1. Introduction
Australia has seen the highest extinction rate for mammals over the last 200 years, with higher impact observed on native marsupial species (Woinarski et al., 2015). Multiple reasons are considered to be the cause for the drastic decline, including environmental stressors such as climate change, heat stress, fringe effects and environmental pollutants (Narayan and Williams, 2016), as well as faunal characteristics of the animals such as body structure and unequal population distribution (McKenzie et al., 2007). In addition, the introduction of feral pest species like cats and invasive predators like foxes has also caused significant damage to native wildlife populations (Dickman, 1996; Frank et al., 2014; Norton et al., 2011; Read & Cunningham, 2010). Forest fire is another contributor to the rapid decline in population of Australian mammals, especially medium-bodied mammals which may be less efficient in finding refuge during fire events (Griffiths & Brook, 2014). After-effects of forest fires also result in marsupial decline, as decreased grass cover makes animals vulnerable to predation and decreases food availability (Lawes et al., 2015).
Marsupials play an essential role in Australian native ecosystem, aiding in many important ecological processes such as the formation of topsoil by continuous digging, dispersal of fungi and mycorrhiza seed, as well as increasing soil mineral composition (Martin, 2003). Many potoroids like long-nosed potoroo and bettongs contribute in improving the soil fertility. A direct decline in soil quality was reported in previous study with the reducing numbers of Australian small mammals (Frankham et al., 2011; Garkaklis et al., 2003).
Given the nonnegligible contribution that these species make to the ecosystem and the aforementioned challenges they face in the wild (Prendergast, 2015), researchers have become increasingly interested in improving captive wildlife managements to benefit Australian marsupial conservation. However, there are many possible constraints that can lead to compromised welfare for the animals in care. Captive environment has abiotic as well as biotic factors like odour, sound levels, lighting intensity, presence or absence of proper enrichments, presence of other dominant individuals which can cause potential stress to the animals (Morgan & Tromborg, 2007). Previous study indicated that capture, restraint, handling, increased human activities, lack of adequate cover and restricted movement in captive environment are all stressful events for wildlife (Schell et al., 2013).
Stress can be defined as a biological response to any noxious environmental stimulus or stressors that causes a disruption in the physiological system that regulates homeostasis (Novak et al., 2013). Depending on the type and intensity of stressors present in the captive environment, the stressors can either cause acute stress (short-term stress) or chronic stress (long-term stress). Acute stress can be beneficial to animals as it helps with generating an ecological response towards the stressors (e.g. prey species escaping predator (Cockrem & Silverin, 2002)). The development of chronic stress, however, can lead to long-term ailments like increased stereotypic behaviours, decreased reproductive performance and reduced immune functions (Bayazit, 2009).
When animal is exposed to a stressor, a series of reactions controlled by the Hypothalamic-pituitary-adrenal (HPA) axis will be initiated. The HPA axis facilitates the secretion of the corticotrophin releasing factor (CRF) from the hypothalamus. CRF then triggers the release of adrenocorticotrophic hormone (ACTH) from the anterior lobe of the pituitary gland (Touma & Palme, 2005). Under the effect of elevated ACTH levels, the adrenal cortex initiates the production of glucocorticoids. Glucocorticoids are responsible for causing a stress response, triggering a series of necessary physiological events, thereby helping animals effectively cope with or escape from the stressor (Narayan et al., 2013). Once the stress response has been initiated and the effect of the stressor has subsided, the body works on resuming its homeostatic balance to its original condition (Charalambous et al., 2021). The activity of the HPA axis is mediated by the concentration of glucocorticoids (cortisol in marsupials) through the negative feedback mechanism (Zschucke et al., 2015). High cortisol levels in blood circulation send a signal to the hypothalamus and the pituitary glands, leading to a reduced production of CRF and ACTH, thereby reducing the levels of cortisol, in turn aiding in maintaining the homeostatic balance of the body (Gjerstad et al., 2018). When animal encounters an acute stressor, the negative feedback mechanism works effectively. Alternatively, if the effects of an acute stressor do not cease, the stressor can get converted into a chronic stressor which may potentially disrupt the negative feedback loop (Charalambous et al., 2021).
Recent years, various methods have been developed to quantify the stress levels in animals by measuring the glucocorticoid metabolites present in body secretions and outgrowth like blood, urine, saliva, faeces, hair (Glenk et al., 2014; Narayan et al., 2012; Narayan et al., 2011; Peric et al., 2013). All the methods involved in the quantification of cortisol levels have their advantages and drawbacks. The collection of blood samples involves physical restraint and the use of sedatives, therefore can be stressful to animals (Novak et al., 2013). While the collection of saliva and urine samples is easier and causes less stress to animals, there is an apparent knowledge gap in prior researches concerning the response of salivary cortisol to different stressors. In case of the urine, the chances of contamination with the faeces should be noticed (Novak et al., 2013).
Another non-invasive method, the measurement of faecal cortisol metabolites (FCM), can be an effective way to quantify stress levels, as it requires no interaction with the animals, striking out the possibility of causing undue stress to animals due to human interactions (Narayan et al., 2012; Palme, 2019). In this study, we quantified faecal glucocorticoid profiles of three Australian marsupial species which belong to the families Potoroidia and Thylacomyidae (Swinston et al., 2020). Due habitat destruction and predation, 8 species from family Potoroidia have gone extinct and the long-nose potoroo is considered as a vulnerable according to IUCN (Frankham et al., 2011). In terms of the bilbies, there has been very limited discussion about the quantification of stress levels, as only a handful of studies have been conducted. (Evans et al., 2013; Narayan et al., 2012; Narayan et al., 2014).
Stress levels can act as an indicator of the welfare status of the animals, and quantifying stress levels can help researchers gain insights on the effectiveness of the management practices in a non-invasive manner (Narayan et al., 2012). In this study, we aim to determine the variations in FCM levels of marsupials through time and present absolute levels across time, sex, age, groupings and housing arrangement for each species. This pilot study will also determine the baseline levels of circulating cortisol by using the iterative baseline approach.
2. Materials and Methods
2.1. Approval
Research was performed in accordance with relevant guidelines and regulations. Formal approval was granted by The University of Queensland Ethics (ACEC) Committee and the research committee at the Hidden Vale Wildlife Centre (approval number: 2022/AE000635).
2.2. Study Site, Animals, Sample Size and Husbandry Practices
The research study was conducted in collaboration with the Hidden Vale Wildlife Centre located in 617 Grandchester Mount Mort Road, Grandchester, Queensland 4340 (GPS coordinates: 27° 43' 0.34'' S, 152° 27' 46'' E). A total of three different species namely Rufous bettongs (Aepyprymnus rufescens) (n=9), Greater bilby (Macrotis lagotis) (n= 5), Long-nosed potoroo (Potorous tridactylus) (n=4) were admitted at the Hidden vale wildlife centre and were sampled in this study to determine the circulating cortisol levels. Animals were housed in various captive settings. Bettongs were housed separately at the outdoor aviaries with the dimensions of (24m x 6m x 7m) which was separated by a partition. Potoroos were housed in pairs in the same type of outdoor aviaries as bettongs. Two pairs of potoroos (one female pair and one breeding pair) were involved in this study. Bilbies were housed separately in small mammal enclosures with the dimensions of (2.5m x 4m x 2m). Animals were fed during the evenings as majority of them were nocturnal. Enclosures were cleaned daily. Regular health check-ups were conducted to evaluate the health condition of the animals.
2.3. Faecal Sample Collection and Storage
Faecal samples were collected from all captive settings on a fixed weekly basis (every Friday at 9:00am) for the duration of 3 months to ensure uniformity in the results and eliminate any possible variations that may occur due to the difference in timings. The enclosures were cleaned daily, allowing the collection of fresh faecal samples. Only visually fresh-looking samples were collected and stored in Ziplock© bags, labelled with the name sex and species of the individual, number of individuals, date, and time of collection. After collection, the samples were immediately transferred to a frozen container until they were transported to the freezers. Samples were stored in freezers at −20°C until further processing and analysis were conducted. For potoroos housed in a paired setting, identification of individual samples was not feasible. To minimise the bias of collection from one individual, faecal sample from the entire enclosure were collected.
2.4. Faecal Sample Processing and Hormone Extraction
Frozen faecal samples were dried in the ovens at 65°C for 24-48 hrs. Once completely dry, the samples were ground manually using a mortar and pestle. 0.1g of the crushed samples were weighted out, transferred into labelled tubes and then stored in a −20°C freezer for further analysis.
The cortisol metabolites extraction from the samples was completed using aqueous 90% ethanol. 1mL of 90% ethanol was added to 0.1g of samples in a test tube. Tubes were then vortexed at medium speed and placed into a water bath (>60°C) for 15 minutes. Then the samples were centrifuged at 2800 rpm for approximately 20 mins using IKA Lab Dancer Vortex and the supernatants were transferred into Eppendorf tubes. The remaining solid faecal residue were suspended for the second time in 2.5mL aqueous 90% ethanol, vortexed, re-centrifuged and the supernatants were transferred into new, clean Eppendorf tubes. Both the supernatants obtained were dried in a fume hood until the alcohol was dried out and the extracted particles were attached to the tube walls. Then the attached particles were reconstituted with 1 mL of assay buffer (39 mM NaH2PO4, 15 mM NaCl and 0.1% bovine albumin, pH 7.0) to be used for further analysis. In order to mix the ethanol extract and the assay buffer, tubes were vortexed using an Eppendorf mini-spin centrifuge for at least 30 s.
2.5. Hormone Analysis
The hormonal analysis of the FCM was carried out by employing a polyclonal anticortisol antiserum (1:15,000), horseradish peroxide (HRP), cortisol standards and conjugated cortisol label diluted to 1:80,000. The extracted samples were assayed in duplicates using the Nunc Maxisorp™ (96 wells). 12 hours prior to the analysis, the plates were coated with diluted cortisol antibody at 4℃. After the incubation, the plates were washed with a plate washing buffer (phosphate-buffered saline along with 0.5 ml/l Tween 20) in an automated plate washer. For all the assays, 50µl of the diluted faecal samples, standard solutions along with the binding internal controls were added into the plate wells. All the wells were loaded with 50µl of HRP before incubating the plate for 2hrs in room temperature. The incubated plates were then washed with wash buffer, 50µl of substrate buffer was added to each well (0.01% tetramethylbenzidine (TMB), hydrogen peroxide and 0.1M acetate citric acid buffer, with the pH of solution maintained at 6.0) and left to incubate for 15-30 minutes. A stopping solution (50 µl of 0.5 mol/l sulphuric acid) was used to stop the reaction based on the colour of the zero wells to obtain an optical density (OD) of 0.8-0.9. Plates were read by an automated microplate plate reader at 450nm with reference readings at 630nm.
2.6. Biological and Laboratory Validation
For biological validation, in the absence of the availability of an ACTH challenge, we relied on the life-history traits of individuals for each species to determine whether the FCM assay was able to differentiate between biological states (e.g. different levels stress loads between males and females of aseasonal species). The absence of an ACTH challenge in our research was due to constraints in animal resources, which influenced our ability to carry out complex procedures such as the ACTH challenge, as well as time limitations and the concurrent involvement of the studied animals in breeding programs in the Hidden Vale Wildlife Centre. While an ACTH challenge could have provided valuable insights, due to these limitations, we prioritized other aspects of our research methodology while ensuring the welfare of the animals involved.
The laboratory validation of the Enzyme-immuno assay (EIA) was demonstrated by making a parallelism between the serially diluted faecal extracts obtained from the pool of faeces for all the above-mentioned species. In this study, we have conducted the validation for the Rufous bettongs and the Long–nosed potoroo.
2.7. Determining Baseline and Peak FCM Concentrations
An iterative baseline approach, as described by Fanson et al. (2017), was used to detect the baseline levels of all the species included in the study. The mean and standard deviation (SD) was calculated using Microsoft Excel 2021©. All the values greater than ‘(mean + 2SD)’ were excluded, and the process of iteration was repeated until all the values were within ‘(mean + 2SD)’. For the analyses of the baselines of potoroos and bettongs, all values exceeding ‘(mean + 2SD)’ were considered as hormonal peaks, whereas in the case of bilbies, we considered all the values exceeding ‘(mean + 1.5SD)’ due to the smaller sample size and the need of ensuring that all the individual peaks were identified accurately (Cope et al., 2022). All the values exceeding ‘(mean + 2SD)’ (for bettongs and potoroos) and ‘(mean + 1.5SD)’ (for bilbies) were then averaged to calculate the peak means for individuals and groups of animals. Peak means were considered to be related with acute stress response.
2.8. Statistical Analysis
Statistical analysis was conducted using the GraphPad Prism. Descriptive statistics was employed to calculate the mean± standard deviation in the FCM values for each individual or groups (Male/female; female/breeding pair). A normality & lognormality test was performed to determine the distribution of data. An ANOVA test was employed to assess the level of significance of the FCM levels between multiple individuals (P<0.05). A t-test was performed to determine the presence of any significant differences (P<0.05) between two individuals or groups. Line charts and scattered dot charts were used to represent the change in the FCM levels over the period of data collection and the range of FCM values for different species respectively.
3. Results
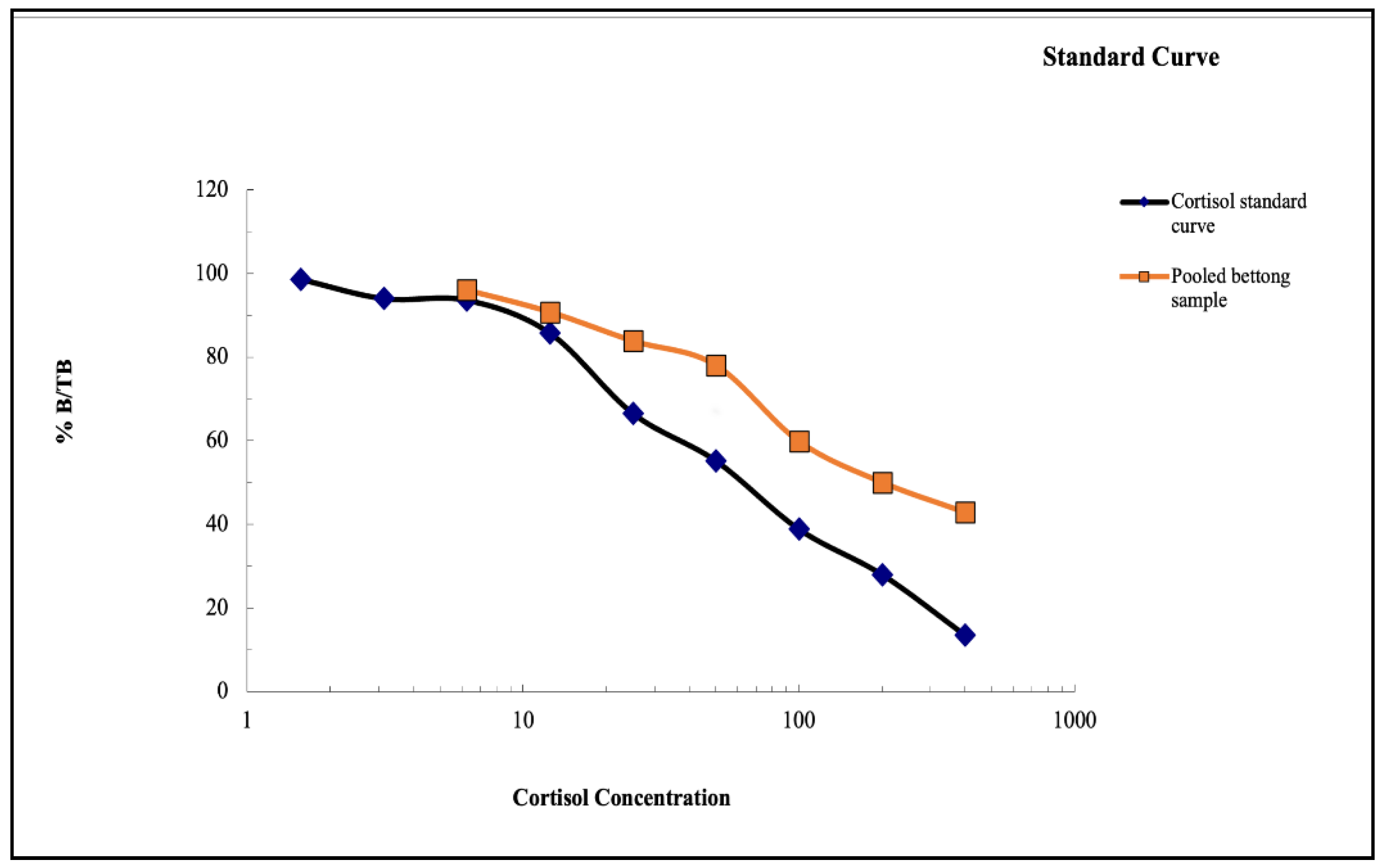
3.1. Laboratory Validation
The serial dilution from the pooled faecal extracts of both the long-nosed potoroo and Rufous bettongs showed a parallel displacement with the cortisol standard curves. (See
Figure 1 and
Figure 2).
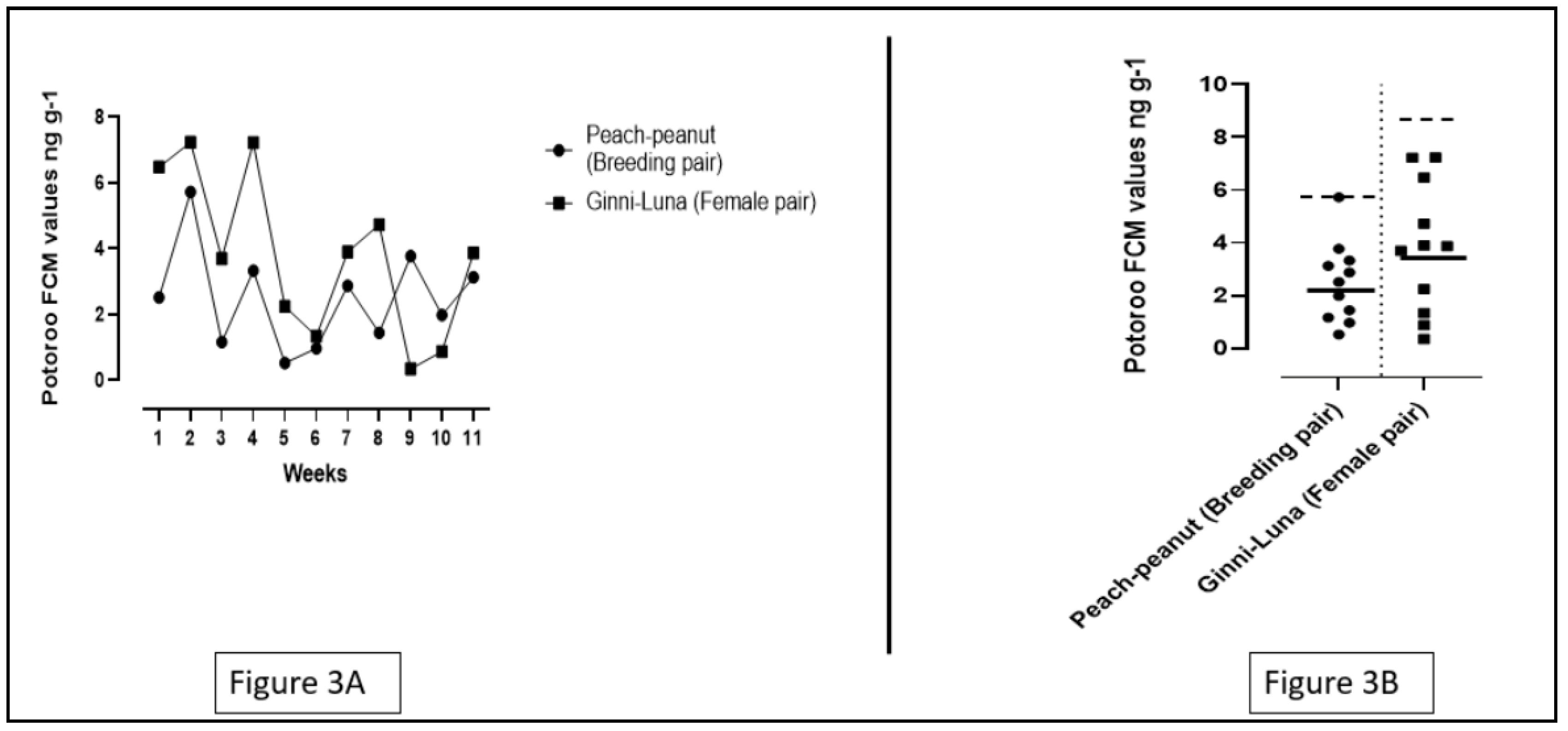
3.2. Comparing the FCM Values and the Mean Baselines in Two Pairs of Potoroos
Data was collected from two pairs of potoroos during a 11-week sample collection period. Presented data suggested that for the breeding pair, the FCM values ranged from 0.525 to 5.725 ng/g, and the mean FCM value was 2.492 ± 1.506 ng/g. For the female pair, the FCM values was in the range of 0.345 to 7.235 ng/g, and the mean FCM value was 3.813 ± 2.461 ng/g (See
Figure 3A). Results from the t-test displayed that no significant differences (T=1.51; p=0.1447; p>0.05) were observed between the mean FCM levels of the breeding pair and the female pair.
Results from the iterative baseline approach depicted a mean baseline of 2.169 ± 1.115 ng/g for the breeding pair. However, the values for the female pair showed no hormonal peaks as no differences were observed in the baseline values and the mean FCM values. Results from the t-test for the baseline values showed no significant differences (T=1.93; p=0.067, p>0.05) between the breeding pair and the female pair (See
Figure 3B). The peak mean values for the breeding pair and female pair were 5.75 ng/g and 8.73 ng/g respectively.
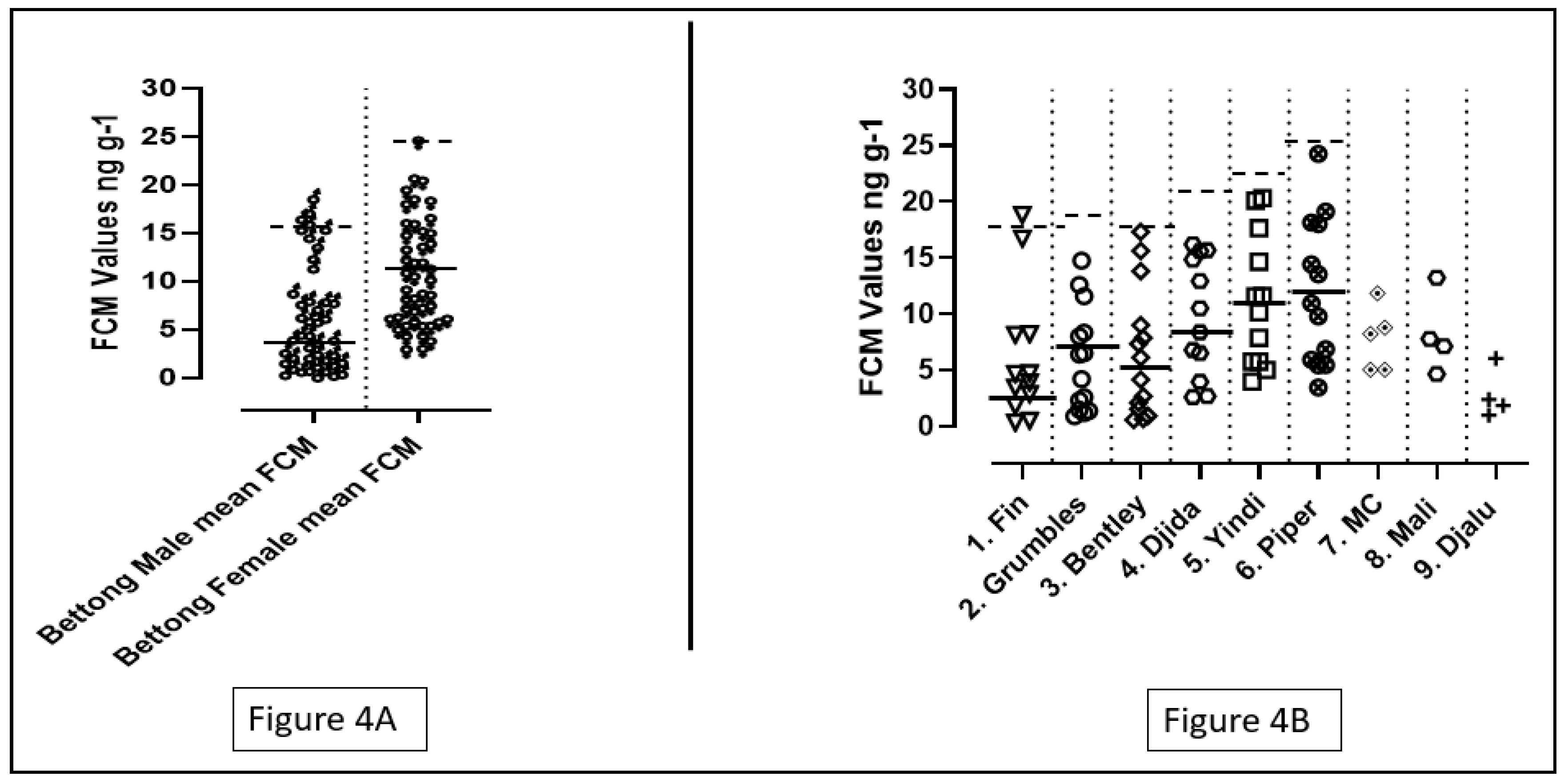
3.3. Comparing the FCM Values and the Mean Baselines in Nine Bettongs
In this study, data was collected from 9 bettong individuals numbered in sequence from 1 to 9. Number 1,2,3,9 were male bettongs and number 4,5,6,7,8 were female bettongs. The FCM values of the four male bettongs ranged from 0.32 to 18.81 ng/g, whereas the FCM values of the five female bettongs ranged from 2.59 to 24.26 ng/g. The male bettongs had a mean FCM value of 6.35 ± 5.55 ng/g and the mean FCM value of female bettongs was 10.40 ± 5.50 ng/g. A t-test was applied to compare the values of male bettongs with the values of their female counterparts, and the results depicted a significant difference in the mean FCM values (p = 0.0002; p<0.05). A significant difference (p=0.014; p<0.05) was also observed on performing an ANOVA test on the individual FCM values of all the bettongs in this study.
Data from 6 bettongs (Number 1-6) were used for the calculation of the individual baseline values. Individuals 7-9 were eliminated from the calculation due to the low number of data points. FCM values of individuals 1-7 were used to calculate the mean baseline values for the male and female bettongs respectively. The male bettongs had a mean baseline value of 4.117 ± 3.155 ng/g. The female bettongs had a mean baseline of 10.27± 5.271 ng/g (See
Figure 4A). Peak means for the male and female bettongs were 15.6±2.08 ng/g and 24.25 ng/g respectively. A significant difference was observed between the baseline levels of male and female bettongs after performing a t-test (p=0.0001; p<0.05). Results of an ANOVA test suggested that there was also a significant difference in the individual baseline values of the bettongs (p=0.0018; p<0.05) (See
Figure 4B).
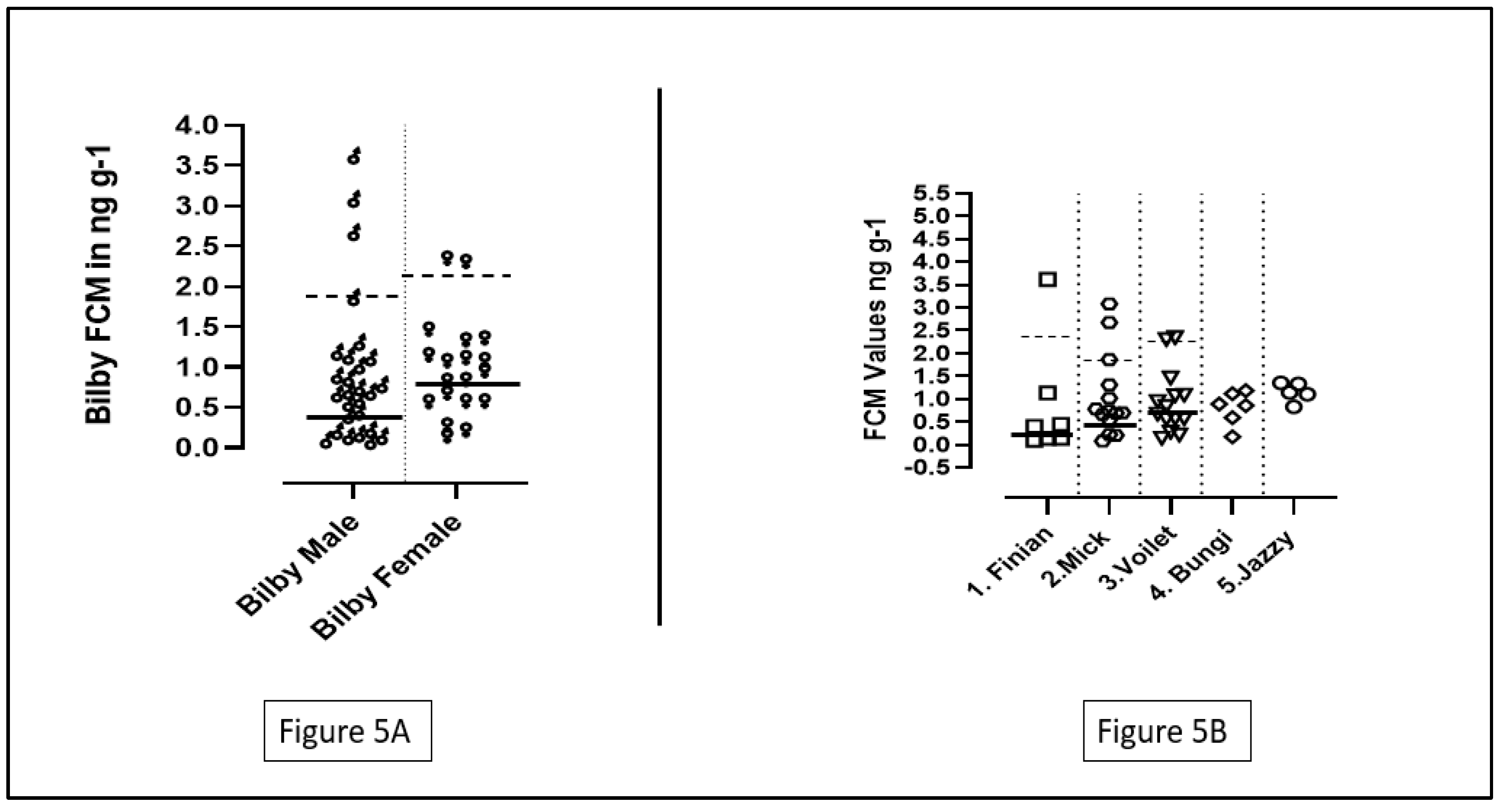
3.3. Comparing the FCM Values and the Mean Baselines in Five Bilbies
A total of 5 bilbies were studied and named from 1 to 5. Number 1,2,4 were males and numbers 3,5 were females. The FCM values of male bilbies ranged from 0.084 to 3.61 ng/g, whereas the females had their FCM values ranging from 0.140 to 2.34 ng/g. The mean FCM value for male bilbies was 0.94 ± 0.90 ng/g, and for females the mean FCM value was 0.99 ± 0.60 ng/g. No significant difference was found between the individual FCM values of the bilbies after performing an ANOVA test (p=0.93; p>0.05). Additionally, the results for the t-test indicated no significant differences between the FCM values of the male and the female bilbies (T=0.20; p=0.83; p>0.05).
Although data collected from all the bilbies were included for the calculation of the mean baselines for the male and female bilbies, only data collected from individuals 1,2,3 were used for the calculation of the individual baselines. Individuals 4 and 5 were excluded from the calculation due to the low number of data points (See
Figure 5B).
Male bilbies had a mean baseline in the range of 0.46 ± 0.26 ng/g while the females had a mean baseline of 0.79 ± 0.37 ng/g (See
Figure 5A). The peak means for males and females were 1.88 ± 0.98 ng/g and 2.03 ± 0.50 ng/g respectively. The result of a t-test suggested that there was a significant difference between the mean baseline FCM values of the male and female bilbies (T=2.83; p=0.0077; p<0.05). However, no significant difference was observed in the baseline FCM values of the individuals 1-3 on performing an ANOVA test (P=0.05; significance is considered at p<0.05).
4. Discussion
In this study, we validated the R4866 EIA for Long-nosed Potoroos and Rufous bettongs. We also presented absolute FCM levels and the baseline levels of all the three marsupial species included, along with detecting and presenting the variations created by factors like sex, grouping and housing conditions.
In this study, no significant difference was observed between the mean FCM levels of the breeding pair and the female pair of potoroos. However, previous research suggests that the social environment has a significant impact on the HPA-axis of the animals, therefore can affect stress levels (Creel et al., 2013). Studies done on another marsupial species, Grey short-tailed opossums, have shown the activation of the female reproductive system in the presence of a male (Fadem, 1985). Additionally, studies conducted on some eutherian species like rats and guinea pigs have found that pairing males and females of the same species can help them better cope with environmental stressors than solitary animals (Sachser et al., 1998; Westenbroek et al., 2005). One possible explanation for the fact that the FCM levels and baseline values of the female pair were not significantly different from the breeding pair in this study could be the limited sample size. Another limitation of the study is that since the potoroos were housed in pairs, quantifying the individual stress levels for the potoroos was not possible. The effect of pairing on the individual FCM levels can be measured by using faecal markers or food dyes as done by Narayan et al. (2012), which might help researchers better understand the effect of pairing in future studies.
There were significant differences in the individual baseline and FCM levels in bettongs, which can be associated to the fact that stress responses are known to display individual differences due to multiple factors like age, physiological state, previous experiences, environmental variables (Hogan et al., 2012). Female bettongs in this study had a significantly higher FCM and baseline value as compared to their male counterparts. Similar patterns were also observed in other marsupial species such as woylies (Hing et al., 2017) and southern brown bandicoots (Dowle et al., 2012). The presences of these differences can be associated with the difference in the endocrine response or the metabolism and excretions of glucorticoids in different gender (Hing et al., 2017; Jensen et al., 2019). Another probable reason for the significant differences lies in the reproductive biology of bettongs. Rufous bettongs are known to breed continuously in captive environments (Claridge et al., 2007; Frederick & Johnson, 1996), and the bettongs at the Hidden Vale Wildlife Centre were involved in continuous breeding programmes. Mating and pregnancy have shown to cause an increase in the adrenal activity in female (Keeley et al., 2012). Having an annual breeding cycle compels the females to make necessary adaptations like increasing foraging pattern and body fat reserves to maintain homeostasis (Bronson, 1985). These strategies pose a higher energic requirement on continuous breeders and lead to the increased activity, higher probability of encountering stressors, ultimately result in the increased stress levels (Bronson, 1985; Dowle et al., 2012).
Amongst all the bettongs, individual 6 (Piper) and individual 1 (Fin) showed the highest and the lowest baseline levels respectively. Multiple factors can be associated with this fact. The amount of time spent in captivity can significantly impact the way in which animals respond to stressors (Hogan et al., 2012). Even though both the individuals were born in the same year (2020), Fin have spent more time in a captive setting than Piper, which might be a possible explanation to the lower baseline level. Fin is a male and Piper is a female bettong, and as mentioned earlier, continuous breeding exerts a higher energetic demand on females, thereby leading to a higher hormonal response (Dowle et al., 2012). Presence of health conditions like diseases or injuries can be stressful and cause significant hike in the baseline stress levels (Bayazit, 2009). The possibility of Piper having diseases or injuries cannot be excluded. One significant hormonal peak was detected in individual 1(Fin). This could be due to the presence of the harsh environmental factors like high temperature and rainfall (Hogan et al., 2012; Hing et al., 2014) as bettongs were housed in open aviaries. Handling for a routine check-up was also a normal protocol at the Hidden Vale Wildlife Centre, which makes it a probable reason for the observed peak.
The baseline values of male bilbies were significantly lower than values of females. Similar to bettongs, bilbies have also been known to breed throughout the year in captivity (Berris et al., 2019). Except high energy demanding and increased chances of encountering stressors (Bronson, 1985; Dowle et al., 2012; Keeley et al., 2012), pregnancy and presence of pouch young might have also led to a slightly higher baseline in individual 3 as compared to individuals 1 and 2. Other primary stressors in captivity including environmental factors, handling, animal interaction and injury/disease (Hing et al., 2014). Chances of environmental variables causing the spike is highly unlikely as bilbies were housed in a covered temperature regulated facility. As for individual 1, animal interactions would likely not have caused the stress responses as he was kept in a separate enclosure. Handling might be a convincing explanation as human handling does induce stress to animals even if they are well acquainted to captivity (Morgan & Tromborg, 2007). In case of individual 2 & 3, animal interaction might have led to hormonal spikes as they were part of a breeding program and were housed together during the initial weeks of the study. As individual 3 was pregnant and had a pouch young at the later weeks of the study, handling for routine check-up was extremely necessary and may have caused the hormonal spike. Handling may have also caused spike in individual 2 as he was shifted to different enclosures couple of times.
One of the reasons for no significant individual differences for bilbies can be associated to the nature of samples collected (Jensen et al., 2019), and the availability of the bilbies at the centre. Individual 1 was transported from Hidden Vale to another centre, restricted our ability to continue collecting his samples. Individual 4 & 5 were added to the study after the 10th week of sample collection. All these reasons might have reduced the sensitivity of the statistical tools.
Since the time of acquisition, history of the animal, health status and husbandry information were not available, outlining the exact cause of the observed baseline levels was not possible, which could act as a limitation of this study. As per the observations made during the sample collection, the above-mentioned reasons might be the closest explanations to the observed acute stress responses for the respective species. This study aims at providing insights into the response of studied animals to stressors within captive environments. Furthermore, this study is also geared towards providing a foundation for future investigations pertaining to stress levels exhibited by these animals in their natural habitats, thereby contributing to the development of comprehensive strategies for animal rewilding initiatives.
5. Conclusions
The aims of this study included validating the R4866 assay for the Long-nose potoroos and Rufous Bettongs, quantifying FCM concentrations in Rufous bettongs, Long-nose potoroo and Greater bilby, and measuring the baseline FCM levels and peak FCM means in all the three species. It was hypothesised that there would be measurable variation observed in the FCM levels of the studied marsupial species with regards to their sex, age, groupings/housing arrangement and sampling period. Statistically, regarding bettongs, significant differences were noted in FCM and baseline levels among individuals and between male and female groups. Potoroos showed no significant difference in FCM and baseline levels between females and breeding pairs. Bilbies exhibited no significant difference in individual FCM and baseline levels, but significant difference was found in baseline levels between the two bilby groups. This study reported a whole range of variations observed in the FCM levels due to factors like sex, housing status and groupings in the studies species. These variations could be caused by factors like social buffering, reproductive biology, human-animal interaction, animal-animal interactions, diseases or injury. This study seeks to delineate the stressors affecting these species the most in a captive environment, in turn helping wildlife managers make practical recommendations to improve the welfare of these species in captivity.
This study was restricted by the relatively limited sample size and limited quantification of individual FCM levels in certain species. Future study should require more accurate and detailed data collection by using advanced technologies such as faecal markers or food dyes to understand the effect of pairing on the individual FCM levels. Furthermore, since management records like the time of acquisition, history of the animal and health were not available, the exact cause of the observed baseline levels was not analysed in this study. Future studies should focus on comparing the laboratory results with management records obtained from wildlife centres to identify the potential stressors in the centres, improve captive wildlife management strategies and improve the quality of life of animals under rehabilitation or involved in the conservation programmes.
Supplementary Materials
The following are available online at Preprints.org, Supplementary file 1.
Author Contributions
Conceptualization, E.N. and H.P.; methodology, E.N. and H.P.; formal analysis, E.N. and H.P.; investigation, E.N. and H.P; resources, E.N.; data curation, E.N. and H.P.; writing—original draft preparation, H.P.; writing—review and editing, E.N. and H.P; supervision, E.N.; project administration, E.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of The University of Queensland (approval number: AE000045).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or supplementary material.
Acknowledgments
We thank the staff at HiddenVale Wildlife Centre for initial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bayazit, V. Evaluation of cortisol and stress in captive animals. Australian Journal of Basic and Applied Sciences 2009, 3, 1022–1031. [Google Scholar]
- Berris, K.K.; Cooper, S.J.; Breed, W.G.; Carthew, S.M. Timing of breeding and female fecundity of the greater bilby (Macrotis lagotis) in the temperate zone of South Australia: Implications for translocations of a previously widespread species. Wildlife Research 2019, 46, 444–453. [Google Scholar] [CrossRef]
- Bronson, F.H. Mammalian reproduction: An ecological perspective. Biology of Reproduction 1985, 32, 1–26. [Google Scholar] [CrossRef]
- Charalambous, R.; Simonato, T.; Peel, M.; Narayan, E.J. Physiological stress in rescued wild koalas (Phascolarctos cinereus) being held in a rehabilitation sanctuary: A pilot study. Animals 2021, 11, 2864. [Google Scholar] [CrossRef]
- Claridge, A.W.; Seebeck, J.H.; Rose, R. 2007. Bettongs, potoroos and the musky rat-kangaroo. CSIRO Publishing Melbourne.
- Cockrem, J.; Silverin, B. Sight of a predator can stimulate a corticosterone response in the great tit (Parus major). General and Comparative Endocrinology 2002, 125, 248–255. [Google Scholar] [CrossRef]
- Cope, H.R.; Keeley, T.; Keong, J.; Smith, D.; Silva, F.R.; Mcarthur, C.; Webster, K.N.; Mella, V.S.; Herbert, C.A. Validation of an enzyme immunoassay to measure faecal glucocorticoid metabolites in common brushtail possums (Trichosurus vulpecula) to evaluate responses to rehabilitation. Animals 2022, 12, 1627. [Google Scholar] [CrossRef]
- Creel, S.; Dantzer, B.; Goymann, W.; Rubenstein, D.R. The ecology of stress: Effects of the social environment. Functional ecology 2013, 27, 66–80. [Google Scholar] [CrossRef]
- Dickman, C.R. Impact of exotic generalist predators on the native fauna of Australia. Wildlife Biology 1996, 2, 185–195. [Google Scholar] [CrossRef]
- Dowle, M.; Webster, K.N.; Deane, E. Faecal glucocorticoid metabolite concentrations in the free-ranging bandicoots (Perameles nasuta and Isoodon obesulus) of northern Sydney. Australian Mammalogy 2012, 35, 1–7. [Google Scholar] [CrossRef]
- Evans, N.; Narayan, E.J.; Hero, J.-M. Effects of natural weathering conditions on faecal cortisol metabolite measurements in the greater bilby (Macrotis lagotis). Australian Journal of Zoology 2013, 61, 351–356. [Google Scholar] [CrossRef]
- Fadem, B.H. Evidence for the activation of female reproduction by males in a marsupial, the gray short-tailed opossum (Monodeiphis domestica). Biology of Reproduction 1985, 33, 112–116. [Google Scholar] [CrossRef]
- Fanson, K.V.; Best, E.C.; Bunce, A.; Fanson, B.G.; Hogan, L.A.; Keeley, T.; Narayan, E.J.; Palme, R.; Parrott, M.L.; Sharp, T.M. One size does not fit all: Monitoring faecal glucocorticoid metabolites in marsupials. General and Comparative Endocrinology 2017, 244, 146–156. [Google Scholar] [CrossRef]
- Frank, A.S.; Johnson, C.N.; Potts, J.M.; Fisher, A.; Lawes, M.J.; Woinarski, J.C.; Tuft, K.; Radford, I.J.; Gordon, I.J.; Collis, M.A. Experimental evidence that feral cats cause local extirpation of small mammals in Australia's tropical savannas. Journal of Applied Ecology 2014, 51, 1486–1493. [Google Scholar] [CrossRef]
- Frankham, G.J.; Reed, R.L.; Fletcher, T.P.; Handasyde, K.A. Population ecology of the long-nosed potoroo (Potorous tridactylus) on French Island, Victoria. Australian Mammalogy 2011, 33, 73–81. [Google Scholar] [CrossRef]
- Frederick, H.; Johnson, C. Social organisation in the rufous bettong, Aepyprymnus rufescens. Australian Journal of Zoology 1996, 44, 9–17. [Google Scholar] [CrossRef]
- Garkaklis, M.J.; Bradley, J.; Wooller, R. The relationship between animal foraging and nutrient patchiness in south-west Australian woodland soils. Soil Research 2003, 41, 665–673. [Google Scholar] [CrossRef]
- Gjerstad, J.K.; Lightman, S.L.; Spiga, F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress 2018, 215, 403–416. [Google Scholar] [CrossRef]
- Glenk, L.M.; Kothgassner, O.D.; Stetina, B.U.; Palme, R.; Kepplinger, B.; Baran, H. Salivary cortisol and behavior in therapy dogs during animal-assisted interventions: A pilot study. Journal of Veterinary Behavior 2014, 9, 98–106. [Google Scholar] [CrossRef]
- Griffiths, A.D.; Brook, B.W. Effect of fire on small mammals: A systematic review. International Journal of Wildland Fire 2014, 23, 1034–1043. [Google Scholar] [CrossRef]
- Hing, S.; Narayan, E.; Thompson, R.A.; Godfrey, S. A review of factors influencing the stress response in Australian marsupials. Conservation Physiology 2014, 2, cou027. [Google Scholar] [CrossRef]
- Hing, S.; Northover, A.S.; Narayan, E.J.; Wayne, A.F.; Jones, K.L.; Keatley, S.; Thompson, R.C.; Godfrey, S.S. Evaluating stress physiology and parasite infection parameters in the translocation of critically endangered woylies (Bettongia penicillata). EcoHealth 2017, 14, 128–138. [Google Scholar] [CrossRef]
- Hogan, L.A.; Lisle, A.T.; Johnston, S.D.; Robertson, H. Non-invasive assessment of stress in captive numbats, Myrmecobius fasciatus (Mammalia: Marsupialia), using faecal cortisol measurement. General and Comparative Endocrinology 2012, 179, 376–383. [Google Scholar] [CrossRef]
- Jensen, M.A.; Moseby, K.E.; Paton, D.C.; Fanson, K.V. Non-invasive monitoring of adrenocortical physiology in a threatened Australian marsupial, the western quoll (Dasyurus geoffroii). Conservation Physiology 2019, 7, coz069. [Google Scholar] [CrossRef]
- Keeley, T.; O’brien, J.; Fanson, B.; Masters, K.; Mcgreevy, P. The reproductive cycle of the Tasmanian devil (Sarcophilus harrisii) and factors associated with reproductive success in captivity. General and Comparative Endocrinology 2012, 176, 182–191. [Google Scholar] [CrossRef]
- Lawes, M.J.; Murphy, B.P.; Fisher, A.; Woinarski, J.C.; Edwards, A.C.; Russell-Smith, J. Small mammals decline with increasing fire extent in northern Australia: Evidence from long-term monitoring in Kakadu National Park. International Journal of Wildland Fire 2015, 24, 712–722. [Google Scholar] [CrossRef]
- Martin, B.G. The role of small ground-foraging mammals in topsoil health and biodiversity: Implications to management and restoration. Ecological Management & Restoration 2003, 4, 114–119. [Google Scholar]
- Mckenzie, N.; Burbidge, A.; Baynes, A.; Brereton, R.; Dickman, C.; Gordon, G.; Gibson, L.; Menkhorst, P.; Robinson, A.; Williams, M. Analysis of factors implicated in the recent decline of Australia's mammal fauna. Journal of Biogeography 2007, 34, 597–611. [Google Scholar] [CrossRef]
- Morgan, K.N.; Tromborg, C.T. Sources of stress in captivity. Applied Animal Behaviour Science 2007, 102, 262–302. [Google Scholar] [CrossRef]
- Narayan, E.J.; Hero, J.-M.; Evans, N.; Nicolson, V.; Mucci, A. Non-invasive evaluation of physiological stress hormone responses in a captive population of the greater bilby Macrotis lagotis. Endangered Species Research 2012, 18, 279–289. [Google Scholar] [CrossRef]
- Narayan, E.J.; Cockrem, J.F.; Hero, J.-M. Urinary corticosterone metabolite responses to capture and captivity in the cane toad (Rhinella marina). General and Comparative Endocrinology 2011, 173, 371–377. [Google Scholar] [CrossRef]
- Narayan, E.J.; Evans, N.; Hero, J.-M. Monitoring physiological stress in semi-free ranging populations of an endangered Australian marsupial, the Greater Bilby (Macrotis lagotis). European journal of wildlife research 2014, 60, 727–735. [Google Scholar] [CrossRef]
- Narayan, E.J.; Webster, K.; Nicolson, V.; Mucci, A.; Hero, J.-M. Non-invasive evaluation of physiological stress in an iconic Australian marsupial: The Koala (Phascolarctos cinereus). General and Comparative Endocrinology 2013, 187, 39–47. [Google Scholar] [CrossRef]
- Narayan, E.J.; Williams, M. Understanding the dynamics of physiological impacts of environmental stressors on Australian marsupials, focus on the koala (Phascolarctos cinereus). BMC zoology 2016, 1, 1–13. [Google Scholar] [CrossRef]
- Norton, M.A.; French, K.; Claridge, A.W. Habitat associations of the long-nosed potoroo (Potorous tridactylus) at multiple spatial scales. Australian Journal of Zoology 2011, 58, 303–316. [Google Scholar] [CrossRef]
- Novak, M.A.; Hamel, A.F.; Kelly, B.J.; Dettmer, A.M.; Meyer, J.S. Stress, the HPA axis, and nonhuman primate well-being: A review. Applied Animal Behaviour Science 2013, 143, 135–149. [Google Scholar] [CrossRef]
- Palme, R. Non-invasive measurement of glucocorticoids: Advances and problems. Physiology & behavior 2019, 199, 229–243. [Google Scholar]
- Peric, T.; Comin, A.; Corazzin, M.; Montillo, M.; Cappa, A.; Campanile, G.; Prandi, A. Hair cortisol concentrations in Holstein-Friesian and crossbreed F1 heifers. Journal of dairy science 2013, 96, 3023–3027. [Google Scholar] [CrossRef]
- Prendergast, K. Australia’s digging mammals as ecosystem engineers: Implications for conservation and ecosystem restoration. The Western Australian Naturalist 2015, 30, 59–81. [Google Scholar]
- Read, J.L.; Cunningham, R. Relative impacts of cattle grazing and feral animals on an Australian arid zone reptile and small mammal assemblage. Austral Ecology 2010, 35, 314–324. [Google Scholar] [CrossRef]
- Sachser, N.; Dürschlag, M.; Hirzel, D. Social relationships and the management of stress. Psychoneuroendocrinology 1998, 23, 891–904. [Google Scholar] [CrossRef]
- Schell, C.J.; Young, J.K.; Lonsdorf, E.V.; Santymire, R.M. Anthropogenic and physiologically induced stress responses in captive coyotes. Journal of Mammalogy 2013, 94, 1131–1140. [Google Scholar] [CrossRef]
- Swinston, P.; Johnston, S.D.; O’hara, P.; Englebright, R.; Keeley, T. Preliminary evaluation of urinary cytology and running wheel activity to detect oestrus and the effect of daily handling on breeding success of fat-tailed dunnarts Sminthopsis crassicaudata. Reproduction, Fertility and Development 2020, 32, 1108–1115. [Google Scholar] [CrossRef]
- Touma, C.; Palme, R. Measuring fecal glucocorticoid metabolites in mammals and birds: The importance of validation. Annals of the New York Academy of Sciences 2005, 1046, 54–74. [Google Scholar] [CrossRef]
- Westenbroek, C.; Snijders, T.; den Boer, J.; Gerrits, M.; Fokkema, D.; Ter Horst, G. Pair-housing of male and female rats during chronic stress exposure results in gender-specific behavioral responses. Hormones and behavior 2005, 47, 620–628. [Google Scholar] [CrossRef]
- Woinarski, J.C.; Burbidge, A.A.; Harrison, P.L. Ongoing unraveling of a continental fauna: Decline and extinction of Australian mammals since European settlement. Proceedings of the National Academy of Sciences 2015, 112, 4531–4540. [Google Scholar] [CrossRef]
- Zschucke, E.; Renneberg, B.; Dimeo, F.; Wüstenberg, T.; Ströhle, A. The stress-buffering effect of acute exercise: Evidence for HPA axis negative feedback. Psychoneuroendocrinology 2015, 51, 414–425. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).