Submitted:
13 June 2024
Posted:
13 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Data and Discussion
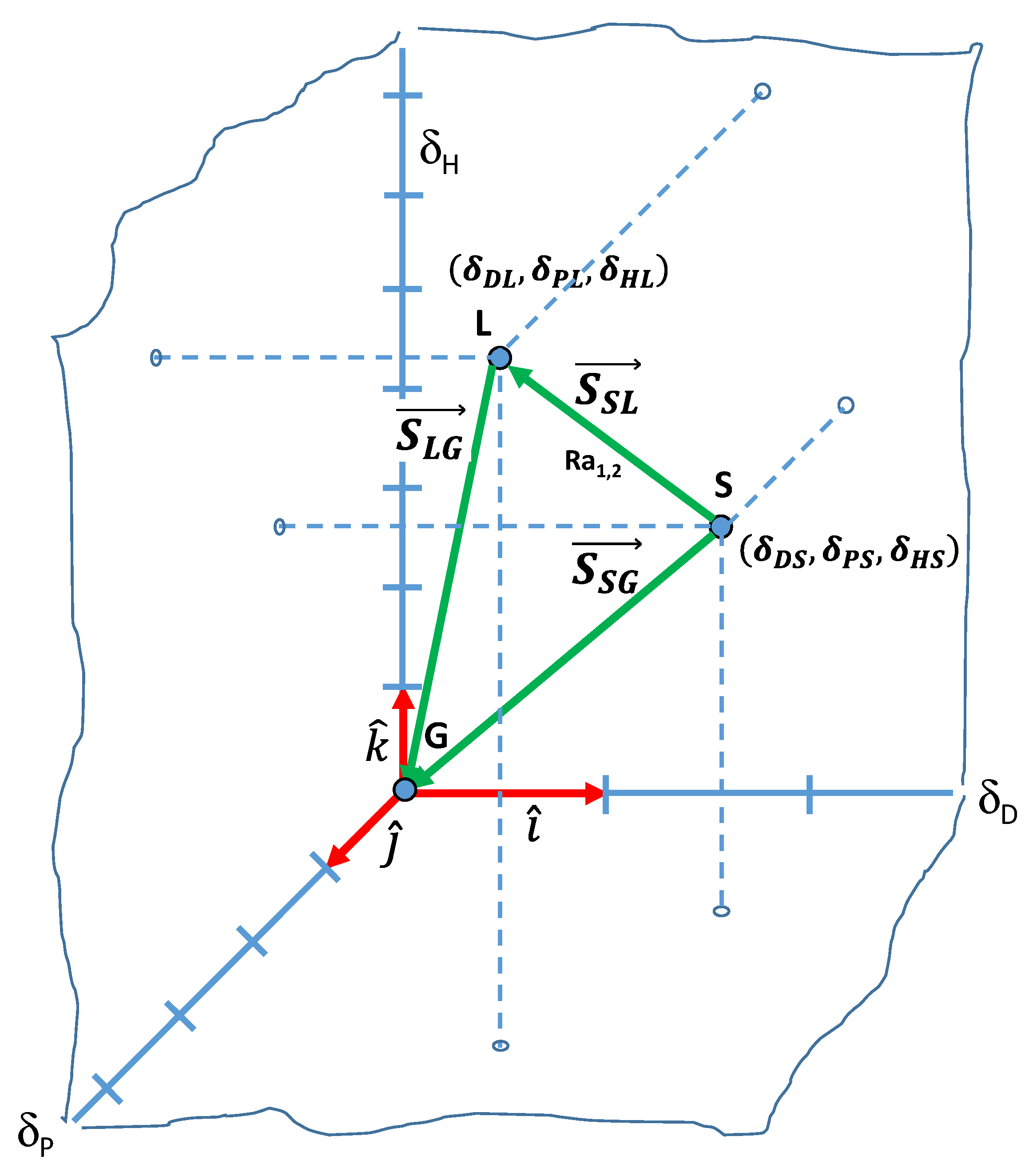
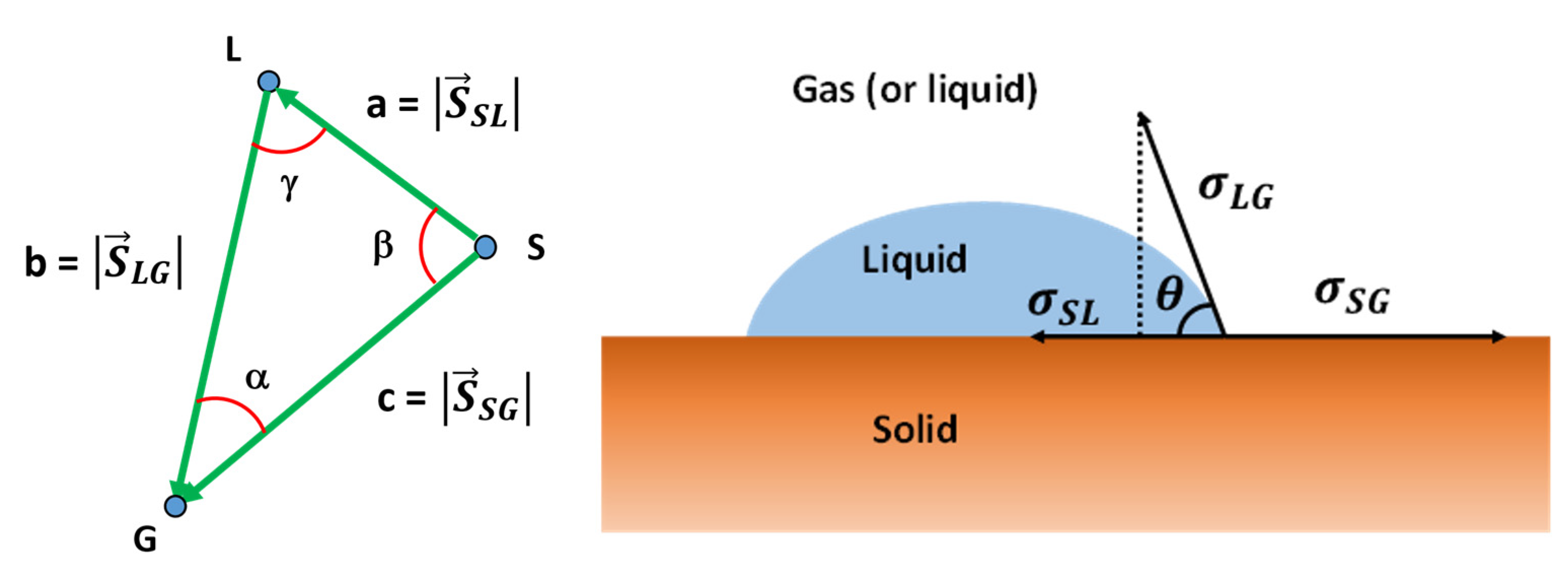
3.1. The Hansen Solubility Parameters Vector Field
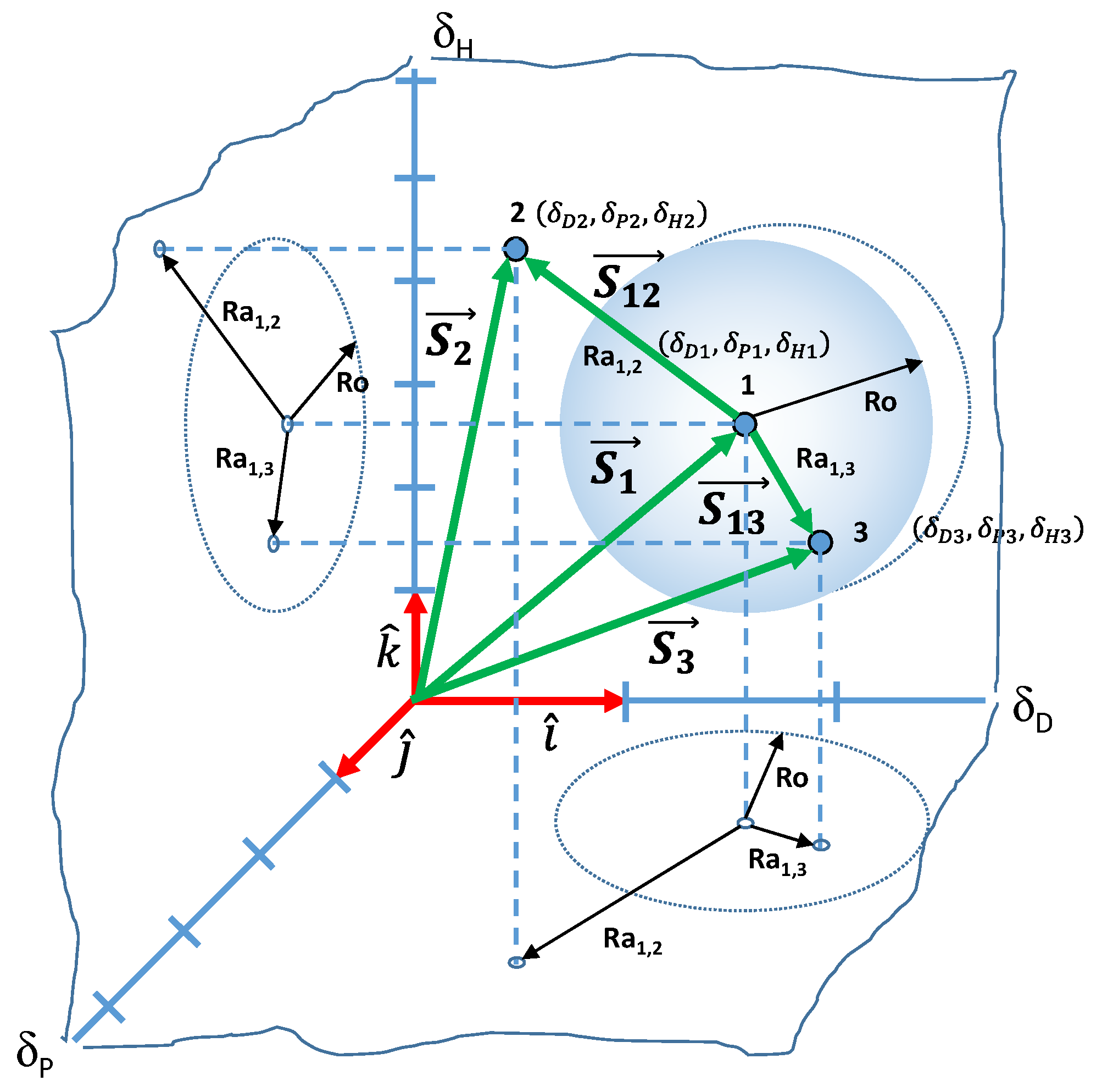
3.1.1. Concepts and Definitions
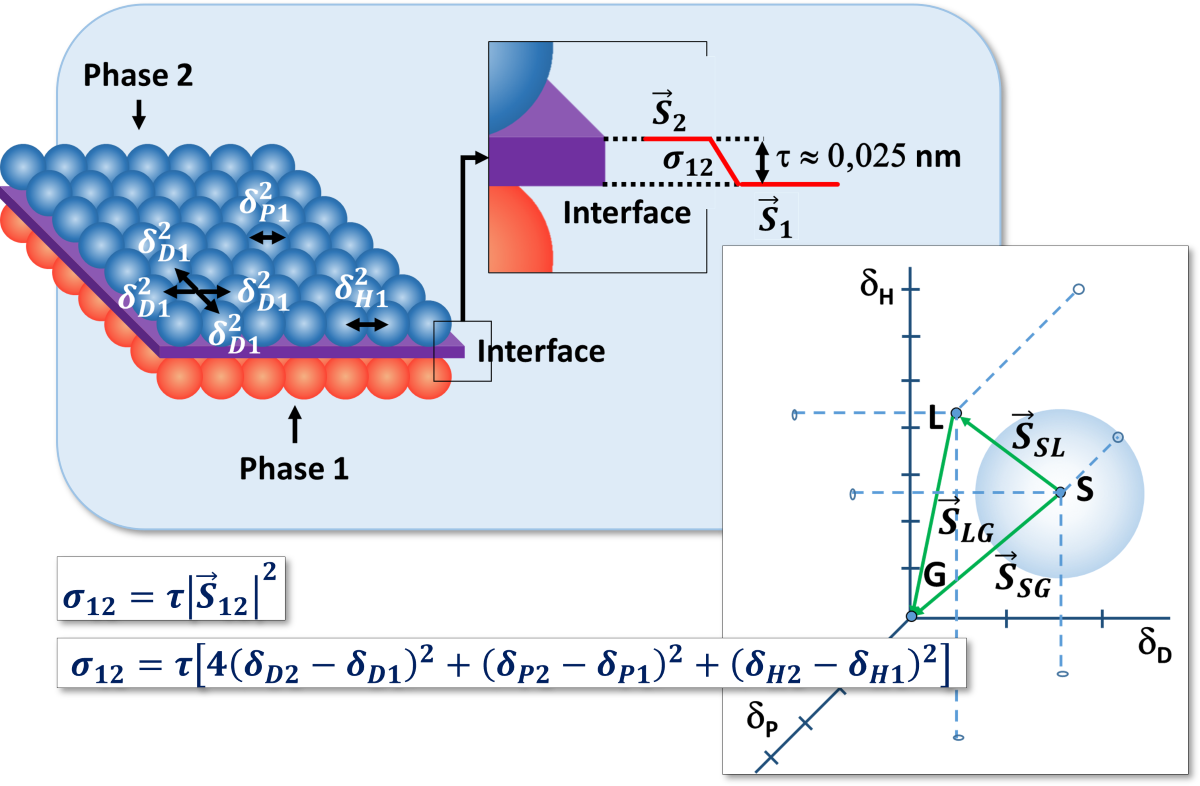
3.1.2. Interaction Vectors and Free Surface Energy
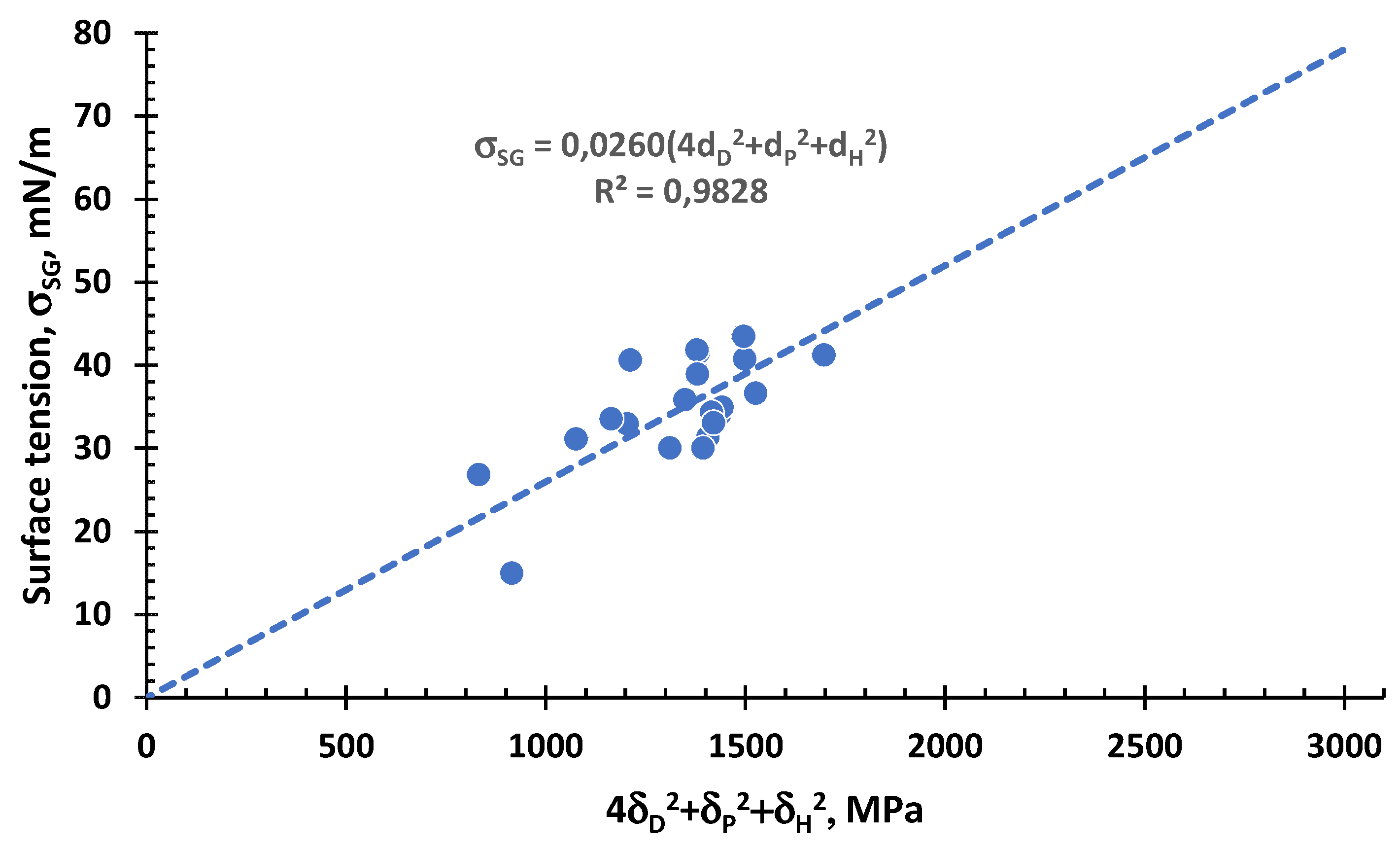
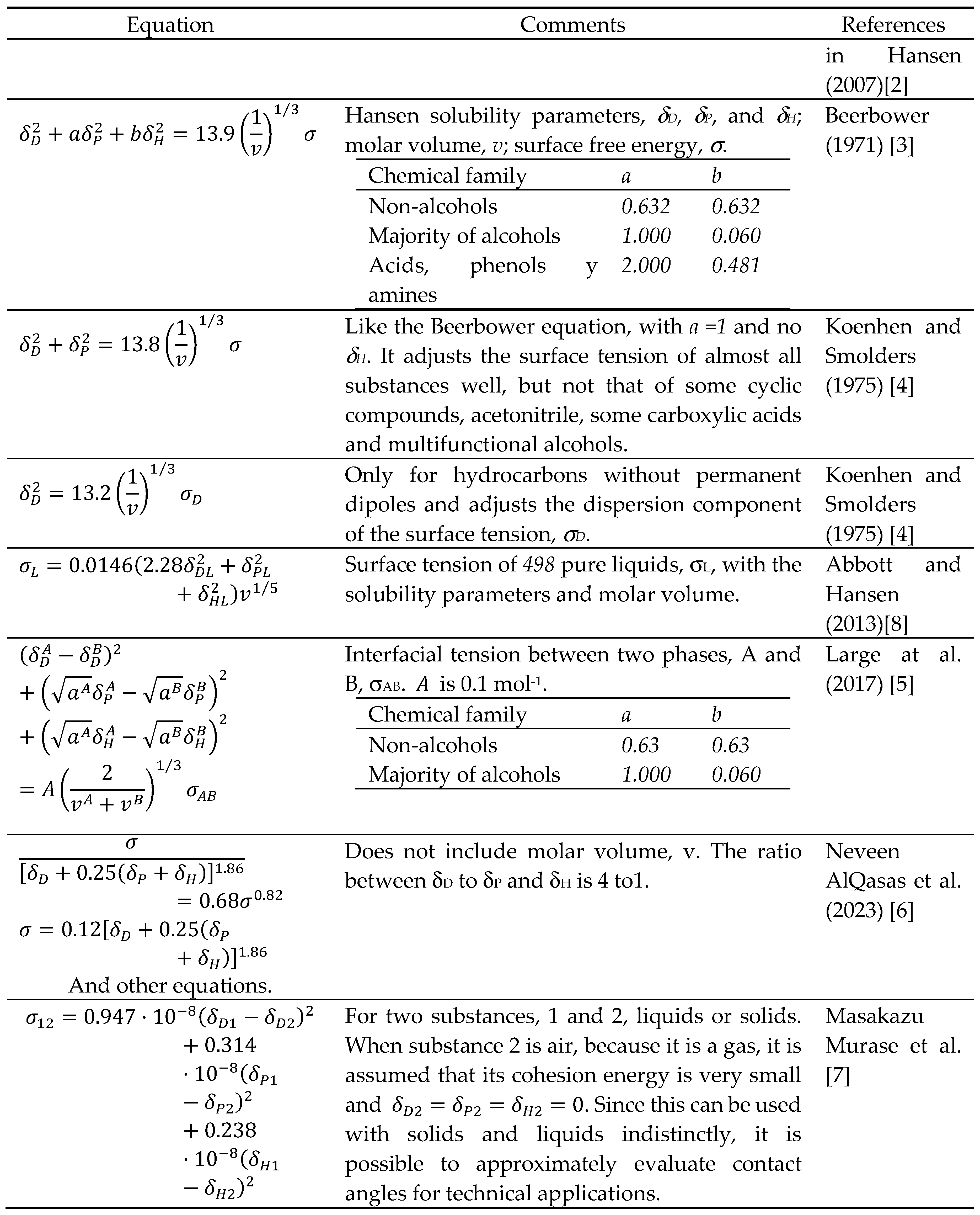
3.2. Surface Tension of Liquids
- (a)
- The liquid is completely non-polar, and its molecules do not present any special orientation at the interface with air, since its molecules are, like the air molecules, non-polar. The only relevant parameter is δD, as δP = 0 and δH = 0. This is the case, for instance, of hydrocarbons like pentane.
- (b)
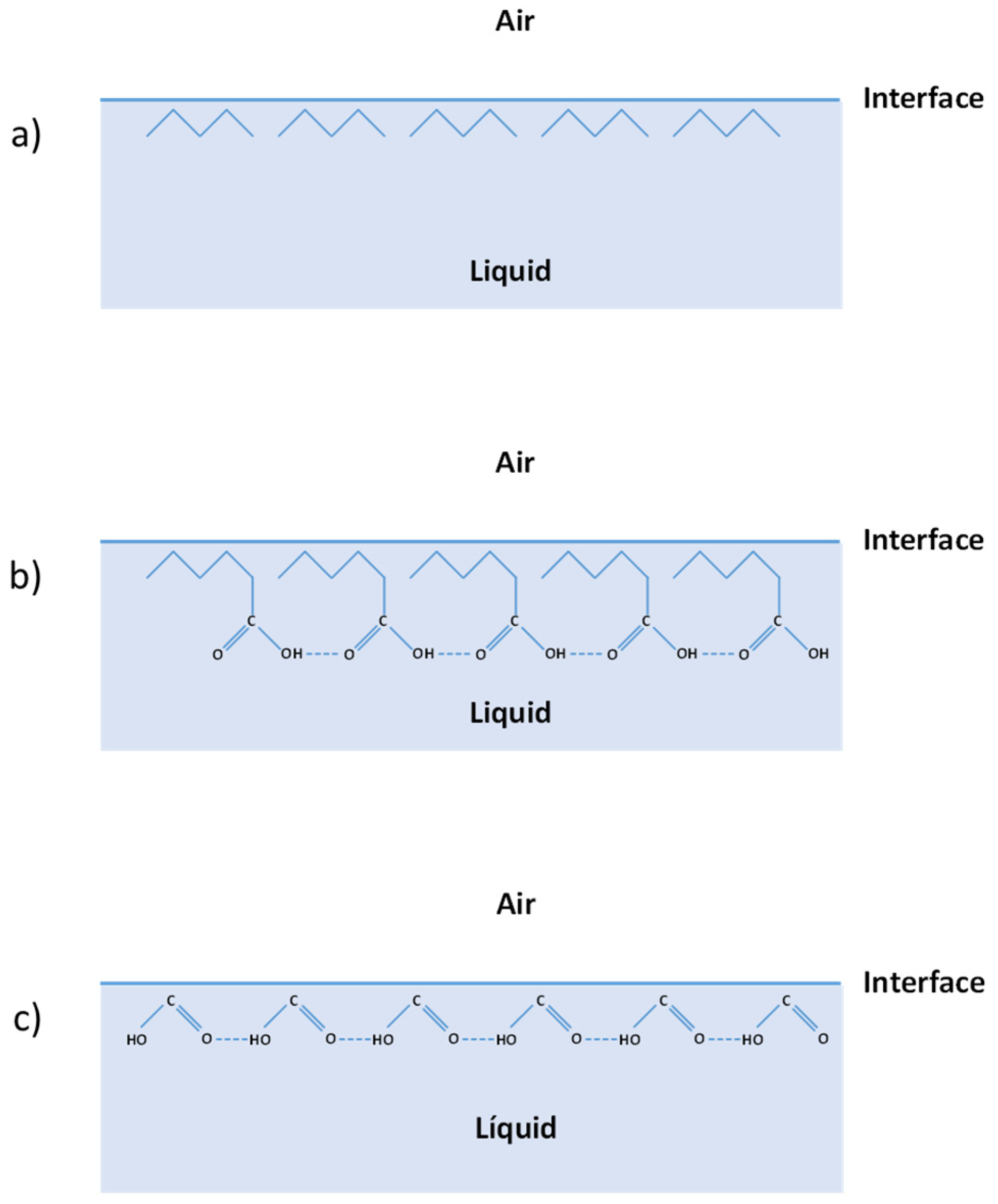
- The liquid is polar, but molecular size is sufficiently large to “hide” its hydrogen bonds in the inner part of the liquid preventing them from interacting with air. The electric dipole moment cannot be “hidden” because it affects the molecule itself. In this case, δH would not be relevant to the air-liquid interaction, while dispersion and polarity would. The Figure 2 shows the example of a carboxylic acid like hexanoic acid. The five-atom carbon chain creates a non-ionic barrier that would conceal the hydrogen bonds.
- (c)
- The liquid is polar, but molecular size is very small, and non-polar hydrocarbon chains are not able to “hide” the hydrogen bonding effect versus the air. In this case, the three solubility parameters–dispersion, polarity, and hydrogen bonding–variables would be relevant. Figure 2 shows the case of formic acid, a small ionic molecule with hydrogen bonds that cannot be concealed.
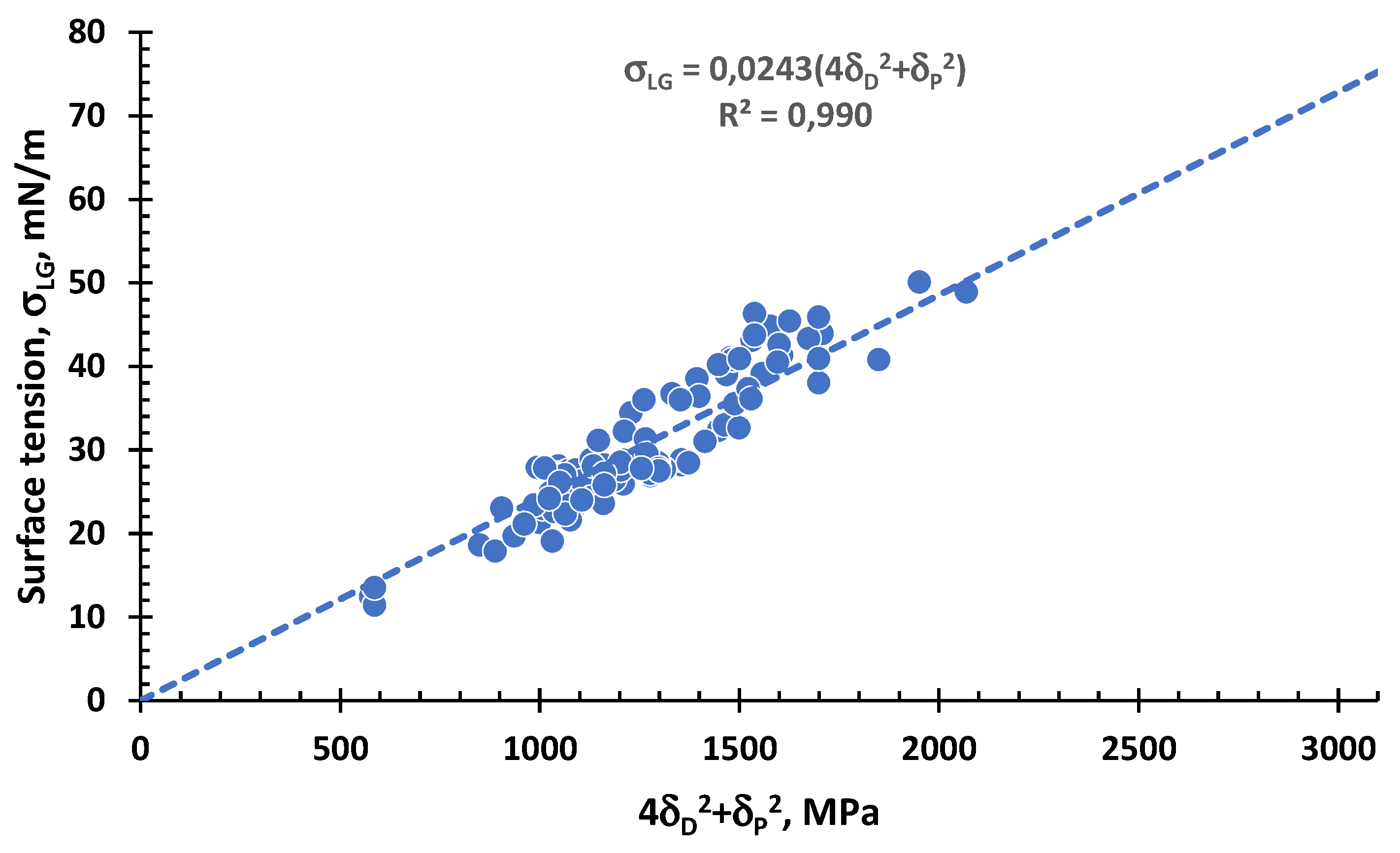
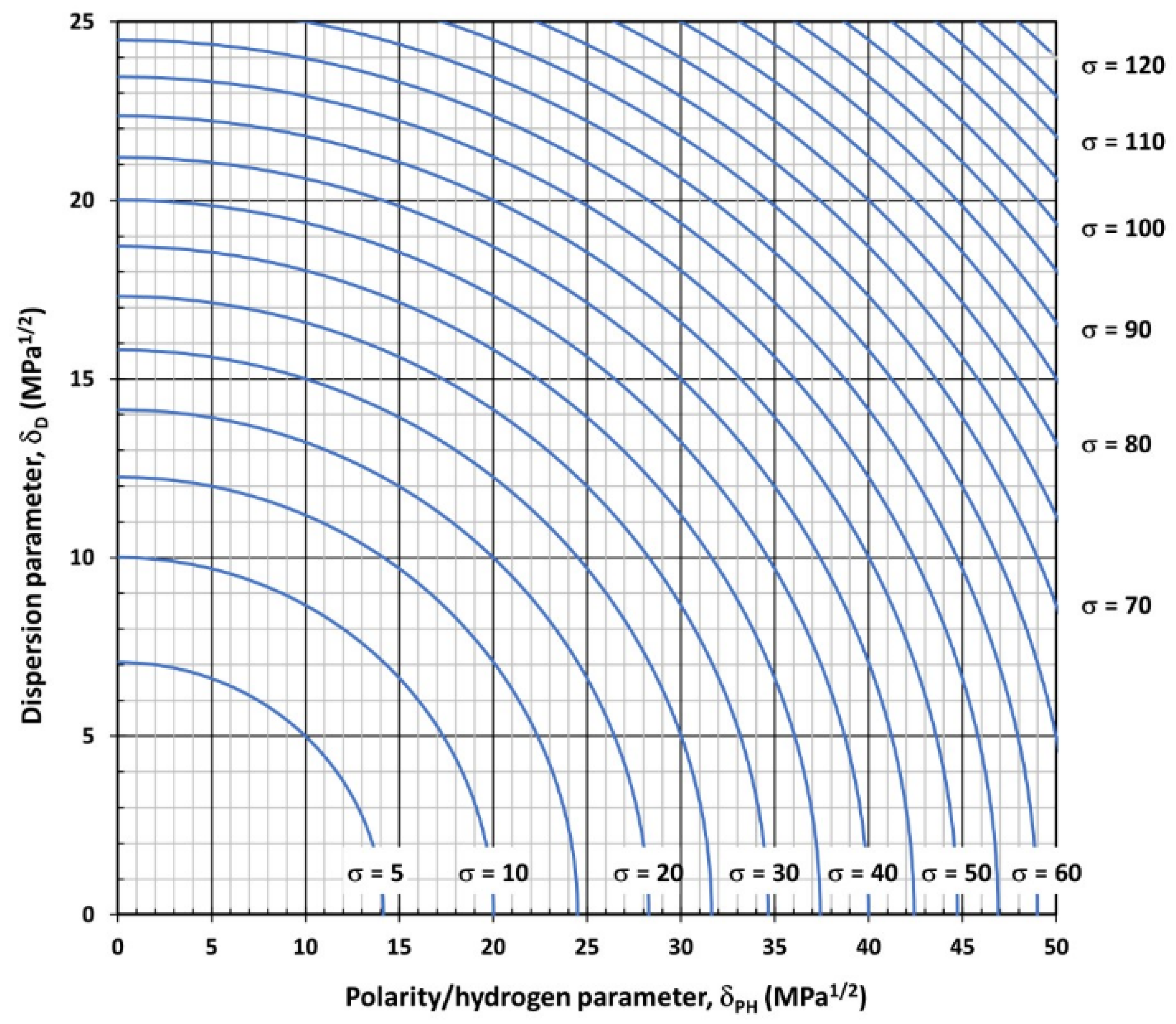
3.2.1. Surface Tension of Non-Ionic Liquids or Ionic with Non-Small Molecular Size as Function of the Interaction Vector
3.2.2. Surface Tension of Ionic or Non-Ionic Liquids with Not-Small Molecular Size as Function of the Interaction Vector
3.3. Surface Free Energy of Solids
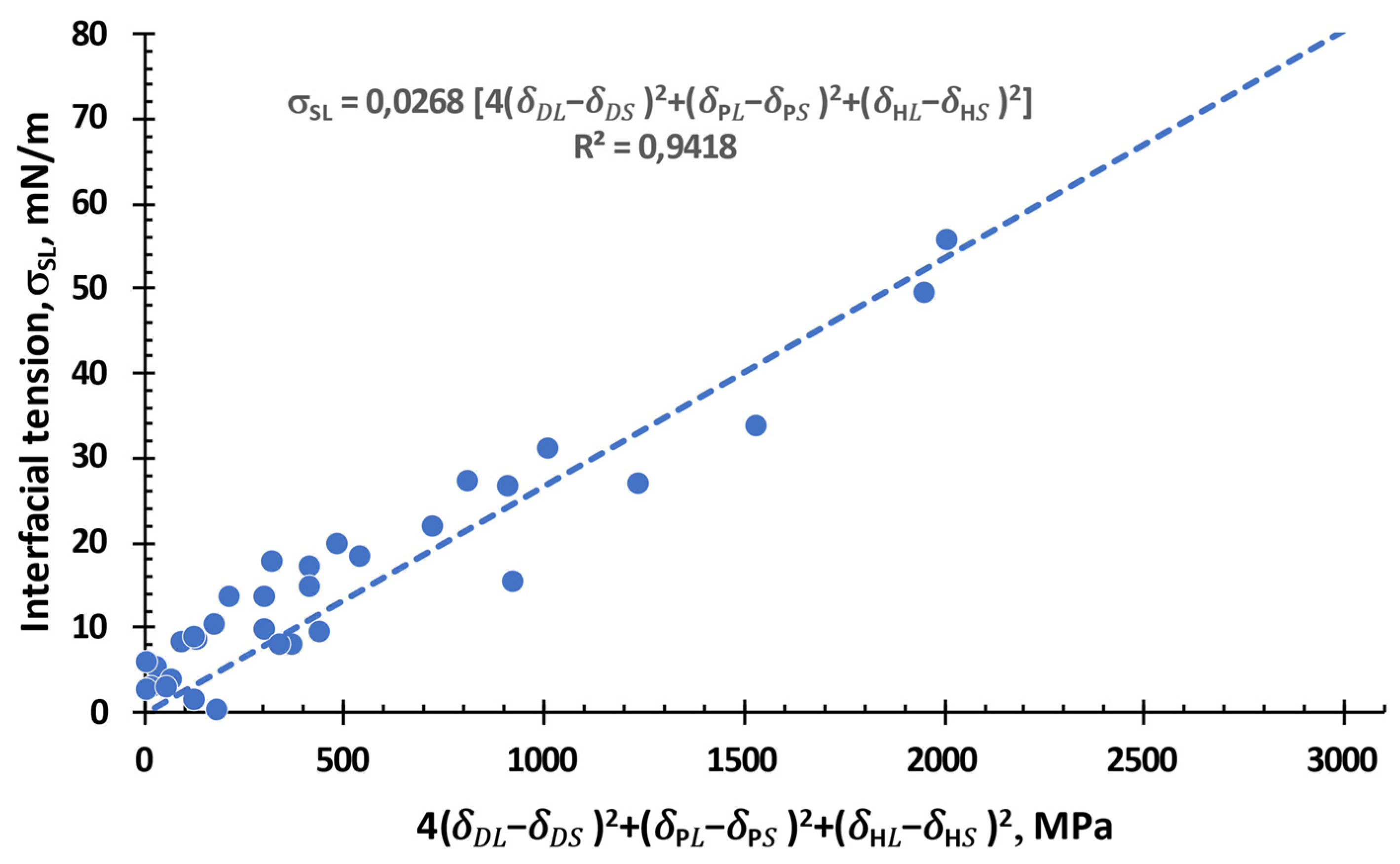
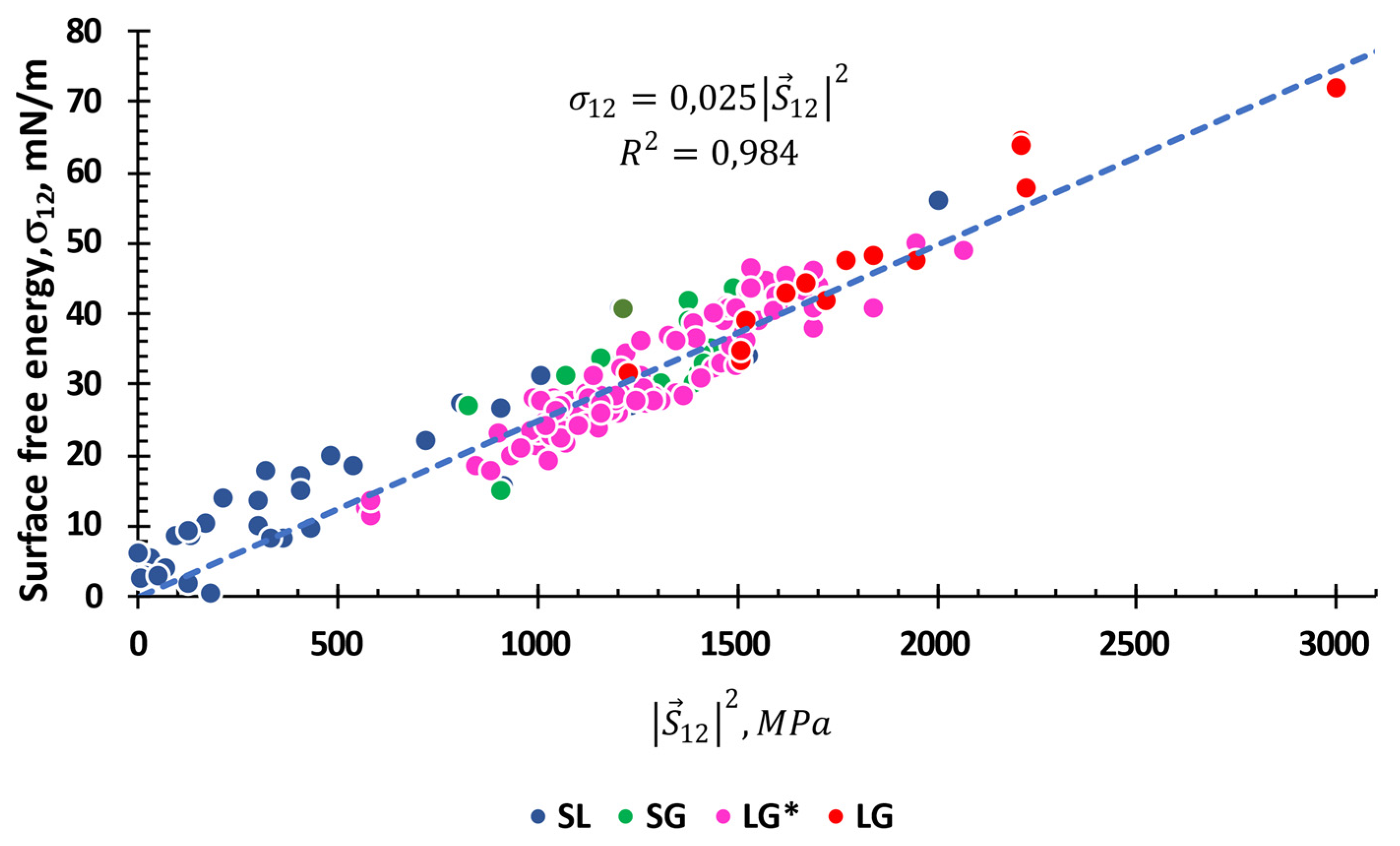
3.4. Interfacial Tension between a Solid and a Liquid, or Any Given two Substances
3.4. Free Surface Energy: General Equation
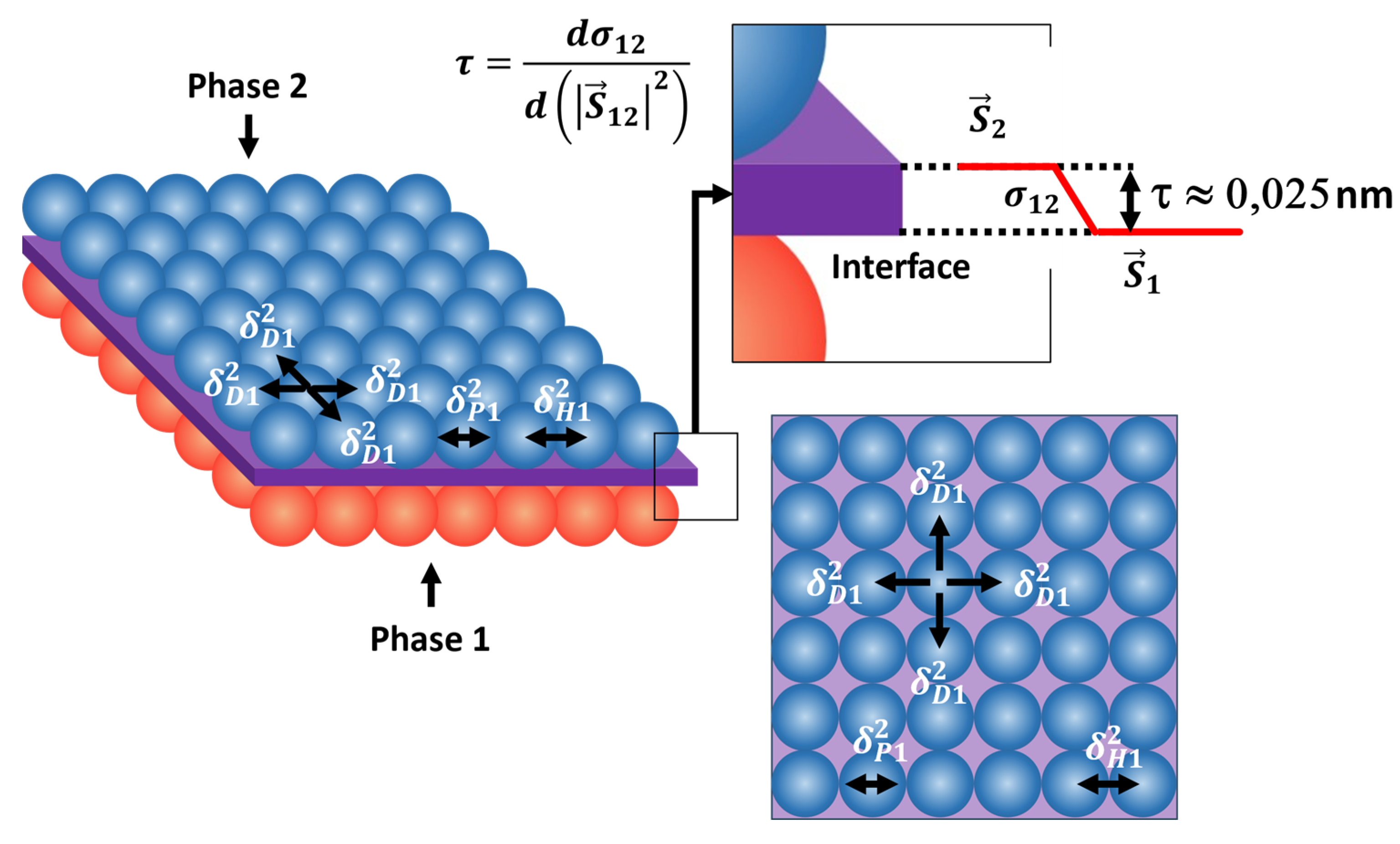
3.5. Physical Meaning of τ Constant
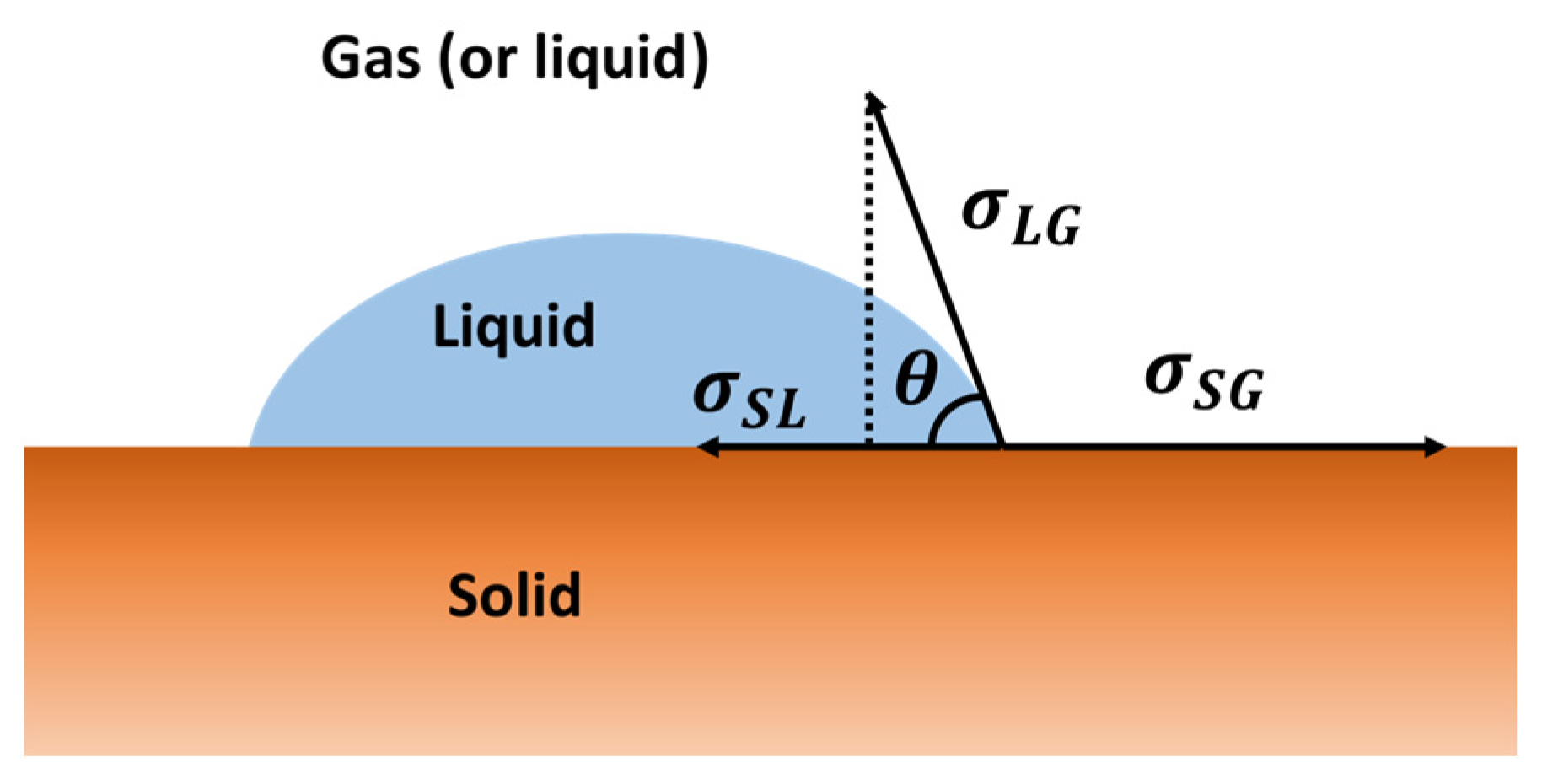
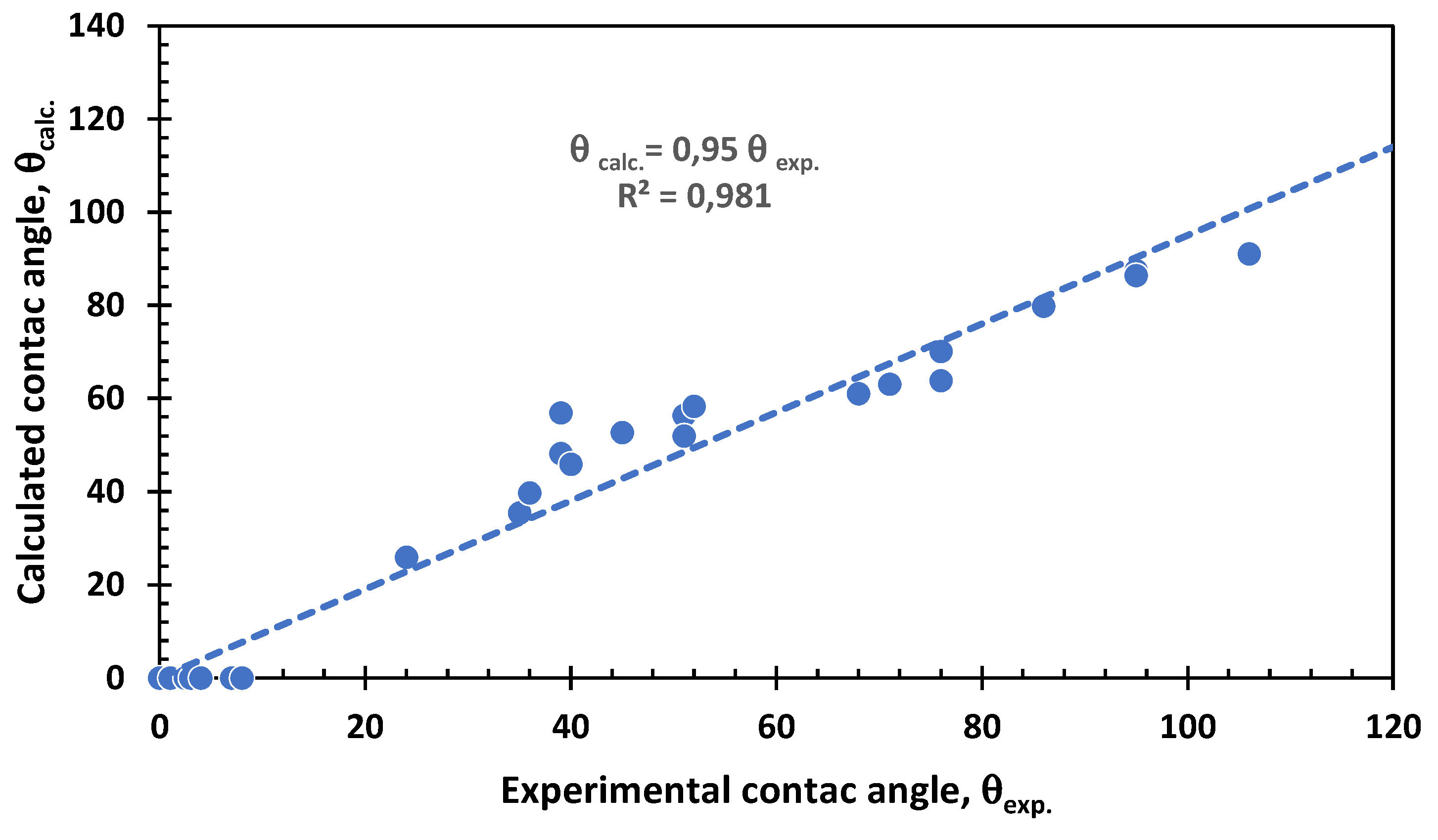
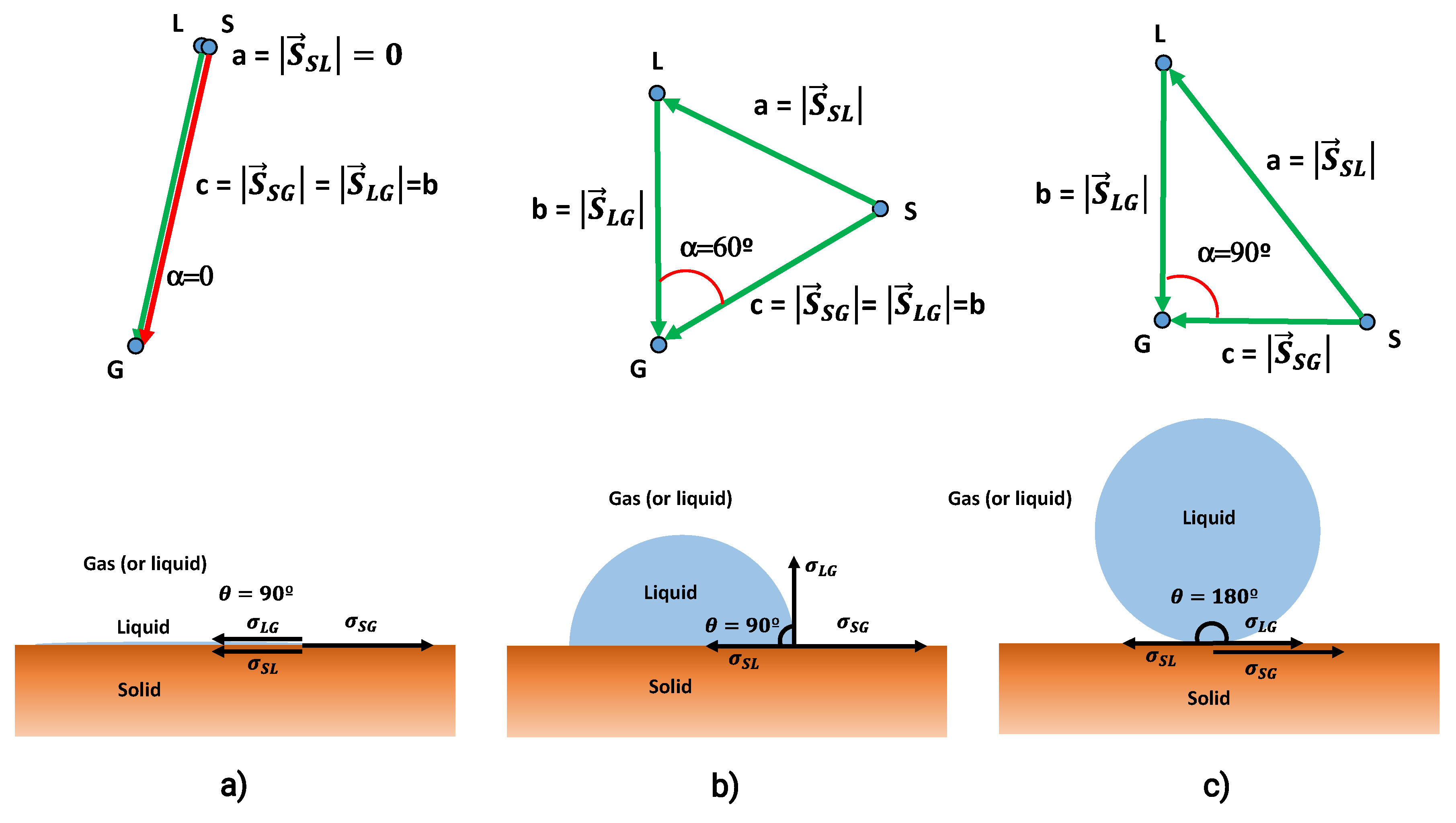
3.6. Contact Angle and the Solubility Parameters Vector Field
3.7. Contact Angle and Interaction Vectors Angle
4. Conclusions
- (a)
- To link, for the first time, the cohesion energy of substances with their free surface energy, as surface or interfacial tension.
- (b)
- To calculate the surface tension and free energy of thousands of substances by knowing their Hansen solubility parameters, which are easily obtained from the molecular structure of substances. Likewise, it is possible to determine the interfacial tension between them with a simple vector calculation.
- (c)
- To theoretically calculate and accurately estimate contact angles that would, in many cases, be hard to experimentally determine. This vector field model saves experimental work and time.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Nº | Substance | Ref. | σLG, mN/m | δD, MPa1/2 | δP, Mpa1/2 | δH, MPa1/2 | 4δD2+δP2, MPa | σLG,cal., mN/m | Error, mN/m |
| 1 | 1,1,2,2-Tetrabromoethane | 2 | 48,9 | 22,6 | 5,1 | 8,2 | 2069,1 | 51,7 | 2,8 |
| 2 | 1,2,3-Tribromopropane | 2 | 44,8 | 19,6 | 6,3 | 6,4 | 1576,3 | 39,4 | -5,4 |
| 3 | 1,3-Diiodopropane | 2 | 46,3 | 19,4 | 5,7 | 4,3 | 1537,9 | 38,4 | -7,9 |
| 4 | 1,4-Dioxane | 2 | 32,3 | 19,0 | 1,8 | 7,4 | 1447,2 | 36,2 | 3,9 |
| 5 | 1-Bromonaphthalene | 2 | 43,9 | 20,6 | 3,1 | 4,1 | 1707,1 | 42,7 | -1,2 |
| 6 | 1-Butanol | 1 | 24,7 | 16,0 | 5,7 | 15,8 | 1056,5 | 26,4 | 1,7 |
| 7 | 1-Chloro-2-Methylpropane | 2 | 21,3 | 15,6 | 5,0 | 2,9 | 998,4 | 25,0 | 3,7 |
| 8 | 1-Chloro-3-Methylbutane | 2 | 23,0 | 15,7 | 4,5 | 2,9 | 1006,2 | 25,2 | 2,2 |
| 9 | 1-Chlorobutane | 2 | 22,5 | 16,2 | 5,5 | 2,0 | 1080,0 | 27,0 | 4,5 |
| 10 | 1-Chloronaphthalene | 2 | 41,3 | 19,9 | 4,9 | 2,5 | 1608,1 | 40,2 | -1,1 |
| 11 | 1-Decanol | 2 | 28,1 | 16,0 | 4,7 | 10,0 | 1046,1 | 26,2 | -1,9 |
| 12 | 1-Methyl Naphthalene | 2 | 38,0 | 20,6 | 0,8 | 4,7 | 1698,1 | 42,5 | 4,5 |
| 13 | 1-Nitropropane | 2 | 28,9 | 16,6 | 12,3 | 5,5 | 1253,5 | 31,3 | 2,4 |
| 14 | 1-Octanol | 2 | 27,2 | 16,0 | 5,0 | 11,9 | 1049,0 | 26,2 | -1,0 |
| 15 | 1-Propanol | 2 | 23,7 | 16,0 | 6,8 | 17,4 | 1070,2 | 26,8 | 3,1 |
| 16 | 1-Propanol | 1 | 27,5 | 16,0 | 6,8 | 17,4 | 1070,2 | 26,8 | -0,7 |
| 17 | 2-Chloro-2-Methyl Propane | 2 | 19,1 | 15,6 | 7,6 | 2,0 | 1031,2 | 25,8 | 6,7 |
| 18 | 2-Phenyl-ethanol | 1 | 41,0 | 19,0 | 5,8 | 7,2 | 1477,6 | 36,9 | -4,1 |
| 19 | 2-Propanol | 2 | 22,6 | 15,8 | 6,1 | 16,4 | 1035,8 | 25,9 | 3,3 |
| 20 | 2-Propanol | 1 | 27,0 | 15,8 | 6,1 | 16,4 | 1035,8 | 25,9 | -1,1 |
| 21 | 3-Methylbutanenitrile | 2 | 25,6 | 15,4 | 9,7 | 4,6 | 1042,7 | 26,1 | 0,5 |
| 22 | Acetic acid | 1 | 23,0 | 14,5 | 8,0 | 13,5 | 905,0 | 22,6 | -0,4 |
| 23 | Acetone | 2 | 24,6 | 15,5 | 10,4 | 7,0 | 1069,2 | 26,7 | 2,1 |
| 24 | Acetone | 1 | 23,3 | 15,5 | 10,4 | 7,0 | 1069,2 | 26,7 | 3,4 |
| 25 | Acetonitrile | 1 | 28,6 | 15,3 | 18,0 | 15,8 | 1260,4 | 31,5 | 2,9 |
| 26 | Aniline | 2 | 43,1 | 19,4 | 5,1 | 10,2 | 1531,5 | 38,3 | -4,8 |
| 27 | Benzene | 2 | 28,2 | 18,4 | 0,0 | 2,0 | 1354,2 | 33,9 | 5,7 |
| 28 | Benzene | 1 | 28,8 | 18,4 | 0,0 | 2,0 | 1354,2 | 33,9 | 5,1 |
| 29 | Benzyl Alcohol | 2 | 38,5 | 18,4 | 6,3 | 13,7 | 1393,9 | 34,8 | -3,7 |
| 30 | Benzyl Benzoate | 2 | 45,4 | 20,0 | 5,1 | 5,2 | 1626,0 | 40,7 | -4,7 |
| 31 | Bromobenzene | 2 | 35,9 | 19,2 | 5,5 | 4,1 | 1504,8 | 37,6 | 1,7 |
| 32 | Bromoform | 2 | 40,8 | 21,4 | 4,1 | 6,1 | 1848,7 | 46,2 | 5,4 |
| 33 | Butanone | 1 | 24,0 | 16,0 | 9,0 | 5,1 | 1105,0 | 27,6 | 3,6 |
| 34 | Butyronitrile | 2 | 27,6 | 15,3 | 12,4 | 5,1 | 1090,1 | 27,3 | -0,3 |
| 35 | Carbon Tetrachloride | 2 | 26,3 | 16,1 | 8,3 | 0,0 | 1105,7 | 27,6 | 1,3 |
| 36 | Chlorobenzene | 2 | 33,0 | 19,0 | 4,3 | 2,0 | 1462,5 | 36,6 | 3,6 |
| 37 | Chloroform | 2 | 26,9 | 17,8 | 3,1 | 5,7 | 1277,0 | 31,9 | 5,0 |
| 38 | Cis-Decahydronaphthalene | 2 | 31,0 | 18,8 | 0,0 | 0,0 | 1413,8 | 35,3 | 4,3 |
| 39 | Cloroform | 1 | 27,2 | 17,8 | 3,1 | 5,7 | 1277,0 | 31,9 | 4,7 |
| 40 | Cyclohexane | 2 | 24,3 | 16,8 | 0,0 | 0,2 | 1129,0 | 28,2 | 3,9 |
| 41 | Cyclohexane | 1 | 28,8 | 16,8 | 0,0 | 0,2 | 1129,0 | 28,2 | -0,6 |
| 42 | Cyclohexanol | 2 | 34,4 | 17,4 | 4,1 | 13,5 | 1227,9 | 30,7 | -3,7 |
| 43 | Cyclohexanone | 1 | 28,2 | 16,8 | 5,7 | 8,0 | 1161,5 | 29,0 | 0,8 |
| 44 | Cyclopentanol | 2 | 32,2 | 17,2 | 5,3 | 12,8 | 1211,5 | 30,3 | -1,9 |
| 45 | Decane | 2 | 23,4 | 15,7 | 0,0 | 0,0 | 986,0 | 24,6 | 1,2 |
| 46 | Dichloromethane | 1 | 27,0 | 17,0 | 7,3 | 7,1 | 1209,3 | 30,2 | 3,2 |
| 47 | Dichloromethane | 2 | 25,9 | 17,0 | 7,3 | 7,1 | 1209,3 | 30,2 | 4,3 |
| 48 | Diethyl ether | 1 | 18,6 | 14,5 | 2,9 | 5,1 | 849,4 | 21,2 | 2,6 |
| 49 | Diethyl Fumarate | 2 | 31,1 | 16,7 | 5,6 | 7,6 | 1146,9 | 28,7 | -2,4 |
| 50 | Diethyl Phthalate | 2 | 36,7 | 17,6 | 9,6 | 4,5 | 1331,2 | 33,3 | -3,4 |
| 51 | Diiodomethane | 2 | 50,1 | 22,0 | 3,9 | 5,5 | 1951,2 | 48,8 | -1,3 |
| 52 | Dimethyl Formamide | 2 | 36,4 | 17,4 | 13,7 | 11,3 | 1398,7 | 35,0 | -1,4 |
| 53 | Dipropylene Glycol Monomethyl Ether | 2 | 27,9 | 15,5 | 5,7 | 11,2 | 993,5 | 24,8 | -3,1 |
| 54 | Dodecane | 2 | 24,9 | 16,0 | 0,0 | 0,0 | 1024,0 | 25,6 | 0,7 |
| 55 | Ethanol | 2 | 21,7 | 15,8 | 8,8 | 19,4 | 1076,0 | 26,9 | 5,2 |
| 56 | Ethanol | 1 | 21,6 | 15,8 | 8,8 | 19,4 | 1076,0 | 26,9 | 5,3 |
| 57 | Ethyl 2-Aminobenzoate | 2 | 39,0 | 18,7 | 8,3 | 7,9 | 1467,7 | 36,7 | -2,3 |
| 58 | Ethyl Acetate | 1 | 24,9 | 15,8 | 5,3 | 7,2 | 1026,7 | 25,7 | 0,8 |
| 59 | Ethyl Benzene | 2 | 28,7 | 17,8 | 0,6 | 1,4 | 1267,7 | 31,7 | 3,0 |
| 60 | Ethyl Bromide | 2 | 23,6 | 16,5 | 8,4 | 2,3 | 1159,6 | 29,0 | 5,4 |
| 61 | Ethylene Dichloride | 2 | 32,6 | 19,0 | 7,4 | 4,1 | 1498,8 | 37,5 | 4,9 |
| 62 | Ethylene Glycol Monoethyl Ether | 2 | 28,1 | 16,2 | 9,2 | 14,3 | 1134,4 | 28,4 | 0,3 |
| 63 | Furfural | 2 | 41,3 | 18,6 | 14,9 | 5,1 | 1605,9 | 40,1 | -1,2 |
| 64 | Heptane | 2 | 19,7 | 15,3 | 0,0 | 0,0 | 936,4 | 23,4 | 3,7 |
| 65 | Hexachloro-1,3-Butadiene | 2 | 35,5 | 19,1 | 5,3 | 0,6 | 1487,3 | 37,2 | 1,7 |
| 66 | Hexadecane | 2 | 27,0 | 16,3 | 0,0 | 0,0 | 1062,8 | 26,6 | -0,4 |
| 67 | Hexane | 2 | 17,9 | 14,9 | 0,0 | 0,0 | 888,0 | 22,2 | 4,3 |
| 68 | Iodobenzene | 2 | 39,1 | 19,5 | 6,0 | 6,1 | 1557,0 | 38,9 | -0,2 |
| 69 | Isopropyl Benzene (Cumene) | 2 | 27,7 | 18,1 | 1,2 | 1,2 | 1311,9 | 32,8 | 5,1 |
| 70 | Limonene | 1 | 26,4 | 17,2 | 1,8 | 4,3 | 1186,6 | 29,7 | 3,3 |
| 71 | Mesitylene | 2 | 28,4 | 18,0 | 0,0 | 0,6 | 1296,0 | 32,4 | 4,0 |
| 72 | Methanol | 2 | 22,3 | 15,1 | 12,3 | 22,3 | 1063,3 | 26,6 | 4,3 |
| 73 | Methyl Anthranilate | 2 | 43,7 | 19,1 | 8,9 | 8,7 | 1538,5 | 38,5 | -5,2 |
| 74 | Methyl Ethyl Ketone | 2 | 24,0 | 16,0 | 9,0 | 5,1 | 1105,0 | 27,6 | 3,6 |
| 75 | m-Nitrotoluene | 2 | 40,8 | 18,9 | 7,3 | 4,0 | 1482,1 | 37,1 | -3,7 |
| 76 | m-Xylene | 2 | 28,3 | 17,6 | 1,0 | 3,1 | 1240,0 | 31,0 | 2,7 |
| 77 | N,N-Dimethyl Acetamide | 2 | 36,0 | 16,8 | 11,5 | 9,4 | 1261,2 | 31,5 | -4,5 |
| 78 | n-Butyl acetate | 1 | 27,8 | 15,8 | 3,7 | 6,3 | 1012,3 | 25,3 | -2,5 |
| 79 | n-Butylbenzene | 2 | 28,7 | 17,4 | 0,1 | 1,1 | 1211,1 | 30,3 | 1,6 |
| 80 | n-Heptane | 1 | 19,7 | 15,3 | 0,0 | 0,0 | 936,4 | 23,4 | 3,7 |
| 81 | Nitrobenzene | 2 | 43,3 | 20,0 | 8,6 | 4,1 | 1674,0 | 41,8 | -1,5 |
| 82 | Nitroethane | 2 | 31,3 | 16,0 | 15,5 | 4,5 | 1264,3 | 31,6 | 0,3 |
| 83 | Nitromethane | 2 | 36,0 | 15,8 | 18,8 | 6,1 | 1352,0 | 33,8 | -2,2 |
| 84 | N-Methyl-2-Pyrrolidone | 2 | 40,2 | 18,0 | 12,3 | 7,2 | 1447,3 | 36,2 | -4,0 |
| 85 | n-Tetradecane | 2 | 26,1 | 16,2 | 0,0 | 0,0 | 1049,8 | 26,2 | 0,1 |
| 86 | Octane | 2 | 21,1 | 15,5 | 0,0 | 0,0 | 961,0 | 24,0 | 2,9 |
| 87 | o-Nitrotoluene | 2 | 40,9 | 19,0 | 7,5 | 4,3 | 1500,3 | 37,5 | -3,4 |
| 88 | o-Xylene | 2 | 29,5 | 17,8 | 1,0 | 3,1 | 1268,4 | 31,7 | 2,2 |
| 89 | p-Cymene | 2 | 27,6 | 17,3 | 2,4 | 2,4 | 1202,9 | 30,1 | 2,5 |
| 90 | Perfluoroheptane | 2 | 12,4 | 12,0 | 0,0 | 0,0 | 576,0 | 14,4 | 2,0 |
| 91 | Perfluorohexane (PFC 5060) | 2 | 11,4 | 12,1 | 0,0 | 0,0 | 585,6 | 14,6 | 3,2 |
| 92 | Perfluorooctane | 2 | 13,5 | 12,1 | 0,8 | 0,3 | 586,3 | 14,7 | 1,2 |
| 93 | Phenyl Isothiocyanate | 2 | 40,9 | 19,4 | 13,9 | 8,5 | 1698,7 | 42,5 | 1,6 |
| 94 | Propylbenzene | 2 | 28,5 | 17,3 | 2,2 | 2,9 | 1202,0 | 30,1 | 1,6 |
| 95 | Pyridine | 2 | 37,3 | 19,0 | 8,8 | 5,9 | 1521,4 | 38,0 | 0,7 |
| 96 | Pyrrole | 2 | 36,1 | 19,2 | 7,4 | 6,7 | 1529,3 | 38,2 | 2,1 |
| 97 | Quinoline | 2 | 42,6 | 19,8 | 5,6 | 5,7 | 1599,5 | 40,0 | -2,6 |
| 98 | Tetrachloroethylene | 1 | 28,4 | 18,3 | 5,7 | 0,0 | 1372,1 | 34,3 | 5,9 |
| 99 | Tetrahydrofuran | 1 | 27,2 | 16,8 | 5,7 | 8,0 | 1161,5 | 29,0 | 1,8 |
| 100 | Tetrahydrofuran | 2 | 25,8 | 16,8 | 5,7 | 8,0 | 1161,5 | 29,0 | 3,2 |
| 101 | Toluene | 2 | 27,8 | 18,0 | 1,4 | 2,0 | 1298,0 | 32,4 | 4,6 |
| 102 | Toluene | 1 | 27,5 | 18,0 | 1,4 | 2,0 | 1298,0 | 32,4 | 4,9 |
| 103 | Tricresyl Phosphate | 2 | 40,5 | 19,0 | 12,3 | 4,5 | 1595,3 | 39,9 | -0,6 |
| 104 | Triethanolamine | 5 | 45,9 | 17,3 | 22,4 | 23,3 | 1698,9 | 42,5 | -3,4 |
| 105 | Undecane | 2 | 24,2 | 16,0 | 0,0 | 0,0 | 1024,0 | 25,6 | 1,4 |
| 106 | Xylene (isomers) | 1 | 27,8 | 17,7 | 1,0 | 3,1 | 1254,2 | 31,4 | 3,6 |
| Nº | Substance | Ref. | σLG, mN/m | δD, MPa1/2 | δP, Mpa1/2 | δH, MPa1/2 | 4δD2+δP2+δH2, MPa | σLG,cal., mN/m | Error, mN/m |
| 107 | 1,5-Pentanediol | 2 | 42,7 | 17,0 | 8,9 | 19,8 | 1627,3 | 40,7 | -2,0 |
| 108 | Diethylene Glycol | 2 | 44,4 | 16,6 | 12,0 | 20,7 | 1674,7 | 41,9 | -2,5 |
| 109 | Diethylene Glycol | 4 | 44,4 | 16,6 | 12,0 | 20,7 | 1674,7 | 41,9 | -2,5 |
| 110 | Dipropylene Glycol | 2 | 33,4 | 16,5 | 10,6 | 17,7 | 1514,7 | 37,9 | 4,5 |
| 111 | Dipropylene Glycol | 1 | 34,8 | 16,5 | 10,6 | 17,7 | 1514,7 | 37,9 | 3,1 |
| 112 | Dimethyl sulfoxide | 1 | 42,2 | 18,4 | 16,4 | 10,2 | 1727,2 | 43,2 | 0,9 |
| 113 | Dimethyl sulfoxide | 3 | 41,8 | 18,4 | 16,4 | 10,2 | 1727,2 | 43,2 | 1,4 |
| 114 | Dimethylformamide | 3 | 39,1 | 17,4 | 13,7 | 11,3 | 1526,4 | 38,2 | -0,9 |
| 115 | Ethanolamine | 2 | 48,3 | 17,0 | 15,5 | 21,2 | 1845,7 | 46,1 | -2,2 |
| 116 | Ethylene Glycol | 2 | 47,3 | 17,0 | 11,0 | 26,0 | 1953,0 | 48,8 | 1,5 |
| 117 | Formamide | 2 | 57,8 | 17,2 | 26,2 | 19,0 | 2230,8 | 55,8 | -2,0 |
| 118 | Formic acid | 3 | 31,4 | 14,3 | 11,9 | 16,6 | 1235,1 | 30,9 | -0,5 |
| 119 | Glycerol | 2 | 64,3 | 17,4 | 12,1 | 29,3 | 2215,9 | 55,4 | -8,9 |
| 120 | Glycerol | 3 | 63,7 | 17,4 | 12,1 | 29,3 | 2215,9 | 55,4 | -8,3 |
| 121 | 3-Hydroxymethylpyridine | 2 | 47,4 | 19,2 | 9,6 | 14,5 | 1777,0 | 44,4 | -3,0 |
| 122 | Water (molecule) | 2 | 72,0 | 15,5 | 16,0 | 42,3 | 3006,3 | 75,2 | 3,2 |
| Nº | Substance | σSG, mN/m | δD, MPa1/2 | δP, Mpa1/2 | δH, MPa1/2 | 4δD2+δP2+δH2, MPa | σSG,cal., mN/m | Error, mN/m |
| 1 | Polyethylene | 33,0 | 17,3 | 1,7 | 2,1 | 1204,5 | 31,3 | -1,7 |
| 2 | Poly(vinyl chloride) | 40,8 | 18,6 | 5,8 | 9,0 | 1498,5 | 39,0 | -1,8 |
| 3 | Poly(vinylidene chloride) | 41,6 | 17,6 | 9,1 | 7,8 | 1382,7 | 35,9 | -5,7 |
| 4 | Poly(vinyl fluoride) | 36,7 | 17,4 | 13,7 | 11,3 | 1526,4 | 39,7 | 3,0 |
| 5 | Poly(vinylidene fluoride) | 31,4 | 17,0 | 12,1 | 10,2 | 1406,5 | 36,6 | 5,2 |
| 6 | Poly(tetrafluoroethylene) | 15,0 | 15,1 | 0,9 | 1,7 | 915,7 | 23,8 | 8,8 |
| 7 | Poly(ethylene terephthalate) | 41,9 | 18,2 | 6,4 | 3,7 | 1379,6 | 35,9 | -6,0 |
| 8 | Poly(methyl methacrylate) | 40,7 | 17,0 | 4,8 | 5,7 | 1211,5 | 31,5 | -9,2 |
| 9 | PA66 | 43,5 | 18,7 | 5,2 | 8,4 | 1496,4 | 38,9 | -4,6 |
| 10 | Polystyrene | 41,3 | 20,5 | 3,1 | 2,6 | 1697,4 | 44,1 | 2,8 |
| 11 | Polychlorotrifluoroethylene | 26,9 | 14,1 | 2,7 | 5,5 | 832,8 | 21,7 | -5,2 |
| 12 | Polypropylene | 30,1 | 18,1 | 1,0 | 0,0 | 1311,4 | 34,1 | 4,0 |
| 13 | Polyisobutylene | 33,6 | 16,9 | 2,5 | 4,0 | 1164,7 | 30,3 | -3,3 |
| 14 | Poly-α-methyl styrene | 39,0 | 18,5 | 2,4 | 2,4 | 1380,5 | 35,9 | -3,1 |
| 15 | Poly-n-butyl methacrylate | 31,2 | 15,9 | 5,5 | 5,9 | 1076,3 | 28,0 | -3,2 |
| 16 | Polycarbonate | 34,2 | 18,4 | 5,9 | 6,9 | 1436,7 | 37,4 | 3,2 |
| 17 | Polyethylmethacrylate | 35,9 | 17,6 | 9,7 | 4,0 | 1349,1 | 35,1 | -0,8 |
| 18 | Graphite | 35,0 | 18,0 | 9,3 | 7,7 | 1441,8 | 37,5 | 2,5 |
| 19 | MoS2 | 34,4 | 18,0 | 9,0 | 6,2 | 1415,4 | 36,8 | 2,4 |
| 20 | WS2 | 33,1 | 17,0 | 9,5 | 13,2 | 1420,5 | 36,9 | 3,8 |
| 21 | BN | 30,1 | 18,0 | 7,0 | 7,0 | 1394,0 | 36,2 | 6,1 |
| HSPS | HSPL | ||||||||||
| Solid | Liquid | σSL, mN/m | δDS, MPa1/2 | δPS, Mpa1/2 | δHS, MPa1/2 | δDL, MPa1/2 | δPL, Mpa1/2 | δHL, MPa1/2 | , MPa | σSL, calc., mN/m | Error, mN/m |
| PS | Water | 49,4 | 20,6 | 2,5 | 1,5 | 15,5 | 16,0 | 42,3 | 1953,8 | 52,8 | 3,4 |
| Formamide | 26,6 | 20,6 | 2,5 | 1,5 | 17,2 | 26,2 | 19,0 | 914,2 | 24,7 | -1,9 | |
| Ethylene Glycol | 21,9 | 20,6 | 2,5 | 1,5 | 17,0 | 11,0 | 26,0 | 726,0 | 19,6 | -2,3 | |
| Benzyl Alcohol | 0,4 | 20,6 | 2,5 | 1,5 | 18,4 | 6,3 | 13,7 | 183,5 | 5,0 | 4,6 | |
| Nitromethane | 8,1 | 20,6 | 2,5 | 1,5 | 15,8 | 18,8 | 5,1 | 369,8 | 10,0 | 1,9 | |
| PMMA | Water | 27,0 | 18,5 | 9,0 | 8,4 | 15,5 | 16,0 | 42,3 | 1235,6 | 33,4 | 6,4 |
| Formamide | 17,2 | 18,5 | 9,0 | 8,4 | 17,2 | 26,2 | 19,0 | 414,1 | 11,2 | -6,0 | |
| Ethylene Glycol | 17,9 | 18,5 | 9,0 | 8,4 | 17,0 | 11,0 | 26,0 | 323,7 | 8,7 | -9,2 | |
| Benzyl Alcohol | 5,3 | 18,5 | 9,0 | 8,4 | 18,4 | 6,3 | 13,7 | 36,0 | 1,0 | -4,3 | |
| 1-Bromonaphtalene | 3,9 | 18,5 | 9,0 | 8,4 | 20,6 | 3,1 | 4,1 | 71,6 | 1,9 | -2,0 | |
| Nitromethane | 8,7 | 18,5 | 9,0 | 8,4 | 15,8 | 18,8 | 5,1 | 134,4 | 3,6 | -5,1 | |
| PA66 | Water | 15,6 | 19,5 | 6,2 | 14,7 | 15,5 | 16,0 | 42,3 | 921,3 | 24,9 | 9,3 |
| Formamide | 9,6 | 19,5 | 6,2 | 14,7 | 17,2 | 26,2 | 19,0 | 438,6 | 11,8 | 2,2 | |
| Ethylene Glycol | 10,4 | 19,5 | 6,2 | 14,7 | 17,0 | 11,0 | 26,0 | 175,4 | 4,7 | -5,7 | |
| Benzyl Alcohol | 6,0 | 19,5 | 6,2 | 14,7 | 18,4 | 6,3 | 13,7 | 5,7 | 0,2 | -5,8 | |
| 1-Bromonaphtalene | 1,7 | 19,5 | 6,2 | 14,7 | 20,6 | 3,1 | 4,1 | 126,9 | 3,4 | 1,7 | |
| Nitromethane | 9,9 | 19,5 | 6,2 | 14,7 | 15,8 | 18,8 | 5,1 | 304,4 | 8,2 | -1,7 | |
| PTFE | Water | 55,8 | 13,6 | 1,7 | 0,0 | 15,5 | 16,0 | 42,3 | 2008,4 | 54,2 | -1,6 |
| Formamide | 31,1 | 13,6 | 1,7 | 0,0 | 17,2 | 26,2 | 19,0 | 1013,6 | 27,4 | -3,7 | |
| Ethylene Glycol | 27,2 | 13,6 | 1,7 | 0,0 | 17,0 | 11,0 | 26,0 | 809,7 | 21,9 | -5,3 | |
| Benzyl Alcohol | 13,6 | 13,6 | 1,7 | 0,0 | 18,4 | 6,3 | 13,7 | 302,8 | 8,2 | -5,4 | |
| 1-Bromonaphtalene | 13,8 | 13,6 | 1,7 | 0,0 | 20,6 | 3,1 | 4,1 | 217,5 | 5,9 | -7,9 | |
| Nitromethane | 8,1 | 13,6 | 1,7 | 0,0 | 15,8 | 18,8 | 5,1 | 338,0 | 9,1 | 1,0 | |
| n-Decane | 2,9 | 13,6 | 1,7 | 0,0 | 15,7 | 0,0 | 0,0 | 21,4 | 0,6 | -2,3 | |
| n-Hexane | 2,7 | 13,6 | 1,7 | 0,0 | 14,9 | 0,0 | 0,0 | 10,2 | 0,3 | -2,4 | |
| Dimethyl sulfoxide | 15,0 | 13,6 | 1,7 | 0,0 | 18,4 | 16,4 | 10,2 | 413,6 | 11,2 | -3,8 | |
| PVB | Water | 33,9 | 17,9 | 8,3 | 4,2 | 15,5 | 16,0 | 42,3 | 1534,2 | 41,4 | 7,5 |
| Formamide | 18,3 | 17,9 | 8,3 | 4,2 | 17,2 | 26,2 | 19,0 | 540,9 | 14,6 | -3,7 | |
| Ethylene Glycol | 20,0 | 17,9 | 8,3 | 4,2 | 17,0 | 11,0 | 26,0 | 486,0 | 13,1 | -6,9 | |
| Benzyl Alcohol | 8,4 | 17,9 | 8,3 | 4,2 | 18,4 | 6,3 | 13,7 | 95,6 | 2,6 | -5,8 | |
| 1-Bromonaphtalene | 3,0 | 17,9 | 8,3 | 4,2 | 20,6 | 3,1 | 4,1 | 56,6 | 1,5 | -1,5 | |
| Nitromethane | 9,1 | 17,9 | 8,3 | 4,2 | 15,8 | 18,8 | 5,1 | 128,1 | 3,5 | -5,6 | |
| Liquid | Solid | θexp. | δD, MPa1/2 | δP, Mpa1/2 | δH, MPa1/2 | θcalc. | |||
| Octane | n-Octacosane | 0 | 15,5 | 0,0 | 0,0 | 961,0 | 1143,8 | 8,0 | 0 |
| 2-Propanol | PMMA | 0 | 15,8 | 6,1 | 16,4 | 1035,8 | 1556,9 | 130,8 | 0 |
| Acetone | PMMA | 0 | 15,5 | 10,4 | 7,0 | 1069,2 | 1556,9 | 39,7 | 0 |
| Benzyl alcohol | PMMA | 0 | 18,4 | 6,3 | 13,7 | 1393,9 | 1556,9 | 56,4 | 0 |
| Bromobenzene | PMMA | 0 | 19,2 | 5,5 | 4,1 | 1504,8 | 1556,9 | 38,1 | 0 |
| Ethyl acetate | PMMA | 0 | 15,8 | 5,3 | 7,2 | 1026,7 | 1556,9 | 59,6 | 0 |
| Octane | PMMA | 0 | 15,5 | 0,0 | 0,0 | 961,0 | 1556,9 | 206,5 | 0 |
| Tetrahydrofuran | PMMA | 0 | 16,8 | 5,7 | 8,0 | 1161,5 | 1556,9 | 37,0 | 0 |
| Chlorobenzene | PMMA | 1 | 19,0 | 4,3 | 2,0 | 1462,5 | 1556,9 | 69,6 | 0 |
| Dodecane | PMMA | 1 | 16,0 | 0,0 | 0,0 | 1024,0 | 1556,9 | 194,9 | 0 |
| Pyridine | PMMA | 1 | 19,0 | 8,8 | 5,9 | 1521,4 | 1556,9 | 6,1 | 0 |
| Ethanol | PMMA | 2,5 | 15,8 | 8,8 | 19,4 | 1076,0 | 1556,9 | 176,6 | 0 |
| Methanol | PMMA | 3 | 15,1 | 12,3 | 22,3 | 1063,3 | 1556,9 | 272,0 | 0 |
| o-xylene | PMMA | 3 | 17,8 | 1,0 | 3,1 | 1268,4 | 1556,9 | 112,9 | 0 |
| Hexadecane | PMMA | 4 | 16,3 | 0,0 | 0,0 | 1062,8 | 1556,9 | 189,0 | 0 |
| Nitroethane | PMMA | 7 | 16,0 | 15,5 | 4,5 | 1264,3 | 1556,9 | 61,7 | 0 |
| Carbon Tetrachloride | PMMA | 8 | 16,1 | 8,3 | 0,0 | 1105,7 | 1556,9 | 87,1 | 0 |
| Cyclohexanol | PMMA | 8 | 17,4 | 4,1 | 13,5 | 1227,9 | 1556,9 | 83,2 | 0 |
| Carbon Tetrachloride | n-Octacosane | 24 | 17,8 | 0,0 | 0,6 | 1267,4 | 1143,8 | 3,5 | 26 |
| Diethylene glycol | PMMA | 35 | 16,6 | 12,0 | 20,7 | 1674,7 | 1556,9 | 192,8 | 35 |
| Cyclohexanol | n-Octacosane | 36 | 17,4 | 4,1 | 13,5 | 1227,9 | 1143,8 | 200,0 | 40 |
| Bromoform | n-Octacosane | 39 | 21,4 | 4,1 | 6,1 | 1848,7 | 1143,8 | 134,7 | 57 |
| Pyridine | n-Octacosane | 39 | 19,0 | 8,8 | 5,9 | 1521,4 | 1143,8 | 129,7 | 48 |
| Nitroethane | n-Octacosane | 40 | 16,0 | 15,5 | 4,5 | 1264,3 | 1143,8 | 263,8 | 46 |
| Nitrobenzene | n-Octacosane | 45 | 20,0 | 8,6 | 4,1 | 1674,0 | 1143,8 | 129,0 | 53 |
| Nitromethane | n-Octacosane | 51 | 15,8 | 18,8 | 6,1 | 1352,0 | 1143,8 | 395,6 | 56 |
| Ethylene Glycol | PMMA | 51 | 17,0 | 11,0 | 26,0 | 1953,0 | 1556,9 | 352,9 | 52 |
| Formamide | PMMA | 52 | 17,2 | 26,2 | 19,0 | 2230,8 | 1556,9 | 386,2 | 58 |
| Glycerol | PMMA | 68 | 17,4 | 12,1 | 29,3 | 2215,9 | 1556,9 | 483,5 | 61 |
| Water | PMMA | 71 | 15,5 | 16,0 | 30,3 | 2135,1 | 1556,9 | 588,9 | 63 |
| Diethylene glycol | n-Octacosane | 76 | 16,6 | 12,0 | 20,7 | 1674,7 | 1143,8 | 572,9 | 70 |
| Dimethyl sulfoxide | n-Octacosane | 76 | 18,4 | 16,4 | 10,2 | 1727,2 | 1143,8 | 381,9 | 64 |
| Ethylene Glycol | n-Octacosane | 86 | 17,0 | 11,0 | 26,0 | 1953,0 | 1143,8 | 797,0 | 80 |
| Formamide | n-Octacosane | 95 | 17,2 | 26,2 | 19,0 | 2230,8 | 1143,8 | 1047,8 | 88 |
| Glycerol | n-Octacosane | 95 | 17,4 | 12,1 | 29,3 | 2215,9 | 1143,8 | 1005,9 | 86 |
| Water | n-Octacosane | 106 | 15,5 | 16,0 | 30,3 | 2135,1 | 1143,8 | 1182,0 | 91 |
References
- Hildebrand, J. H.; Scott, R. L. The solubility of Nonelectrolytes; Reinhold: New York, 1950. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters. A User's Handbook; CRC Press. Taylor & Francis Group: Boca Ratón, 2007. [Google Scholar]
- Beerbower, A. Surface Free Energy: A New Relationship to Bulk Energies. Journal of Colloid and Interface Science 1971, 35, 126–131. [Google Scholar] [CrossRef]
- Koenhen, D. M.; Smolders, C. A. The determination of Solubility Parameters of Solvents and Polymers by Means of Correlations with other Physical Quantities. Journal of Applied Polymer Science 1975, 19, 1163–1179. [Google Scholar] [CrossRef]
- Large, M. J.; Ogilvie, S. P.; King, A. A.; Dalton, A. B. Understanding Solvent Spreading for Langmuir Deposition of Nanomaterial Films: A Hansen Solubility Parameter Approach. Langmuir 2017, 33, 14766–14771. [Google Scholar] [CrossRef] [PubMed]
- AlQasas, N.; Eskhan, A.; Johnson, D. Hansen Solubility Parameters from Surface Measurements: A Comparison of Different Methods. Surfaces and Interfaces 2023, 36, 102594. [Google Scholar] [CrossRef]
- Murase, M.; Nakamura, D. Hansen Solubility Parameters for Directly Dealing with Surface and Interfacial Phenomena. Langmuir 2023, 39, 10475–10484. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.; Hansen, C.M.; Yamamoto, H.; Valpey, R. S. Hansen Solubility Parameters in Practice, Complete with eBook, Software and Data. 2013. [Google Scholar]
- Harkins, W. D.; Brown, F. E. The Determination of Surface Tension (Free Surface Energy) and the Weight of Falling Drops: The Surface Tension of Water and Benzene by the Capillary Method. Journal of the American Chemical Society 1919, 41, 499–525. [Google Scholar] [CrossRef]
- Yu, W.; Hou, W. Correlations of Surface Free Energy and Solubility Parameters for Solid Substances. Journal of Colloid and Interface Science 2019, 544, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Panzer, J. Components of Solid Surface Free Energy from Wetting Measurements. Journal of Colloid and Interface Science 1973, 44, 142–161. [Google Scholar] [CrossRef]
- Gibbs, J. W. On the equilibrium of heterogeneus substances. American Journal of Science 1878, s3-16, 441–458. [Google Scholar] [CrossRef]
- Guggenheim, E. A. The Thermodynamics of Interfaces in Systems of Several Componets. Transactions of the Farady Society 1940, 35, 397–411. [Google Scholar] [CrossRef]
- Dadashev, R. K.; Elimkhanov, D. Z.; Kutuev, R. A.; Umarkhadzhiev, K. S. Concentration Dependence of the Distance Between Different Positions of the Gibbs Dividing Surface in Two-Component Solutions. Russian Journal of Physical Chemistry A 2021, 95, 2290–2294. [Google Scholar] [CrossRef]
- Cho, J. Pressure Coefficient of Interfacial Tension for Polymer Blends. Macromolecular Research 2013, 21, 1360–1365. [Google Scholar] [CrossRef]
- Cho, J. Superposition in Flory-Huggins X and Interfacial Tension for Compresible Polymer Blends. Macro Letters 2013, 2, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.; Schmid, F.; Müller, M.; Binder, K. "Intrinsic" Profiles and Capillary Waves at Homopolymer Interfaces: A Monte Carlo Study. Physical Review E 1999, 59, 728–738. [Google Scholar] [CrossRef]
- Singh, M. Survismeter, 2-in-1 For Viscosity and Surface Tension Measurement, an Excellent Invention for Industrial Proliferation of Surface Forces in Liquids. Surface review and Letters 2007, 14, 973–983. [Google Scholar] [CrossRef]
- Gaur, A. P.; Sahoo, S.; Ahmadi, M.; Dash, S. P.; Guinel, M. J. F.; Katiyar, R. S. Surface Energy Engineering for Tunable Wettability through Controlled Synthesis of MoS2. Nano Letters 2014, 14, 4314–4321. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





