Submitted:
15 June 2024
Posted:
17 June 2024
You are already at the latest version
Abstract
Keywords:
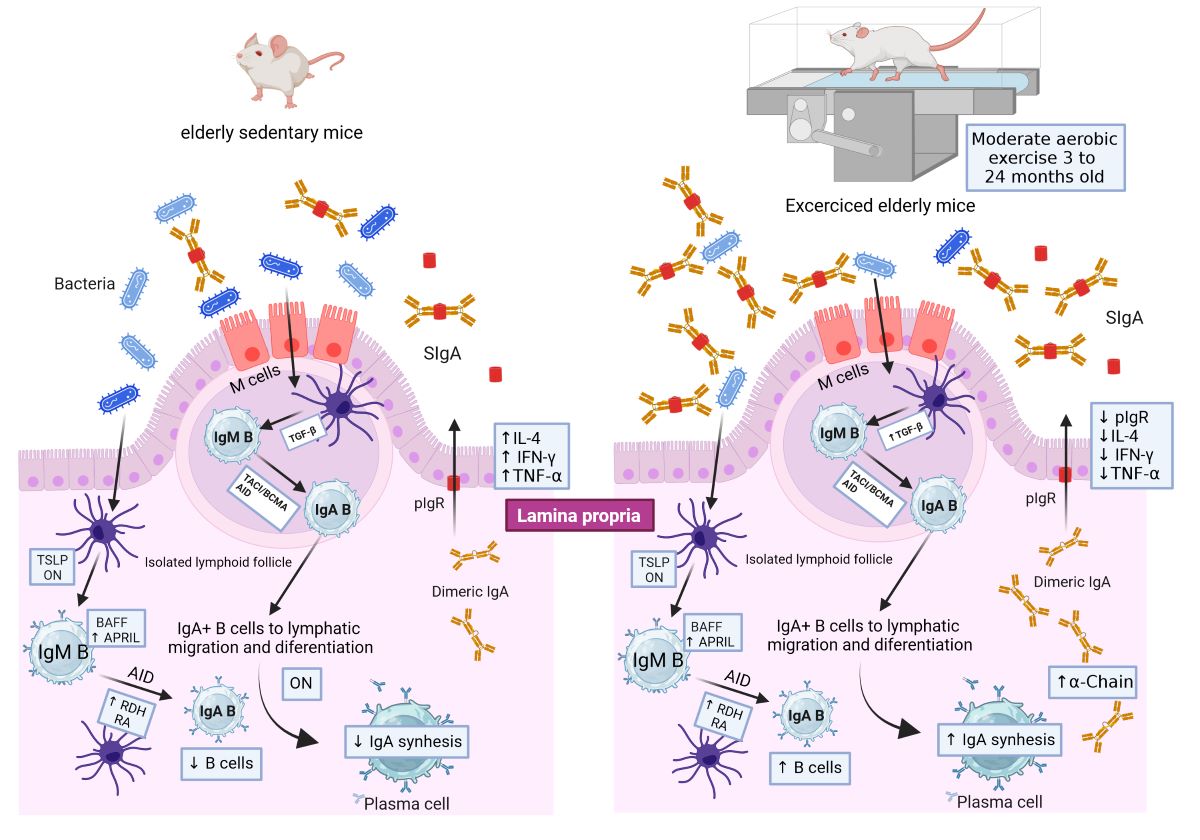
1. Introduction
2. Results
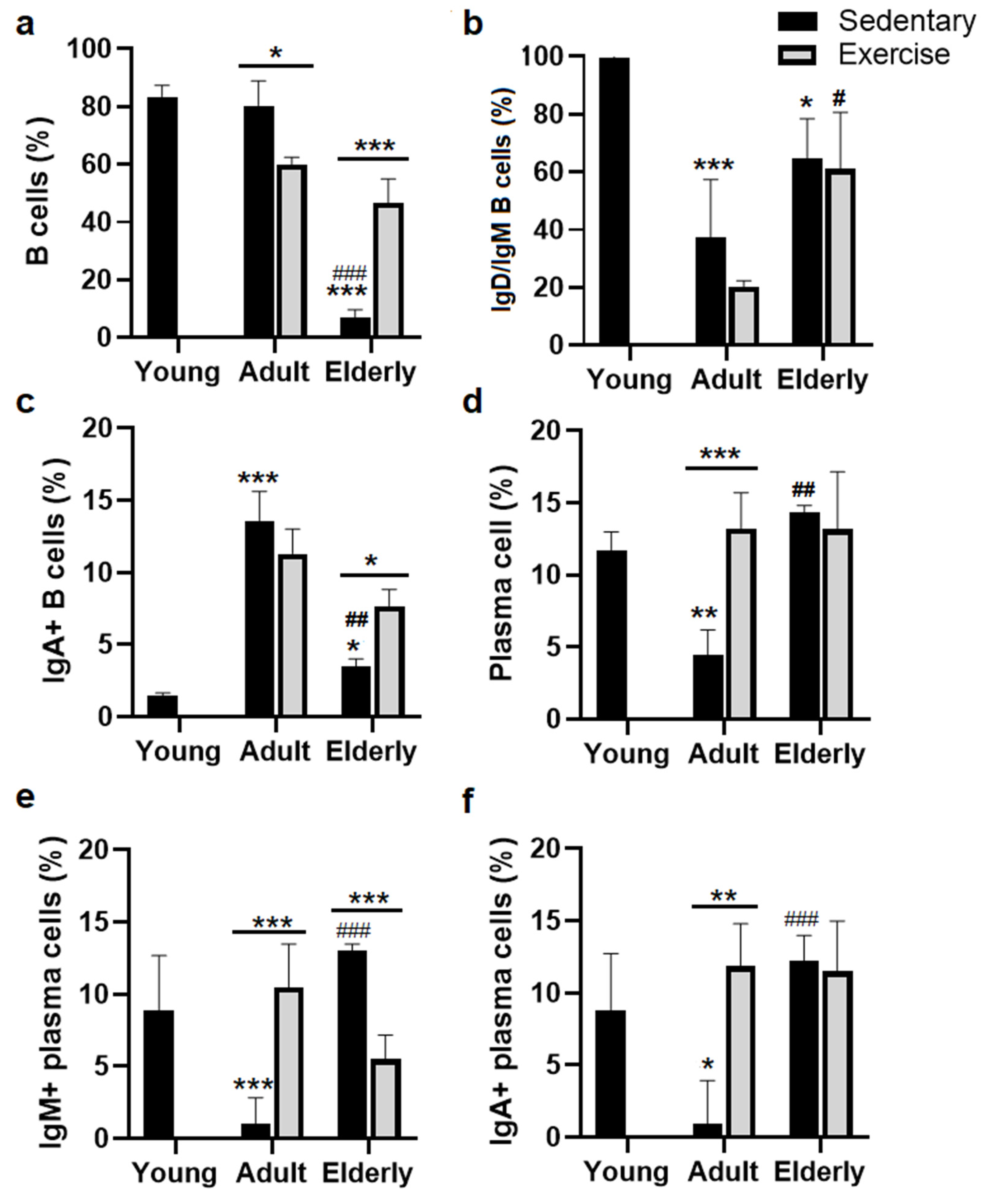
2.1. Exercise Increased total B Cells in Senile Mice.
2.2. Exercise Increased the IgA+ B Cells in Senile Mice
2.3. Exercise Increased the α-Chain and APRIL mRNA in Adult and Senile Mice
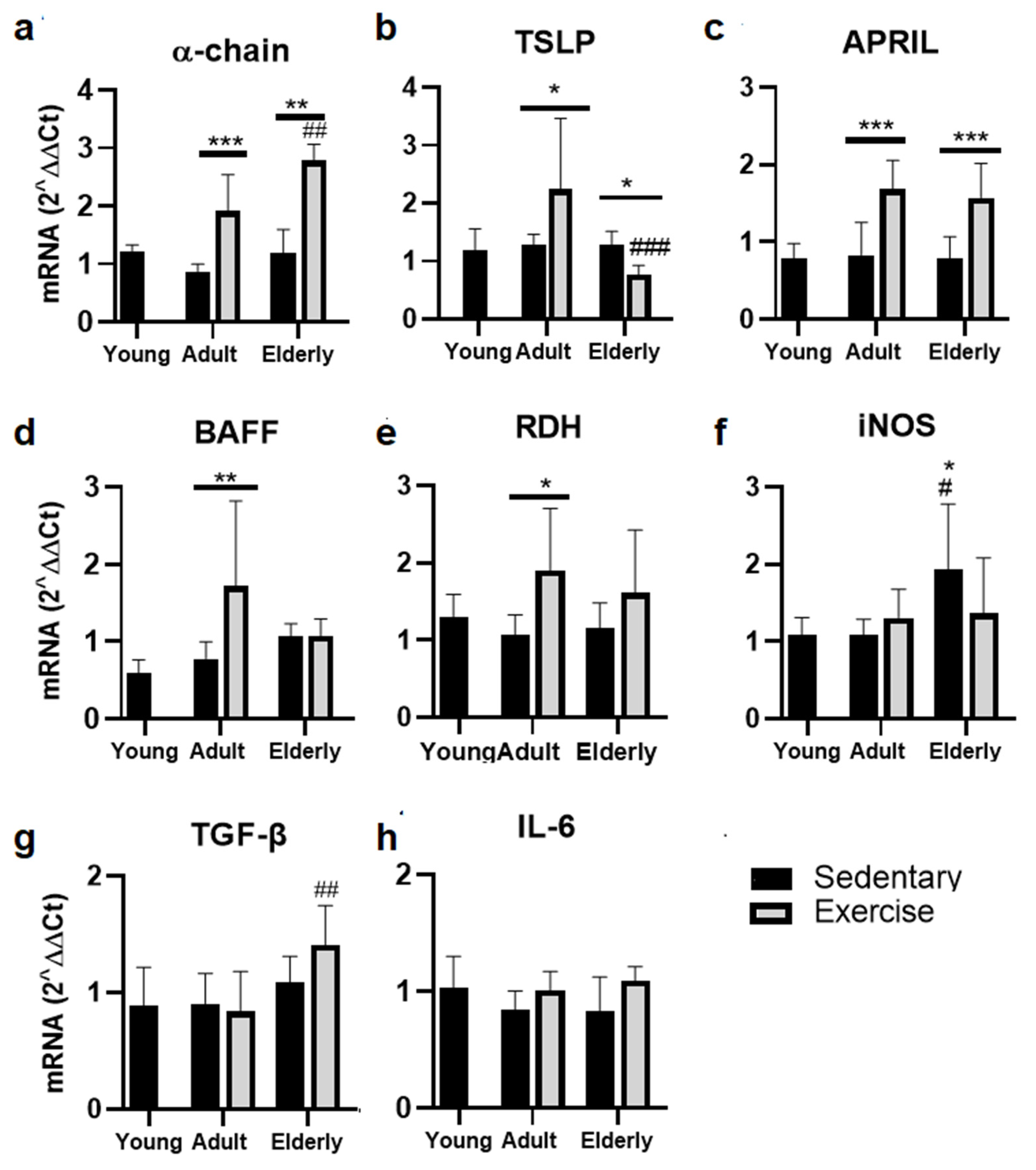
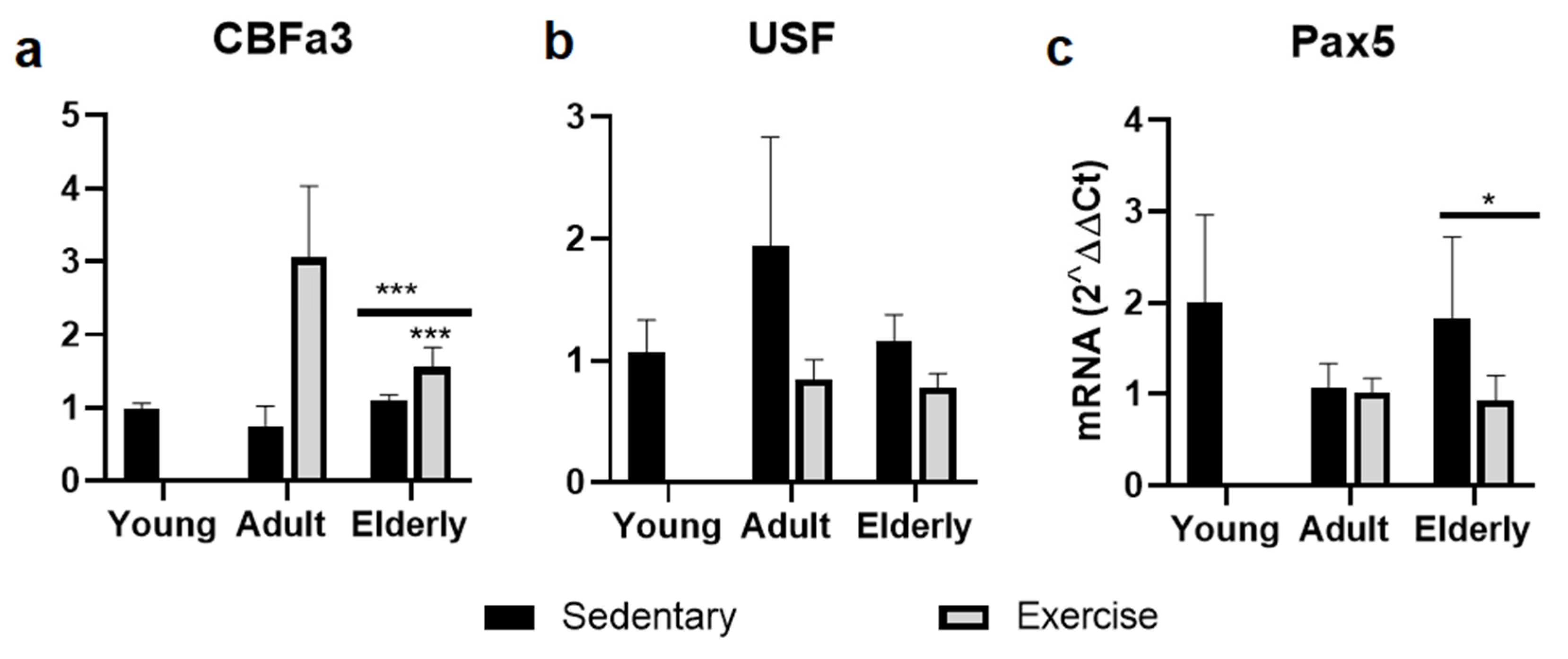
2.4. Factors Related to B cells Maduration and Diferentiation
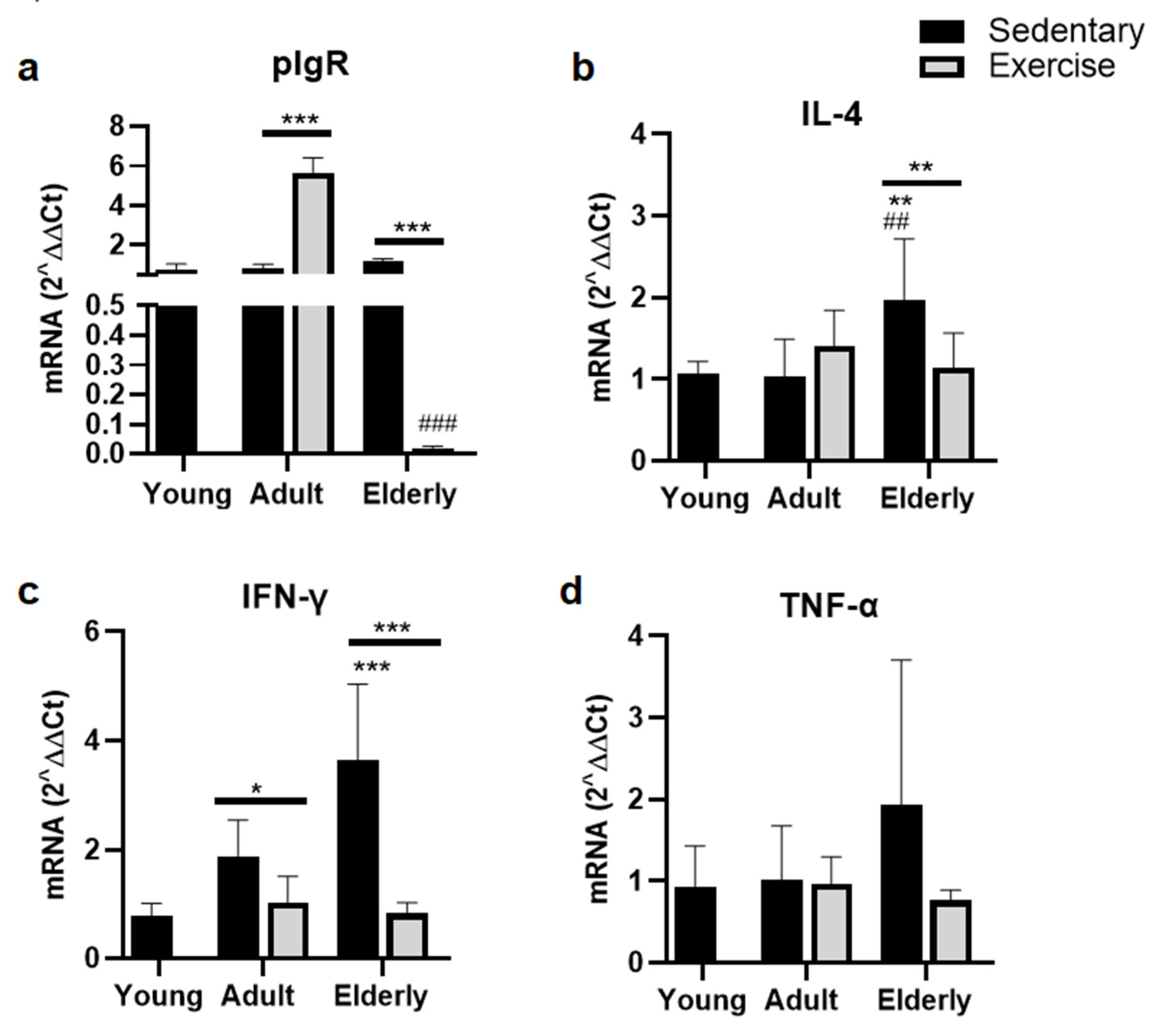
2.5. Exercise Suppressed Parameters of pIgR for IgA-Transcytosis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Moderate Aerobic Exercise Protocol
4.4. Sampling
4.5. Lymphoid Sub-Populations in Lamina Propria by Cytometry
4.6. Relative Expression of mRNA Assay
4.7. Statistical Analysis
5. Conclusions
Authors contribution
Funding Statement
Data Availability
Acknowledgments
Conflicts of Interest
References
- J. M. Woof & M. A. Kerr, The function of immunoglobulin A in immunity. The Journal of Pathology, 208 (2006) 270–282. [CrossRef]
- H. Tezuka & T. Ohteki, Regulation of IgA Production by Intestinal Dendritic Cells and Related Cells. Frontiers in Immunology, 10 (2019) 1891. [CrossRef]
- J. J. Bunker, S. A. Erickson, T. M. Flynn, C. Henry, J. C. Koval, M. Meisel, B. Jabri, D. A. Antonopoulos, P. C. Wilson, & A. Bendelac, Natural polyreactive IgA antibodies coat the intestinal microbiota. Science (New York, N.Y.), 358 (2017) eaan6619. [CrossRef]
- E. D. León & M. P. Francino, Roles of Secretory Immunoglobulin A in Host-Microbiota Interactions in the Gut Ecosystem. Frontiers in Microbiology, 13 (2022) 880484. [CrossRef]
- C. Lindner, B. Wahl, L. Föhse, S. Suerbaum, A. J. Macpherson, I. Prinz, & O. Pabst, Age, microbiota, and T cells shape diverse individual IgA repertoires in the intestine. The Journal of Experimental Medicine, 209 (2012) 365–377. [CrossRef]
- F. Barone, P. Patel, J. D. Sanderson, & J. Spencer, Gut-associated lymphoid tissue contains the molecular machinery to support T-cell-dependent and T-cell-independent class switch recombination. Mucosal Immunology, 2 (2009) 495–503. [CrossRef]
- S. Uematsu, K. Fujimoto, M. H. Jang, B.-G. Yang, Y.-J. Jung, M. Nishiyama, S. Sato, T. Tsujimura, M. Yamamoto, Y. Yokota, H. Kiyono, M. Miyasaka, K. J. Ishii, & S. Akira, Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nature Immunology, 9 (2008) 769–776. [CrossRef]
- Y.-J. Liu, TSLP in epithelial cell and dendritic cell cross talk. Advances in Immunology, 101 (2009) 1–25. [CrossRef]
- A. Delogu, A. Schebesta, Q. Sun, K. Aschenbrenner, T. Perlot, & M. Busslinger, Gene repression by Pax5 in B cells is essential for blood cell homeostasis and is reversed in plasma cells. Immunity, 24 (2006) 269–281. [CrossRef]
- Pabst & E. Slack, IgA and the intestinal microbiota: the importance of being specific. Mucosal Immunology, 13 (2020) 12–21. [CrossRef]
- S. Yuan, J. Yang, Y. Jian, Y. Lei, S. Yao, Z. Hu, X. Liu, C. Tang, & W. Liu, Treadmill Exercise Modulates Intestinal Microbes and Suppresses LPS Displacement to Alleviate Neuroinflammation in the Brains of APP/PS1 Mice. Nutrients, 14 (2022) 4134. [CrossRef]
- H. Sugahara, S. Okai, T. Odamaki, C. B. Wong, K. Kato, E. Mitsuyama, J.-Z. Xiao, & R. Shinkura, Decreased Taxon-Specific IgA Response in Relation to the Changes of Gut Microbiota Composition in the Elderly. Frontiers in Microbiology, 8 (2017) 1757. [CrossRef]
- B. van der Lugt, A. A. van Beek, S. Aalvink, B. Meijer, B. Sovran, W. P. Vermeij, R. M. C. Brandt, W. M. de Vos, H. F. J. Savelkoul, W. T. Steegenga, & C. Belzer, Akkermansia muciniphila ameliorates the age-related decline in colonic mucus thickness and attenuates immune activation in accelerated aging Ercc1 -/Δ7 mice. Immunity & Ageing: I & A, 16 (2019) 6. [CrossRef]
- W. Yang, Y. Liu, G. Yang, B. Meng, Z. Yi, G. Yang, M. Chen, P. Hou, H. Wang, & X. Xu, Moderate-Intensity Physical Exercise Affects the Exercise Performance and Gut Microbiota of Mice. Frontiers in Cellular and Infection Microbiology, 11 (2021) 712381. [CrossRef]
- A. Aiello, F. Farzaneh, G. Candore, C. Caruso, S. Davinelli, C. M. Gambino, M. E. Ligotti, N. Zareian, & G. Accardi, Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Frontiers in Immunology, 10 (2019) 2247. [CrossRef]
- M. Sellami, N. L. Bragazzi, B. Aboghaba, & M. A. Elrayess, The Impact of Acute and Chronic Exercise on Immunoglobulins and Cytokines in Elderly: Insights From a Critical Review of the Literature. Frontiers in Immunology, 12 (2021) 631873. [CrossRef]
- A. Jafarzadeh, M. Sadeghi, G. A. Karam, & R. Vazirinejad, Salivary IgA and IgE levels in healthy subjects: relation to age and gender. Brazilian Oral Research, 24 (2010) 21–27. [CrossRef]
- D. A. Sullivan, J. P. Vaerman, & C. Soo, Influence of severe protein malnutrition on rat lacrimal, salivary and gastrointestinal immune expression during development, adulthood and ageing. Immunology, 78 (1993) 308–317.
- S. Senda, E. Cheng, & H. Kawanishi, Aging-associated changes in murine intestinal immunoglobulin A and M secretions. Scandinavian Journal of Immunology, 27 (1988) 157–164. [CrossRef]
- D. L. Schmucker, C. K. Daniels, R. K. Wang, & K. Smith, Mucosal immune response to cholera toxin in ageing rats. I. Antibody and antibody-containing cell response. Immunology, 64 (1988) 691–695.
- K. Thoreux, R. L. Owen, & D. L. Schmucker, Intestinal lymphocyte number, migration and antibody secretion in young and old rats. Immunology, 101 (2000) 161–167. [CrossRef]
- T. Yanagihara, Y. Kumagai, Y. Norose, I. Moro, M. Nanno, M. Murakami, & H. Takahashi, Age-dependent decrease of polymeric Ig receptor expression and IgA elevation in ddY mice: a possible cause of IgA nephropathy. Laboratory Investigation; a Journal of Technical Methods and Pathology, 84 (2004) 63–70. [CrossRef]
- D. Wilson, T. Jackson, E. Sapey, & J. M. Lord, Frailty and sarcopenia: The potential role of an aged immune system. Ageing Research Reviews, 36 (2017) 1–10. [CrossRef]
- A. L. de Araújo, L. C. R. Silva, J. R. Fernandes, & G. Benard, Preventing or reversing immunosenescence: can exercise be an immunotherapy? Immunotherapy, 5 (2013) 879–893. [CrossRef]
- R. J. Simpson, T. W. Lowder, G. Spielmann, A. B. Bigley, E. C. LaVoy, & H. Kunz, Exercise and the aging immune system. Ageing Research Reviews, 11 (2012) 404–420. [CrossRef]
- M.-E. Drago-Serrano, M. Godínez-Victoria, E. Lara-Padilla, A. A. Resendiz-Albor, H. Reyna-Garfias, I. M. Arciniega-Martínez, A. Kormanovski-Kovsova, & R. Campos-Rodriguez, Moderate exercise enhances expression of SIgA in mouse ileum. International Journal of Sports Medicine, 33 (2012) 1020–1025. [CrossRef]
- M. Viloria, E. Lara-Padilla, R. Campos-Rodríguez, A. Jarillo-Luna, H. Reyna-Garfias, P. López-Sánchez, V. Rivera-Aguilar, A. Salas-Casas, F. J. Berral de la Rosa, & E. García-Latorre, Effect of moderate exercise on IgA levels and lymphocyte count in mouse intestine. Immunological Investigations, 40 (2011) 640–656. [CrossRef]
- M. Godínez-Victoria, M. E. Drago-Serrano, H. Reyna-Garfias, M. Viloria, E. Lara-Padilla, A. A. Resendiz-Albor, L. E. Sánchez-Torres, T. R. Cruz-Hernández, & R. Campos-Rodriguez, Effects on secretory IgA levels in small intestine of mice that underwent moderate exercise training followed by a bout of strenuous swimming exercise. Brain, Behavior, and Immunity, 26 (2012) 1300–1309. [CrossRef]
- J. A. Sierra-Ramírez, L. Saucedo-Bueno, A. L. García-Hernández, A. Martínez-Dávalos, C. Rodríguez-López, M. E. Drago-Serrano, & M. Godínez-Victoria, Moderate aerobic exercise on bone quality changes associated with aging and oxidative stress in BALB/c mice. Journal of Biomechanics, 135 (2022) 111035. [CrossRef]
- M. D. Cook, J. M. Allen, B. D. Pence, M. A. Wallig, H. R. Gaskins, B. A. White, & J. A. Woods, Exercise and gut immune function: evidence of alterations in colon immune cell homeostasis and microbiome characteristics with exercise training. Immunology and Cell Biology, 94 (2016) 158–163. [CrossRef]
- R. Divella, G. DE Palma, A. Tufaro, G. Pelagio, G. Gadaleta-Caldarola, R. Bringiotti, & A. Paradiso, Diet, Probiotics and Physical Activity: The Right Allies for a Healthy Microbiota. Anticancer Research, 41 (2021) 2759–2772. [CrossRef]
- K. G. McDonald, M. R. Leach, C. Huang, C. Wang, & R. D. Newberry, Aging impacts isolated lymphoid follicle development and function. Immunity & Ageing: I & A, 8 (2011) 1. [CrossRef]
- A. J. Hernández-Urbán, M. E. Drago-Serrano, A. Cruz-Baquero, A. L. García-Hernández, I. M. Arciniega-Martínez, J. Pacheco-Yépez, F. Guzmán-Mejía, & M. Godínez-Victoria, Exercise improves intestinal IgA production by T-dependent cell pathway in adults but not in aged mice. Frontiers in Endocrinology, 14 (2023) 1190547. [CrossRef]
- M. Godínez-Victoria, R. Campos-Rodriguez, V. Rivera-Aguilar, E. Lara-Padilla, J. Pacheco-Yepez, R. A. Jarillo-Luna, & M. E. Drago-Serrano, Intermittent fasting promotes bacterial clearance and intestinal IgA production in Salmonella typhimurium-infected mice. Scandinavian Journal of Immunology, 79 (2014) 315–324. [CrossRef]
- D. Frasca & B. B. Blomberg, Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology, 17 (2016) 7–19. [CrossRef]
- B. Zhao, Z. Xia, B. Yang, Y. Guo, R. Zhou, M. Gu, M. Liu, Q. Li, W. Bai, J. Huang, X. Zhang, C. Zhu, K. T. Leung, C. Chen, & J. Dong, USP7 promotes IgA class switching through stabilizing RUNX3 for germline transcription activation. Cell Reports, 43 (2024) 114194. [CrossRef]
- J. L. Gommerman, O. L. Rojas, & J. H. Fritz, Re-thinking the functions of IgA(+) plasma cells. Gut Microbes, 5 (2014) 652–662. [CrossRef]
- F.-E. Johansen & P. Brandtzaeg, Transcriptional regulation of the mucosal IgA system. Trends in Immunology, 25 (2004) 150–157. [CrossRef]
- C. K. Daniels, D. L. Schmucker, H. Bazin, & A. L. Jones, Immunoglobulin A receptor of rat small intestinal enterocytes is unaffected by aging. Gastroenterology, 94 (1988) 1432–1440. [CrossRef]
- T. Shibuya, T. Kaburagi, R. Nagai, & S. Oshiro, The effects of moderate exercise on secretory IgA production in mice depends on dietary carbohydrate intake. Journal of Clinical Biochemistry and Nutrition, 57 (2015) 44–49. [CrossRef]
- M. E. C. Bruno, R. B. West, T. A. Schneeman, E. H. Bresnick, & C. S. Kaetzel, Upstream stimulatory factor but not c-Myc enhances transcription of the human polymeric immunoglobulin receptor gene. Molecular Immunology, 40 (2004) 695–708. [CrossRef]
- H. Kawanishi & J. Kiely, Immune-related alterations in aged gut-associated lymphoid tissues in mice. Digestive Diseases and Sciences, 34 (1989) 175–184. [CrossRef]
- A. E. Gaffey, C. S. Bergeman, L. A. Clark, & M. M. Wirth, Aging and the HPA axis: Stress and resilience in older adults. Neuroscience and Biobehavioral Reviews, 68 (2016) 928–945. [CrossRef]
- A. Reséndiz-Albor, H. Reina-Garfias, S. Rojas-Hernández, A. Jarillo-Luna, V. Rivera-Aguilar, A. Miliar-García, & R. Campos-Rodríguez, Regionalization of pIgR expression in the mucosa of mouse small intestine. Immunology Letters, 128 (2010) 59–67. [CrossRef]
- K. J. Livak & T. D. Schmittgen, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif.), 25 (2001) 402–408. [CrossRef]
| Gen | Sequence 5´→3´ | ID* | |
|---|---|---|---|
| Forward | Reverse | ||
| TSLP | cag ctt gtc tcc tga aaa tcg | aaa tgt ttt gtc ggg gag tg | NM_021367.2 |
| BAFF | atg cgg aag gca gat tga | tgc atc ttt tgc tac cct ga | NM_001347309.1 |
| APRIL | agc tgg gca ctg agc ttt ac | aag ttg gcc tcg aac tca tc | NM_023517.2 |
| iNOS | ctt ttc cta tgg ggc aaa aa | ctg gaa ctc tgg gct gtc a | NM_010927.4 |
| RDH11 | tgt act tgg tca cgc caa aa | ccg gga agc tga aca tta ga | NM_021557.5 |
| TGF-β1 | tgg agc aac atg tgg aac tc | gtc agc agc cgg tta cca | NM_011577.2 |
| IL-6 | gct acc aaa ctg gat ata atc agg a | cca ggt agc tat ggt act cca gaa | NM_031168.2 |
| CBFa3 | gct ctc tca gca cca cga g | tca ggt ctg agg agc ctt g | NM_019732.2 |
| USF-1 | tca aga ggt ggg aaa gga tg | cat tgg gcc ccc ttc tac | NM_001305676.1 |
| Pax5 | cct ggg agt gaa ttt tct gg | tgg gga acc tcc aag aat c | NM_008782.2 |
| GAPDH | aag agg gat gct gcc ctt ac | cca ttt tgt cta cgg gac ga | NM_001289726.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





