1. Introduction
Stress is an inherent facet of life, and the responses it elicits can have both beneficial and detrimental effects on an organism’s survival. To mitigate the detrimental effects of stress, it is essential to understand the stress response at both the behavioral and biological levels, especially its regulatory processes. Unlike in humans and various vertebrates, stress in crocodilians has not been thoroughly investigated from a neurological, behavioral, and psychological perspective [
1].
Crocodilians are the world’s largest extant reptiles with the Nile crocodile,
Crocodylus niloticus, being the second largest member of the Crocodylidae family [
2]. Nile crocodiles in Africa faced near extinction by the mid-1970s due to their highly valued hides [
3]. Their populations have however made a strong recovery, in part due to trade restrictions legislation [
4], and the significant contribution of commercial crocodilian farms (CCFs; [
3,
5,
6]). CCFs have reduced the need for wild harvesting of crocodilia by supplying the textile industry and traditional markets with hides and other crocodilian products in a sustainable manner [
4,
6].
Crocodilians, especially farmed crocodiles, are highly stress-sensitive which exerts detrimental effects on their welfare and the commercial value of their hides [
7,
8]. It is therefore crucial for CCFs to remain free of diseases, promote optimal husbandry conditions, and reduce environmental stress [
2]. In fact, unexpected deaths on a CCF are often directly related to sub-optimal husbandry conditions [
9]. Therefore, for CCFs to remain economically viable and to sustain their positive impact on the conservation of these animals, sound husbandry techniques and management practices are required [
2]. Unfortunately, the development of appropriate and modernized husbandry guidelines, particularly those focused on stress mitigation, is impeded by the limited understanding of the biological and behavioral stress axis of crocodilians.
As in mammals that present with highly complex stress response mechanisms, reptiles such as crocodilians attempt to mitigate a stressor through inherent allostatic responses. Allostasis is a dynamic homeostatic process that draws on physiological and behavioral changes aimed at learned adaptation and survival [
10,
11]. An allostatic response differs between acute and chronic stressors. The stress response in crocodiles (see
Figure 1) is essentially regulated via the sympathetic adrenomedullary (SAM) axis and the hypothalamic-pituitary-adrenal (HPA) axis [
10,
12], or more specifically in crocodiles, fish, and amphibians, the hypothalamic-pituitary-interrenal (HPI) axis. The acute response is mediated by the SAM axis and is dedicated to the animal’s immediate survival requirements while neglecting certain needs, such as energy storage or reproduction [
10,
13]. On the other hand, extended responses involve the HPA/HPI axis and elevation of glucocorticoids. The latter results in prolonged reproductive and immune suppression, as well as muscle atrophy and maladaptive behaviors when chronically elevated or when released in an unregulated fashion. If these effects persist without intervention, they collectively contribute to allostatic load [
11,
14], which is counterproductive to survival. Importantly, corticosterone feeds back negatively on the HPI-axis at the level of the hypothalamus and pituitary to prevent excessive release of corticosterone [
15,
16].
In crocodilians, the SAM axis involves the release of adrenaline and noradrenaline from the steroidogenic tissues in the adrenal glands [
10,
16], which induce hyperglycemia, increase blood flow to essential limbs and organs, and increase heart rate and respiration [
17,
18]. The HPI axis is activated by physiological, environmental, or physical stressors [
13,
19] that trigger the sequential release of corticotropin-releasing hormone (CRH) from the hypothalamus, adrenocorticotropic hormone (ACTH) from the anterior pituitary [
4,
19], and corticosterone from the adrenal glands (see
Figure 1). Corticosterone is the principal stress hormone in crocodilians [18-20]. It induces glycolysis, gluconeogenesis, glycogenolysis, and proteolysis, to mobilize energy substrates [
21,
22], leading to increased blood glucose and lactate, its metabolic by-product. Lactate, and lactic acidosis, is produced during strenuous exercise and is potentially harmful [
23]. Dehydroepiandrosterone (DHEA) is a metabolic intermediate in gonadal steroidogenesis [
19,
20,
24] that exerts anti-stress effects such as improved immunological functioning, reduced anxiety, and improved mood [
20,
24]. Corticosterone decreases inflammatory mediators, decreases specific immune cells [
25], e.g., lymphocytes, basophils, eosinophils, monocytes, macrophages, while increasing heterophils (analogous to mammalian neutrophils) [
26]. The heterophil-to-lymphocyte ratio (HLR) highlights the effects of stress on the immune system, specifically the time-dependent downstream effects of corticosterone [
10,
27].
Allostatic loads in stressed crocodiles presents with failure to reproduce and to thrive, anxiety or agitation, and an increased susceptibility to infection [
28]. While the immediate effects of SAM axis activation are evident, the enduring effects of HPI axis activation ensure long-term homeostasis and recovery, but at the same time can be severely disabling to the animal if left unchecked. Lance and Elsey [
7] first studied the crocodilian stress response using multiple biomarkers at multiple time points. They reported the acute reaction of catecholamines in American alligators (
Alligator mississippiensis) and their association with corticosterone, glucose, and white blood cells. Similarly, Franklin
, et al. [
29] and Pfitzer [
18] studied the effects of handling techniques, i.e., physical restraint versus electrical immobilization, on corticosterone, glucose, lactate, and various hematological markers in estuarine crocodiles (
Crocodylus porosus) and Nile crocodiles, respectively, with Pfitzer [
18] also studying alanine aminotransferase, alkaline phosphatase, and creatinine kinase. More recently, Duncan
, et al. [
30] compared corticosterone, glucose, lactate, and calcium levels in
Melanosuchus niger and
Caiman crocodilus, identifying differences in lactate clearance rates as an important aspect of crocodilian post-capture recovery [
23]. Finger [
31], in turn, studied the immune aspects of the stress response in estuarine crocodiles, while Lance and Elsey [
32] studied the interactions between the reproductive system and stress in American alligators.
Given the above-described biological changes that happen during acute and chronic stress, these biological agent provocateurs are linked to an array of behavioral manifestations that at first are adaptive and gradually become maladaptive [
8,
33]. In mammals and humans, the interaction between glucocorticoids, monoamines, and other stress-related neuropeptides is linked to various neuropsychiatric and anxiety manifestations [
33,
34]. Crocodilians housed in captivity encounter numerous environmental, anthropogenic, and biological stressors that can result in diminished welfare and substantial economic losses to farmers [
35,
36]. This phenomenon has not been investigated in crocodilians, although are most often seen by farmers and animal caretakers following exposure to both acute and chronic stressors, presenting as excessive lithophagy (ingestion of stones), anorexia, hydrophobia, runting, and piling [
8,
37].
Considering the limited and outdated literature and the intricacies of the crocodilian stress response, further understanding is necessary by applying an array of stress biomarkers and assessing how their response changes over time post-stress. By examining standard acute and chronic stress biomarkers, such as monoamines, metabolic and immunological markers, and glucocorticoids in conjunction with a novel biomarker, i.e., DHEA, we will attempt to elucidate new connections within the stress response and thus provide a deeper understanding of this physiological process and how it informs on the presence of adaptive versus maladaptive behavior. This will enable future researchers, conservationists, farmers, and animal welfare specialists to identify novel and effective ways to mitigate the damaging effects of uncontrolled stress in this species.
3. Results
3.1. Noradrenaline and Adrenaline
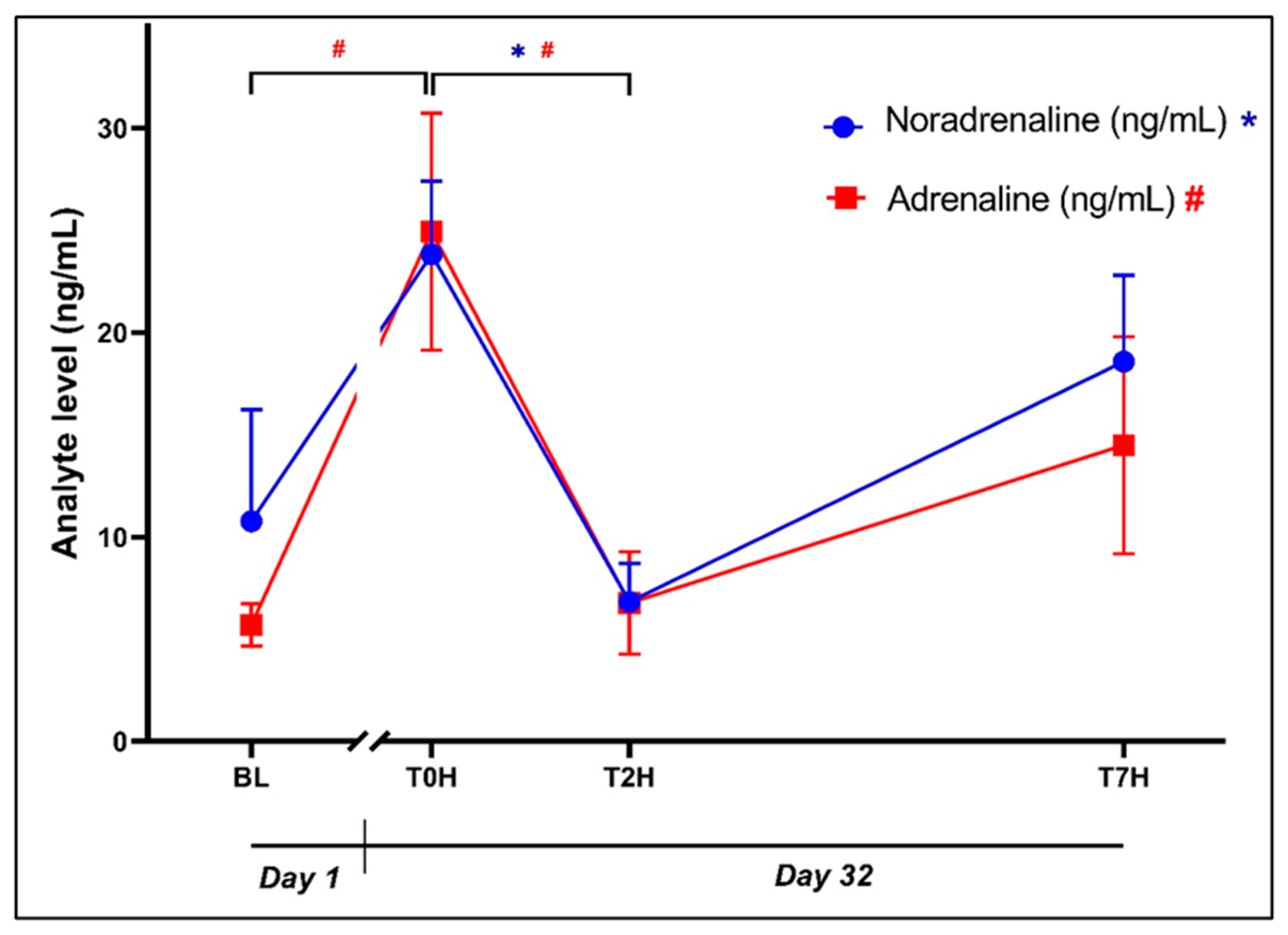
The initial ANOVA revealed that there was a statistically significant effect of time for plasma noradrenaline (Error! Reference source not found.: F3, 42 = 3.77, p = 0.018) and adrenaline (Error! Reference source not found.: F3, 56 = 4.56, p = 0.006) concentrations. Although BL and T0H concentrations were comparable (10.77 ± 21.15 ng/mL vs. 23.82 ± 13.85 ng/mL, p = 0.10, d = 0.86 [0.11; 1.61]), noradrenaline concentration at T2H (6.83 ± 7.27 ng/mL) was significantly increased, compared to that at T0H (p = 0.02, d = 1.11 [0.35; 1.88]). As for plasma adrenaline, a statistically significant difference was identified between BL and T0H (5.70 ± 4.01 ng/mL vs. 24.93 ± 22.49 ng/mL, p = 0.01, 1.19 [0.43; 1.96]), whereafter it decreased towards T2H (6.76 ± 9.71 ng/mL, p = 0.02, d = 1.13 [0.37; 1.89]).
Figure 2.
Graphical representation of the change in noradrenaline and adrenaline levels post stress, over the time periods, as indicated. Plasma concentrations (ng/mL) of noradrenaline (blue dots; *p < 0.05) and adrenaline (red squares; #p < 0.05) over time. Baseline blood withdrawal (BL; Day 1); Day 32 blood withdrawals with 7-hour transport simulation stress: blood withdrawal immediately post-capture (T0H); blood withdrawal two hours post-capture; blood withdrawal seven hours post-capture (T7H). Error bars indicate the standard error of the mean (SEM).
Figure 2.
Graphical representation of the change in noradrenaline and adrenaline levels post stress, over the time periods, as indicated. Plasma concentrations (ng/mL) of noradrenaline (blue dots; *p < 0.05) and adrenaline (red squares; #p < 0.05) over time. Baseline blood withdrawal (BL; Day 1); Day 32 blood withdrawals with 7-hour transport simulation stress: blood withdrawal immediately post-capture (T0H); blood withdrawal two hours post-capture; blood withdrawal seven hours post-capture (T7H). Error bars indicate the standard error of the mean (SEM).
3.2. Corticosterone and Dehydroepiandrosterone (DHEA)
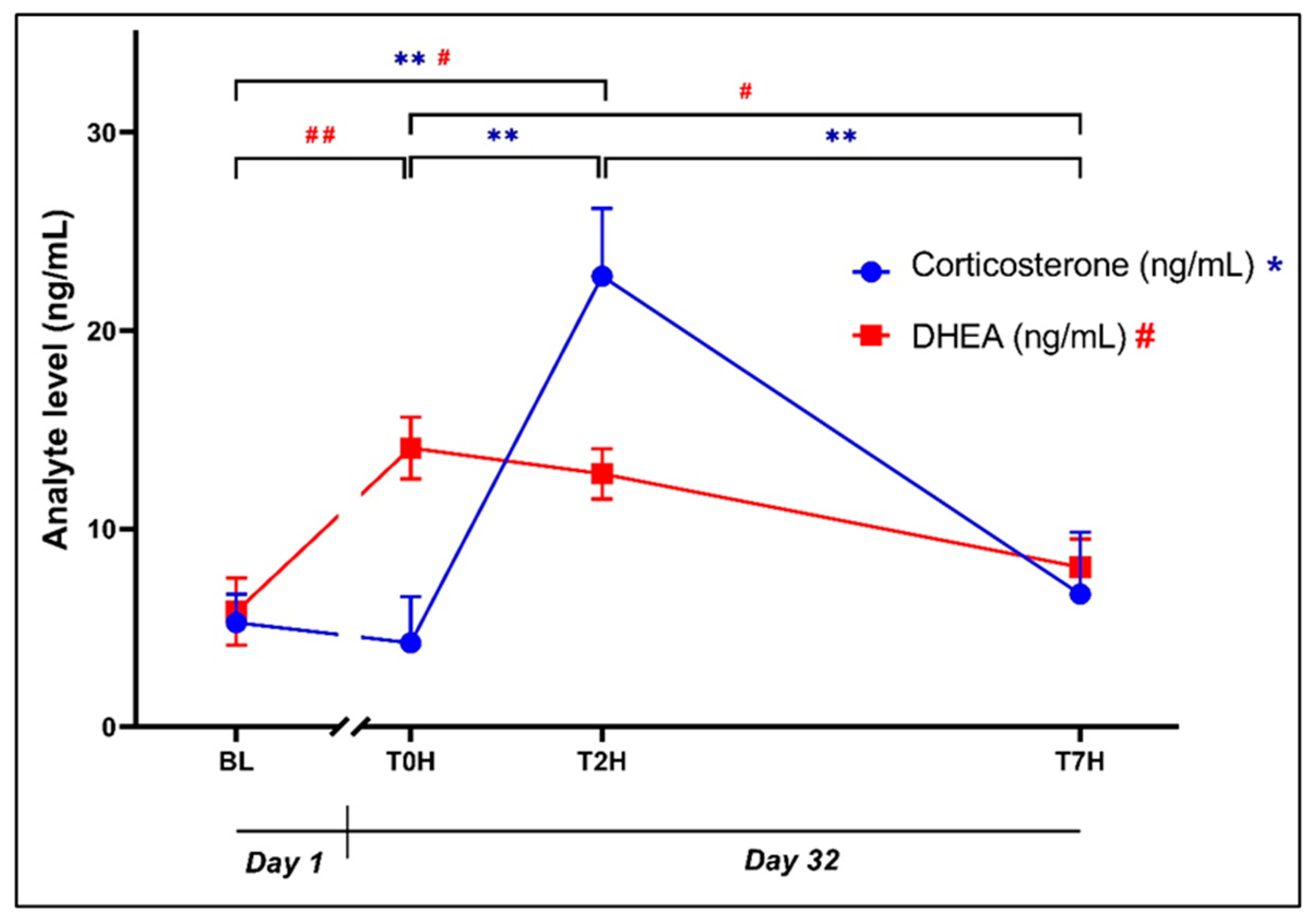
The initial ANOVA on plasma data revealed statistical significance for the effect of time on plasma corticosterone (Error! Reference source not found.: F3, 56 = 10.52, p < 0.0001) and DHEA (Error! Reference source not found.: F3, 56 = 6.78, p = 0.0006) concentrations. Although BL corticosterone concentrations were comparable to T0H (5.25 ± 5.56 ng/mL vs. 4.23 ± 8.99 ng/mL, p = 0.99, d = 0.10 [-0.63; 0.83]), DHEA concentrations were significantly increased between BL and T0H (5.81 ± 6.55 ng/mL vs. 14.08 ± 6.04 ng/mL, p = 0.002, d = 1.43 [0.65; 2.21]).
Plasma corticosterone concentrations at T2H (22.73 ± 13.26 ng/mL) were also significantly higher than levels at BL (5.25 ± 5.56 ng/mL, p = 0.0002, d = 1.68 [0.88; 2.48]), T0H (4.23 ± 8.99 ng/mL, p = 0.0001, d = 1.77 [0.97; 2.58]) and T7H (6.68 ± 12.13 ng/mL, p = 0.0007, d = 1.54 [0.75; 2.33]).
Plasma DHEA concentrations were significantly increased between BL versus T2H (5.81 ± 6.55 ng/mL vs. 12.76 ± 4.95 ng/mL, p = 0.01, d = 1.20 [0.43; 1.97]), and significantly decreased between T0H and T7H (14.08 ± 6.04 ng/mL vs. 8.05 ± 5.50 ng/mL, p = 0.002, d = 1.43 [0.65; 2.21]).
Figure 3.
Change in corticosterone and DHEA levels post stress, over the time periods as indicated. Plasma concentrations (ng/mL) of corticosterone (blue dots; **p < 0.001) and dehydroepiandrosterone (DHEA; red squares; #p < 0.05; ##p < 0.001) over time. Baseline blood withdrawal (BL; Day 1); Day 32 blood withdrawals with 7-hour transport simulation stress: blood withdrawal immediately post-capture (T0H); blood withdrawal two hours post-capture; blood withdrawal seven hours post-capture (T7H). Error bars represent the calculated SEM.
Figure 3.
Change in corticosterone and DHEA levels post stress, over the time periods as indicated. Plasma concentrations (ng/mL) of corticosterone (blue dots; **p < 0.001) and dehydroepiandrosterone (DHEA; red squares; #p < 0.05; ##p < 0.001) over time. Baseline blood withdrawal (BL; Day 1); Day 32 blood withdrawals with 7-hour transport simulation stress: blood withdrawal immediately post-capture (T0H); blood withdrawal two hours post-capture; blood withdrawal seven hours post-capture (T7H). Error bars represent the calculated SEM.
3.3. Corticosterone-to-DHEA (CORT:DHEA) Ratio
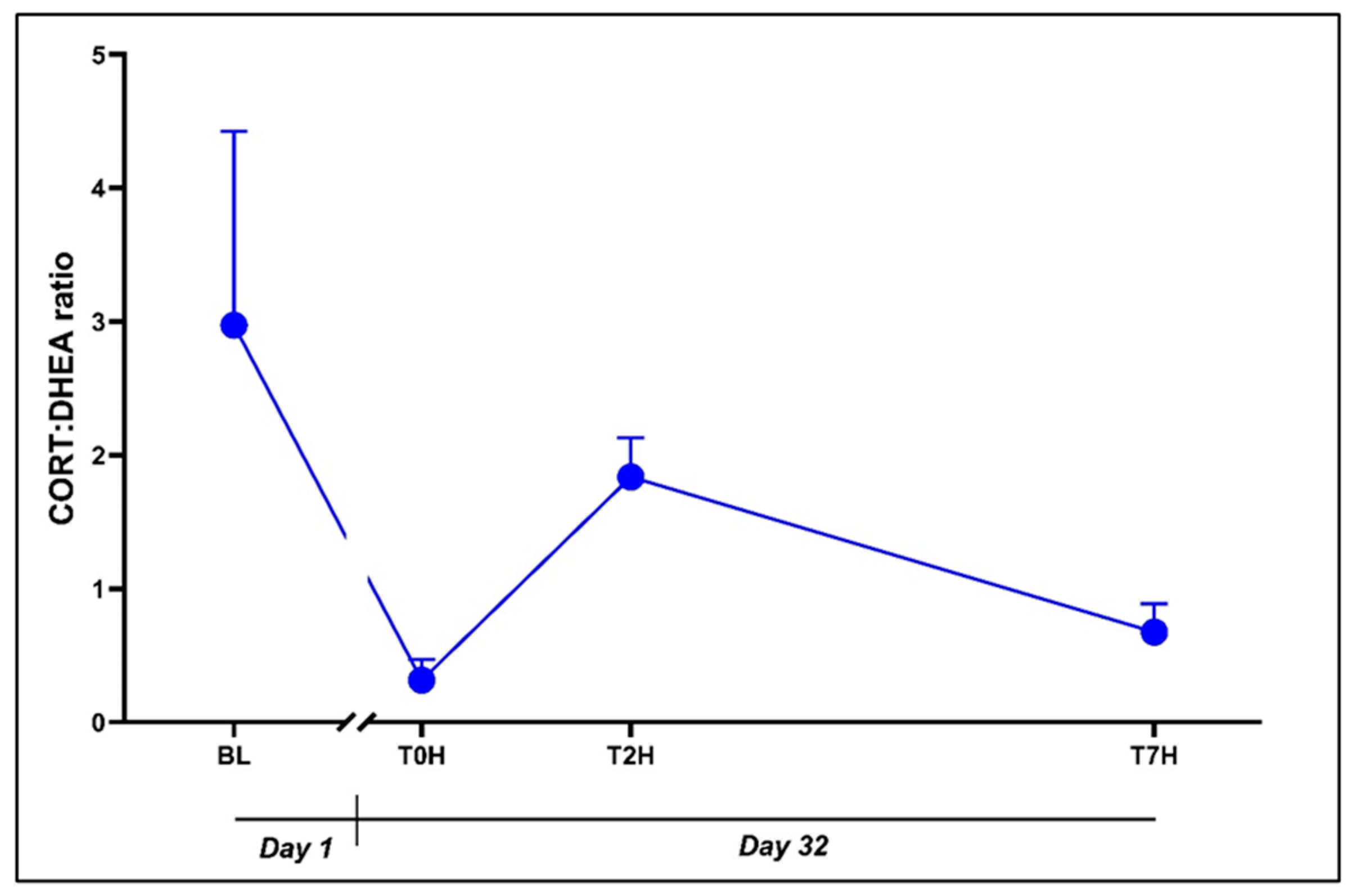
There was no influence of time on the CORT:DHEA ratio (Error! Reference source not found.: F3, 53 = 2.573, p = 0.06), as indicated by the initial ANOVA. This is supported by the comparable values between the different time points. Interestingly, T0H values strongly trended to decrease from BL values (0.31 ± 0.61 vs. 2.97 ± 5.62, p = 0.07, d = 0.91 [0.16; 1.67]).
Figure 4.
Change in the CORT:DHEA ratio post stress over the time periods, as indicated. Base-line blood withdrawal (BL; Day 1); Day 32 blood withdrawals with 7-hour transport simulation stress: blood withdrawal immediately post-capture (T0H); blood with-drawal.
Figure 4.
Change in the CORT:DHEA ratio post stress over the time periods, as indicated. Base-line blood withdrawal (BL; Day 1); Day 32 blood withdrawals with 7-hour transport simulation stress: blood withdrawal immediately post-capture (T0H); blood with-drawal.
3.4. Glucose and Lactate
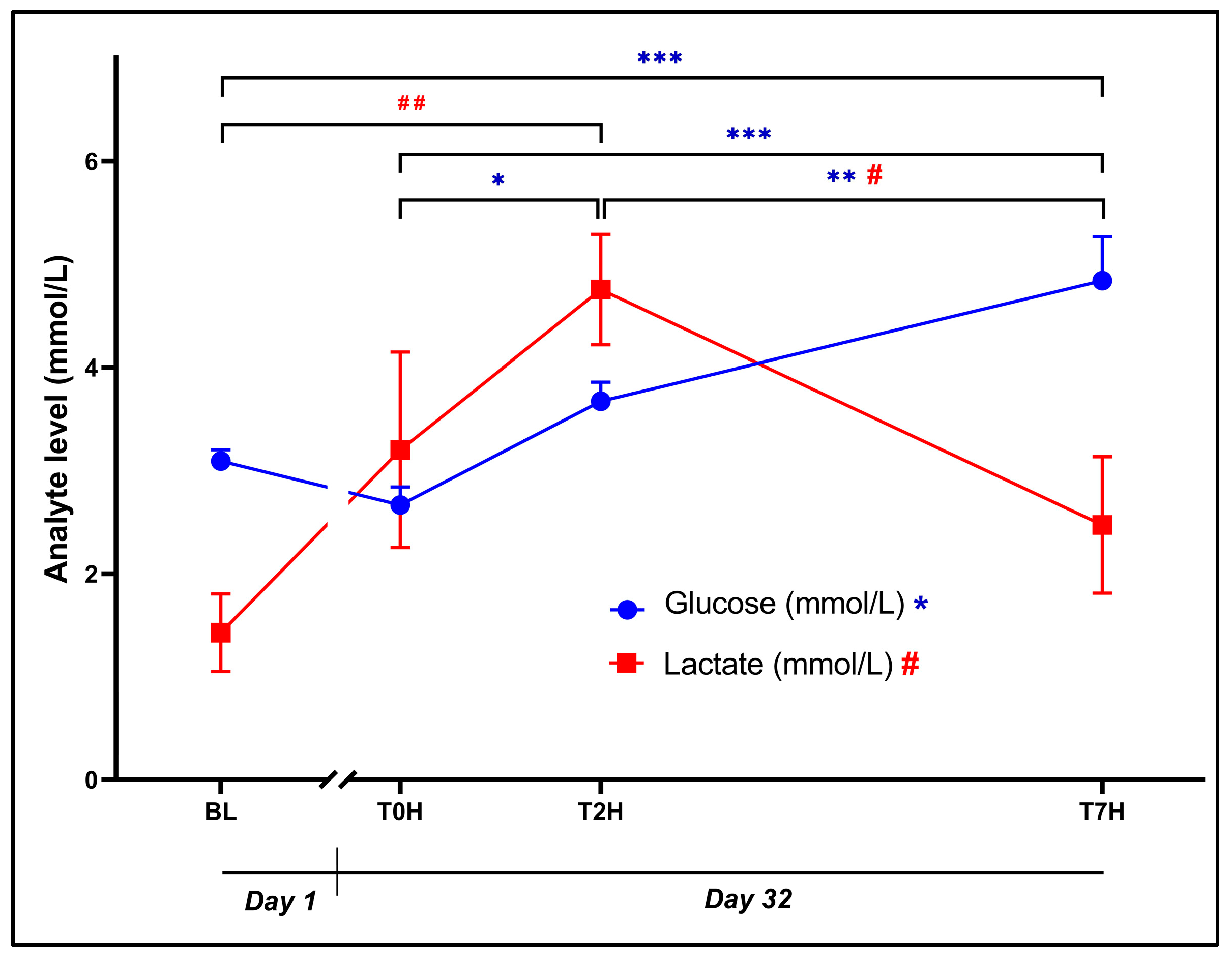
The ANOVA indicated a statistically significant effect of time for plasma glucose (Error! Reference source not found.: F3, 42 = 24.51, p < 0.0001) and lactate (Error! Reference source not found.: F3, 42 = 6.31, p = 0.001), despite both glucose (3.07 ± 0.42 mmol/L vs. 2.65 ± 0.67, p = 0.40, d = 0.58 [-0.17; 1.33]) and lactate (1.43 ± 1.46 mmol/L vs. 3.20 ± 3.67 mmol/L, p = 0.13, d = 0.82 [0.07; 1.58]) concentrations being comparable (non-significant) between BL and T0H.
Average glucose concentration increased significantly from T0H to T2H (3.65 ± 0.71 mmol/L, p = 0.003, d = 1.35 [0.57; 2.14]). Also, GLUC concentration at T7H (34.83 ± 1.64 mmol/L) was significantly higher than at BL (p < 0.0001, d = 2.37 [1.50; 3.24]), at T0H (p < 0.0001, d = 2.95 [2.00; 3.89]), and at T2H (p = 0.001, d = 1.60 [0.79; 2.40]).
As for lactate, concentrations increased significantly from BL to T2H (4.75 ± 2.08 mmol/L, p = 0.001, d = 1.54 [0.75; 2.34]), whereafter it returned to baseline levels at T7H (2.47 ± 2.56 mmol/L, p = 0.03, d = 1.06 [0.29; 1.82]).
Figure 5.
Change in glucose and lactate levels post stress over the time periods, as indicated. Plasma concentrations (mmol/L) of glucose (blue dots; *p < 0.05; **p < 0.001; *** p < 0.0001) and dehydroepiandrosterone (red squares; #p < 0.05; ##p < 0.001) over time. Baseline blood withdrawal (BL; Day 1); Day 32 blood withdrawals with 7-hour transport simulation stress: blood withdrawal immediately post-capture (T0H); blood withdrawal two hours post-capture; blood withdrawal seven hours post-capture (T7H). Error bars represent the calculated SEM.
Figure 5.
Change in glucose and lactate levels post stress over the time periods, as indicated. Plasma concentrations (mmol/L) of glucose (blue dots; *p < 0.05; **p < 0.001; *** p < 0.0001) and dehydroepiandrosterone (red squares; #p < 0.05; ##p < 0.001) over time. Baseline blood withdrawal (BL; Day 1); Day 32 blood withdrawals with 7-hour transport simulation stress: blood withdrawal immediately post-capture (T0H); blood withdrawal two hours post-capture; blood withdrawal seven hours post-capture (T7H). Error bars represent the calculated SEM.
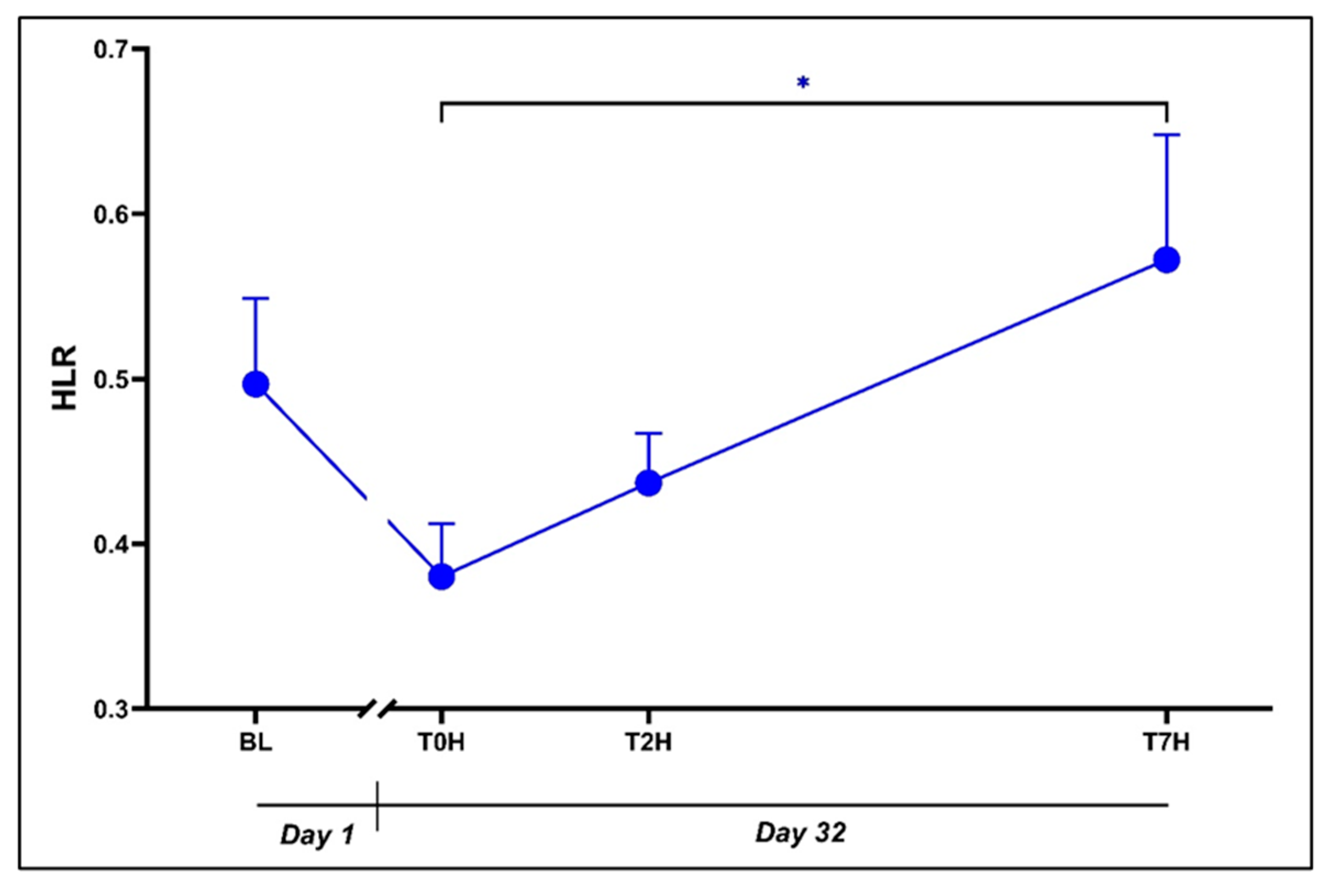
3.5. Heterophil-to-Lymphocyte Ratio (HLR)
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
Figure 6.
Change in HLR levels post stress over the time periods, as indicated. Baseline blood withdrawal (BL; Day 1); Day 32 blood withdrawals with 7-hour transport simulation stress: blood withdrawal immediately post-capture (T0H); blood withdrawal two hours post-capture; blood withdrawal seven hours post-capture (T7H). *p < 0.05. Error bars represent the calculated SEM.
Figure 6.
Change in HLR levels post stress over the time periods, as indicated. Baseline blood withdrawal (BL; Day 1); Day 32 blood withdrawals with 7-hour transport simulation stress: blood withdrawal immediately post-capture (T0H); blood withdrawal two hours post-capture; blood withdrawal seven hours post-capture (T7H). *p < 0.05. Error bars represent the calculated SEM.
3.6. Correlations
Only biomarker correlations that achieved statistical significance are presented in Error! Reference source not found.. Significance for Spearman’s r was set at varying levels of significance. A weak significance being 0.1 ≥ r > 0.3, a moderate significance being 0.3 ≥ r > 0.5, and a strong significance being r ≥ 0.5. This applies to all correlations with the addition of a 95 % CI that should not include 0.
1 DHEA: Dehydroepiandrosterone; 2 HLR: Heterophil-to-lymphocyte ratio
4. Discussion
The biomarkers analyzed were found to be valid indicators of acute stress, with noradrenaline and adrenaline demonstrating rapid reactivity to a stressor, while noticeable effects on HLR were only seen over time. The corticosterone response displayed a range of downstream effects that translates to a varying response dependent on the biomarker assayed. Thus, we observed correlations between DHEA and lactate following the acute response, whilst stress displayed temporal effects on glucose and HLR. Finally, acute stress was associated with fluctuations in the CORT:DHEA ratio, underscoring its reactivity and swift response to a stressor and validating its inclusion in investigations pertaining to the crocodilian stress response.
Captive and wild animals experience different types of stressors but are dependent on the same basic adaptive mechanisms to ensure survival [
48]. The initial factors involved are the SAM axis and its effector hormones noradrenaline and adrenaline. Both have very short half-lives, viz, 10 to 100 seconds [
49], and manifest change transiently. In fact, their concentrations increase within a few minutes (< 2 min) following an acute environmental stressor [
10] and show a rapid decline within 60 minutes [
19]. On the other hand, the HPI axis may take up to 15 min post stress to show an increase in corticosterone, with up to an hour before many of its distal effects become manifest [
15,
16]. This duration of effect is dependent on the duration and severity of the stressor and the integrity of the HPI negative feedback system after the dissipation of the stressor [
15,
16]. Catecholamines ensure immediate organism survival, while HPI axis hormones ensure long-term adaptation and homeostasis maintenance [
10,
13]. Previous studies [
7,
29,
30] have focused on the crocodilian stress response, examining plasma monoamines, metabolic markers like glucose and lactate, and glucocorticoids such as corticosterone. Apart from assessing multiple biomarkers, here DHEA was also explored both in its independent capacity and when utilized in conjunction with corticosterone, i.e., CORT: DHEA. Moreover, the study utilized animals as their own control versus that over time, enhancing baseline integrity.
An interesting pattern emerged regarding the catecholamines (see Error! Reference source not found.). While low concentrations of both noradrenaline and adrenaline were observed on Day 1 (BL pre-stress blood collection), as could be expected, baseline measurement on Day 32 that preceded the stressor (T0H) showed these values to have increased nearly 3-fold. Due to their short plasma half-life [
49], these effects are unlikely to represent an adverse response to the previous four weeks of habituation/acclimatization, but rather to an acute pre-emptive increase in catecholamine levels prior to the application of the stressor. Indeed, these findings concur with that of Lance and Elsey [
7] who described a similar pre-stress increase in adrenaline. The underlying mechanisms are speculative but may represent some form of pre-cognition during this habituation-pre-stress period. Further study is warranted.
Considering noradrenaline and adrenaline (see Error! Reference source not found.), both catecholamines followed a similar zig-zag pattern across the blood collection points, with a near significant trend in their absolute concentrations (p ≤ 0.05; dNA(BL vs. T7H) = -0.51; dAdr(BL vs. T7H = -0.55) at any of the collection points, reaffirming their shared release pattern. Notable differences within each analyte included the difference between T0H and T2H for both noradrenaline (p = 0.02, d = 1.11 [0.35; 1.88]) and adrenaline (p = 0.02, d = 1.13 [0.37; 1.89]), possibly indicating that T0H might have been collected near the peak of their release. There was however a non-significant, yet notable, difference between T2H and T7H (dNA = 0.77; dAdr = -0.48) for both monoamines. The latter being a possible secondary peak stemming from resistance to handling following the dissipation of the effects of electrical immobilization.
Measuring noradrenaline and adrenaline are often maligned as stand-alone biomarkers of the response to stress due to their short biological half-lives and rapid release patterns. Nevertheless, these catecholamines remain essential when investigating the acute stress response of crocodilians. When interpreted together with complementary analytes such as corticosterone, glucose, lactate, and immune-inflammatory markers, e.g., HLR, they provide a clearer picture of the stress response over time. This progression extends from its immediate “fight-or-flight” response to its more complex temporal effects that determine the eventual adaptive or maladaptive capacity of the animal.
By stimulating aerobic glycolysis, gluconeogenesis, and glycogenolysis [
17,
50], noradrenaline and adrenaline are hyperglycemic hormones. This, together with the delayed but more sustained metabolic influences of corticosterone, provides the muscles and supportive structures with much-needed energy substrates that can be broken down and utilized during the “fight-or-flight” response [
10]. The impact of catecholamines on the glycemic levels in the body therefore manifests significantly faster (“acute” effects) than that of corticosterone (“late” effects) [
50]. This supports the pattern of plasma glucose levels depicted in Error! Reference source not found.. Here we observed a non-significant decrease in plasma glucose between BL and T0H (
d = 0.58), suggesting the potential absence of a perceived chronic stressor and, consequently, the lack of sustained elevation in corticosterone levels. However, this might also be attributed to naturally low blood glucose levels resulting from the limited feed ingested during the treatment period (see Error! Reference source not found.). Studying the three collection points on Day 32, plasma glucose increased progressively and significantly with each interval/sampling point. As with other animals and humans, we expected to see an initial increase in blood glucose following the acute stressor, especially since noradrenaline and adrenaline tended to be elevated. This, however, was not the case, possibly due to differences related to the slow metabolism of crocodiles/reptiles compared to mammals [
4,
8]. While the acute hyperglycemic effects of noradrenaline and adrenaline may have been overlooked due to the timing of blood samples, the “late” or temporal effects of corticosterone, as evidenced by the gradual increase in glucose levels over time (T2H to T7H), could be responsible for this phenomenon and hence associated with the patterns of corticosterone activity, as discussed later. Anticipated hyperglycemia, stemming from the pre-emptive surge in catecholamines presented earlier, was not evident in the initial blood collection (T0H). This absence may be attributed to a delayed onset resulting from multi-system activation and their resulting effects on various metabolic processes, in particular the temporal effects of corticosterone on glucose homeostasis that manifest later [
17,
50].
With an increase in blood glucose forming an essential part of the acute stress response, the importance of monitoring lactate becomes evident. Hyperlactatemia is well known to be accompanied by adrenaline-induced hyperglycemia due to the release of lactate from muscle tissues for use by the liver to produce glucose [
30,
50]. Together with pyruvate, lactate is a by-product of glucose metabolism produced under conditions of anaerobic metabolism [
29,
50]. Crocodilians rely significantly on anaerobic metabolism during rigorous muscle utilization such as when captured, or during naturally demanding physical activities such as hunting or territorial disputes with other crocodilians [
23,
51]. Even though small quantities of lactate are safe, if left unchecked it can result in lactic acidosis, and ultimately result in potentially fatal metabolic acidosis [
23,
52].
A strong correlation exists between lactate, glucose, and corticosterone [
23,
53], although this was only partly the case in our investigation (see Error! Reference source not found. and Error! Reference source not found.). Despite a non-significant increase in lactate (
d = -0.72) between T0H and T2H, this trajectory coincided with a simultaneous significant increase in glucose (Error! Reference source not found.) and corticosterone (Error! Reference source not found.) during the same time interval. This increase also followed the initial increase in catecholamines (Error! Reference source not found.), very likely due to increased lactate levels resulting from the exhaustion of capture and restraint at T0H. Furthermore, a significant decrease in lactate was observed between T2H and T7H (
p = 0.03). This reduction suggests an effective breakdown of lactate, thus contributing to the restoration of homeostasis. Intriguingly, the moderate positive correlation of lactate with corticosterone (
r = 0.40; Error! Reference source not found.) reaffirms the extensive interplay between different biomarkers in the stress response. Despite not having a direct relationship, lactate and corticosterone exhibit similar trajectories, possibly due to their connections with shared biomarkers like glucose, noradrenaline, and adrenaline [
18].
The immune system and its subsequent inflammatory responses are especially affected by glucocorticoids [
13,
31]. The effect of corticosterone is mainly immunosuppressive and ultimately damaging if sustained for extended periods [
54], expressed by the immunosuppressive biomarker HLR. Increased HLR suggests reduced lymphocytes and an indicator of stress-induced immunosuppression [
27,
55], attributable to corticosterone decreasing circulating lymphocytes (lymphocytopenia) and increasing heterophils (heterophilia; [
13,
26]). These actions result in a weakened inflammatory response as well as increased susceptibility to infection [
28]. HLR is regarded as a better measure of chronic as opposed to acute stress [
18], although here we have observed both acute and chronic changes to the HLRs in acutely stressed crocodiles (see Error! Reference source not found.).
A slightly decreased HLR between BL and T0H (
d = 0.761) could suggest a small improvement in the acclimation status of the animals following the standard housing period (Day 1 to Day 32), although the absence of a statistically significant difference does not fully support this assumption (
p = 0.17). A significant exponential increase in HLRs was however seen in the hours immediately after the acute stressor, viz. T0H vs. T7H (p = 0.007; d = 1.25). Importantly, these sequential and opposing findings correspond with the effects of corticosterone and DHEA. Between T0H and T2H we noted a nonsignificant trend towards an increase in HLRs (
p = 0.74;
d = 0.37). While speculative, this suggests a potential association with the initial immunosuppressive effects of corticosterone. Thereafter, a further increase in HLR was noted between T2H and T7H (
p = 0.09;
d = 0.88) and, while again presumptive, resembles the temporal immunosuppressive effects of corticosterone. This trend coincided with the waning concentration of DHEA, potentially indicating the dissipation of its immunostimulatory effects. In support of this work, Lance and Elsey [
7] also report an acute increase in the HLR ratio following an acute stressor.
Glucocorticoids, such as corticosterone, are seen as golden standard biomarkers when investigating acute and chronic stress responses [
10,
30]. Here corticosterone levels (Error! Reference source not found.) increased significantly between blood collection at T0H to that of T2H (
p = 0.001), followed by a significant decrease at T2H versus T7H (
p = 0.007). The absence of significant differences between BL and T0H (
p = 0.993) could indicate the absence of chronically stressed individuals, further highlighting the gradual rise of corticosterone plasma levels over time. Taken together these corticosterone changes not only depict the presence of a rapidly responsive HPI axis to a stressor but also one that is under tight control following the dissipation of the situational stressor, resulting in appropriate negative feedback on the HPI axis and lowering of plasma corticosterone levels. Interestingly, our study design and results emulate that observed in American alligators [
7,
29], thus providing validity for the interpretation of other co-measured biomarkers.
Importantly, the reduction in plasma corticosterone between T2H and T7H does not necessarily imply a parallel diminishment in the physiological effects of corticosterone despite its reduced plasma levels. Corticosterone binding to its nuclear DNA receptor represents an immediate early gene expression event that sets in motion important metabolic, immune, and other physiological effects that are slow/“late” to begin yet are sustained over time [
22,
28]. These processes cause temporal/lasting effects even when the plasma concentrations are low, unlike the effects seen from catecholamines [
56]. This corresponds with the sustained increases seen in glucose (Error! Reference source not found.) and the HLR (Error! Reference source not found.) as well as the positive correlation (
r = 0.476) between these two biomarkers (
Table 1).
Glucocorticoids, like corticosterone, also have their limitations when viewed in isolation and are thus frequently used in combination with other biomarkers to bolster translational capacity [
20]. Investigative problems do however arise with the inability of corticosterone to differentiate between detrimental/prolonged and beneficial/essential releases of glucocorticoids [
57], and to tease out dysfunctional states of the HPI axis [
54]. This can be addressed by adding other HPA/HPI-derived biomarkers, such as DHEA. The value of DHEA as a stress biomarker in crocodilians remains to be realized [
54], despite being implicated in adaptive or species-appropriate behaviors such as aggression and territorial behavior [
20]. DHEA is a “glucocorticoid antagonist” and immunostimulant, among others (see Whitham, Bryant and Miller [
20] and Dutheil
, et al. [
58] for review), and is sensitive to the effects of chronic stressors. Since dysregulation of the HPI axis leads to a reduction in DHEA, it may be used to detect and mitigate the possible causes and effects of chronic stress.
In humans DHEA, like noradrenaline and adrenaline, is released from the adrenal glands with levels rising and diminishing rapidly [
59] and hence may represent an early anti-stress or stress-coping hormone. As noted, while the role of DHEA in crocodilians is unknown, these characteristics could account for the significantly increased DHEA levels observed between BL and T0H (
p = 0.002), suggesting a pre-emptive release as earlier observed with the catecholamines (Error! Reference source not found.). Its gradual decrease between T0H and T7H (
p = 0.03) is not only inversely proportional to HLR levels over the same period but also to glucose levels. Both these effects correspond to declining DHEA levels following its initial release, whereby the antagonistic action of DHEA on glucocorticoids weakens [
20] leading to a subsequent increase in HLR (Error! Reference source not found.) and glucose (Error! Reference source not found.).
The significant positive correlation between corticosterone and DHEA (
r =0.32; Error! Reference source not found.
) led us to further investigate the concurrent use of these biomarkers in the context of stress, especially the use of the CORT:DHEA ratio (Error! Reference source not found.). By depicting net glucocorticoid activity instead of the levels of each glucocorticoid alone, this ratio demonstrates physiological homeostasis and informs on inherent HPI axis dysfunction [
20]. It has therefore been connected to immune function/immunosenescence, treatment-resistant depression, anxiety, and stressful life events in humans [
20], hence of potential value in neuropsychiatric conditions. Research conducted in livestock has revealed that DHEA analysis is useful in assessing stress under transportation conditions, environmental factors, and husbandry [
58,
60]. As described here, increased CORT:DHEA ratio was associated with an activation of the stress response (i.e., increasing corticosterone), with acutely increased DHEA levels representing the body’s attempt to reverse this imbalance, reduce stress, and restore homeostasis.
Although the CORT:DHEA ratio (Error! Reference source not found.) did not show any significant interactions, except for a non-significant decrease (
p = 0.07;
d = 0.91) between BL and T0H, this response can represent an unabated net glucocorticoid effect in the animal. Again, regardless of the insignificance between the Day 32 collection points, the return of the ratio to near pre-stress levels (T0H) is indicative of a healthy and functional stress response and negative feedback system in these crocodiles. Lance and Elsey [
7] described a possible biphasic response to stress via corticosterone with a significantly higher peak emerging after 48 hours post-stressor, whereas Franklin, Davis, Peucker, Stephenson, Mayer, Whittier, Lever and Grigg [
29] observed this same pattern to a lesser extent at 24 hours post-stressor. Neither of the latter two studies assessed DHEA and hence the CORT:DHEA ratio. By using the CORT:DHEA ratio, we have attained a deeper understanding of this extended response to stress that could be utilized when monitoring crocodilian welfare and when considering or assessing treatment options.
Some limitations should be noted in this study. Crocodiles are ectothermic poikilotherms and are particularly susceptible to seasonal changes. Since the study was conducted during late summer, it is possible that warmer conditions might yield less variation and a more robust stress response. Here, brumation could have compromised the maximal stress response, so that assessing stress-related anorexia would strengthen the findings. Finally, data could have benefitted by increasing the number of sampling times, although at the risk of damaging the blood vessel (post-occipital spinal venous sinus).