Preprint
Article
Mechanically homogenized expanded graphite/ cobalt(II) rhenate(VII) – Co(ReO4)2, as electrodes in hybrid symmetric supercapacitors
Altmetrics
Downloads
78
Views
30
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
13 June 2024
Posted:
14 June 2024
You are already at the latest version
Alerts
Abstract
Mechanically homogenized composite of expanded graphite and cobalt(II) rhenate(VII) has been described. Cobalt(II) rhenate(VII) was obtained in a reaction of perrhenic acid with cobalt(II) nitrate. A simple mortar homogenization method was used to enhance intercalation of cobalt species within carbon matrix. The specific capacity of composite was enhanced by 50% in comparison to bare expanded graphite. The electrochemical characteristic was significantly improved including better cyclability, lower resistance of electrode material, lower iR drop, with respect to expanded graphite without cobalt(II) rhenate(VII) active species. Expanded graphite with its unique specific surface area and pore size diameter was proved as potential and cheap carbon support.
Keywords:
Subject: Chemistry and Materials Science - Electrochemistry
1. Introduction
A continuing increase in the electrical energy demand, which is observed in recent years altogether with rapid industrialization, leads to advanced research of more efficient energy storage solutions. On the other hand an excessive consumption of natural resources (oil, coal, gas) is not in accordance with European Union climate policy. Development of highly advanced energy storage technologies may reduce fossil fuel consumption and the greenhouse effect. Efficient storage of the electrical energy would also prevent power outages, observed all over the world [1,2]. The most important modern energy storage device are lithium-ion batteries and supercapacitors. There are some constructional differences between both groups, however, in recent years the technological progress has blurred these differences. Generally, the main features of supercapacitors, in contrary to lithium ion batteries, are the possibility of fast charging/discharging, so the quick delivery of huge energy within a short time. Consequently the main advantages are high power density, long cycle life, relatively simple construction from easily accessible materials, eco-friendliness (no disposal issues) and high coulombic efficiency. Electrochemical supercapacitors are the promising energy storage materials that due to long cycle life and high-power density may be applied in many areas of everyday living, i.e., back-up memories, traffic warning signals, security and alarm systems, smoke detectors engine starting and acceleration, and load lifting [3]. The perspective electrode materials for supercapacitors should meet numerous requirements like high electrical conductivity, chemical inertness, gas or liquid adsorption permission, relatively high surface area, and state of material, whether it is powder, fiber or monolith. Regarding these criteria the active carbons seem to be the most adequate materials. Within applied electrolytes, aqueous, organic or ionic liquids can be found. Each groups has their pros and cons. Aqueous electrolyte either alkali hydroxides or sulfates (most commonly used) are relatively cheap and easily available but the drawback is a voltage window limited to 1.2 V. Organic electrolytes show broader stability range (2–2.5 V) but suffer from flammability, volatility and toxicity. Ionic liquids may give voltage window as high as 4V, but their preparation is complex and expensive, and still insufficient for industrial applications.
The capacitance of supercapacitor is proportional to the surface area of the electrode material. The higher specific surface area, the higher charge contact interface therefore, chemically and electrochemically inert carbons are considered as best choice for matrices. Further increase in capacitance may be achieved by intercalation of an active species like conducting polymers, metal nanoparticles, and metal oxides. Such composite containing eg. metal oxide on carbons are generally classified as conversion electrodes with capacitance arising from typical charge separation and pseudocapacitance caused by Faradaic reactions [4]. Redox reactions between electrolyte and metal oxide can significantly increase the specific capacitance. Within many proposals of metal oxide–carbon composites, transition metal oxides are privileged. Transition metals may offer several possible oxidation states. Consequently, during charge/discharge cycles such a material is able to store much more electrolyte ions. Refractory metals like molybdenum, tungsten and rhenium seem to be very attractive and perspective components of supercapacitors electrodes, which is mainly attributed to oxidation states from +2 to +6 and numerous polymeric like structures [5,6]. These groups, including oxides and oxo-hydroxides of tungsten, molybdenum, and rhenium were marginally examined. Combination of active carbon with ammonium perrhenate allowed to produce an electrode material with an enhanced specific capacitance around 60F/g [7]. Similar results were obtained by reacting multiwalled carbon nanotubes with potassium permanganate and ammonium perrhenate [8]. ReS2 was also examined as a potential pseudocapacitive material, presenting high Coulombic efficiency and dramatic increase for initial hundreds of cycles [9]. Material with a high specific capacitance was produced by combination of ternary hybrid of MoS2–ReS2/rGO [10]. Rhenium sulphide-based structures may result in specific capacity in the range from 85F/g up to 190F/g, depending on synthesis method (either sonochemical or hydrothermal) [11,12]. Rhenium nanoparticles decorated on biomass-derived active carbon was tested in applications of electrochemical sensing and supercapacitor resulting in high energy density and good long-term stability [13]. Owing to this huge irreversible capacity of lithium rhenium oxide it was proposed as a good candidate for positive activated carbon electrode of a lithium-ion capacitor to be used for pre-lithiating the graphite negative electrode [14]. The biggest drawbacks of rhenium compounds application are limited availability of rhenium and its high market price in comparison to other metals of interest like nickel, cobalt, lithium, manganese, zinc, iron. However, despite these facts the interest in rhenium application can be still found in literature and new ideas of its potential application is under review.
In this report the possibility to produce rhenium-doped carbon electrodes was examined, using popular rhenium compounds synthesized within the progress of RenMet project activities. The center of Hydroelectrometallurgy at LUKASIEWICZ – IMN, Poland, is a highly qualified R&D group specialized in rhenium chemistry, including recovery of rhenium from various materials and production of vast portfolio of rhenium compounds.
2. Materials and Methods
2.1. Carbon matrix
Thermally-expanded graphite was produced in thermal treatment of graphite oxide. It was chosen as a carbon matrix for the intercalated active species. Although structural defects generated in thermal shock significantly limit the Brunauer Emmet Teller (BET) specific surface area the expanded graphite has got much bigger pore size in comparison to other modified carbon precursors, which is important factor regarding intercalation of large perrhenate molecules [3]. Synthetic graphite was oxidized by Hummers method mixing 10 g synthetic graphite with 230cm3 95–97% sulfuric acid (Acros Organics) in a round-bottomed flask equipped with a reflux and thermometer and ice-cooled at the temperature close to 273K [15]. Then 5g sodium nitrate and 30g potassium permanganate were added. Produced graphite oxide slurry was diluted with deionized water, washed several times by decantation and vacuum-filtrated using two filter papers and dried at 110°C. Produced graphite oxide powder was placed in a tubular furnace purged with inert gas (N2). Temperature was increased above 700⁰C for 10minutes. It allowed desorbing the oxygen containing groups and expanding the material to give a fluffy, light carbon powder (EXPANDED).
2.1. Metal salt
Cobalt(II) rhenate(VII) was chosen as a potential candidate for energy storage material. It was synthesized based on equation:
2 HReO4 + Co(NO3)2 → Co(ReO4)2 + 2 HNO3
50cm3 perrhenic acid of a concentration 200 g Re/dm3 was mixed with cobalt(II) nitrate(V) solution in 10% stochiometric excess. Solution was vigorously mixed and evaporated at 90⁰C. Solid product was dried at 120⁰C for 3 days to produce constant mass violet product.
2.1. Electrochemical characterization
Experiments were carried out using two-electrode symmetric system with electrolytic nickel current collectors, with electrode materials pasted on it. This was separated with a membrane (micro glass fiber paper, MGC, Ahlstrom Munksjo) soaked with 6M KOH. High-precision balance (Mettler Toledo AB 204 S) was used to weigh mass of electrode materials. Carbon-cobalt(II) perrhenate(VII) composite for the electrochemical analysis was prepared using simple solid state mixing 0.08 g of expanded graphite, 0.02g cobalt(II) perrhenate(VII), and 0.02g polytetrafluoroethylene, in mortar agate. The whole assembly was pressed in a poly(methyl methacrylate) casing. One current collector was mounted with the working electrode while the latter with both reference and counter electrode. Autolab PGSTAT 302 N workstation was used to perform cyclic voltammetry (to evaluate cycle life of the electrode) in a potential window 0 – 1 V at scan rate 500 mV/s; galvanostatic charge/discharge (GC) at current density in the range 0.1 - 2 A/g (to evaluate the specific capacity), and the electrochemical impedance spectroscopy (EIS) in the frequency range 100 kHz –100mHz with the amplitude of sinusoidal voltage signal equal 10 mV (to determine the resistance of the electrode processes, and the charge transfer characteristics).
2.1. Characterization
Elemental analysis was performed using atomic absorption spectroscopy (FAAS - iCE 3000 Series AA spectrometer Thermo Scientific), while quantitative analysis using X-ray diffractometry (Rigaku MiniFlex 600 equipped with Cu tube as a source of Kα radiation with wave length 1.5406 Å).
3. Results
Qualitative analysis revealed that cobalt species was in fact mixture of cobalt bis(rhenate(VII)) – Co(ReO4)2 (01-089-6940), commonly known as cobalt(II) rhenate(VII) or cobalt(II) perrhenate, and cobalt rhenium oxide hydrate – Co(H2O)4(ReO4)2 (01-088-1324). The diffraction pattern was presented in a Figure 1.
Table 16. 60 and 25.50 were attributed to hydrated form of cobalt salt.
Quantitative analysis of obtained cobalt rhenium species showed that contents of cobalt and rhenium, were 10.4% and 66.8%, respectively, which was in line with stoichiometry, 10.5% and 66.5%, respectively.
Expanded graphite and Co-modified expanded graphite were electrochemically analyzed to show their characteristics and advantages of Co (ReO4)2 application. Cyclic voltamperometry was used to specify charge discharge behavior emphasizing the loss in the specific capacity between the first and last work cycle. Supercapacitor should be quickly charged and discharged with box-like shape of CV curves. Curves obtained for examined carbon matrix and composite were presented in a Figure 2.
Cyclic voltammetry showed that in both cases shape of curves is not ideal with uneven charge and discharge. Additionally for bare carbon matrix there were significant operational discrepancies from cycle to cycle, indicating loss of the specific capacity in 1000cycles period. On the other hand intercalation of cobalt(II) rhenate(VII) allowed to limit capacity deterioration in a cyclic mode. The specific capacity was calculated from CV curves using equation 1:

where V1, V2 are initial and final potential, I is current intensity, V is voltage, v is scan rate, m mass of electrode, ΔV is a potential window.
Capacity retention was calculated for both materials with respect to the first and the last CV cycle. The intercalation of cobalt rhenium species allowed to diminish capacity loss from 11% for EXPANDED to 7% for EXP_CoPR, after 1000 cycles.
Galvanostatic charge/discharge is the most reliable technique to evaluate the specific capacity of electrode material. Capacity was calculated from equation 2:

where is current intensity of discharge, t is discharge time, m mass of electrode, ΔV is a potential window. The specific capacity of bare expanded graphite was as high as 52F/g at current density 0.2A/g, while after modification with cobalt rhenium species this has increased up to 78F/g at 0.3A/g. Results showed that simple mechanical intercalation of cobalt(II) rhenate(VII) allowed to enhance the specific capacity by 50%. Additionally, the path of charge and discharge curves was more symmetric after active species addition, and the iR drop well-observed in EXPANDED curves did not appear in EXP_CoPR (Figure 3).
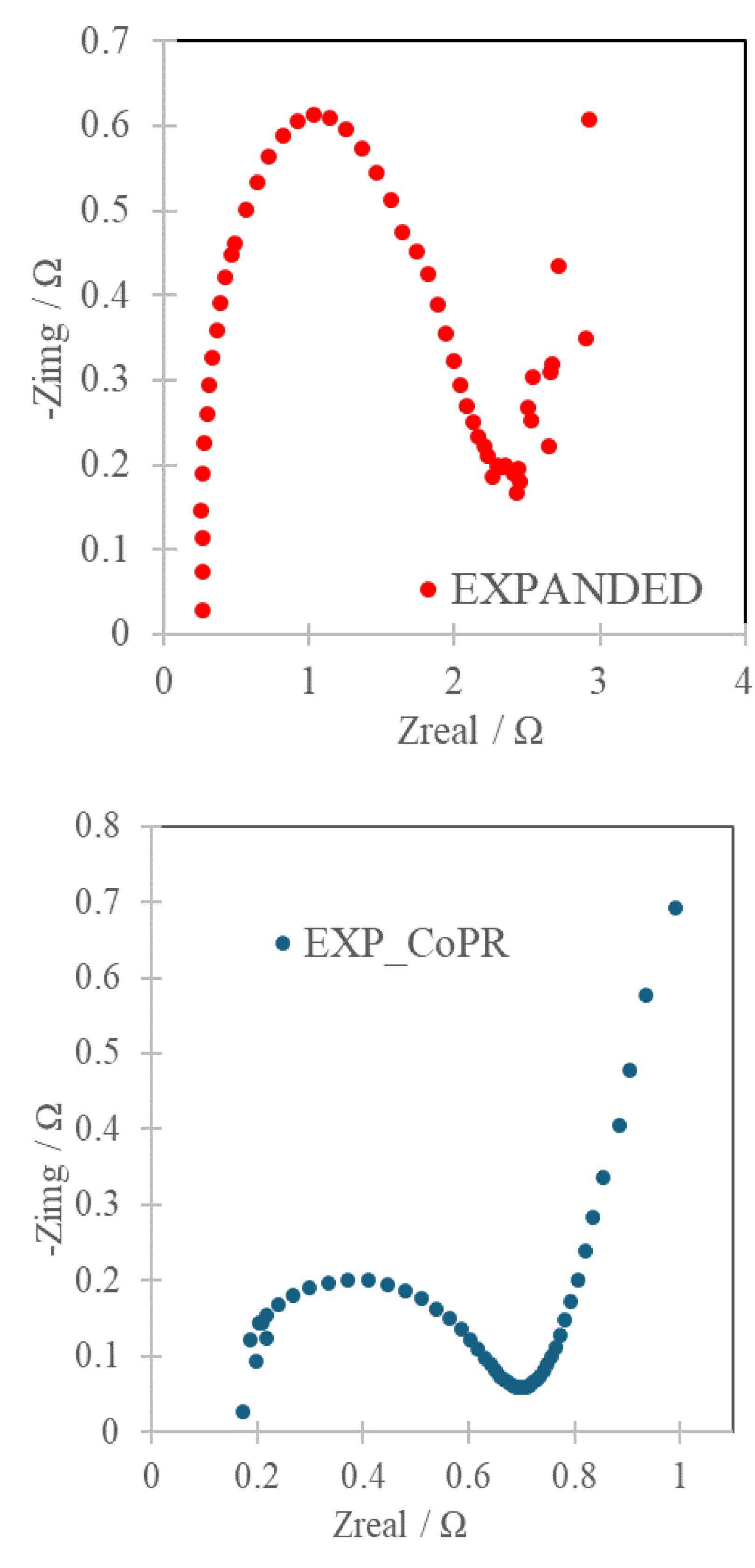
EIS was used to characterize the resistance of processes within electrode. The relation of real and imaginary component of impedance, shortly called the Nyquist plot, is a powerful tool to characterize electrodes for energy storage applications. Electrode material that is governed by electrostatic and chemical processes is represented by two components, namely the semicircle in the high frequency region attributed to the Faradaic charge transfer resistance and linear part in a low-frequency region indicating a pure capacitive behavior and representing the ion diffusion in the electrode structure (Figure 4).
It allowed to estimate the resistance combined with the electrode RS, and the one combined with charge transfer RCT. These were 0.26Ω and 0.17Ω (RS) and 2.43Ω and 0.71Ω (RCT) for EXPANDED and EXP_CoPR, respectively. While the total resistivity component was 2.92Ω and 0.99Ω for EXPANDED and EXP_CoPR. Smaller loop and steep curve in the low frequency region are the consequences of better Warburg diffusion and consequently enhanced mass transfer from the electrolyte to electrode interface in the EXP_CoPR.
4. Conclusions
This paper explores the possibility of cobalt(II) rhenate(VII) application in hybrid symmetric aqueous electrolyte-based carbon supercapacitors. It was showed that mechanical homogenization of thermally expanded graphite with active species of cobalt rhenium compound may enhance the specific capacity by 50%. The forces exerted on graphite oxide structure by desorbing oxygen-containing groups are so high that materials with a big pore size diameter, voids, and interlayer distance is produced. Thus the resulting thermally expanded graphite can accommodate big molecules like rhenate(VII) anions, without detrimental effect on electrolyte diffusion through material during charge/discharge cycles. Consequently, an improvement in the specific capacity and total resistance of electrode reactions were observed.
Author Contributions
Conceptualization, M.C.; methodology, M.C. and K.P.; validation, K.L.S.; formal analysis, J.M. and K.P.; investigation, M.C. and Ł.H.; resources, K.P. and J.M.; data curation, M.C.; writing—original draft preparation, M.C.; writing—review and editing, K.L.S. and Ł.H.; visualization, M.C.; supervision, K.L.S.; project administration, K.L.S.; funding acquisition, K.L.S. All authors have read and agreed to the published version of the manuscript.
Funding
The work was funded by the Norwegian Financial Mechanism 2014–2021—Small Grant 2020 NOR/SGS//RenMet/0049/2020-00 (11/PE/0146/21), entitled: Innovative hydrometallurgical technologies for the production of rhenium compounds from recycled waste materials for catalysis, electromobility, aviation and defense industry.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to patent application and project contract.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chang, S.E.; McDaniels, T.L.; JMikawoz, J.; Peterson, K. Infrastructure failure interdependencies in extreme events: Power outage consequences in the 1998 Ice Storm. Nat. Hazards 2007, 41, 337. [Google Scholar] [CrossRef]
- Hines, P.; Apt, J.; Talukdar, S. Large blackouts in North America: Historical trends and policy implications. Energy Policy 2009, 37, 5249. [Google Scholar] [CrossRef]
- Ciszewski, M.; Koszorek, A.; Radko, T.; Szatkowski, P.; Janas, D. Review of the Selected Carbon-Based Materials for Symmetric Supercapacitor Application. Journal of Electronic Materials 2019, 48. [Google Scholar] [CrossRef]
- Buglione, L.; Chng, E.L.K.; Ambrosi, A.; Sofer, Z.; Pumera, M. Graphene materials preparation methods have dramatic influence upon their capacitance. Electrochem Commun 2012, 14, 5–8. [Google Scholar] [CrossRef]
- Tytko, K.H.; Fleischmann, W.D.; Gras, D.; Warkentin, E. Mo Molybdenum, Hydrous Molybdates of Groups VA to VIS Metals. Springer-Verlag, Berlin Heidelberg, 1985.
- Shabalin, I.L. (2014). Rhenium. In: Ultra-High Temperature Materials I. Springer, Dordrecht. (2014) 317 - 358.
- Ciszewski, M.; Koszorek, A.; Hawełek, Ł.; Osadnik, M.; Szleper, K.; Drzazga, M. Active Carbon Modified by Rhenium Species as a Perspective Supercapacitor Electrode. Electrochem 2020, 1, 278–285. [Google Scholar] [CrossRef]
- Korusenko, P.M.; Nesov, S.N. Composite Based on Multi-Walled Carbon Nanotubes and Manganese Oxide with Rhenium Additive for Supercapacitors: Structural and Electrochemical Studies. Appl. Sci. 2022, 12, 12827. [Google Scholar] [CrossRef]
- Ghosh, K.; Ng, S.; Iffelsberger, C.; Pumera, M. ReS2: A High-Rate Pseudocapacitive Energy Storage Material. ACS Appl. Energy Mater. 2020, 3, 10261–10269. [Google Scholar]
- Salarizadeh, P.; Askari, M.B. MoS2–ReS2/rGO: A novel ternary hybrid nanostructure as a pseudocapacitive energy storage material. Journal of Alloys and Compounds 2021, 874, 159886. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Mariappan, V.K.; Sathyaseelan, A. Kim Solar driven renewable energy storage using rhenium disulfide nanostructure based rechargeable supercapacitors. Mater. Chem. Front. 2020, 4, 3290–3301. [Google Scholar] [CrossRef]
- Pataniya, P.M.; Dabhi, S.; Patel, V.; Sumesh, C.K. Liquid phase exfoliated ReS2 nanocrystals on paper based electrodes for hydrogen evolution and supercapacitor applications. Surfaces and Interfaces 2022, 34, 102318. [Google Scholar] [CrossRef]
- Veerakumar, P.; Rajkumar, C.; Chen, S.-M.; Thirumalraj, B.; Lin, K.-C. Activated porous carbon supported rhenium composites as electrode materials for electrocatalytic and supercapacitor applications. Electrochimica Acta 2018, 271, 433–447. [Google Scholar] [CrossRef]
- Jeżowski, P.; Fic, K.; Crosnier, O.; Brousse, T.; Béguin, F. Lithium rhenium(vii) oxide as a novel material for graphite pre-lithiation in high performance lithium-ion capacitors. J. Mater. Chem. A 2016, 4, 12609–12615. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J Am. Chem. Soc. 1958, 80, 1339–1339. [Google Scholar] [CrossRef]
Figure 1.
XRD pattern of active cobalt rhenium species.
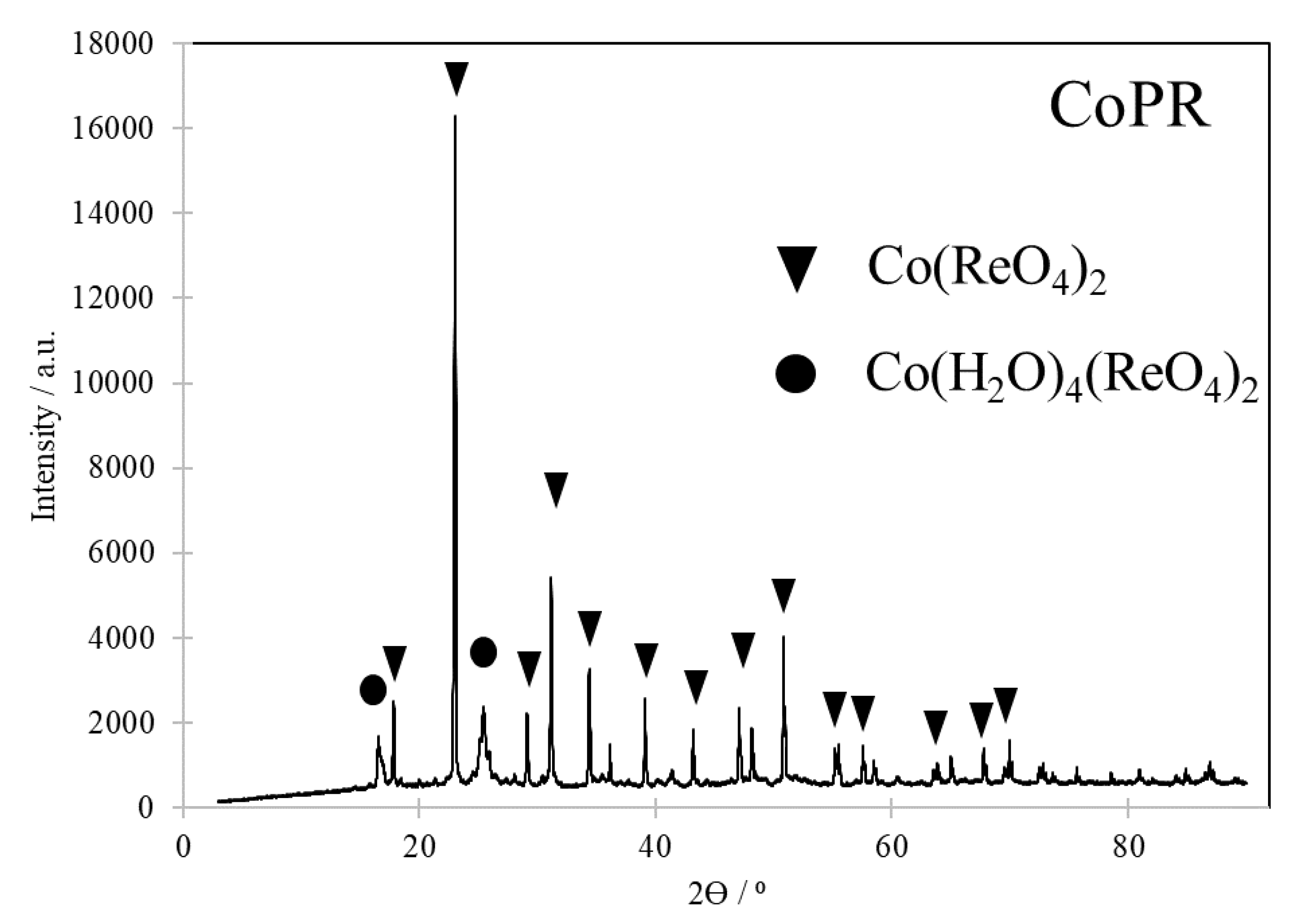
Figure 2.
CV curves of carbon matrix and Co-modified carbon.
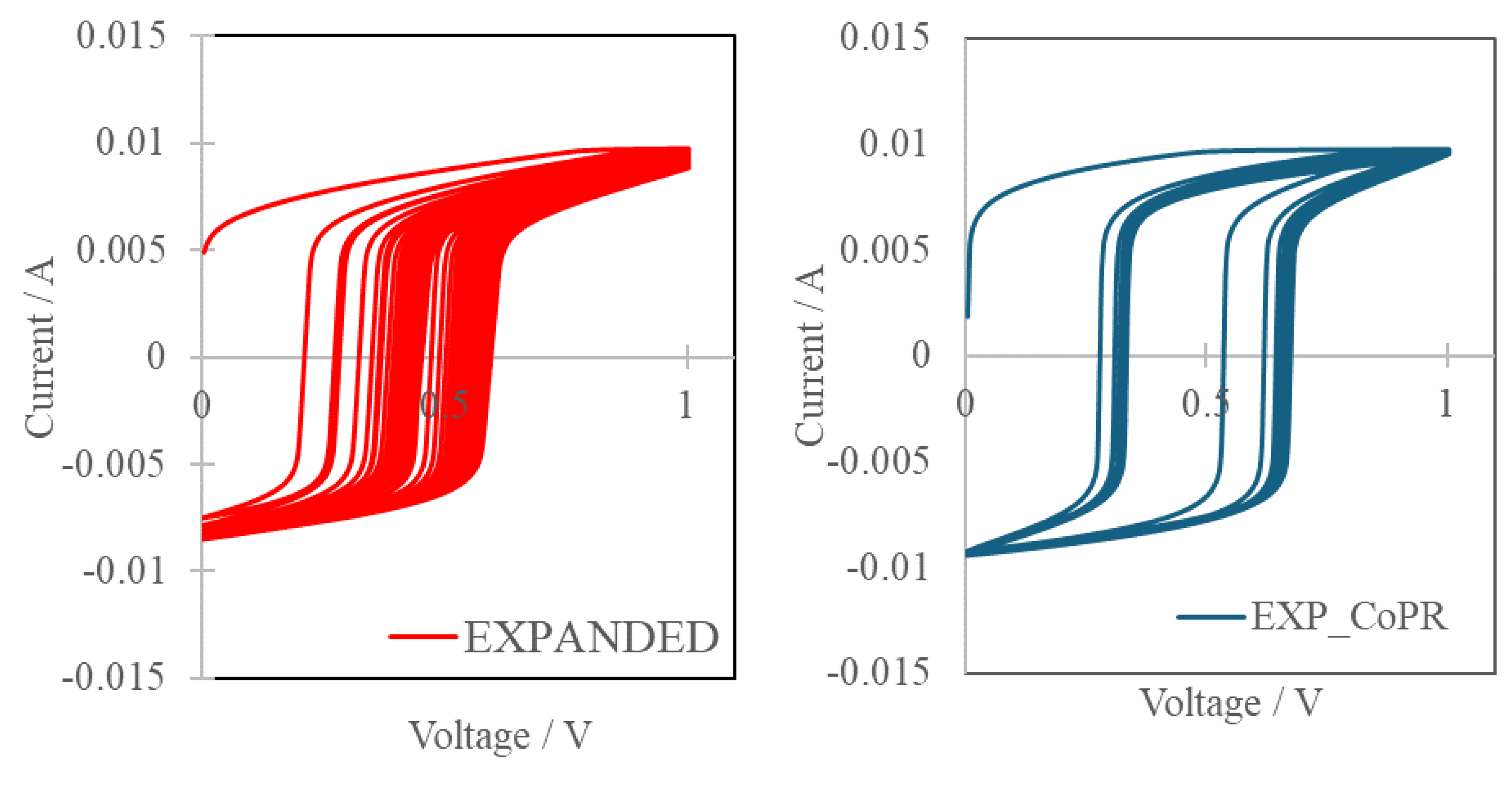
Figure 3.
GC/GD curves of carbon matrix and Co-modified carbon.
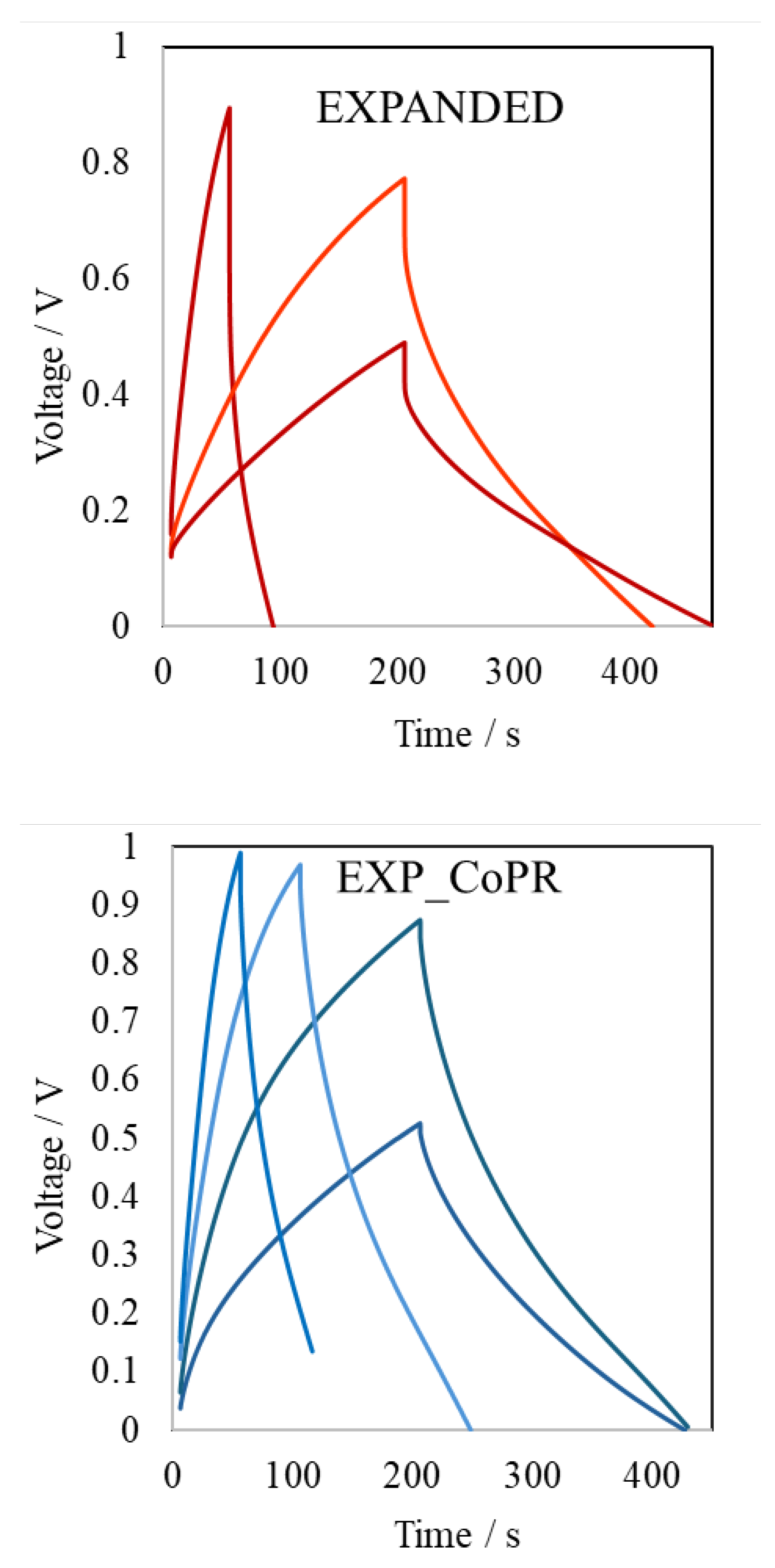
Figure 4.
Nyquist plots of carbon matrix and Co-modified carbon.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated






