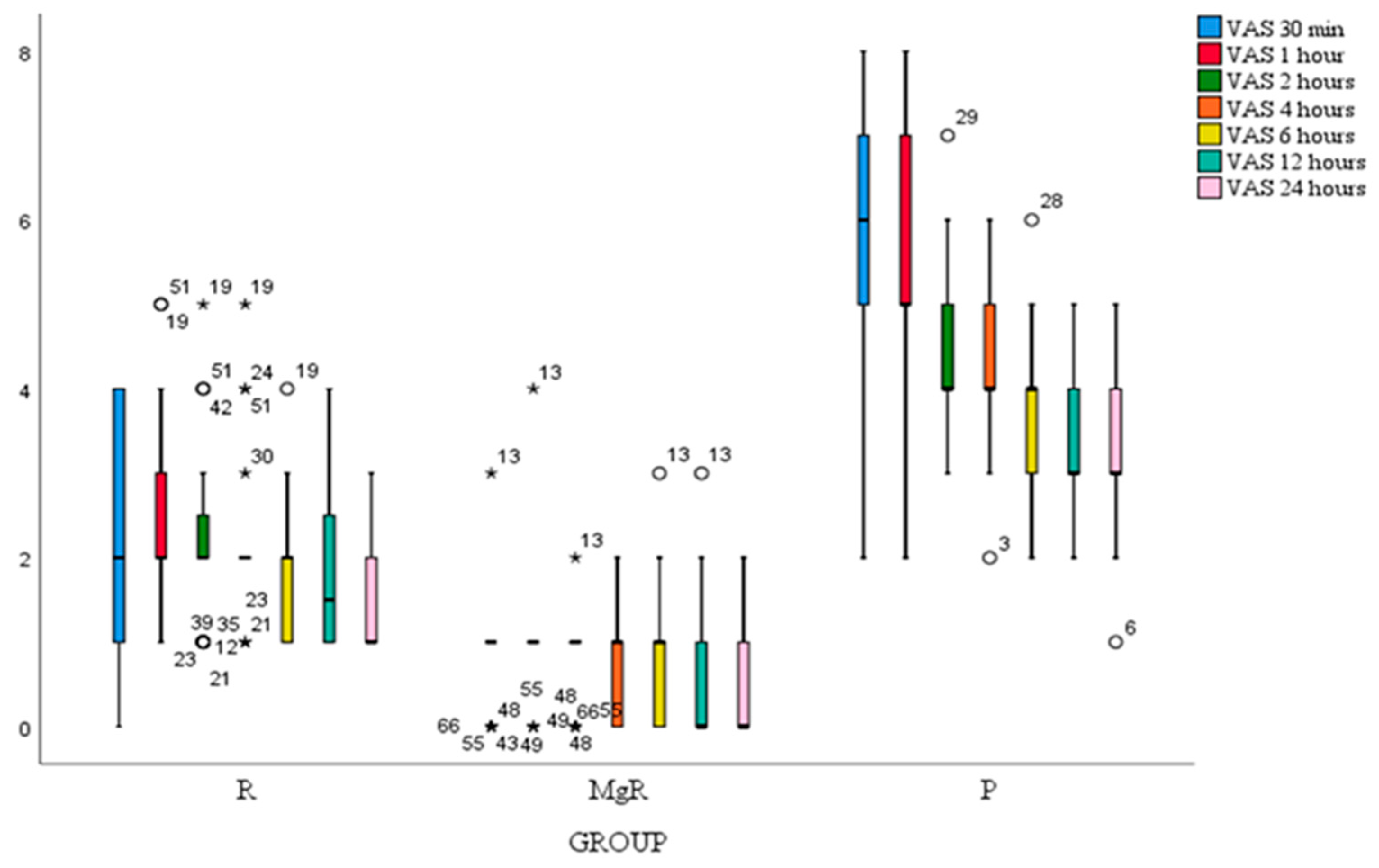
VAS score at rest of all study patients decreased significantly after thyroidectomy at all-time points. Statistical significance was noted when comparing 1st to 2nd, 4th to 6th, and 6th to 8th postoperative hour (
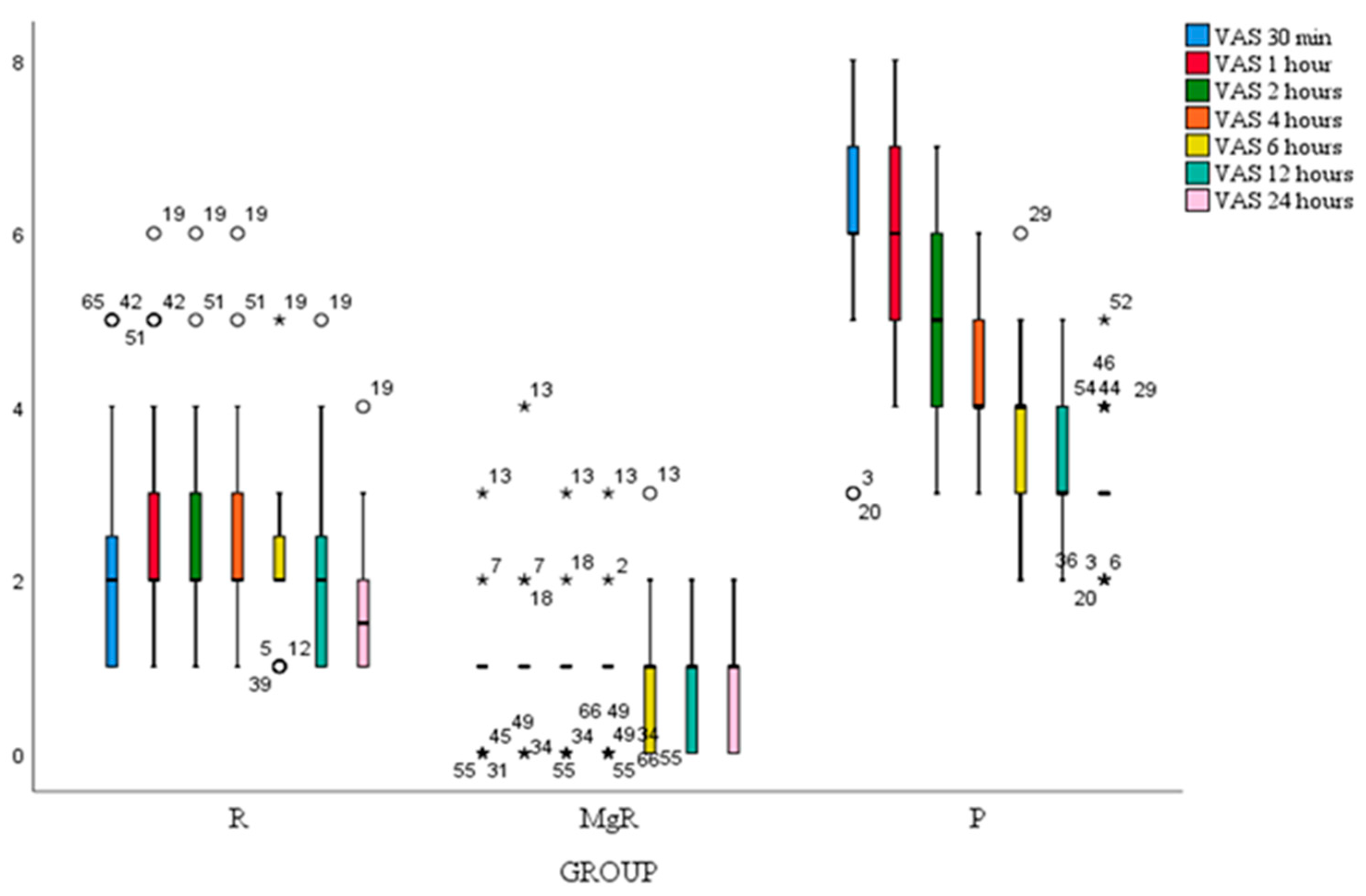
Figure 2). Besides the 1st postoperative hour, VAS score at movement decreased significantly after thyroidectomy at all-time points. (
Figure 3). VAS scores at rest and movement were significantly lower for group MgR at all the evaluated time points (
Figure 2).
3.3. Total Analgesic Requirements
Patients in group P received their first analgesic drug within the first postoperative hour, while the time to first analgesic request was significantly elongated in groups R, and RMg (
Table 2). The time for analgesic demand was significantly lower in the RMg group compared to other groups and in group R compared to placebo.
3.4. Inflammatory Markers Release
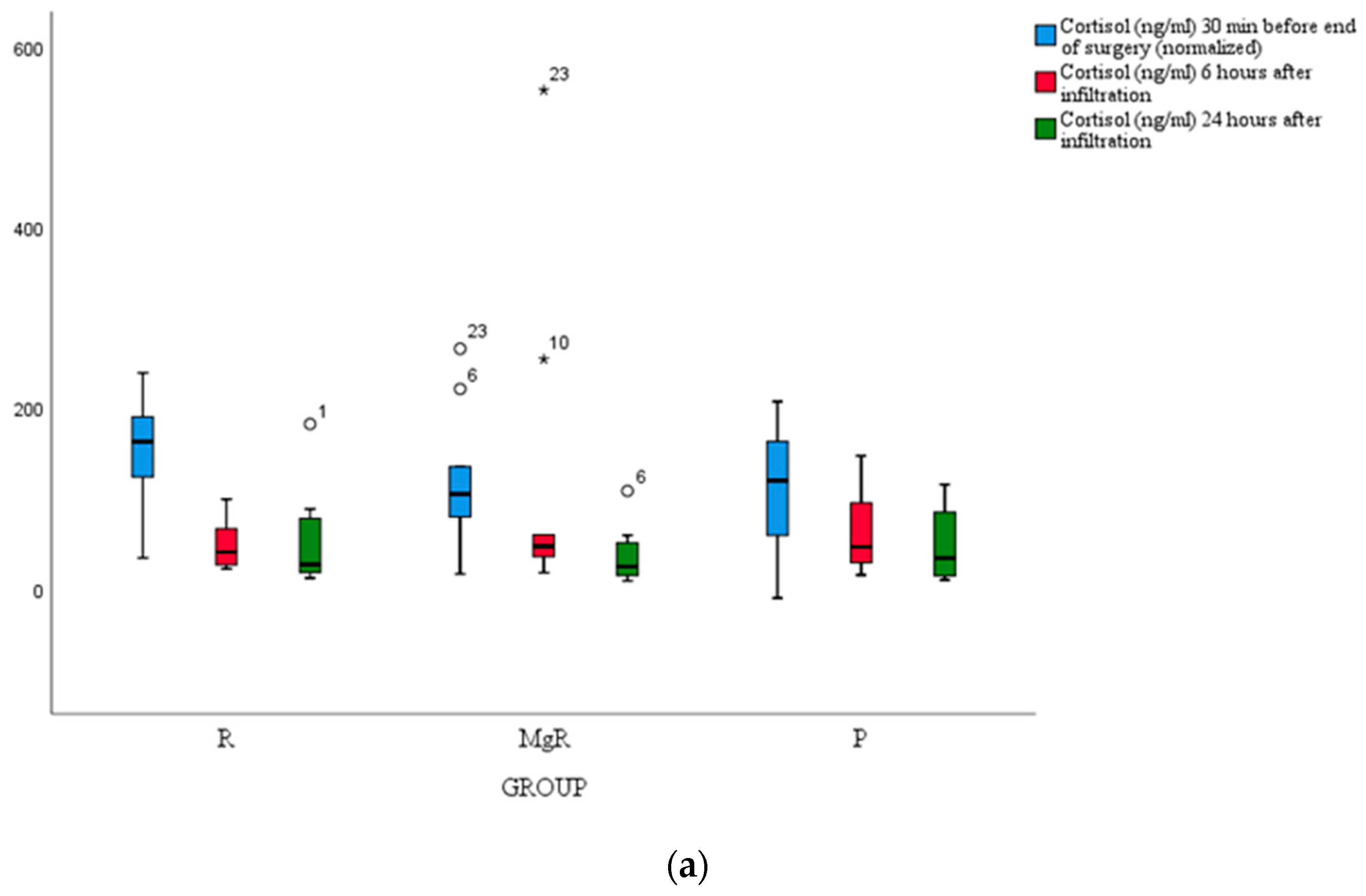
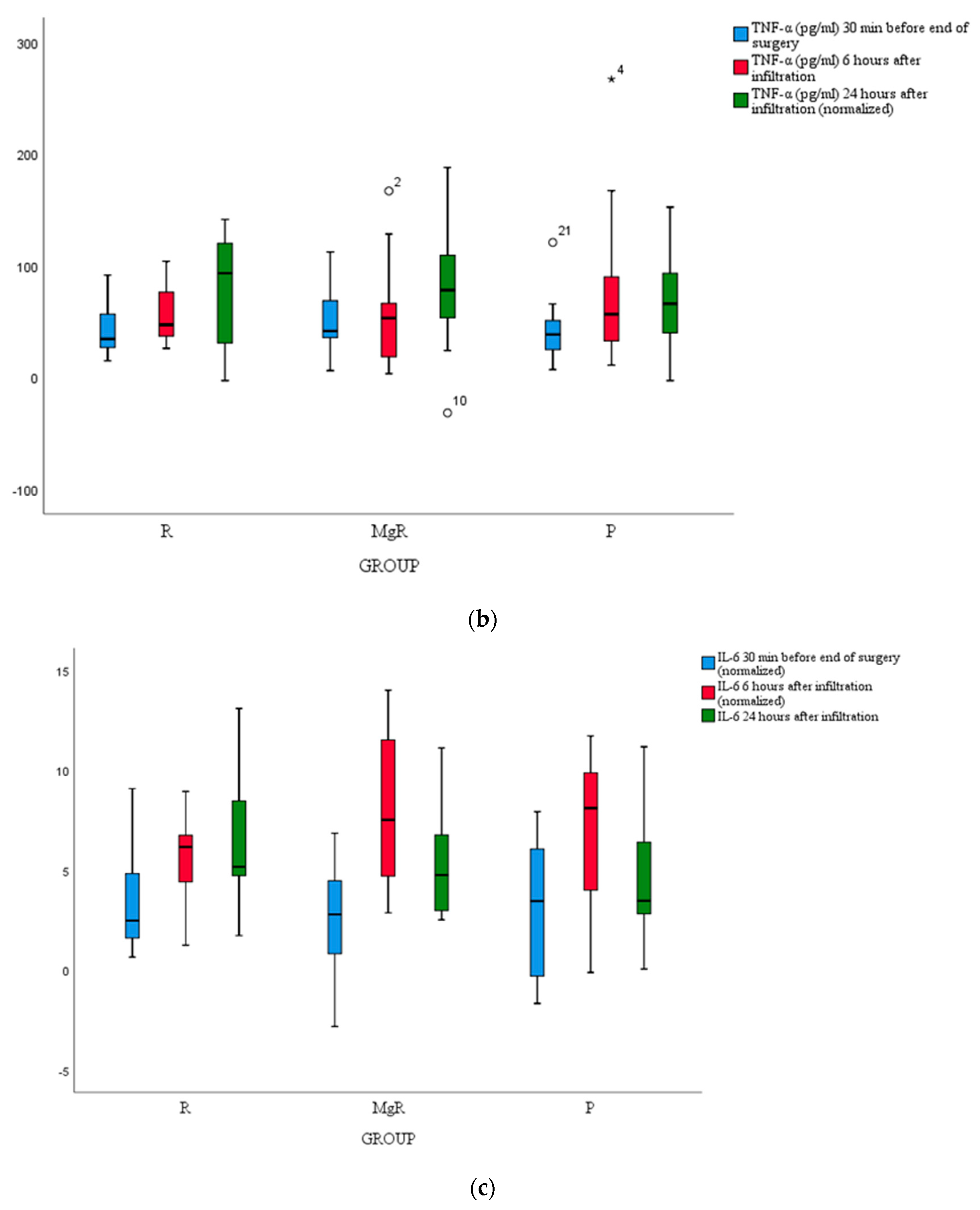
No statistically significant difference between groups was detected for cortisol, IL-6, and TNF-a levels (
Figure 4a, b, c).
3.5. Postoperative Complications
The most commonly reported adverse effect in the study was sore throat but no between groups difference was detected. However, the incidence of nausea and vomiting was similar between the treatment groups (
Table 3).
No immediate or short-term case of mortality was encountered, while wound complications at the injection sites were absent in this study group. Regarding local infiltration agents, no patient developed complications after infiltration.
3.6. Patients’ Satisfaction
Based on the median overall satisfaction score based on a 5-point scale, pairwise comparisons revealed a statistically significant difference (chi-square (2,66) = 16.00, p < 0.001). Patients in group RMg (5(1)) reported better overall satisfaction than patients in the placebo group (3 (1.5)), while a marginal statistical difference was found between patients in the R group (4, (1.75)) and patients in the RMg group.
4. Discussion
Postoperative discomfort following thyroid surgery is elicited by multiple causes. Incisional pain especially in the first postoperative day is the major component of this discomfort. The complexity of surgical maneuvers, odynophagia due to endotracheal intubation, and neck soreness caused by the hyperextension position are also incriminated. 9 In the context of Enhanced Recovery After Surgery (ERAS) protocols, which aim to reduce hospital length of stay, while providing faster recovery and functional restoration of the patient, the implementation of a structured analgesic protocol is of paramount importance. 11
Pain after thyroid surgery is poorly assessed by many physicians, misjudging the small incision. Although these procedures are characterized by short duration-, surgery-related pain is maximized one hour postoperatively and starts to decline 3 hours later.10 Postoperative pain may delay the patient's discharge as well as his return to everyday life.
Pain relief is most commonly achieved by administering NSAIDs and/or opioid analgesics. However, NSAIDs have been associated with potential cardiovascular events, postoperative bleeding, and renal impairment 12 while opioids are linked to side effects such as sedation, dizziness, nausea, vomiting, constipation, physical dependence, tolerance, and respiratory depression. 13
Based on the fact that the main innervation of thyroidectomy’s field originates from the superficial branches of the cervical plexus, regional techniques such as local anesthesia, and bilateral superficial and/or deep cervical plexus block, could represent a promising method. Local wound infiltration bears the advantage of being a safe and easy-to-implement procedure. It seems that thyroidectomy wound infiltration elongates the time frame until rescue analgesia. Local anesthetics used for thyroidectomy wound infiltration include the amides bupivacaine, lidocaine, and ropivacaine.
Ropivacaine is a long-acting amide local anesthetic, which reversibly binds and inactivates sodium channels in the open state, inhibiting sodium ion influx in nerve fibers and blocking the propagation of action potentials.15 Compared to other local anesthetics, it is less likely to penetrate large myelinated motor fibers acting selectively on the nociceptive A, B, and C fibers. Being manufactured as a pure S(-) enantiomer is characterized by significantly less cardiotoxicity and neurotoxicity.15,16
Thyroidectomy wound infiltration with ropivacaine has been studied in a few randomized clinical trials. Motamed et al17 compared thyroidectomy wound infiltration at the end of surgery with ropivacaine versus placebo and they observed a significant reduction in pain scales as well as opioid consumption during the patient's stay in the recovery room but not in the ward. Their observation was attributed to the low ropivacaine dose (20mg) used for wound infiltration. Comparing local infiltration with ropivacaine (75 mg), bupivacaine (50 mg), and placebo, Ayman et al18 observed a reduction in postoperative pain with ropivacaine but not with bupivacaine. This effect, however, was significant only during the first postoperative hour.18 Contrary to previous findings, Miu et al,14 could not prove any significant analgesic benefit with ropivacaine infiltration (75mg) or placebo at the end of thyroid surgery.
It appears that the efficacy of ropivacaine is present but limited when given alone. Both the efficacy and the duration of the method depend on surgical incision length, as well as the topical agent used.4 In the same clinical setting, the use of NSAIDs as adjuncts to ropivacaine infiltration promoted an enhanced analgesic effect.11,19
In our trial, we tried to achieve a long-lasting infiltrative technique by supplementing ropivacaine with magnesium sulfate. Magnesium sulfate reduces the NMDA receptor binding capacity preventing central sensitization to peripheral pain stimulus while preventing the established hyperalgesia. 20 It is even hypothesized to antagonize opioid-induced hyperalgesia as the analgesic effect appears to be better when remifentanil is administered in the anesthetic protocol rather than desflurane.21 The effects of magnesium sulfate on postoperative pain and opioid consumption have been studied in recent years in a variety of surgical procedures. It has been administered by various routes, including oral, intravenous, intrathecal, and epidural.
Regarding wound infiltration, magnesium sulfate has been applied, alone or as an adjunct, to prostatectomy, caesarian section, lumbar discectomy, and inguinal hernia repair procedures.
In laparoscopic prostatectomy cases, wound infiltration with magnesium achieved a superior analgesic effect compared to placebo, while in inguinal hernia repair, magnesium was less effective compared to local anesthetics infiltration at reducing opioid requirements.21,22 Similarly, in pediatric adenectomy, the combination of magnesium with levobupivacaine or ropivacaine reduced postoperative pain and the incidence of laryngospasm compared to the administration of levobupivacaine or ropivacaine alone.23,24 However, different results were obtained by Sun et al. They assumed that the addition of epinephrine to their solution masked the analgesic effect of magnesium.25
Notably, in lumbar discectomy procedures a superior nociceptive effect was attributed to the combined administration of ropivacaine or bupivacaine plus magnesium sulfate. 27 More recently, Dave et al applied the combination of bupivacaine and magnesium sulfate in rectal procedures. 28 Such operations require a deep level of anesthesia since the anorectal region is innervated from both the autonomic nervous system and the animal nervous system while postoperatively patients experience severe pain and discomfort. It was found that during the first 24 hours, the combined administration led to superior results in terms of pain management, less opioid consumption, and pain during defecation.
The supplementary use of magnesium sulfate in peri-articular injection drug mixtures after total arthroplasty procedures has also provided promising findings in terms of early postoperative pain control.29,30 Noteworthy enough is that no adverse effect was observed in any of the local infiltration studies with magnesium sulfate.
In the present study, we attempted to evaluate the analgesic efficacy of the tested infiltrative agents by assessing the magnitude of inflammatory markers' release namely cortisol, TNF-α, and IL-6, but we failed to detect any notable difference between study groups. A plausible explanation for this finding could be the fact that inflammatory response modulation was a secondary study endpoint, and presumably, our study was underpowered for this effect. Over and above, it appears that thyroidectomy-induced stress response is trivial considering that the average VAS score at rest and movement was maintained at clinically acceptable levels throughout the study course.14,31,32
Several limitations could be acknowledged in the present study. A possible drawback could be the absence of serum magnesium level determination as well as the recording of subsequent magnesium-related adverse effects. Secondly, the scale used to assess pain was a subjective scale and could be associated with bias since patients may interpret sore throat or neck pain as incisional pain. Finally, by conducting a single-center study our findings reflect the effect of this practice in a defined population subjected to a standardized surgical practice.
5. Conclusions
In conclusion, local wound infiltration with magnesium sulfate as an adjunct to ropivacaine can enhance the postoperative analgesic effect in comparison to either ropivacaine or placebo following total thyroidectomy, further demonstrating an advanced safety profile. Future large-scale studies are mandatory to enlighten the role of magnesium sulfate as an adjunct analgesic regimen for wound infiltration in this clinical setting.
Author Contributions
Stiliani Laskou: Data curation, writing – original draft. Georgia Tsaousi: Writing – review and editing, formal analysis. Chryssa Pourzitaki: Formal analysis. Georgios Papazisis: Validation. Isaak Kesisoglou: Resources. Konstantinos Sapalidis: Supervision, Project administration.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Aristotle University of Thessaloniki 18.01.2022/3.468.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Terris DJ, Snyder S, Carneiro-Pla D, et al. American Thyroid Association statement on outpatient thyroidectomy. Thyroid 2013;23(10):1193-1202.
- Bianchini C, Malago M, Crema L, Aimoni C, Matarazzo T, Bortolazzi S, et al. Post-operative pain management in head and neck cancer patients: predictive factors and efficacy of therapy, Acta Otorhinolaryngol. Ital. 2016;36:91.
- Kylie, J. Nabata, Rachel Guo, Anne Nguyen, Jill A. Osborn, Sam M. Wiseman. Superiority of non-opioid postoperative pain management after thyroid and parathyroid operations: A systematic review and meta-analysis, Surgical Oncology 2022;41: 101731.
- Laskou S, Tsaousi G, Pourzitaki C, Loukipoudi L, Papazisis G, Kesisoglou I, Sapalidis K. Local Wound Infiltration for Thyroidectomized Patients in the Era of Multimodal Analgesia. Medicina (Kaunas). 2023 Sep 14;59(9):1662. PMCID: PMC10534959. [CrossRef] [PubMed]
- Tauzin-Fin P, Sesay M, Svartz L, Krol-Houdek MC, Maurette P. Wound infiltration with magnesium sulphate and ropivacaine mixture reduces postoperative tramadol requirements after radical prostatectomy. Acta Anaesthesiol Scand. 2009 Apr;53(4):464-9. Epub 2009 Feb 18. [CrossRef] [PubMed]
- Eldaba AA, Amr YM, Sobhy RA. Effect of wound infiltration with bupivacaine or lower dose bupivacaine/magnesium versus placebo for postoperative analgesia after cesarean section. Anesth Essays Res 2013;7:336–340.
- Kundra S, Singh RM, Singh G, Singh T, Jarewal V, et al. Efficacy of Magnesium Sulphate as an Adjunct to Ropivacaine in Local Infiltration for Postoperative Pain Following Lower Segment Caesarean Section. J Clin Diagn Res. 2016 Apr;10(4):18-22.
- Donadi P, Moningi S, Gopinath R. Comparison of bupivacaine anδ bupivacaine plus magnesium sulphate infiltration for postoperative analgesia in patients undergoing lumbar laminectomy: a prospective randomised double-blinded controlled study. J Neuroanaesth Crit Care 2014;01:183–187.
- Lacoste L, Thomas D, Kraimps JL, Chabin M, Ingrand P, Barbier J, Fusciardi J. Postthyroidectomy analgesia: morphine, buprenorphine, or bupivacaine? J Clin Anesth 1997; 9: 189– 193.
- Ayman M, Materazzi G, Bericotti M, Rago R, Nidal Y,Miccoli P. Bupivacaine 0.5% versus ropivacaine 0.75% wound infiltration to decrease postoperative pain in total thyroidectomy, a prospective controlled study. Minerva Chir. 2012;67(6):511–6.
- Li, X., Yu L., Yang J., Tan H. Multimodal analgesia with ropivacaine wound infiltration and intravenous flurbiprofen axetil provides enhanced analgesic effects after radical thyroidectomy: A randomized controlled trial. BMC Anesthesiol. 2019;19:167.
- Ghlichloo I, Gerriets V. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) [Updated 2023 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK547742/.
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain Physician. 2008 Mar;11(2 Suppl):S105-20. [PubMed]
- Miu, M., Royer C., Gaillat C., Schaup B., Menegaux F., Langeron O., Riou B., Aubrun F. Lack of Analgesic Effect Induced by Ropivacaine Wound Infiltration in Thyroid Surgery: A Randomized, Double-Blind, Placebo-Controlled Trial. Anesth. Analg. 2016;122:559–564. [CrossRef]
- George AM, Liu M. Ropivacaine. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532924/.
- Graf BM, Abraham I, Eberbach N, Kunst G, Stowe DF, Martin E. Differences in cardiotoxicity of bupivacaine and ropivacaine are the result of physicochemical and stereoselective properties. Anesthesiology. 2002 Jun;96(6):1427-34.
- Motamed, C., Merle J.C., Combes X., Yahkou L., Saidi N.E., Degranges P., Dhonneur G. Postthyroidectomy pain control using ropivacaine wound infiltration after intraoperative remifentanil: A prospective double blind randomized controlled study. Acute Pain. 2007;9:119–123. [CrossRef]
- Ayman M, Materazzi G, Bericotti M, Rago R, Nidal Y, Miccoli P. Bupivacaine 0.5% versus ropivacaine 0.75% wound infiltration to decrease postoperative pain in total thyroidectomy, a prospective controlled study. MINERVA cHIR 2012;67:511-516.
- Karamanlioglu, B., Turan A., Memis D., Kaya G., Ozata S., Ture M. Infiltration with ropivacaine plus lornoxicam reduces postoperative pain and opioid consumption. Can. J. Anaesth. 2005;52:1047–1053. [CrossRef]
- Ko SH, Lim HR, Kim DC, Han YJ, Choe H, et al. Magnesium sulfate does not reduce postoperative analgesic requirements. Anaesthesiology. 2001;95(3):640-646.
- Lee C, Song Y-K, Jeong H-M, et al. The effects of magnesium sulfate infiltration on perioperative opioid consumption and opioid-induced hyperalgesia in patients undergoing robot-assisted laparoscopic prostatectomy with remifentanil-based anesthesia. Korean J Anesthesiol 2011;61:244–50.
- Razavi SS, Peyvandi H, Badrkhani Jam AR, Safari F, Teymourian H, et al. Magnesium Versus Bupivacaine Infiltration in Controlling Postoperative Pain in Inguinal Hernia Repair. Anesth Pain Med. 2015 Dec 5;5(6):e30643. [CrossRef]
- Karaaslan K, Yilmaz F, Gulcu N, Sarpkaya A, Colak C, Kocoglu H. The effects of levobupivacaine versus levobupivacaine plus magnesium infiltration on postoperative analgesia and laryngospasm in pediatric tonsillectomy patients. Int J Pediatr Otorhinolaryngol 2008;72:675–81. [CrossRef]
- Derbel R, Achour I, Thabet W, Chakroun A, Zouch I, Charfeddine I. Addition of magnesium sulfate to bupivacaine improves analgesic efficacy after tonsillectomy: A randomized trial and a CONSORT analysis. Eur Ann Otorhinolaryngol Head Neck Dis. 2022 Nov;139(6):327-331. Epub 2022 Jun 7. [CrossRef] [PubMed]
- Sun J, Wu X, Zhao X, Chen F, Wang W. Pre-emptive peritonsillar infiltration of magnesium sulphate and ropivacaine vs. ropivacaine or magnesium alone for relief of post-adenotonsillectomy pain in children. Int J Pediatr Otorhinolaryngol 2015;79:499–503. [CrossRef]
- Sane S, Mahdkhah A, Golabi P, Hesami SA, Kazemi Haki B. Comparison the effect of bupivacaine plus magnesium sulfate with ropivacaine plus magnesium sulfate infiltration on postoperative pain in patients undergoing lumbar laminectomy with general anesthesia. Br J Neurosurg. 2020 Dec 17:1-4. Epub ahead of print. [CrossRef] [PubMed]
- Hazarika R, Parua S, Choudhury D, Barooah RK. Comparison of Bupivacaine Plus Magnesium Sulfate and Ropivacaine Plus Magnesium Sulfate Infiltration for Postoperative Analgesia in Patients Undergoing Lumbar Laminectomy: A Randomized Double-blinded Study. Anesth Essays Res. 2017 Jul-Sep;11(3):686-691. PMCID: PMC5594791. [CrossRef] [PubMed]
- Dave S, Gopalakrishnan K, Krishnan S, Natarajan N. Analgesic Efficacy of Addition of Magnesium Sulfate to Bupivacaine in Wound Infiltration Technique in Perianal Surgeries. Anesth Essays Res. 2022 Apr-Jun;16(2):250-254. Epub 2022 Oct 7. PMCID: PMC9701323. [CrossRef] [PubMed]
- Wang, Qiuru MD*; Zhao, Chengcheng MM*; Hu, Jian MM; Ma, Ting MM; Yang, Jing MD, PhD; Kang, Pengde MD, PhD. Efficacy of a Modified Cocktail for Periarticular Local Infiltration Analgesia in Total Knee Arthroplasty: A Prospective, Double-Blinded, Randomized Controlled Trial. The Journal of Bone and Joint Surgery 2023: 105(5):p 354-362.
- Zhao C, Wang L, Chen L, Wang Q, Kang P. Effects of magnesium sulfate on periarticular infiltration analgesia in total knee arthroplasty: a prospective, double-blind, randomized controlled trial. J Orthop Surg Res. 2023 Apr 14;18(1):301. PMCID: PMC10105472. [CrossRef] [PubMed]
- Kilba¸s, Z.; Mente¸s, M.Ö.; Harlak, A.; Yi ˘ git, T.; Balkan, S.M.; Co¸sar, A.; Öztürk, E.; Kozak, O.; Tufan, C.T. Efficacy of wound infiltration with lornoxicam for postoperative analgesia following thyroidectomy: A prospective, randomized, double-blind study. Turk. J. Med. Sci. 2015, 45, 700–705. [Google Scholar] [CrossRef]
- Yücel A, Yazıcı A, Müderris T, Gül F. Comparison of lornoxicam and low-dose tramadol for management of post-thyroidectomy pain. Agri. 2016 Oct;28(4):183-189. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).