Submitted:
14 June 2024
Posted:
19 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
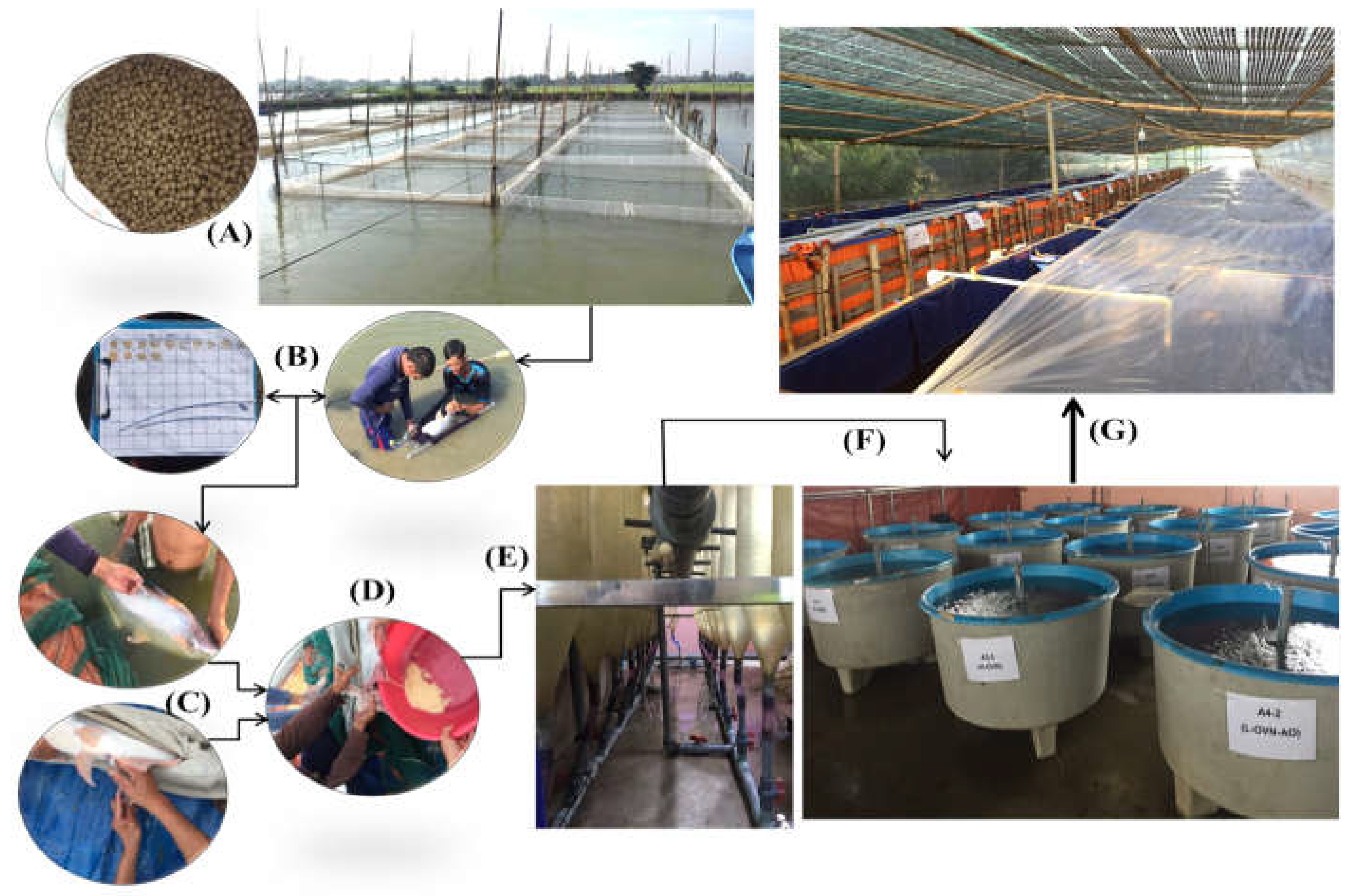
2.1. Study Site and Research Layout
2.2. Pond Preparation and Management of the Broodstock
2.3. Selection Criteria of Broodstock
2.4. Experimental Diet Preparation and Feeding Practice
2.5. Broodstock Experimental Design
2.6. Induced Spawning and Larvae Rearing Practices
2.6.1. Mature Broodstock Fish Selection for Induced Spawning
2.6.2. Induced Stripping Practices of Broodstock
2.6.3. Eggs Incubation and Larvae Fish Nursing Practices
2.7. The Experiments of Larvae to Fingerling Rearing
2.8. Water Quality Monitoring
2.9. Chemical Analysis
2.10. Calculation
2.11. Statistical Analysis
3. Results
3.1. Chemical Composition and Essential Amino Acid Content of Feed Ingredients and Diets
3.2. Growth Performance Indices of Broodstock
3.3. Reproductive Breeding, Hatching, and Early Life-Stage Development
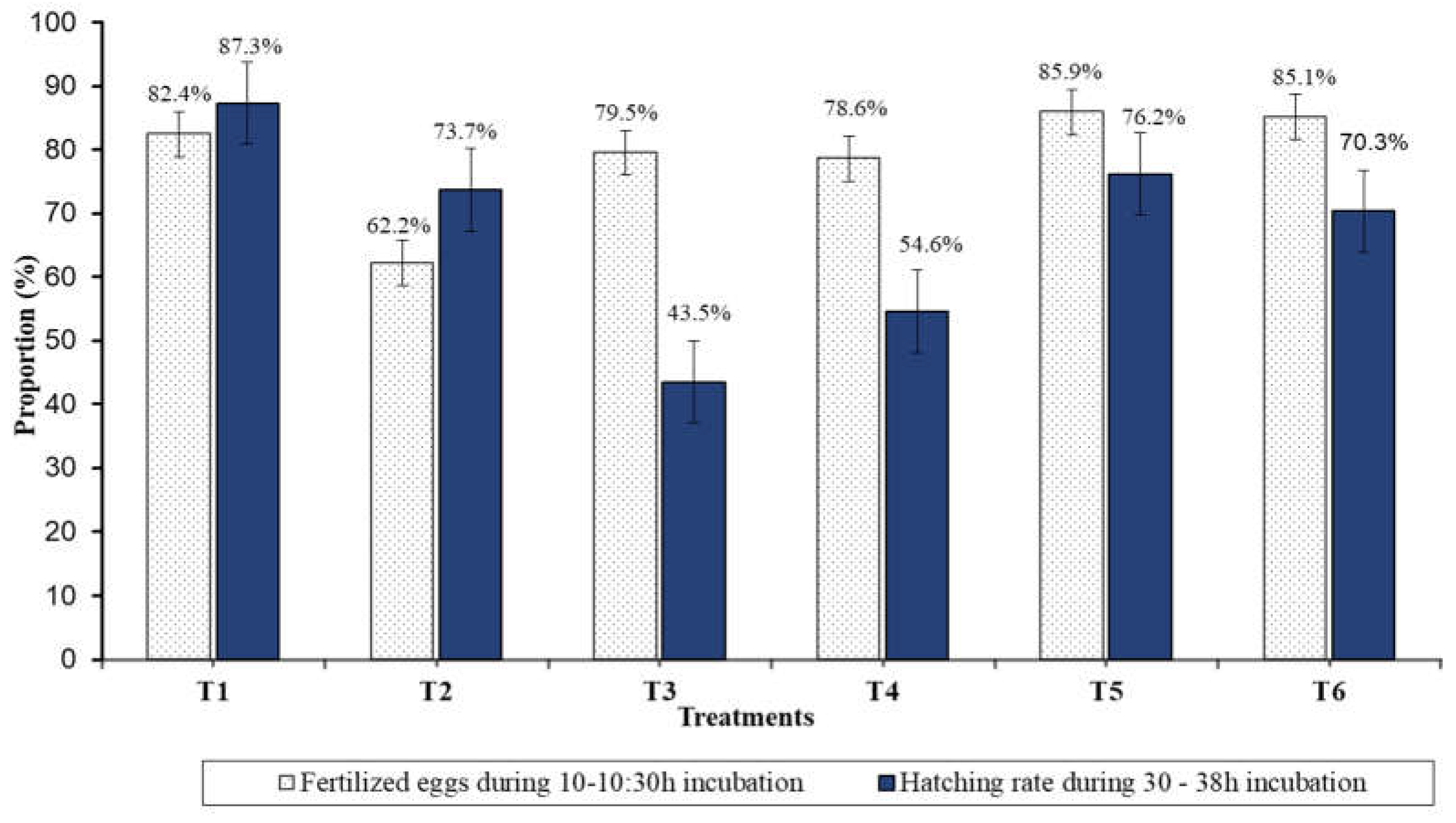
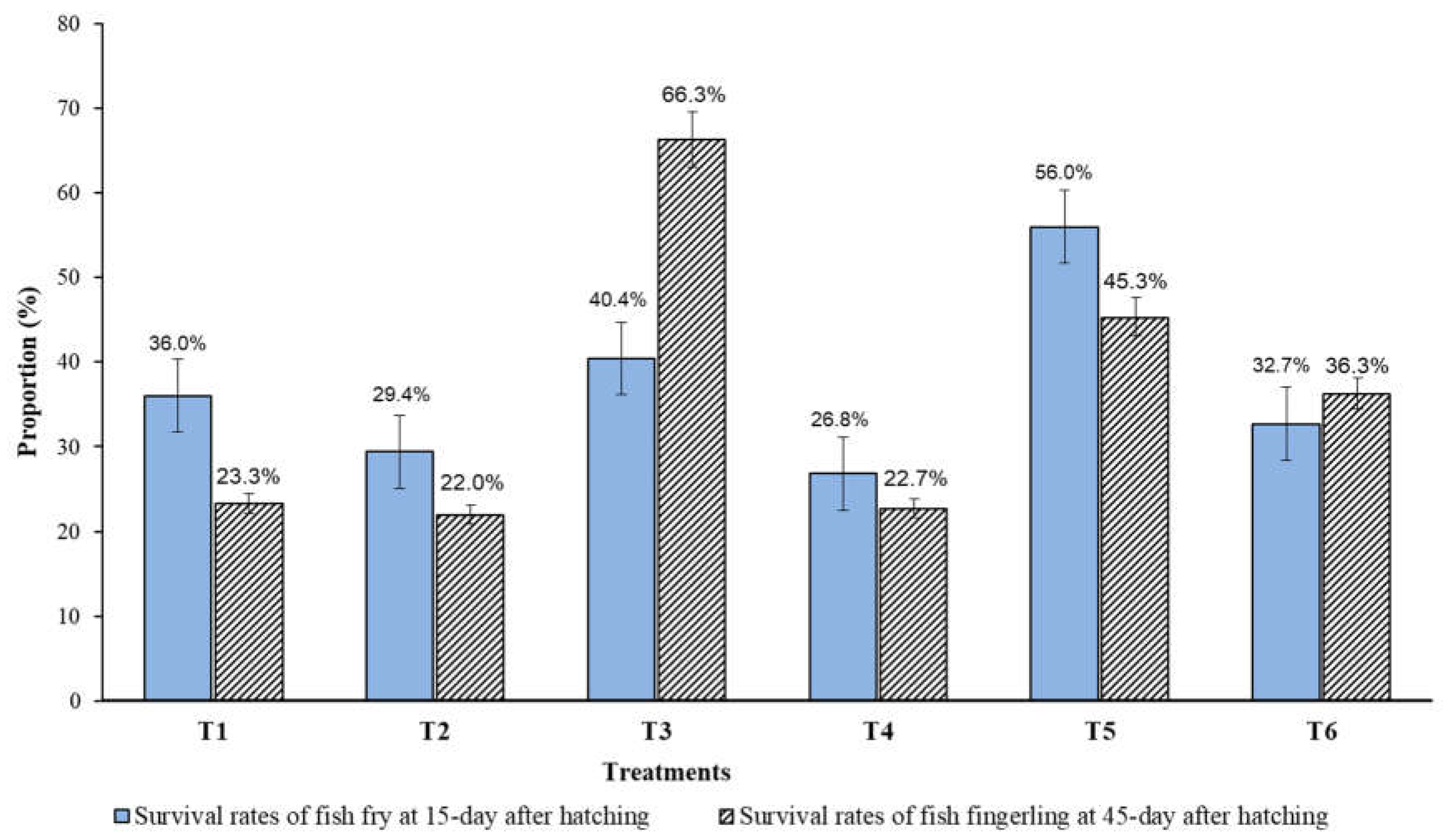
3.4. Growth Performance and Survival Rate of Fingerling Reared at 30 and 45 Days after Hatching
3.5. Water Quality Monitoring
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abidin, M.Z.; Hashim R, Chong SCA. Influence of dietary protein levels on growth and egg quality in broodstock female Bagrid catfish (Mystus nemurus Cuv. & Val.). Aquacult Res. 2006, 37, 416–418. [CrossRef]
- Adewolu, M.A.; Benfey, T.J. Growth, nutrient utilization and body composition of juvenile Bagrid catfish, Chrysichthys nigrodigitatus (Actinopterygii: Siluriformes: Claroteidae), fed different dietary crude protein levels. Acta Ichthyologica Et Piscatoria. 2009, 39, 95–101. [Google Scholar] [CrossRef]
- Ahmad, A.W.; Hassan, S.; Banat, F. An overview of microalgae biomass as a sustainable aquaculture feed ingredient: food security and circular economy. Bioengineered. 2022, 13, 9521–9547. [Google Scholar] [CrossRef]
- Ali, M.; Jauncey, K. Approaches to optimizing dietary protein to energy ratio for African catfish Clarias gariepinus (Burchell, 1822). Aquacult Nutr. 2005, 11, 95–101. [Google Scholar] [CrossRef]
- AOAC. Animal feeds. Chapter 4. In: Official methods of analysis. Association of official analytical chemists international (ed. by P.A. Cunniff). AOAC, Arlington, VA, USA 1, VI (16th (ed.)). 1997, 1102.
- Astiasarán, I.; Ansorena, D. In gourmet and health-promoting specialty oils. (Eds, Moreau, R.A. and Kamal-Eldin, A.) AOCS Press. 2009, 491–513. [CrossRef]
- Baidya, A.P.; Senoo, S. Decline in fertilization and hatching rates of Patin, (Pangasius hypophthalmus) after ovulation. Aquacult Sci. 2003, 51, 407–415. [Google Scholar] [CrossRef]
- Berlinsky, D.L.; Kenter, L.W.; Reading, B.J.; Goetz, F.W. Chapter 1 - Regulating reproductive cycles for captive spawning. In Fish Physi, Vol. 38 (Eds, Benfey, T.J., Farrell, A.P. and Brauner, C.J.) Academic Press. 2020, 1–52.
- Bruce, M.; Oyen, F.; Bell, G.; Asturiano, J.F.; Farndalem, B.; Carrillo, M.; Zanuy, S.; Ramos, J.; Bromage, N. Development of broodstock diets for the European Sea Bass (Dicentrarchus labrax) with special emphasis on the importance of n−3 and n−6 highly unsaturated fatty acid to reproductive performance. Aquaculture. 1999, 177, 85–97. [Google Scholar] [CrossRef]
- Bui, T.M.; Lam, P.T.; Ingram, B.A.; Thuy, N.T.T.; Gooley, G.J.; Hao, N.V.; Phuong, N.T.; De Silva, S.S. Seed production practices of Striped catfish (Pangasianodon hypophthalmus) in the Mekong Delta region, Vietnam. Aquaculture. 2010, 306, 92–100. [Google Scholar] [CrossRef]
- Bui, T.M.; Phuong, N.T.; Nguyen, G.H.; De Silva, S.S. Fry and fingerling transportation in the Striped catfish (Pangasianodon hypophthalmus) farming sector, Mekong Delta, Vietnam: A pivotal link in the production chain. Aquaculture. 2013, 388, 70–75. [Google Scholar] [CrossRef]
- Cacot, P. Description of the sexual cycle related to the environment and set up of the artificial propagation in Pangasius bocourti (Sauvage 1880) and Pangasius hypophthalmus (Sauvage 1878) reared in floating cages and in ponds in the Mekong Delta. In: Legendre M., Pariselle, A. (eds). The Biological Diversity and Aquaculture of Clariid and Pangasiid catfishes in South East Asia. Proceedings of the mid-term workshop of the ‘Catfish Asia Project’, 11–15 May 1998. Cantho, Vietnam.1999, 71–89.
- Cacot, P.; Legendre, M.; Dan, T.Q.; Tung, L.T.; Liem, P.T.; Mariojouls, C.; Lazard, J. Induced ovulation of Pangasius bocourti (Sauvage, 1880) with a progressive hCG treatment. Aquaculture. 2002, 213, 199–206. [Google Scholar] [CrossRef]
- Chand, B.K.; Singh, M.K.; Mandal, B. Studies on the breeding of Pangasius sutchi using different inducing agents. J. Appl Aquacult. 2011, 23, 32–40. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Han, D.; Zhu, X.; Xie, S.; Han, D.; Hu, Q. Two filamentous microalgae as feed ingredients improved flesh quality and enhanced antioxidant capacity and immunity of the gibel carp (Carassius auratus gibelio). Aquacult Nutr. 2019, 25, 1145–1155. [Google Scholar] [CrossRef]
- Chuapoehuk, W.; Pothisoong, T. Protein requirements of catfish fry, Pangasius sutchi, Fowler. In: Finfish Nutrition in Asia: Methodological approaches to research development. Proceedings of the Asian Finfish Nutrition Workshop. Singapore, 23_26, Ottawa. 1985, 103–106.
- Colombo, S.M. In Fish Physiology, Vol. 38 (Eds, Benfey, T.J., Farrell, A.P. and Brauner, C.J.). Academic Press. 2020, 53–82. [CrossRef]
- Craig, S.R.; Gardner, T.R.; Carnevali, O. In Marine Ornamental Species. Aquaculture. 2017, 139–158. [Google Scholar] [CrossRef]
- D-Fish. Vietnam shrimp 2021: Farming output increases, exports are estimated at 3.8 billion USD (Reported on December 10, 2021). Website of the Directorate of Fisheries, Vietnam. Accessed on 03rd March, 2022.
- Da, C.T.; Hung, L.T.; Berg, H.; Lindberg, J.E.; Lundh, T. Evaluation of potential feed sources, and technical and economic considerations of small-scale commercial striped catfish (Pangasius hypothalamus) pond farming systems in the Mekong Delta of Vietnam. Aquacult Res. 2011, 2, 1–13. [Google Scholar] [CrossRef]
- Da, C.T.; Lundh, T.; Lindberg, J.E. Evaluation of local feed resources as alternatives to fish meal in terms of growth performance, feed utilisation and biological indices of striped catfish (Pangasianodon hypophthalmus) fingerlings. Aquaculture. 2012, 364, 150–156. [Google Scholar] [CrossRef]
- Datta, S.N.; Ansal, M.D. Induced breeding of Asian striped catfish (Pangasianodon hypophthalmus) under farmer participatory mode in Punjab. J. Krishi Vigyan, 2020. [Google Scholar] [CrossRef]
- Datta, S.N.; Singh, A.; Jassal, G.; Pandey, A. A study on induced breeding, embryonic and larval development of Pangasianodon hypophthalmus in semi-arid agro-climate. J Envi Biol. 2018, 39, 671–676. [Google Scholar] [CrossRef]
- Estrada-Godinez, J.A.; Rodríguez-Montes, De. Oca. G.A.; Bañuelos-Vargas, M.I.; Martínez-Montaño, E.; Pacheco-Marges, Md.R.; Román-Reyes, J.C. Effect of feeding rate and hormonal treatments on the condition factor and the reproductive performance of the catfish, Pangasianodon hypophthalmus. J. Appl Aquacult. [CrossRef]
- Fagbenro, O.A. Quantitative dietary protein requirements of Clarias isheriensis (Sydenham 1980) (Clariidae) fingerlings. J. Appl Ichthy. 1992, 8, 164–169. [Google Scholar] [CrossRef]
- Fernández-Palacios, H.; Izquierdo, M.S.; Robaina, L.; Valencia, A.; Salhi, M.; Vergara, J. Effect of n − 3 HUFA level in broodstock diets on egg quality of gilthead sea bream (Sparus aurata L.). Aquaculture. 1995, 132, 325–337. [Google Scholar] [CrossRef]
- Gatlin, D.M.; Poe, W.E.; Wilson, R.P. Protein and energy requirements of fingerling channel catfish for maintenance and maximum growth. J Nutr. 1986, 116–2121. [Google Scholar]
- Ha, H.P.; Nguyen, T.T.T.; Poompuang, S.; Na-Nakorn, U. Microsatellites revealed no genetic differentiation between hatchery and contemporary wild populations of Striped catfish, Pangasianodon hypophthalmus (Sauvage 1878) in Vietnam. Aquaculture. 2009, 291, 154–160. [Google Scholar] [CrossRef]
- Haas, S.; Bauer, J.L.; Adakli, A.; Meyer, S.; Lippemeier, S.; Schwarz, K.; Schulz, C. Marine microalgae Pavlova viridis and Nannochloropsis sp. as n-3 PUFA source in diets for juvenile European sea bass (Dicentrarchus labrax L.). J. Appl Phyc, 1011. [Google Scholar]
- Hossain, M.R.A.; Rahman, B.M.S. Thai Pangas (Pangasius sutchi) in Bangladesh: A review. Inter J Busi, Soc Sci Res. 2014, 1, 98–106. [Google Scholar]
- Hung, L.T.; Liem, P.T.; Tu, H.T.; Mariojouls, C. Comparing growth and protein requirements for fingerlings of three catfish of the Mekong River (Pangasius bocourti, Pangagasius hypothalmus and Pangasius conchophilus). J Aquacult in Trop. 2002, 17, 325–335. [Google Scholar]
- Hung, L.T.; Thanh, N.T.; Pham, M.A.; Browdy, C.L. A comparison of the effect of dietary fungal phytase and dicalcium phosphate supplementation on growth performances, feed and phosphorus utilization of tra catfish juveniles (Pangasianodon hypophthalmus Sauvage, 1878). Aquacult Nutr. 2015, 21, 10–17. [Google Scholar] [CrossRef]
- Ibrahem, M.D.; Mohamed, M.F.; Ibrahim, M.A. The role of spirulina platensis (Arthrospira platensis) in Growth and Immunity of Nile Tilapia (Oreochromis niloticus) and Its Resistance to bacterial infection. J Agricult Sci. 2013, 5, 109. [Google Scholar] [CrossRef]
- Izquierdo, M.S.; Fernández-Palacios, H.; Tacon, A.G.J. In reproductive biotechnology in Finfish Aquaculture (Eds, Lee, C.-S. and Donaldson, E.M.) Elsevier. Amsterdam, 2001, 25–42. [CrossRef]
- Izquierdo, M.S.; Turkmen, S.; Montero, D.; Zamorano, M.J.; Afonso, J.M.; Karalazos, V.; Fernández-Palacios, H. Nutritional programming through broodstock diets to improve utilization of very low fishmeal and fish oil diets in gilthead sea bream. Aquaculture. 2015, 449, 18–26. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Forster, I.P.; Dominy, W.G. Effects of supplementing two species of marine algae or their fractions to a formulated diet on growth, survival and composition of shrimp (Litopenaeus vannamei). Aquaculture. 2009, 292, 237–243. [Google Scholar] [CrossRef]
- Kabir, M.A.; Ghaedi, A.; Talpur, A.D.; Hashim, R. Effect of dietary protein levels on reproductive development and distribution of amino acids in the body tissues of female Pangasianodon hypophthalmus (Sauvage, 1878) broodstock in captivity. Aquacult Res. 2015, 46, 1736–1747. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, Y.; Ji, W.X. Growth, feed utilization and body composition of Asian catfish (Pangasius hypophthalmus) fed at different dietary protein and lipid levels. Aquacult Nutr. 2011, 17, 578–584. [Google Scholar] [CrossRef]
- Mejri, S.; Audet, C.; Vandenberg, G.W.; Parrish, C.C.; Tremblay, R. Biochemical egg quality in a captive walleye (Sander vitreus) broodstock population relative to ovulation timing following hormonal treatment. Aquaculture. 2014, 431, 99–106. [Google Scholar] [CrossRef]
- Mejri, S.; Tremblay, R.; Vandenberg, G.; Moren, M.; Khemis, I.B.; Audet, C. Differences in nutrient content of eggs and larvae as indicators for improvement of broodstock nutrition in walleye (Sander vitreus) production. Canad J Zool. 2017, 95, 299–310. [Google Scholar] [CrossRef]
- Miller, M.R.; Nichols, P.D.; Carter, C.G. n-3 Oil sources for use in aquaculture – alternatives to the unsustainable harvest of wild fish. Nutr Res Rev. 2008, 21, 85–96. [Google Scholar] [CrossRef]
- Na-Nakorn, U.; Moeikum, T. Genetic diversity of domesticated stocks of striped catfish, Pangasianodon hypophthalmus (Sauvage 1878), in Thailand: Relevance to broodstock management regimes. Aquaculture, 2009, 297, 70–77. [Google Scholar] [CrossRef]
- Nagappan, S.; Das, P.; AbdulQuadir, M.; Thaher, M.; Khan, S.; Mahata, C.; Al-Jabri, H.; Vatland, A.K.; Kumar, G. Potential of microalgae as a sustainable feed ingredient for aquaculture. J Biotech. 2021, 341, 1–20. [Google Scholar] [CrossRef]
- Ng WK, Soon SC, Hashim R. The dietary protein requirement of a bagrid catfish, Mystus nemurus, determined using semipurified diets of varying protein level. Aquacult Nutr. 2001, 7, 45–51. [CrossRef]
- NRC. Nutrient requirements of fish and shrimp. National Research Council of the National Academies. Washington, D.C (U.S.) 2011, 363, http://www.nap.edu/openbook.php–record_id=13039.
- Pérez, M.J.; Rodríguez, C.; Cejas, J.R.; Martín, M.V.; Jerez, S.; Lorenzo, A. Lipid and fatty acid content in wild white seabream (Diplodus sargus) broodstock at different stages of the reproductive cycle. Comp Biochem Physiol B Bioc Mol Biol. 2007, 146, 187–96. [Google Scholar] [CrossRef]
- Phan, T. L, Tam, B,M.; Thuy, N.T.T.; Geoff, G.J.; Brett, I.A.; Hao, N.V.; Phuong, N.T.; Silva, S.S.D. Current status of farming practices of striped catfish, Pangasianodon hypophthalmus in the Mekong Delta, Vietnam. Aquaculture. 2009, 296, 227–236. [Google Scholar] [CrossRef]
- Phumee, P.; Hashim, R.; Aliyu-Paiko, M.; Shu-Chien, A.C. Effects of dietary protein and lipid content on growth performance and biological indices of iridescent Shark (Pangasius hypophthalmus, Sauvage 1878) fry. Aqua Res. 2009, 40(4), 456–463. [Google Scholar] [CrossRef]
- Phuong, N.T.; Tam, B.M.; Nguyen, T.A.; De Silva, S. In Advances in Aquaculture hatchery technology (Eds, Allan, G. and Burnell, G.). Woodhead Publishing. [CrossRef]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Applied Micr Biotec. 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; He, L.; Wang, Y.; Li, D.; Chen, W.; Ye, J. Growth performance, fatty acid composition, and lipid metabolism are altered in groupers (Epinephelus coioides) by dietary fish oil replacement with palm oil. Animal Nutr. 2022, 8, 102–113. [Google Scholar] [CrossRef]
- Quintero, H.; Davis, A.D. Broodstock nutrition: enhancement of egg quality in Channel catfish. Nutrición Acuícola: Investigación y Desarrollo, Universidad Autónoma de Nuevo León, San Nicolás de los Garza, Nuevo León, México. ISBN 978-607-27-0593-7, 2015, 259–273.
- Rahman, M.A.; Ullah, M.R.; Kabir, M.A.; Alam, M.A.; Rahman M, Hossen MF. Artificial propagation of indigenous Yellowtail catfish (Pangasius pangasius): Experiences and challenges. Aquaculture. 2020, 523, 1–7. [CrossRef]
- Sah, U.; Wagle, S.K.; Mehta, S.N.; Mukhiya, Y.K. Preliminary observations on breeding and fry rearing of Pangas (Pangasius hypophthalmus) in eastern terai region of Nepal. Inter J Fish Aquatic Res. 2018, 3, 14–16. [Google Scholar]
- Salhi, M.; Bessonart, M.; Chediak, G.; Bellagamba, M.; Carnevia, D. Growth, feed utilization and body composition of black catfish, Rhamdia quelen, fry fed diets containing different protein and energy levels. Aquaculture. 2004, 231, 435–444. [Google Scholar] [CrossRef]
- Shah, M.R.; Lutzu, G.A.; Alam, A.; Sarker, P.; Kabir Chowdhury, M.A.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in aquafeeds for a sustainable aquaculture industry. J Appl Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- Sink, T.D.; Lochmann, R.T.; Pohlenz, C.; Buentello, A.; Gatlin, D. Effects of dietary protein source and protein–lipid source interaction on Channel catfish (Ictalurus punctatus) egg biochemical composition, egg production and quality, and fry hatching percentage and performance. Aquaculture. 2010, 298, 251–259. [Google Scholar] [CrossRef]
- Ślusarczyk, J.; Adamska, E.; Czerwik-Marcinkowska, J. Fungi and algae as sources of medicinal and other biologically active compounds: A Revi Nutri. 2021, 13, 31–78. 13. [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J Biosc Bioe. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Lu, J.; Yoshizaki, G.; Satoh, S. Effect on the growth and body composition of juvenile tilapia (Oreochromis niloticus) fed raw Spirulina. Fish Sci. 2002, 68(1), 34–40. [Google Scholar] [CrossRef]
- Thuy, N.T.T. Patterns of use and exchange of genetic resources of the striped catfish, Pangasianodon hypophthalmus (Sauvage 1878). Rev Aquacult. 2009, 1, 224–231. [Google Scholar] [CrossRef]
- Trong, T.Q.; Hao, N.V.; Griffiths, D. Status of Pangasiid aquaculture in Vietnam. MRC Technical Paper No. 2, Mekong River Commission, Phnom Penh. 2002, 2, 16. [Google Scholar]
- Va´zquez-Ortiz, F.A.; Caire, G.; Huguere-Ciapara, I.; Herna´ndez, G. High-performance liquid chromatographic determination of free amino acid in shrimp. J Liq Chromatogr, 1995, 18, 2059–2068. [Google Scholar] [CrossRef]
- Vu NU, Pham TH, Huynh PV, Huynh TG. Importance of the freshwater rotifer Brachionus angularis for improved survival rate of early life-history stages of Pangasius catfish (Pangasianodon hypophthalmus). Aquacult Res. 2021, 52, 783–792. [CrossRef]
- Watanabe, T.; Vassallo-Agius, R. Broodstock nutrition research on marine finfish in Japan. Aquaculture. 2003, 227, 35–61. [Google Scholar] [CrossRef]
- White, R.L.; Ryan, R.A. Long-term cultivation of algae in open-raceway ponds: Lessons from the field. Industr Biotech. 2015, 11, 213–220. [Google Scholar] [CrossRef]
- Wilson, R.P.; Halver, J.E. Protein and amino acid requirements of fishes. Ann Rev Nutr. 1986, 6, 225–244. [Google Scholar] [CrossRef] [PubMed]
| Raw materials | Experimental diets | |||||
|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | |
| Fish meal (578 g/kg CP) | 75.0 | 75.0 | 75.0 | 75.0 | 75.0 | 75.0 |
| Poultry by-product meal (648 g/kg CP) | 184.1 | 184.1 | 184.1 | 184.1 | 184.1 | 184.1 |
| Wheat flour (158 g/kg CP) | 288.1 | 288.1 | 288.1 | 289.2 | 289.2 | 288.0 |
| Soybean meal (490 g/kg CP) | 360.0 | 360.0 | 360.0 | 360.0 | 360.0 | 360.0 |
| Soybean oil | 38.9 | 38.9 | 38.9 | 47.6 | 47.6 | 47.2 |
| Fish oil | 40.0 | 40.0 | 40.0 | 17.6 | 17.6 | 17.6 |
| Choline chloride | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Mineral premixa | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Vitamin premix (Rovimix 2020)b | 6.0 | − | − | − | − | − |
| Vitamin premix (L-OVN)c | − | 6.0 | − | 6.0 | − | − |
| Vitamin premix (H-OVN)d | − | − | 6.0 | − | 6.0 | 6.0 |
| Algal oile | − | − | − | 12.6 | 12.6 | 12.6 |
| Fungal oil | − | − | − | − | − | 1.5 |
| Actual chemical composition (g kg−1 DM) | ||||||
| Dry matter | 901.0 | 904.1 | 885.2 | 897.0 | 895.3 | 930.2 |
| Crude protein | 350.1 | 350.5 | 350.0 | 351.5 | 358.5 | 351.5 |
| Crude fat | 65.2 | 66.8 | 65.2 | 68.4 | 73.8 | 69.8 |
| Crude fibre | 28.9 | 34.4 | 48.8 | 46.4 | 40.9 | 44.5 |
| Ash | 103 | 110 | 109 | 110 | 110 | 118 |
| Feed Ingredients | ||||
|---|---|---|---|---|
| Soybean meal | Wheat flour | Fish meal | Poultry byproduct meal | |
| DM | 895 | 888 | 911 | 953 |
| CP | 490 | 158 | 578 | 648 |
| Lipid | 12.0 | 13.0 | 70.0 | 72.0 |
| Ash | 58.0 | 15.0 | 185 | 259 |
| Crude fibre | 26.0 | 4.0 | 4.0 | 26.0 |
| Essential amino acids | ||||
| Histidine | 13.5 | 3.0 | 8.2 | 6.9 |
| Isoleucine | 22.8 | 4.8 | 19.3 | 11.3 |
| Leucine | 38.3 | 9.0 | 37.4 | 23.5 |
| Lysine | 30.8 | 2.9 | 25.1 | 19.9 |
| Methionine | 2.4 | 1.6 | 8.3 | 4.9 |
| Phenylalanine | 24.8 | 5.9 | 0.2 | 13.0 |
| Valine | 24.0 | 6.2 | 27.1 | 17.2 |
| Threonine | 19.2 | 3.7 | 19.5 | 12.0 |
| Total | 175.8 | 37.1 | 145.1 | 108.7 |
| Essential amino acids | Experimental Diets | |||||
|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | |
| Histidine | 6.4 | 6.4 | 6.4 | 6.4 | 6.4 | 6.4 |
| Isoleucine | 12.2 | 12.2 | 12.2 | 12.2 | 12.2 | 12.2 |
| Leucine | 23.8 | 23.8 | 23.8 | 23.8 | 23.8 | 23.8 |
| Lysine | 17.2 | 17.2 | 17.2 | 17.3 | 17.3 | 17.3 |
| Methionine | 4.6 | 4.6 | 4.6 | 4.6 | 4.6 | 4.6 |
| Phenylalanine | 7.7 | 7.7 | 7.7 | 7.7 | 7.8 | 7.8 |
| Valine | 16.9 | 16.9 | 16.9 | 17.0 | 17.0 | 17.0 |
| Threonine | 12.1 | 12.1 | 12.1 | 12.1 | 12.1 | 12.1 |
| Total | 101.0 | 101.0 | 101.0 | 101.1 | 101.3 | 101.3 |
| Indices | Experimental Treatments | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | |||
| Growth performance indices of brooder fish | ||||||||
| Initial body weight (kg) | 4.4±0.7 | 5.0±0.9 | 5.2±0.9 | 4.9±0.8 | 5.0±0.1 | 4.7±0.1 | 0.841 | |
| Final body weight (kg) | 6.2±0.9b | 6.1±0.7b | 6.0±0.01b | 5.6±0.6c | 7.0±1.1a | 6.0±0.2 | 0.024 | |
| Weight gain (kg) | 1.8±0.2ab | 1.0±0.3b | 0.9±0.9bc | 0.6±0.3c | 2.0±0.9a | 1.3±0.2ab | 0.027 | |
| Daily weight gain (kg) | 0.04±0.01ab | 0.02±0.01b | 0.02±0.02bc | 0.02±0.01c | 0.10±0.02a | 0.02±0.01ab | 0.027 | |
| Specific growth rate (SGR%) | 0.5±0.4ab | 0.3±0.2b | 0.3±0.2bc | 0.2±0.1c | 0.6±0.2a | 0.4±0.01ab | 0.027 | |
| Food conversion ratio (FCR) | 1.8±0.3 | 1.8±0.4 | 1.7±0.9 | 1.8±0.2 | 1.6±0.1 | 1.7±0.3 | 0.100 | |
| Reproductive performance indices | ||||||||
| Egg size (µm) | Before injecting hCG | 1.0±0.1 | 0.9±0.01 | 0.9±0.1 | 1.0±0.01 | 1.0±0.1 | 0.9±0.1 | 0.07 |
| After injecting hCG | 1.1±0.03ab | 1.1±0.1b | 1.0±0.3c | 1.0±0.5bc | 1.1±0.3a | 1.0±0.1ab | 0.001 | |
| Gonad weight (g/fish) | 750.1±54.8a | 366.7±100.1b | 400.0±109.5ab | 466.7±264.6ab | 754.02±248.1a | 500.1±100.6ab | 0.004 | |
| Gonad somatic index (GSI%) | 12.2±0.98a | 6.0±1.7b | 6.7 ± 1.8ab | 8.4±4.8ab | 12.8±0.8a | 8.4±0.1ab | 0.027 | |
| Relative fecundity index (egg/kg) | 152,158±7,467a | 90,014±2,467b | 104,267±7,381ab | 133,642±1,503a | 199,512±7,467a | 119,748±1,166ab | 0.022 | |
| Total number of eggs in female ovary (egg) | 1,227,000±107.4a | 546,383±303.7b | 625,600±452.3ab | 744,387±119.9 ab | 1,057,500±232.1a | 712,500±106.9ab | 0.047 | |
| Indices | Experimental Treatments | |||||
|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | T5 | T6 | |
| Body indices of fingerling reared at 15 days (n = 15,000 larvae/tank) | ||||||
| BWG (mg) | 12.1±0.1 c | 11.11±0.01d | 15.6±0.1a | 14.4±0.2b | 14.3±0.2b | 12.9±0.2c |
| Length (mm) | 14.9±0.4bc | 14.82 ± 0.4c | 15.4±0.5a | 15.2±0.4ab | 15.1±0.4bc | 15.0±0.4bc |
| DWG (mg) | 0.6±0.01d | 0.60±0.01e | 0.8±0.01a | 0.8±0.01b | 0.8±0.01b | 0.6±0.01cd |
| SGR% | 8.5±0.6c | 10.05±0.7a | 8.2±0.4c | 9.2±0.9b | 9.1±0.6b | 8.6±1.0c |
| Body indices of fingerling reared at 30 days (n = 2,000 fry/tank) | ||||||
| BWG (mg) | 63.0±35.2b | 147.0±24.5a | 41.0±9.4d | 23.0±15.2e | 47.0±11.3c | 39.0±18.7d |
| Length (mm) | 25.0±2.2a | 22.0±2.0ab | 18.3±0.5b | 23.6±0.6a | 18.0±0.4b | 24.0±1.4a |
| DWG (mg/d) | 4.9±0.3b | 11.3±1.5a | 3.2±0.8d | 1.7±0.9e | 3.6±1.5c | 3.0±19d |
| SGR (%) | 6.9±1.2c | 10.6±2.2a | 4.4±1.6e | 2.6±1.7f | 6.4±3.0d | 4.7±2.1e |
| Body indices of fingerling reared at 45 days (n = 2,000 fry/tank) | ||||||
| BWG (mg) | 947.0±64.0a | 313.0±58.7d | 708.0±53.2b | 431.0±66.4c | 304.0±51.2d | 499.0±98.8c |
| Length (mm) | 60.1±1.7a | 41.5±1.2b | 41.5±0.9b | 56.5±1.0a | 37.0±0.7b | 57.5±1.3a |
| DWG (mg/d) | 79.0±1.5a | 26.0±2.3d | 59.0±3.3b | 36.0±2.9c | 25.0±3.2d | 42.0±5.4c |
| SGR (%) | 6.8±0.7b | 2.7±0.6d | 9.0±1.5a | 4.0±2.2c | 4.12±2.2c | 4.0±1.8c |
| Total number of survival larvae (fish) | 466.7±12.7b | 439.3±94.7b | 1326.3±560.5a | 413.3±242.7b | 781.3±173.2b | 603.7±251.7b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





