1. Introduction
Trauma is one of the leading causes of death, especially in the young population. There is a wide spectrum of thoracic traumas ranging from simple isolated rib fractures to life-threatening injuries. The extent of the damage is related to the disruption of cardiac and pulmonary physiology. is directly proportional (1). Pulmonary contusion (PC), a multifaceted clinical entity is characterized by hemorrhage into the interstitial space and alveoli within the lung and accompanying tissue reaction such as leukocyte infiltration and edema. Although it usually occurs with blunt thoracic trauma, it can also occur with specific penetrating injuries such as gunshot wounds. PC is an important cause of mortality and morbidity in patients with blunt chest trauma and multiple body trauma (2).
In PC that develops after blunt chest trauma, there is an increase in proinflammatory cytokines (such as IL-1β, IL-6, TNF-α, etc. and NO) and oxidative stress agents after the innate immune response. PC pathophysiology including inflammation is a risk factor for the development of acute lung injury and acute respiratory distress. Acute lung injury in human and animal models has been shown to be characterized by an intense inflammatory response in the lung parenchyma. The natural inflammatory response triggered by direct or indirect trauma to the lungs includes leukocyte activation in the blood, macrophage activation in the tissue, production of different mediator series including cytokines, chemokines, oxygen radicals, arachidonic acid metabolites, complement and coagulation cascade (3).
Endothelin-1 (ET-1) binds to endothelin receptors (A and B) in the pulmonary vasculature and causes vasoconstriction. Bosentan, ambrisentan and masitentan are endothelin receptor antagonists used in the treatment of pulmonary arterial hypertension (PAH) (4). Efficacy of bosentan in the BREATHE-1 study has been shown (5). In this study, bosentan prevented worsening of 6-minute walk distance (6MWD) distance, although not as much as in idiopathic (IPAH) patients.
Since bosentan, which is an inhibitor of both ET-A and ET-B receptors, may play an important role in inflammatory diseases and act as a regulator in various steps of inflammatory and oxidation/reduction reactions, it was thought that it may be effective in preventing or reversing the inflammatory reactions that develop after lung contusion caused by blunt thoracic trauma. In this study, we investigated the effects of bosentan on ET-1, hypoxia-inducible factor-1 (HIF-1), nuclear factor-kappa B (NF-κB), tumor necrosis factor (TNF)-α as inflammation markers, pro-oxidant antioxidant balance (PAB) and total antioxidant capacity (TAC) levels as oxidative stress parameters in lung tissues of rats in experimental model of PC induced by blunt thoracic trauma.
2. Materials and Methods
All experiments were approved by the Yeditepe University (2017/601) Ethics Committee for Animal Experiments and followed the NIH Guide for the Care and Use of Laboratory Animals. This work is derived from Gonca Gercel’s Specialist’s Thesis (Investigation of bosentan’s effects on pulmonary contusion created by blunt thoracic trauma in rats) (6)
2.1. Animals
Thirty-seven Sprague-Dawley rats, weighing 200 to 250 g, were included in this study under standard laboratory conditions (22 ± 1°C, 12-hour light/dark cycle). They were fed with standard rat chow and tap water ad libitum.
2.2. Experimental Protocol
Thirty-seven animals were randomly divided into 5 groups, and all 4 groups, except for the control group, were performed to PC. In the first group, C: control group (n=6) consisted of unprocessed and untreated. The second group, PC3 (n=8) underwent of 3 days PC performed. The third group, PC-B3 (n=8) received 100 mg/kg bosentan was given orally once a day for 3 days. The fourth group, PC7 group (n=7) underwent of 7 days PC performed, and the fifth group PC-B7 (n=8) received 100 mg/kg bosentan was given orally once a day for 7 days.
The rats were anesthetized with intraperitoneal ketamine (50 mg/kg) and xylazine (15 mg/kg). PC was induced by dropping a cylindrical metal weight (0.4 kg) from a specified distance (60 cm) through a stainless-steel tube onto the right hemithorax. In this way, the trauma was standardized by applying 2.35 J energy on the chest according to the formula E=mgh (E=energy [joule], m=mass of the cylinder [kg], g= gravity constant [9.8 m/s2], and h=height [meter]). This is a modified form of the model defined by Raghavendran et al. (7).
2.3. Biochemical Analysis Lung Tissue
Lung tissue samples were collected for subsequent biochemical and snap-frozen in liquid nitrogen and then stored at -80 0C. The lung tissues were homogenized using 20% phosphate buffer via the homogenizer (Next Advance Bullet Blender Storm 24). The homogenates were centrifuged for 10 minutes at 3.000xg at 40°C to remove the debris. Supernatant was taken for determination of ET-1, NF-κB, TNF-α, HIF-1α, TAC, and PAB. All samples were examined twice.
The levels of ET-1, NF-κB, TNF-α, and HIF-1α were measured using commercially available ELISA (MyBioSource, Inc. in San Diego, CA, USA) and the manufacturer’s instructions were followed for the assessment.
2.4. Measurement of Lung Tissue Prooxidant-Antioxidant Balance (PAB)
Tissue PAB was measured with the method of Alamdari et al., (8) with slight modifications. The oxidation-reduction indicators used in this method were 3,3′,5,5′-tetramethylbenzidine (TMB) and TMB cations, which have different optical and electrochemical properties. The coefficients of intra- and inter-assay variation were 5.0% (n = 20) and 6.2% (n = 20), respectively.
2.5. Measurement of Ferric Reducing Antioxidant Power (FRAP)
The antioxidant status of the serum samples was measured with the FRAP assay, which is a redox-linked colorimetric method that uses reductant antioxidants (9). The coefficients of intra- and inter-assay variation were 4.9% (n = 20) and 6.1% (n = 20), respectively.
2.6. Statistical Analysis
The statistical analysis was conducted using JASP 0.18.3. Descriptive statistical methods, including the calculation of the mean and standard deviation, were employed in the evaluation of the study data. The analysis of variance (ANOVA) test was used for the comparison of groups, with the Tukey test serving as a post-hoc test. The threshold for statistical significance was set at p < 0.05.
3. Results
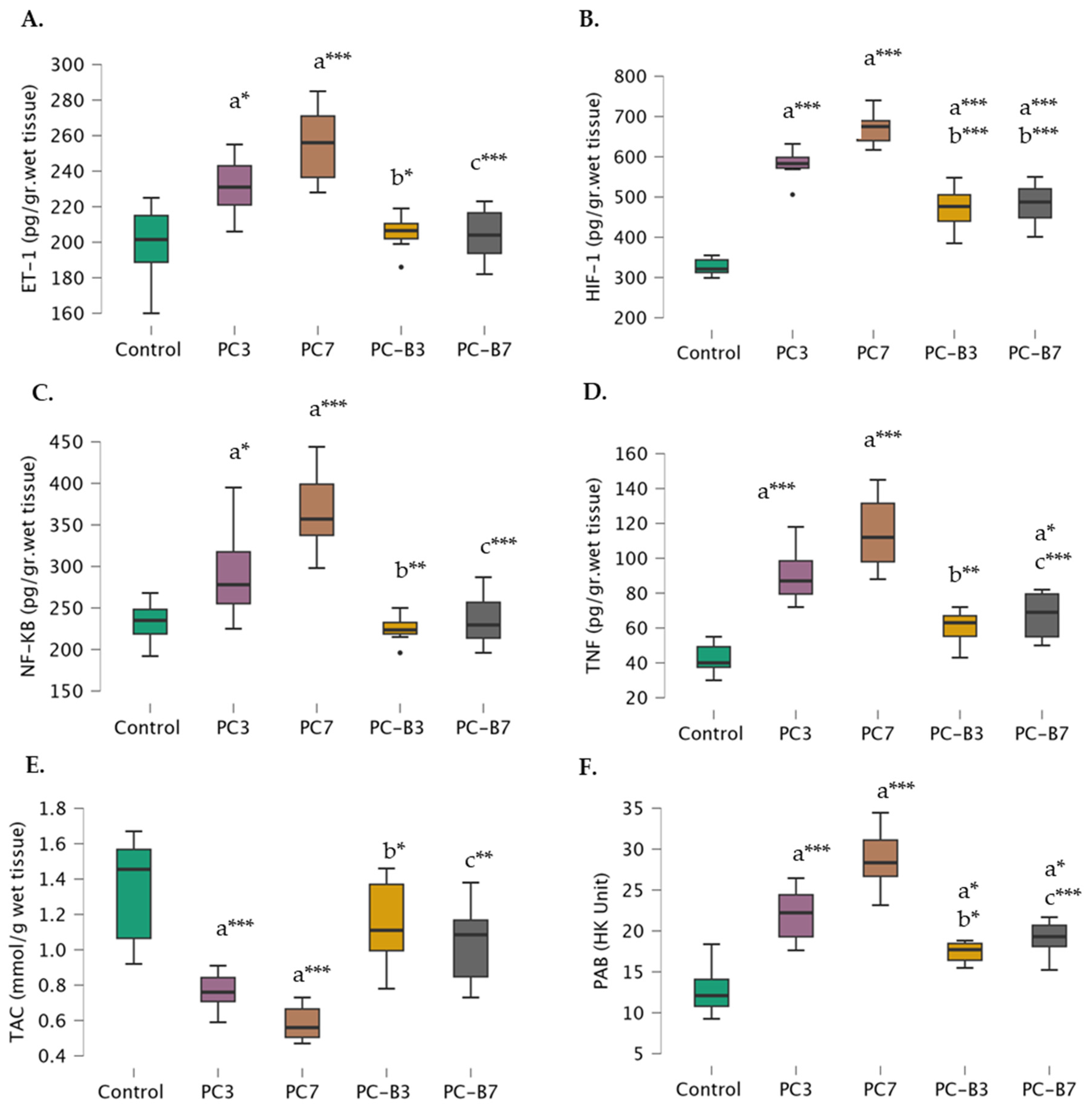
As anticipated, ET-1 levels were found to be significantly higher in the PC-3 (231.38±16.58) and PC-7 groups (254.86±22.05) compared to control group (198.67±23.58). When comparing the bosentan-treated groups, it was observed that the PC-B3 group (205.50±10.14) exhibited significantly lower ET-1 levels than the PC-3 group, whereas the PC-B7 group (204.00 ±14.46) displayed significantly lower ET-1 levels than the PC-7 group. Furthermore, there was no significant difference in ET-1 levels between the PC-B3 and PC-B7 groups and the control group (
Figure 1A).
Additionally, the levels of HIF-1 were found to be lower in the control group (326.17 ± 22.15), PC-3 group (581.75 ± 37.44), and PC-7 group (670.14 ± 42.16), respectively. The level of this factor was found to be lower in the PC-B3 group (470.00 ± 56.10) than in the PC-3 group. Moreover, the level of HIF-1 was found to be lower in the PC-B7 group (670.14±42.16) compared to the PC-7 group. However, its levels were still higher in both the PC-B3 and PC-B7 groups than in the control group (
Figure 1B).
NF-κß levels were also elevated in the control (232.67±26.91), PC-3 (293.63 ± 53.16), and PC-7 (367.43 ± 56.10) groups, respectively. However, a notable discrepancy was observed between the Bosentan-treated groups. In fact, the levels in the PC-B3 group (224.75 ± 16.26) were found to be comparable to the control level and significantly lower than those observed in the PC-3 group. Similarly, in the PC-B7 group (235.00 ± 31.66), the levels were comparable to the control levels and significantly lower than those observed in the PC-7 group (
Figure 1C).
TNF-α levels exhibited an increase in the PC-3 (90.25 ± 15.77), and PC-7 (114.86 ± 21.62) groups yet exhibited a notable decline in the PC-B3 group (60.50 ± 10.01) in comparison to the PC-3 group and a further decline in the PC-B7 group (67.25 ± 13.31) in comparison to the PC-7 group. Notably, while no significant difference was observed between the PC-B3 and controls, the PC-B7 group exhibited a higher level of TNF-α than the controls (
Figure 1D).
The TAC exhibited a gradual decline in the control (1.34±0.32), PC-3 (0.79±0.16), and PC-7 (0.59±0.11) groups, while no significant difference was observed between the control and PC-B3 (1.15±0.25) and PC-B7 (1.04±0.23) groups. The PC-B3 group exhibited a significantly higher value than the PC-3 group, while the PC-B7 group demonstrated a significantly higher value than the PC-7 group (
Figure 1E).
PAB levels exhibited a gradual increase in the control (12.82±3.27), PC-3 (21.98±3.24), and PC-7 (28.79±3.82) groups, with levels being higher in the PC-B3 (17.44±1.24) and PC-B7 (19.13±2.14) groups compared to the control. However, they remained lower in the PC-B3 group compared to the PC-3 group and, in the PC-B7 group compared to the PC-7 group (
Figure 1F).
4. Discussion
Pulmonary contusion (PC) is a common injury after blunt thoracic trauma with increased morbidity and mortality (10,11). Our aim in this study is to contribute to the research on the correlations between systemic inflammatory responses and oxidative stress induced processes in the lung tissue. In this respect, our study is important in that bosentan was tested in order to correct PC-induced lung injury induced by blunt thoracic trauma and the results were compared with each other. The major findings of the current study are i) a significant increase was found in lung tissue ET-1, NF-κB, TNF-α, HIF-1α and PAB levels in all of the traumatized groups compared to the control group, while a significant decrease was found in TAC levels. ii) after bosentan administration, ET-1, NF-κB, TNF-α, HIF-1α and PAB levels decreased significantly while TAC levels increased. iii) there was no significant difference in ET-1 levels between the given bosentan groups and the control group. Our study showed that the use of bosentan may have protective effects by reducing the severity of lung contusion, inflammatory and oxidation/reduction reactions in rats with PC induced by blunt trauma.
Inflammation can be defined as tissue damage or the body’s unique response in the presence of inflammatory stimuli. In current study, ET-1 levels were found to be significantly higher in the PC groups compared to control group. The PC-B3 and PC-B7 groups exhibited significantly lower ET-1 levels than the PC-3 and PC-7 groups. Furthermore, there was no significant difference in ET-1 levels between the bosentan--treated groups and the control group. ET-1, one of the important endothelial synthesis products in systemic inflammation, has a primary role in vascular tone regulation due to its tonic vasoconstrictor effect. ET-1-dependent constriction in response to vasodilator stimuli has a slow onset and persists for hours or even days. In addition to its vasoactive properties, it stimulates smooth muscle cell proliferation, contributes to vascular remodeling and leukocyte adhesion. Thus, it plays an important role in inflammation and atherogenesis (16). Therefore, both ET-A and ET-B receptor antagonist bosanten may provide therapeutic potential to reduce inflammatory responses in lung tissues in blunt thoracic trauma. Thus, although ET-1 was first identified in the vasculature, the effects of ET-1 go far beyond blood pressure control and appear to be essentially relevant to all major organs. While ET-1 serves many essential functions in the body, it is important to note that its dysregulation or overproduction can contribute to pathological conditions. Understanding the role of ET-1 is crucial for both basic science research and the development of therapeutic interventions in various diseases related to vascular function and inflammation. Researchers continue to study the complex regulatory mechanisms of ET-1 and its potential as a therapeutic target. While it is clear that many aspects of blunt thoracic trauma remain unexplained and ET-1 may play a role, this requires further investigation (16). There is no study investigating ET-1 levels in lung tissues of rats in experimental model of pulmonary contusion (PC) induced by blunt thoracic trauma. Researchers continue to study the complex regulatory mechanisms of ET-1 and its potential as a therapeutic target.
One of the most important factors determining the prognosis after lung contusion is the inflammatory response. The main factor in the formation of endothelial/epithelial damage is neutrophils (13). Many changes occur in the lung parenchyma after contusion. These include hemorrhage, edema, and consolidation, which cause impaired ventilation/perfusion ratio, hypoventilation, and decreased compliance resulting in hypoxia (14). Activation of HIF-1α has a decisive role in cell proliferation and apoptosis. It is able to do this through its regulatory roles on ion changes in cells, transporters, circulating hormones and their receptors (15,16). In current study, the levels of HIF-1 and NF-κB were found to be elevated in the control group, PC-3 group, and PC-7 group. The level of HIF-1 and NF-κB was also found to be lower in the treated groups. However, their levels were still higher in treated groups than in the control group. There is a well-known link between hypoxia and inflammation. Prominent among factors other than hypoxia that activate HIF-1α is NF-κB. NF-κB, in turn, can upregulate the transcription of HIFs (17,18). In current study, although no correlation was found between ET-1, NF-κB and HIF-1α, transcriptional regulation of numerous hypoxia-responsive genes, including vascular endothelial growth factor (VEGF) and ET-1, is via the critical mediator of hypoxia-induced transcription, HIF-1α (19). In biopsies of invasive breast cancer, the expression of ET-1, ET-RA and ET-RB is associated with increased VEGF expression and vascularity (20). Bosentan inhibits tumour vascularisation and bone metastasis in an immunocompetent skinfold chamber model of breast carcinoma cell metastasis (21). Panchal et al. (22) reported that new derivatives (17d, 16j and 16h) of bosentan, an endothelin receptor antagonist, can dose-dependently reduce HIF-1α levels in vivo studies using monocrotaline (MCT) induced PAH in rat model. PC often presents with hypoxemia, reduced lung compliance, and tachycardia. There are currently no specific pharmacological treatments (23). Suresh et al. (23) PC results in profound global hypoxia with HIF-1α activation and subsequent upregulation of proinflammatory mediators, including IL-1β and IL-6. Our and these results indicate that blockade of HIF-1α activation with compounds represents a targeted therapy for blunt force trauma resulting in PC. Hypoxia is a direct consequence of PC and acid aspiration that prompts some patients to require mechanical ventilation (23).
TNF-α has been shown to play a key pathophysiologic role in different models of acute lung injury (24,25). Lung injury directly activates neutrophils and macrophages, which release proinflammatory cytokines such as TNF-α, and also affects other inflammatory products released by neutrophils. It also affects the relationship between neutrophils and vascular endothelium, leading to an increase in neutrophil-dependent vascular permeability (26) . Chu et al. (25) showed that TNF-α concentration increased in broncho alveolar lavage (BAL) fluid and plasma in their acute lung injury models. In current study, TNF-α levels were found to be significantly increased in contusion-induced rats. This result proves once again that TNF-α plays a role in contusion-induced lung injury. The results of the analysis showed that TNF-α levels measured 3 days after contusion increased further after 7 days. This suggests that TNF-a-mediated mechanisms are active in the early response after contusion, accompanied by a rapid progression of the inflammatory process. Bosentan had a significant effect on TNF-α caused a decline. Bosentan suppresses TNF-α, a mediator that plays a key role in the inflammatory response induced by contusion, thus reflecting at least through this mechanism that it attenuates the destructive effects of contusion. Donate ve ark (27) reported that bosentan treatment also reduced joint damage, leukocyte infiltration and pro-inflammatory cytokine levels (IL-1β, TNFα and IL-17) in the joint tissues. Bellisai et al. (28) showed that a decrease of profibrotic and proinflammatory cytokines levels in humans during treatment with Bosentan. The degree of systemic inflammation and subsequent immunosuppression increases in relation with the rates of development of pulmonary organ failure (29).
It is known that alveolar macrophages can produce potent reactive oxygen and nitrogen species (ROS, RNS) called superoxide radicals and peroxynitrite in conditions such as PC leading to acute lung injury (6,30). These released ROS can cause oxidative damage through lipid peroxidation, protein oxidation and DNA damage. In current study, there was a significant increase in PAB and TAC levels in PC developing after blunt chest trauma. ROS, RNT contribute to inflammatory damage through both direct and indirect effects (31). It was found that Bosentan, a potent ET receptor antagonist, decreased oxidative stress by decreasing PAB levels and increasing TAC levels in lung tissue. This result supports the positive effects of bosentan on the antioxidant system in PC.
4.1. Limitation of Study
Other metabolic and hemodynamic markers such as blood gas analysis and oxygen saturation monitoring could not be evaluated in our study. Çalışmamızda arter kan gazı analizi yapılamaması ve saturasyonun monitorize edilememesi nedeniyle arteriyel parsiyel oksijen basıncı ve karbondioksit basıncı değerlendirilememiştir.
In conclusion, PC has been shown to cause oxidative damage. ET-1 receptors may play an important role in PC-induced vasoconstriction and this vasoconstriction could be reversed experimentally by inhibiting ET-1 receptors. Inhibition of ET-1 receptors with bosentan prevented contusion and ET-1 levels decreased in the lung. Bosentan, a potent ET receptor antagonist, has been found to reduce oxidative stress and inflammation in lung tissue. Bosentan was found to have anti-inflammatory and antioxidant effects in pulmonary contusion caused by blunt thoracic trauma. The low and high bosentan doses used in our study are within the recommended dose ranges according to the PC diagnosis and treatment guideline, and studies with higher doses and long-term effects would be appropriate. Bosentan antagonizes the deleterious effects and hypoxic vasoconstriction of endothelin in contusion-induced lung injury and exhibits vasodilating, antiproliferative, anti-inflammatory, and anti-oxidant effects. Accordingly, although Bosentan shows multifactorial properties in PC, dose selection in humans for clinical use should be patient-based. If supported by similar studies, bosentan can be used in both pulmonary and emergency clinics to reduce ischemic complications, inflammation, oxidative stress in some diseases that may be accompanied by ischemia.
Author Contributions
NU: SD, GG, BA, NFM, and HU conceptualized and designed this study. GG performed data acquisition. SD performed the statistical analyses. NU, SD, GG, BA, NFM, and HU drafted the manuscript. All authors finalized the manuscript. NU and HU supervised the entire process. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was received for conducting this study.
Institutional Review Board Statement
All experiments were approved by the Yeditepe University (2017/601) Ethics Committee for Animal Experiments and followed the NIH Guide for the Care and Use of Laboratory Animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data underlying this article are available in the article. If needed, please contact the corresponding author. The email address is huzun59@hotmail.com.
Conflicts of Interest
The authors declare no competing interests.
References
- Dogrul, B.N.; Kiliccalan, I.; Asci, E.S.; Peker, S.C. Blunt trauma related chest wall and pulmonary injuries: An overview. Chin. J. Traumatol. 2020, 23, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Rendeki S, Molnár TF. Pulmonary contusion. J Thorac Dis. 2019;11(Suppl 2):S141-S151.
- Krishnan Raghavendran, Robert H. Notter, Bruce A. Davidson, Jadwiga D. Helinski, Steven L. Kunkel, And Paul R. Knight. Lung Contusion: Inflammatory Mechanisms And Interaction With Other Injuries. Shock Vol. 32, No. 2, Pp. 122-130, 2009.
- Chester, A.H.; Yacoub, M.H. The role of endothelin-1 in pulmonary arterial hypertension. Glob. Cardiol. Sci. Pr. 2014, 2014, 62–78. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.J.; Badesch, D.B.; Barst, R.J.; Galiè, N.; Black, C.M.; Keogh, A.; Pulido, T.; Frost, A.; Roux, S.; Leconte, I.; et al. Bosentan Therapy for Pulmonary Arterial Hypertension. New Engl. J. Med. 2002, 346, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Gercel, G.; Aksu, B.; Ozkanli, S.; Uzun, H.; Aksu, F.; Ozatman, E.; Durakbaşa, U. Investigation of Bosentan's Effects on Pulmonary Contusion Created by Blunt Thoracic Trauma in Rats. Eur. J. Pediatr. Surg. 2020, 30, 071–078. [Google Scholar] [CrossRef] [PubMed]
- Raghavendran, K.; Davidson, B.A.; Helinski, J.D.; Marschke, C.J.; Manderscheid, P.; Woytash, J.A.; Notter, R.H.; Knight, P.R. A Rat Model for Isolated Bilateral Lung Contusion from Blunt Chest Trauma. Obstet. Anesthesia Dig. 2005, 101, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Alamdari, D.H.; Ghayour-Mobarhan, M.; Tavallaie, S.; Parizadeh, M.R.; Moohebati, M.; Ghafoori, F.; Kazemi-Bajestani, S.M.R.; Paletas, K.; Pegiou, T.; Koliakos, G. Prooxidant–antioxidant balance as a new risk factor in patients with angiographically defined coronary artery disease. Clin. Biochem. 2008, 41, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Parohan, M.; Sadeghi, A.; Khatibi, S.R.; Nasiri, M.; Milajerdi, A.; Khodadost, M.; Sadeghi, O. Dietary total antioxidant capacity and risk of cancer: a systematic review and meta-analysis on observational studies. Crit. Rev. Oncol. 2019, 138, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Wu Zq, Lei Yq. Advances In The Research Of Lung Injury After Chest Impact Injury. J Mod Clin Med. 2010;136:83-85.
- Bamvita, J.-M.; Bergeron, E.; Lavoie, A.; Ratte, S.; Clas, D. The Impact of Premorbid Conditions on Temporal Pattern and Location of Adult Blunt Trauma Hospital Deaths. J. Trauma: Inj. Infect. Crit. Care 2007, 63, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Banecki, K.M.R.M.; Dora, K.A. Endothelin-1 in Health and Disease. Int. J. Mol. Sci. 2023, 24, 11295. [Google Scholar] [CrossRef]
- Bellingan, G.J. The pulmonary physician in critical care * 6: The pathogenesis of ALI/ARDS. Thorax 2002, 57, 540–546. [Google Scholar] [CrossRef]
- Gavelli, G.; Canini, R.; Bertaccini, P.; Battista, G.; Bnà, C.; Fattori, R. Traumatic injuries: imaging of thoracic injuries. Eur. Radiol. 2002, 12, 1273–1294. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Yang, Y.; Fu, Q.; Wang, X.; Liu, Y.; Zeng, Q.; Li, Y.; Gao, S.; Bao, L.; Liu, S.; et al. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J. Appl. Physiol. 1985, 88, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, W.; Schmitt, R.; Rosenberger, C.; Münchenhagen, P.; Gröne, H.-J.; Frei, U.; Warnecke, C.; Bachmann, S.; Wiesener, M.; Willam, C.; et al. Expression of hypoxia-inducible transcription factors in developing human and rat kidneys. Kidney Int. 2006, 69, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Rius J, Guma M, Schachtrup C, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453(7196):807-811.
- van Uden P, Kenneth NS, Rocha S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem J. 2008;412(3):477-484.
- Wenger, RH. Mammalian oxygen sensing, signalling and gene regulation. J Exp Biol. 2000;203(Pt 8):1253-1263.
- Wülfing, P.; Kersting, C.; Tio, J.; Fischer, R.-J.; Wülfing, C.; Poremba, C.; Diallo, R.; Böcker, W.; Kiesel, L. Endothelin-1-, Endothelin-A-, and Endothelin-B-Receptor Expression Is Correlated with Vascular Endothelial Growth Factor Expression and Angiogenesis in Breast Cancer. Clin. Cancer Res. 2004, 10, 2393–2400. [Google Scholar] [CrossRef] [PubMed]
- Dréau, D.; Karaa, A.; Culberson, C.; Wyan, H.; McKillop, I.H.; Clemens, M.G. Bosentan® inhibits tumor vascularization and bone metastasis in an immunocompetent skin-fold chamber model of breast carcinoma cell metastasis. Clin. Exp. Metastasis 2006, 23, 41–53. [Google Scholar] [CrossRef]
- Panchal, J.; Jaiswal, S.; Jain, S.; Kumawat, J.; Sharma, A.; Jain, P.; Jain, S.; Verma, K.; Dwivedi, J.; Sharma, S. Development of novel bosentan analogues as endothelin receptor antagonists for pulmonary arterial hypertension. Eur. J. Med. Chem. 2023, 259, 115681. [Google Scholar] [CrossRef] [PubMed]
- Suresh, M.V.; Balijepalli, S.; Solanki, S.; Aktay, S.; Choudhary, K.; Shah, Y.M.; Raghavendran, K. Hypoxia-Inducible Factor 1α and Its Role in Lung Injury: Adaptive or Maladaptive. Inflammation 2023, 46, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Knöferl, M.W.; Liener, U.C.; Seitz, D.H.; Perl, M.; Brückner, U.B.; Kinzl, L.; Gebhard, F. Cardiopulmonary, Histological, and Inflammatory Alterations After Lung Contusion in a Novel Mouse Model of Blunt Chest Trauma. Shock 2003, 19, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.-J.; Li, M.-H.; Hsu, C.-W.; Tsai, S.-H.; Lin, S.-H.; Huang, K.-L. Influence of hyperbaric oxygen on tumor necrosis factor-α and nitric oxide production in endotoxin-induced acute lung injury in rats. Pulm. Pharmacol. Ther. 2007, 20, 684–690. [Google Scholar] [CrossRef]
- Sträter, J.; Walczak, H.; Krammer, P.H.; Möller, P. Simultaneous in situ detection of mRNA and apoptotic cells by combined hybridization and TUNEL. J. Histochem. Cytochem. 1996, 44, 1497–1499. [Google Scholar] [CrossRef]
- Donate PB, Cunha TM, Verri WA Jr, et al. Bosentan, an endothelin receptor antagonist, ameliorates collagen-induced arthritis: the role of TNF-α in the induction of endothelin system genes. Inflamm Res. 2012;61(4):337-348.
- Bellisai, F.; Morozzi, G.; Scaccia, F.; Chellini, F.; Simpatico, A.; Pecetti, G.; Galeazzi, M. Evaluation of the Effect of Bosentan Treatment on Proinflammatory Cytokine Serum Levels in Patients Affected by Systemic Sclerosis. Int. J. Immunopathol. Pharmacol. 2011, 24, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Papae Hc, Tsukamoto T, Kobbe P, Et Al. Assesment Of The Clinical Course With Inflammatory Parameters. Injury 2007;38:1358-64.
- Ischiropoulos, H.; Zhu, L.; Beckman, J.S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch. Biochem. Biophys. 1992, 298, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Iqbal M, Cohen R, Marzouk K, Liu S. Time course of nitric oxide, peroxynitrite and antioxidants in the endotoxemic heart. Crit Care Med 2002;30:1291-1296.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).