Submitted:
13 June 2024
Posted:
14 June 2024
You are already at the latest version
Abstract
Keywords:
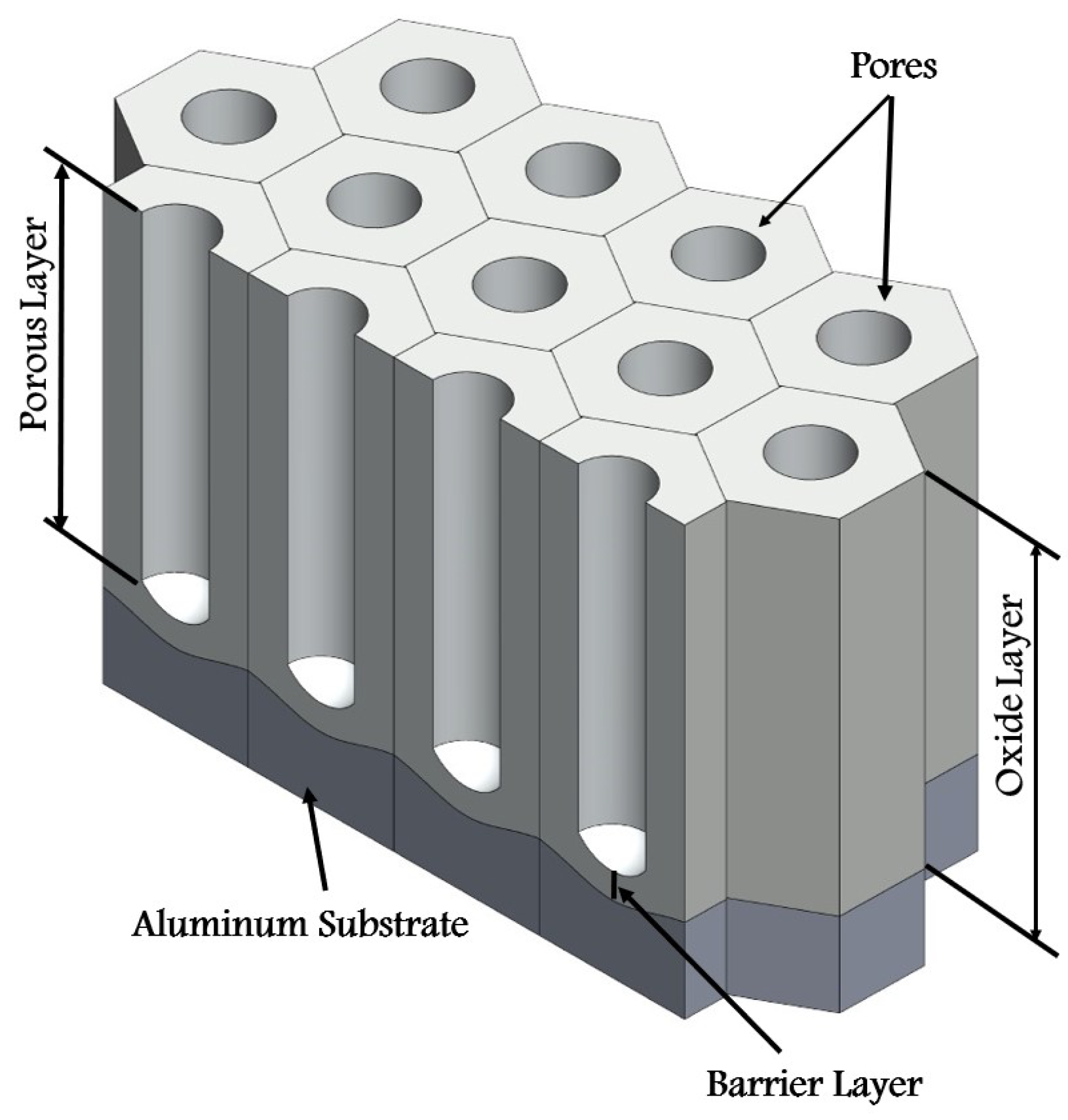
1. Introduction
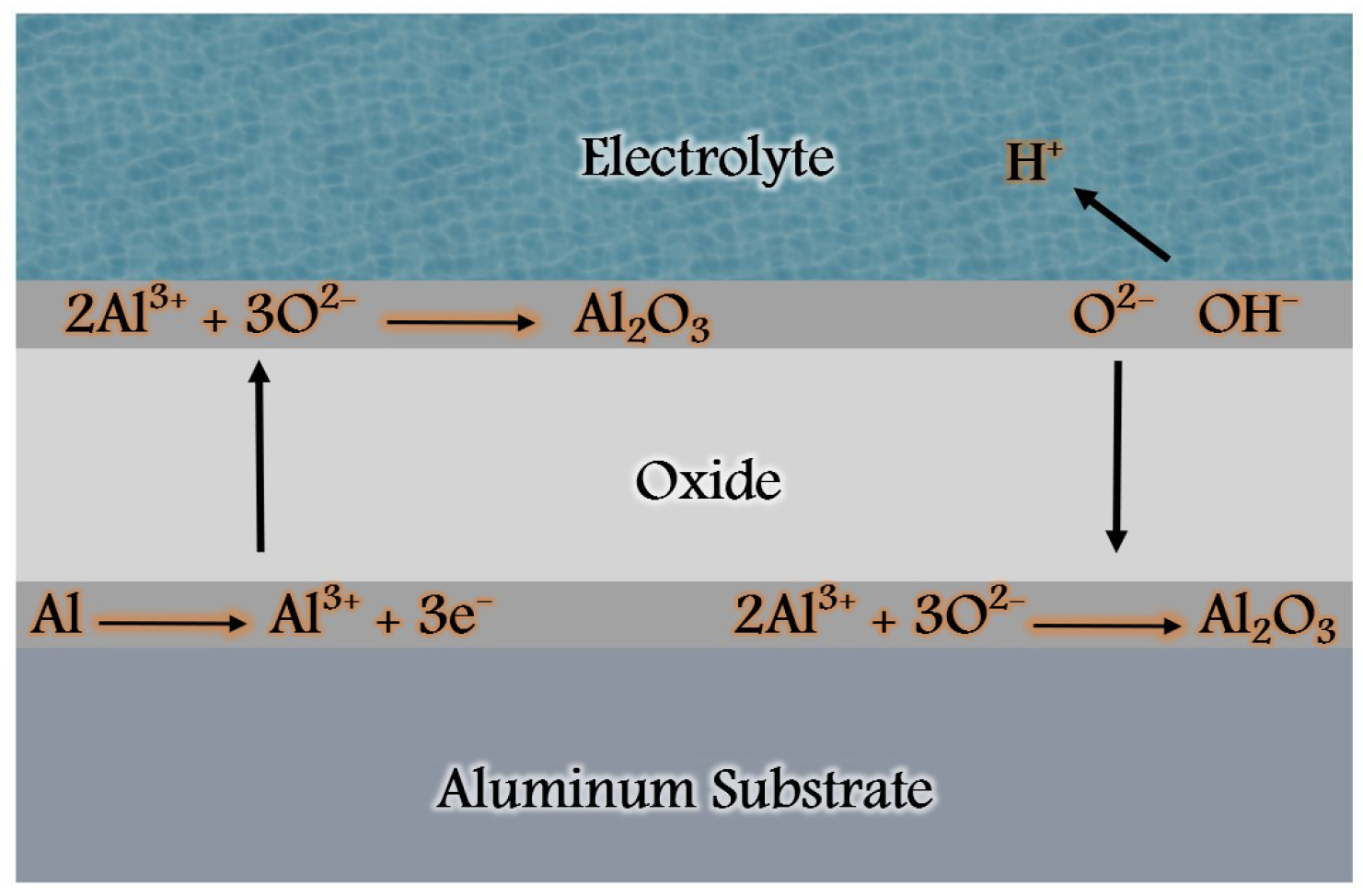
- Initially, aluminum cations (Al3+) are generated from the aluminum substrate acting as the anode.
- In the presence of a strong electric field, aluminum cations undergo migration towards the cathode, while anions present in the aqueous solution (such as O2-, OH-, and electrolyte anions) move in the opposite direction. At the interfaces of the metal/oxide and oxide/electrolyte, the Al3+ cations react with the anions, giving rise to the formation of aluminum oxide (Al2O3).2Al3+ + 3O2- = Al2O3 (at the metal/oxide interface)2Al3+ + 3H2O= Al2O3+ 6H+ (at the oxide/electrolyte interface)
- Simultaneously, at the oxide/electrolyte interface, the aluminum oxide has the potential to dissolve within the electrolyte, leading to the creation of a porous structure. This chemical dissolution process is governed by the equation:Al2O3 + 6H+ = 2Al3+ + 3H2O
2. Effect of the Alloy Second-Phase Particles
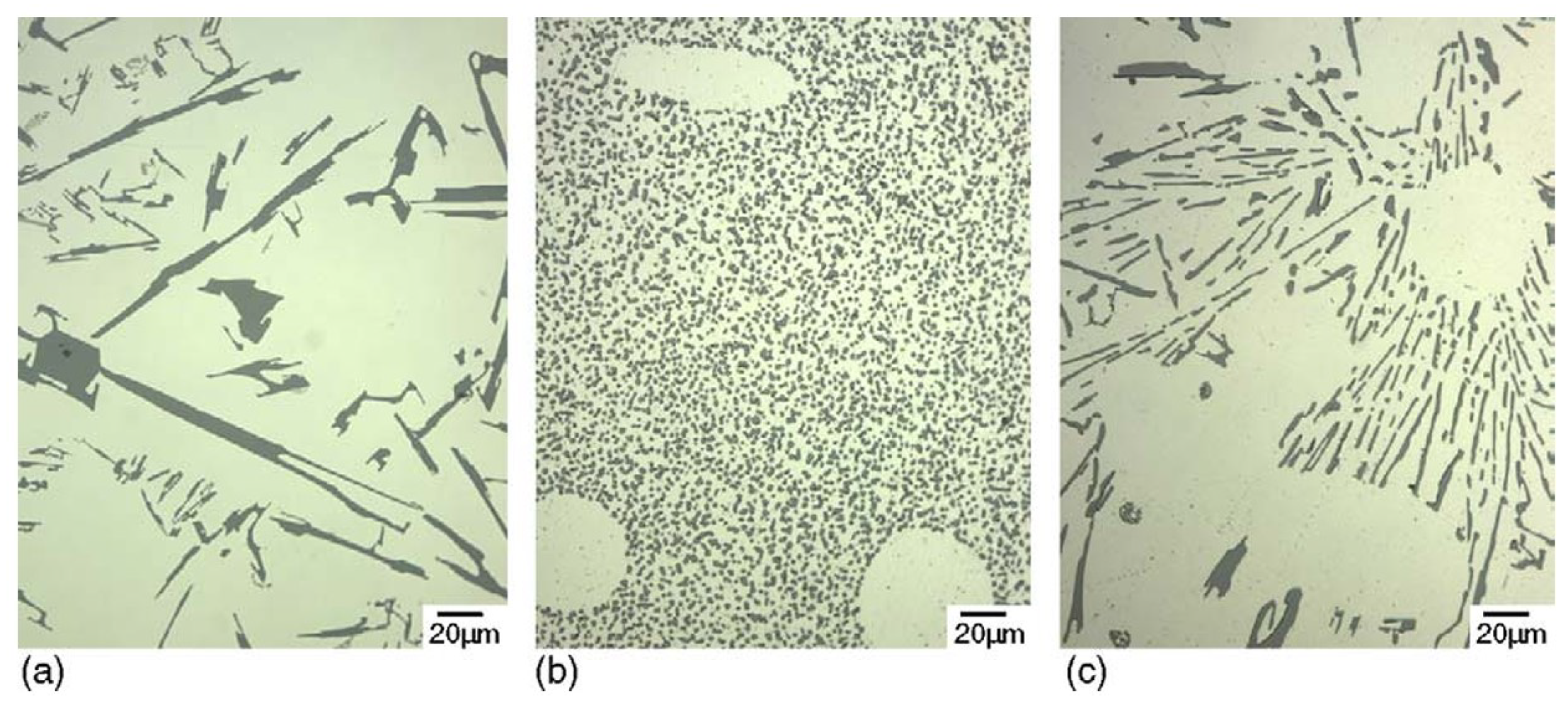
2.1. Silicon Particles
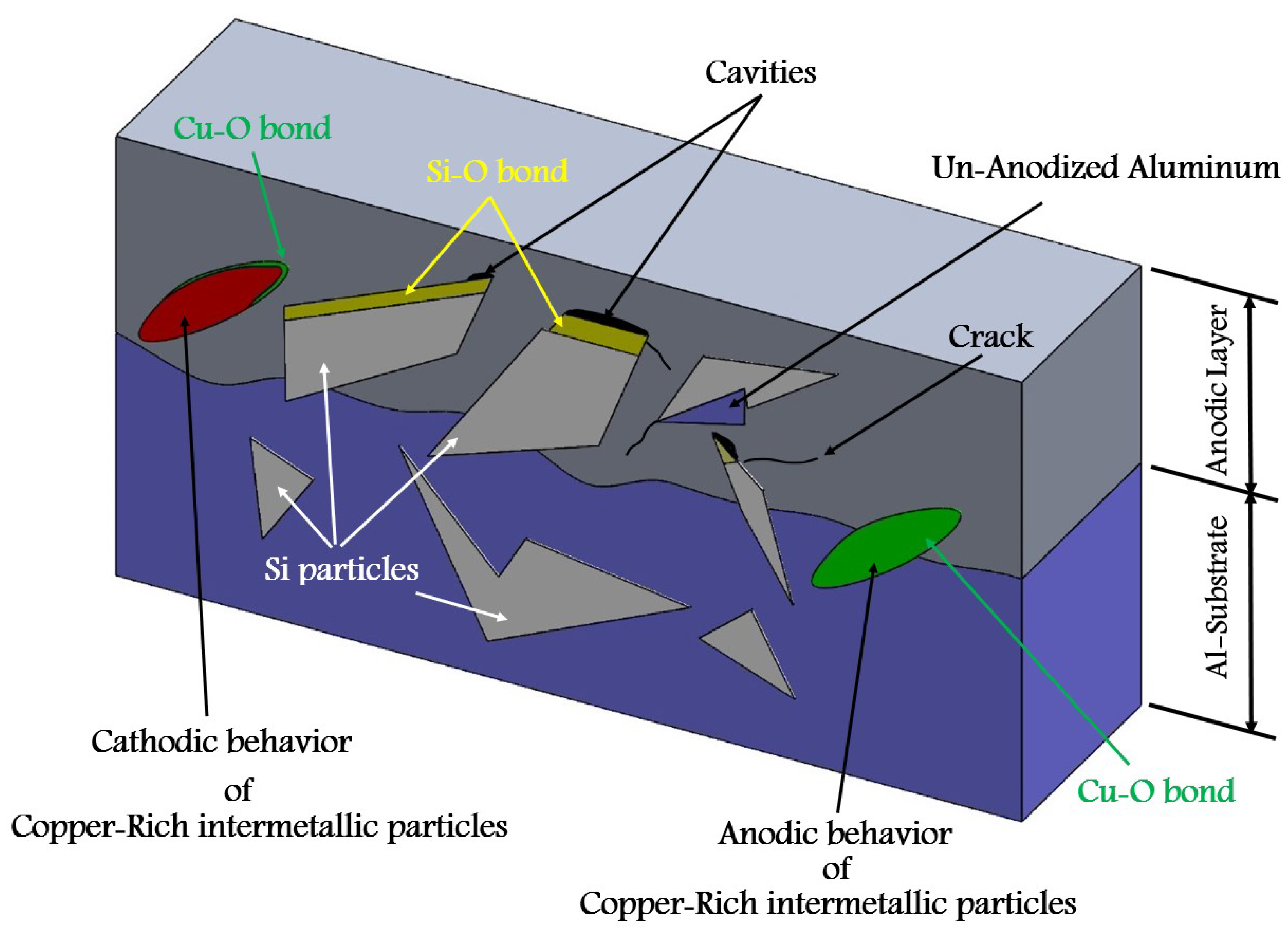
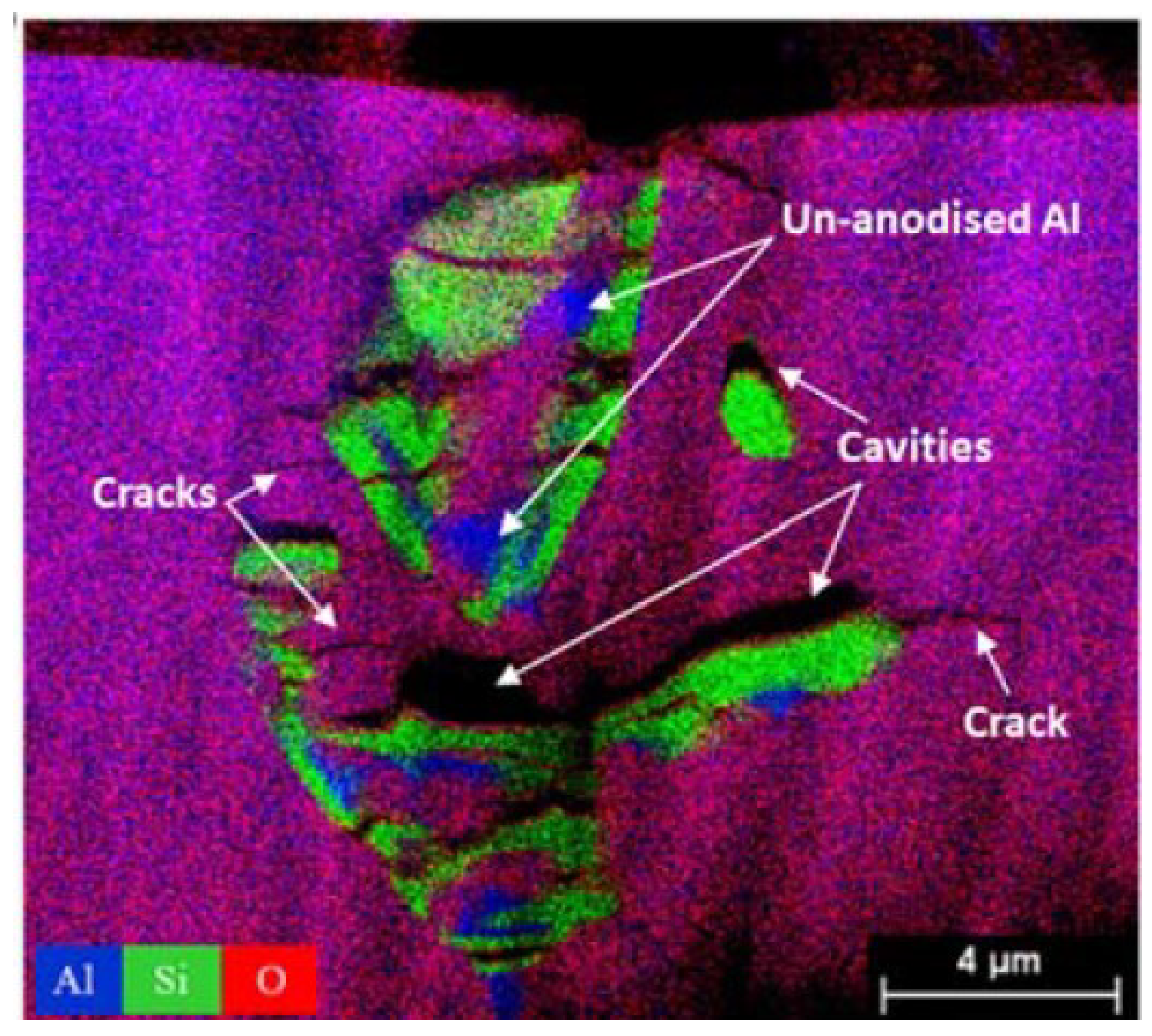
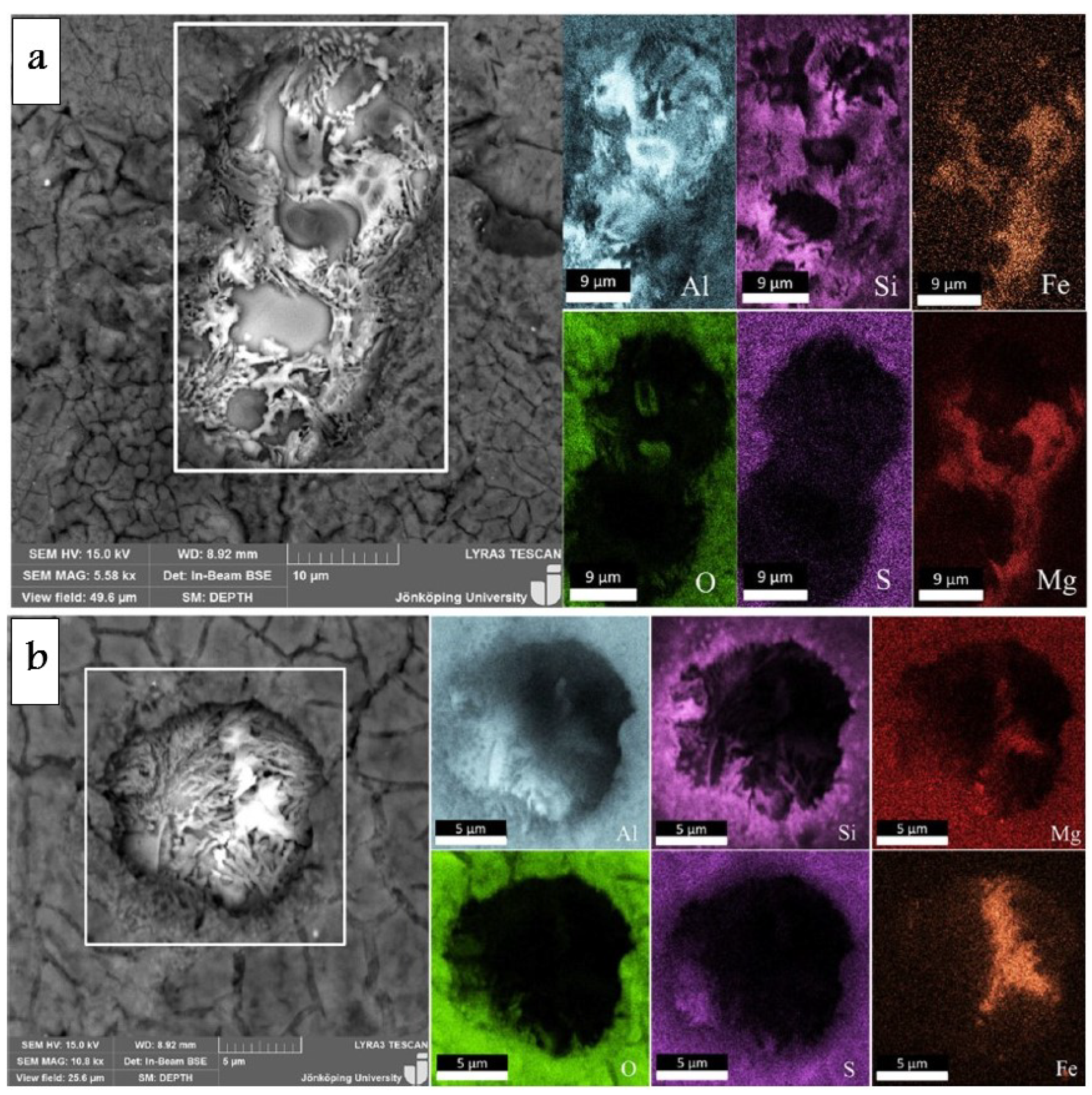
- Formation of oxygen gas-filled voids: When the oxide front interacts with the silicon phase, it leads to the generation of both SiO2 and gaseous oxygen due to the semiconductor properties of the Si-O bond. Consequently, oxygen gas-filled voids emerge in the aluminum substrate in proximity to the Si particles [35,36,37] as depicted in Figure 3.
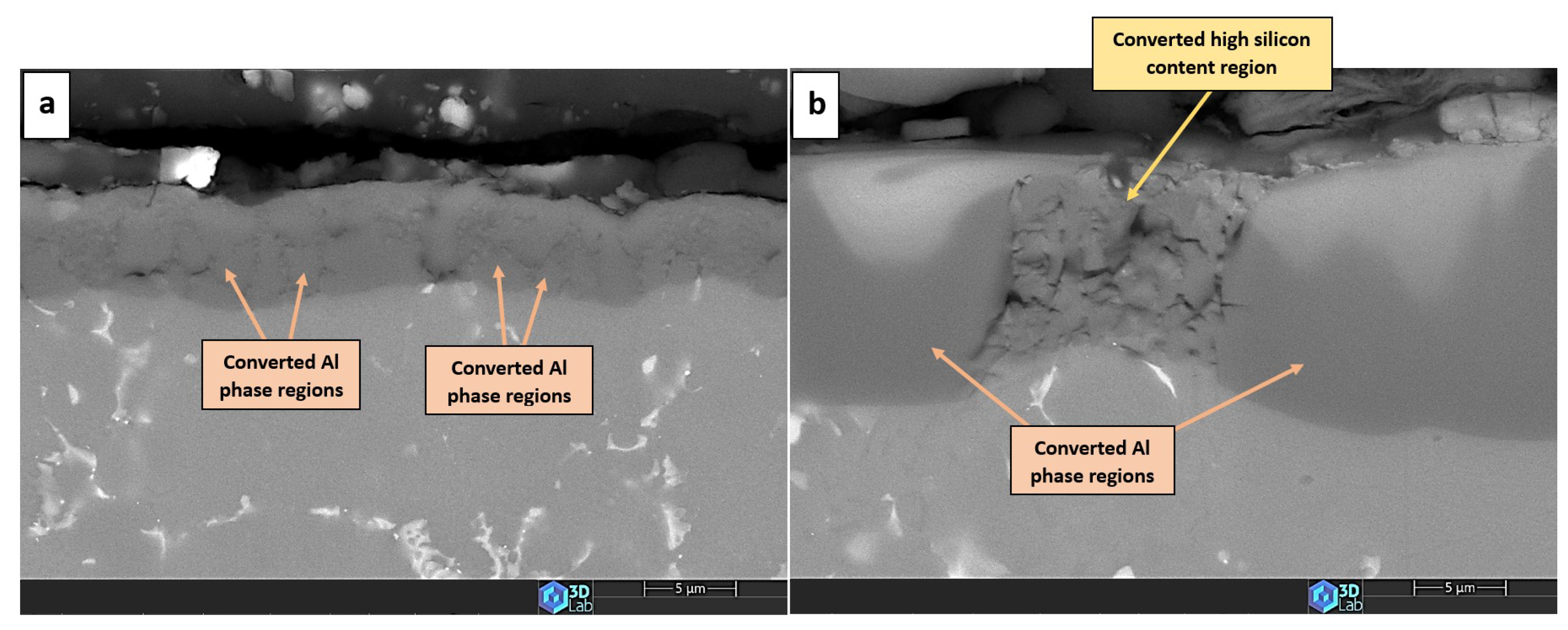
- Creation of un-anodized zones: Un-anodized zones form when the oxide front fails to entirely encircle the Si phase, possibly due to its shape or reduced spacing between particles. Consequently, the eutectic silicon phase acts as a barrier shielding the adjacent Al-matrix, preventing it from being reached by the oxide front and thus remaining un-anodized. Residual metallic Al phase is predominantly detected beneath or amid coarse and interconnected Si eutectic particles [36,37].
2.2. Iron-Rich Intermetallic Particles
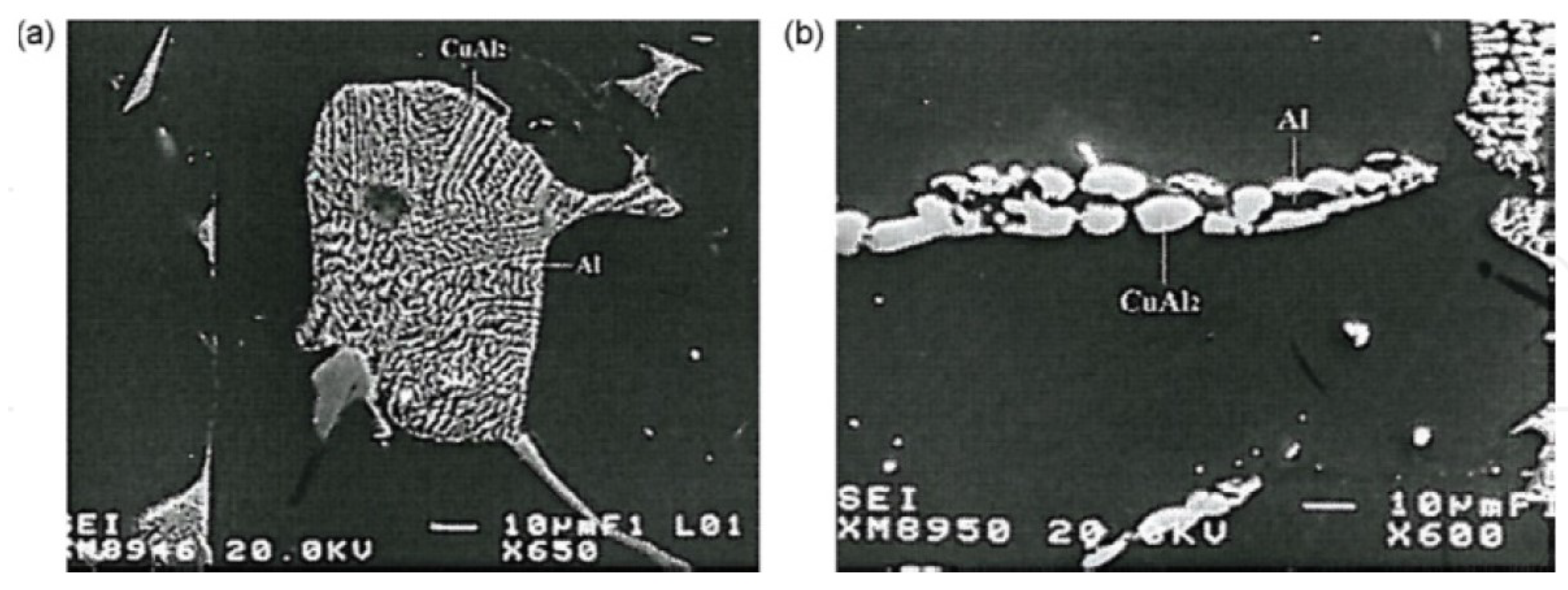
2.3. Copper-Rich Intermetallic Particles
3. Influence of Processing Prior to the Anodizing Process
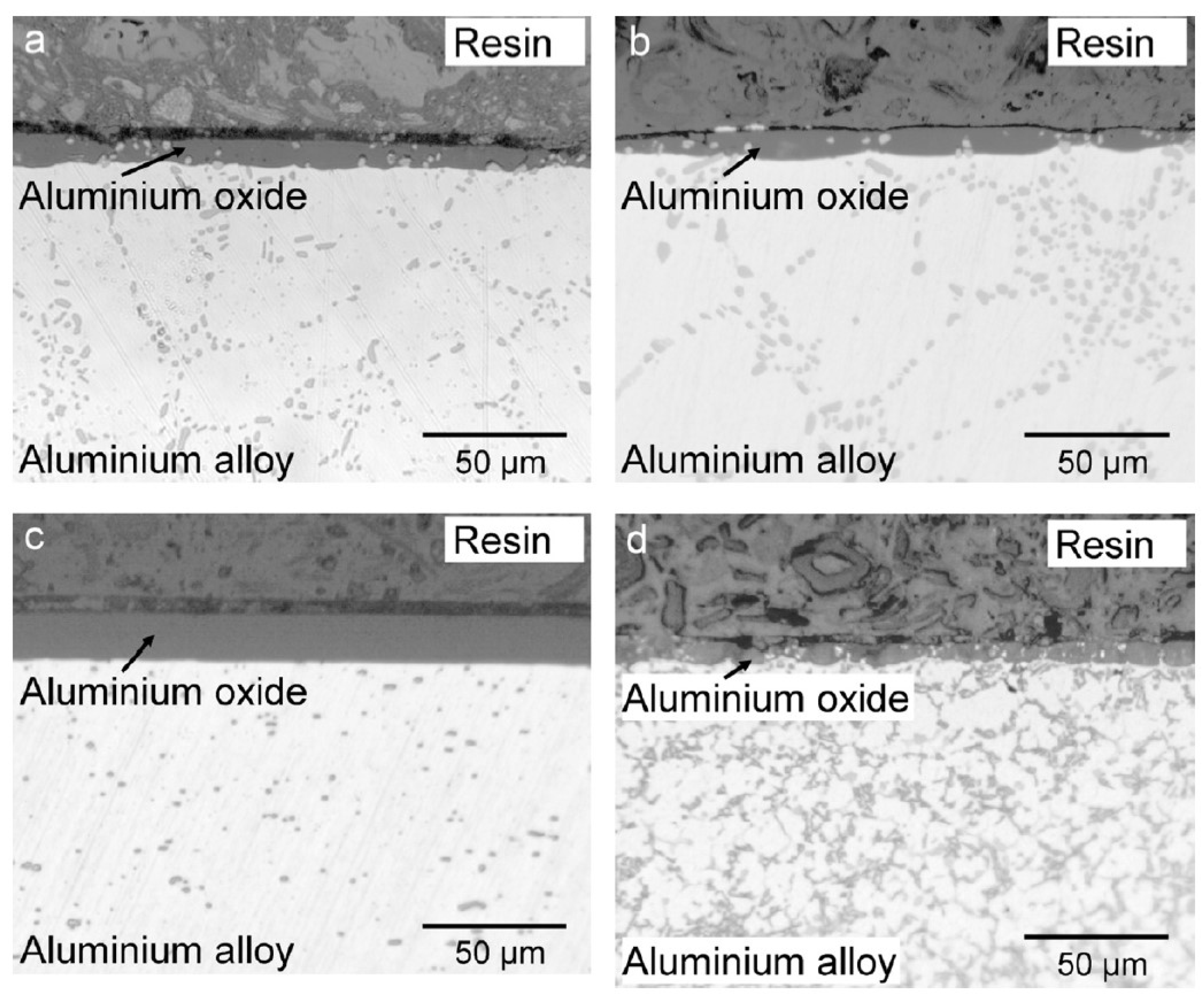
3.1. Casting Process
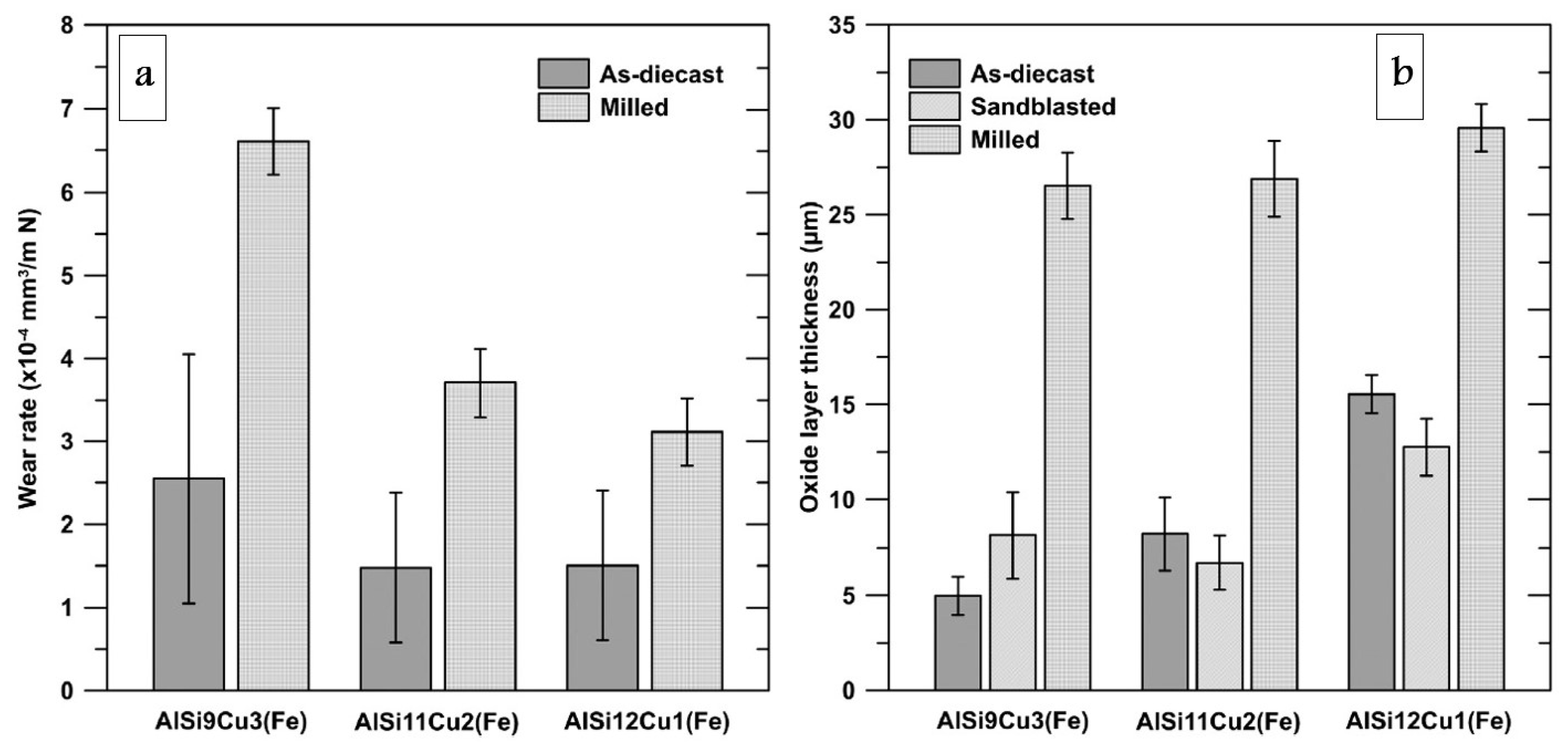
3.2. Machining Operations
4. Influence of Anodizing Parameters
4.1. Electrolyte
4.2. Anodizing Duration and Electrolyte Temperature
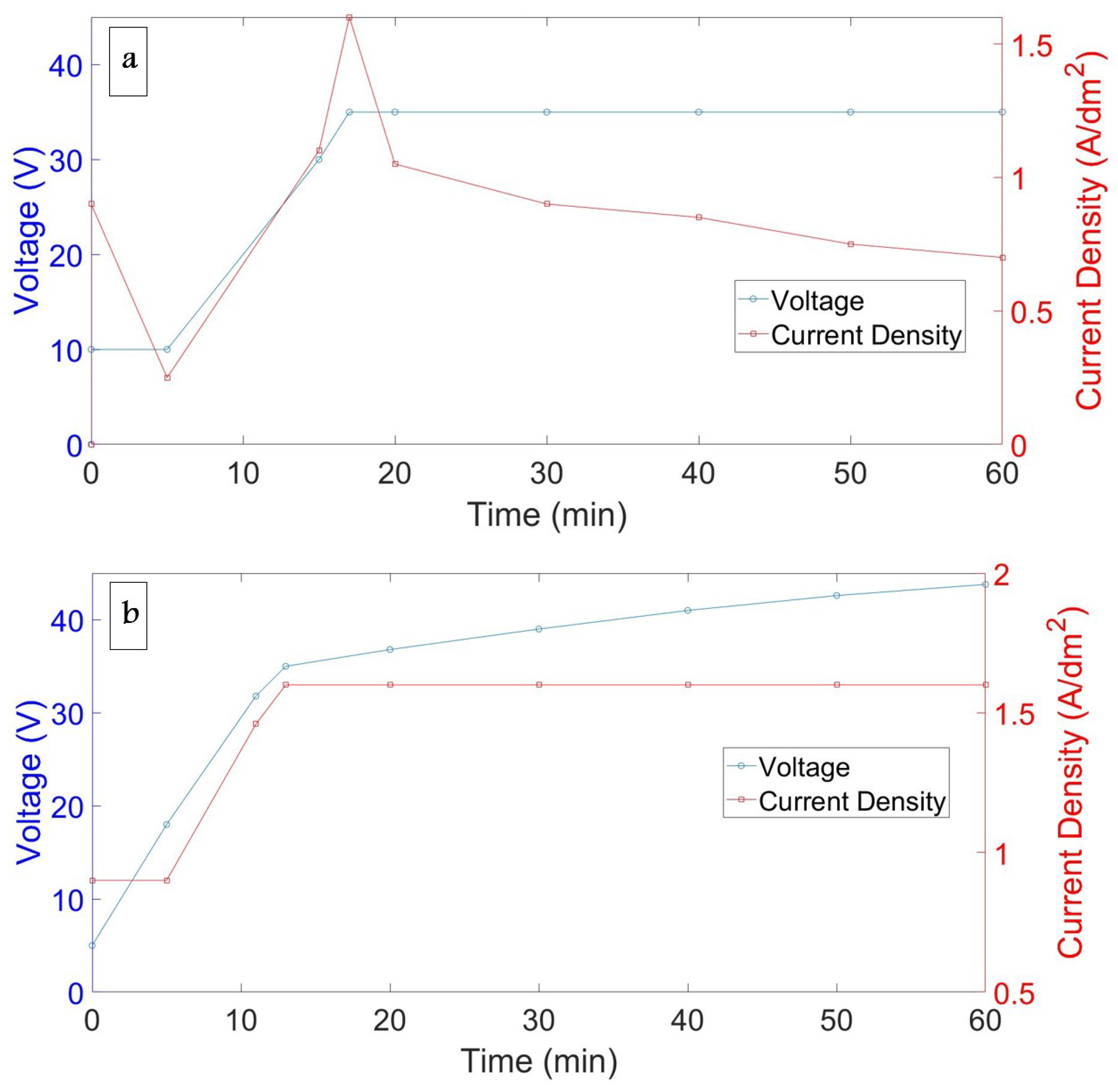
4.3. Voltage and Current
5. Influence of Post-Treatment
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Asensio-Lozano, Juan, Beatriz Suárez-Peña, and George F. Vander Voort. Effect of processing steps on the mechanical properties and surface appearance of 6063 aluminium extruded products. Materials 2014, 7, 4224–4242. [CrossRef]
- Runge, Jude Mary. The metallurgy of anodizing aluminum; Springer International Publishing: Cham, 2018. [Google Scholar]
- Diggle, John W., Thomas C. Downie, and C. W. Goulding. Anodic oxide films on aluminum. Chemical Reviews 1969, 69, 365–405. [CrossRef]
- Thompson, G. E. Porous anodic alumina: fabrication, characterization and applications. Thin solid films 1997, 297, 192–201. [Google Scholar] [CrossRef]
- O’sullivan, J. P.; Wood, G. C. The morphology and mechanism of formation of porous anodic films on aluminium. Proceedings of the Royal Society of London. A. Mathematical and Physical Sciences 1970, 317, 511–543. [Google Scholar]
- Valiev, R. Z.; Zhilyaev, A. P.; Langdon, T. G. Bulk nanostructured materials: fundamentals and applications; John Wiley & Sons, 2013. [Google Scholar]
- Abrahami, S.T. Cr (VI)-free pre-treatments for adhesive bonding of aerospace aluminium alloys. Delft University of Technology 2016. [Google Scholar]
- Voon, C. H., M. N. Derman, U. Hashim, K. R. Ahmad, and K. L. Foo. Effect of temperature of oxalic acid on the fabrication of porous anodic alumina from Al-Mn alloys. Journal of Nanomaterials 2013, 40.
- Paz Martínez-Viademonte, Mariana, Shoshan T. Abrahami, Theodor Hack, Malte Burchardt, and Herman Terryn. A review on anodizing of aerospace aluminum alloys for corrosion protection. Coatings 2020, 10, 1106. [CrossRef]
- Sheasby, P. G., S. Wernick, and R. Pinner. Surface treatment and finishing of aluminum and its alloys. Volumes 1 and 2. 1987.
- Emel, Razzouk, Dániel Koncz-Horváth, and Tamás I. Török. Anodic film changes during anodizing commercial pure aluminum. Doktorandusz Almanach PhD Students Almanach 2023, 34.
- Thompson, G. E.; Wood, G. C. Anodic Films on Aluminium. In Treatise on Materials Science and Technology; Elsevier, 1983; vol. 23, pp. 205–329. [Google Scholar]
- Takahashi, H.; Nagayama, M. The determination of the porosity of anodic oxide films on aluminium by the pore-filling method. Corrosion Science 1978, 18, 911–925. [Google Scholar] [CrossRef]
- Chesterfield, L.; Runge, J. Connecting theory to practice, the science of successfully anodizing aluminum die castings. In Proceedings of the 19th Annual Technical Conference and Exposition of the Aluminum Anodizers Council; 2010. [Google Scholar]
- Chen, Jiqiang, Feng Wen, Chao Liu, Weirong Li, Qiongyu Zhou, Wencai Zhu, Yinghui Zhang, and Renguo Guan. The microstructure and property of Al–Si alloy improved by the Sc-microalloying and Y2O3 nano-particles. Science and Technology of Advanced Materials 2021, 22, 205–217. [CrossRef]
- Huang, Jia-Min, Hai-Dong Zhao, and Zhen-Ming Chen. Microstructure and properties of A356 alloy wheels fabricated by low-pressure die casting with local squeeze. Journal of Materials Engineering and Performance 2019, 28, 2137–2146. [CrossRef]
- Martin, John H., Brennan D. Yahata, Jacob M. Hundley, Justin A. Mayer, Tobias A. Schaedler, and Tresa M. Pollock. 3D printing of high-strength aluminium alloys. Nature 2017, 549, 365–369.
- Cabrini, Marina, Sergio Lorenzi, Tommaso Pastore, Cristian Testa, Diego Manfredi, Massimo Lorusso, Flaviana Calignano, Matteo Pavese, and Francesco Andreatta. Corrosion behavior of AlSi10Mg alloy produced by laser powder bed fusion under chloride exposure. Corrosion Science 2019, 152, 101–108. [CrossRef]
- Rogov, Aleksey B., Huiling Lyu, Allan Matthews, and Aleksey Yerokhin. AC plasma electrolytic oxidation of additively manufactured and cast AlSi12 alloys. Surface and Coatings Technology 2020, 399, 126116. [CrossRef]
- Pezzato, L., M. Dabalà, Silvia Gross, and K. Brunelli. Effect of microstructure and porosity of AlSi10Mg alloy produced by selective laser melting on the corrosion properties of plasma electrolytic oxidation coatings. Surface and Coatings Technology 2020, 404, 126477. [Google Scholar] [CrossRef]
- Zhu, Baiwei, and Caterina Zanella. Hardness and corrosion behaviour of anodised Al-Si produced by rheocasting. Materials & Design 2019, 173, 107764.
- Lien, Huai-Hsun, Jyoti Mazumder, Jian Wang, and Amit Misra. Microstructure evolution and high density of nanotwinned ultrafine Si in hypereutectic Al-Si alloy by laser surface remelting. Materials Characterization 2020, 161, 110147. [CrossRef]
- Mora-Sanchez, H., R. Del Olmo, J. Rams, B. Torres, M. Mohedano, E. Matykina, and R. Arrabal. Hard anodizing and plasma electrolytic oxidation of an additively manufactured Al-Si alloy. Surface and Coatings Technology 2021, 420, 127339. [CrossRef]
- Wang, Ping, Qun Ma, Qianqian Yuwen, and Jianping Li. The differences in the formation mechanism of PEO and CPED composited ceramic coatings on Al-12Si alloy. Journal of Alloys and Compounds 2019, 788, 61–66. [CrossRef]
- Konieczny, J., L. A. Dobrzański, K. Labisz, and J. Duszczyk. The influence of cast method and anodizing parameters on structure and layer thickness of aluminium alloys. Journal of Materials Processing Technology 2004, 157, 718–723.
- Tsangaraki-Kaplanoglou, I., S. Theohari, Th Dimogerontakis, Yar-Ming Wang, Hong-Hsiang Harry Kuo, and Sheila Kia. Effect of alloy types on the anodizing process of aluminum. Surface and Coatings Technology 2006, 200, 2634–2641. [Google Scholar] [CrossRef]
- Shin, J. S.; Kim, B. H.; Lee, S. M. Effects of physical melt treatments on microstructural evolution and anodizing characteristics of Al-Si casting alloys. In Materials Science Forum; Trans Tech Publications Ltd., 2011; vol. 695, pp. 243–246. [Google Scholar]
- Chauke, Levy, Heinrich Möller, and Gonasagren Govender. Anodising of aluminium alloy plates from different series produced by rheo-high pressure die casting. Solid State Phenomena 2015, 217, 247–252.
- Aluminum and Magnesium Alloys Annual, vol. 02.02. ASTM standards. 2018.
- Arrabal, R., B. Mingo, A. Pardo, M. Mohedano, E. Matykina, and I. Rodríguez. Pitting corrosion of rheocast A356 aluminium alloy in 3.5 wt.% NaCl solution. Corrosion Science 2013, 73, 342–355. [CrossRef]
- Mingo, Beatriz, R. Arrabal, A. Pardo, E. Matykina, and P. Skeldon. 3D study of intermetallics and their effect on the corrosion morphology of rheocast aluminium alloy. Materials Characterization 2016, 112, 122–128. [CrossRef]
- Samuel, A. M., and F. H. Samuel. Modification of iron intermetallics by magnesium and strontium in Al-Si alloys. International Journal of Cast Metals Research 1997, 10, 147–157. [CrossRef]
- Fratila-Apachitei, L. E., J. Duszczyk, and L. Katgerman. Vickers microhardness of AlSi (Cu) anodic oxide layers formed in H2SO4 at low temperature. Surface and Coatings Technology 2003, 165, 309–315. [Google Scholar] [CrossRef]
- Zhang, Fan, Cem Örnek, Jan-Olov Nilsson, and Jinshan Pan. Anodisation of aluminium alloy AA7075–Influence of intermetallic particles on anodic oxide growth. Corrosion Science 2020, 164, 108319. [CrossRef]
- Fratila-Apachitei, L. E., F. D. Tichelaar, G. E. Thompson, Herman Terryn, P. Skeldon, J. Duszczyk, and L. Katgerman. A transmission electron microscopy study of hard anodic oxide layers on AlSi (Cu) alloys. Electrochimica Acta 2004, 49, 3169–3177. [CrossRef]
- Zhu, Baiwei, Salem Seifeddine, Per OÅ Persson, Anders EW Jarfors, Peter Leisner, and Caterina Zanella. A study of formation and growth of the anodised surface layer on cast Al-Si alloys based on different analytical techniques. Materials & design 2016, 101, 254–262.
- Razzouk, Emel, Dániel Koncz-Horváth, and Tamás I. Török. Microstructure Effects on Anodizing High-Silicon Aluminium Alloy AlSi12Cu1 (Fe) under Various Surface Conditions and Power Modes. Crystals 2024, 14, 352. [CrossRef]
- Fratila-Apachitei, L. E., J. Duszczyk, and L. Katgerman. Voltage transients and morphology of AlSi (Cu) anodic oxide layers formed in H2SO4 at low temperature. Surface and Coatings Technology 2002, 157, 80–94.
- Fratila-Apachitei, L. E., H. Terryn, P. Skeldon, G. E. Thompson, J. Duszczyk, and L. Katgerman. Influence of substrate microstructure on the growth of anodic oxide layers. Electrochimica Acta 2004, 49, 1127–1140. [CrossRef]
- Li, X., X. Nie, L. Wang, and D. O. Northwood. Corrosion protection properties of anodic oxide coatings on an Al–Si alloy. Surface and Coatings Technology 2005, 200, 1994–2000. [CrossRef]
- Mukhopadhyay, A. K., and A. K. Sharma. Influence of Fe-bearing particles and nature of electrolyte on the hard anodizing behaviour of AA 7075 extrusion products. Surface and Coatings Technology 1997, 92, 212–220. [CrossRef]
- Meyers, C. W.; Hinton, K. H.; Chou, J. S. Towards the optimization of heat-treatment in aluminium alloys. In Materials Science Forum; Trans Tech Publications Ltd., 1992; vol. 102, pp. 75–84. [Google Scholar]
- Dahle, A. K., Kazuhiro Nogita, S. D. McDonald, C. Dinnis, and L. Lu. Eutectic modification and microstructure development in Al–Si Alloys. Materials Science and Engineering: A 2005, 413, 243–248.
- Xu, Shan-Liang, Hai-Long Jia, Min Zha, Xiao-Li Zhou, Dan Gao, Pin-Kui Ma, and Dawei Wang. The effect of B and Sb on the corrosion behavior of T6-treated Al–Si–Mg alloys. Journal of Materials Research and Technology 2024, 30, 3611–3621. [CrossRef]
- Dinnis, Cameron M., Arne K. Dahle, and John A. Taylor. Three-dimensional analysis of eutectic grains in hypoeutectic Al–Si alloys. Materials Science and Engineering: A 2005, 392, 440–448. [CrossRef]
- Riddar, Frida, Sture Hogmark, and Å. Kassman Rudolphi. Comparison of anodised aluminium surfaces from four fabrication methods. Journal of Materials Processing Technology 2012, 212, 2272–2281. [CrossRef]
- Zhu, Baiwei, and Caterina Zanella. Influence of Fe-rich intermetallics and their segregation on anodising properties of Al-Si-Mg rheocast alloys. Surface and Coatings Technology 2021, 422, 127570. [CrossRef]
- Chauke, Levy, Kalenda Mutombo, and Gonasagren Govender. Corrosion behaviour of the anodised A356 aluminium alloy produced by the rheo-high pressure die casting process. Advanced Materials Research 2014, 1019, 67–73. [CrossRef]
- Menargues, S., J. A. Picas, E. Martin, M. T. Baile, M. Campillo, and A. Forn. Surface finish effect on the anodizing behaviour of Al-Si components obtained by sub-liquidus casting process. International Journal of Material Forming 2010, 3, 767–770. [CrossRef]
- Ceschini, Lorella, Iuri Boromei, Alessandro Morri, Salem Seifeddine, and Ingvar L. Svensson. Effect of Fe content and microstructural features on the tensile and fatigue properties of the Al–Si10–Cu2 alloy. Materials & Design (1980-2015) 2012, 36, 522–528.
- Jariyaboon, Manthana, Per Møller, Rafal E. Dunin-Borkowski, and Rajan Ambat. FIB-SEM investigation of trapped intermetallic particles in anodic oxide films on AA1050 aluminium. Anti-Corrosion Methods and Materials 2011, 58, 173–178. [CrossRef]
- Shimizu, K., G. M. Brown, K. Kobayashi, P. Skeldon, G. E. Thompson, and G. C. Wood. Ultramicrotomy—a route towards the enhanced understanding of the corrosion and filming behaviour of aluminium and its alloys. Corrosion science 1998, 40, 1049–1072. [CrossRef]
- Zhu, B. Casting and anodising of Al alloys-Alloy design, manufacturing process and material properties. PhD diss., Jönköping University, School of Engineering.
- Zhang, Fan, Jan-Olov Nilsson, and Jinshan Pan. In situ and operando AFM and EIS studies of anodization of Al 6060: influence of intermetallic particles. Journal of The Electrochemical Society 2016, 163, C609. [CrossRef]
- Wu, H., Y. Ma, W. Huang, X. Zhou, K. Li, Y. Liao, Z. Wang, Z. Liang, and L. Liu. Effect of iron-containing intermetallic particles on film structure and corrosion resistance of anodized AA2099 alloy. Journal of the Electrochemical Society 2018, 165, C573. [CrossRef]
- Mohamed, A. M. A.; Samuel, F. H. A review on the heat treatment of Al-Si-Cu/Mg casting alloys. Heat Treatment-Conventional and Novel Applications 2012, 55–72. [Google Scholar]
- Li, Z., A. M. Samuel, F. H. Samuel, C. Ravindran, and S. Valtierra. Effect of alloying elements on the segregation and dissolution of CuAl 2 phase in Al-Si-Cu 319 alloys. Journal of materials science 2003, 38, 1203–1218. [CrossRef]
- Zhou, X., G. E. Thompson, H. Habazaki, M. A. Paez, K. Shimizu, P. Skeldon, and G. C. Wood. Morphological development of oxygen bubbles in anodic alumina. Journal of the Electrochemical Society 2000, 147, 1747.
- Shimizu, K., K. Kobayashi, P. Skeldon, G. E. Thompson, and G. C. Wood. An atomic force microscopy study of the corrosion and filming behaviour of aluminium. Corrosion science 1997, 39, 701–718. [CrossRef]
- Scampone, Giulia, and Giulio Timelli. Anodizing Al–Si foundry alloys: A critical review. Advanced Engineering Materials 2022, 24, 2101480. [CrossRef]
- Gastón-García, B., E. García-Lecina, M. Díaz-Fuentes, J. A. Díez, and C. Müller. Sulphuric acid anodising of EN AC-46500 cast aluminium alloy. Transactions of the IMF 2011, 89, 312–319. [CrossRef]
- Meng, Qingjiang, and G. S. Frankel. Effect of Cu content on corrosion behavior of 7xxx series aluminum alloys. Journal of the Electrochemical Society 2004, 151, B271. [CrossRef]
- Saenz de Miera, M., M. Curioni, P. Skeldon, and G. E. Thompson. Preferential anodic oxidation of second-phase constituents during anodising of AA2024-T3 and AA7075-T6 alloys. Surface and Interface Analysis: An International Journal devoted to the development and application of techniques for the analysis of surfaces, interfaces and thin films 2010, 42, 241–246. [CrossRef]
- Birbilis, Nick, Mary K. Cavanaugh, and Rudolph G. Buchheit. Electrochemical behavior and localized corrosion associated with Al7Cu2Fe particles in aluminum alloy 7075-T651. Corrosion Science 2006, 48, 4202–4215. [CrossRef]
- Labisz, K., L. A. Dobrzański, and J. Konieczny. Anodization of cast aluminium alloys produced by different casting methods. Archives of Foundry Engineering 2008, 8, 45–50.
- Caliari, Daniele, Giulio Timelli, Borja Zabala, and Amaya Igartua. Microstructural and tribological investigations of diecast and hard anodized AlSiCu alloys. Surface and Coatings Technology 2018, 352, 462–473. [CrossRef]
- Chauke, L.; Möller, H.; Curle, U. A.; Govender, G. Anodising of Al-Mg-Si-(Cu) alloys produced by R-HPDC. In Materials Science Forum<; Trans Tech Publications Ltd, 2013; vol. 765, pp. 658–662. [Google Scholar]
- Caliari, D., G. Timelli, T. Salata, G. Cavagnini, S. Maestri, and A. Manfredini. Influence of microstructure and surface finishing on the hard anodizing of diecast Al-Si-Cu alloys. La Metallurgia Italiana 2019, 111, 23–31.
- Shin, J. S.; Kim, B. H.; Lee, S. M. A study on Surface Oxidation Coating Characteristic of Al-Si Casting Alloys. In Materials Science Forum; Trans Tech Publications Ltd, 2012; vol. 724, pp. 173–177. [Google Scholar]
- Mandich, N. V. Surface Preparation of Metals Prior to Plating. I: Metal Finishing 2003, 101, 8–10. [Google Scholar]
- Mohamed, A. Growth mechanism of porous anodic films formed on aluminium in sulphuric acid; The University of Manchester (United Kingdom), 2010. [Google Scholar]
- Yerokhin, A. L., X. Nie, A. Leyland, A. Matthews, and S. J. Dowey. Plasma electrolysis for surface engineering. Surface and coatings technology 1999, 122, 73–93.
- Guezmil, M., W. Bensalah, A. Khalladi, K. Elleuch, M. De-Petris Wery, and H. F. Ayedi. Effect of test parameters on the friction behaviour of anodized aluminium alloy. International Scholarly Research Notices 2014.
- Fratila-Apachitei, L. E., J. Duszczyk, and L. Katgerman. AlSi (Cu) anodic oxide layers formed in H2SO4 at low temperature using different current waveforms. Surface and Coatings Technology 2003, 165, 232–240.
- Davis, K. Material Review: Alumina (Al 2 O 3). School of Doctoral Studies European Union Journal 2010, 2. [Google Scholar]
- Auerkari, P. Mechanical and physical properties of engineering alumina ceramics. 1996. [Google Scholar]
- Makhlouf, M. M.; Apelian, D. Casting characteristics of aluminum die casting alloys; No. DOE/ID/13716; Worcester Polytechnic Institute (US), 2002. [Google Scholar]
- Arora, Rama, Suresh Kumar, Gurmel Singh, and O. P. Pandey. Influence of particle size and temperature on the wear properties of rutile-reinforced aluminium metal matrix composite. Journal of Composite Materials 2015, 49, 843–852. [CrossRef]
- Long, Alastair, David Thornhill, Cecil Armstrong, and David Watson. Predicting die life from die temperature for high pressure dies casting aluminium alloy. Applied thermal engineering 2012, 44, 100–107. [CrossRef]
- Sheasby, P. G.; Pinner, R. Introduction: Aluminium, its properties, alloys and finishes. Met. Finish 2001, 435–450. [Google Scholar]
- Eessaa, Ashraf K., and A. M. El-Shamy. Review on fabrication, characterization, and applications of porous anodic aluminum oxide films with tunable pore sizes for emerging technologies. Microelectronic Engineering 2023, 112061.
- Dong, H. Surface Engineering of Light Alloys: Aluminium. Magnesium and Titanium Alloys. 2010, 243–247. [Google Scholar]
- Talbot, D. E.; Talbot, J. D. Corrosion science and technology; CRC press, 2018. [Google Scholar]
- Jessensky, O., F. Müller, and U. Gösele. Self-organized formation of hexagonal pore structures in anodic alumina. Journal of the Electrochemical Society 1998, 145, 3735. [CrossRef]
- Chiang, Ming-Hung, Chi-Chen Yeh, and Chien-Liang Lee. Improvement in the abrasive wear resistance of an aluminum alloy casting for a continuously-variable transmission using heat treatment and pulsed anodizing. Wear 2020, 442, 203137.
- Romdhane, Anas Ben, Delphine Veys-Renaux, Mouhamadou Moustapha NDiaye, Stéphanie Bruyère, Khaled Elleuch, and Emmanuel Rocca. Anodizing of AS12 alloy in alkaline media. Applied Surface Science 2022, 572, 151436.
- Shang, Yan, Linshan Wang, Dun Niu, Zhaoyue Liu, Yuhong Wang, and Changsheng Liu. Effects of additive for anodizing electrolyte on anodic film of high silicon aluminum alloy. International Journal of Electrochemical Science 2016, 11, 1549–1557. [CrossRef]
- Raffin, Florian, Jacques Echouard, and Polina Volovitch. Influence of the anodizing time on the microstructure and immersion stability of tartaric-sulfuric acid anodized aluminum alloys. Metals 2023, 13, 993. [CrossRef]
- Aerts, Tom, Th Dimogerontakis, Iris De Graeve, Jan Fransaer, and H. Terryn. Influence of the anodizing temperature on the porosity and the mechanical properties of the porous anodic oxide film. Surface and Coatings Technology 2007, 201, 7310–7317. [CrossRef]
- Caliari, D. Development and optimization of surface hardening treatments and anodizing processes. 2018. [Google Scholar]
- Fratila-Apachitei, L. E., Iris De Graeve, I. Apachitei, H. Terryn, and J. Duszczyk. Electrode temperature evolution during anodic oxidation of AlSi (Cu) alloys studied in the wall-jet reactor. Surface and Coatings Technology 2006, 200, 5343–5353. [CrossRef]
- Fratila-Apachitei, L. E., I. Apachitei, and J. Duszczyk. Thermal effects associated with hard anodizing of cast aluminum alloys. Journal of applied electrochemistry 2006, 36, 481–486. [CrossRef]
- Li, Feiyue, Lan Zhang, and Robert M. Metzger. On the growth of highly ordered pores in anodized aluminum oxide. Chemistry of materials 1998, 10, 2470–2480. [CrossRef]
- Hao, Xue-Long, Ning Zhao, Hong-Hai Jin, Wen Ma, and Dong-Hui Zhang. Nickel-free sealing technology for anodic oxidation film of aluminum alloy at room temperature. Rare Metals 2021, 40, 968–974. [CrossRef]
- Yu, Shanwen, Lishi Wang, Chonggang Wu, Tao Feng, Yihang Cheng, Zhixiang Bu, and Shiqi Zhu. Studies on the corrosion performance of an effective and novel sealing anodic oxide coating. Journal of Alloys and Compounds 2020, 817, 153257. [CrossRef]
- Ono, Sachiko, and Hidetaka Asoh. Mechanism of hot water sealing of anodic films formed on aluminum. Corrosion Science 2021, 181, 109221. [CrossRef]
- Scampone, Giulia, and Giulio Timelli. The Influence of Sealing Processes and Machining Operations on the Scratch and Wear Resistance of Anodized AlSi9Cu3 (Fe) Diecasting Alloy. JOM 2024, 76, 196–208. [CrossRef]
- Fernández-López, P., S. A. Alves, A. López-Ortega, J. T. San José-Lombera, and R. Bayón. High performance tribological coatings on a secondary cast Al–Si alloy generated by Plasma Electrolytic Oxidation. Ceramics International 2021, 47, 31238–31250. [CrossRef]
- Kaseem, Mosab, Siti Fatimah, Nisa Nashrah, and Young Gun Ko. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Progress in materials science 2021, 117, 100735. [CrossRef]
- Matykina, E., R. Arrabal, P. Skeldon, and G. E. Thompson. Incorporation of zirconia nanoparticles into coatings formed on aluminium by AC plasma electrolytic oxidation. Journal of Applied Electrochemistry 2008, 38, 1375–1383. [CrossRef]
- Mohedano, M., E. Matykina, R. Arrabal, B. Mingo, and A. Pardo. PEO of pre-anodized Al–Si alloys: Corrosion properties and influence of sealings. Applied Surface Science 2015, 346, 57–67. [CrossRef]
- Mohedano, Marta, E. Lopez, Beatriz Mingo, S. Moon, Endzhe Matykina, and Raul Arrabal. Energy consumption, wear and corrosion of PEO coatings on preanodized Al alloy: The influence of current and frequency. Journal of Materials Research and Technology 2022, 21, 2061–2075. [CrossRef]

| Temperature °C | Phase precipitated | Suffix |
|---|---|---|
| 650 | Primary Al15(Mn, Fe)3Si2 (sludge) | Pre-dendrite |
| Aluminum dendrite and | Dendritic | |
| 600 | Al15(Mn, Fe)3Si2 and/or | Post-dendritic |
| Al5FeSi | Pre-eutectic | |
| Eutectic Al+Si | Eutectic | |
| 550 | and Al5FeSi | Co-eutectic |
| Mg5Si | Post-eutectic | |
| 500 | Al2Cu and more complex phases | Post-eutectic |
| Non-Acid | Chemical formula | Conc., (M) | pH |
|---|---|---|---|
| Ammonium Adipate | NH4OCO | 150 g/L | 6.4 |
| (CH2)4COONH4 | |||
| Sodium Borate | Na2B4O7 | 2.2 | 7 |
| Sodium Chromate | Na2CrO4 | 0.1 | 10 |
| Sodium Hydrogen Phosphate | Na2HPO4 | 0.1 | 9.4 |
| Sodium Hydroxide | NaOH | 0.01 , 0.03 & 0.1 | Not specified |
| Sodium Sulfate | Na2SO4 | 0.1 | 5.8 |
| Acid | Conc., (M) | Voltage, (Volts) | Pore Size, (nm) | Time, (hours) |
|---|---|---|---|---|
| 0.25 | 60 | 75 | 8.8 | |
| 0.3 | 40 | Not specified | Variable | |
| 0.3 | 40 | 80 | 8, Variable | |
| 0.3 | 40 | 50 | 10.5 min | |
| 0.3 | 60 | 80 | 3.8 | |
| Oxalic | 0.3 | 40 | 40- 50 | 40 min, 2 |
| 0.3 | 40, 50 | 20, 35 | Variable | |
| 0.3 | 30 | 40 | 8, 10 | |
| 0.4 | 40 | 50 | 8, 10 | |
| 0.5 | 50 | 80 | 8, 10 | |
| 0.3 | 40 | 22 | 12, 4, 8, 12 & 16 | |
| Not specified | 195 | 200 | variable | |
| 0.4 | 5 to 40 | 20 to 75 | 1 step/variable | |
| 0.4 | 80 | 80 | 1 step | |
| Phosphoric | 0.42 | 87 to 117 | 64 to 79 | 1 step/Variable |
| 0.5 | 18 | 70 | 4, variable | |
| 2.4 | 15 to 25 | 13 to 27 | 2-step/variable | |
| Sulfuric | Not specified | 12, 25, 40 | 25, 50, 100 | Not specified |
| 0.3 | 25 | 20 | 12, 4, 8, 12 & 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




