1. Introduction
Fossil fuels have been generally accepted as the primary energy source for the next 50 years, and the CO
2 emissions generated from these fuels severely impact climate change [
1]. This problem requires the use of developed net-zero carbon technologies in the future. The 2015 U.N. Climate Change conference in Paris witnessed the agreement of two extremely important long-term goals: significant reduction in the generated CO
2 emissions across all industries and achieving net greenhouse gas neutrality. The process of net-zero carbon refers to the cognitive balance between the generation of anthropogenic emissions from all industrial sources and the continuous removal from sinks by the next 50 years [
1,
2,
3].
Contrastingly, the growing global population is predicted to significantly increase the demand for energy supply during the coming decades. This prediction necessitates the emergence of efficient renewable energy alternatives. A prolific solution to the demanding challenges of energy supply and emission is using captured carbon dioxide as a valuable industrial feedstock for synthesizing numerous fuels and chemicals, which provides added value [
4]. CO
2 capture and utilization concepts concerning climate change and carbon management are frequently employed. Carbon capture and storage (CCS) refers to technologies that extract carbon dioxide from selective gas streams and compress the gas to its supercritical state. The supercritical gas is then sequestered in geological formations such as oceans or depleted hydrocarbon formations. Its high cost has hindered the Large-scale deployment of CCS despite government incentives, regulatory policies, and promises of mitigating large volumes of CO2 [
5].
Capturing and compressing the CO
2 gas accounts for about 75% of the total cost of CCS [
6]. Employing CCS technologies can reduce emissions from power generation industries and other industrial applications such as cement, oil refining, and biofuel. This analysis is from the 2013 report from the International Energy Agency (IEA) [
7] It was also predicted that about 3000 CCS projects will be implemented globally, storing over 7000 million tons of CO
2 annually [
8].
Recently, carbon capture and utilization technologies have garnered significant attention as a more suitable substitute for the permanent sequestration of CO
2 [
9]. The conversion of the captured CO
2 to valuable industrial and petrochemical products has been proven to be a viable option. As an alternative to traditional petrochemical feedstocks, CCU technologies treat the captured carbon dioxide as a renewable source [
10]. The thermodynamic stability of CO
2 makes it extremely difficult to convert and use in chemical reactions, even with the large benefits that CCU offers over CCS [
11].
The main goal of this study is to present a review of the latest advancements in carbon capture and utilization technologies, with significant emphasis on carbon management. This review also gives a broad overview of the opportunities and challenges to expect in the future. By exploring the technological advances, potential benefits, and challenges associated with these approaches, this research provides valuable insights to the ongoing discussion on sustainable carbon utilization and management.
2. Available Options for CO2 Capture: Analyzing the Challenges and Opportunities
Reducing the carbon intensity of power generation and CO
2 capture technologies can directly relate to post-combustion and precombustion processes. Post-combustion carbon capture refers to removing carbon dioxide from flue gas streams, while precombustion is the systematic development of sophisticated reduced carbon-intensive combustion mechanisms. Processes describing precombustion systems comprise integrated gasification combined cycle (IGCC) and oxyfuel combustion, which utilizes purified oxygen fuel [
12]. Results from recent techno-economic analyses have shown that to reduce electrical costs and increase combustion efficiency significantly, energy-intensive CO
2 capture technologies need to be employed.
The capture technology suitable for a given industry can be determined from the source of carbon dioxide used in the industry and the type of industrial process involved. The source of CO
2 generation plays a pivotal role in determining the energy costs associated with CO
2 capture. In industries such as petrochemical production plants, highly concentrated CO
2 effluents are produced, while power plants produce lower concentrations of carbon dioxide, necessitating greater energy for recovery [
13]. However, the highest source of CO
2 comes from the latter, which poses a huge challenge for the energy sector. Furthermore, supercritical carbon dioxide combustion processes that utilize re-generated carbon dioxide and function using its supercritical state are considered effective in addressing anthropogenic emissions.
Compared to the traditional steam cycle, utilizing supercritical CO
2 as an operating fluid in a power cycle has increased the plant’s energy efficiency in several dynamic contexts. Studies have also demonstrated that using carbon dioxide as a working fluid instead of oil increases steam turbine efficiency [
14]. The disadvantage of this method is that purified oxygen is required for use. Moreover, liquid and gaseous CO
2 recirculation are two possible routes. The former entails liquid CO
2 that has undergone cryogenically treated CO
2. Around 45% of global CO
2 emissions are typically attributed to power plants alone, which presents substantial potential for CCU and CCS alternatives to capture CO
2. Compared with other options, the commercial Implementation of CO
2 capture technologies by post-combustion presents a greater economic influence on the cost reduction of CO
2 capture [
15]. Considering the best-case approach, utilizing a newly developed power plant equipped with advanced post-combustion capture technologies for capture would cost an estimated 56 USD per ton, resulting in a 62% energy penalty for the plant. Commercial applications of carbon capture already exist in the natural gas and chemical industries [
10].
In the recently completed Boundary Dam 110 MW Power Station, SaskPower presented the industrial scale capture of post-combustion CO
2 from coal-fired flue gas [
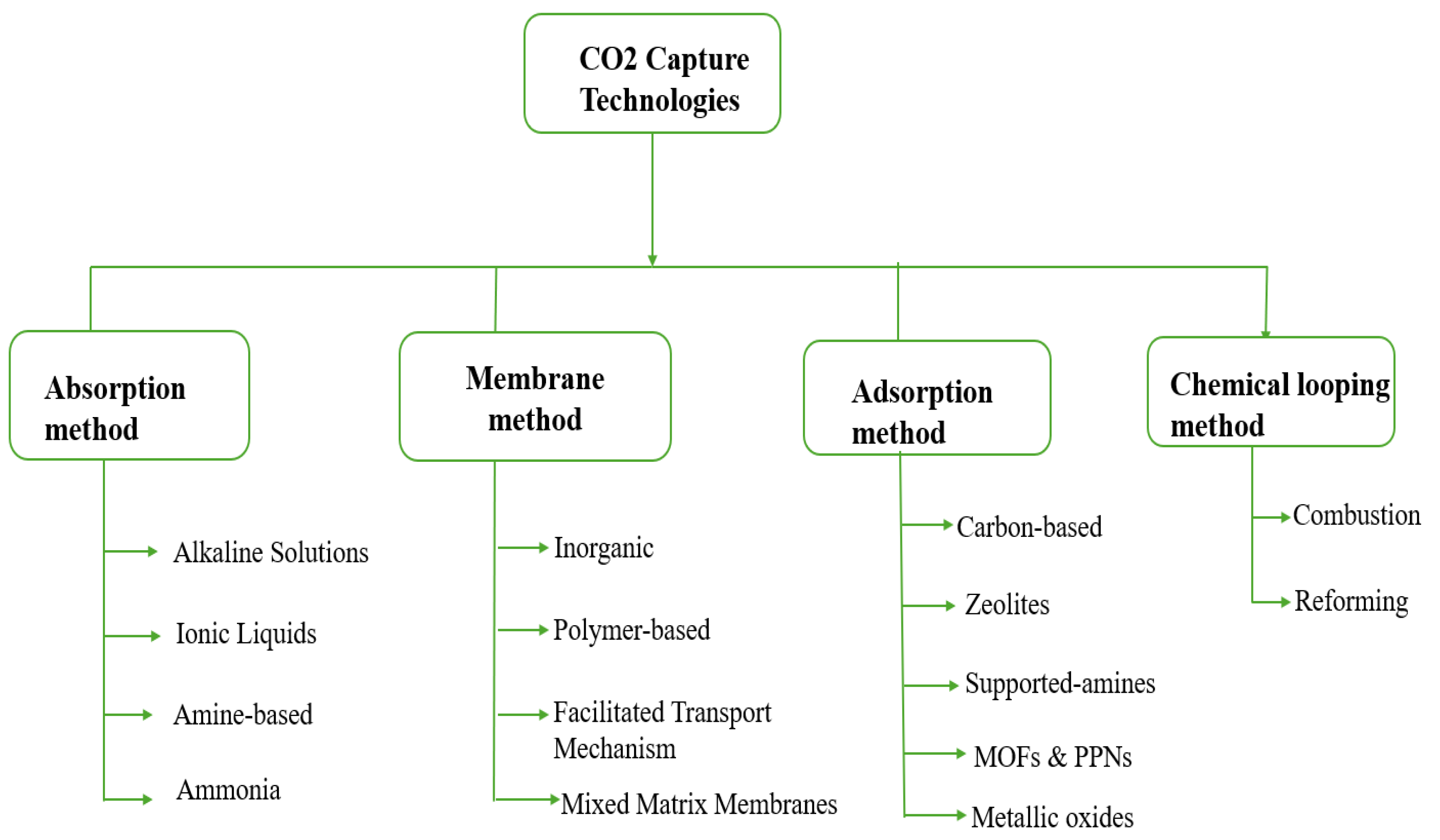
16]. The different capture processes that have been studied over the last few decades in both industry and academia are presented in
Figure 1. These processes will be further explained in the next section of this study.
2.1. CO2 Capture Technologies: By Absorption
CO
2 capture by absorption is the most developed separation technique available in petrochemical industries. This technology has been heavily utilized for precombustion capture and post-combustion capture. The most widely recognized technique for post-combustion CO
2 capture used in several industries is the chemical Absorption of aqueous ammonia and amine-based solvents. Commercial physical absorption technologies available for petrochemical and industrial applications include Rectisol, Fluor, Purisol, and Selexol [
17,
18,
19,
20].
However, their low working capacity is the primary barrier to their widespread application in CO
2 capture processes. The huge energy requirements for solvent regeneration are responsible for the high energy penalties associated with absorption processes despite absorption being a well-researched and mature separation technique that is very efficient in CO
2 capture. Although heat integration helps to lower energy consumption in some industries, such as power plants, it is impossible to achieve reduced energy consumption in `other industries like cement, iron, or steel [
21,
22,
23]. Furthermore, other known operational constraints pose serious obstacles, i.e., corrosion and a high-water makeup volume.
Chemical absorption usually depends on thermal swing regeneration; therefore, developing effective absorption-based CO
2 capture processes depends on the choice of solvents with the best possible integration of thermal and physical features. Chemical solvents such as piperazine (PZ) and its byproducts are superior chemical solvent substitutes due to their low chemical reactivity, quick reaction kinetics, and, most importantly, low regeneration energy [
24]. An additional method to improve the functionality of ionic liquids (ILs) is to include functional groups such as carboxylate anions, amine, and amino acid groups. Adding molecular groups to ILs could eventually result in using ILs as CO
2 absorption solvents [
4].
Another crucial factor to consider is the disposition between the reaction kinetics and the heat generated during the reaction process. While using thermally stable solvents has significantly reduced separation processes, solvents with heat of absorption greater than 60 KJ/mole are more effective in reducing energy consumption during chemical absorption. The stability of chemical solvents is often decreased by poisoned impurities, predominantly found in flue gas and other effluent streams [
5]As a result, it is important to consider resistance to solvent oxidation and impurity tolerance as significant performance indicators when developing new solvents for CO
2 absorption technologies [
25].
Process improvements are just as important for the next generation of absorption technology to be scaled up as advances in material development and the selection of energy-efficient solvents. Accordingly, innovative CO2 capture solutions could be provided by emerging absorption technologies with enhanced process configurations that provide effective heat integration strategies. Some of these heat integration strategies include intercooled absorbers and inter-heated strippers.
2.2. CO2 Capture Technologies: Membrane Separation Process
The use of membranes as a technique for gas separation provides a method that is considered more environmentally and energy-friendly than other separation techniques. A pressure gradient through the membrane fuels the degree of permeation in CO
2 capture technologies by membrane separation. This process is commonly conducted continuously and uniformly. Some important factors that determine the gas separation performance of the membrane include its material, configuration, design, and operational constraints [
26,
27,
28,
29]. Several studies have been conducted on membrane separation to remove CO
2 from the waste gas streams of power plants. The utilization of membrane technology for CO
2 capture by post-combustion is difficult because of the low pressure of flue gas streams. Inorganic membranes have been proven to withstand high-temperature conditions and often portray good mechanical stability. However, their economic constraints hinder the large-scale implementation of these membranes.
Contrastingly, membranes are more efficient in processes such as multistage operations and stream re-generation, often considered obstacles in membrane separation. Several porous inorganic membranes were previously analyzed for CO
2 capture from flue gas and other discharge streams. Despite having mechanical stability and the ability to tolerate high temperatures, inorganic membranes are expensive, which prevents them from being widely used. Inorganic membranes are yet to be utilized in large-scale processes and are still not scaled up. The primary obstacles to the widespread use of inorganic membranes remain the huge cost of fabrication as well as their durability and reliability [
30,
31,
32,
33].
On the other hand, easily formulated polymer-based membranes embodied in concentric-fiber units have proven to be excellent alternatives for the industrial application of membrane separation on a large scale. Furthermore, considering the case of polymer-based synthesized concentric-fiber membranes arranged in units, inorganic membranes cannot match the packing efficiency provided by polymer-based membranes. Nevertheless, polymer-based composite membranes perform poorly in separation compared to inorganic membranes.
The process of CO
2 capture using available polymer-based membranes is affected by factors such as low CO
2/N
2 selectivity, susceptibility to impurities, and molecular stability, particularly for operations requiring high-pressure conditions. Polymer-based membranes must maintain a relatively high permeability and a minimum selectivity ratio of 200 for CO
2 over N
2 to be economically viable for post-combustion CO
2 capture [
34]. High permeability lowers the investment cost of the membrane separation process because it disregards the requirement for a large membrane surface area to achieve adequate separation performance. Polymer-based membranes are used in the process of natural gas sweetening. Membrane Technology & Research, Inc (MTR) has achieved a CO
2 capture rate of 90% from an 880 MW coal-fired power plant using a membrane-based experimental-scale process [
29]. The high selectivity of facilitated transport membranes (FTM) has been demonstrated. However, these membranes have long-term stability issues and can be poisoned by minute amounts of acidic gases in the flue gas stream.
Concentric-fiber membranes, as opposed to spirally configured membranes, offer more compact modules, high surface area-to-volume ratios, and the ideal configuration for high rates of production amongst the various commercially available types of membranes [
30,
35]. Advanced membrane development is made possible through composite concentric fiber membranes, which are made of a highly porous polymer-based substructure [
30]. This polymer-based substructure is often supported by a fine selective layer with a diameter of less than a micrometer [
36]. Mixed matrix membranes (MMM) are formed from the dispersion of highly selective molecular-sieve particles. The scaling advantage of polymer-based membranes is integrated with the separation efficiency of molecular-sieve materials to serve as a promising contactor.
MMMs offer a solution beyond the recognized compromise threshold of polymer-based membranes and the prevailing challenges related to the cost and processing of inorganic membranes. They are currently conceptual and will not be used in industries anytime soon. In addition, their existing fabrications are expensive and intricate. Future developments in membrane CO
2 capture should consequently concentrate on composite membranes that can outperform the best membranes presently commercially available by utilizing both polymer-based and inorganic components [
37,
38,
39,
40]An efficient understanding of the challenges regarding developing complex integrated systems for emerging CO
2 membrane separation technologies requires enhancing integrated systems through various configurations and re-enforcing the material systems and the process methodologies.
2.3. CO2 Capture Technologies: By Adsorption
Many industries adopt the method of capturing carbon dioxide through porous solid materials as an effective approach to removing carbon dioxide from their flue gas emissions selectively. Several absorbents have been tested and studied to extract CO
2 from the flue gas streams of precombustion and post-combustion processes. In industrial applications, absorbents fall into two categories: high-temperature materials and low-temperature materials. The latter refers to carbonaceous materials such as molecular sieve, activated carbon, and graphene, while the higher temperature materials include calcium oxides and most double salts [
41,
42,
43,
44,
45].
Most available low-temperature materials are physisorbents in nature, while high-temperature are classified as chemisorbents. Anchored amines are among the current low-temperature adsorbents available; they interact strongly with CO
2 as physisorbents [
46]. The chemical properties of the adsorbent determine the efficacy and the economics of the adsorption processes. These properties must be considered in addition to system design and operational requirements; absorbents must satisfy certain requirements to be effective for industrial-scale separation in any gas separation process [
47,
48]Some metrics to measure the efficiency of the adsorption separation process include high selectivity and working capacity, durability, and rapid kinetics.
To achieve the maximum capture efficiency of any adsorption process, the physiochemical characteristics of the adsorbents are not the only crucial parameters that require optimization. Other critical parameters that can be optimized include cycle configuration, cycle time, operating conditions, and number of beds [
46]. In most laboratory-scale studies, the practical conditions performance of adsorptive CO
2 capture has often been overlooked. The main challenge inhibiting the large-spread use of MOF materials is their extensive production scale and water stability despite their exceptional capacity and selectivity towards CO
2.
Developing hybrid adsorbents such as MOF-functionalized amines may solve the issues with traditional absorbents and provide a highly successful and affordable capture method for post-combustion capture processes [
49]. The process of CO
2 capture by adsorption using PSA has garnered significant interest because it is economical and has low energy requirements. However, using this method for low CO
2 recovery is still a challenge. These scale-up difficulties might be overcome by cutting-edge methods that provide options for heat management. The heat management processes include monolithic structures with ideal thermal management or concentric-fiber adsorbents using a cooling agent engrafted in the bore [
50]. To effectively enhance the energy reduction process and further shorten the time required for cooling in the TSA operation. Design modifications incorporating innovative indirect heating processes such as heat exchangers, heating coils, and heating jackets should be implemented in adsorption separation techniques. It is possible to overcome the challenges of the absorption processes using adsorption-based separation, but the required or suggested technologies are still in the development phase and are not economical [
50,
51,
52,
53].
Moreover, extensive operation has yet to be fully implemented. It is important to align the assessment of the efficiency and process considerations of high-performing adsorbents with their design, development, and evaluation process. Furthermore, the absorbent features that will be employed should be considered in any cyclic process’s design and optimization [
48,
54].
2.4. CO2 Capture Technologies: By Chemical Looping
The two primary methods through which carbon dioxide and water are naturally separated from flue gas streams are known as chemical-looping combustion (CLC) and chemical-looping reforming (CLR) [
55]. These processes are economical CO
2 capture methods due to thermal energy requirements. During the reaction, they can significantly reduce the formation of NO
x. Combining IGCC with chemical looping produces syngas as a byproduct of CO
2 captured by precombustion. The large-scale applications of these technologies heavily rely on the availability of appropriate oxygen carriers because they rely on metal oxides to transport oxygen between fuel reactors and the air [
56].
Transition metal oxides are important for the chemical looping process due to their physical and chemical properties and environmental and economic impacts. The most important characteristics to consider are their reactivity in their processes’ reduction and oxidation cycle. Also, the transition metal oxides must be fully combusted to attain optimal combustion efficiency. The oxidation catalysts discussed above can only partially meet specified requirements [
55].
While high pressures may offer advantages for CCS (carbon capture and storage) applications, overcoming the challenge of achieving high overall efficiency through high-pressure operation is another obstacle in the chemical-looping processes. According to recent energy analysis, it has been established that the efficiency penalty associated with the calcium-looping post-combustion capture process can often be as low as 6-8% [
57,
58,
59]Including heat-recovery beds to transfer heat between the CO
2 stream and the solid particles entering the calciner is a significant improvement, resulting in decreased energy penalties.
Some pilot-scale studies are currently being conducted. However, most of the available chemical technologies being used in the energy generation sector are still in the experimental or initial development stages [
60,
61,
62]. It is anticipated that these technologies will not be fully deployed by 2030 [
7]. It is important to address the technical challenges resulting from materials development and process optimization to advance the employment of present innovative chemical looping technologies. Reduction of equipment costs and a significant increase in capture efficiency can be attained by utilizing new chemical-looping processes based on the principles of metallic oxides such as calcium and copper. These metal oxides can integrate exothermic and endothermic chemical reactions within a single solid matrix.
2.5. CO2 Capture Technologies: By Direct Capture from Air
The selective removal of carbon dioxide directly from the environment is referred to as direct air capture (DAC). Recently, this process has been of interest to researchers due to its potential to reduce the difficulties associated with moving significant amounts of carbon dioxide from localized emission sources to locations considered appropriate for geological sequestration. Furthermore, if widely used, DAC can lower atmospheric CO
2 levels, unlike traditional capture methods targeting only larger point source emitters, thereby reducing the CO
2 accumulation rate [
32,
63,
64]. The idea behind DAC is practically related to adsorption-based CO
2 capture because the CO
2 in the air is diluted at about 400 ppm. Nevertheless, there are significant technological obstacles to overcome.
It is important to utilize materials with efficient binding affinities and high CO2/N2 selectivity during DAC processes due to the extremely low concentration of CO
2. Some of these materials include MOFs, alkali-based carbonates, and a variety of aqueous oxides, such as sodium and potassium oxide solutions [
32]. Although a high-performance material may be suitable for CO
2 capture from large point sources, it might not be efficient for DAC processes [
64]. Current thermodynamic studies have shown that as the absorption enthalpy increases at low carbon dioxide concentrations, the TSA process becomes more energy-efficient than the PSA process for DAC technology applications. The estimated cost of applying DAC technology is higher than capturing CO
2 from large point sources. Clarifying the underlying assumptions is necessary to address the significant uncertainty in the DAC process design factors and cost analysis.
Furthermore, inexpensive, and durable materials are required for air capture applications on an extensive basis. It is important to minimize the cost associated with the adoption and commercial Implementation of any DAC process in its conceptual stages. A possible means of improving the viability of the DAC process is to minimize the energy requirements by using distributed renewable energy sources such as thermal energy derived from solar systems.
2.6. CO2 Capture Technologies: By Hybrid processes
A viable method for providing an economical and durable capture path is hybrid separation, which merges two or more subsystems for capture. Several applications have employed hybrid processes, particularly in gas separations. Hybrid processes aim to reduce the overall cost of separation while improving separation efficiency by using dual or multiple separation systems in series or parallel configurations. Numerous studies have been conducted to determine the viability of diverse hybrid solutions for CO
2 capture, examples being membrane-PSA and membrane distillation [
65].
American Air Liquide recently developed a promising hybrid system that could minimize energy costs. The system utilizes hybrid membrane-cryogenic distillation technology to capture CO
2 at sub-ambient temperatures of -50°C to -20°C [
66]. This hybrid process is designed to improve the efficiency and selective capacity of the membrane module by reducing the energy and investment costs associated with the CO
2 capture process. The widespread adoption of this technology on a large scale is dependent on a successful heat-integration strategy, which requires the cooling of all feed gases to sub-ambient temperature conditions [
65,
67,
68,
69].
The high-pressure membrane permeation is the main concept behind the success of hybrid membrane systems, as it is used in pressurizing and absorbing high-pressure materials. Moreover, membrane permeation might be added to the blowdown phase of PSA system cycles to allow the operating pressure of the PSA to catalyze efficient membrane permeation. These two solutions can help reduce the cost of employing heavy-duty pumps. Another process that can efficiently reduce the energy expenditure for CO
2 capture is using a unified system called the hybrid pressure-temperature swing adsorption process (PTSA) [
70,
71]. This system can operate at significantly moderate pressure and temperature conditions, reducing energy costs [
66].
The deep vacuum required in PSA to produce highly concentrated CO
2 under elevated temperature conditions is significantly reduced using the PTSA setup. Using the PTSA setup results in economical operating conditions, rapid cycles, and, most importantly, the durability of the adsorbents. The goal of designing PTSA systems is to achieve an efficient heat and mass transfer process during the respective phases of desorption and adsorption [
71].
Therefore, it is imperative to consider that hybrid processes operate by combining dual or multiple CO2 capture paths as innovative methods to enhance the economics and efficiency of separation, given the present state of innovative technologies. Understanding and commercializing these hybrid systems will require extensive research that considers uncertainty factors and examines them from the perspective of viability, process design, and, most importantly, environmental, and economic considerations.
2.7. Overall Comparison of CO2 Capture Technologies: Advantages and Disadvantages
An in-depth analysis of various CO
2 capture technologies has been presented, encompassing absorption, membrane separation, adsorption, chemical looping, direct air capture, and hybrid processes.
Table 1 summarizes the advantages and disadvantages of each technology.
In general, the landscape of CO2 capture technologies presents a spectrum of advantages and disadvantages, each catering to specific industrial needs. Despite significant advancements, most technologies are still in the development phase, awaiting widespread application. Bridging the gap between material properties and process performance is crucial for successful implementation. Integration of the expertise of material scientists and engineers is paramount to unravel the intricate correlation between material properties and hybrid process parameters. This interdisciplinary approach is fundamental for designing next-generation capture technologies that are distinctive, comprehensive, and aligned with environmental and economic considerations. The continuous collaboration between these fields will be instrumental in unlocking the full potential of CO2 capture, bringing us closer to sustainable and efficient solutions for mitigating climate change.
3. Evaluating the Challenges and opportunities Encountered in CO2 Utilization Processes
CO
2 utilization across the years has been considered a major practical means of providing renewable energy sources for manufacturing several useful byproducts. CO
2 utilization must be safe, environmentally responsible, and commercially feasible [
73]. The primary usage pathways for CO
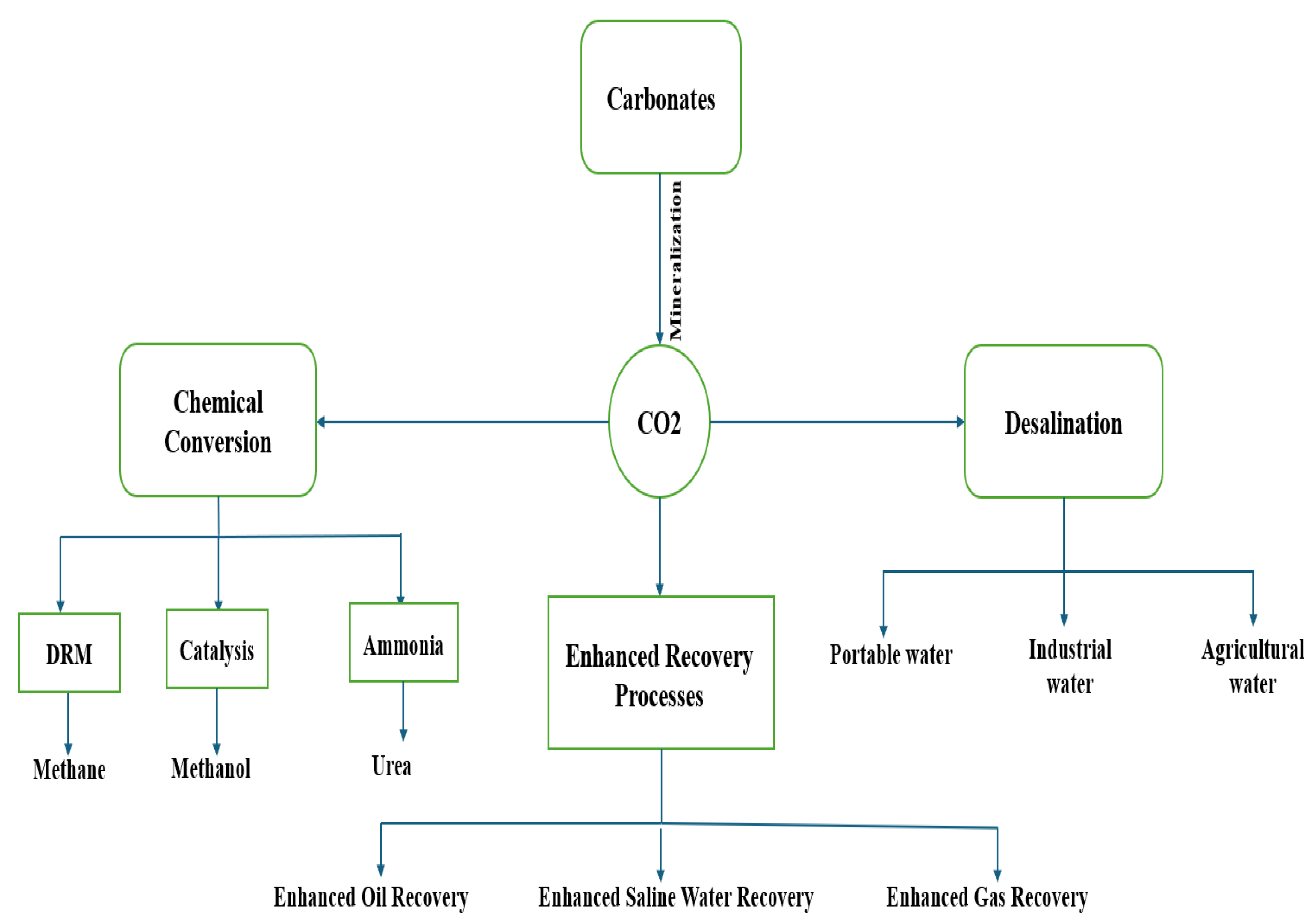
2 can be classified into enhanced oil recovery (EOR), chemical conversion, mineralization, and desalination processes.
Figure 2 depicts the diverse applications of carbon dioxide. The US Department of Energy classifies CO
2 utilization technologies into three main branches: EOR, mineralization, and the production of cement-polycarbonate plastics.
The ammonia synthesis process primarily generates CO
2, as CO
2 is produced as a byproduct. CO
2 is also produced from fermenting and synthesizing ethylene oxide in crude oil refineries [
4]CO
2 is used as a raw material in various processes such as preserving food, beverage industry, water treatment, petrochemical processes, and EOR [
5,
74]. Nevertheless, currently, industries only utilize about 1% of the total carbon dioxide emitted globally as raw [
75,
76]. The captured CO
2 should be used to produce valuable fine chemicals and transportation fuels.
3.1. Utilization of CO2 for Enhanced Oil Recovery Process
CO
2 is utilized to improve the recovery of valuable subsurface products. Depending on the type of formation and reserves, it can be employed in oil reservoirs as CO
2-Enhanced Oil Recovery (CO
2-EOR), in gas formations as CO
2-Enhanced Gas Recovery (CO
2-EGR), and in saline aquifers as CO
2-Enhanced Deep Saline Water/Brine Recovery (CO
2-EWR) [
77]. Herein, the diverse applications of CO
2 in the enhanced recovery process, encompassing various geological formations. The utilization of CO
2 in these recovery methods involves distinct operational and process mechanisms. Numerous studies have delved into these aspects, providing valuable insights into the efficacy and intricacies of CO
2-enhanced recovery across different domains [
78,
79].
3.1.1. CO2-Enhanced Oil Recovery (CO2-EOR)
Enhanced oil recovery involves the injection of substances into the reservoir to restore formation pressure and liberate any trapped hydrocarbons [
80]. In the case of the CO
2-EOR process, the crude oil is released from the reservoir formation by the injection of CO
2. Once the mixture of CO
2 and crude oil is brought to the surface, the separated CO
2 from the well effluent is reinjected into the formation to commence the cycle. Compared to other conventional oil recovery techniques, this process frequently produces more barrels of oil per reservoir [
81,
82]CO
2 flooding is a widely embraced and effective technique for enhanced oil recovery (EOR). Its effectiveness is attributed to CO
2’s role in simplifying oil extraction to the surface, achieved through its expansion and the reduction in oil density [
77].
While naturally occurring CO
2 is used in most CO
2-EOR systems, recent studies have focused on using the carbon dioxide extracted from potentially harmful industrial gas streams [
83]. There are two main techniques for conducting CO
2-EOR processes, namely water alternating gas (WAG) and continuous gas injection (CGI) [
84]. The former produces higher oil recovery. An intermediate hydrocarbon, like propane, can be added to CO
2-EOR to increase recovery efficiency by increasing the diffusion coefficient and displacement efficiency [
85].
In general, the effectiveness of the CO
2-EOR process hinges significantly on the temperature and pressure conditions within the reservoir formation [
86]. CO
2 EOR techniques are often faced with several challenges. For instance, the fluid characteristics and capillary pressure of the reservoir formation reduce the efficiency of the CO
2 flooding process because of the heterogeneity of the hydrocarbon formation between drilled wells [
87]. In addition, several factors are required for the successful execution of CO
2-EOR processes, such as production logs, fluid production rates, and compensated neutron logs (CNL) [
87]. Despite these limitations, many research studies have been on the efficiency of CO
2- EOR & EGR processes, which are projected to increase. Practically, the technology of CO
2-Enhanced oil and gas recovery is a promising strategy that can be applied to most types of reservoirs. However, EOR processes account for only about 3% of the global CO
2 utilization. The number of hydrocarbon formations utilizing CO
2 for enhanced oil recovery is steadily increasing even though the price of CO
2 has significantly reduced its advancement in EOR applications [
88,
89,
90].
3.1.2. CO2-Enhanced Gas Recovery (CO2-EGR)
CO
2-enhanced gas recovery (CO
2-EGR) is a mechanism employed in the energy industry to enhance natural gas extraction from mature reservoirs. This technique involves the injection of carbon dioxide (CO
2) into depleted oil and gas fields, leveraging the unique properties of CO
2 to stimulate increased hydrocarbon production [
78]. As the injected CO
2 interacts with the reservoir fluids and rock formations, it serves multiple purposes. Firstly, CO
2 acts as a displacement agent, improving sweep efficiency by reducing the reservoir’s residual oil and gas saturation [
91]. Secondly, the injected CO
2 alters the properties of the reservoir fluids, reducing oil viscosity and enhancing the mobility of hydrocarbons. This process extends the productive life of mature fields and contributes to the efficient utilization of existing energy resources [
82].
Beyond its role in enhanced gas recovery, CO2-EGR is crucial in carbon capture and storage (CCS) efforts. The injected CO
2 is sequestered underground, preventing its release into the atmosphere, and mitigating greenhouse gas emissions. This dual benefit of enhancing gas recovery while simultaneously addressing environmental concerns aligns CO
2-EGR with the broader objectives of sustainable energy practices [
92]. Ongoing research in this field focuses on optimizing injection strategies, understanding reservoir interactions, and developing technologies that maximize hydrocarbon recovery and environmental impact mitigation [
86,
93]. As the energy industry evolves towards cleaner practices, CO
2-EGR is a promising approach combining enhanced resource extraction with environmental responsibility.
3.1.3. CO2-Enhanced Water/Brine Recovery (CO2-EWR)
CO2-enhanced water or brine recovery (CO
2-EWR) is an innovative process utilized in subsurface resource management, particularly in geothermal energy and unconventional oil and gas extraction. Unlike traditional enhanced oil recovery (EOR) methods that primarily focus on hydrocarbon extraction, CO
2-EWR involves injecting carbon dioxide (CO
2) into underground formations to improve the recovery of water or brine resources [
78,
81]This technique is particularly relevant in geothermal reservoirs, where the injection of CO
2 can enhance fluid circulation, increase permeability, and improve heat transfer efficiency [
94]. In unconventional oil and gas, CO
2-EWR can be applied to optimize water recovery, ensuring more sustainable and efficient use of water resources in hydraulic fracturing operations [
95].
The CO
2-EWR process relies on carbon dioxide’s unique properties to influence the subsurface fluids’ physical and chemical characteristics. The injected CO
2 can alter the viscosity of water or brine, promoting enhanced fluid flow and, in turn, improving the overall recovery rates [
96]Additionally, the method contributes to carbon capture and storage (CCS) by sequestering CO
2 underground, addressing environmental concerns associated with greenhouse gas emissions. As researchers delve deeper into the potential applications and optimization of CO2-EWR, it represents a promising avenue for achieving more sustainable practices in subsurface resource extraction, balancing the imperative for resource recovery with environmental responsibility [
15,
97].
3.2. CO2 Utilization: Conversion of CO2 into Fuels and Petrochemicals
The use of CO
2 is predicted to help overcome the several challenges of implementing CCS on a large scale, such as high financial costs, commerciality, and durability. It also makes the CO
2 capture process beneficial and provides the potential to partially replace fossil fuels as the primary source of energy [
20]. It may also pave the way for generating sustainable technologies complementing present fossil fuel resources.
3.2.1. CO2 Utilization: Conversion of CO2 into Fuels
The optimal method of CO
2 utilization is its conversion to fuels. Harnessing captured CO
2 as a feedstock can produce several compounds, including methane, methanol, syngas, and alkanes. Some industries that use the fuel produced from CO
2 include fuel cells, powerplant and transportation industries [
5]. There are many ways for CO
2 to be used to produce fuels. Given its thermodynamic stability, substantial heat and a considerable catalyst supply are essential for achieving significant fuel production through CO2 utilization [
98]. The production of fuels from captured CO2 primarily involves two crucial processes: hydrogenation and dry reforming of methane (DRM) [
99].
The hydrogenation of CO
2 is a promising approach for using CO
2 due to its potential to successfully recycle CO2, store hydrogen, fuel generation, and, most specifically, its capacity to resolve the challenge of electrical energy storage [
75]. The dry reforming of methane is regarded as one of the most efficient routes for the Fischer-Tropsch (FT) process, which produces methanol as a byproduct as well as other significant liquid fuels [
38,
73,
100,
101]. One of the major challenges to consider in the hydrogenation of CO
2 is the source of the hydrogen from fossil fuels because it could lead to an increase in CO
2 emissions into the atmosphere [
102,
103,
104].
To effectively reduce the amount of CO
2 emission generated from hydrogenation, renewable energy sources such as solar, biomass, and wind can be considered suitable substitutes for fossil fuels [
4]. The low volumetric gas density of methane is the property that makes it inapplicable in the transportation industry [
98]. More specifically, methane has a relatively high global warming potential. It will be economically and environmentally inappropriate for the CO
2 capture process to produce large amounts of methane because it is already abundant in natural gas and other hydrocarbon gases. Therefore, it is more beneficial to produce methanol through CO
2 hydrogenation [
105,
106].
Activating hydrocarbon bonds over the existing copper-based catalysts to produce methanol is very difficult. The catalysts previously tested for this process have yet to yield good results. Methanol has found widespread use in petrochemical industries, combustion engines, and the production of organic solvents. Despite its extensive applications, methanol production contributes only a marginal 0.1% difference in CO
2 emissions [
105]. The reverse water-gas-shift (RWGS) reaction is pivotal for CO
2 utilization, transforming carbon dioxide into carbon monoxide. This resulting carbon monoxide plays a crucial role as a raw material in synthesizing methanol and hydrocarbon fuels through the Fischer-Tropsch (FT) reaction [
106]. Two main challenges impeding the commercial-scale implementation of methanol production are the endothermic nature of the reverse water-gas-shift (RWGS) reaction and the low conversion rates observed under moderate temperature conditions.
Another major challenge in commercial methanol production is the production of active catalysts that can maximize production and enhance reaction kinetics. The dry reforming of methane has recently directed researchers’ focus on using CO
2 to produce syngas [
107,
108,
109]. Syngas produced from the DRM process typically have a higher concentration than those generated from partial oxidation and steam reforming [
100,
110]. The process of DRM produces only about 2% unreacted methane, which is significantly lower than steam reforming. As a result, DRM can be used at inaccessible natural gas sites to generate liquid fuels [
74].
Several research studies have been conducted on the viability of the DRM reaction using silica, alumina, and lanthanum oxide as supports for nickel, ruthenium, nickel-carbonyl, iridium, and rhodium [
70]. Despite advancements in developing highly reactive catalysts with optimal stability for dry reforming of methane, finding a suitable catalyst for this reaction remains challenging. The issue arises because the process of deactivation through coke formation is unavoidable under high-temperature [
110,
111,
112,
113].
The oxidative hydrogenation of light alkanes to alkenes, utilizing carbon dioxide as a mild oxidant, represents a promising method with the potential to decrease coke formation, which helps maintain the stability of catalysts under high-temperature conditions [
7,
36,
114,
115,
116]. Furthermore, by eliminating hydrogen through the RWGS reaction, carbon dioxide improves the equilibrium process of the aerobic dehydrogenation of lighter alkanes [
117]. It is essential to regulate the temperature conditions because high heat could result in the over-oxidation of the olefins, thereby producing carbon oxides and significantly reducing selectivity [
118]. Carbon dioxide is also used as an oxygen compound in the redox cycle process. The role of carbon dioxide in the ODA process is directly influenced by the reducibility of the reactive metals and their supporting materials. It is also significantly influenced by the mechanism of the reaction [
119]. The catalysts that have been previously analyzed are characterized by poor stability despite being highly reactive.
Therefore, the primary challenge in the use of the captured CO2 from industrial activities as a feedstock to produce synthetic fuel is the process of designing and developing innovative catalysts that demonstrate chemical durability and structural stability as well as high catalytic reactivity amongst several other reaction considerations and resistance to coke formation.
3.2.2. CO2 Utilization: Conversion of CO2 into Petrochemicals
Carbon dioxide can be utilized as a primary feedstock for developing a wide range of fine chemicals despite being used for synthetic fuel production. Urea is the most popular fertilizer derived from CO
2 [
5,
120]. It has also been extensively utilized as a feedstock for synthesizing polymers, medications, and other important petrochemicals such as urea resins and melamine [
121].
Organic carbonates are another important class of petrochemicals produced from capturing CO
2. Some of these chemicals include linear acyclic molecules, diethyl carbonate (DEC, cyclohexane carbonate (CC), and bisphenol polycarbonate (BPA-PC), amongst several others which can be applied in several operations, i.e., pharmaceuticals, production of lubricants, catalytic reactions, and agrochemicals [
73,
76].
This process has experienced several challenges because it is more efficient at elevated temperature and pressure conditions and has a large catalyst inventory requirement. Moreover, a significant challenge in this process involves the separation of the catalyst from the reaction products [
73,
76]. AI-based catalysts, readily available in the market, have found extensive use in producing polycarbonates through the chemical reaction of carbon dioxide with epoxides. However, it is important to note that these catalysts, despite their widespread use, are not considered environmentally friendly [
74]. The oxidative carboxylation process has been presented as a viable, environmentally friendly alternative solution. Polyurethane is an important substance produced from the reaction of CO
2 with cyclic amines [
74].
A significant chemical obtained through carbon dioxide utilization is formic acid. There is a growing interest in the hydrogenation of CO
2 to produce formic acid, mainly due to its intermediate reaction conditions. Other appealing factors include the absence of byproduct formation, the ability to store hydrogen in the liquid phase, and the simple breakdown of formic acid into its hydrogen and CO
2 components [
75,
122]. Another method of producing biodiesel and other petrochemicals derived from biomass is the process of the biological utilization of carbon dioxide. This method produces a variety of valuable byproducts and has the potential for a more rapid growth rate and reduced growth cycle [
123].
To eliminate contaminants such as sulfuric oxide, nitrous oxides, and notable heavy metals considered harmful to the development of organisms such as microalgae, captured CO
2 should be purified before use as feedstock in photobioreactors [
124]. Aside from EOR processes, several industries have utilized CO
2 as a technological fluid that does not turn into chemicals. These industries include beverages, dry cleaning, food preservation, air conditioning, and solvents [
5,
73,
75] Approximately 50Mt/year of CO
2 is typically consumed by EOR processes, while the food and beverage industries consume about 8Mt per year [
125]. The suggested laboratory-scale technologies are yet to be industrially commercialized despite the available commercial market for converting captured CO
2 into fuels and petrochemicals. This limitation is because the researched materials are economical to produce but must be chemically stable. The conversion rates of CO
2 and the total generation of its primary products are quite low and need to satisfy commercial use requirements. Furthermore, knowledge about the reaction mechanisms influencing the chemical conversions of carbon dioxide is still in the conceptual stages. In this field, the process evaluation and operation requirements are not considered.
3.3. Process of CO2 Mineralization
Carbon dioxide can be stored non-geologically or through mineral carbonation when exposed to metallic oxides like calcium and magnesium [
126]. The carbonation of calcium and magnesium silicates, occurring spontaneously through a slow and thermodynamically favored reaction with atmospheric CO
2, is classified as a natural process known as natural weathering [
5]. It is highly possible to artificially improve the carbonation kinetics by increasing temperature and injection of fluids using a higher concentration of carbon dioxide.
The primary obstacle to scaling up the mineralization process remains the slow kinetics, even with major efforts to speed up this reaction [
5]. Furthermore, to attain more than 80% carbonation efficiency, this process requires relatively high pressures of approximately 10-15 MPa and temperature conditions of 150 °C–600 °C [
5]. Other requirements for high carbonation efficiency include extraction, processing, and transportation of the rock formations. Also, the carbonation reaction takes a long time, ranging from about 6-24 hours and the rocks need to be mined in diameters of about 37mm. Large plant sizes and the demanding requirements of additives for the successful extraction of reactive species and disposal of reaction byproducts are the factors that result in high penalty costs [
127].
The process of mineralization is a type of sequestration since its main aim is the permanent storage of CO
2. Unlike the geological sequestration of CO
2, which sometimes experiences leakages, carbonates are safe and stabilized [
128]. The exothermic characteristics of the mineralization reaction process, coupled with the geothermal gradient of the formation, can ultimately lead to a reduction in energy consumption. Furthermore, flue gas captured from industrial facilities can be directly utilized for mineralization processes, as purified CO
2 is not required [
125].
Indirect CO
2 storage, sometimes called indirect carbonation, can be employed in several industrial reactors to overcome the dire challenge of technological and operational limitations of the process of direct carbonation. By utilizing this method, reduced time and mild conditions can yield high carbonation efficiency and purity [
128,
129]. The process also yields various products, including silica, iron oxide, and carbonates of magnesium and calcium, which can offset its expenses. Nonetheless, because of this process’s complexity, each step’s operating conditions should be independently optimized [
5]. Furthermore, the high energy costs are a major barrier preventing its commercialization. Other materials, such as sodium hydroxide, acetic acids, and ammonium salts, are employed in this process as substitutes for hydrochloric acid because they can effectively reduce energy [
126,
128]. Using more sophisticated materials to accelerate the reaction kinetics increases process efficiency [
128,
129,
130]. The most beneficial method is the process of indirect mineral carbonation, which may soon experience a significant increase in size [
81]. In-situ mineralization in basaltic rock has grown significantly in just about two years of applying the indirect mineral carbonation method [
131]. Further studies in this area should concentrate on the potential of upgrading wastes generated from alkali metals into highly commercial products through carbonation, for example, the precipitation of highly concentrated CaCO
3 [
129,
130].
3.4. Process of Desalination and Water Production
Another promising approach for utilizing captured CO
2 involves converting brine to water and removing total dissolved solids (TDS) [
132,
133]. In areas where potable water is scarce, water from this reaction can be used [
122]. Due to financial limitations, most industrial desalination plants do not utilize CO
2 during desalination processes. However, innovative technologies are currently being developed to provide affordable and effective utilization of CO
2. During the exposure of ammonia-treated seawater to carbon dioxide, weak bonds are produced, resulting in the separation of the ions in the water phase [
110]
Due to their weight, the resulting products, NA
2CO
2 and NH
4CL, can readily sink to the bottom of the container. The two options available for recycling NH
4CL are heat and calcium oxide or using NH4CL to produce ammonia and chlorine [
30,
134]. Another desalination method is the hydrate formation method, which produces hydrates by separating the salts from the water using carbon dioxide. In employing this method, the CO
2 can be utilized in either the gaseous or liquid phases. Subsequently, the CO
2 hydrates formed are disposed of into the ocean or other water bodies [
135]. The forward osmosis process involving ammonia and carbon dioxide represents another significant desalination method that uses carbon dioxide [
133].
As a desalination method, reverse osmosis relies on hydraulic pressure as its primary driving force. In this process, osmotic pressure is leveraged, and brine and fresh water are separated through a “draw” solution. One major challenge with this process is the quantity of brine waste generated during desalination [
136]. Aside from that, the local ecosystem can become unstable due to metal corrosion, solvent chemical residues, and high salt concentrations [
137]. Carbonation, filtration, and recovery are the three primary units suggested for use with chloride and amine compounds to address these challenges [
138]
It is unlikely that the desalination process will achieve commerciality without a substantial cost benefit. The economic implications of producing treated water with around 21 ppm TDS from approximately 233,000 ppm of brine solution while fully utilizing the three primary stages of CO2-based crystalline desalination processes, are predicted to be about 3.17 USD per kilo-gallon, as presented by a 2013 DOE report [
139]. The price may differ dramatically depending on the origin of the brine. The economic costs of successfully conducting the desalination process are presently higher than the cost of agricultural water. As a result, the technology of CO
2-based desalination is yet to be commercially appealing.
Specifically, it is quite uncertain that this technology will be applied in agricultural operations due to the high costs of producing treated water through CO2-based desalination. Therefore, while CO2 remains a promising option for desalination, the financial implications of producing potable water using CO2-based technologies are comparatively higher than alternative options.
3.5. Challenges and Opportunities in CO2 Utilization
The outlook for carbon-utilization technologies appears promising, presenting abundant prospects across various sectors. The focus has shifted from mere sequestration in saline waterbodies to viewing captured CO2 as a valuable and sustainable energy resource.
3.5.1. Challenges
- ➢
Affordability: The widespread adoption of CO2 utilization technologies hinges on their affordability. The economic viability of these innovations will play a crucial role in determining their acceptance and integration into existing industrial processes.
- ➢
Efficiency concerns: The current challenges lie in the efficiency of CO2 utilization technologies. Researchers are actively working to address concerns related to high costs and low efficiency, aiming to enhance the overall performance and competitiveness of these processes.
- ➢
imited Utilization: Despite capturing CO2 from flue gas streams, its utilization in energy production and material synthesis remains relatively low. The main hindrances to broader adoption are the anticipated high costs and the efficiency constraints of existing technologies.
3.5.2. Opportunities
- ➢
Diverse applications: There is a considerable opportunity for CO2 utilization in multiple sectors, offering a versatile approach to address energy needs sustainably. This shift in perspective unlocks potential applications beyond traditional sequestration practices.
- ➢
Emerging technologies: Ongoing advancements in CO2 utilization technologies, including innovative processes discussed earlier, signify a transformative phase. These emerging technologies hold the promise of producing petrochemicals and fuels from captured CO2, marking a paradigm shift in the utilization of greenhouse gases.
- ➢
Economic momentum: The economic aspect of CO2 utilization is gaining momentum as a key research area. The potential for economic benefits, including the production of valuable materials, is driving interest and investment in these technologies.
In summary, CO2 utilization is marked by promising opportunities and ongoing challenges. The transition from viewing CO2 solely as a sequestered compound to recognizing its potential as a valuable energy resource underscores a paradigm shift in sustainability practices. The trajectory of these technologies will be shaped by the delicate balance between addressing challenges and leveraging emerging opportunities.
4. Evaluating the Synergy between CO2 Capture and Utilization Processes
Integrating CO
2 capture and utilization mitigates the elevated energy costs associated with the direct production of fuels and petrochemicals from flue gas streams, particularly when conducted under the same temperature conditions. This process intensification holds the promise of yielding cleaner and more energy-efficient technologies. Previously, there have been several applications of the concept of integrated CO
2 capture and utilization process in gas separation and reaction. For example, membrane reactors (MRs) integrate chemical reactions using membrane separation. Membrane reactors can effectively expedite a reaction process and facilitate equilibrium reactions on the product side by eliminating at least one process from the reaction zone [
140,
141,
142]
The sorption-enhanced reaction (SER) represents a similar concept, combining the processes of adsorption and reaction into a unified unit. Specifically, the water-gas-shift (WGS) reaction is employed in SER applications to generate highly concentrated hydrogen, demonstrating its broad utility [
143,
144,
145,
146,
147]. The thermodynamically constrained WGS process can function at relatively high-temperature conditions (approximately 350°C), having favorable reaction kinetics because of the in-situ capture of CO
2 [
146]. These innovative concepts could be used in various industries to capture and utilize CO
2 simultaneously.
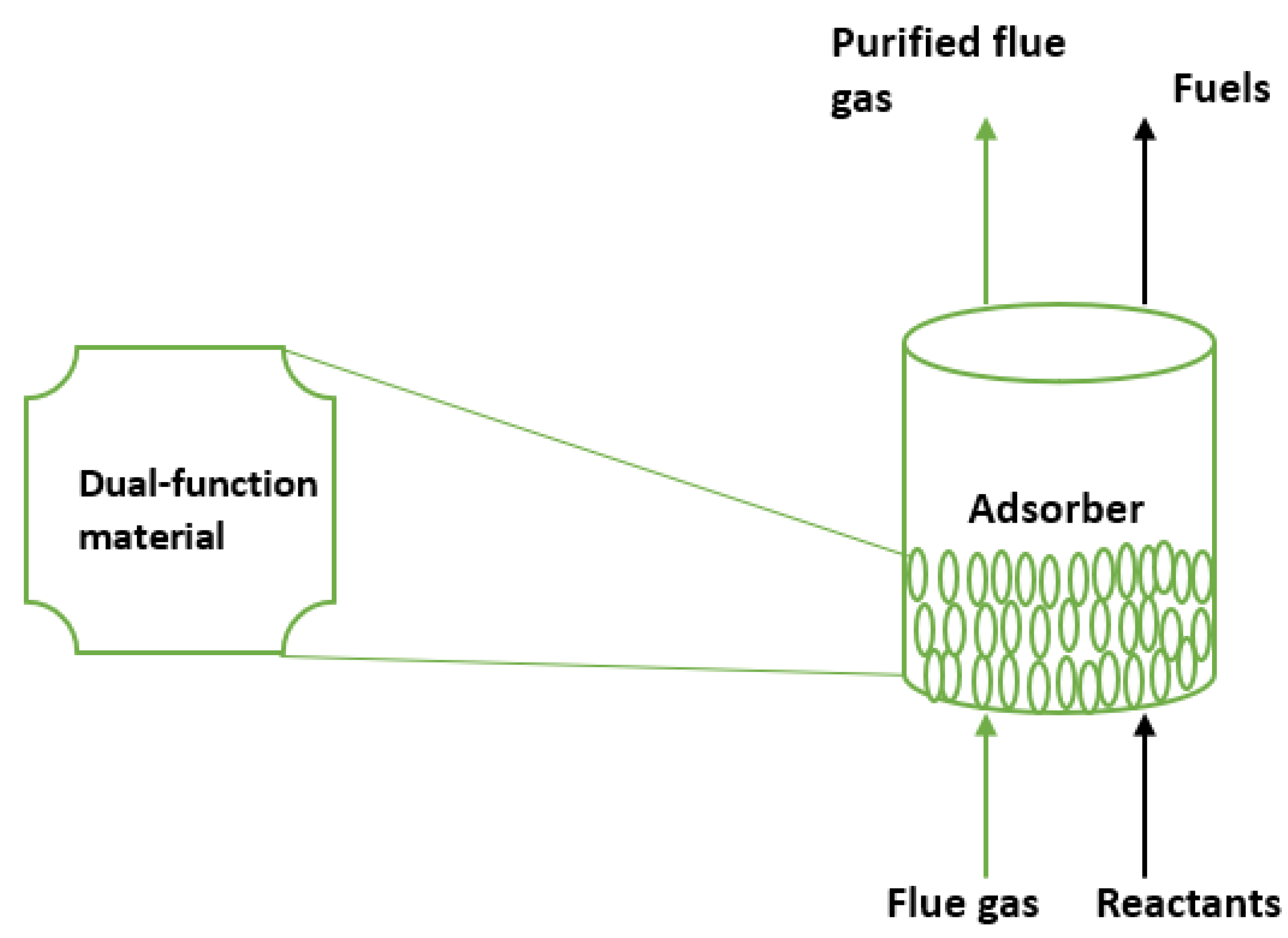
Previously, syngas (CO and H
2) production has been commercially possible by directly converting industrial flue gases into chemicals and fuels using a dual-function material. Syngas is a vital reactant for methanol synthesis [
70,
148,
149]. Tri-reforming of methane is a process where supported nickel catalysts work in concert to reform CO
2 in a single reactor at about 850 C, steam reform methane, and partially oxidize methane. Synthetic methane has been created by the in-situ capture and carbon dioxide methanation utilizing a double-function material, usually a monolith [
49,
70,
150]. Another recently documented instance of a combined capture and reduction process is the hydrogenation of carbon dioxide [
151]. A further illustration of this hybrid process recently proven at an experimental scale in the United States is the process of the simultaneous capture and mineralization of carbon dioxide from the combustion of flue gas using coal [
152]
Figure 3.
Schematic of combined carbon capture utilization process [
153].
Figure 3.
Schematic of combined carbon capture utilization process [
153].
The solutions to the global energy and environmental issues that are currently being experienced may be found in such integrated systems. Process engineering and material science developments are required to create new in situ capture-conversion technology. Considering the distinctive characteristics of the adsorption and catalytic processes, developing highly efficient and economical technologies requires thoroughly examining the fundamentals of composite adsorbents and catalysts, operating conditions, and process considerations. Additionally, the resistance of the materials to impurities, particularly catalysts, may present a problem for the direct use of waste gas streams. Therefore, measures should be taken to guarantee the efficiency of the material over an extended period of use.
5. Conclusion
This review provides a comprehensive overview of the current state and future potential of CO2 capture and utilization technologies. Notably, recent years have witnessed substantial advancements in the innovative design and development of various carbon capture and utilization technologies. Despite this obvious growth, most available technological options are still conceptual. Both cases will undoubtedly witness a reduction in the energy required for the capture and utilization processes with the commercial introduction of innovative materials that perform better than traditional materials in each technique.
Process performance considerations should be integrated with materials research and development to assess their potential in practical settings. This integrated perspective on materials and processes, coupled with effective communication between materials scientists and engineers, is crucial for advancing the development of carbon capture and utilization technologies. Providing a factual assessment of performance and addressing uncertainties in cost estimation are essential steps in aligning with the requirements for successful commercial implementation.
For several emerging CCU technologies, the viability of adoption ultimately depends on cost-effectiveness. The durability of the materials utilized in most carbon capture and utilization processes remains a crucial factor influencing the process economics and system performance. Hence, producing fuels and petrochemicals, especially using renewable energy resources, can mitigate overall costs associated with CO2 utilization. This approach reduces expenses and provides a sustainable means of generating value-added products. Most technologies can be improved more economically by system integration and process intensification, but other relevant factors, such as the complexity of the operation, should also be considered.
Their thermodynamic analyses have illustrated hybrid processes’ energy efficiency and cost-effectiveness by reducing capital and operating costs. Therefore, future research studies should focus on hybrid processes that provide simultaneous CO2 capture and utilization strategies and integrate CO2-capture intermediate systems. Although the developing hybrid systems share several characteristics with the emerging generation of carbon capture and utilization technologies, extensive development, research, process scale-up, and synergistic assessment studies are still required before these developing technologies can be made commercially available soon. Any feasibility study should include other factors about life-cycle analysis, risk assessment, and environmental effects.
Acknowledgments
The authors appreciate the Distinguished Graduate Student Fellowship Award from Texas Tech University, United States.
Conflicts of Interest
The authors state that they do not have any known financial interests or personal relationships that could have influenced the work reported in this paper.
References
- Keerthana KB, Wu SW, Wu ME, Kokulnathan T. The United States Energy Consumption and Carbon Dioxide Emissions: A Comprehensive Forecast Using a Regression Model. Sustainability 2023, Vol 15, Page 7932 2023;15:7932. [CrossRef]
- Keerthana KB, Wu SW, Wu ME, Kokulnathan T. The United States Energy Consumption and Carbon Dioxide Emissions: A Comprehensive Forecast Using a Regression Model. Sustainability 2023, Vol 15, Page 7932 2023;15:7932. [CrossRef]
- Schwarz-Herion, O. The impact of the climate change discussion on society, science, culture, and politics: From The Limits to Growth via the Paris agreement to a binding global policy? The Impact of Climate Change on Our Life: The Questions of Sustainability 2018:1–32. [CrossRef]
- Markewitz P, Kuckshinrichs W, Leitner W, Linssen J, Zapp P, Bongartz R, et al. Worldwide innovations in the development of carbon capture technologies and the utilization of CO2. Energy Environ Sci 2012;5:7281–305. [CrossRef]
- Boot-Handford ME, Abanades JC, Anthony EJ, Blunt MJ, Brandani S, Mac Dowell N, et al. Carbon capture and storage update. Energy Environ Sci 2013;7:130–89. [CrossRef]
- Rubin ES, Davison JE, Herzog HJ. The cost of CO2 capture and storage. International Journal of Greenhouse Gas Control 2015;40:378–400. [CrossRef]
- Qiu HH, Liu LG. A Study on the Evolution of Carbon Capture and Storage Technology Based on Knowledge Mapping. Energies 2018, Vol 11, Page 1103 2018;11:1103. [CrossRef]
- Kamkeng ADN, Wang M, Hu J, Du W, Qian F. Transformation technologies for CO2 utilisation: Current status, challenges and future prospects. Chemical Engineering Journal 2021;409:128138. [CrossRef]
- Eyitayo SI, Okere CJ, Hussain A, Gamadi T, Watson MC. Synergistic sustainability: Future potential of integrating produced water and CO2 for enhanced carbon capture, utilization, and storage (CCUS). J Environ Manage 2024;351:119713. [CrossRef]
- Miller DC, Litynski JT, Brickett LA, Morreale BD. Toward transformational carbon capture systems. AIChE Journal 2016;62:2–10. [CrossRef]
- Zhao Y, Ho WSW. CO2-selective membranes containing sterically hindered amines for CO2/H2 separation. Ind Eng Chem Res 2013;52:8774–82. [CrossRef]
- Songolzadeh M, Soleimani M, Takht Ravanchi M, Songolzadeh R. Carbon dioxide separation from flue gases: A technological review emphasizing reduction in greenhouse gas emissions. The Scientific World Journal 2014;2014. [CrossRef]
- Abotalib M, Zhao F, Clarens A. Deployment of a Geographical Information System Life Cycle Assessment Integrated Framework for Exploring the Opportunities and Challenges of Enhanced Oil Recovery Using Industrial CO2 Supply in the United States. ACS Sustain Chem Eng 2016;4:4743–51. [CrossRef]
- Ahn Y, Bae SJ, Kim M, Cho SK, Baik S, Lee JI, et al. Review of supercritical CO2 power cycle technology and current status of research and development. Nuclear Engineering and Technology 2015;47:647–61. [CrossRef]
- Ali M, Jha NK, Pal N, Keshavarz A, Hoteit H, Sarmadivaleh M. Recent advances in carbon dioxide geological storage, experimental procedures, influencing parameters, and future outlook. Earth Sci Rev 2022;225:103895. [CrossRef]
- Patricia LARKIN | Senior Research Associate | PhD, Population Health | University of Ottawa, Ottawa | Institute for Science, Society and Policy | Research profile n.d. https://www.researchgate.net/profile/Patricia-Larkin (accessed January 29, 2024).
- Arstad B, Fjellvåg H, Kongshaug KO, Swang O, Blom R. Amine functionalised metal organic frameworks (MOFs) as adsorbents for carbon dioxide. Adsorption 2008;14:755–62. [CrossRef]
- Builes S, López-Aranguren P, Fraile J, Vega LF, Domingo C. Analysis of CO2 adsorption in amine-functionalized porous silicas by molecular simulations. Energy and Fuels 2015;29:3855–62. [CrossRef]
- Kong Y, Shen X, Fan M, Yang M, Cui S. Dynamic capture of low-concentration CO2 on amine hybrid silsesquioxane aerogel. Chemical Engineering Journal 2016;283:1059–68. [CrossRef]
- Goeppert A, Czaun M, Surya Prakash GK, Olah GA. Air as the renewable carbon source of the future: an overview of CO2 capture from the atmosphere. Energy Environ Sci 2012;5:7833–53. [CrossRef]
- Bates ED, Mayton RD, Ntai I, Davis JH. CO2 capture by a task-specific ionic liquid. J Am Chem Soc 2002;124:926–7. [CrossRef]
- Cadena C, Anthony JL, Shah JK, Morrow TI, Brennecke JF, Maginn EJ. Why is CO2 so Soluble in Imidazolium-Based Ionic Liquids? J Am Chem Soc 2004;126:5300–8. [CrossRef]
- Corvo MC, Sardinha J, Casimiro T, Marin G, Seferin M, Einloft S, et al. A Rational Approach to CO 2 Capture by Imidazolium Ionic Liquids: Tuning CO 2 Solubility by Cation Alkyl Branching. ChemSusChem 2015;8:1935–46. [CrossRef]
- Rochelle, GT. Amine Scrubbing for CO2 Capture. Science (1979) 2009;325:1652–4. [CrossRef]
- Godin J, Liu W, Ren S, Xu CC. Advances in recovery and utilization of carbon dioxide: A brief review. J Environ Chem Eng 2021;9:105644. [CrossRef]
- Han S, Huang Y, Watanabe T, Dai Y, Walton KS, Nair S, et al. High-throughput screening of metal-organic frameworks for CO 2 separation. ACS Comb Sci 2012;14:263–7. [CrossRef]
- Huang Q, Eić M. Commercial adsorbents as benchmark materials for separation of carbon dioxide and nitrogen by vacuum swing adsorption process. Sep Purif Technol 2013;103:203–15. [CrossRef]
- Japip S, Wang H, Xiao Y, Chung TS. Highly permeable zeolitic imidazolate framework (ZIF)-71 nano-particles enhanced polyimide membranes for gas separation. J Memb Sci 2014;467:162–74. [CrossRef]
- Merkel TC, Lin H, Wei X, Baker R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J Memb Sci 2010;359:126–39. [CrossRef]
- Koros, WJ. Evolving Beyond the Thermal Age of Separation Processes: Membranes Can Lead the Way 2004. [CrossRef]
- Kosuri MR, Koros WJ. Asymmetric hollow fiber membranes for separation of CO2 from hydrocarbons and fluorocarbons at high-pressure conditions relevant to C 2F4 polymerization. Ind Eng Chem Res 2009;48:10577–83. [CrossRef]
- Lively RP, Realff MJ. On thermodynamic separation efficiency: Adsorption processes. AIChE Journal 2016;62:3699–705. [CrossRef]
- Rownaghi AA, Kant A, Li X, Thakkar H, Hajari A, He Y, et al. Aminosilane-Grafted Zirconia–Titiania–Silica Nanoparticles/Torlon Hollow Fiber Composites for CO 2 Capture. ChemSusChem 2016;9:1166–77. [CrossRef]
- Favre, E. Carbon dioxide recovery from post-combustion processes: Can gas permeation membranes compete with absorption? J Memb Sci 2007;294:50–9. [CrossRef]
- Kim TJ, Vrålstad H, Sandru M, Hägg MB. Separation performance of PVAm composite membrane for CO2 capture at various pH levels. J Memb Sci 2013;428:218–24. [CrossRef]
- Cheng Y, Zhang F, Zhang Y, Miao C, Hua W, Yue Y, et al. Oxidative dehydrogenation of ethane with CO2 over Cr supported on submicron ZSM-5 zeolite. Chinese Journal of Catalysis 2015;36:1242–8. [CrossRef]
- Bae TH, Long JR. CO2/N2 separations with mixed-matrix membranes containing Mg2(dobdc) nanocrystals. Energy Environ Sci 2013;6:3565–9. [CrossRef]
- Fan M, Abdullah AZ, Bhatia S. Catalytic Technology for Carbon Dioxide Reforming of Methane to Synthesis Gas. ChemCatChem 2009;1:192–208. [CrossRef]
- Tanh Jeazet HB, Staudt C, Janiak C. Metal–organic frameworks in mixed- matrix membranes for gas separation. Dalton Transactions 2012;41:14003–27. [CrossRef]
- Labreche Y, Lively RP, Rezaei F, Chen G, Jones CW, Koros WJ. Post-spinning infusion of poly(ethyleneimine) into polymer/silica hollow fiber sorbents for carbon dioxide capture. Chemical Engineering Journal 2013;221:166–75. [CrossRef]
- Chue KT, Kim JN, Yoo YJ, Cho SH, Yang RT. Comparison of Activated Carbon and Zeolite 13X for CO2 Recovery from Flue Gas by Pressure Swing Adsorption. Ind Eng Chem Res 1995;34:591–8.
- Liu H, Guo P, Regueira T, Wang Z, Du J, Chen G. Irreversible Change of the Pore Structure of ZIF-8 in Carbon Dioxide Capture with Water Coexistence. Journal of Physical Chemistry C 2016;120:13287–94. [CrossRef]
- Patel HA, Karadas F, Canlier A, Park J, Deniz E, Jung Y, et al. High capacity carbon dioxide adsorption by inexpensive covalent organic polymers. J Mater Chem 2012;22:8431–7. [CrossRef]
- Phan A, Doonan CJ, Uribe-Romo FJ, Knobler CB, Okeeffe M, Yaghi OM. Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks. Acc Chem Res 2010;43:58–67. [CrossRef]
- Reich TE, Behera S, Jackson KT, Jena P, El-Kaderi HM. Highly selective CO2/CH4 gas uptake by a halogen-decorated borazine-linked polymer. J Mater Chem 2012;22:13524–8. [CrossRef]
- Choi S, Drese JH, Jones CW. Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources. ChemSusChem 2009;2:796–854. [CrossRef]
- Hauchhum L, Mahanta P. Carbon dioxide adsorption on zeolites and activated carbon by pressure swing adsorption in a fixed bed. International Journal of Energy and Environmental Engineering 2014;5:349–56. [CrossRef]
- Principles of Adsorption and Adsorption Processes—Douglas M. Ruthven—Google Books n.d. https://books.google.com/books?hl=en&lr=&id=u7wq21njR3UC&oi=fnd&pg=PR17&dq=Principles+of+adsorption+and+adsorption+processes.&ots=wdStQpDetX&sig=bKK6mVjzEQ5ZmJQYCcW7I9OEr5I#v=onepage&q=Principles%20of%20adsorption%20and%20adsorption%20processes.&f=false (accessed January 29, 2024).
- Li R, Dalton /, Gao X-J, Zheng G. The difference in the CO 2 adsorption capacities of different functionalized pillar-layered metal–organic frameworks (MOFs). Dalton Transactions 2021;50:9310–6. [CrossRef]
- Rezaei F, Webley P. Structured adsorbents in gas separation processes. Sep Purif Technol 2010;70:243–56. [CrossRef]
- Lively RP, Chance RR, Kelley BT, Deckman HW, Drese JH, Jones CW, et al. Hollow fiber adsorbents for CO 2 removal from flue gas. Ind Eng Chem Res 2009;48:7314–24. [CrossRef]
- Lively RP, Chance RR, Koros WJ. Enabling low-cost CO2 capture via heat integration. Ind Eng Chem Res 2010;49:7550–62. [CrossRef]
- Rezaei F, Lively RP, Labreche Y, Chen G, Fan Y, Koros WJ, et al. Aminosilane-grafted polymer/silica hollow fiber adsorbents for CO 2 capture from flue gas. ACS Appl Mater Interfaces 2013;5:3921–31. [CrossRef]
- Kärger J, Ruthven DM. Self-diffusion and diffusive transport in zeolite crystals. Stud Surf Sci Catal 1997;105:1843–50. [CrossRef]
- Hossain MM, de Lasa HI. Chemical-looping combustion (CLC) for inherent CO2 separations—a review. Chem Eng Sci 2008;63:4433–51. [CrossRef]
- Review on global CCUS technology and application—Google Scholar n.d. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C44&q=Review+on+global+CCUS+technology+and+application&btnG= (accessed January 29, 2024).
- Martínez A, Lara Y, Lisbona P, Romeo LM. Energy penalty reduction in the calcium looping cycle. International Journal of Greenhouse Gas Control 2012;7:74–81. [CrossRef]
- Martínez I, Murillo R, Grasa G, Carlos Abanades J. Integration of a Ca looping system for CO 2 capture in existing power plants. AIChE Journal 2011;57:2599–607. [CrossRef]
- Romeo LM, Usón S, Valero A, Escosa JM. Exergy analysis as a tool for the integration of very complex energy systems: The case of carbonation/calcination CO2 systems in existing coal power plants. International Journal of Greenhouse Gas Control 2010;4:647–54. [CrossRef]
- Charitos A, Hawthorne C, Bidwe AR, Sivalingam S, Schuster A, Spliethoff H, et al. Parametric investigation of the calcium looping process for CO2 capture in a 10 kWth dual fluidized bed. International Journal of Greenhouse Gas Control 2010;4:776–84. [CrossRef]
- Rodríguez N, Alonso M, Abanades JC. Experimental investigation of a circulating fluidized-bed reactor to capture CO 2 with CaO. AIChE Journal 2011;57:1356–66. [CrossRef]
- Symonds RT, Lu DY, Manovic V, Anthony EJ. Pilot-scale study of CO 2 capture by CaO-based sorbents in the presence of steam and SO 2. Ind Eng Chem Res 2012;51:7177–84. [CrossRef]
- Lackner KS, Brennan S, Matter JM, Park A-HA, Wright A, van der Zwaan B. The urgency of the development of CO 2 capture from ambient air. Proceedings of the National Academy of Sciences 2012;109:13156–62. [CrossRef]
- Sanz-Pérez ES, Murdock CR, Didas SA, Jones CW. Direct Capture of CO2 from Ambient Air. Chem Rev 2016;116:11840–76. [CrossRef]
- Zhao L, Primabudi E, Stolten D. Investigation of a Hybrid System for Post-Combustion Capture. Energy Procedia 2014;63:1756–72. [CrossRef]
- Hasse D, Ma J, Kulkarni S, Terrien P, Tranier JP, Sanders E, et al. CO2 Capture by Cold Membrane Operation. Energy Procedia 2014;63:186–93. [CrossRef]
- Belaissaoui B, Le Moullec Y, Willson D, Favre E. Hybrid membrane cryogenic process for post-combustion CO2 capture. J Memb Sci 2012;415–416:424–34. [CrossRef]
- Esteves IAAC, Mota JPB. Simulation of a new hybrid membrane/pressure swing adsorption process for gas separation. Desalination 2002;148:275–80. [CrossRef]
- Scholz M, Frank B, Stockmeier F, Falß S, Wessling M. Techno-economic analysis of hybrid processes for biogas upgrading. Ind Eng Chem Res 2013;52:16929–38. [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal Today 2006;115:2–32. [CrossRef]
- Wurzbacher JA, Gebald C, Steinfeld A. Separation of CO2 from air by temperature-vacuum swing adsorption using diamine-functionalized silica gel. Energy Environ Sci 2011;4:3584–92. [CrossRef]
- Lively RP, Chance RR, Kelley BT, Deckman HW, Drese JH, Jones CW, et al. Hollow Fiber Adsorbents for CO 2 Removal from Flue Gas. Ind Eng Chem Res 2009;48:7314–24. [CrossRef]
- Aresta, M. Carbon dioxide as chemical feedstock 2010:394.
- Aresta M, Dibenedetto A. Utilisation of CO2 as a chemical feedstock: opportunities and challenges. Dalton Transactions 2007:2975–92. [CrossRef]
- Aresta M, Dibenedetto A, Angelini A. Catalysis for the valorization of exhaust carbon: From CO2 to chemicals, materials, and fuels. technological use of CO2. Chem Rev 2014;114:1709–42. [CrossRef]
- Review on global CCUS technology and application.—Google Scholar n.d. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C44&q=Review+on+global+CCUS+technology+and+application.&btnG= (accessed January 30, 2024).
- Tunio SQ, Tunio AH, Ghirano NA, Mohamed Z, Adawy E. Comparison of Different Enhanced Oil Recovery Techniques for Better Oil Productivity. Int J Appl Sci Technol 2011;1.
- Edouard MN, Okere CJ, Ejike C, Dong P, Suliman MAM. Comparative numerical study on the co-optimization of CO2 storage and utilization in EOR, EGR, and EWR: Implications for CCUS project development. Appl Energy 2023;347:121448. [CrossRef]
- Safi R, Agarwal RK, Banerjee S. Numerical simulation and optimization of CO2 utilization for enhanced oil recovery from depleted reservoirs. Chem Eng Sci 2016;144:30–8. [CrossRef]
- Ighalo JO, Nwabueze QA. Production and Economic Analysis of Enhanced Oil Recovery (EOR) by Water Flooding: A Case Study of Reservoir OD-48 in The Niger Delta Article Open Access Production and Economic Analysis of Enhanced Oil Recovery (EOR) by Wate r Flooding: A Case Study of Reservoir OD-48 in The Niger Delta n.d.
- Bao W, Li H, Zhang Y. Experimental investigation of enhanced carbonation by solvent extraction for indirect CO2 mineral sequestration. Greenhouse Gases: Science and Technology 2014;4:785–99. [CrossRef]
- Azzolina NA, Nakles D V., Gorecki CD, Peck WD, Ayash SC, Melzer LS, et al. CO2 storage associated with CO2 enhanced oil recovery: A statistical analysis of historical operations. International Journal of Greenhouse Gas Control 2015;37:384–97. [CrossRef]
- Perera MSA, Gamage RP, Rathnaweera TD, Ranathunga AS, Koay A, Choi X. A Review of CO2-Enhanced Oil Recovery with a Simulated Sensitivity Analysis. Energies 2016, Vol 9, Page 481 2016;9:481. [CrossRef]
- Gozalpour F, Ren SR, Tohidi B. CO2 Eor and Storage in Oil Reservoir. Oil & Gas Science and Technology 2005;60:537–46. [CrossRef]
- Cho J, Kim TH, Lee KS. Modeling of CO2 EOR Process Combined with Intermediate Hydrocarbon Solvents for Higher Recovery Efficiency. Society of Petroleum Engineers—SPE EOR Conference at Oil and Gas West Asia, OGWA 2016 2016. [CrossRef]
- Celia MA, Bachu S, Nordbotten JM, Bandilla KW. Status of CO 2 storage in deep saline aquifers with emphasis on modeling approaches and practical simulations. Water Resour Res 2015;51:6846–92. [CrossRef]
- Panda M, Nottingham D, Lenig D. Systematic Surveillance Techniques for a Large Miscible WAG Flood. SPE Reservoir Evaluation & Engineering 2011;14:299–309. [CrossRef]
- Al Hajeri S, Negahban S, Al-Yafei G, Al Basry A. Design and Implementation of the First CO2-EOR Pilot in Abu Dhabi, UAE. SPE EOR Conference at Oil and Gas West Asia 2010, OGWA—EOR Challenges, Experiences and Opportunities in the Middle East 2010:587–95. [CrossRef]
- Ampomah W, Balch RS, Grigg RB, Will R, Dai Z, White MD. Farnsworth Field CO2-EOR Project: Performance Case History. SPE—DOE Improved Oil Recovery Symposium Proceedings 2016;2016-January. [CrossRef]
- Pizarro JODSA, Branco CCM. Challenges in Implementing an EOR Project in the Pre-Salt Province in Deep Offshore Brasil. Society of Petroleum Engineers—SPE EOR Conference at Oil and Gas West Asia 2012, OGWA—EOR: Building Towards Sustainable Growth 2012;2:954–66. [CrossRef]
- Klimkowski Ł, Nagy S, Papiernik B, Orlic B, Kempka T. Numerical Simulations of Enhanced Gas Recovery at the Załęcze Gas Field in Poland Confirm High CO 2 Storage Capacity and Mechanical Integrity. Oil & Gas Science and Technology—Revue d’IFP Energies Nouvelles 2015;70:655–80. [CrossRef]
- Ren B, Duncan IJ. Reservoir simulation of carbon storage associated with CO2 EOR in residual oil zones, San Andres formation of West Texas, Permian Basin, USA. Energy 2019;167:391–401. [CrossRef]
- Allen MJ, Faulkner DR, Worden RH, Rice-Birchall E, Katirtsidis N, Utley JEP. Geomechanical and petrographic assessment of a CO2 storage site: Application to the Acorn CO2 Storage Site, offshore United Kingdom. International Journal of Greenhouse Gas Control 2020;94:102923. [CrossRef]
- Iglauer, S. CO2-Water-Rock Wettability: Variability, Influencing Factors, and Implications for CO2 Geostorage. Acc Chem Res 2017;50:1134–42. [CrossRef]
- Saira, Janna F, Le-Hussain F. Effectiveness of modified CO2 injection at improving oil recovery and CO2 storage—Review and simulations. Energy Reports 2020;6:1922–41. [CrossRef]
- Ajayi T, Gomes JS, Bera A. A review of CO2 storage in geological formations emphasizing modeling, monitoring and capacity estimation approaches. Petroleum Science 2019 16:5 2019;16:1028–63. [CrossRef]
- Verma Y, Vishal V, Ranjith PG. Sensitivity Analysis of Geomechanical Constraints in CO2 Storage to Screen Potential Sites in Deep Saline Aquifers. Frontiers in Climate 2021;3. [CrossRef]
- Hu B, Guild C, Suib SL. Thermal, electrochemical, and photochemical conversion of CO2 to fuels and value-added products. Journal of CO2 Utilization 2013;1:18–27. [CrossRef]
- Driverless Vehicles: Opportunity for Further Greenhouse Gas Emission Reductions on JSTOR n.d. https://www.jstor.org/stable/26353861 (accessed January 29, 2024).
- Okere CJ, Sheng JJ. Review on clean hydrogen generation from petroleum reservoirs: Fundamentals, mechanisms, and field applications. Int J Hydrogen Energy 2023;48:38188–222. [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal Today 2006;115:2–32. [CrossRef]
- Graciani J, Mudiyanselage K, Xu F, Baber AE, Evans J, Senanayake SD, et al. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science (1979) 2014;345:546–50. [CrossRef]
- Laurenczy G, Laurenczy G. Hydrogen Storage and Delivery: The Carbon Dioxide—Formic Acid Couple. Chimia (Aarau) 2011;65:663. [CrossRef]
- Matsubu JC, Yang VN, Christopher P. Isolated metal active site concentration and stability control catalytic CO2 reduction selectivity. J Am Chem Soc 2015;137:3076–84. [CrossRef]
- Bansode A, Urakawa A. Towards full one-pass conversion of carbon dioxide to methanol and methanol-derived products. J Catal 2014;309:66–70. [CrossRef]
- Porosoff MD, Yan B, Chen JG. Catalytic reduction of CO 2 by H 2 for synthesis of CO, methanol and hydrocarbons: challenges and opportunities. Energy Environ Sci 2016;9:62–73. [CrossRef]
- Thermochemical Processing of Biomass: Conversion into Fuels, Chemicals and Power—Google Books n.d. https://books.google.com/books?hl=en&lr=&id=AsOKDwAAQBAJ&oi=fnd&pg=PR15&dq=Thermochemical+processing+of+biomass:+conversion+into+fuels,+chemicals+and+power&ots=bGv8DAbA47&sig=szpfm4vOLxIXnOrCzO7iIeMwJtA#v=onepage&q=Thermochemical%20processing%20of%20biomass%3A%20conversion%20into%20fuels%2C%20chemicals%20and%20power&f=false (accessed January 29, 2024).
- Gangadharan P, Kanchi KC, Lou HH. Evaluation of the economic and environmental impact of combining dry reforming with steam reforming of methane. Chemical Engineering Research and Design 2012;90:1956–68. [CrossRef]
- Rodriguez-Santiago, V. Utilization of carbon dioxide from coalfired power plant for the production of value-added products 2006.
- F. Arajo O de Q, de Medeiros JL, Maria R. CO2 Utilization: A Process Systems Engineering Vision. CO2 Sequestration and Valorization 2014. [CrossRef]
- Ozkan M, Nayak SP, Ruiz AD, Jiang W. Current status and pillars of direct air capture technologies. IScience 2022;25. [CrossRef]
- Kahle LCS, Roussière T, Maier L, Herrera Delgado K, Wasserschaff G, Schunk SA, et al. Methane dry reforming at high temperature and elevated pressure: Impact of gas-phase reactions. Ind Eng Chem Res 2013;52:11920–30. [CrossRef]
- Ross JRH. Natural gas reforming and CO2 mitigation. Catal Today 2005;100:151–8. [CrossRef]
- Estes DP, Copéret C. The Role of Proton Transfer in Heterogeneous Transformations of Hydrocarbons. Chimia (Aarau) 2015;69:321. [CrossRef]
- Koirala R, Buechel R, Krumeich F, Pratsinis SE, Baiker A. Oxidative dehydrogenation of ethane with CO2 over flame-made Ga-loaded TiO2. ACS Catal 2015;5:690–702. [CrossRef]
- Urlan F, Marcu IC, Sandulescu I. Oxidative dehydrogenation of n-butane over titanium pyrophosphate catalysts in the presence of carbon dioxide. Catal Commun 2008;9:2403–6. [CrossRef]
- Müller K, Baumgärtner A, Mokrushina L, Arlt W. Increasing the Equilibrium Yield of Oxidative Dehydrogenation with CO 2 by Secondary Reactions. Chem Eng Technol 2014;37:1261–4. [CrossRef]
- Wang S, Zhu ZH. Catalytic conversion of alkanes to olefins by carbon dioxide oxidative dehydrogenation—A review. Energy and Fuels 2004;18:1126–39. [CrossRef]
- Ansari MB, Park SE. Carbon dioxide utilization as a soft oxidant and promoter in catalysis. Energy Environ Sci 2012;5:9419–37. [CrossRef]
- MacDowell N, Florin N, Buchard A, Hallett J, Galindo A, Jackson G, et al. An overview of CO2 capture technologies. Energy Environ Sci 2010;3:1645–69. [CrossRef]
- Carbon Management: Implications for R&D in the Chemical Sciences and Technology—National Research Council, Division on Earth and Life Studies, Board on Chemical Sciences and Technology, Chemical Sciences Roundtable—Google Books n.d. https://books.google.com/books?hl=en&lr=&id=BQ1Lt4yEKskC&oi=fnd&pg=PT13&dq=Carbon+Management:+Implications+for+R+%26+D+in+the+Chemical+Sciences+and+Technology+(A+Workshop+Report+to+the+Chemical+Sciences+Roundtable).&ots=MGzYopqM10&sig=VvEZ00ModX1zruDYWTQqN-PAvUs#v=onepage&q=Carbon%20Management%3A%20Implications%20for%20R%20%26%20D%20in%20the%20Chemical%20Sciences%20and%20Technology%20(A%20Workshop%20Report%20to%20the%20Chemical%20Sciences%20Roundtable).&f=false (accessed January 29, 2024).
- Carbon Capture and Utilisation in the green economy—Google Scholar n.d. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C44&q=Carbon+Capture+and+Utilisation+in+the+green+economy&btnG= (accessed January 30, 2024).
- EngeboØ, Angunn, Ahmed, Nada, Bureau-Cauchois, Gaëlle. Evaluation of Carbon Dioxide Utilisation Concepts: A Quick and Complete Methodology. Energy Procedia 2014;63:8010–6. [CrossRef]
- Meylan FD, Moreau V, Erkman S. CO2 utilization in the perspective of industrial ecology, an overview. Journal of CO2 Utilization 2015;12:101–8. [CrossRef]
- Special report on carbon dioxide capture and storage—Google Scholar n.d. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C44&q=Special+report+on+carbon+dioxide+capture+and+storage&btnG= (accessed January 29, 2024).
- Cuéllar-Franca RM, Azapagic A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. Journal of CO2 Utilization 2015;9:82–102. [CrossRef]
- Sanna A, Uibu M, Caramanna G, Kuusik R, Maroto-Valer MM. A review of mineral carbonation technologies to sequester CO 2. Chem Soc Rev 2014;43:8049–80. [CrossRef]
- Pan SY, Chiang A, Chang EE, Lin YP, Kim H, Chiang PC. An Innovative Approach to Integrated Carbon Mineralization and Waste Utilization: A Review. Aerosol Air Qual Res 2015;15:1072–91. [CrossRef]
- Sanna A, Hall MR, Maroto-Valer M. Post-processing pathways in carbon capture and storage by mineral carbonation (CCSM) towards the introduction of carbon neutral materials. Energy Environ Sci 2012;5:7781–96. [CrossRef]
- Huijgen WJJ. Carbon Dioxide Sequestration by Mineral Carbonation n.d.
- Matter JM, Stute M, Snæbjörnsdottir S, Oelkers EH, Gislason SR, Aradottir ES, et al. Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions. Science (1979) 2016;352:1312–4. [CrossRef]
- Bijl DL, Bogaart PW, Kram T, de Vries BJM, van Vuuren DP. Long-term water demand for electricity, industry and households. Environ Sci Policy 2016;55:75–86. [CrossRef]
- McCutheon_McGinnis_Elimelech.pdf | Enhanced Reader n.d.
- Max MD, Sheps K, Tatro SR, Brazel L, Osegovic JP. SEAWATER DESALINATION AS A BENEFICIAL FACTOR OF CO2 SEQUESTRATION. 2008. [CrossRef]
- Method and apparatus for efficient injection of CO2 in oceans 2001.
- Dawoud, MA. Environmental Impacts of Seawater Desalination: Arabian Gulf Case Study. Article in International Journal of Environment and Sustainability 2012. [CrossRef]
- Development of an Environmental Impact Assessment and Decision Support...—Sabine Latteman—Google Books n.d. https://books.google.com/books?hl=en&lr=&id=2kXLBQAAQBAJ&oi=fnd&pg=PP1&dq=Development+of+an+environmental+impact+assessment+and+decision+support+system+for+seawater+desalination+plants&ots=vYhITysE5M&sig=uHI8Zk7SiNppVoIwWHPFaodyHpU#v=onepage&q=Development%20of%20an%20environmental%20impact%20assessment%20and%20decision%20support%20system%20for%20seawater%20desalination%20plants&f=false (accessed , 2024).
- Dindi A, Quang DV, Abu-Zahra MRM. Simultaneous carbon dioxide capture and utilization using thermal desalination reject brine. Appl Energy 2015;154:298–308. [CrossRef]
- Ampelli C, Perathoner S, Centi G. CO2 utilization: an enabling element to move to a resource- and energy-efficient chemical and fuel production. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 2015;373. [CrossRef]
- Avila AM, Yu Z, Fazli S, Sawada JA, Kuznicki SM. Hydrogen-selective natural mordenite in a membrane reactor for ethane dehydrogenation. Microporous and Mesoporous Materials 2014;190:301–8. [CrossRef]
- Buonomenna MG, Yave W, Golemme G. Some approaches for high performance polymer based membranes for gas separation: block copolymers, carbon molecular sieves and mixed matrix membranes. RSC Adv 2012;2:10745–73. [CrossRef]
- Chen H, Zhang H, Yan Y. Catalytic combustion of volatile organic compounds over a structured zeolite membrane reactor. Ind Eng Chem Res 2013;52:12819–26. [CrossRef]
- Duyar MS, Farrauto RJ, Castaldi MJ, Yegulalp TM. In Situ CO 2 Capture Using CaO/γ-Al 2 O 3 Washcoated Monoliths for Sorption Enhanced Water Gas Shift Reaction. Ind Eng Chem Res 2014;53:1064–72. [CrossRef]
- Carvill BT, Hufton JR, Anand M, Sircar S. Sorption-enhanced reaction process. AIChE Journal 1996;42:2765–72. [CrossRef]
- Hufton JR, Mayorga S, Sircar S. Sorption-enhanced reaction process for hydrogen production. AIChE Journal 1999;45:248–56. [CrossRef]
- Stevens RW, Shamsi A, Carpenter S, Siriwardane R. Sorption-enhanced water gas shift reaction by sodium-promoted calcium oxides. Fuel 2010;89:1280–6. [CrossRef]
- Van Selow ER, Cobden PD, Verbraeken PA, Hufton JR, Van Den Brink RW. Carbon capture by sorption-enhanced water-gas shift reaction process using hydrotalcite-based material. Ind Eng Chem Res 2009;48:4184–93. [CrossRef]
- Minutillo M, Perna A. A novel approach for treatment of CO2 from fossil fired power plants. Part B: The energy suitability of integrated tri-reforming power plants (ITRPPs) for methanol production. Int J Hydrogen Energy 2010;35:7012–20. [CrossRef]
- Song C, Pan W. Tri-reforming of methane: a novel concept for catalytic production of industrially useful synthesis gas with desired H2/CO ratios. Catal Today 2004;98:463–84. [CrossRef]
- Duyar MS, Wang S, Arellano-Treviño MA, Farrauto RJ. CO2 utilization with a novel dual function material (DFM) for capture and catalytic conversion to synthetic natural gas: An update. Journal of CO2 Utilization 2016;15:65–71. [CrossRef]
- Lao DB, Galan BR, Linehan JC, Heldebrant DJ. The steps of activating a prospective CO2 hydrogenation catalyst with combined CO2 capture and reduction. Green Chemistry 2016;18:4871–4. [CrossRef]
- Reddy KJ, John S, Weber H, Argyle MD, Bhattacharyya P, Taylor DT, et al. Simultaneous capture and mineralization of coal combustion flue gas carbon dioxide (CO2). Energy Procedia 2011;4:1574–83. [CrossRef]
- Al-Mamoori A, Krishnamurthy A, Rownaghi AA, Rezaei F. Carbon Capture and Utilization Update. Energy Technology 2017;5:834–49. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).