Submitted:
12 June 2024
Posted:
13 June 2024
You are already at the latest version
Abstract
Keywords:
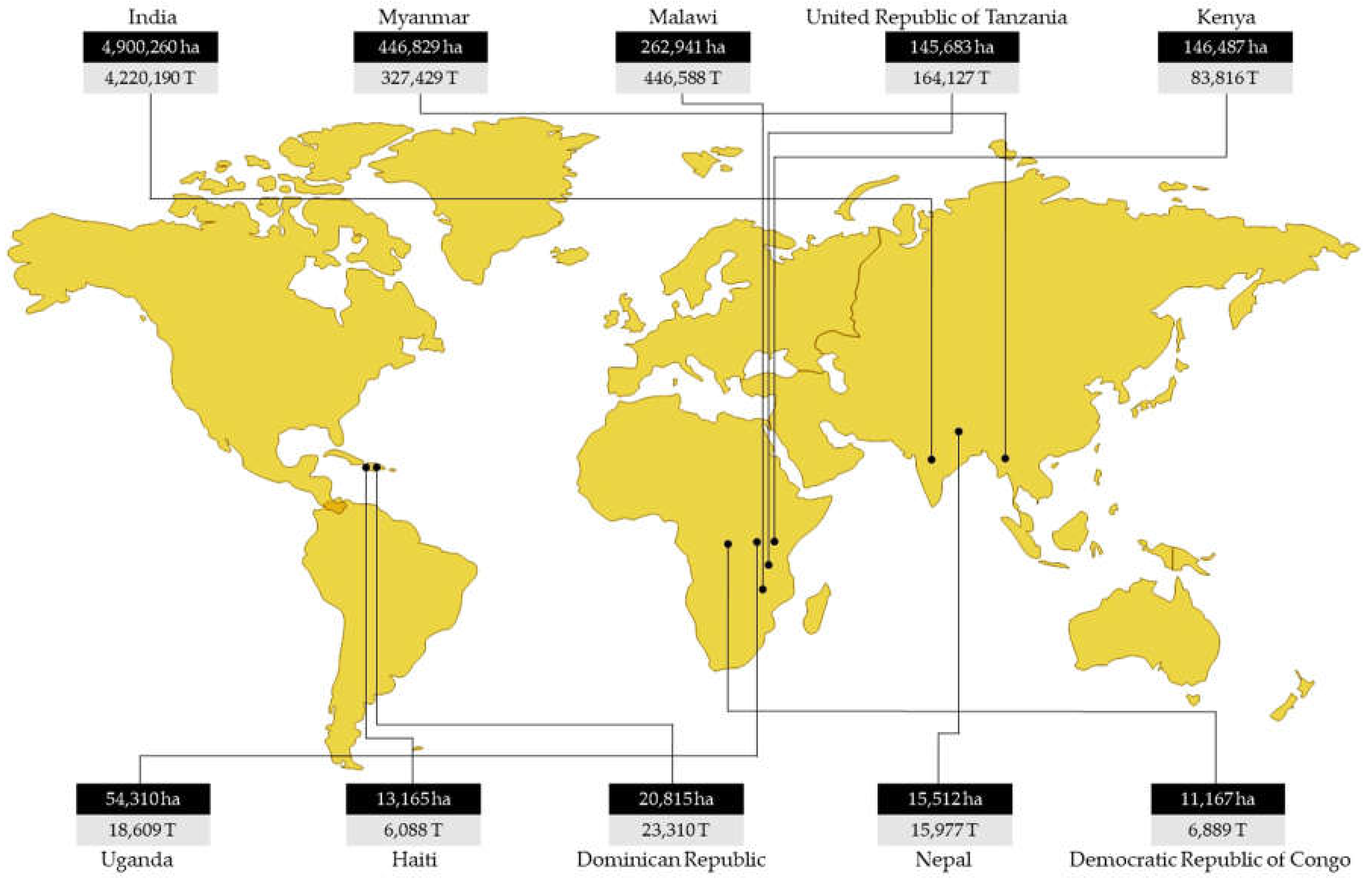
1. The Crop
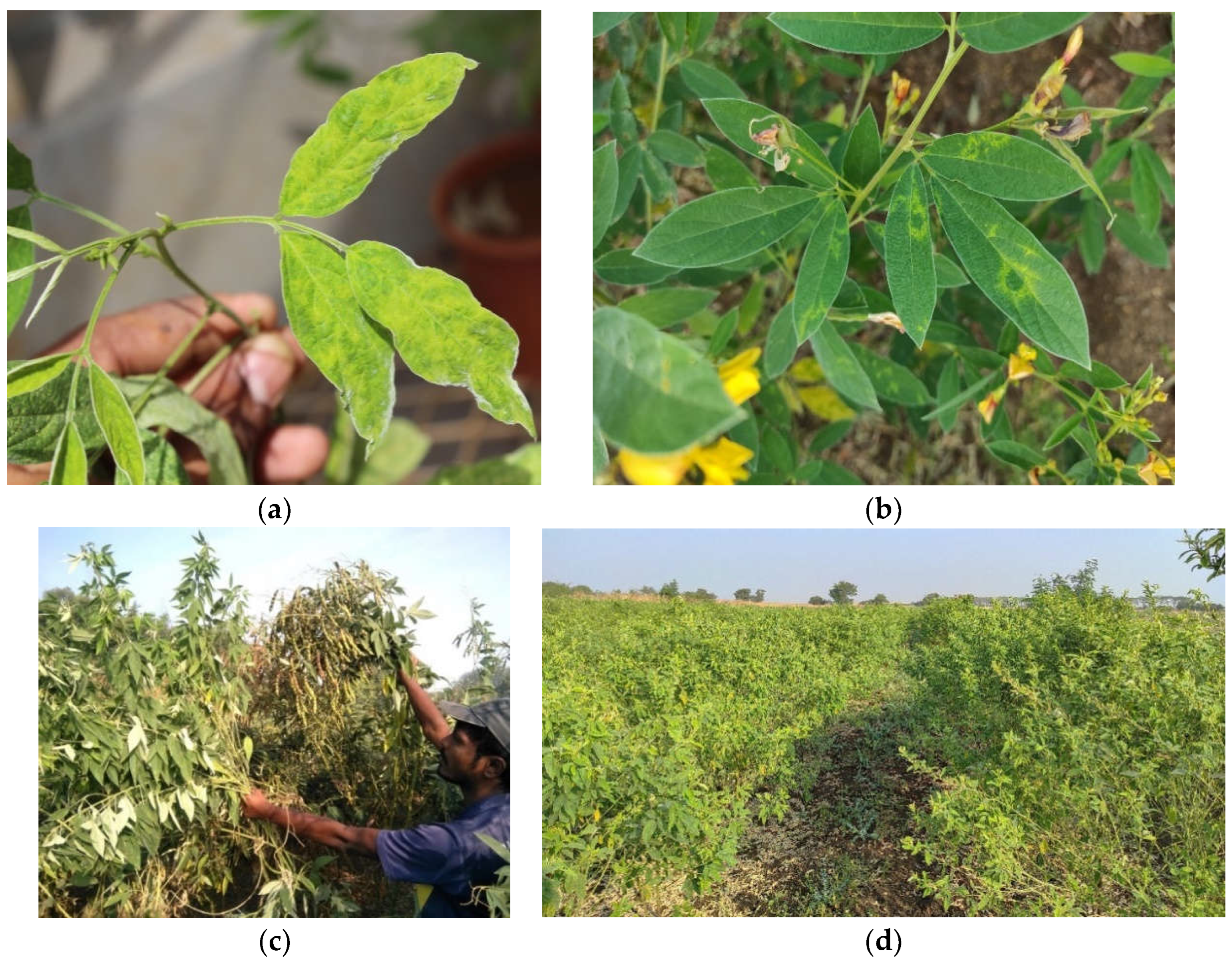
2. Sterility Mosaic Disease (SMD)
2.1. The Vector-Aceria cajani
2.2. SMD Transmission
2.3. SMD Etiology and Detection
2.4. PPSMV Genome Organization and Genetic Relationship
3. Epidemiology of SMD
4. Screening Techniques for SMD Resistance
4.1. Leaf Stapling Method
4.2. Infector Hedge/Row Method
4.3. Petiole-Grafting
5. SMD Management
6. Application of Molecular Markers
7. Future Line of Work
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Van der Maesen, L.J.G. Pigeonpea: origin, history, evolution and taxonomy. In The pigeonpea; Nene, Y.L., Hall, S.D., Sheila, V.K., Eds.; CAB International: Wallingford, UK, 1990; pp. 15–46. [Google Scholar]
- Food and Agriculture Organization (FAO) STAT. 2022. Available online: https://www.fao.org/faostat/en/#data/QCL.
- Faris, D.G.; Saxena, K.B.; Mazumdar, S.; Singh, U. Vegetable pigeonpea: a compromising crop for India. Patancheru, A.P. India, ICRISAT. 1987. [Google Scholar]
- Saxena, K.B.; Kumar, R.V.; Rao, P.V. Pigeonpea nutrition and its improvement. J. Crop Prod. 2002, 5, 227–260. [Google Scholar] [CrossRef]
- Jones, A.T.; Kumar, P.L.; Saxena, K.B.; Kulkarni, N.K.; Muniyappa, V.; Waliyar, F. Sterility mosaic disease- the “Green Plague” of pigeonpea. Advances in understanding the etiology, transmission and control of a major virus disease. Plant Dis. 2004, 88, 436–445. [Google Scholar] [CrossRef]
- Kumar, P.L.; Jones, A.T.; Waliyar, F. Biology, etiology and management of pigeonpea sterility mosaic disease. Annu. Rev. Plant. Pathol. 2004, 3, 77–100. [Google Scholar]
- Varshney, R.K.; Chen, W.; Li, Y.; Bharti, A.K.; Saxena, R.K.; Schlueter, J.A.; Donoghue, M.T.; Azam, S.; Fan, G.; Whaley, A.M.; Farmer, A.D.; Sheridan Iwata, A.; Tuteja, R.; Penmetsa, R.V.; Wu, W.; Upadhyaya, H.D.; Yang, S.P.; Shah, T.; Saxena, K.B.; Michael, T.; McCombie, W.R.; Yang, B.; Zhang, G.; Yang, H.; Wang, J.; Spillane, C.; Cook, D.R.; May, G.D.; Xu, X.; Jackson, S. Draft genome sequence of pigeonpea (Cajanus cajan), an orphan legume crop of resource-poor farmers. Nat. Biotechnol. 2012, 30, 83–89. [Google Scholar] [CrossRef]
- Sayiprathap, B.R.; Patibanda, A.K.; Prasanna Kumari, V.; Jayalalitha, K.; Srinivasa Rao, V.; Sharma, M.; Sudini, H.K. Prevalence of sterility mosaic disease (SMD) and variability in Pigeonpea sterility mosaic virus (PPSMV) in southern India. Indian Phytopathol. 2020, 73, 741–750. [Google Scholar] [CrossRef]
- Sharma, M.; Rathore, A.; Mangala, U.N.; Ghosh, R.; Sharma, S.; Upadhyay, H.D.; Pande, S. New sources of resistance to Fusarium wilt and sterility mosaic disease in a mini-core collection of pigeonpea germplasm. Eur. J. Plant Pathol. 2012, 133, 707–714. [Google Scholar] [CrossRef]
- Sharma, M.; Telangre, R.; Ghosh, R.; Pande, S. Multi-environment field testing to identify broad, stable resistance to sterility mosaic disease of pigeonpea. J. Gen. Plant Pathol. 2015, 81, 249–259. [Google Scholar] [CrossRef]
- Mitra, M. Report of the Imperial Mycologist. Scientific report of the agricultural research institute, Pusa, New Delhi, India. 19: 58-71. Mosaic of pigeonpea. In Plant Diseases of International Importance: Diseases of Cereals and Pulses; Singh, U.S., Mukhopadhyay, A.N., Kumar, J., Chaube, H.S., Eds.; Prentice Hall, New Jersey, 1931. [Google Scholar]
- Reddy, M.V.; Raju, T.N.; Lenne, J.M. Diseases of pigeonpea. In The pathology of food and pasture legumes; Allen, D.J., Lenne, J.M., Eds.; CAB International: Wallingford; UK, 1998; pp. 517–558. [Google Scholar]
- Nene, Y.L.; Sheila, V.K.; Sharma, S.B. A world list of chickpea (Cicer arietinum L.) and pigeonpea (Cajanus Cajan (L.)) pathogens. Legume Pathology Progress Report-7. ICRISAT Publication: Patancheru, Andhra Pradesh, India, 1996. [Google Scholar]
- Su, U. Plant disease in Burma. Int. Bull. Plant Prot. 1931, 5, 141. [Google Scholar]
- Newton, W.; Peiris, T.W.L. Virus disease of plant in Cylon. Plant Prot. Bull. FAO. 1953, 2, 17–21. [Google Scholar]
- Chaohong, Z.; Saxena, K.B.; Li, Z.; Zong, X.; Yang, S. Pigeonpea diseases in China. Int. Chickpea Pigeonpea Newsl. 2001, 8, 39–40. [Google Scholar]
- Kannaiyan, J.; Nene, Y.L.; Reddy, M.V.; Ryan, J.G.; Raju, T.N. Prevalence of pigeonpea diseases and associated crop losses in Asia, Africa and the Americas. Trop. Pest Manag. 1984, 30, 62–71. [Google Scholar] [CrossRef]
- Zote, K.K.; Mali, VR.; Mayee, C.D.; Kulkarni, S.V.; Mote, T.S. Outbreak of sterility mosaic of pigeonpea In Marathwada region of Maharashtra, India. Int. Pigeonpea Newsl. 1991, 14, 19–21. [Google Scholar]
- Singh, J.; Raghuraman, M. Emerging scenario of important mite pest in north India. Zoosymposia. 2011, 6, 170–179. [Google Scholar] [CrossRef]
- Alam, M. Arhar sterility. In Proceedings of the 20th Annual Meeting of the Indian Science Congress, Poona. 1933, 43, 15–16. [Google Scholar]
- Capoor, S.P. Observations on the sterility disease of pigeonpea. Indian J. Agri. Sci. 1952, 22, 271–274. [Google Scholar]
- Nene, Y.L. A survey of viral diseases of pulse crops in Uttar Pradesh. Experimental Station Bulletin; G. B. Pant University Agriculture and Technology: Pantnagar, Uttar Pradesh, 1972; pp. 4–191. [Google Scholar]
- Reddy, A.S.; Kulkarni, N.K.; Kumar, P.L.; Jones, A.T.; Muniyappa, V.; Reddy, D.V.R. A new graft-inoculation method for screening for resistance to Pigeonpea sterility mosaic virus. Int. Chickpea Pigeonpea Newsl. 2002, 9, 44–46. [Google Scholar]
- Patil, B.P.; Kumar, P.L. Pigeonpea sterility mosaic virus: a legume infecting Emaravirus from South Asia. Mol. Plant Pathol. 2015, 16, 775–786. [Google Scholar] [CrossRef]
- Kumar, S.; Subbarao, B.L.; Vipin, H. Molecular characterization of Emaraviruses associated with pigeonpea sterility mosaic disease. Sci. Rep. 2017, 7, 11831. [Google Scholar] [CrossRef]
- Dahal, G.; Neupane, K.R. Incidence and effect of sterility mosaic disease of pigeonpea in Nepal. Int. Pigeonpea Newsl. 1991, 13, 23–24. [Google Scholar]
- Singh, A.K.; Rathi, Y.P.S.; Agrawal, K.C. Sterility mosaic of pigeonpea: A challenge of 20th Century. Indian J. Virol. 1999, 15, 85–92. [Google Scholar]
- Reddy, M.V.; Nene, Y.L. Influence of sterility mosaic resistant pigeonpeas on multiplication of the mite vector. Indian Phytopathol. 1980, 33, 61–63. [Google Scholar]
- Kumar, P.L.; Jones, A.T.; Sreenivasulu, P.; Reddy, D.V.R. Unfolding the mystery of the causal agent of pigeonpea sterility mosaic disease. In Proceedings of the International Conference on Life Sciences in Next Milleniurnm, 11–14 December 1999; University of Hyderbad: India. [Google Scholar]
- Kumar, P.L.; Jones, A.T.; Sreenivasulu, P.; Fenton, B.; Reddy, D.V.R. Characterization of a virus from pigeonpea with affinities to species in the genus Aureusvirus, Family Tombusviridae. Plant dis. 2001, 85, 208–215. [Google Scholar] [CrossRef]
- Kumar, P.L.; Jones, A.T.; Duccan, G.; Roberts, I.M.; Reddy, D.V.R. Characterization of a novel mite transmitted virus associated with pigeonpea sterility mosaic disease. Phytopathol. 2001, 91, 51. [Google Scholar]
- Reddy, M.V.; Beniwal, S.P.S.; Sheila, V.K.; Sithananthan, S.; Nene, Y.L. Role of eriophyid mite (Aceria cajani) in transmission and spread of sterility mosaic of pigeonpea. In Progress in Acarology; Channbasavanna, G.P., Virakthmath, C.A., Eds.; Oxfard and IEH Publishing Co.: New Dellhi, India, 1989. [Google Scholar]
- Reddy, M.V.; Raju, T.N.; Ghanekar, A.M.; Amin, K.S.; Arjunan, G.; Ashtaputhra, J.V.; Sinha, B.K.; Muniyappa, V.; Reddy, S.V.; Guptha, R.P.; Kausalya, G. Variability in sterility mosaic pathogen of pigeonpea in India. Indian Phytopathol. 1993, 46, 206–212. [Google Scholar]
- Kulkarni, N.K.; Reddy, A.S.; Kumar, P.L.; Vijaynarasimha, J.; Rangaswamy, K.T.; Reddy, L.J.; Saxena, K.B.; Jones, A.T.; Reddy, D.V.R. Broad-based resistance to pigeonpea sterility mosaic disease in the accessions of Cajanus scarabaeoides. Indian J. Plant Prot. 2003, 31, 6–11. [Google Scholar]
- Sayiprathap, B.R.; Patibanda, A.K.; Prasanna Kumari, V.; Jayalalitha, K.; Ramappa, H.K.; Rajeswari, E.; Karthiba, L.; Saratbabu, K.; Sharma, M.; Sudini, H.K. Salient findings on host range, resistance screening, and molecular studies on sterility mosaic disease of pigeonpea induced by Pigeonpea sterility mosaic viruses (PPSMV-I and PPSMV-II). Front. Microbiol. 2022, 13, 838047. [Google Scholar] [CrossRef]
- Reddy, M.V.; Kannaiyan, J.; Nene, Y.L. Increased susceptibility of sterility mosaic infected pigeonpea to powdery mildew. Int. J. Trop. Plant. Dis. 1984, 2, 35–40. [Google Scholar]
- Sithanantham, S.; Reddy, M.V.; Rao, R. Increased damage by the spider mite. Schizotetranychus cajani in pigeonpea plants affected by sterility mosaic. In Progress in Acarology; Channabasavanna, G.P., Viraktamath, C.A., Eds.; Oxford and IHB Publishing Co.: India, 1989; Volume 2, pp. 11–14. [Google Scholar]
- Channabasavanna, G.P.A. Contribution of the knowledge of Indian eriophyid mites (Eriophyoidae: Trombidiformes: Acarina). Thesis, University of Agricultural Sciences, Bangalore, India, 1966. [Google Scholar]
- Kandaswamy, T.K.; Ramakrishnan, K. A epiphytotic of pigeonpea sterility mosaic at Coimbatore. Madras Agric. J. 1960, 47, 440–441. [Google Scholar]
- Seth, M.L. Transmission of pigeonpea sterility by an eriophyid mite. Indian Phytopathol. 1962, 15, 225–227. [Google Scholar]
- Ghanekar, A.M.; Sheila, V.K.; Beniwal, S.P.S.; Reddy, M.V.; Nene, Y.L. Sterility mosaic of pigeonpea. In Plant Diseases of International Importance: Diseases of Cereals and Pulses; Singh, U.S., Mukhopadhyay, A.N., Kumar, J., Chaube., H.S., Eds.; Prentice Hall, New Jersey, 1992; Volume 1, pp. 415–428. [Google Scholar]
- Reddy, M.V.; Reddy, D.V.R.; Sacks, W.R. Epidemiology and management of sterility mosaic disease of pigeonpea. Proceedings of International Symposium. Rose Rosette and other Eriophyid Mite-Transmitted Plant Disease Agents of Uncertain Etiology, 19–21 May 1994; Iowa State University: United States. [Google Scholar]
- Kulkarni, N.K.; Kumar, P.L.; Muniyappa, V.; Jones, A.T.; Reddy, D.V.R. Transmission of Pigeonpea sterility mosaic virus by the eriophyid mite, Aceria cajani (Acari: Arthropoda). Plant Dis. 2002, 86, 1297–1302. [Google Scholar] [CrossRef]
- Oldfield, G.N.; Reddy, M.V.; Nene, Y.L.; Reed, W. Preliminary studies of the eriophyid vector of sterility mosaic. Int. Chickpea Pigeonpea Newsl. 1981, 1, 25–27. [Google Scholar]
- Sheila, V.K.; Manohar, S.K.; Nene, Y.L. Biology and morphology of Aceria cajani. Int. Pigeonpea Newsl. 1988, 7, 28. [Google Scholar]
- Muniyappa, V.; Nangia, N. Pigeonpea cultivars and selections for resistance to sterility mosaic in relation to prevalence of eriophyid mite Aceria cajani Channabasavanna. Trop. Grain Legume Bull. 1982, 25, 28–30. [Google Scholar]
- Dhar, V.; Rathore, Y.S. Pattern of population distribution of Aceria cajani on pigeonpea plant. Indian J. Pulses Res. 1995, 7, 137–143. [Google Scholar]
- Kumar, P.L.; Fenton, B.; Duncan, G.; Jones, A.T.; Sreenivasulu, P.; Reddy, D.V.R. Assessment of variation in Aceria cajani (Acari: Eriophyidae) using analysis of nuclear rDNA ITS regions and scanning electron microscopy: Implications for the variability observed in host plant resistance to pigeonpea sterility mosaic disease. Ann. Appl. Biol. 2001, 139, 61–73. [Google Scholar] [CrossRef]
- Pallavi, M.S.; Ramappa, H.K. Population dynomics of pigronpea sterility mosaic virus vector Aceria cajani. Mysore J. Agric. Sci. 2014, 48, 394–399. [Google Scholar]
- Reddy, M.V.; Sheila, V.K.; Murthy, A.K.; Padma, P. Mechanism of resistance to Aceria cajani in pigeonpea. Int. J. Trop. Plant Dis. 1995, 13, 51–57. [Google Scholar]
- Kulkarni, N.K.; Kumar, P.L.; Muniyappa, V.; Jones, A.T.; Reddy, D.V.R. Studies on the natural and experimental host range of Pigeonpea sterility mosaic virus. J. Mycol. Plant Pathol. 2003, 33, 141–145. [Google Scholar]
- Reddy, M.V.; Arjunan, N.; Muniyappa, V. Survival of pigeonpea sterility mosaic pathogen and its vector during summer in southern India. Int. Pigeonpea newsl. 1990, 11, 16–17. [Google Scholar]
- Singh, A.K.; Rathi, Y.P.S. Epidemiology of vector of Pigeonpea sterility mosaic virus. Indian J. Virol. 1997, 13, 143–145. [Google Scholar]
- Kaushik, D.; Srivastava, S.; Nath, B.C.; Chauhan, V.B.; Singh, R. Correlation between mite population (Aceria cajani) and environmental factors causing sterility mosaic disease of pigeon pea. Int. J. Life Sci. 2013, 1, 228–232. [Google Scholar]
- Latha, T.K.S.; Doraiswamy, S. Detection of Pigeonpea sterility mosaic virus, the causal agent of sterility mosaic disease of pigeonpea in viruliferous mite vector by DAS-ELISA and DIBA. Arch. Phytopathol. Plant Prot. 2008, 41, 537–542. [Google Scholar] [CrossRef]
- Kumar, P.L.; Latha, T.K.S.; Kulkarni, N.K.; Raghavendra, N.; Saxena, K.B.; Waliyar, F.; Rangaswamy, K.T.; Muniyappa, V.; Doriswamy, S.; Jones, A.T. Broad-based resistance to pigeonpea sterility mosaic disease in wild relatives of pigeonpea (Cajanus: Phaseoleae). Ann. Appl. Biol. 2005, 146, 371–379. [Google Scholar] [CrossRef]
- Kumar, P.L.; Duncan, G.H.; Roberts, I.M.; Jones, A.T.; Reddy, D.V.R. Cytopathology of Pigeonpea sterility mosaic virus in pigeonpea and Nicotiana benthamiana: Similarities with those of eriophyid mite-borne agents of undefined aetiology. Ann. Appl. Biol. 2002, 140, 87–96. [Google Scholar] [CrossRef]
- Kumar, P.L.; Jones, A.T.; Reddy, D.V.R. Mechanical transmission of Pigeonpea sterility mosaic virus. J. Mycol. Plant Pathol. 2002, 32, 88–89. [Google Scholar]
- Jones, A.T. Experimental transmission of viruses in diagnosis. In Diagnosis of plant virus diseases; Matthews, R.E.F., Ed.; CRC Press: Boca Raton, Florida, USA, 1993; pp. 49–72. [Google Scholar]
- Manjunatha, L. Detection and characterization of Pigeonpea sterility mosaic virus (PPSMV) and its management. Ph. D. Thesis, University of Agricultural Sciences, Bengaluru, India, 2012. [Google Scholar]
- Nene, Y.L. Sterility mosaic of pigeonpea: the challenge continues. Indian J. Mycol. Plant Pathol. 1995, 25, 1–11. [Google Scholar]
- Kumar, P.L.; Jones, A.T.; Reddy, D.V.R. ELISA for the detection of pigeonpea sterility mosaic virus. Patancheru 502 324, Andhra Pradesh, India: International Crops Research Institute for the Semi-Arid Tropics. 2000. Available online: http://www.icrisat.org/text/research/grep/homepage/archives_cd/frame.htm.
- Kumar, P.L.; Jones, A.T.; Reddy, D.V.R. A novel mite-transmitted virus with a divided RNA genome closely associated with pigeonpea sterility mosaic disease. Phytopathol. 2003, 93, 71–81. [Google Scholar]
- Kumar, P.L.; Jones, A.T.; Waliyar, F. Serological and nucleic acid based methods for detection of plant viruses: Methods manual. ICRISAT: Patancheru, A.P., India, 2004. [Google Scholar]
- Elbeaino, T.; Digiaro, M.; Uppala, M.; Sudini, H.K. Deep sequencing of Pigeonpea sterility mosaic virus discloses five RNA segments related to Emaraviruses. Virus Res. 2014, 188, 27–31. [Google Scholar] [CrossRef]
- Elbeaino, T.; Digiaro, M.; Uppala, M.; Sudini, H.K. Deep sequencing of dsRNAs recovered from mosaic-diseased pigeonpea reveals the presence of a novel Emaravirus, Pigeonpea sterility mosaic virus 2. Arch. Virol. 2015, 160, 2019–2029. [Google Scholar]
- Patil, B.P.; Meenakshi, D.; Ritesh, M. Variability of Emaravirus species associated with sterility mosaic disease of pigeonpea in India provides evidence of segment reassortment. Viruses 2017, 9, 183. [Google Scholar] [CrossRef]
- Latha, T.K.S.; Kuamr, P.L. Preliminary characterization of Coimbatore isolate of Pigeonpea sterility mosaic virus. Madras Agri. J. 2015, 102, 344–347. [Google Scholar]
- Tatineni, S.; McMechan, A.J.; Wosula, E.N.; Wegulo, S.N.; Graybosch, R.A.; French, R.; Hein, G.L. An eriophyid mite-transmitted plant virus contains eight genomic RNA segments with unusual heterogenicity in the nucleocapsid protein. J. Virol. 2014, 88, 11834–11845. [Google Scholar] [CrossRef]
- Sailaja, K. Biological and molecular characterization of Pigeonpea sterility mosaic virus. M.Sc Thesis, Acharya N.G. Ranga Agricultural University, Lam, Guntur, India, 2016. [Google Scholar]
- Padule, D.N.; Mewase, A.G.; Patel, B.P. Survey for the incidence of Fusarium wilt and other diseases of pigeonpea in central part of western Maharashtra. J. Maharashtra Agri. University. 1982, 118, 57–70. [Google Scholar]
- Paul, R.K.; Vennila, S.; Yadav, S.K.; Bhat, M.N.; Kumar, M.; Chandra, P.; Paul, A.K.; Prabhakar, M. Weather based forecasting of sterility mosaic disease in pigeonpea (Cajanus cajan) using machina learning techniques and hybrid models. Indian J. Agric. Sci. 2020, 90, 1952–1958. [Google Scholar] [CrossRef]
- Nene, Y.L.; Reddy, M.V. A new technique to screen pigeonpea for resistance to sterility mosaic. Trop. Grain Legume Bull. 1976, 5, 23–24. [Google Scholar]
- Nene, Y.L.; Reddy, M.V. Screening for resistance to sterility mosaic of pigeonpea. Plant. Dis. Rep. 1976, 66, 1034–1036. [Google Scholar]
- Nene, Y.L.; Kannaiyan, J.; Haware, M.P.; Reddy, M.V. Pigeonpea diseases: Resistance-screening techniques. Information Bulletin. ICRISAT, Patancheru, Andhra Pradesh, India. 1981, 1, 1–14. [Google Scholar]
- Pandey, S.; Sharma, M.; Guvvala, G.; Telangre, R. High throughput phenotyping of pigeonpea diseases: stepwise identification of host plant resistance. Information Bulletin; ICRISAT: Patancheru, Andhra Pradesh, India, 2012; p. 93. [Google Scholar]
- AICRP on Pigeonpea Annual Report 2020-21, All India Coordinated Research Project on Pigeonpea, ICAR-Indian Institute of Pulses Research, Kanpur-208024, pp. 406.
- Singh, A.K. Sterility mosaic of pigeonpea: etiology, epidemiology and management. Ph.D. Thesis, G. B. Pant University of Agriculture and Technology, Uttar Pradesh, India, 1992. [Google Scholar]
- De, R.K.; Dhar, V.; Rathore, Y.S. Control of mosaic in pigeonpea with insecticides and acaricides. Indian J. Pulses Res. 1996, 11, 87–89. [Google Scholar]
- Rathi, Y.P.S. Temik treatment of pigeonpea seeds for prevention of sterility mosaic. Acta Bot. Indica. 1997, 90–91. [Google Scholar]
- Ramakrishnan, K.; Kandaswamy, T.K. Investigation on virus disease of pulse crops in Tamil Nadu. Final Technical Report; Tamil Nadu Agricultural University: Coimbatore, Tamil Nadu, India, 1972. [Google Scholar]
- Narayanaswamy, C.A. Studies on Aceria cajani (Acari: Eriophyidae), mite vector of sterility mosaic disease with special reference to varietal screening and its interplant distribution. M.Sc Thesis, University of Agricultural Sciences, Banglore, India, 2004. [Google Scholar]
- Manjunatha, L.; Ramappa, H.K.; Mahantesha, S.R.V.; Gowda, M.B.; Rajappa, P.V.; Kavitha, T.R. Management of sterility mosaic disease (SMD) of pigeonpea. Plant Arch. 2012, 12, 1007–1012. [Google Scholar]
- Sudharani, S.; Amaresh, Y.S.; Naik, M.K.; Arunkumar, H.; Sunkad, G. Management of sterility mosaic disease through vector management. Indian J. Agric. Res. 2017, 51, 498–501. [Google Scholar]
- Rajeswari, E.; Smitha, K.P.; Kamalakannan, A.; Alice, D.; Kannan Bapu, J.R. Management of pigeonpea sterility mosaic disease. Legume Res. 2016, 39, 648–650. [Google Scholar] [CrossRef]
- Maurya, R.K.; Kumar, B.; Kumar, R.; Singh, M. Transmission of Pigeonpea sterility mosaic virus and management of sterility mosaic disease of pigeonpea by different acaricides under middle IGP of Bihar. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 3711–3716. [Google Scholar] [CrossRef]
- Gurha, S.N.; Singh, D.N.; Gopal, L.K. Effect of population density on the incidence of sterility and yellow mosaic disease of pigeonpea. Int. Pigeonpea Newsl. 1983, 2, 47–48. [Google Scholar]
- Bhatnagar, V.S.; Nene, Y.L.; Jadhaw, D.R. Sterility mosaic disease of pigeonpea in sorghum-based cropping system. Int. Pigeonpea Newsl. 1984, 3, 37–38. [Google Scholar]
- Nene, Y.L.; Reddy, M.V.; Beniwal, S.P.S.; Mahmood, M.; Zote, K.K.; Singh, R.N.; Sivaprakasam, K. Multilocational testing of pigeonpea for broad-based resistance sterility mosaic in India. Indian Phytopathol. 1989, 42, 444–448. [Google Scholar]
- Subramanian, K.S.; Samuel, G.S.; Janarthanan, R.; Kandaswamy, T.K. Studies on the varietal resistance of pigeonpea (Cajanus cajan) to sterility mosaic disease. Madras Agric. J. 1973, 60, 38–40. [Google Scholar]
- Rathi, Y.P.S. Studies on sterility mosaic disease of pigeonpea. Final technical report; GBPU &AT: Pantanagar, U.P., India, 1983. [Google Scholar]
- Singh, A.B.; Singh, D.P.; Singh, N.P.; Kumar, R. Evaluation of pigeonpea germplasm for resistance to sterility mosaic. Int. Chickpea Pigeonpea Newsl. 1995, 2, 69–70. [Google Scholar]
- Amin, K.S.; Reddy, M.V.; Nene, Y.L.; Raju, T.N.; Shukla, P.; Zote, K.K.; Arjunan, G.; Bendre, J.N.; Rathi, Y.P.S.; Sinha, B.K.; Gupta, R.P.; Anilkumar, T.B.; Chauhan, V.B.; Singh, G.; Jha, D.K.; Gangadharan, K. Multi-location evaluation of pigeonpea (Cajanus cajan) for broad based resistance to sterility mosaic disease in India. Indian J. Agri. Sci. 1993, 63, 542–546. [Google Scholar]
- Reddy, M.V.; Jain, K.C.; Chauhan, Y.S.; Singh, L. Wilt and sterility mosaic disease resistant pigeonpea genotype ICPL 87119 benefits farmers in Medak district of Andhra Pradesh, India. Int. Chickpea Pigeonpea Newsl. 1995, 2, 71–72. [Google Scholar]
- Rangaswamy, K.T.; Sulladmath, V.V.; Byre, G.M. Reaction of certain pigeonpea entries and varieties to sterility mosaic disease. Abs. Symposium on economically important diseases of crop plants, IPS southern chapter, University of Agricultural Sciences, Bangalore. 1997, 18–20. [Google Scholar]
- Nagaraj, K.M.; Chikkadevaiah, Muniyappa, V.; Rangaswamy, K.T.; Kumar, P.L. Evaluation of pigeonpea genotypes for resistance to Pigeonpea sterility mosaic virus-B isolate. Indian J. Plant Prot. 2006, 34, 216–220. [Google Scholar]
- Sharma, M.; Rathore, A.; Mangala, U.N.; Ghosh, R.; Sharma, S.; Upadhyay, H.D.; Pande, S. New source of resistance to fusarium wilt and sterility mosaic disease in a mini-core collection of pigeonpea germplasm. Eur J. Plant Pathol. 2012, 133, 707–714. [Google Scholar] [CrossRef]
- Singh, N.; Tyagi, R.K.; Pandey, C. Genetic resources of pigeonpea (Cajanus cajan): conservation for use. National Bureau of Plant Genetic Resources, New Delhi. 2013. [Google Scholar]
- Jaggal, L.G.; Patil, B.R.; Salimath, P.M.; Madhusudhan, K.; Patil, M.S.; Udikeri, S.S. Evaluation of mini core accessions of pigeonpea (Cajanus cajan (L.) Millsp.) against sterility mosaic disease and fusarium wilt. Karnataka J. Agric. Sci, 2014; 27, 337–339. [Google Scholar]
- Bhaskar, A.V. Screening of pigeonpea genotypes against wilt and sterility mosaic disease in Telangana state, India. Indian J. Agric. Res. 2016, 50, 172–176. [Google Scholar] [CrossRef]
- Tharageshwari, L.M.; Hemavathy, A.T.; Jayamani, P.; Karthiba, L. Evaluation of pigeonpea (Cajanus cajan) genotypes against pigeonpea sterility mosaic disease. Electron. J. Plant Breed. 2019, 10, 727–731. [Google Scholar] [CrossRef]
- International Crops Research Institute for the Semi-Arid Tropics (ICRISAT). 2021. Available online: https://www.icrisat.org/new-high-yielding-pigeonpea-variety-set-for-release-in-india-more-to-come/.
- Dhanushasree, M.A.; Thanga, H.; Gnanamalar, R.P.; Karthiba, L. Evaluation of Pigeonpea Genotypes against Sterility Mosaic Virus (PPSMV) Disease. Biol. Forum Int. J. 2022, 14, 1263–1268. [Google Scholar]
- Mediga, K.R.; Sunkad, G.; Kulkarni, S.; Chandran, U.S.S.; Ghosh, R.; Kshirsagar, D.; Sonnappa, M.; Katravath, S.; Parthasarathy, A.; Sharma, M. Multi-environment testing based G×E interactions reveal stable host-plant resistance against sterility mosaic disease in pigeonpea. Agron 2023, 13, 2859. [Google Scholar] [CrossRef]
- Seednet India Portal, Ministry of Agriculture and Farmers Welfare. Available online: https://seednet.gov.in/.
- Project coordinator’s report, Annual group meet on pigeonpea, 2015-16, ICAR, IIPR, Kanpur, pp. 272.
- Remanandan, P. The wild gene pool of Cajanus at ICRISAT: present and future. Proc. Int. Workshop on Pigeonpea, 15–19 December 1980; ICRISAT: Patancheru 502324 AP, India. [Google Scholar]
- Singh, V.K.; Khan, A.W.; Saxena, R.K.; Kumar, V.; Kale, S.M.; Sinha, P.; Chitikineni, A.; Pazhamala, L.T.; Garg, V.; Sharma, M.; Sameer Kumar, C.V.; Parupalli, P.; Vechalapu, S.; Patil, S.; Muniswamy, S.; Ghanta, A.; Yamini, K.N.; Dharmaraj, P.S.; Varshney, R.K. Next-generation sequencing for identification of candidate genes for Fusarium wilt and sterility mosaic disease in pigeonpea (Cajanus cajan). Plant Biotechnol. J. 2016, 14, 1183–1194. [Google Scholar] [CrossRef]
- Saxena, R.K.; Hake, A.; Bohra, A.; Khan, A.W.; Hingane, H.; Sultana, R.; Singh, I.P.; Naik, S.S.J.; Varshney, R.K. A diagnostic marker kit for Fusarium wilt and sterility mosaic diseases resistance in pigeonpea. Theor. Appl. Genet. 2021, 134, 367–379. [Google Scholar] [CrossRef]
- Patil, P.G.; Byregowda, M.; Patil, B.R.; Das, A.; Reena, M.G.A.; Sowjanya, M.S.; Shashidhar, H.E. Microsatellite markers linked to sterility mosaic disease resistance in pigeonpea (Cajanus cajan (L.) Millsp.). Legume Genom. Genet. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Prasanthi, L.R.; Reddy, B.V.B.; Rani, K.R.; Sivaprasad, Y.; Rajeswari, T.; Reddy, K.R. RAPD and SCAR markers linked to the sterility mosaic disease resistance gene in pigeonpea (Cajanus cajan (L.) Millisp.). Asian Australasian J. Plant Sci. Biotecnol 2009, 16–20. [Google Scholar]
- Gnanesh, B.N.; Bohra, A.; Sharma, M.; Byregowda, M.; Pande, S.; Wesley, V.; Saxena, R.K.; Saxena, K.B.; Kavi Kishor, P.B.; Varshney, R.K. Genetic mapping and quantitative trait locus analysis of resistance to sterility mosaic disease in pigeonpea (Cajanus cajan (L.) Milisp.). Field Crops Res. 2011, 123, 53–61. [Google Scholar] [CrossRef]
- Saxena, R.K.; Sandip, M.K.; Kumar, V.; Parupali, S.; Joshi, S.; Singh, V.; Garg, V.; Das, R.R.; Sharma, M.; Yamini, K.N.; Ghanta, A.; Rathore, A.; Sameerkumar, C.V.; Saxena, K.B.; Varshney, R.K. Genotyping-by-sequencing of three mapping populations for identification of candidate genomic regions for resistance to sterility mosaic disease in pigeonpea. Sci. Rep. 2017, 7, 1813. [Google Scholar] [CrossRef]
- Cao, Y.; Zhou, H.; Zhou, X.; Li, F. Control of plant viruses by CRISPR/Cas system-mediated adaptive immunity. Front. Microbiol. 2020, 11, 593700. [Google Scholar] [CrossRef]



| Sl. No. | Variety | Patancheru | Dholi | Varanasi | Badnapur | Rahuri | Bengaluru | Coimbatore | Dharwad | Tirupati | Vijayapura | Warangal |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bahar | R | R | R | R | R | R | R | R | HS | R | R |
| 2 | BRG 1 | R | R | R | R | R | R | MR | R | R | MR | R |
| 3 | BRG 2 | R | R | R | R | R | S | MR | R | MR | MR | R |
| 4 | BRG 3 | R | R | R | R | R | R | R | R | R | MR | R |
| 5 | BRG 5 | * | R | R | R | MR | S | R | MR | R | MR | R |
| 6 | BSMR 736 | R | R | MR | R | R | R | MR | R | R | R | R |
| 7 | ICP 7035 | R | MR | R | R | R | R | R | R | HS | R | S |
| 8 | IPA 8F | R | R | R | R | HS | R | R | R | R | R | R |
| 9 | Purple 1 | MR | R | HS | R | R | S | R | MR | HS | R | S |
| 10 | ICP 2376 | R | R | R | R | R | MR | R | S | HS | R | S |
| 11 | ICP 8863 | HS | HS | HS | HS | HS | HS | S | HS | HS | MR | HS |
| Sl. No. | Genotypes/Cultivars | Reference |
|---|---|---|
| 1. | NP (WR) 15, P-435, P-1100, P-1289, P-1778 and P-2621 | [81] |
| 2. | L-3 and P-4875 | [90] |
| 3. | ICRISAT-3783, 6986, 1137, 2719, 7119, HY-3c, ICP-7035 and Atylosia lineata | [74] |
| 4. | ICRISAT-3784, 5449, 6497, 7035, 7119, Pant 8-76, 8-77, E-41, P-4785 and L-26 | [91] |
| 5. | ICP-7035, 3782, 6986, 6997, 7119, 7197, 7867, 7942 and 8136 | [28] |
| 6. | ICP 7378, 2S2 | [46] |
| 7. | Bahar, ICPLC-88046, DA-35, K32-1, Pusa-14, 19, Gant-9005, DA-I 1, 32, 33 | [92] |
| 8. | ICP 7035 | [93] |
| 9. | ICPL 787119 | [94] |
| 10. | ICP 7035 and HY3C | [95] |
| 11. | ICP 7035, MAL 14, and MAL 19 | [96] |
| 12. | ICP’s 7869, 9045, 11015, 11230, 11281, 11910, and 14976. Combined resistance to FW and SMD-ICP 6739, 8860, 11015, 13304, 14638, and 14819 | [97] |
| 13. | BRG3, IPA8F, IPA 15-F, GT 101, ICP 7035, ICPL 87091 and JKM 189 | [60] |
| 14. | ICPL 85010, IC245198, IC45768, IC73313, IC73336, IC73342, IC73340, IC73347, IC73727, IC73731, IC73735, IC73739, IC73841, IC73879, IC73880, IC73914, IC73925, IC73747, IC245198, IC45768, IC73332, IC74126, IC74084, IC74107, IC74123, Sehore, DPPA84-61-3, DPPA84-8-3, ICP786 (IC306500), ICP4395, ICP6997, ICP10976, CP10977, ICP7035 (IC306496), ICP8862, MA97, Rampur, Amar, Bahar, Bageshwari, PantA3, PantA104, PantA8505, PantA8508, Narendra arhar 1, comp 1-ESR6, Hy 3C, Maruti, BSMR1, BSMR2, BSMR604, BSMR736, Bhawanisagar 1, NPRR-1, ICP6997, MA3, LRG36, S1-3, ICPL88034(IC245153), DPPA85-3(IC527890), DPPA85-14(IC527891), ICPL7197, ICPL7264, PUSA14, USA15, PUSA17, PUSA18, Purple-1, PR 5149, PI 397430, KA-32-1, ICPL342, ICPL88034, DPPA85-2, DPPA85-15, BWR159, MA3, MA6, KPL44, AWR1,KAWR2, KAWR7, KAWR9, KAWR73, | [98] |
| 15. | ICP772, ICP939, ICP995, ICP1071, ICP1126, ICP1156, ICP1273, ICP1279, ICP2577, ICP2698, ICP2746, ICP3451, ICP3576, ICP4029, ICP4167, ICP4307, ICP7426, ICP4575, ICP4715, ICP6128, ICP6370, ICP6668, ICP6739, ICP6845, ICP6859, ICP6929, ICP7076, ICP7148, ICP7223, ICP6049, ICP7803, ICP7869, ICP8012, ICP8255, ICP8266, ICP8602, ICP8793, ICP8860, ICP8949, ICP9045, ICP9336, ICP9655, ICP10094, ICP10228, ICP11321, ICP10447, ICP11015, ICP11059, ICP11230, ICP11281, ICP11627, ICP11823, ICP11910, ICP12105, ICP12123, ICP12410, ICP12654, ICP13011, ICP13139, ICP13191, ICP13244, ICP13270, ICP13304, ICP13359, ICP13431, ICP13577, ICP13579, ICP13633, ICP13662, ICP13884, ICP14116, ICP14120, ICP14147, ICP14155, ICP14545, ICP14569, ICP14638, ICP14701, ICP14722, ICP14801, ICP14819, ICP14832, ICP14903, ICP14976, ICP15049, ICP15068, ICP15107, ICP15161, ICP15185, ICP12142, ICP16264 and ICP163 | [99] |
| 16. | ICPL 20094, ICPL 20106, ICPL 20098 and ICPL 20115 | [10] |
| 17. | ICPL-87119, ICPL-2376, BDN-2, PT-4-307, CORG-9701, BSMR-736, GRG-811 and BSMR-853 | [100] |
| 18. | DPP 2-89, DPP 3-182, IC 22557 and ICP 3666 | [101] |
| 19. | TDRG 59 (ICPL 99050) and Bheema | [102] |
| 20. | BWR 153 and CRG 16-07 | [103] |
| 21. | ICPL-16086 and ICPL-16087 | [35] |
| 22. | NAM 2082, NAM 2088, NAM 2089, NAM 2162, GRG 11, TS-3R-58-53-2, GRG 152, KRG 33, BSMR 736, AND ICPL 87119 | [104] |
| State | Varieties |
|---|---|
| Andhra Pradesh | Laxmi, LRG-41, LRG-38, WRG-27, WRG-53, Bahar, Pusa-9, NDA 1, WRG 65, Surya (MRG 1004) |
| Bihar | MA-6, Ajad, DA-11, IPA-203, Bahar, Pusa-9, Narendra Arhar-2 |
| Madhya Pradesh | JKM-189, TJT-501, JKM-7, TT-401, BSMR-175, ICPL-87119, BSMR-736 |
| Chhattisgarh | Rajiv Lochan, MA-3, ICPL-87119, Vipula, BSMR-853 |
| Gujarat | GT-100, GT-101, Banas, BDN-2, BSMR-853, AGT 2 |
| Haryana | Paras, Pusa-992, UPAS-120, AL-201, Manak, Pusa-855, PAU-881 |
| Karnataka | Vamban-3, CORG-9701, ICPL-84031, BRG-2, Maruti (ICP-8863), WRP-1, Asha (ICPL 87119), TS-3, KM 7 |
| Maharashtra | BDN-711, BSMR-736, AKT-8811, PKV Tara, Vipula, BDN-708, Asha, BSMR 175, Vaishali (BSMR 853) |
| Punjab | AL-201, PAU-881, Pusa-992, Upas-120 |
| Uttar Pradesh | Pradesh Bahar, NDA-1, NDA-2, Amar, MA-6, MAL-13, IPA-203, UPAS 120 |
| Rajasthan | UPAS-120, PA-291, Pusa-992, Asha (ICPL-87119), VLA-1 |
| Tamil Nadu | Co-6, CORG-9701, Vamban-3, ICPL-151, Vamban 1, Vamban 2 |
| Jharkhand | Bahar, Asha, MA-3 |
| Uttarakhand | VLA-1, PA-291, UPAS 120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





