1. Introduction
The development of society worldwide has led to the growing demand to maximize natural resources in a sustainable way, thus the use of waste derived by-products from the agri-food industry has become a key aspect in terms of circular economy (Frazzini et al., 2023). In this sense, during the last decades, certain nut crops such as walnut (Juglans regia L.) and hazelnut (Corylus avellana L.) have generated significant interest due to their shells as main by-products from industry (Manterola-Barroso et al., 2024). In production terms, the nut industry plays an important role in the global agricultural economy (USDA, 2022). Global nut production has grown over the past decade, with 4.6 million tons (t) in the 2019/20 season, where walnut and hazelnuts represent 21% and 11%, respectively (USDA, 2022; Nuts Dried Fruits Statistical Yearbook, 2023). Overall, the value of nut supply has increased steadily over the past decade, at an average rate of $USD 1.9 billion per year, reaching $USD 35.6 billion in 2019/20 (Du et al., 2021; FAOSTAT, 2020). In the case of hazelnut, the annual world production exceeds one million t and Chile has become the main hazelnut producer in the southern hemisphere with an off-season nut supply of approximately 52,949 t in 2022/23 (Agrichile-Ferrero Hazelnut Company, 2023). About 90% of hazelnuts produced in Chile are marketed without shells (unshelled) (Agrichile-Ferrero Hazelnut Company, 2023), whereas for walnuts, the exportation volume of both peeled or shelled nuts was approximately 191,972 t for the year 2023, with estimated increase to 214,344 in 2025 (ChileNut, 2023). This nut tree represents the main species established and destined for the nut market in Chile and the world, with a global production of over 3.7 million t, experiencing an increase of 23% in the last ten years (FAOSTAT, 2020). In fact, Chilean walnut exportation reached around 160,000 t and U$ 474.8 M in 2023, being walnuts in shell the main exported product in terms of value, with shipments totaling 97,371 t during 2023 (ChileNut, 2023). Moreover, around 50% of exports correspond to shelled walnuts, while the shells from the other 50% remain in the country of origin (ChileNut, 2023). The weight value (w/w) of the shell of both nuts is between 50 to 60% of yield in relation to the kernel (40-50%) (Manterola-Barroso et al., 2022; Meriño-Gergichevich et al., 2021). Thus, a huge amount of annual by-product biomass is available for its potential use (Manterola-Barroso et al., 2024). Currently, as a cheap raw material, nutshells biomass is mainly used as an heat energy source for heaters conferring a low value-added (Demirkaya et al., 2019, Rivas et al., 2020) without considering their high phenolic and lignin content. In fact, there are limited studies on the nutshell use in industrial applications (Manterola-Barroso et al., 2024). The utilization of hazelnut shells for the extraction of phenolic compounds has been explored with the purpose to obtain naturally valued additives to some extent. Contini et al. (2012) reported that phenolic compounds in shells processed at different roasting temperatures and times, were about 2.7 mg g−1 DW for cultivars Tonda Gentile Romana, Tonda di Giffoni, Tonda Gentile delle Langhe and Tombul. Furthermore, Manterola-Barroso et al. (2022) reported total phenolic content (TPC) and oxygen radical absorbance capacity (ORAC) mean values of 180 mg GAE (gallic acid equivalents) g−1 DW and 2119.7 μmol TE g−1 DW in Tonda di Giffoni shell samples, respectively, demonstrating their huge antioxidant potential. In terms of TPC and antioxidant potential 31.79 mg GAE g−1 DW and TEAC (Trolox Equivalent Antioxidant Capacity) values around 18.86 mg TE g−1 DW, respectively, were reported for walnut shell methanol extracts by Queirós et al. (2020). In fact, there are limited studies on its use in industrial applications (Manterola-Barroso et al., 2022). Polyphenolic compounds found in plants play a variety of roles, such as color, antimicrobial and antifungal action and antioxidant protection against free radicals (Crozier et al., 2007; Friedman et al., 2007). Moreover, there is a close relationship between antioxidant compounds such as polyphenols, phenolic acids and anthocyanidins with color of fruits such as berries(Jensen et al., 2008). Therefore, it would be of great interest to determine if there is a link between color and antioxidant parameters in shells of species such as hazelnut and walnut. Although, there are no available studies that generate a relationship between both parameters in nutshells. However, Jensen et al. (2008) proposed a method of prediction of wine color from phenolic profiles in red grapes, demonstrating the high relationship between grape anthocyanins and wine color (r=0.961) and the importance of anthocyanins, phenols and flavanols in the color intensity of young wines. In this sense, colorimeters have been extensively used in the food industry due to their cheap and simple measurements of food color (Kara and Erçelebi, 2013).
On the other hand, in relation to its structural conformation these shells are composed by hemicelluloses (25-30%), cellulose (26-32%), lignin (40-43%) and extractives (3.3-4%) (Barbu et al., 2017). Lignin is composed by phenolic polymers endowed with potent antioxidant properties that are finding increasing applications in a variety of fields (Argenziano et al., 2022). In relation to walnut shells, Queirós et al. (2020) reported a chemical composition of walnut shell conformed by 10.6% total extractives, 29.9% lignin, and 49.7% polysaccharides, whereas Domingos et al. (2022) reported 35% of lignin and 55.2% holocellulose (30.4 and 24.9% as α-cellulose and hemicelluloses respectively). Today, involved in a global campaign of circular economy and in a sustainable atmosphere, the main nut industrial waste is starting a new road to industrial re-incorporation as a new waste material, potential applications that could help in CO2 emission reduction (Baran et al., 2020). This study contributes to increasing knowledge about the potential of nut agro-industry by-products for sustainable use. Therefore, the aim of this work was to evaluate the potential of hazelnut and walnut shells from southern Chile agro-industrial by-products as a source of phenolic compounds with ORAC antioxidant capacity effect in order to reveal their association with color and microstructure.
2. Materials and Methods
2.1. Sample Collection and Processing
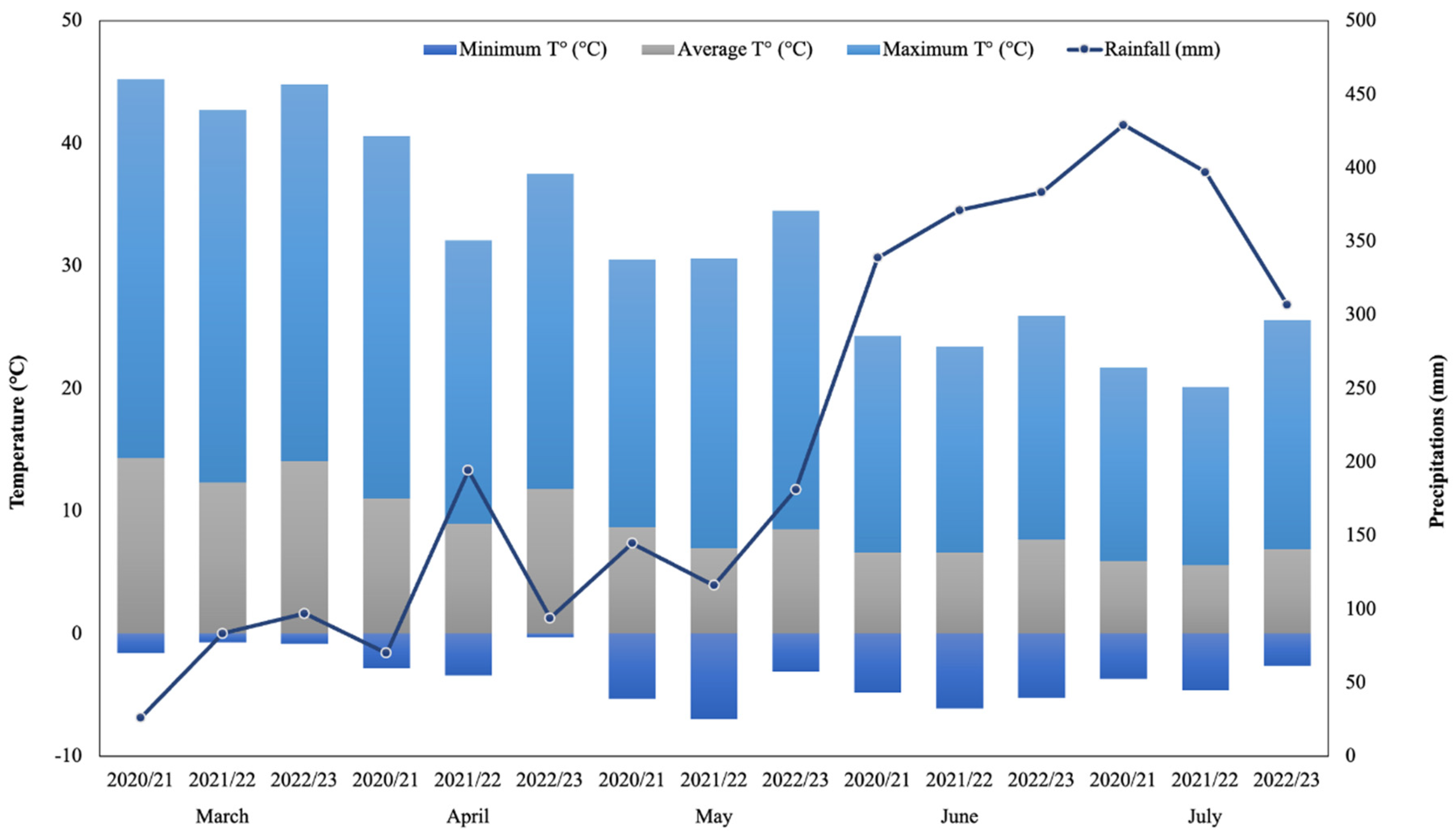
During 2020/21, 2021/22 and 2022/23 productive seasons, hazelnut (cv. Tonda di Giffoni) and walnut (cv. Franquette) were collected from commercial orchards located in Radal (39º 10′ 06” S, 72º 19′ 11” W; Altitude: 168 m.a.s.l.) and “Campo Experimental Maquehue” (38° 50′ 28.5” S, 72° 41′ 40.4” W; Altitude: 200 m.a.s.l.) respectively, in La Araucanía region, Chile. The meteorological regime showed an expected behavior according to the evaluated area (mostly temperate localities) where precipitation shows higher volumes and frequencies during June and July for three studied seasons. Maximum temperatures (30.9°C) occurred during the 2022/23 season while minimum temperatures (‒7°C) was measured in May of 2021/22 season (
Figure 1). In both locations the nuts harvest was performed from late March to the beginning of April with two vacuum backpacks (Cifarelli S.p.A V1200, Voghera, Italy). Then, the collected nuts were gently transported to the Laboratory of Plant Physiology and Nutrition in Fruits Crops (BIOREN-UFRO) and weighted in an I-Thermo G163L thermo balance (Bell Engineering, Russia) to determinate field humidity. The samples were afterwards washed with deionized water and prepared for the stabilization process at 40ºC for around 72 h in forced-air oven (Heratherm OGS100, Thermo Scientific, Waltham, MA, USA; Memmert UF-55 Büchenbach, Germany) to be stabilized at 6% of humidity and stored at room temperature (20ºC) until further analysis. Samples were subjected to a mechanical peeling process using a traditional nutcracker (IDS, Chile). For of total phenolic content and oxygen radical absorbance capacity (ORAC) determinations, shells were milled and sieved (<1mm) in an ultra-centrifugal mill (Retsch ZM 200, Haan, Germany), obtaining a powder. The whole shell was used to evaluate color and microstructural properties.
2.2. Extraction of Phenolic Compounds
Extraction was performed according method described by Yuan et al. (2018). Shell powder (5g), sieved at 35 ASTM), was suspended on 20 mL aqueous methanol (70% v/v), and the samples were sonicated (Elma S10H, Hohentwiel, Germany) at 37 khz for 30 min in iced water (4ºC). Later, the samples were centrifuged (Eppendorf, 5804, Hamburg, Germany) at 8.000g (4ºC) for 10 min and then shaken at 200 rpm (15°C) in darkness for 18h (Orbital Shaker ZHWY-100B Zhicheng, Shanghai, China). After this procedure, the solution was re-centrifuged at 8.000g for 10 min. Finally, the samples were filtered through a 0.45 µm pore size (BIOFIL Syringe Driven, Guangzhou, China) and stored at ‒20ºC until analysis. The pellet was re-suspended for a re-extraction under identical conditions (only 6h), resulting in a total extract volume of 40 mL. The extracts were used for biochemical determinations (TPC and ORAC).
2.3. Total Phenolic Content Determination
The total phenolic content was quantified by Folin Ciocalteu methodology performed by Singleton and Rossi (1965) with minor modifications, using Na₂CO₃ (Sodium carbonate) (20% w/v) (Merck KGaA, Darmstadt, Germany) and the above-mentioned extract of shell powder samples diluted (1:10 v/v). The analysis was performed by a TECAN Infinite® 200 PRO NanoQuant multi-plate reader (Männedorf, Switzerland) at 765 nm of wavelength. The results were expressed as mg of gallic acid equivalents (GAE) g‒1 of dry weight (DW) (Merck KGaA, Darmstadt, Germany) following a calibration curve between 0 to 750 µg mL−1 of Gallic Acid.
2.4. Oxygen Radical Absorbance Capacity (ORAC)
To determine the antioxidant capacity of hazelnut and walnut shells, the “Methodology for the determination of ORAC antioxidant capacity in the pericarp of European hazelnut (Corylus Avellana L.) fruits” protocol was used, ownership of the Universidad de La Frontera, registered in the Chilean Department of Intellectual Rights (DDI), Nº 2021-A-8614 and adapted for walnut samples. The ORAC was analyzed in a multi-mode reader (Synergy H1 Hybrid, Biotek, Vermont, United States) with five replicates. The working solutions used for the determination were AAPH (153mM) (Sigma-Aldrich, St. Louis, United States), and the 70 nM sodium fluorescein (Sigma-Aldrich, St. Louis, United States). After preliminary tests, the extracts were diluted 1:1000 and a Trolox calibration curve (0 to 300µM) was applied. The analyses were carried out using the Gen5™ software, at 37ºC. 25μL of each sample was loaded, standard or blank in 96-wells black TPC micro-plates, filling the external wells with 250μL of distilled H2O, and then inserting the loaded plate into the multi-plate reader. Then, injector 1 immediately dispensed 150μL of Fluorescein (70 nM, followed by orbital shaking for 10 seconds at maximum intensity and incubated for 15 min). Subsequently, 25 μL of AAPH was injected through injector 2, followed by orbital shaking for 50s at maximum intensity, then kinetic readings were generated every 60 sec for a total of 2.5 hr. Finally, the data were calculated using two equations to calculate the area under the curve (AUC).
2.5. Color Determination
The superficial color was determined by photo-colorimetry using a handheld colorimeter model CR400, Minolta Co (Tokyo, Japan). Color functions were calculated for the CIELAB or CIE (Commission Internationale d’Eclairage) (1976) as L*a*b* uniform color space based on modified methodology described by Lixia et al. (2013). Measurements were performed using a Petri plate containing enough powder to reach a height of 1 cm (20g approximately of each nutshell powder sample), and five replicates were measured (four cardinal points and the central one of each plate). White paper was placed under the plates. Finally, a mathematical difference (Delta E or ΔE) between the parameters (L*, a* and *b) of each sample were calculated considering differences (Δ) between the first seasons to the last one and among all the calculated color parameters, according the following equation for L*, a* and b* values:
Where:
ΔL* = Difference in light and dark (+ brighter − darker).
Δa* = Difference in red and green (+ red − green).
Δb* = Difference in yellow and blue (+ yellow − blue).
ΔE = Total color difference between L*, a* and b* parameters.
2.6. Nutshell Microstructural Analysis
For microscopy aims, whole shell samples were used (n=9 for each species).
2.6.1. Scanning Electron Microscopy (VP-SEM)
The nutshell samples were adhered to the sample holder with double-sided carbon tape. Visualization was performed using a chemical contrast detector (Backscatter, BSE) in variable pressure without any further sputtering under the following parameters: 15 KV energy, 40 Pa pressure, WD 10 mm in scanning electron microscope (Hitachi SU3500, Tokyo, Japan), and images were acquired and analyzed with Hitachi software controller.
2.6.2. Confocal Laser Scanning Microscopy (CLSM)
To obtain the cellulose and lignin relative content, their organization and apparent distribution in nutshell samples, a fluorescence assay was performed. Transversal sections of nutshell of approximately 0.3 x 0.3 mm size were prepared. The samples were stained with safranin 0.1% for lignin (λ excitation/emission, 546/590 nm), or congo red 1% for cellulose (λ excitation/emission, 633/700 nm), and both were incubated for 30 minutes at 25ºC in the dark room. Subsequently, the nutshell samples were washed with deionized water three times to remove excess dyes. The samples were mounted on fluodish plates, and were visualized at 20x magnification by confocal laser scanning microscopy FV1000 Olympus. Images were captured at every 2.5 μm for a total of 50 μm and Fv10 Ver 2.0c software was used to analyze the images and quantify the relative fluorescence intensity.
2.7. Experimental Design and Statistical Analyses
The experimental design was a factorial completely randomized design where the factor was the season. All analyses were done with five biological replicates per species per season and the data were submitted to normality test (Kruskall-Wallis). An One-way analysis of variance (ANOVA) was performed to determine the differences among seasons. A Tukey post-hoc test was implemented to establish mean differences at 5%. A Pearson’s correlation test was performed to determine the linear relationship between the analyzed dependent variables.
3. Results
In this study, the properties of walnut and hazelnut shells were assessed during three seasons (2020/21, 2021/22 and 2022/23) with the aim to evaluate if they can be used as beneficial by-products of nut processing instead of being treated as waste material.
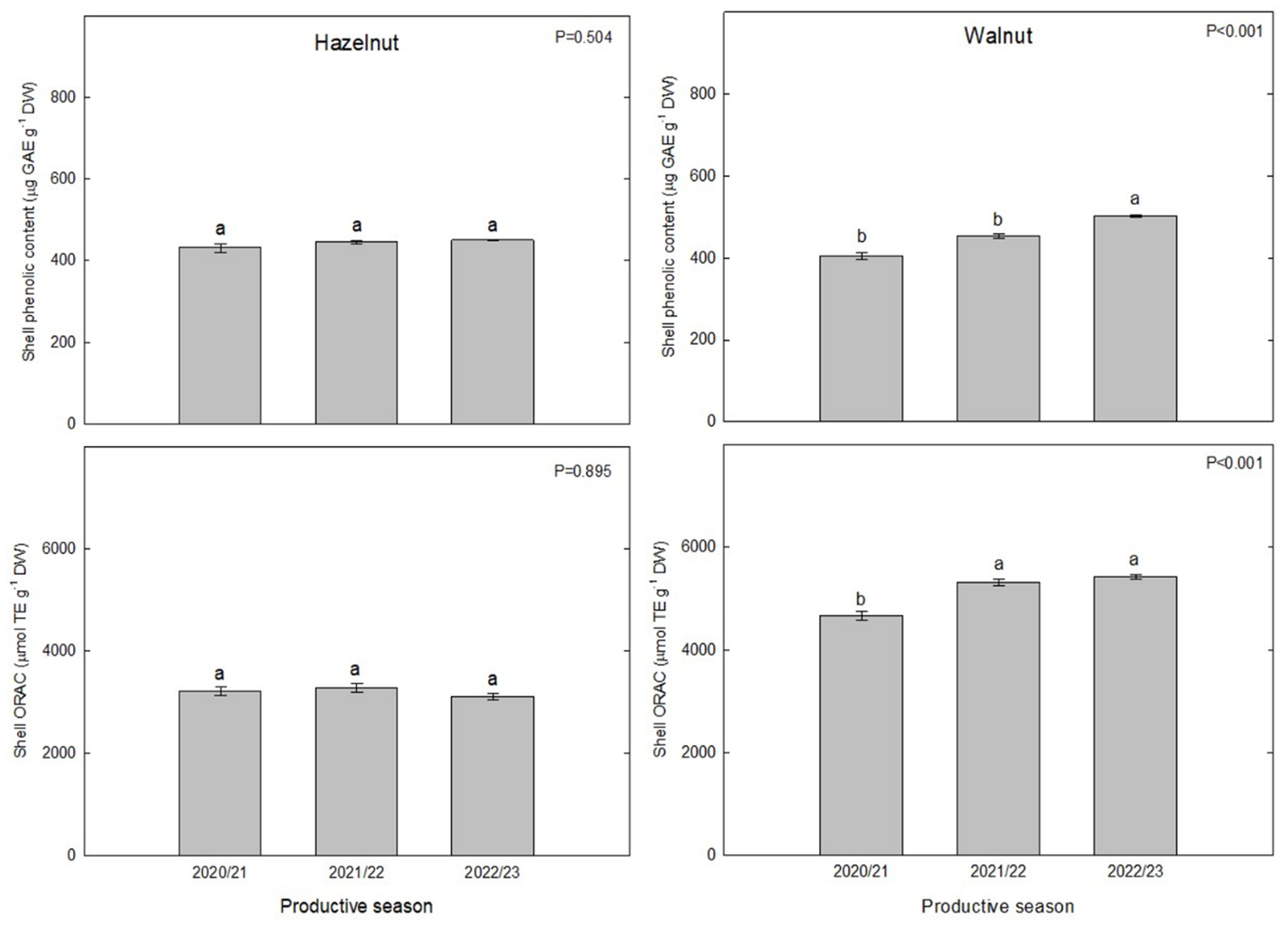
3.1. Total Phenolic Content
The analyses of total phenolics content (TPC) showed overall similar amounts between hazelnut and walnut shells (in gallic acid equivalents). In TPC of hazelnut samples had a mean of 0.42 mg GAE g
‒1 in 2020/21 season, similarly to values obtained in the following season (2021/22), around 0.44 mg GAE g
‒1 (
Figure 2). Moreover, in the last productive season (2022/23) the values determined were quite similar (mean 0.45 mg GAE g
‒1). Finally, the values obtained for the three evaluated seasons did not show significant differences among themselves. The TPC of walnut shells was similar to hazelnut, although it showed greater seasonal variability. The TPC values in walnut shells in 2020/21 were lower than in 2021/22 season and the same was observed for the 2022/23 season which increased significantly compared to the previous season. Trends showed a 10.5% increase from 2020/21 to 2021/22, whereas for 2022/23 an 11% increase was determined (ranges from 0.36 to 0.51 mg GAE g
‒1 DW) (
Figure 2).
3.2. Oxygen Radical Absorbance Capacity
To determinate the antioxidant capacity of hazelnut and walnut shells, ORAC method was used. Overall, ORAC values were higher for walnut than for hazelnut extracts, in all three seasons investigated. For hazelnut, the mean ORAC values varied slightly between the seasons (3,217 µmol TE g
‒1 DW, 3,282 µmol TE g
‒1 DW and 3,100 µmol TE g
‒1 DW for 2020/21, 2021/22 and 2022/23 seasons, respectively,
Figure 2). These values of antioxidant capacity are also a little higher than evaluated by Manterola-Barroso et al. (2022), who reported a mean of 2,119 µmol TE g
‒1 DW in control trees from Southern Chile hazelnut shell extracts. Regarding walnut shells, the values ranged between 4,600 and 5,500 µmol TE g
‒1 DW. The highest ORAC values were obtained in 2021/22 and 2022/23.
3.3. Nutshell Color
It is well-known the relationship between certain antioxidant compounds and their nature of origin with color parameters, as well as certain pigments in fresh fruits, however, in nuts we don’t have available studies regarding to this relationship. In our study, Luminosity (L*) parameters were not significantly changed in hazelnut or walnut shell over the three seasons evaluated (2020/21, 2021/22 and 2022/23). Nevertheless, in hazelnut shell, the parameters a* and b* showed a significant increase (P ≤ 0.05) of 6.7 and 8.4%, respectively for the 2022/23 season in relation to the two previous ones. Therefore, hazelnut shells exhibited a redder (a*) and yellower (b*) chromatic parameter at the same time, although the lightness parameter (L*) was not changed (
Table 1). In walnut shell samples, L* was significantly increased (19.4%) for the 2021/22 season and 20.9% for the 2022/23 season. In relation to a* and b*, these parameters increased progressively from the first to the last evaluated season, increasing on average by 13% for a* and 6.6% for b* from 2020/21 to 2022/23. Consequently, in chromatic terms, the shells exhibited an increase in chromatic luminosity and a slight trend towards red and yellow (
Table 1). Finally, the aforementioned parameters can be explained in a better way by understanding the chroma changes that occurred through the calculation of ΔE (
Table 1).
ΔE was determined for each season evaluated for each species (hazelnut and walnut). In this regard,
ΔE had an increasing trend from the first to the third season in both species, with more significant increase in walnut (
Table 1).
3.4. Pearson’s Correlation between Antioxidants and Color Parameters
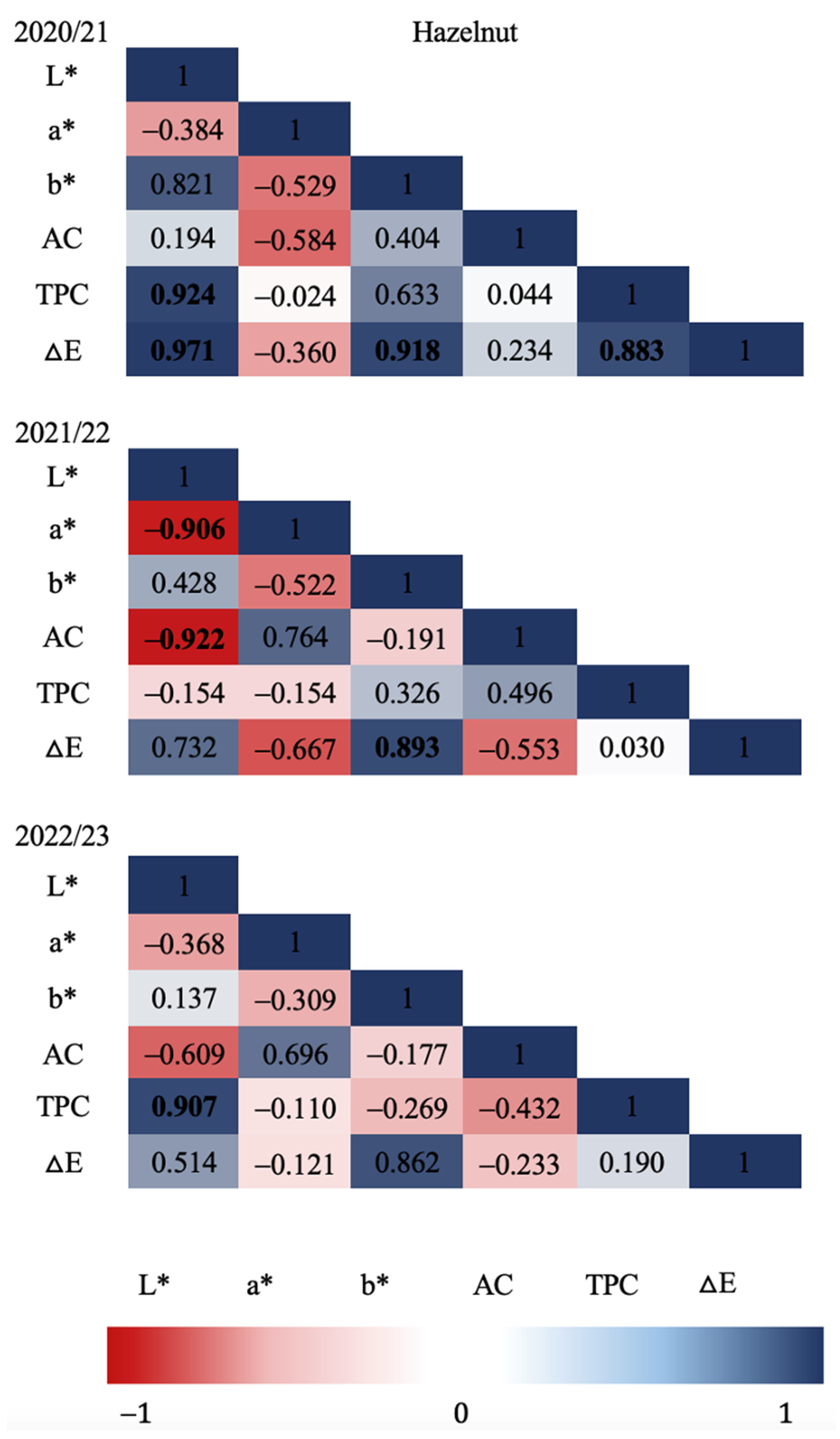
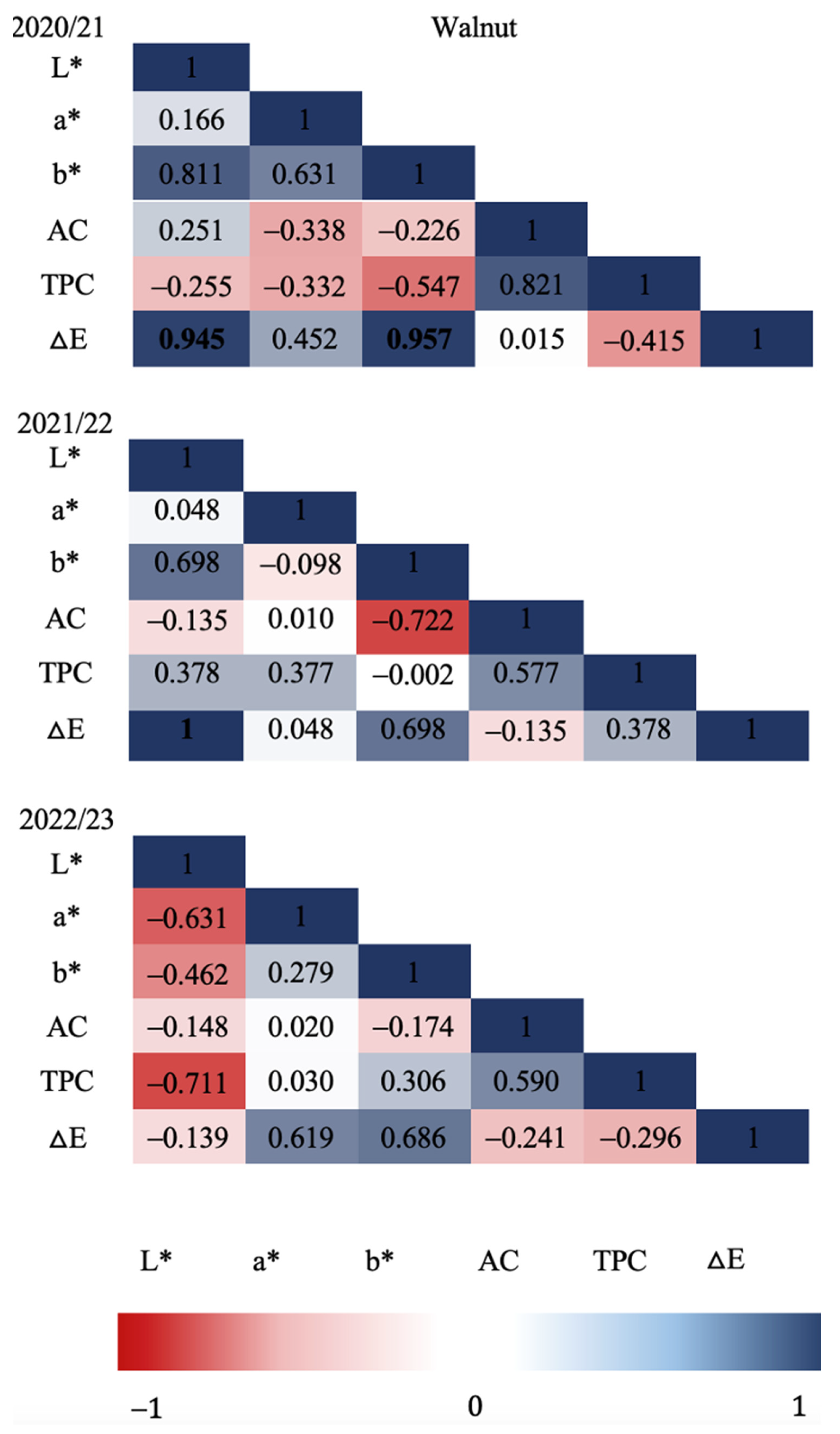
Figure 3 and
Figure 4 show the Pearson correlation coefficients in relation to all the variables evaluated in each production season for hazelnut shell samples. Strong significant correlations (P≤0.05) were found for the 2020/21 season between TPC and L* (CIE L*a*b* brightness parameter), and TPC and
ΔE (colorimetric change parameter) as well as for
ΔE with L* and with b*. For the 2021/22 season, strong significant correlations were determined, although mostly negative, as in the case of AC and a* with L*, determining a possible relationship of a* with other types of antioxidants present in European hazelnut shells. In relation to the color difference,
ΔE resulted in a positive correlation with b* showing a color change possibly related to a more yellow chroma. Regarding the last season evaluated (2022/23) no significant correlation was found between the variables evaluated. Significant correlations (P≤0.05) between
ΔE and L* and b* were found for walnut shells (
Figure 4) during the 2020/21 season, indicating a relationship between chroma difference with a higher lightness (L*) and possibly toward a more yellowish chroma. Similarly, during the 2021/22 season, a perfect positive correlation (1.00) was determined between
ΔE and L*, indicating a trend of chroma change towards a higher luminosity. In relation to the 2022/23 season, no significant correlations were found between any of the response variables evaluated in walnut shells.
3.5. Microstructure
3.5.1. Scanning Electron Microscopy (VP-SEM) of nutshell profiles
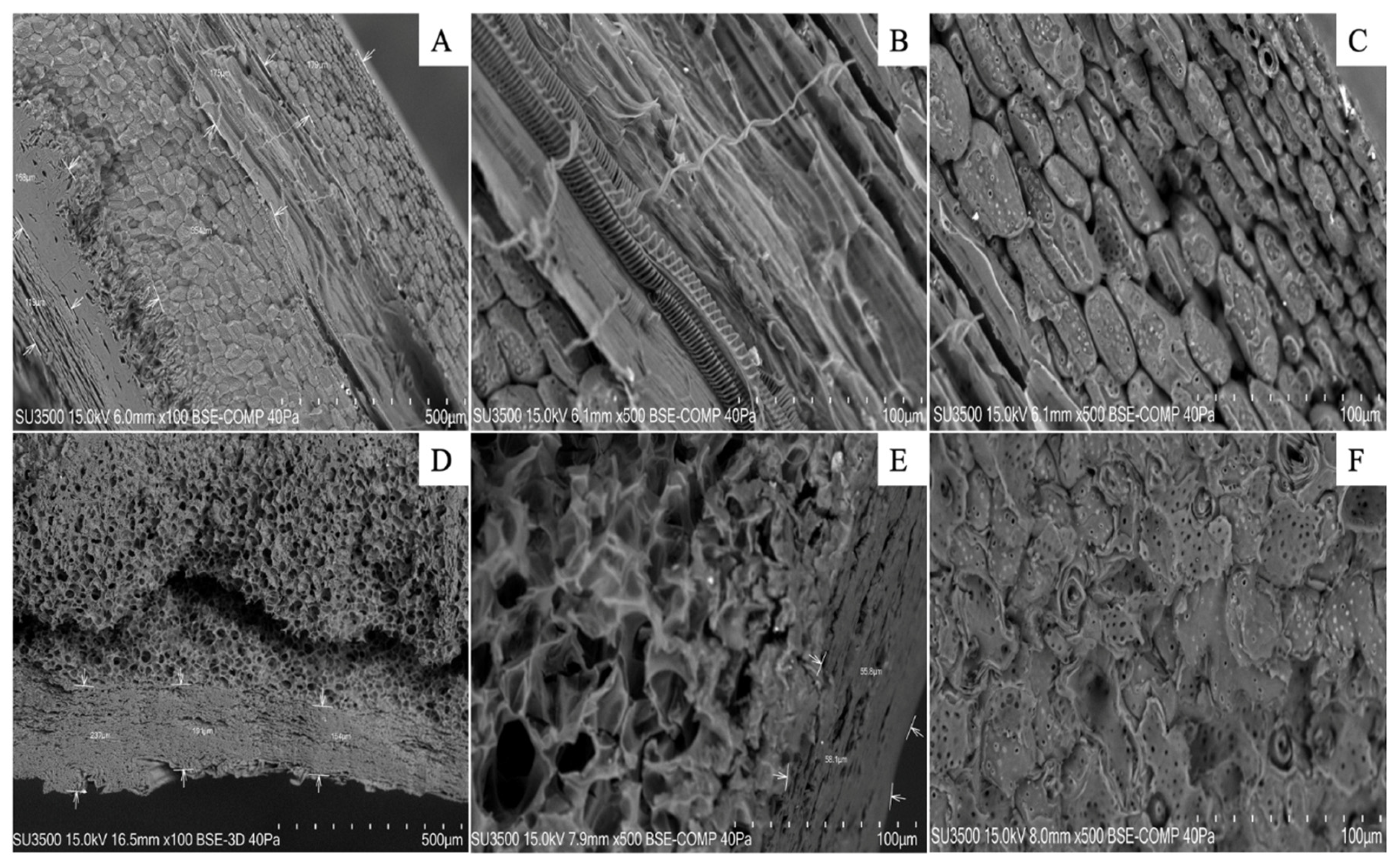
In
Figure 5A six different cellular layers are denoted which make the total profile of the hazelnut shell and represent different types of vegetal tissues. The observed thickness of the layers ranged between 179 μm and 175 μm for the first two, and 354 μm for the central ones. However, those closest to the core ranged between 168 μm and 119 μm in thickness. Conversely, the walnut shell profile was composed of only three cell layers with similar thickness to those closest to the exterior (between 154 μm and 237μm). Nevertheless, the layer closest to the nucleus was much thinner, around 55 μm. Each of the walnut layers was thicker than the hazelnut shells and with a higher porosity. The endocarp of the walnuts consists of stone cells, most with very thick cell walls, but towards the interior the cell walls become thinner and with relatively large pits (
Figure 5D). Also, a zone of flattened parenchyma cells could be observed inside the endocarp (
Figure 5E).
3.5.2. Lignin and Cellulose Accumulation Patterns in Nutshell Profiles Obtained by Confocal Laser Scanning Microscopy (CLSM)
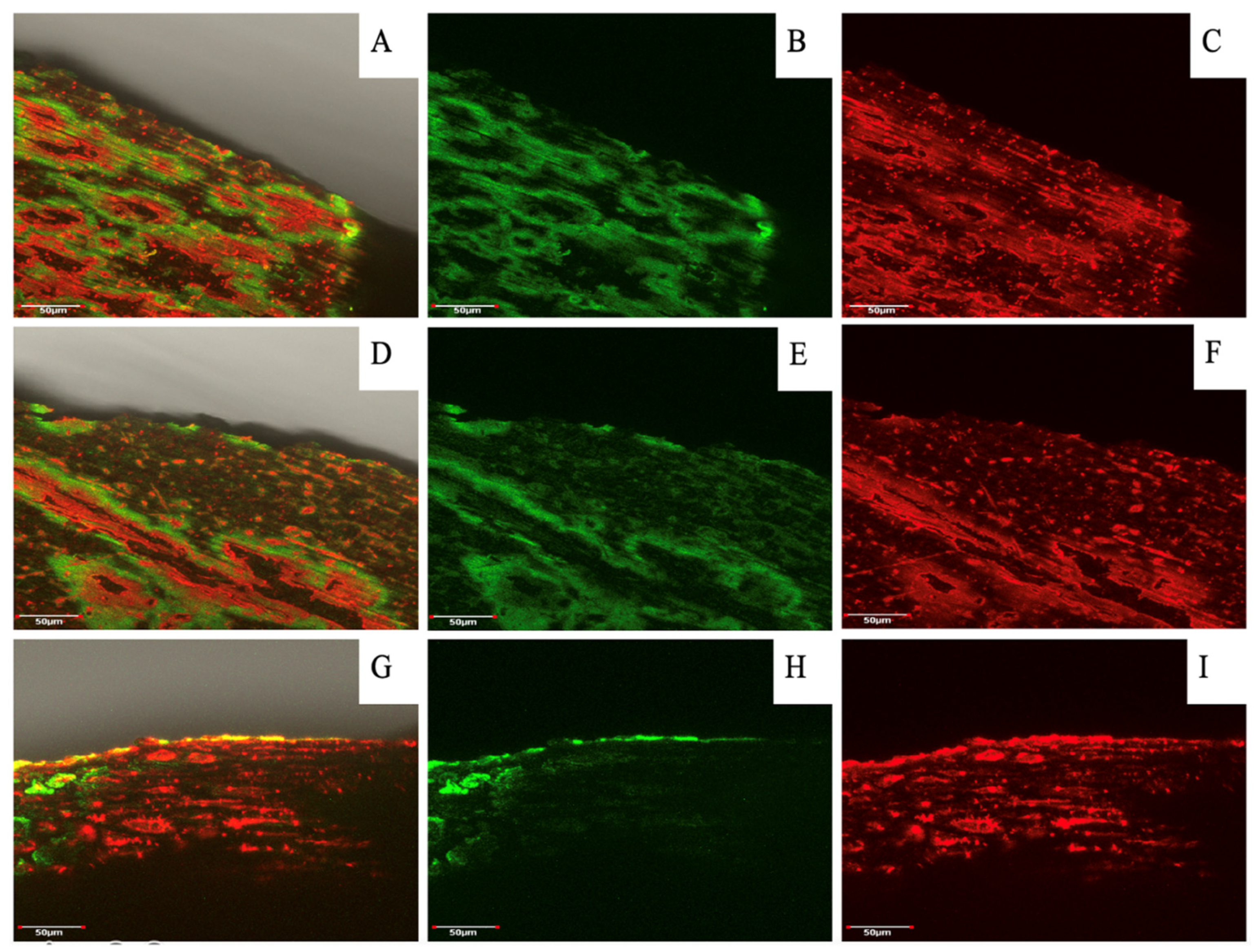
In addition to morphological observations, confocal microscopy provided insight into the composition of the shells in terms of lignin and cellulose distribution and accumulation. Comparing the images of SEM (
Figure 5) and lignin and cellulose staining (
Figure 6A,D,G) in hazelnut shell samples it was visible that the parenchyma cells contained huge amounts of cellulose while the vascular bundles, and cell walls, were the main points of lignin accumulation. An overlap between vascular and parenchyma tissues and the formation of three-dimensional shapes, could explain the distribution of the different fluorescence signals observed.
In relation to the effect of the three evaluated seasons on the relative fluorescence of lignin and cellulose in hazelnut shells, there was a slight decrease in lignin fluorescence and a greater decrease in cellulose fluorescence for the 2022/23 season compared to the two previous seasons. Whereas, the fluorescence in walnut shells had a fairly similar relative fluorescence of lignin and cellulose in the 2020/21 and 2022/23 seasons. However, in the 2021/22 season there was a higher fluorescence of lignin (green) and a lower fluorescence of celluloses (red). With respect to different seasons, the accumulation of lignin was the lowest in the 2022/23 season.
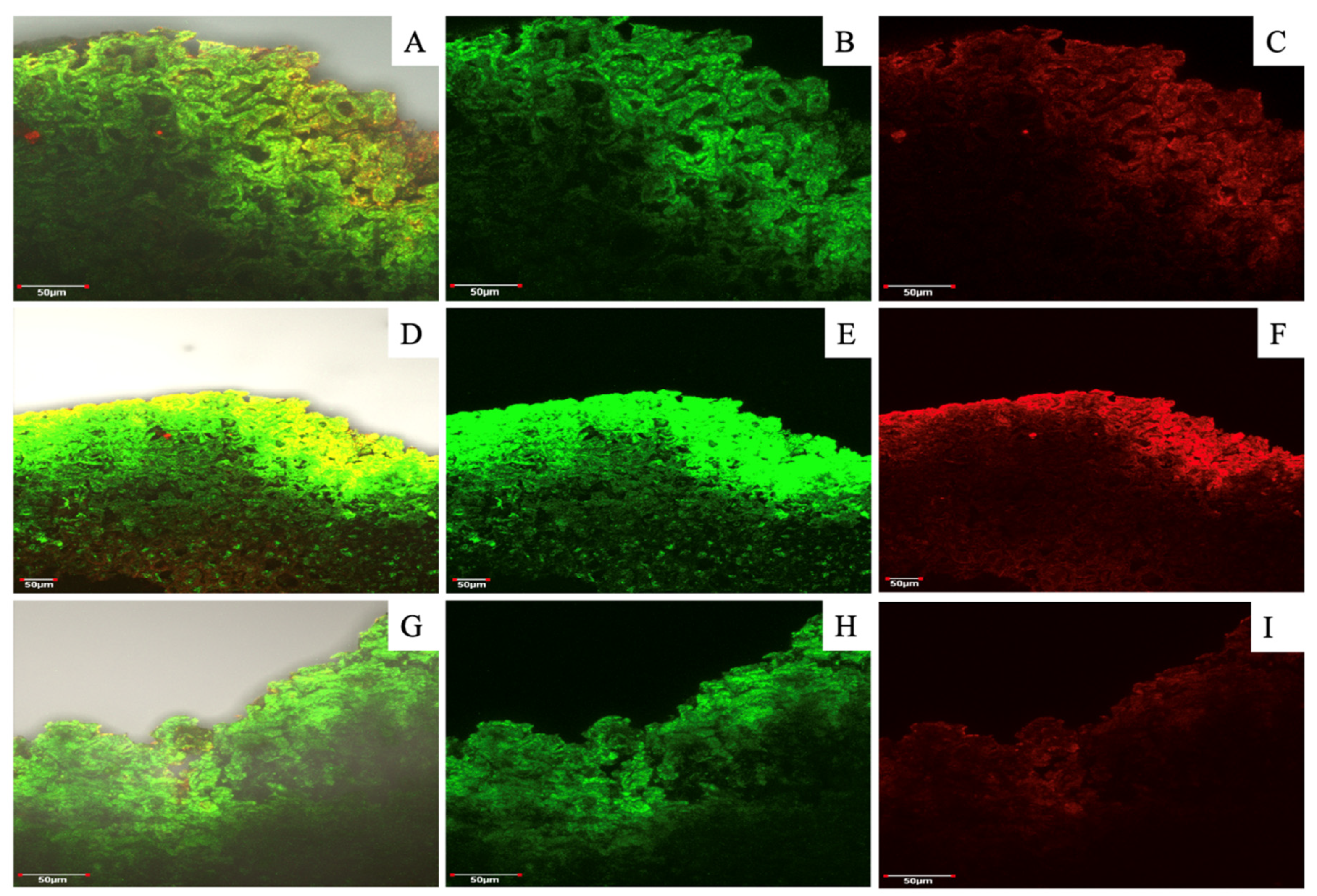
In relation to the walnut shell profile (
Figure 7) a different order and distribution of lignin and cellulose was observed compared to hazelnut. In the walnut shell, both compounds seemed to co-localize. Probably the surface layers contained the major relative concentrations of lignin and the deeper layer contained cellulose that visually appears to be sparse, or this is effect is due to confocal microscopy measurement which shows better the surface layers while the deeper ones don’t manifest very well.
4. Discussion
The present work revealed the potential of valorization of useful phytochemical features from agro-food by-products (nutshells) which could be used subsequently as a new alternative for construction materials design and development.
4.1. Total Phenolic Content and Antioxidant Capacity
Results are within the range in relation to the data reported by Contini et al. (2012), who estimated the content of phenolic compounds in the shells to around 2.7 mg GAE g‒1 DW for hazelnut cultivars Tonda Gentile Romana, Tonda di Giffoni, Tonda Gentile delle Langhe and Tombul and those reported by Manterola-Barroso et al. (2022)(between 0.1 to 0.28 mg GAE g‒1 DW). Moreover, values as high as around 19.38 mg GAE g‒1 have been reported for hazelnut shell extracts by Esposito et al. (2017). The values of TPC in walnut are in the range reported for these nutshells by Queirós et al. (2019) who reported TPC values around 31.79 mg GAE g‒1 for walnut shells and similarly Jalili et al. (2012) reported values ranging from 13.4 to 30.1 mg GAE g‒1 for seven cultivars of the same species planted in Iran. The content of phenolics can be determined by the genotype, time of harvest as well as abiotic and biotic factors (Manterola-Barroso et al., 2024). In fact, Manterola-Barroso et al. (2022) and Meriño-Gergichevich et al. (2021) demonstrated that the location and the agronomical management significantly affected the TPC content and other antioxidant features in hazelnut shell. Moreover, it is important to monitor the parameters of interest in time course due to variability of environmental factors, as shown in this study.
Finally, comparing the data obtained to other nutshells investigated, Prakash et al., 2018 reported total phenolic content (mg GAE g‒1) in cashew nut (Anacardium occidentale) shell cake, ranged from 32.97 to 35.82 mg GAE g‒1 results significantly higher than those determined in this study. Moreover. They proposed an extraction method (360 min extraction time, shaking + gentle heating conditions and methanol extraction solution) which is quite similar to the used in the present investigation. Conversely was the reported by Chandrasekara and Shahidi et al. (2011) who studied the effect of roasting on phenolic content of cashew nut testa and resulted ranges of total soluble phenolic content between 269 to 347 GAE mg g‒1 of defatted meal for commercial cashew nut (unknow genotype) testa extracts. Moreover, the increase in antioxidant capacity in walnut was correlated with the increase in the total phenolic content.
On the other hand, although TPC was similar between walnut and hazelnut shells, higher ORAC in walnut indicates that either the composition of phenolic compounds or the presence of other low molecular weight antioxidants (e.g phenolic acids, flavanols, anthocyanins and proanthocyanins) contributes to high antioxidant activity in those shells. These results together with the few variations between production seasons show a great stability of the antioxidant capacity in shells of both nuts, not increasing by more than 20% and not decreasing over time by more than 2%. To our knowledge, there is scarce or no available or published data regarding ORAC antioxidant capacity in nutshell samples or extracts. The difference between TPC and ORAC values indicates the need to evaluate the antioxidant properties of shells on different levels to be able to recommend the most suitable use.
ORAC values were higher for walnut than for hazelnut extracts, in all three studied seasons. Moreover, are also a little higher than the evaluated by Manterola-Barroso et al. (2022), who reported a mean of 2,119 µmol TE g‒1 DW in hazelnut trees (Tonda di Giffoni) planted in four localities of Southern Chile with different environmental conditions. The TPC was similar between hazelnut and walnut shells and the difference between TPC and ORAC values, indicates the need to determinate the phenolic compound involved in the hazelnut and walnut shells. To our knowledge, there is scarce published data regarding ORAC antioxidant capacity in hazelnut and walnut samples or extracts. Therefore, in relation to other nutshells, Cardullo et al. (2021) reported ORAC values around 1340 µmol TE g‒1 DW for Italian pistachio (Pistacia vera cv. Bronte) shell extract. While the ORAC values obtained by Chandrasekara and Shahidi et al. (2011) showed values around 74 mmol of TE g‒1 of defatted meal. Although, these values were obtained measuring ORAC of the phenolic extract, which explain the higher quantity of ORAC activity. Moreover, they determined very a strong positive relationship between ORAC and TPC (r2 = 0.977; P<0.001) between TPC and ORAC as well as the correlation resulted in this study for hazelnut and walnut shells.
On the other hand, Griffin and Dean et al. (2017) studied the nutritional and antioxidant composition of skin-on cashew nuts. They reported ORAC values around 42 μg TE g‒1. The increase of this value with respect to that obtained in skin-off cashew nut samples (85.8% lower) demonstrate the high antioxidant load present in the testa and consequently in the nut shells. Finally, Chirinos et al. (2016) reported ORAC maximum values for Sacha inchi (Plukenetia volubilis L.) shell samples of 192.6 μmol TE g‒1 by an extraction solution of ethanol/acetone/water/acetic acid (40/40/10/1, v/v/v/v). Therefore, reaffirming the efficacy of methanolic extracts for the extraction of antioxidant compounds.
4.2. Relationship between Antioxidants and Nutshell Color
In the case of hazelnut shells, a relationship between these parameters was found, although further studies are needed to understand the relationship between certain types of antioxidant compounds and colorimetric parameters, where compounds such as anthocyanins are probably involved in the coloring process of hazelnut shells (Jensen et al., 2008; Suriano et al., 2021). Suriano et al. (2021) observed a significant difference (around 300%) for TPC in diverse maize genotypes, ranging from 1359 ±34.4 μg CE (catechin equivalents) g‒1 DW of a yellow genotype to 4047 ± 26.8 μg CE g‒1 DW for a purple maize genotype (VA1245w x Morado). On the other hand, CIE Lab parameters were correlated by principal component analysis (PCA), resulting in a high correlation between phenolic compounds (flavanols and anthocyanidins) and VA1245w x Morado maize. This correlation, related to the results of our research, allows us to find a relationship between these phenolic compounds and certain darker color patterns linked to L* and b* parameters. On the other hand, Jensen et al. (2008) have demonstrated the high relationship between grape anthocyanins and total wine color (r=0.96) and the importance of anthocyanins, total phenols and flavanols in the color intensity of young wines, factors that actually are widely studied in fruits. Nevertheless, there are scarce reports of color assignment in nutshells and, to our knowledge, none for hazelnut and walnut shell samples. No correlations were found between colorimetric and antioxidant parameters in walnut shells (for all studied seasons). Although, for hazelnut shell interesting positive correlations were found between L* and TPC in two of the evaluated seasons, allowing us to explore a potential link between phenolic compounds and luminosity color parameter (L*), which is in agreement with several authors (Jensen et al., 2008; Suriano et al., 2022 and Suriano et al., 2021) whose have reported the participation of these compounds in browning process of fruits. In addition, it allows us to inquire an interaction and reactions between different antioxidants for the conformation and/or change of coloration parameters in function of time.
4.3. Nutshell Microstructure (SEM-VP and CLSM)
In relation to the physical and morphological features of studied hazelnut and walnut shells, the first one shows six different cellular layers which compose the total profile of the nutshell and represent different types of tissues. In the case of walnut shell profile, it was composed of only three cell layers, each of them thicker than the hazelnut ones and structurally with a higher porosity. In hazelnut shell samples parenchyma cells may contain huge amounts of cellulose while the vascular bundles have major contents of lignin and possibly, they are overlapping in three-dimensional shapes, which would explain the distribution of observed fluorescence signals. Relating the above to fluorescence assays it is possible to explain the overlapping of fluorescence by visualizing the images corresponding to MERGE, where the fluorescence intensity, that actually correspond to lignin, is observed on the surface possibly meaning an over-positioning of tissues rich in this compound over tissues related to cellulose presence. In this sense, walnut shell profile presented an aspect quite similar to that obtained by Nicolás-Bermúdez et al. (2018) who reported morphological and micromechanical characterization of Mexican pecan nut (
Carya illinoinensis) shells. The thick porous layers of walnut shell have a high potential for both the scientific community and industry, as shown for pecan nut shells which are used as activated carbon and have an excellent performance as bio-adsorbent of heavy metal ions such as Zn
2+, Cd
2+, Ni
2+ and Cu
2+ (Aguayo-Villareal et al., 2017; Zazycki et al., 2018). Other authors have analyzed the microstructure of commercial walnut shells and have been able to identify two layers of tissues (dense tissue and porous one) in the walnut shell profile (approximately 1 cm) (Antreich et al., 2019; Xiao et al., 2021). Quite similar to what was reported in this study, except for a third layer that was considered as a surface in this case. Moreover, Cortat et al. (2021) investigated the macadamia shell powder by SEM before milling and after being sieved by a 35 ASTM (American Society for Testing Material). They found particles with a rough surface and some irregular-shaped flake morphology and also a high porosity (
Figure 5), this justified by the lignocellulosic material. Regarding other nutshells evaluated, Andrade et al. (2021) demonstrated by SEM the surface of samples of pecan nut shells planted in Brazil (unknown genotype). They reported nutshell regions with pores whereas others showed an amorphous material, probably with high hemicellulose accumulation. Results consistent with those observed in both hazelnut and walnut shells by Sonego et al. (2020) and Sonego et al. (2021) who reported hierarchical levels of organization in the Brazilian nut (
Bertholletia excelsa) shells. In addition, these authors found similar microstructural features as reported in our study for studied walnut related to the distribution of cell layers in the nutshell. In relation to possible further applications of some nutshells in function of its microstructure, pecan nutshell has reported as a potential lignocellulosic fiber for polymeric composites development (Andrade et al., 2021) and as bio-absorbent of certain compounds (Aguayo-Villareal et al., 2017; Zazycki et al., 2018). With ecofriendly purpose for bio composites manufacturing, commercial Indian pistachio shell samples were analyzed by SEM and showed particles with a rough surface and some irregular-shaped flake morphology added to a high porosity (Balasundar et al., 2019) that was concomitant with our finds in hazelnut shells surface (
Figure 5) studied during three seasons.
Finally, these results reported above agree on certain common characteristics among several types of nut shells (pecan nut, cashew nut, macadamia nut, hazelnut and walnut), such as highly porous microstructure, in addition to cellular layers of irregular and agglomerated shapes, which could be explained by the high amount of structural and hard components, such as lignocellulosic compounds.
This study provided insight into antioxidant features, colorimetric parameters, morphology and structure of hazelnut and walnut shells from La Araucanía region through three productive seasons given their potential valorization as a residual material of the agro-industrial processing. The chemical composition of the nutshells showing high cellulose and lignin content as well as rich phenolic content and antioxidant capacity, suggests a potential application of these by-products in the biotechnological processes. However, further investigations regarding hemicelluloses and lignin fractionation and concentration will support such circular bio-economy pathways.
5. Conclusions
Regarding to the effect of the seasons evaluated on the antioxidant capacity and total phenolic compounds it was possible to establish certain differences between the evaluated seasons. In relation to walnut shell, an increasing tendency of both TPC and ORAC was observed throughout the evaluated seasons. The results point to the high potential of hazelnut and nutshell by-products for further use, based on the stability of the features investigated (hazelnut) or even their increase (walnut) in three consecutive years.
In both hazelnut and walnut, the color change parameter (ΔE) showed a significant upward increase according to the chronology of the studied seasons, while a* and b* showed a significant inclination towards a reddish and yellowish coloration in both nutshells.
The relative fluorescence of lignin and cellulose in hazelnut and walnut shells did not show significant differences across the seasons evaluated, indicating an equal distribution of these compounds across different production seasons and showing complex overlapping of layers rich in these compounds (3D shapes). In terms of microstructure in both shells, the number of cell layers was determined and the cell types were described, while high porosity of walnut shell was observed. However, more studies are needed to determine the relationship between microstructure and antioxidant compounds in order to provide future applications of walnut and hazelnut shells for industrial purposes.
Finally, the present work revealed the potential of valorization of useful phytochemical features from agro-food by-products (nutshells) which could be used subsequently as a new alternative for construction materials design and development.
Author Contributions
Conceptualization, C.M.-B. and C.M.-G.; methodology, C.M.-B., C.M.-G., K.G., D.A., E.S., and D.P.; formal analysis, C.M.-B., K.G. and C.M.-G.; writing-original draft, C.M.-B. C.M.-G. and F.M.; Investigation, C.M.-B., K.G., D.A., D.P. and C.M.-G.; Visualization and project administration, C.M.-B. and C.M.-G.; funding acquisition, C.M.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Fondef VIU20P0027 and Fondecyt 11160762, projects from the Agencia Nacional de Investigación y Desarrollo (ANID) and CORFO-16PTECFS-66647 from Corporación de Fomento de la Producción (CORFO). DIUFRO DI22-0045 and partially by DI22-2001 from Dirección de Investigación. The authors thank the Doctoral Program in Science of Natural Resources and to the Scientific and Technological Bioresource Nucleus (BIOREN-UFRO) from Universidad de La Frontera for their technical support. F.M was supported by grant KOROLID, CZ.02.1.01/0.0/0.0/15_003/0000336 and the Czech Academy of Sciences, RVO 60077344.
Acknowledgments
The authors would like to thank Scientific and Technological Bioresource Nucleus (BIOREN-UFRO) and its professional staff for providing us with help and knowledge about specific equipment and processes for this research; Frutícola Agrichile S.A.; Doctoral Program in Science of Natural Resources of the Universidad de La Frontera; VIU20P0027, CORFO 16PTECFS-66647, Fondecyt 11160762, DIUFRO DI22-0045 and DI22-2001 projects and their human and professional formation units; The Laboratory of Physiology and Plant Nutrition for Fruit Trees and its wonderful scientific and professional staff for their confidence and for making this original article possible.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Agrichile—Ferrero Hazelnut Company. Available online: https://agrichile.cl/avellano-europeo/. (Accessed on 12th June 2024).
- Aguayo-Villarreal, I. A., Bonilla-Petriciolet, A., and Muñiz-Valencia, R. (2017). Preparation of activated carbons from pecan nutshell and their application in the antagonistic adsorption of heavy metal ions. J. Mol. Struct, 230, 686-695. [CrossRef]
- Antreich, S. J., Xiao, N., Huss, J. C., Horbelt, N., Eder, M., Weinkamer, R., and Gierlinger, N. (2019). The puzzle of the walnut shell: a novel cell type with interlocked packing. J. Adv. Sci, 6(16), 1900644.
- Argenziano, R., Moccia, F., Esposito, R., D’Errico, G., Panzella, L., and Napolitano, A. (2022) Recovery of lignin with potent antioxidant properties from shells of edible nuts by a green ball milling/deep eutectic solvent (des) based protocol. Antioxidants, 11(10), 1860.
- Balasundar, P., Narayanasamy, P., Senthil, S., Al-Dhabi, N. A., Prithivirajan, R., Kumar, T., Ramkumar, and Bhat, K. S. (2019). Physico-chemical study of pistachio (Pistacia vera) nutshell particles as a bio-filler for eco-friendly composites. Mater. Res. Express, 6(10), 105339.
- Baran, Y., Gökçe, H. S., and Durmaz, M. (2020). Physical and mechanical properties of cement containing regional hazelnut shell ash wastes. J. Clean. Prod, 259, 120965.
- Barbu, M.C.; Reh, R., and Çavdar, A.D. (2017). Non-Wood Lignocellulosic Composites in Materials Science and Engineering: Concepts, Methodologies, Tools, and Applications; IGI Global: Hershey, PA, USA, 2017; pp. 947–977.
- Barczewski, M., Sałasińska, K., and Szulc, J. (2019). Application of sunflower husk, hazelnut shell and walnut shell as waste agricultural fillers for epoxy-based composites: A study into mechanical behavior related to structural and rheological properties. Polym. Test, 75, 1-11. [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E. and Berset, C.L.W.T. (1995). Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol, 28, 25–30.
- Cardullo, N., Leanza, M., Muccilli, V., and Tringali, C. (2021). Valorization of agri-food waste from pistachio hard shells: Extraction of polyphenols as natural antioxidants. Resources, 10(5), 45.
- Chandrasekara, N., and Shahidi, F. (2011). Effect of roasting on phenolic content and antioxidant activities of whole cashew nuts, kernels, and testa. J. Agric. Food Chem, 59(9), 5006-5014.
- ChileNut, Reportes de Embarques. (2023). Available online: https://www.chilenut.cl/our-harvest/ (Accessed on 26th December 2023).
- Chirinos, R., Necochea, O., Pedreschi, R., and Campos, D. (2016). Sacha inchi (Plukenetia volubilis L.) shell: an alternative source of phenolic compounds and antioxidants. Int. J. Food Sci. Technol., 51(4), 986-993.
- CIREN and ODEPA. Catastro Frutícola y Principales Resultados, Región de La Araucanía (2023). Available online: https://www.odepa.gob.cl/wp-content/uploads/2023/11/RegionMetropolitana-0911.pdf. Accessed on 25th December 2023). Debe ser referenciado como documento, no como página WEB.
- Contini, M.; Baccelloni, S.; Massantini, R., and Anelli, G. (2008) Extraction of natural antioxidants from hazelnut (Corylus avellana L.) shell and skin wastes by long maceration at room temperature. Food Chem. 110, 659–669. [CrossRef]
- Contini, M.; Baccelloni, S.; Frangipane, M.T.; Merendino, N., and Massantini, R. (2012) Increasing espresso coffee brew antioxidant capacity using phenolic extract recovered from hazelnut skin waste. J. Funct. Foods 4, 137–146.
- Cortat, L. O., Zanini, N. C., Barbosa, R. F., de Souza, A. G., Rosa, D. S., and Mulinari, D. R. (2021). A sustainable perspective for macadamia nutshell residues revalorization by green composites development. J. Polym. Environ, 29, 3210-3226.
- Crozier, A.; Yokota, T.; Jaganath, I. B.; Marks, S.; Saltmarsh, M.;Clifford, M. N. (2007). Secondary metabolites in fruits, vegetables, beverages and other plant-based dietary components. Plant Secondary Metabolites; Blackwell Publishing: Oxford, U.K, 2007; pp 208-302.
- De Prá Andrade, M., Piazza, D., and Poletto, M. (2021). Pecan nutshell: morphological, chemical and thermal characterization. J. Mater. Res. Technol, 13, 2229-2238.
- Demirkaya, E., Dal, O., and Yüksel, A. (2019). Liquefaction of waste hazelnut shell by using sub-and supercritical solvents as a reaction medium. J. Supercrit. Fluids, 150, 11-20.
- Domingos, I., Ferreira, J., Cruz-Lopes, L. P., and Esteves, B. (2022). Liquefaction and chemical composition of walnut shells. Open Agric, 7(1), 249 256.
- Du, F., and Tan, T. (2021). Recent Studies in Mechanical Properties of Selected Hard Shelled Seeds: A Review. JOM, 73(6), 1723-1735.
- Esposito T, Sansone F, Franceschelli S, Del Gaudio P, Picerno P, Aquino RP, and Mencherini, T. (2017). Hazelnut (Corylus avellana L.) shells extract: phenolic composition, antioxidant effect and cytotoxic activity on human cancer cell lines. Int J Mol Sci 18(2):392.
- FAOSTAT. (2020). Agriculture data. Available online: https://www.fao.org/faostat/en/#data/QCL (Accessed on February 09th, 2024).
- Frazzini, S., Zuorro, A., Panseri, S., Pavlovic, R., Sgoifo Rossi, C. A., and Rossi, L. (2023). Repurposing Hazelnut Waste Products for a Sustainable Economy: A Metabolomic Analysis of Cuticles and Shells to Highlight Their Antioxidant Potential and Inhibitory Activity against Verocytotoxic Escherichia coli. Sustainability, 15(4), 3268.
- Friedman, M. (2007). Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res, 51, 116-134.
- Griffin, L. E., and Dean, L. L. (2017). Nutrient composition of raw, dry-roasted, and skin-on cashew Nuts. J. Food Res, 6(6), 13-28.
- Held, P. (2006). Performing Oxygen Radical Absorbance Capacity Assays with SynergyTM HT. Application Note. BioTek Instruments. Inc. Winooski, VT.
- Jalili A, Heydari R, Sadeghzade A, and Alipour S (2012) Reducing power and radical scavenging activities of phenolic extracts from Juglans regia hulls and shells. Afr J Biotechnol 11(37):9040–9047.
- Jensen, J. S., Demiray, S., Egebo, M., and Meyer, A. S. (2008). Prediction of wine color attributes from the phenolic profiles of red grapes (Vitis vinifera). J. Agric. Food Chem, 56(3), 1105-1115. [CrossRef]
- Kara, Ş., and Erçelebi, E. A. (2013). Thermal degradation kinetics of anthocyanins and visual color of Urmu mulberry (Morus nigra L.). J. Food Eng, 116(2), 541-547.
- Lixia, H., Bo, L., and Shaojin, W. (2015). Kinetics of color degradation of chestnut kernel during thermal treatment and storage. Int. J. Agric. Biol. Eng, 8(4), 106-115.
- Manterola-Barroso, C., Godoy, K., Alarcón, D., Padilla, D., and Meriño-Gergichevich, C. (2022). Antioxidants in Shell and Nut Yield Components after Ca, Mg and K Preharvest Spraying on Hazelnut Plantations in Southern Chile. Plants, 11(24), 3536.
- Manterola-Barroso, C., Padilla-Contreras, D., Ondrasek, G., Horvatinec, J., Gavilán-Cuicui, G., and Meriño-Gergichevich, C. (2024). Hazelnut and Walnut Nutshell Features, as Emerging Added Value By-Products of the Nut Industry: A Review. Plants, 13(7), 1034.
- Matějka, V., Fu, Z., Kukutschová, J., Qi, S., Jiang, S., Zhang, X., and Lu, Y. (2013). Jute fibers and powderized hazelnut shells as natural fillers in non-asbestos organic non-metallic friction composites. Mater. Des, 51, 847-853.
- Meriño-Gergichevich, C., Luengo-Escobar, A., Alarcón, D., Reyes-Díaz, M., Ondrasek, G., Morina, F., and Ogass, K. (2021). Combined spraying of boron and zinc during fruit set and premature stage improves yield and fruit quality of European hazelnut cv. Tonda di Giffoni. Front. Plant Sci, 12, 984.
- Mittler, R.; Vanderauwera, S.; Gollery, M. and van Breusegem, F. (2004). Reactive oxygen gene network of plants. Trends Plant Sci, 9, 490–498.
- Müller, A.K.; Helms, U.; Rohrer, C.; Möhler, M.; Hellwig, F.; Glei, M.; Schwerdtle, T.; Lorkowski, S., and Dawczynski, C. (2020). Nutrient composition of different hazelnut cultivars grown in Germany. Foods, 9, 1596.
- Nicolás-Bermúdez, J., Arzate-Vázquez, I., Chanona-Pérez, J. J., Méndez-Méndez, J. V., Rodríguez-Castro, G. A., and Martínez-Gutiérrez, H. (2018). Morphological and micromechanical characterization of calcium oxalate (CaOx) crystals embedded in the pecan nutshell (Carya illinoinensis). Plant. Physiol. Biochem, 132, 566-570. [CrossRef]
- Nuts Dried Fruits Statistical Yearbook. International Nut and Dried Fruit Council. (2023). Available online: https://inc.nutfruit.org/technical-projects/. Accessed on 25th April 2023.
- Prakash, A., Vadivel, V., Banu, S. F., Nithyanand, P., Lalitha, C., and Brindha, P. (2018). Evaluation of antioxidant and antimicrobial properties of solvent extracts of agro-food by-products (cashew nut shell, coconut shell and groundnut hull). Agric. Nat. Resour, 52(5), 451-459.
- Queirós, C. S., Cardoso, S., Lourenço, A., Ferreira, J., Miranda, I., Lourenço, M. J. V., and Pereira, H. (2020). Characterization of walnut, almond, and pine nut shells regarding chemical composition and extract composition. Biomass Convers. Biorefin, 10(1), 175-188.
- Rice-Evans, C., Miller, N.J. and Paganga, G. (1996) Structure antioxidant activity relationships of flavonoids and phenolic acids, Free Rad. Biol. Med. 20, 933-956.
- Rivas, S., Moure, A., and Parajó, J. C. (2020). Pre-treatment of hazelnut shells as a key strategy for the solubilization and valorization of hemicelluloses into bioactive compounds. Agronomy, 10(6), 760.
- Sadzawka, A.; Carrasco, M.; Demanet, R.; Flores, H.; Grez, R.; Mora, M.L. and Neaman, A. (2007). Métodos de análisis de tejidos vegetales. Ser. Actas INIA, 40, 140.
- Sette, P., Fernandez, A., Soria, J., Rodriguez, R., Salvatori, D., and Mazza, G. (2020). Integral valorization of fruit waste from wine and cider industries. J. Clean Prod, 242, 118486.
- Silvestri, C.; Bacchetta, L.; Bellincontro, A.; Cristofori, V. Advances in cultivar choice, hazelnut orchard management and nuts storage for enhancing product quality and safety: An overview. J. Sci. Food Agric 2020, 101, 27–43. [Google Scholar]
- Singleton, V.L. and Rossi, J.A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158.
- Sonego, M., Fleck, C., and Pessan, L. A. (2020). Hierarchical levels of organization of the Brazil nut mesocarp. Sci. Rep, 10(1), 6786.
- Sonego, M., Madia, M., Eder, M., Fleck, C., and Pessan, L. A. (2021). Microstructural features influencing the mechanical performance of the Brazil nut (Bertholletia excelsa) mesocarp. J. Mech. Behav. Biomed, 116, 104306.
- Suriano, S., Balconi, C., Valoti, P., and Redaelli, R. (2021). Comparison of total polyphenols, profile anthocyanins, color analysis, carotenoids and tocols in pigmented maize. LWT Food Sci. Technol, 144, 111257.
- USDA, Tree nuts: World Markets and Trade. (2022). https://usda.library.cornell.edu/concern/publications/tm70mv16z?locale=en (Accessed on November 03rd, 2022).
- Wu, D., and Sun, D. W. (2013). Colour measurements by computer vision for food quality control–A review. Trends in JFST, 29(1), 5-20.
- Xiao, N., Felhofer, M., Antreich, S. J., Huss, J. C., Mayer, K., Singh, A., Bock, P., and Gierlinger, N. (2021). Twist and lock: nutshell structures for high strength and energy absorption. R. Soc. Open Sci, 8(8), 210399.
- Yuan, B., Lu, M., Eskridge, K. M., Isom, L. D., and Hanna, M. A. (2018). Extraction, identification, and quantification of antioxidant phenolics from hazelnut (Corylus avellana L.) shells. Food Chem, 244, 7-15. [CrossRef]
- Zazycki, M. A., Godinho, M., Perondi, D., Foletto, E. L., Collazzo, G. C., and Dotto, G. L. (2018). New biochar from pecan nutshells as an alternative adsorbent for removing reactive red 141 from aqueous solutions. J. Clean. Prod, 171, 57-65.
Figure 1.
Weather parameters among studied seasons in Freire, La Araucanía region, Chile, which includes the two evaluated localities: Campo Experimental Maquehue (38° 50′ 28.5” S, 72° 41′ 40.4” W; Altitude: 200 m.a.s.l.) and Radal (39º 10′ 06” S, 72º 19′ 11” W; Altitude: 168 m.a.s.l.). Data were provided by Frutícola Agrichile S.A—Ferrero Hazelnut Company.
Figure 1.
Weather parameters among studied seasons in Freire, La Araucanía region, Chile, which includes the two evaluated localities: Campo Experimental Maquehue (38° 50′ 28.5” S, 72° 41′ 40.4” W; Altitude: 200 m.a.s.l.) and Radal (39º 10′ 06” S, 72º 19′ 11” W; Altitude: 168 m.a.s.l.). Data were provided by Frutícola Agrichile S.A—Ferrero Hazelnut Company.
Figure 2.
The total phenolic content (µg GAE g−1 DW) and ORAC (µmol TE g‒1 DW) in hazelnut (Tonda di Giffoni) and walnut (Franquette) shell samples from La Araucanía region orchards. Bars represent the average of five replicates ± S.E. Different letters indicate statistical differences (P ≤ 0.05) between three different productive seasons.
Figure 2.
The total phenolic content (µg GAE g−1 DW) and ORAC (µmol TE g‒1 DW) in hazelnut (Tonda di Giffoni) and walnut (Franquette) shell samples from La Araucanía region orchards. Bars represent the average of five replicates ± S.E. Different letters indicate statistical differences (P ≤ 0.05) between three different productive seasons.
Figure 3.
Hazelnut shell Pearson’s correlation coefficient matrix among seasons and all variables. Correlation was calculated for three productive seasons (2020/21, 2021/22 and 2022/23). Pearson’s coefficients that are significant at P≤0.05 are indicated by bold numbers. Positive and negative correlations are distinguished by blue and red colors respectively.
Figure 3.
Hazelnut shell Pearson’s correlation coefficient matrix among seasons and all variables. Correlation was calculated for three productive seasons (2020/21, 2021/22 and 2022/23). Pearson’s coefficients that are significant at P≤0.05 are indicated by bold numbers. Positive and negative correlations are distinguished by blue and red colors respectively.
Figure 4.
Walnut shell Pearson’s correlation coefficient matrix among seasons and all variables. Correlation was calculated for three productive seasons (2020/21, 2021/22 and 2022/23). Pearson’s coefficients that are significant at P≤0.05 are indicated by bold numbers. Positive and negative correlations are distinguished by blue and red colors respectively.
Figure 4.
Walnut shell Pearson’s correlation coefficient matrix among seasons and all variables. Correlation was calculated for three productive seasons (2020/21, 2021/22 and 2022/23). Pearson’s coefficients that are significant at P≤0.05 are indicated by bold numbers. Positive and negative correlations are distinguished by blue and red colors respectively.
Figure 5.
Scanning electron microscopy (SEM) visualization of nutshell profiles (hazelnut A-C, and walnut D-F). Images were zoomed to 500 µm (A and D) and to 100 µm (B, C, E and F). The arrows point to different cell layers in each sample. Analyses were carried out at Microscopy and Flow Cytometry Unit, BIOREN-UFRO, Universidad de La Frontera, Temuco, Chile.
Figure 5.
Scanning electron microscopy (SEM) visualization of nutshell profiles (hazelnut A-C, and walnut D-F). Images were zoomed to 500 µm (A and D) and to 100 µm (B, C, E and F). The arrows point to different cell layers in each sample. Analyses were carried out at Microscopy and Flow Cytometry Unit, BIOREN-UFRO, Universidad de La Frontera, Temuco, Chile.
Figure 6.
Hazelnut shell profiles after dyeing and fluorescence visualization assay by laser confocal microscopy. Figure A, D and G correspond to a MERGE (overlap of both dying solutions) of nutshell profiles in relation to three seasons (2020/21, 2021/22 and 2022/23 respectively), while figures B, C, E, F, H and I correspond to the same picture with separate dyeing. Staining solution used were “red congo” (red) for cellulose and “safranin” (green) for lignin. Analyses were carried out at Microscopy and Flow Cytometry Unit, BIOREN-UFRO, Universidad de La Frontera.
Figure 6.
Hazelnut shell profiles after dyeing and fluorescence visualization assay by laser confocal microscopy. Figure A, D and G correspond to a MERGE (overlap of both dying solutions) of nutshell profiles in relation to three seasons (2020/21, 2021/22 and 2022/23 respectively), while figures B, C, E, F, H and I correspond to the same picture with separate dyeing. Staining solution used were “red congo” (red) for cellulose and “safranin” (green) for lignin. Analyses were carried out at Microscopy and Flow Cytometry Unit, BIOREN-UFRO, Universidad de La Frontera.
Figure 7.
Walnut shell profiles after dyeing and fluorescence visualization assay by laser confocal microscopy. Figures A, D and G correspond to a MERGE (overlap of both dying solutions) of nutshell profiles in relation to three seasons (2020/21, 2021/22 and 2022/23 respectively), while figures B, C, E, F, H and I correspond to the same picture with separate dyeing. Staining solution used were “red congo” (red) for cellulose and “safranin” (green) for lignin. Analyses were carried out at Microscopy and Flow Cytometry Unit, BIOREN-UFRO, Universidad de La Frontera, Temuco, Chile.
Figure 7.
Walnut shell profiles after dyeing and fluorescence visualization assay by laser confocal microscopy. Figures A, D and G correspond to a MERGE (overlap of both dying solutions) of nutshell profiles in relation to three seasons (2020/21, 2021/22 and 2022/23 respectively), while figures B, C, E, F, H and I correspond to the same picture with separate dyeing. Staining solution used were “red congo” (red) for cellulose and “safranin” (green) for lignin. Analyses were carried out at Microscopy and Flow Cytometry Unit, BIOREN-UFRO, Universidad de La Frontera, Temuco, Chile.
Table 1.
Colorimetric parameters (L*, a*, b* and ΔE) obtained for hazelnut and walnut shell samples in relation to three productive seasons, data was obtained in CIE L*a*b* color scale. Where: L*=luminosity, a*= red/green coordinates (+a indicates red, −a indicates green), b*= coordinates yellow/blue (+b indicates yellow, −b indicates blue) while ΔE correspond to the difference between colorimetric parameters. Values represent the average of five replicates ± S.E. Different low letters indicate statistical differences (P≤0.05) between the three seasons.
Table 1.
Colorimetric parameters (L*, a*, b* and ΔE) obtained for hazelnut and walnut shell samples in relation to three productive seasons, data was obtained in CIE L*a*b* color scale. Where: L*=luminosity, a*= red/green coordinates (+a indicates red, −a indicates green), b*= coordinates yellow/blue (+b indicates yellow, −b indicates blue) while ΔE correspond to the difference between colorimetric parameters. Values represent the average of five replicates ± S.E. Different low letters indicate statistical differences (P≤0.05) between the three seasons.
| Species |
Season |
L* |
a* |
b* |
ΔE |
| Hazelnut |
2020/21 |
51.33±0.35a |
7.71±0.03b |
21.59±0.12b |
54.95±0.26b |
| 2021/22 |
52.03±0.14a |
7.82±0.04b |
21.85±0.06b |
55.68±0.06b |
| 2022/23 |
52.31±0.10a |
8.32±0.05a |
22.37±0.08a |
56.84±0.12a |
| |
|
|
|
|
|
| Walnut |
2020/21 |
46.83±0.42b |
4.58±0.03c |
23.13±2.32b |
55.65±0.27c |
| 2021/22 |
58.07±0.47a |
4.85±0.04b |
24.20±0.18b |
58.09±0.35b |
| 2022/23 |
59.15±0.50a |
5.41±0.06a |
25.32±0.06a |
60.31±0.12a |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).