Submitted:
14 June 2024
Posted:
14 June 2024
You are already at the latest version
Abstract
Keywords:
1. Why Chlamydomonas and Why Its Consortia?
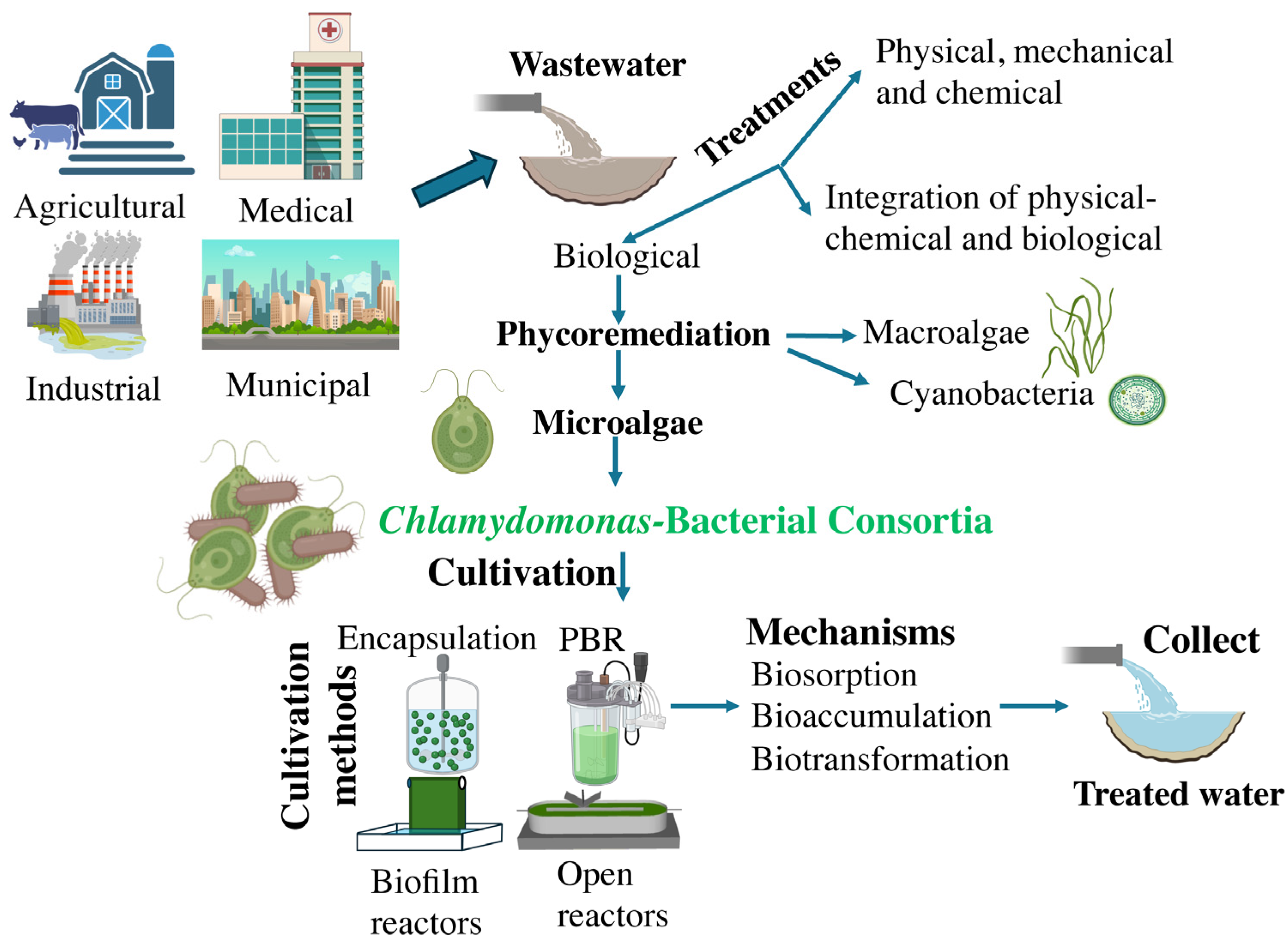
2. Wastewater Types and Composition
3. Wastewater Treatment Methods
4. Microalgae Cultivation Methods
4.1. Open Reactors
4.2. Closed Photobioreactors
4.3. Biofilm Reactors
4.4. Encapsulation
5. Main Mechanisms and Molecules Bioremediated by Chlamydomonas
5.1. Biosorption
5.2. Bioaccumulation
5.3. Biotransformation
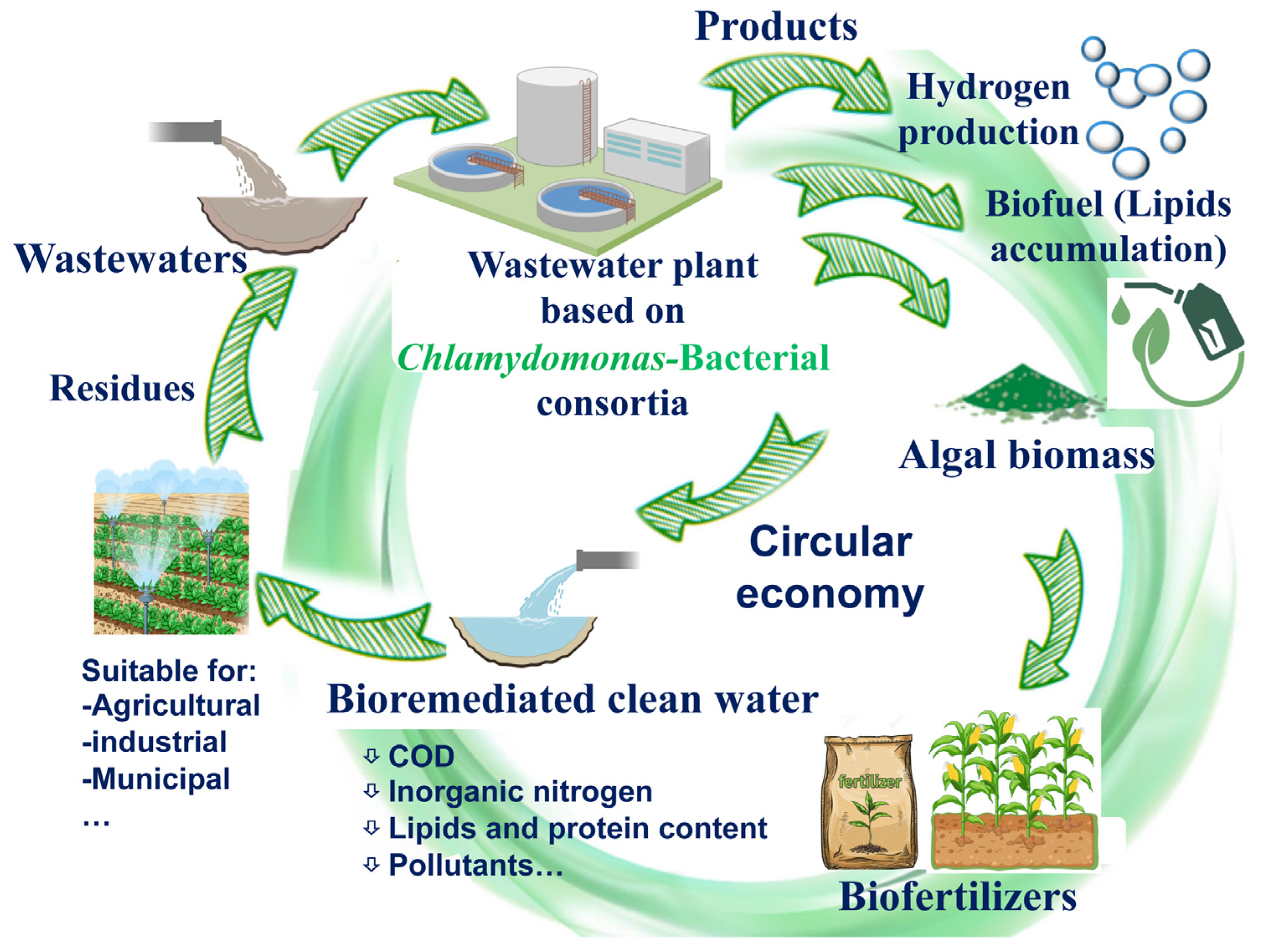
6. Chlamydomonas-Bacterial Consortia for Bioremediation
7. Chlamydomonas-Bacterial Consortia for Biomass and Bio-Product Generation
7.1. Biomass
7.2. Biofuels
7.2.1. Biohydrogen
7.2.2. Lipids
7.3. Biofertilizers
8. Future Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rani, S.; Gunjyal, N.; Ojha, C.S.P.; Singh, R.P. Review of Challenges for Algae-Based Wastewater Treatment: Strain Selection, Wastewater Characteristics, Abiotic, and Biotic Factors. J. Hazardous, Toxic, Radioact. Waste 2021, 25, 03120004. [Google Scholar] [CrossRef]
- Görgényi, J.; T-Krasznai, E.; Lukács, Á.; Kókai, Z.; B-Béres, V.; Várbíró, G.; Ács, É.; Kiss, K.T.; Tóthmérész, B.; Borics, G. Functional Properties of Planktic Microalgae Determine Their Habitat Selection. Hydrobiologia 2024, 851, 801–821. [Google Scholar] [CrossRef]
- Ochoa de Alda, J.A.G.; Esteban, R.; Diago, M.L.; Houmard, J. The Plastid Ancestor Originated among One of the Major Cyanobacterial Lineages. Nat. Commun. 2014, 5, 4937. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, S.S.U.H.; Yapa, N.; Karunarathna, S.C.; Suwannarach, N. Perceived Intensification in Harmful Algal Blooms Is a Wave of Cumulative Threat to the Aquatic Ecosystems. Biology (Basel). 2022, 11, 852. [Google Scholar] [CrossRef] [PubMed]
- Gerotto, C.; Norici, A.; Giordano, M. Toward Enhanced Fixation of CO2 in Aquatic Biomass: Focus on Microalgae. Front. Energy Res. 2020, 8, 213. [Google Scholar] [CrossRef]
- Olguín, E.J.; Sánchez-Galván, G.; Arias-Olguín, I.I.; Melo, F.J.; González-Portela, R.E.; Cruz, L.; De Philippis, R.; Adessi, A. Microalgae-Based Biorefineries: Challenges and Future Trends to Produce Carbohydrate Enriched Biomass, High-Added Value Products and Bioactive Compounds. Biology (Basel). 2022, 11, 1146. [Google Scholar] [CrossRef] [PubMed]
- Mavrommatis, A.; Tsiplakou, E.; Zerva, A.; Pantiora, P.D.; Georgakis, N.D.; Tsintzou, G.P.; Madesis, P.; Labrou, N.E. Microalgae as a Sustainable Source of Antioxidants in Animal Nutrition, Health and Livestock Development. Antioxidants 2023, 12, 1882. [Google Scholar] [CrossRef]
- Satya, A.D.M.; Cheah, W.Y.; Yazdi, S.K.; Cheng, Y.S.; Khoo, K.S.; Vo, D.V.N.; Bui, X.D.; Vithanage, M.; Show, P.L. Progress on Microalgae Cultivation in Wastewater for Bioremediation and Circular Bioeconomy. Environ. Res. 2023, 218, 114948. [Google Scholar] [CrossRef]
- Sasso, S.; Stibor, H.; Mittag, M.; Grossman, A.R. From Molecular Manipulation of Domesticated Chlamydomonas Reinhardtii to Survival in Nature. Elife 2018, 7, e39233. [Google Scholar] [CrossRef]
- Merchant, S.S.; Prochnik, S.E.; Vallon, O.; Harris, E.H.; Karpowicz, S.J.; Witman, G.B.; Terry, A.; Salamov, A.; Fritz-Laylin, L.K.; Maréchal-Drouard, L.; et al. The Chlamydomonas Genome Reveals the Evolution of Key Animal and Plant Functions. Science 2007, 318, 245–250. [Google Scholar] [CrossRef]
- Kelterborn, S.; Boehning, F.; Sizova, I.; Baidukova, O.; Evers, H.; Hegemann, P. Gene Editing in Green Alga Chlamydomonas Reinhardtii via CRISPR-Cas9 Ribonucleoproteins. Plant Synth. Biol. Methods Mol. Biol. 2022, 2379, 45–65. [Google Scholar] [CrossRef]
- Schroda, M.; Remacle, C. Molecular Advancements Establishing Chlamydomonas as a Host for Biotechnological Exploitation. Front. Plant Sci. 2022, 13, 911483. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, R.; Kim, B.-H.; Cho, D.-H.; Oh, H.-M.; Kim, H.-S. Algae-Bacteria Interactions: Evolution, Ecology and Emerging Applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Nayak, M.; Ghosh, A. A Review on Co-Culturing of Microalgae: A Greener Strategy towards Sustainable Biofuels Production. Sci. Total Environ. 2022, 802, 149765. [Google Scholar] [CrossRef] [PubMed]
- Bell, W.; Mitchell, R. Chemotactic and Growth Responses of Marine Bacteria to Algal Extracellular Products. Biol. Bull. 1972, 143, 265–277. [Google Scholar] [CrossRef]
- Hasnain, M.; Zainab, R.; Ali, F.; Abideen, Z.; Yong, J.W.H.; El-Keblawy, A.; Hashmi, S.; Radicetti, E. Utilization of Microalgal-Bacterial Energy Nexus Improves CO2 Sequestration and Remediation of Wastewater Pollutants for Beneficial Environmental Services. Ecotoxicol. Environ. Saf. 2023, 267, 115646. [Google Scholar] [CrossRef] [PubMed]
- Venkataram, S.; Kuo, H.-Y.; Hom, E.F.Y.; Kryazhimskiy, S. Mutualism-Enhancing Mutations Dominate Early Adaptation in a Two-Species Microbial Community. Nat. Ecol. Evol. 2023, 7, 143–154. [Google Scholar] [CrossRef]
- Calatrava, V.; Tejada-Jimenez, M.; Sanz-Luque, E.; Fernandez, E.; Galvan, A.; Llamas, A. Chlamydomonas Reinhardtii, a Reference Organism to Study Algal–Microbial Interactions: Why Can’t They Be Friends? Plants 2023, 12, 788. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, Q.; Zhou, R.; Feng, J.; Zhang, K.; Li, X.; Ma, X.; Dietrich, A.M. Occurrence of Free Amino Acids in the Source Waters of Zhejiang Province, China, and Their Removal and Transformation in Drinking Water Systems. Water (Switzerland) 2020, 12, 73. [Google Scholar] [CrossRef]
- Gaur, N.; Dutta, D.; Singh, A.; Dubey, R.; Kamboj, D.V. Recent Advances in the Elimination of Persistent Organic Pollutants by Photocatalysis. Front. Environ. Sci. 2022, 10, 872514. [Google Scholar] [CrossRef]
- Ahamed, A.; Ge, L.; Zhao, K.; Veksha, A.; Bobacka, J.; Lisak, G. Environmental Footprint of Voltammetric Sensors Based on Screen-Printed Electrodes: An Assessment towards “Green” Sensor Manufacturing. Chemosphere 2021, 278, 130462. [Google Scholar] [CrossRef] [PubMed]
- Kargol, A.K.; Burrell, S.R.; Chakraborty, I.; Gough, H.L. Synthetic Wastewater Prepared from Readily Available Materials: Characteristics and Economics. PLOS Water 2023, 2, e0000178. [Google Scholar] [CrossRef]
- Ali, I.; Naz, I.; Peng, C.; Abd-Elsalam, K.A.; Khan, Z.M.; Islam, T.; Pervez, R.; Amjed, M.A.; Tehrim, A.; Perveen, I.; et al. Sources, Classifications, Constituents, and Available Treatment Technologies for Various Types of Wastewater: An Overview. Aquananotechnology 2021, 11–46. [Google Scholar] [CrossRef]
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Biruntha, M.; Govarthanan, M.; Karmegam, N. Removal of Emerging Micropollutants Originating from Pharmaceuticals and Personal Care Products (PPCPs) in Water and Wastewater by Advanced Oxidation Processes: A Review. Environ. Technol. Innov. 2021, 23, 101757. [Google Scholar] [CrossRef]
- Marlina, E. ; Purwanto Electro-Fenton for Industrial Wastewater Treatment: A Review. E3S Web Conf. 2019, 125, 03003. [Google Scholar]
- Talukdar, A.; Kundu, P.; Bhattacharya, S.; Dutta, N. Microplastic Contamination in Wastewater: Sources, Distribution, Detection and Remediation through Physical and Chemical-Biological Methods. Sci. Total Environ. 2024, 916, 170254. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.A. Wastewater Treatment and Reuse for Sustainable Water Resources Management: A Systematic Literature Review. Sustainability 2023, 15, 10940. [Google Scholar] [CrossRef]
- Yadav, G.; Shanmugam, S.; Sivaramakrishnan, R.; Kumar, D.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A.; Rajendran, K. Mechanism and Challenges behind Algae as a Wastewater Treatment Choice for Bioenergy Production and Beyond. Fuel 2021, 285, 119093. [Google Scholar] [CrossRef]
- Razaviarani, V.; Arab, G.; Lerdwanawattana, N.; Gadia, Y. Algal Biomass Dual Roles in Phycoremediation of Wastewater and Production of Bioenergy and Value-Added Products. Int. J. Environ. Sci. Technol. 2023, 20, 8199–8216. [Google Scholar] [CrossRef]
- Dayana Priyadharshini, S.; Suresh Babu, P.; Manikandan, S.; Subbaiya, R.; Govarthanan, M.; Karmegam, N. Phycoremediation of Wastewater for Pollutant Removal: A Green Approach to Environmental Protection and Long-Term Remediation. Environ. Pollut. 2021, 290, 117989. [Google Scholar] [CrossRef]
- Barboza-Rodríguez, R.; Rodríguez-Jasso, R.M.; Rosero-Chasoy, G.; Rosales Aguado, M.L.; Ruiz, H.A. Photobioreactor Configurations in Cultivating Microalgae Biomass for Biorefinery. Bioresour. Technol. 2024, 394, 130208. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.G.G.; Alonso Gómez, V.; Muñoz Torre, R.; de Godos Crespo, I. Scale-down of High-Rate Algae Ponds Systems for Urban Wastewater Reuse. J. Water Process Eng. 2023, 56, 104342. [Google Scholar] [CrossRef]
- Jebali, A.; Acién, F.G.; Rodriguez Barradas, E.; Olguín, E.J.; Sayadi, S.; Molina Grima, E. Pilot-Scale Outdoor Production of Scenedesmus Sp. in Raceways Using Flue Gases and Centrate from Anaerobic Digestion as the Sole Culture Medium. Bioresour. Technol. 2018, 262, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Keeley, R.; Zalivina, N.; Halfhide, T.; Scott, K.; Zhang, Q.; van der Steen, P.; Ergas, S.J. Advances in Algal-Prokaryotic Wastewater Treatment: A Review of Nitrogen Transformations, Reactor Configurations and Molecular Tools. J. Environ. Manage. 2018, 217, 845–857. [Google Scholar] [CrossRef] [PubMed]
- de Godos, I.; Arbib, Z.; Lara, E.; Rogalla, F. Evaluation of High Rate Algae Ponds for Treatment of Anaerobically Digested Wastewater: Effect of CO2 Addition and Modification of Dilution Rate. Bioresour. Technol. 2016, 220, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Huang, C.Y.; Chang, J.S. Pollution Prevention and Waste Phycoremediation by Algal-Based Wastewater Treatment Technologies: The Applications of High-Rate Algal Ponds (HRAPs) and Algal Turf Scrubber (ATS). J. Environ. Manage. 2021, 296, 113193. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, C.; Molinuevo-Salces, B.; García-González, M.C. Nitrogen Transformations under Different Conditions in Open Ponds by Means of Microalgae-Bacteria Consortium Treating Pig Slurry. Bioresour. Technol. 2011, 102, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Grönlund, E.; Hanaeus, J.; Johansson, E.; Falk, S. Performance of an Experimental Wastewater Treatment High-Rate Algal Pond in Subarctic Climate. Water Environ. Res. a Res. Publ. Water Environ. Fed. 2010, 82, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Godos, I. de; Blanco, S.; García-Encina, P.A.; Becares, E.; Muñoz, R. Long-Term Operation of High Rate Algal Ponds for the Bioremediation of Piggery Wastewaters at High Loading Rates. Bioresour. Technol. 2009, 100, 4332–4339. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Phan, D.; Spierling, R.E.; Kopachevsky, A.M.; Bouwer, E.J.; Lundquist, T.J.; Betenbaugh, M.J. Production of Lipid-Containing Algal-Bacterial Polyculture in Wastewater and Biomethanation of Lipid Extracted Residues: Enhancing Methane Yield through Hydrothermal Pretreatment and Relieving Solvent Toxicity through Co-Digestion. Sci. Total Environ. 2019, 653, 1377–1394. [Google Scholar] [CrossRef]
- Villalba, M.R.; Cervera, R.; Sánchez, J. Green Solutions for Urban Sustainability: Photobioreactors for Algae Cultivation on Façades and Artificial Trees. Buildings 2023, 13, 1541. [Google Scholar] [CrossRef]
- Ugwu, C.U.; Aoyagi, H.; Uchiyama, H. Photobioreactors for Mass Cultivation of Algae. Bioresour. Technol. 2008, 99, 4021–4028. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Carvajal, G.D.; Taidi, B.; Jarrahi, M. Towards a Low Energy, Stirless Photobioreactor Using Photosynthetic Motile Microalgae. Algal Res. 2024, 77, 103350. [Google Scholar] [CrossRef]
- Rodríguez-Bolaños, M.; Vargas-Romero, G.; Jaguer-García, G.; Aguilar-Gonzalez, Z.I.; Lagos-Romero, V.; Miranda-Astudillo, H. V. Antares I: A Modular Photobioreactor Suitable for Photosynthesis and Bioenergetics Research. Appl. Biochem. Biotechnol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.; Fernández, J.; Acién, F.G.; Chisti, Y. Tubular Photobioreactor Design for Algal Cultures. J. Biotechnol. 2001, 92, 113–131. [Google Scholar] [CrossRef]
- Bilanovic, D.; Holland, M.; Starosvetsky, J.; Armon, R. Co-Cultivation of Microalgae and Nitrifiers for Higher Biomass Production and Better Carbon Capture. Bioresour. Technol. 2016, 220, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Mennerich, A.; Urban, B. Municipal Wastewater Treatment and Biomass Accumulation with a Wastewater-Born and Settleable Algal-Bacterial Culture. Water Res. 2011, 45, 3351–3358. [Google Scholar] [CrossRef] [PubMed]
- Moreno Osorio, J.H.; Pollio, A.; Frunzo, L.; Lens, P.N.L.; Esposito, G. A Review of Microalgal Biofilm Technologies: Definition, Applications, Settings and Analysis. Front. Chem. Eng. 2021, 3, 737710. [Google Scholar] [CrossRef]
- Kesaano, M.; Sims, R.C. Algal Biofilm Based Technology for Wastewater Treatment. Algal Res. 2014, 5, 231–240. [Google Scholar] [CrossRef]
- Kreis, C.T.; Grangier, A.; Bäumchen, O. In Vivo Adhesion Force Measurements of Chlamydomonas on Model Substrates. Soft Matter 2019, 15, 3027–3035. [Google Scholar] [CrossRef]
- Catalan, R.E.; Fragkopoulos, A.A.; von Trott, N.; Kelterborn, S.; Baidukova, O.; Hegemann, P.; Bäumchen, O. Light-Regulated Adsorption and Desorption of Chlamydomonas Cells at Surfaces. Soft Matter 2023, 19, 306. [Google Scholar] [CrossRef] [PubMed]
- Schaedig, E.; Cantrell, M.; Urban, C.; Zhao, X.; Greene, D.; Dancer, J.; Gross, M.; Sebesta, J.; Chou, K.J.; Grabowy, J.; et al. Isolation of Phosphorus-Hyperaccumulating Microalgae from Revolving Algal Biofilm (RAB) Wastewater Treatment Systems. Front. Microbiol. 2023, 14, 1219318. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yu, T.; Xie, Y.; Chen, J.; Ho, S.H.; Wang, Y.; Huang, F. Attached Culture of Chlamydomonas Sp. JSC4 for Biofilm Production and TN/TP/Cu(II) Removal. Biochem. Eng. J. 2019, 141, 1–9. [Google Scholar] [CrossRef]
- Posadas, E.; García-Encina, P.A.; Soltau, A.; Domínguez, A.; Díaz, I.; Muñoz, R. Carbon and Nutrient Removal from Centrates and Domestic Wastewater Using Algal-Bacterial Biofilm Bioreactors. Bioresour. Technol. 2013, 139, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, L.; Wang, J.; Chen, Y.; Gao, X.; Zhang, Z.; Liu, T. Attached Cultivation for Improving the Biomass Productivity of Spirulina Platensis. Bioresour. Technol. 2015, 181, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yang, Z.H.; Li, C.; Zeng, G.M.; Ma, D.H.; Zhou, L. A Novel Algal Biofilm Membrane Photobioreactor for Attached Microalgae Growth and Nutrients Removal from Secondary Effluent. Bioresour. Technol. 2015, 179, 8–12. [Google Scholar] [CrossRef]
- Vieira, M. V.; Pastrana, L.M.; Fuciños, P. Microalgae Encapsulation Systems for Food, Pharmaceutical and Cosmetics Applications. Mar. Drugs 2020, 18, 644. [Google Scholar] [CrossRef]
- Han, M.; Zhang, C.; Ho, S.H. Immobilized Microalgal System: An Achievable Idea for Upgrading Current Microalgal Wastewater Treatment. Environ. Sci. Ecotechnology 2023, 14, 100227. [Google Scholar] [CrossRef]
- Han, M.; Xie, P.; Ren, N.; Ho, S.H. Cytoprotective Alginate Microcapsule Serves as a Shield for Microalgal Encapsulation Defensing Sulfamethoxazole Threats and Safeguarding Nutrient Recovery. J. Hazard. Mater. 2024, 465, 133454. [Google Scholar] [CrossRef]
- Weng, Y.; Yang, G.; Li, Y.; Xu, L.; Chen, X.; Song, H.; Zhao, C.X. Alginate-Based Materials for Enzyme Encapsulation. Adv. Colloid Interface Sci. 2023, 318, 102957. [Google Scholar] [CrossRef]
- de-Bashan, L.E.; Bashan, Y. Immobilized Microalgae for Removing Pollutants: Review of Practical Aspects. Bioresour. Technol. 2010, 101, 1611–1627. [Google Scholar] [CrossRef]
- Nazos, T.T.; Ghanotakis, D.F. Biodegradation of Phenol by Alginate Immobilized Chlamydomonas Reinhardtii Cells. Arch. Microbiol. 2021, 203, 5805–5816. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, D.; Im, S.J.; Jang, A. Optimization of Alginate Bead Size Immobilized with Chlorella Vulgaris and Chlamydomonas Reinhardtii for Nutrient Removal. Bioresour. Technol. 2020, 302, 122891. [Google Scholar] [CrossRef]
- Homburg, S.V.; Kruse, O.; Patel, A. V. Growth and Photosynthetic Activity of Chlamydomonas Reinhardtii Entrapped in Lens-Shaped Silica Hydrogels. J. Biotechnol. 2019, 302, 58–66. [Google Scholar] [CrossRef]
- Zhang, B.B.; Wang, L.; Charles, V.; Rooke, J.C.; Su, B.L. Robust and Biocompatible Hybrid Matrix with Controllable Permeability for Microalgae Encapsulation. ACS Appl. Mater. Interfaces 2016, 8, 8939–8946. [Google Scholar] [CrossRef]
- Mandsberg, N.K.; Liao, W.; Yamanouchi, Y.A.; Boisen, A.; Ejima, H. Encapsulation of Chlamydomonas Reinhardtii into a Metal-Phenolic Network. Algal Res. 2022, 61, 102569. [Google Scholar] [CrossRef]
- Therien, J.B.; Zadvornyy, O.A.; Posewitz, M.C.; Bryant, D.A.; Peters, J.W. Growth of Chlamydomonas Reinhardtii in Acetate-Free Medium When Co-Cultured with Alginate-Encapsulated, Acetate-Producing Strains of Synechococcus Sp. PCC 7002. Biotechnol. Biofuels 2014, 7, 154. [Google Scholar] [CrossRef]
- Kumar, R.; Goyal, D. Comparative Biosorption of Pb 2+ by Live Algal Consortium and Immobilized Dead Biomass from Aqueous Solution. Indian J. Exp. Biol. 2009, 46, 690–694. [Google Scholar]
- Choix, F.J.; Guadalupe López-Cisneros, C.; Oscar Méndez-Acosta, H. Azospirillum Brasilense Increases CO2 Fixation on Microalgae Scenedesmus Obliquus, Chlorella Vulgaris, and Chlamydomonas Reinhardtii Cultured on High CO2 Concentrations. Microb. Ecol. 2018, 76, 430–442. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Mehariya, S.; Bhatia, R.K.; Kumar, M.; Pugazhendhi, A.; Awasthi, M.K.; Atabani, A.E.; Kumar, G.; Kim, W.; Seo, S.O.; et al. Wastewater Based Microalgal Biorefinery for Bioenergy Production: Progress and Challenges. Sci. Total Environ. 2021, 751, 141599. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.S. Bioremediation of Heavy Metals Using Microalgae: Recent Advances and Mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Xi, Y.; Han, B.; Kong, F.; You, T.; Bi, R.; Zeng, X.; Wang, S.; Jia, Y. Enhancement of Arsenic Uptake and Accumulation in Green Microalga Chlamydomonas Reinhardtii through Heterologous Expression of the Phosphate Transporter DsPht1. J. Hazard. Mater. 2023, 459, 132130. [Google Scholar] [CrossRef]
- Saavedra, R.; Muñoz, R.; Taboada, M.E.; Vega, M.; Bolado, S. Comparative Uptake Study of Arsenic, Boron, Copper, Manganese and Zinc from Water by Different Green Microalgae. Bioresour. Technol. 2018, 263, 49–57. [Google Scholar] [CrossRef]
- Nam, S.H.; Kwak, J. Il; An, Y.J. Assessing Applicability of the Paper-Disc Method Used in Combination with Flow Cytometry to Evaluate Algal Toxicity. Environ. Pollut. 2018, 234, 979–987. [Google Scholar] [CrossRef]
- Baselga-Cervera, B.; García-Balboa, C.; Díaz-Alejo, H.M.; Costas, E.; López-Rodas, V. Rapid Colonization of Uranium Mining-Impacted Waters, the Biodiversity of Successful Lineages of Phytoplankton Extremophiles. Microb. Ecol. 2020, 79, 576–587. [Google Scholar] [CrossRef]
- Ibuot, A.; Webster, R.E.; Williams, L.E.; Pittman, J.K. Increased Metal Tolerance and Bioaccumulation of Zinc and Cadmium in Chlamydomonas Reinhardtii Expressing a AtHMA4 C-Terminal Domain Protein. Biotechnol. Bioeng. 2020, 117, 2996–3005. [Google Scholar] [CrossRef]
- Hoyos, B.S.; Hernandez-Tenorio, F.; Miranda, A.M.; Villanueva-Mejía, D.F.; Sáez, A.A. Systematic Analysis of Genes Related to Selenium Bioaccumulation in Microalgae: A Review. Biology (Basel). 2023, 12, 703. [Google Scholar] [CrossRef]
- Wei, S.; Cao, J.; Ma, X.; Ping, J.; Zhang, C.; Ke, T.; Zhang, Y.; Tao, Y.; Chen, L. The Simultaneous Removal of the Combined Pollutants of Hexavalent Chromium and O-Nitrophenol by Chlamydomonas Reinhardtii. Ecotoxicol. Environ. Saf. 2020, 198, 110648. [Google Scholar] [CrossRef]
- Jin, Z.P.; Luo, K.; Zhang, S.; Zheng, Q.; Yang, H. Bioaccumulation and Catabolism of Prometryne in Green Algae. Chemosphere 2012, 87, 278–284. [Google Scholar] [CrossRef]
- Xiong, J.Q.; Kurade, M.B.; Abou-Shanab, R.A.I.; Ji, M.K.; Choi, J.; Kim, J.O.; Jeon, B.H. Biodegradation of Carbamazepine Using Freshwater Microalgae Chlamydomonas Mexicana and Scenedesmus Obliquus and the Determination of Its Metabolic Fate. Bioresour. Technol. 2016, 205, 183–190. [Google Scholar] [CrossRef]
- Touliabah, H.E.S.; El-Sheekh, M.M.; Ismail, M.M.; El-Kassas, H. A Review of Microalgae-and Cyanobacteria-Based Biodegradation of Organic Pollutants. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Li, S.; Wang, P.; Zhang, C.; Zhou, X.; Yin, Z.; Hu, T.; Hu, D.; Liu, C.; Zhu, L. Influence of Polystyrene Microplastics on the Growth, Photosynthetic Efficiency and Aggregation of Freshwater Microalgae Chlamydomonas Reinhardtii. Sci. Total Environ. 2020, 714, 136767. [Google Scholar] [CrossRef]
- Luo, J.; Deng, J.; Cui, L.; Chang, P.; Dai, X.; Yang, C.; Li, N.; Ren, Z.; Zhang, X. The Potential Assessment of Green Alga Chlamydomonas Reinhardtii CC-503 in the Biodegradation of Benz(a)Anthracene and the Related Mechanism Analysis. Chemosphere 2020, 249, 126097. [Google Scholar] [CrossRef]
- Wan, L.; Wu, Y.; Ding, H.; Zhang, W. Toxicity, Biodegradation, and Metabolic Fate of Organophosphorus Pesticide Trichlorfon on the Freshwater Algae Chlamydomonas Reinhardtii. J. Agric. Food Chem. 2020, 68, 1645–1653. [Google Scholar] [CrossRef]
- Carbó, M.; Chaturvedi, P.; Álvarez, A.; Pineda-Cevallos, D.; Ghatak, A.; González, P.R.; Cañal, M.J.; Weckwerth, W.; Valledor, L. Ferroptosis Is the Key Cellular Process Mediating Bisphenol A Responses in Chlamydomonas and a Promising Target for Enhancing Microalgae-Based Bioremediation. J. Hazard. Mater. 2023, 448, 130997. [Google Scholar] [CrossRef]
- Seoane, M.; Conde-Pérez, K.; Esperanza, M.; Cid, Á.; Rioboo, C. Unravelling Joint Cytotoxicity of Ibuprofen and Oxytetracycline on Chlamydomonas Reinhardtii Using a Programmed Cell Death-Related Biomarkers Panel. Aquat. Toxicol. 2023, 257, 106455. [Google Scholar] [CrossRef]
- Li, Z.; Dong, S.; Huang, F.; Lin, L.; Hu, Z.; Zheng, Y. Toxicological Effects of Microplastics and Sulfadiazine on the Microalgae Chlamydomonas Reinhardtii. Front. Microbiol. 2022, 13, 865768. [Google Scholar] [CrossRef]
- Sobieh, S.S.; Abed El-Gammal, R.; El-Kheir, W.S.A.; El-Sheimy, A.A.; Said, A.A.; El-Ayouty, Y.M. Heterologous Expression of Cyanobacterial Cyanase Gene (CYN) in Microalga Chlamydomonas Reinhardtii for Bioremediation of Cyanide Pollution. Biology (Basel). 2022, 11, 1420. [Google Scholar] [CrossRef]
- Dai, C.; Wang, F. Potential Applications of Microalgae–Bacteria Consortia in Wastewater Treatment and Biorefinery. Bioresour. Technol. 2024, 393, 130019. [Google Scholar] [CrossRef]
- Gururani, P.; Bhatnagar, P.; Kumar, V.; Vlaskin, M.S.; Grigorenko, A. V. Algal Consortiums: A Novel and Integrated Approach for Wastewater Treatment. Water (Switzerland) 2022, 14, 3784. [Google Scholar] [CrossRef]
- Chang, Y.L.; Nagarajan, D.; Chen, J.H.; Yen Chen, C.; Wu, Y.J.; Whang, L.M.; Lee, D.J.; Chang, J.S. Microalgae-Bacteria Consortia for the Treatment of Raw Dairy Manure Wastewater Using a Novel Two-Stage Process: Process Optimization and Bacterial Community Analysis. Chem. Eng. J. 2023, 473, 145388. [Google Scholar] [CrossRef]
- Zambrano, J.; García-Encina, P.A.; Jiménez, J.J.; Ciardi, M.; Bolado-Rodríguez, S.; Irusta-Mata, R. Removal of Veterinary Antibiotics in Swine Manure Wastewater Using Microalgae–Bacteria Consortia in a Pilot Scale Photobioreactor. Environ. Technol. Innov. 2023, 31, 103190. [Google Scholar] [CrossRef]
- Zambrano, J.; García-Encina, P.A.; Hernández, F.; Botero-Coy, A.M.; Jiménez, J.J.; Irusta-Mata, R. Kinetics of the Removal Mechanisms of Veterinary Antibiotics in Synthetic Wastewater Using Microalgae–Bacteria Consortia. Environ. Technol. Innov. 2023, 29, 103031. [Google Scholar] [CrossRef]
- Bhatt, P.; Bhandari, G.; Bhatt, K.; Simsek, H. Microalgae-Based Removal of Pollutants from Wastewaters: Occurrence, Toxicity and Circular Economy. Chemosphere 2022, 306, 135576. [Google Scholar] [CrossRef]
- Mora-Salguero, D.; Vives Florez, M.J.; Husserl Orjuela, J.; Fernández-Niño, M.; González Barrios, A.F. Evaluation of the Phenol Degradation Capacity of Microalgae-Bacteria Consortia from the Bay of Cartagena, Colombia. TecnoLógicas 2019, 22, 149–158. [Google Scholar] [CrossRef]
- Torres, M.; Gonzalez-Ballester, D.; Gomez-Osuna, A.; Galván, A.; Fernandez, E.; Dubini, A. Chlamydomonas-Methylobacterium Oryzae Cooperation Leads to Increased Biomass, Nitrogen Removal, and Hydrogen Production. Bioresour. Technol. 2022, 352, 127088. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.Y.; Klotz, M.G. The Nitrogen Cycle. Curr Biol 2016, 26, R94–8. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Simsek, H. Bioavailability of Wastewater Derived Dissolved Organic Nitrogen to Green Microalgae Selenastrum Capricornutum, Chlamydomonas Reinhardtii, and Chlorella Vulgaris with/without Presence of Bacteria. J. Environ. Sci. 2017, 57, 346–355. [Google Scholar] [CrossRef]
- Sun, J.; Khan, E.; Simsek, S.; Ohm, J.-B.; Simsek, H. Bioavailability of Dissolved Organic Nitrogen (DON) in Wastewaters from Animal Feedlots and Storage Lagoons. Chemosphere 2017, 186, 695–701. [Google Scholar] [CrossRef]
- Yang, Q.; Jie, S.; Lei, P.; Gan, M.; He, P.; Zhu, J.; Zhou, Q. Effect of Anthropogenic Disturbances on the Microbial Relationship during Bioremediation of Heavy Metal-Contaminated Sediment. Microorganisms 2023, 11, 1185. [Google Scholar] [CrossRef]
- Mitra, M.; Nguyen, K.M.A.K.; Box, T.W.; Gilpin, J.S.; Hamby, S.R.; Berry, T.L.; Duckett, E.H. Isolation and Characterization of a Novel Sphingobium Strain Variant That Uses Biohazardous Saturated and Aromatic Compounds as Sole Carbon. F1000Research 2022, 9, 767. [Google Scholar] [CrossRef] [PubMed]
- Mitra, M.; Nguyen, K.M.A.K.; Box, T.W.; Gilpin, J.S.; Hamby, S.R.; Berry, T.L.; Duckett, E.H. Isolation and Characterization of a Novel Bacterial Strain from a Tris-Acetate-Phosphate Agar Medium Plate of the Green Micro-Alga Chlamydomonas Reinhardtii That Can Utilize Common Environmental Pollutants as a Carbon Source. F1000Research 2022, 9, 656. [Google Scholar] [CrossRef] [PubMed]
- Mitra, M.; Nguyen, K.M.-A.-K.; Box, T.W.; Berry, T.L.; Fujita, M. Isolation and Characterization of a Heavy Metal- and Antibiotic-Tolerant Novel Bacterial Strain from a Contaminated Culture Plate of Chlamydomonas Reinhardtii, a Green Micro-Alga. F1000Research 2023, 10, 533. [Google Scholar] [CrossRef]
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Khan, T.M.Y.; Nghiem, L.D.; Ong, H.C.; et al. Microalgae Biomass as a Sustainable Source for Biofuel, Biochemical and Biobased Value-Added Products: An Integrated Biorefinery Concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- Sivaramakrishnan, R.; Suresh, S.; Kanwal, S.; Ramadoss, G.; Ramprakash, B.; Incharoensakdi, A. Microalgal Biorefinery Concepts’ Developments for Biofuel and Bioproducts: Current Perspective and Bottlenecks. Int. J. Mol. Sci. 2022, 23, 2623. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; W. Hassan, S.; Banat, F. An Overview of Microalgae Biomass as a Sustainable Aquaculture Feed Ingredient: Food Security and Circular Economy. Bioengineered 2022, 13, 9521–9547. [Google Scholar] [CrossRef] [PubMed]
- Bunbury, F.; Deery, E.; Sayer, A.P.; Bhardwaj, V.; Harrison, E.L.; Warren, M.J.; Smith, A.G. Exploring the Onset of B 12-Based Mutualisms Using a Recently Evolved Chlamydomonas Auxotroph and B12-Producing Bacteria. Environ. Microbiol. 2022, 24, 3134–3147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, M.-Y.; Khan, N.; Tan, L.-L.; Yang, S.; Naveed, M. Sustainability Potentials, Utilization, and Bioengineering of Plant Growth-Promoting Methylobacterium for Sustainable Agriculture. Sustainability 2021, 13, 3941–12. [Google Scholar] [CrossRef]
- Calatrava, V.; Hom, E.F. .; Llamas, Á.; Fernández, E.; Galván, A. OK, Thanks! A New Mutualism between Chlamydomonas and Methylobacteria Facilitates Growth on Amino Acids and Peptides. FEMS Microbiol. Lett. 2018, 365, fny021. [Google Scholar] [CrossRef]
- Llamas, A.; Leon-Miranda, E.; Tejada-Jimenez, M. Microalgal and Nitrogen-Fixing Bacterial Consortia: From Interaction to Biotechnological Potential. Plants 2023, 12, 2476. [Google Scholar] [CrossRef]
- Romano, I.; Ventorino, V.; Pepe, O. Effectiveness of Plant Beneficial Microbes: Overview of the Methodological Approaches for the Assessment of Root Colonization and Persistence. Front. Plant Sci. 2020, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Gyurjan, I.; Koranyi, P.; Paless, G.Y. Ultrastructural Analysis of an Artificial Alga-Bacterium Endosymbiosis After Prolonged Cultivation. Symbiosis 1992, 14, 475–468. [Google Scholar]
- Preininger, E.; Ponyi, T.; Sarkadi, L.; Nyitrai, P.; Gyurjan, I. Long-Living Azotobacter-Chlamydomonas Association as a Model System for Plant-Microbe Interactions. Symbiosis 2006, 42, 45–50. [Google Scholar]
- Chaos-Hernández, D.; Reynel-Ávila, H.E.; Bonilla-Petriciolet, A.; Villalobos-Delgado, F.J. Extraction Methods of Algae Oils for the Production of Third Generation Biofuels – A Review. Chemosphere 2023, 341, 139856. [Google Scholar] [CrossRef]
- Daneshvar, E.; Sik Ok, Y.; Tavakoli, S.; Sarkar, B.; Shaheen, S.M.; Hong, H.; Luo, Y.; Rinklebe, J.; Song, H.; Bhatnagar, A. Insights into Upstream Processing of Microalgae: A Review. Bioresour. Technol. 2021, 329, 124870. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Lyu, S.; An, Y.; Lu, J.; Gjermansen, C.; Schramm, A. Microalgae–Bacteria Symbiosis in Microalgal Growth and Biofuel Production: A Review. J. Appl. Microbiol. 2019, 126, 359–368. [Google Scholar] [CrossRef]
- Nirmala, N.; Praveen, G.; AmitKumar, S.; SundarRajan, P.S.; Baskaran, A.; Priyadharsini, P.; SanjayKumar, S.P.; Dawn, S.S.; Pavithra, K.G.; Arun, J.; et al. A Review on Biological Biohydrogen Production: Outlook on Genetic Strain Enhancements, Reactor Model and Techno-Economics Analysis. Sci. Total Environ. 2023, 896, 165143. [Google Scholar] [CrossRef]
- Frenkel, A.W. Hydrogen Evolution by the Flagellate Green Alga, Chlamydomonas Moewusii. Arch Biochem Biophys 1951, 38, 219–230. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Q.; Yu, D. The Future of Hydrogen Energy: Bio-Hydrogen Production Technology. Int. J. Hydrogen Energy 2022, 47, 33677–33698. [Google Scholar] [CrossRef]
- King, S.J.; Jerkovic, A.; Brown, L.J.; Petroll, K.; Willows, R.D. Synthetic Biology for Improved Hydrogen Production in Chlamydomonas Reinhardtii. Microb. Biotechnol. 2022, 15, 1946–1965. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Erbes, D.L.; Riederer-Henderson, M.A.; Peavey, D.G.; Gibbs, M. H2 Metabolism in Photosynthetic Organisms: I. Dark H2 Evolution and Uptake by Algae and Mosses. Plant Physiol 1975, 56, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Melis, A.; Zhang, L.; Forestier, M.; Ghirardi, M.L.; Seibert, M. Sustained Photobiological Hydrogen Gas Production upon Reversible Inactivation of Oxygen Evolution in the Green Alga Chlamydomonas Reinhardtii. Plant Physiol. 2000, 122, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Fakhimi, N.; Gonzalez-Ballester, D.; Fernández, E.; Galván, A.; Dubini, A. Algae-Bacteria Consortia as a Strategy to Enhance H2 Production. Cells 2020, 9, 1353. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, X.; Wang, Q. Effect of Co-Cultivation of Chlamydomonas Reinhardtii with Azotobacter Chroococcum on Hydrogen Production. Int. J. Hydrogen Energy 2017, 42, 22713–22719. [Google Scholar] [CrossRef]
- Wu, S.; Li, X.; Yu, J.; Wang, Q. Increased Hydrogen Production in Co-Culture of Chlamydomonas Reinhardtii and Bradyrhizobium Japonicum. Bioresour. Technol. 2012, 123, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Fakhimi, N.; Dubini, A.; Tavakoli, O.; González-Ballester, D. Acetic Acid Is Key for Synergetic Hydrogen Production in Chlamydomonas-Bacteria Co-Cultures. Bioresour. Technol. 2019, 289, 121648. [Google Scholar] [CrossRef] [PubMed]
- Fakhimi, N.; Tavakoli, O.; Marashi, S.A.; Moghimi, H.; Mehrnia, M.R.; Dubini, A.; González-Ballester, D. Acetic Acid Uptake Rate Controls H2 Production in Chlamydomonas-Bacteria Co-Cultures. Algal Res. 2019, 42, 101605. [Google Scholar] [CrossRef]
- Yu, Q.; He, J.; Zhao, Q.; Wang, X.; Zhi, Y.; Li, X.; Li, X.; Li, L.; Ge, B. Regulation of Nitrogen Source for Enhanced Photobiological H2 Production by Co-Culture of Chlamydomonas Reinhardtii and Mesorhizobium Sangaii. Algal Res. 2021, 58, 102422. [Google Scholar] [CrossRef]
- Iqbal, K.; Saxena, A.; Pande, P.; Tiwari, A.; Chandra Joshi, N.; Varma, A.; Mishra, A. Microalgae-Bacterial Granular Consortium: Striding towards Sustainable Production of Biohydrogen Coupled with Wastewater Treatment. Bioresour. Technol. 2022, 354, 127203. [Google Scholar] [CrossRef]
- Rezvani, F.; Sarrafzadeh, M.H. Autotrophic Granulation of Hydrogen Consumer Denitrifiers and Microalgae for Nitrate Removal from Drinking Water Resources at Different Hydraulic Retention Times. J. Environ. Manage. 2020, 268, 110674. [Google Scholar] [CrossRef]
- Wirth, R.; Lakatos, G.; Maróti, G.; Bagi, Z.; Minárovics, J.; Nagy, K.; Kondorosi, É.; Rákhely, G.; Kovács, K.L. Exploitation of Algal-Bacterial Associations in a Two-Stage Biohydrogen and Biogas Generation Process. Biotechnol. Biofuels 2015, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Hena, S.; Fatimah, S.; Tabassum, S. Cultivation of Algae Consortium in a Dairy Farm Wastewater for Biodiesel Production. Water Resour. Ind. 2015, 10, 1–14. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, X.; Wang, Q. Enhanced Lipid Production in Chlamydomonas Reinhardtii by Co-Culturing With Azotobacter Chroococcum. Front. Plant Sci. 2018, 9, 741. [Google Scholar] [CrossRef]
- Yee, C.S.; Okomoda, V.T.; Hashim, F.; Waiho, K.; Abdullah, S.R.S.; Alamanjo, C.; Hasan, H.A.; Mustafa, E.M.; Kasan, N.A. Marine Microalgae Co-Cultured with Floc-Forming Bacterium: Insight into Growth and Lipid Productivity. PeerJ 2021, 9, 11217. [Google Scholar] [CrossRef]
- Toyama, T.; Kasuya, M.; Hanaoka, T.; Kobayashi, N.; Tanaka, Y.; Inoue, D.; Sei, K.; Morikawa, M.; Mori, K. Growth Promotion of Three Microalgae, Chlamydomonas Reinhardtii, Chlorella Vulgaris and Euglena Gracilis, by in Situ Indigenous Bacteria in Wastewater Effluent. Biotechnol. Biofuels 2018, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.M.; Hernandez-Tenorio, F.; Villalta, F.; Vargas, G.J.; Sáez, A.A. Advances in the Development of Biofertilizers and Biostimulants from Microalgae. Biology (Basel). 2024, 13. [Google Scholar] [CrossRef]
- Martini, F.; Beghini, G.; Zanin, L.; Varanini, Z.; Zamboni, A.; Ballottari, M. The Potential Use of Chlamydomonas Reinhardtii and Chlorella Sorokiniana as Biostimulants on Maize Plants. Algal Res. 2021, 60, 102515. [Google Scholar] [CrossRef] [PubMed]
- Metting, B. Population Dynamics of Chlamydomonas Sajao and Its Influence on Soil Aggregate Stabilization in the Field. Appl. Environ. Microbiol. 1986, 51, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Sido, M.Y.; Tian, Y.; Wang, X.; Wang, X. Application of Microalgae Chlamydomonas Applanata M9V and Chlorella Vulgaris S3 for Wheat Growth Promotion and as Urea Alternatives. Front. Microbiol. 2022, 13, 1035791. [Google Scholar] [CrossRef]
- Gitau, M.M.; Farkas, A.; Balla, B.; Ördög, V.; Futó, Z.; Maróti, G. Strain-Specific Biostimulant Effects of Chlorella and Chlamydomonas Green Microalgae on Medicago Truncatula. Plants 2021, 10, 1060. [Google Scholar] [CrossRef]
- Mutale-Joan, C.; Redouane, B.; Najib, E.; Yassine, K.; Lyamlouli, K.; Laila, S.; Zeroual, Y.; Hicham, E.A. Screening of Microalgae Liquid Extracts for Their Bio Stimulant Properties on Plant Growth, Nutrient Uptake and Metabolite Profile of Solanum Lycopersicum L. Sci. Rep. 2020, 10, 2820. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Ördög, V.; Staden, J. Van; Jäger, K. Cytokinin-and Auxin-like Activity in Cyanophyta and Microalgae. J. Appl. Phycol. 2002, 14, 215–221. [Google Scholar] [CrossRef]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, Soil and Plants: A Critical Review of Microalgae as Renewable Resources for Agriculture. Algal Res. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Keswani, C.; Satyendra, &; Singh, P. ; Cueto, L.; García-Estrada, C.; Mezaache-Aichour, S.; Glare, T.R.; Borriss, R.; Surya, &; Angel Blázquez, M.; et al. Auxins of Microbial Origin and Their Use in Agriculture. Appl. Microbiol. Biotechnol. 2020, 104, 8549–8565. [Google Scholar] [PubMed]
- Amin, S.A.; Hmelo, L.R.; van Tol, H.M.; Durham, B.P.; Carlson, L.T.; Heal, K.R.; Morales, R.L.; Berthiaume, C.T.; Parker, M.S.; Djunaedi, B.; et al. Interaction and Signalling between a Cosmopolitan Phytoplankton and Associated Bacteria. Nature 2015, 522, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Palombella, A.L.; Dutcher, S.K. Identification of the Gene Encoding the Tryptophan Synthase-Subunit from Chlamydomonas Reinhardtii. Plant Physiol 1998, 117, 455–464. [Google Scholar] [CrossRef]
- Calatrava, V.; Hom, E.F.Y.; Guan, Q.; Llamas, A.; Fernández, E.; Galván, A. Genetic Evidence for Algal Auxin Production in Chlamydomonas and Its Role in Algal-Bacterial Mutualism. iScience 2024, 27, 108762. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The Rhizosphere Microbiome: Significance of Plant Beneficial, Plant Pathogenic, and Human Pathogenic Microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




