1. Introduction
Osteochondritis dissecans (OCD) is an idiopathic, focal, subchondral-bone abnormality that can cause instability or detachment of a bone fragment and overlying articular cartilage, with subsequent progression to osteoarthritis [
1]. OCD is usually regarded as either juvenile OCD (occurring with an open epiphyseal plate) or adult OCD (after the physis has closed) [
2]. Overall incidence rates in adults per 100, 000 person-years are 3.42 for all OCD, and 1.21 for knee OCD [
3]. Juvenile OCD is more common in those aged from 6-19 years with an incidence of 9.5 per 100,000, with an increased risk in those who are >12 years-old [
4].
The classic site of knee OCD is the posterior-lateral aspect of the medial condyle (63.6%) compared with inferior central-aspect of the lateral condyle (32.5%), inferomedial aspect of the patella (1.5% to 10%) and trochlea less than 1% [
4,
5]. The etiology of OCD is incompletely understood. A recent systematic review presented that OCD can be caused by biological and/or mechanical factors; where biological etiological factors include genetic, ossification deficit and endocrine hypothesis. Mechanical etiological factors include hypothesis of injury and overuse, tibial spine impingement, presence of discoid meniscus and biomechanical alteration [
6].
Symptoms depend on the lesion location and stage. Stable lesions can cause nonspecific symptoms, including vague or intermittent pain, whereas unstable lesions or loose bodies can cause mechanical symptoms, including catching or locking. Restricted range of motion and joint effusion may signify unstable lesions or loose bodies [
7].
Radiography is the first step. An antero-posterior view, a lateral view, and a tunnel view with the knee flexed at 60°C should be obtained. A skyline view is required if an OCD lesion of the patella or trochlea is suspected [
1]. MRI is the method of choice as the second step in an imaging workup [
2]. T1- weighted sequences allow lesion size measurement, while T2-weighted sequences with cartilage mapping, provide information on articular cartilage integrity and reactive marrow edema adjacent to the affected subchondral bone [
7].
Operative treatments for cartilage defects can be separated into palliative, reparative, restorative, and reconstructive procedures. Palliative procedures include lesion debridement and loose body removal, with the single goal of alleviating mechanical symptoms. Reparative procedures include microfracture to achieve bone marrow stimulation. Reconstructive procedures include mosaicplasty or osteochondral autograft transplantation (OAT), and matrix-induced autologous chondrocytes implantation (MACI), where the purpose of the treatment is to fill the articular defect with hyaline like cartilage tissue [
8].
Treatment for adult knee OCD with lesions classified as stage III, requires a treatment to fill the articular defect, however not all approaches have reported the save favorable outcomes. Microfractures, however this treatment has shown a low repair rate, due to a resultant unstable fibrocartilage that usually degenerates in less than 5 years [
9,
10]. Mosaicplasty, consists of transplanting a healthy osteochondral area to another affected area, is suitable in lesions smaller than 2 cm
2, however, it can place load-bearing cartilage in places where friction predominates, leaving an area without cartilage, which could potentially lead to the generation of fibrosis [
11]. MACI is considered a third-generation technique for cartilage repair that consists of two stages; an initial arthroscopy is done to perform a cartilage biopsy, followed by open or arthroscopic surgery to implant the cultured chondrocytes into the cartilage defect. Even Though, clinical benefits associated to increased survival of the graft, patient satisfaction, and faster return to daily activities have been documented with the use of MACI, there is a lack in the consensus related to this is still not the treatment of choice for a specific surgical technique, times of intervention, and this can be translated into an absence of standardized treatment. Therefore, our purpose is to share our experience with the use of MACI, with the hope to contribute to a full standardization of this treatment to improve clinical outcomes in a significant number of patients affected by this condition.
2. Case Presentation Section
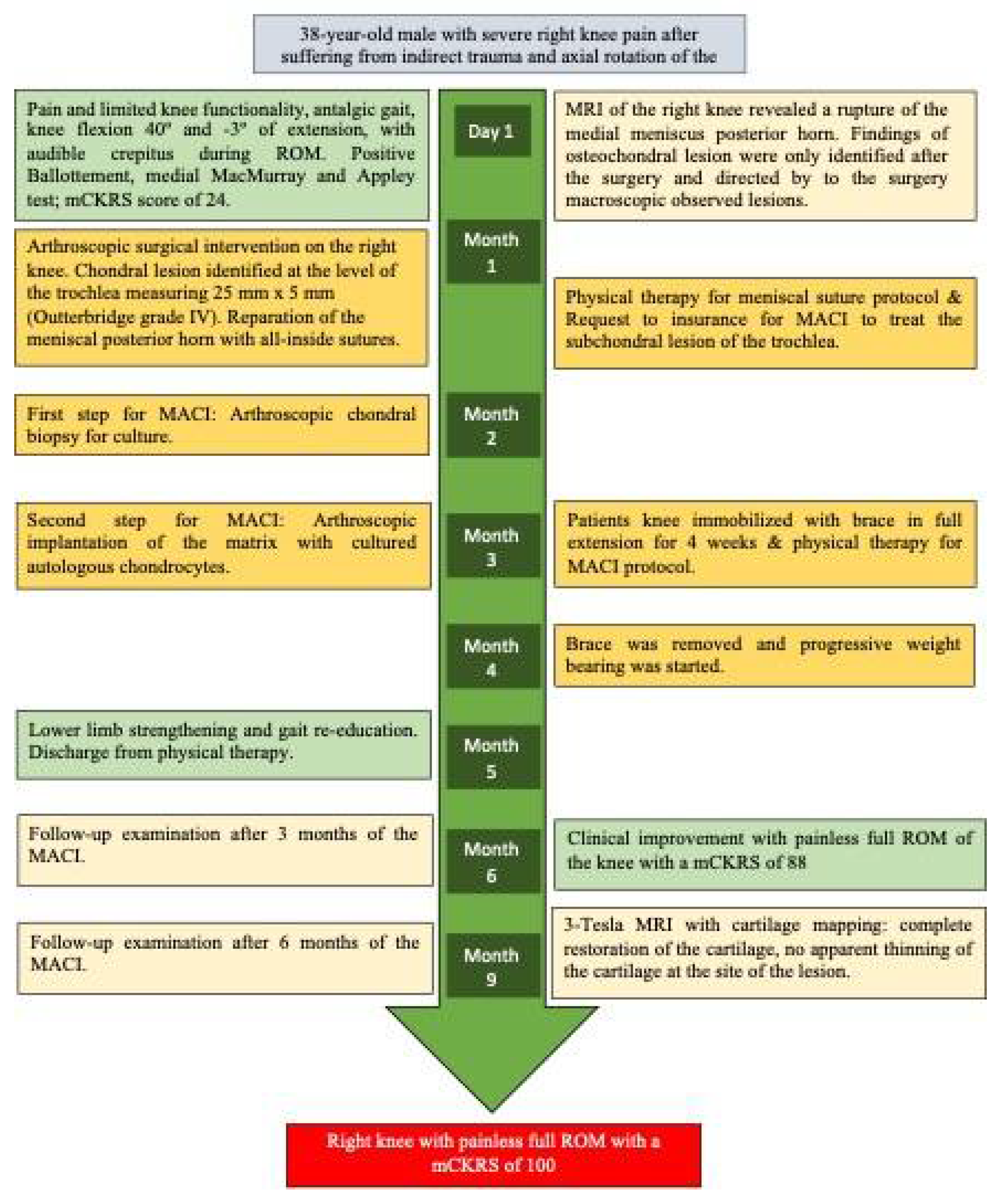
A 38-year-old male presented to our clinic with severe right knee pain after suffering from an indirect trauma and axial rotation of the knee, the pain limited knee functionality, impeding the patient to walk for the past two days. Upon evaluation the patient presented an antalgic gait at the expense of the right knee with pain in the patellofemoral region and in the medial intra articular line, the knee exploration revealed ranges of motion (ROM) 40º of knee flexion and -3º of extension due to pain, crepitus was noted during ROM of the knee. Ballottement test was positive for knee effusion, medial McMurray and Appley tests were positive; whilst Lachmann anterior & posterior test were negative (
Figure 1).
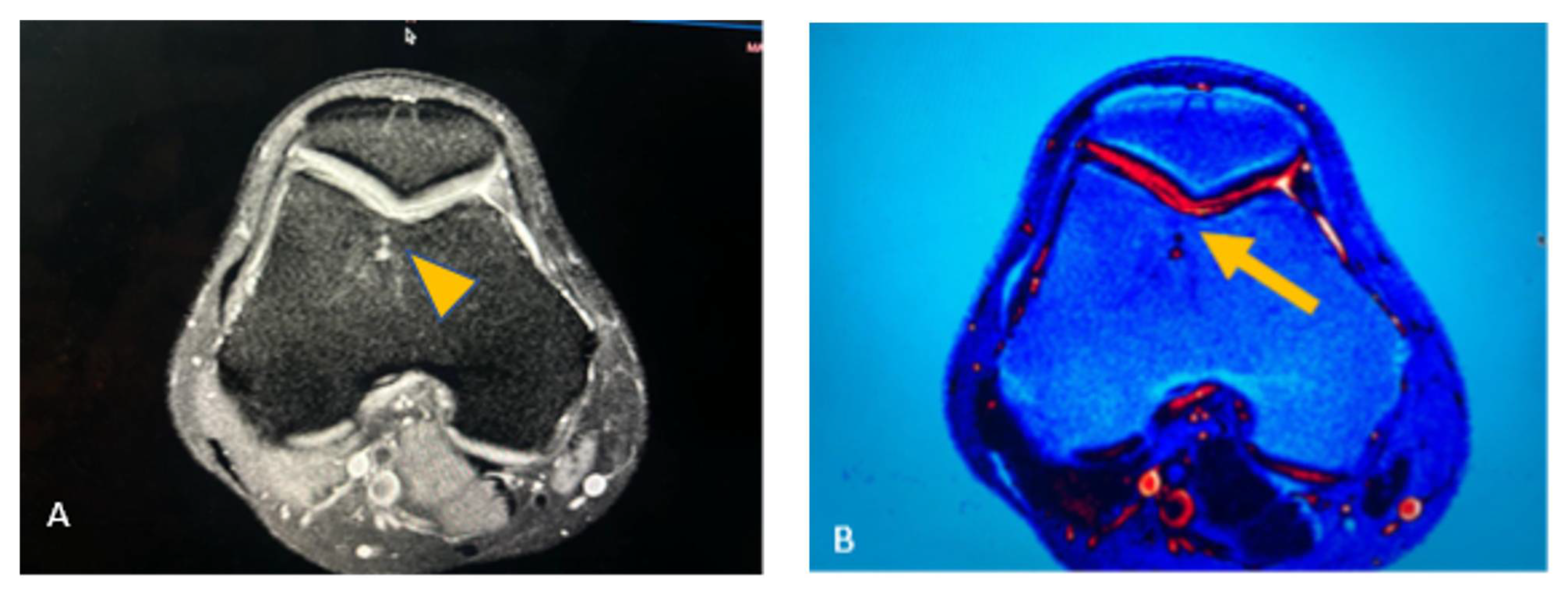
A knee MRI was ordered to identify the type and extent of the lesion. Findings revealed a medial meniscal posterior horn tear, without evidence of osteochondral lesion, nevertheless, in a postoperative inspection of the images, we found changes in the trochlea are of the knee suggestive of osteochondral lesion (
Figure 2). The preoperative diagnosis was medial meniscal posterior horn tear of the right knee.
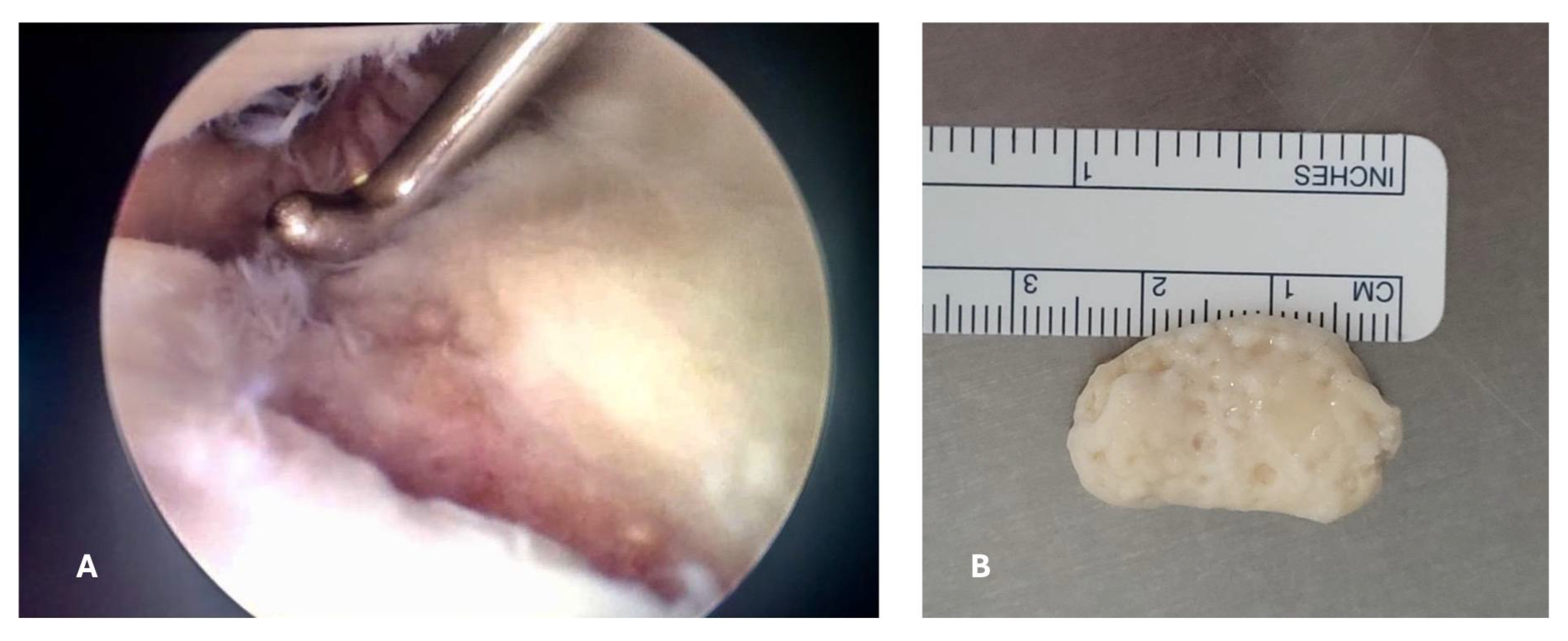
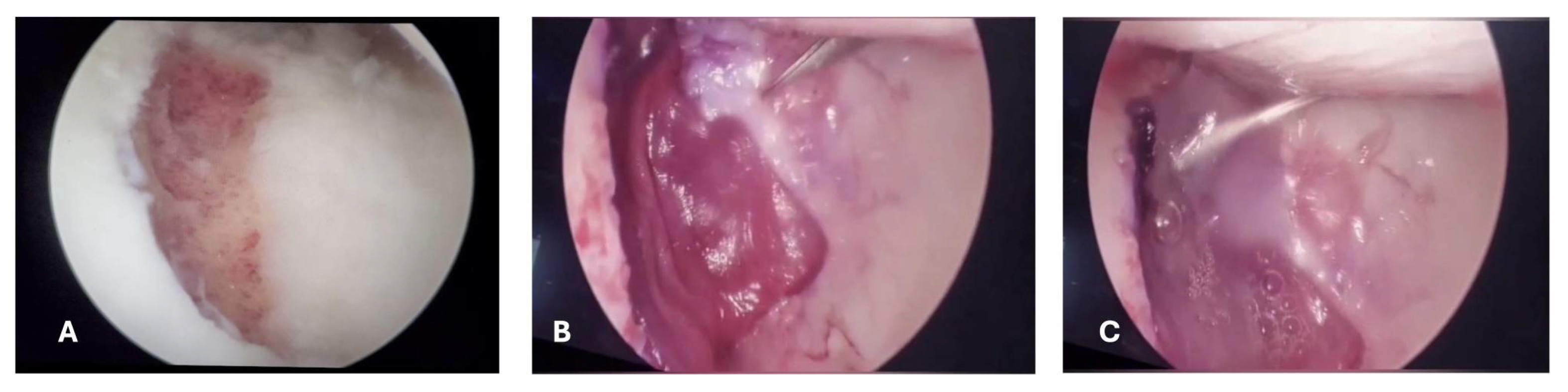
An arthroscopic surgical intervention was performed on the right knee of the patient for reparation of the medial meniscal posterior horn tear, during the procedure, a chondral lesion was identified at the level of the trochlea measuring 25mmx15mm involving the cartilage full depth with exposure of the subchondral bone (Outterbridge grade IV); additionally, a free chondral body of similar dimensions was identified (
Figure 3).
A trans operative diagnosis of osteochondritis dissecans of the knee grade IV in the Diapola Classification [
12] was established. The chondral body was removed and sent for histopathologic study, afterwards the meniscus repair was conducted with the use of all-in meniscal sutures before closure of arthroscopic portals a joint drainage (Drenovac) was placed, and wounds were closed. Patient was discharged the same day of the surgery with etoricoxib 120 mg once daily, and levofloxacin 750 mg once daily to prevent wound infections for 7 days.
After considering the extent of the chondral lesion (> 2cm2), a young age of the patient, and evidence related to long-term benefits with the use of MACI, we offered this type of intervention for chondral repair treatment, however before obtaining a biopsy to be used for chondrocytes culture, the insurance approval was needed.
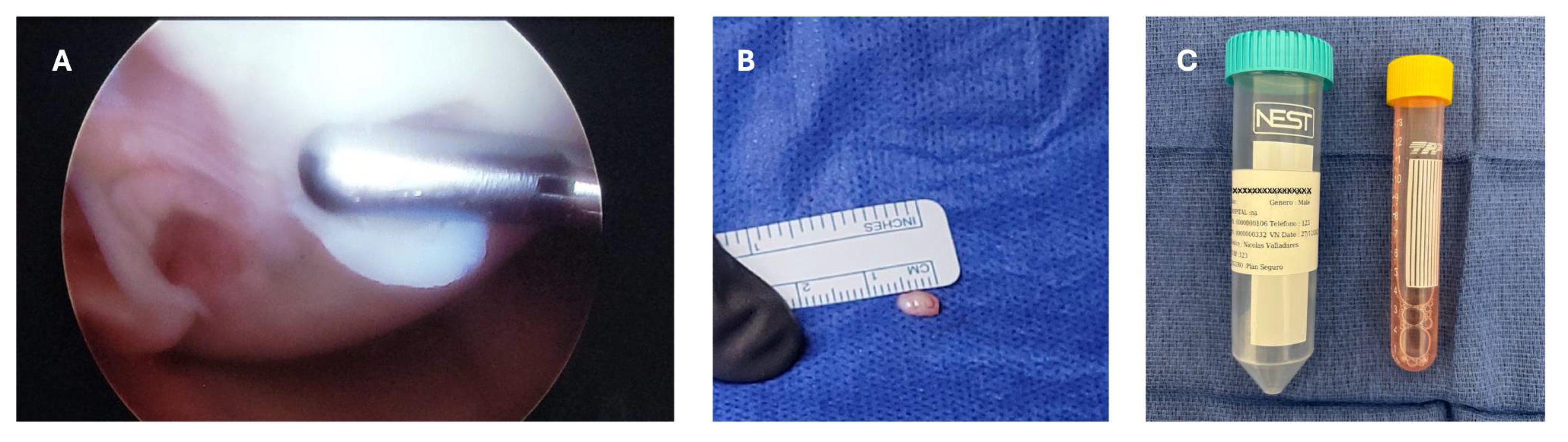
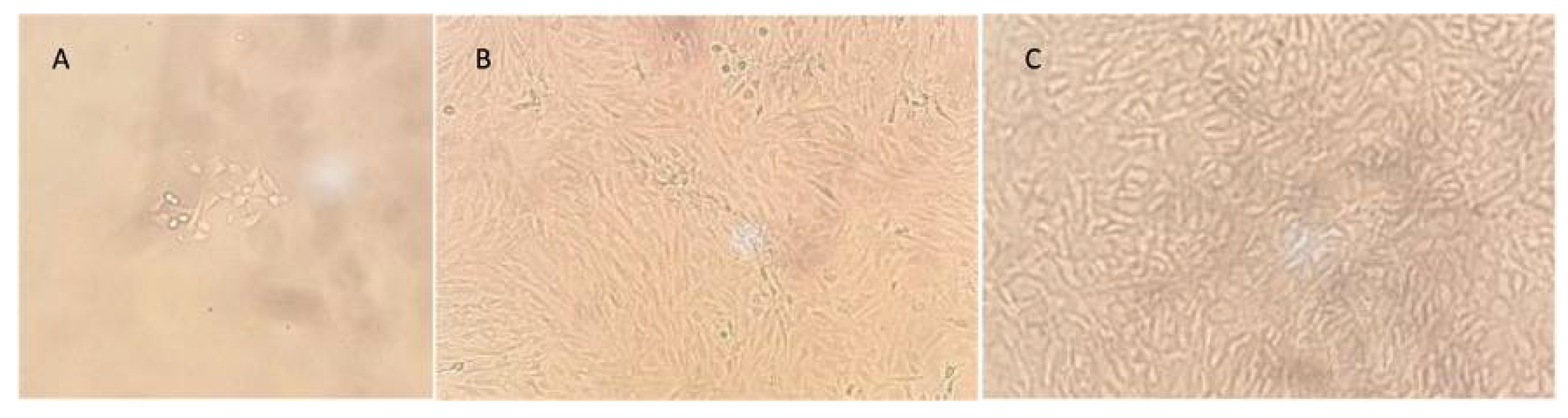
The patient started with physical therapy for pain and inflammation management, as well as for the meniscal suture protocol, which consist in progressive mobilization with partial progressive weight bearing movement. The patient completed the meniscal repair protocol, an approval was obtained from the insurance company and was scheduled for a second arthroscopic procedure with the aim of obtaining a cartilage biopsy for chondrocyte culture. A small cartilage sample was taken from the non-weight bearing area of the trochlea using and arthroscopic chondrotome and then transferred into a serum-free transport medium (chondrocyte medium I) and sent to Gencell Biotechnollogy in Mexico City for culturing and processing (
Figure 4).
Chondrocytes were isolated by enzyme digestion and resuspended into chondrocyte medium I, with 10% bovine fetal serum. Cells were transferred several times until a confluence of 100% was reached with 5 million cell count per cm
2 (
Figure 5).
Cells were transferred (3-5 million) into a collagen I/III matrix (Chondro-Gide
®). The matrix was packed, sealed, and sent to be placed in the patient within the next 24 hours. Three weeks after the first intervention, the patient was readmitted to apply the matrix with cultured autologous chondrocytes. An arthroscopy surgery was performed in a tourniquet-controlled bloodless field, intra articular arthroscopic retractors are placed to expose the defect, the chondral lesion was excised with a chondrotome, the subchondral bone is prepped until having a flat and uniform layer of bleeding bone with a ring of normal surrounding cartilage. The MACI (chondroMATRIX
®) was cut to size, to allow for correct membrane integration, then fixed with fibrin glue (Tissucol) on the top of the MACI and finally prominent edges are trimmed (
Figure 6).
Towards the end of the surgery, the knee was flexed and extended to confirm stability of the implant. After the surgery, the patient’s knee was immobilized with a brace for 4 weeks before starting again with physical therapy with MACI protocol and continuous passive motion. At 6 weeks after the surgery a complete ROM was achieved, and mobility of the knee was completed. Lower limb strengthening and gait re-education was completed 2 weeks after. Upon completing all objectives, the patient was discharged from physical therapy.
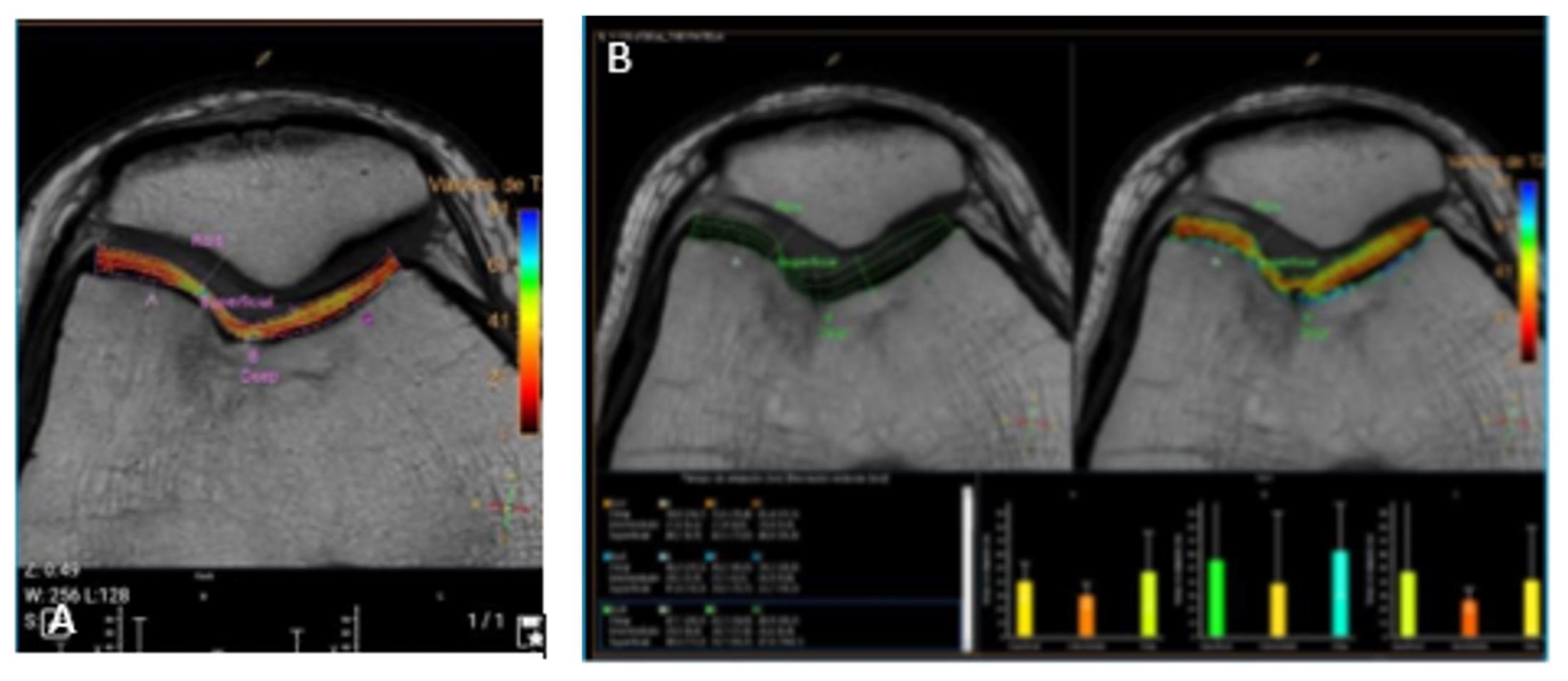
Follow-up examinations were performed at 3 and 6 months after the surgery. Clinical improvement was evident at 3 months after the surgery with progressive movement and less pain of the right knee at this time the mCKRS was of 88. At 6 months after the surgery patient presented with significant clinical improvement, with absence of pain, complete ROM, and functionality with a mCKRS of 100, at this time a 3-Tesla control MRI of the right knee was ordered, showing the complete regeneration of the chondral defect, without a trace of injury or thinning of the cartilage compared to healthy cartilage (
Figure 7). At this time, the patient was able to perform daily activities and sports without limitations or pain.
3. Discussion
In the present case the diagnosis of OCD was suspected during the initial evaluation and confirmed during the first arthroscopic intervention.
Many surgical options have been proposed for the treatment of stage III and IV OCD lesions; However, there is an ongoing debate related to long-term results when different techniques are compared. Additionally, some factors have been previously associated with poor outcomes, including incongruence of the fragment or too many fragmented loose bodies [
13].
Autologous chondrocytes implantation (ACI) has been reported as a good alternative to treat large lesions with decent performance, over the use of mosaicplasty in the past [
11,
14]. However, some problems became apparent with this technique, such as the treatment of the subchondral bone, hypertrophy of the periosteal flap and dedifferentiation of chondrocytes. This gave rise to additional alternatives, such as the matrix-supported transplantation of autologous chondrocytes (MACI).
Since, the matrix supplies the natural environment of chondrocytes, it will provide an environment with adequate physical and biochemical conditions, in addition to providing a parallel columnar arrangement like that of the natural articular cartilage [
15]. It has been observed that the implementation of MACI as a technique for the repair of chondral defects has significantly improved quality of life in patients, with high satisfaction rates as well as greater than 10-year graft survival [
16] and for the treatment of OCD with up to 36 months, with better results when compared to osteoarticular transfer systems (OAT) [
17].
Compared to other techniques used to repair chondral lesions, there is a significant improvement in the quality of life of people who have undergone a MACI procedure.
Several studies have been conducted in the past to analyze MACI performance in comparison to other techniques for cartilage repair. This superiority has not been shown in a very clear form, mostly due to a lack of consensus regarding the evaluation of postoperative clinical outcomes with respect to a minimal clinically important difference. For this purpose, a metanalysis was conducted to determine the proportion of available cartilage repair studies that either meet or exceed minimal clinically important difference (MCID), a measurement of whether changes in patient-reported outcome scores reflect meaningful improvement for the patient [
18]. After the conduction of this metanalysis it was evident in terms of the International Knee Documentation Committee (IKDC) and visual analog scale for pain (VAS) to report on MCID, the microfractures technique met MCID values for all outcome scores at short- and midterm follow-up, except for pain at the midterm; while, osteoarticular transfer systems (OAT) met MCID values for all outcome scores at short, midterm and long-term follow-up (except for VAS in the long term), and for MACI MCID values were met at all times, plus an extended maintenance of clinical benefits. This benefit observed, especially in the long-term follow-up and beyond, is what started the dialogue to consider MACI as a superior alternative procedure. However, the studies included in this metanalysis might be heterogenic with regards to patient characteristics and may have biased the results, another important limitation of this study, is that microfractures is typically used to treat smaller cartilage lesions [
19], and by not including the size of the lesion in this analysis we can have selection bias determining this results in favor of this procedure, although it wasn’t the best approach.
In this regard, a study compared the long-term follow-up in patients who underwent cartilage repair after failure of conservative treatment, patients were treated with either MACI or mosaicplasty. Patients were followed for a 12-year period, at the end of the study, both interventions presented with satisfactory clinical and MRI evaluations; however, for larger lesions (> 2 cm
2) MACI presented with less failures and therefore lower risk for reoperation compared to mosaicplasty [
20]. Although groups of treatment in this study were similar in terms of demographics and cartilage lesion features, the decision for treatment was not randomized, and therefore, this study might be subject to selection bias.
A randomized controlled trials, with the purpose to examine the clinical efficacy and safety after in a mid-term follow up (5 years) with MACI in comparison to microfractures for patients with symptomatic cartilage defects of the knee, found superior improvements with MACI, consistent in the knee injury and osteoarthritis outcome score (KOOS) pain and function domains after the surgery and maintained over a 5-year follow-up, superiority was also observed in function, with improvements in daily living and quality of life at 2 years of follow-up. While the MRI evaluation showed similar improvement related to defect filling for both groups of treatment [
21].
With respect to the treatment of unstable OCD in adults, a recent literature review performed a qualitative analysis of different treatment options and included information from follow-ups, which ranged from 2 to 17 years. The internal fixation surgical technique had acceptable rates of radiographic union and patient reported outcome measures in skeletally immature patients. For adults with large lesions, MACI, and osteochondral autograft transplantation (OAT) were found to have the best performance. Although, in comparison to MACI, OAT had higher conversion to arthroplasty [
22].
A recent long-term prospective study that followed 87 patients with OCD in the tibiofemoral and patellofemoral regions treated with MACI, over a 10-year follow-up found an increased graft survival compared to microfracture. Treatment with MACI was also associated with high patient satisfaction rates [
16].
To be noted, one of the common criticisms of cell-based repair techniques is their propensity for graft hypertrophy, which can lead to mechanical symptoms, pain, and the need for re-operation. However, a network meta-analysis provided conclusive evidence (with a high degree of statistical certainty) that both second generation ACI and MACI significantly reduce the risk of graft hypertrophy in comparison with OAT and Microfractures [
23].
The transplantation of cultured autologous chondrocytes, in a collagen scaffold, is a technique that allows for the reproduction of autologous cartilage in-vitro, to regenerate an extensive, full-thickness chondral lesion. Avoiding degeneration and the requirement for prostheses in the medium-term follow up. Derived from variations in techniques, and clinical characteristics of patients intervened with MACI, some previous studies have reported unsuccessful cases among their cohorts, which has allowed for the identification of risk factors associated with poor outcomes, especially in the short-term follow-up including: a long period of pain, the presence of a functional disorder, and larger defects (> 6 cm2) [
24]. Even Though, our patient had a recent previous surgery it was for the repair of the meniscal tear, and not related to the chondral lesion; a long period of pain and functional disorder, as well as a larger defect (> 6 cm
2) was not present. The absence of risk factors, in this case, might have been associated with a good clinical response.
The MACI procedure is indicated for isolated chondral and osteochondral symptomatic defects between 2-10 cm
2, or with affection of the cartilage full thickness with no significative osteoarthritis [
25]. MACI has two main disadvantages compared to other chondral repair techniques. The first is a need of two surgical procedures, one for taking the cartilage sample and the second to apply the graft [
26]. The second disadvantage is the cost of the procedure, estimated up to 3 more times comparted to a scaffold alone or mosaicplasty.
Nevertheless, it has been demonstrated that specially for the treatment of medium size lesions (>4 cm
2), and in association with long-term follow-up evaluations, MACI has demonstrated a more cost-effective approach in comparison to microfractures [
27]. Specially by analyzing the quality of the resulting cartilage, which might be an additional consideration in young patients.